Abstract
Carbon is one of the most abundant minerals in the universe. The world’s energy needs are being unmet due to the exponential rise in population. Since its inception 20 years ago, carbon and its allotropes, including fullerenes, carbon nanotubes, and graphene, have been marketed as potential energy storage and generation materials. By solving important issues like accumulation and inadequate thermodynamic compatibility, carbon fiber, expanded graphite, and carbon nanotubes are promising functional materials that can be used to improve the performance of bipolar plates further. There are several potential uses for carbon-based nanomaterials (CBNMs) in the energy area. This mini-review provides an overview of the synthetic routes employed for producing CBNMs, categorizing them based on their types, elucidating their diverse applications in fuel energy systems, and emphasising the uses of CBNMs in energy. The advantages and disadvantages of several synthetic processes have been examined and compared. The types of CBNMs, like carbon nanotubes, graphene, carbon dots, and fullerenes, are explored in terms of their unique structural properties and fabrication methods. Furthermore, the utilization of CBNMs in fuel energy systems, such as fuel cells, energy storage devices, and catalysis, is comprehensively reviewed.
Highlights
Carbon-based nanomaterials show promise in fuel energy applications, with a focus on fuel cells, energy storage devices, and catalysis.
The mini-review provides a comprehensive overview of synthetic routes for carbon-based nanomaterial production.
The study explores diverse carbon-based nanomaterials, including carbon nanotubes, graphene, carbon dots, and fullerenes.
A critical analysis of synthetic processes, like arc discharge, and chemical vapor deposition, reveals their respective advantages and disadvantages.
1 Introduction
Carbon stands out as a fundamental and essential element in the fabric of our universe, occupying a unique place in its composition. It is the 17th most abundant element on Earth’s surface, the fourth most abundant element in our solar system, and the sixth most abundant element in the universe overall. 1 Studies estimate its relative abundance ranges between 180 and 270 parts per million. Notably, carbon holds the distinction of being the second most prevalent element in the human body, trailing only oxygen and constituting approximately 18 % of an individual’s body weight. 2 One of the remarkable characteristics of carbon is its capacity to manifest a diverse array of metastable phases, many of which can emerge under ambient conditions while maintaining substantial kinetic stability. 3 Despite its scarcity in the Earth’s crust, comprising only 0.2 % of the planet’s total mass. 4 Elemental carbon is pivotal due to its unique capacity to form bonds with light elements. This catenation ability of carbon has been instrumental in expanding the realms of chemistry and biology, ultimately facilitating the emergence of life’s wonders. With the world’s rising energy demand, developing more efficient and environmentally friendly methods of producing and storing energy is crucial. It has also become critical to meet our energy needs with reliable, cost-effective technology. Making dependable, efficient, scalable, and inexpensive catalysts is essential for this goal. Evidence suggests that carbon-based materials can significantly enhance the efficiency and dependability of energy conversion and storage technologies. 5 When it comes to energy storage, carbon compounds play an essential function as conductive agents, electrode materials, and so on. Carbon nanomaterials, including fullerene, carbon nanotubes, and graphene, have unique morphologies and structures and exhibit probable electrical, chemical, and physical properties in comparison to more conventional carbon materials like graphite and carbon black. 6 , 7
There is a wide range of chemistry and structure among carbon-based nanomaterials. Elements that makeup lithium alloys include carbon and a wide variety of compounds, including oxides, chalcogenides, carbides, and more. Quantum dots, nanowires, nanotubes, nanobelts, nanoflakes, and nanosheets are all part of this. Technologies like structural energy storage technology and wearable energy storage are made possible by combining these chemically diverse nanoscales building blocks with lithium ions and other substances that are not achievable with conventional materials. The development of wearable, flexible, and foldable energy storage devices is made possible by carbon-based nanomaterials, which have a high surface-to-volume ratio and are compatible with sophisticated production techniques, including printing, spray coating, and roll-to-roll assembly. 8 , 9 , 10
Thus, carbon science is currently at the forefront of research, particularly in nanoscience, materials science, engineering, and technology. Within these disciplines, carbon nanostructures, since the unveiling of diverse carbon-based nanostructures, such as fullerenes in 1985, carbon nanotubes in 1991, and graphene in 2004, there has been a surge of interest in carbon-based nanomaterials within the realm of nanotechnology, 11 encompass a diverse array of low-dimensional allotropes, including graphite, activated carbon, carbon nanotubes, carbon nanofibers, the C60 family of Buckyballs, polyromantic molecules, and graphene. 12 In recent years, there has been a significant surge in research focusing on carbon-based nanomaterials, including carbon nanotubes, graphene and its derivatives, fullerenes, and other carbon allotropes at the nanoscale. This exponential growth is attributed to the vast potential for modifying and customizing carbon nanomaterials. Their small size, which approaches that of fundamental coupled with their substantial specific surface area, high electrical and thermal conductivity, distinctive optical properties, and outstanding mechanical properties, open up diverse applications. 13 Graphene, a recently discovered carbon allotrope, features a single layer of atoms arranged in a 2D honeycomb lattice, offering exceptional strength. It serves as a critical precursor for synthesizing other carbon nanoparticles. Through oxidation, graphene transforms into graphene oxide, leading to derivatives like reduced graphene oxide (rGO) and graphene quantum dots (GQDs), which find diverse applications in various operations. 14 These categories include zero-dimensional nanostructures, carbon dots (CDs), one-dimensional nanomaterials such as carbon nanotubes (CNTs) and carbon nanofibers (CNFs), and two-dimensional nanomaterials like graphene. In the contemporary era, nanotechnology has garnered significant attention for its ability to produce novel materials with exceptional properties. Nanostructures offer advantages such as superior directionality, high surface area, and flexibility, rendering them suitable for a broad spectrum of applications. 15 , 16 Consequently, researchers from various scientific fields are deeply intrigued by these materials, recognizing their pivotal role in driving advancements across numerous technologies. This versatility opens up new avenues in chemistry for nanomaterials design, facilitating the development of functional nanoparticle arrays for catalytic applications 17 , 18 , 19 to enhance the effective harnessing of renewable energy sources like solar, wind, waves, and biomass, a range of energy conversion and storage technologies have been developed, which include supercapacitors, fuel cells, compressed hydrogen storage, and batteries. 20
The exploration of clean energy technologies remains a significant area of investigation within carbon nanomaterials. One promising avenue is hydrogen fuel, which offers an eco-friendly alternative to traditional fossil fuels. 21 , 22 However, challenges persist, particularly the susceptibility of metal catalysts to impurities during hydrogen production processes. Therefore, the effective separation of hydrogen from other gases like carbon monoxide, carbon dioxide, methane, nitrogen, and water vapor becomes imperative. 23 , 24 In addressing this challenge, Muraru and Ionita introduced a novel approach employing a rotating partially double-walled carbon nanotube membrane for hydrogen separation. They evaluated the membrane’s performance under varying angular velocities through molecular dynamics simulations. Their findings underscored the significant influence of angular velocity on hydrogen separation efficiency via the rotating nanotube, highlighting the potential of this innovative technique. 25 This summary will go into further detail regarding fuel energy’s current and future state related to carbon-based nanomaterials. 26 The imperative to develop innovative energy sources becomes evident against accelerating global energy consumption. This surge is fueled by rapid economic expansion, population growth, and increasing reliance on energy-dependent appliances worldwide. 27 According to predictions, the global energy supply will have to be doubled by the year 2050. 28 Considering this, research into modern energy storage and conversion technologies, like batteries and supercapacitors, is proceeding rapidly. In response to the ever-increasing demand, these technologies are the subject of substantial research on a global scale. Emerging as a critical tool in materials science and engineering, nanotechnology provides new ways to address this problem. 29 , 30 Carbon nanoparticles have shown promise as technology enablers for next-generation energy storage and conversion systems. The purpose of this article is to focus on the use of carbon-based nanomaterials to overcome energy shortages through energy storage devices such as batteries and supercapacitors. 31
2 Synthetic methods for carbon-based nanomaterials
The synthesis portion will discuss the different synthesis techniques for preparing carbon-based nanomaterials. The term “constructive technique” refers to the bottom-up approach, which contrasts the top-down method. The bottom-up method produces nanomaterials (NMs) with precisely controlled dimensions, chemical structure, and shape, enabling atoms and molecules to self-assemble and grow. Figure 1 shows some synthetic techniques to synthesize CNMs. 32

Shows some crucial methods used for the synthesis of CNMs.
2.1 Solvothermal/hydrothermal synthesis
Various methods are employed to synthesize carbon dots (CDs), including hydrothermal/solvothermal, microwave, electrochemical, micro-plasma, and chemical oxidation techniques. In hydrothermal synthesis, water is the primary solvent, whereas the solvothermal technique employs solvents other than water. 33 , 34 These methods apply to producing graphene and its composites by reducing graphene oxide. Additionally, nanofibers, carbon nanotubes 35 and their composites can be synthesized using hydrothermal/solvothermal technology, wherein a carbon source and catalyst are utilized to regulate morphology and properties. 36 , 37 CDs synthesized through different methods exhibit varying properties. 38 Both “top-down” and “bottom-up” approaches are common ways to describe these methods. Electrochemical synthesis, chemical oxidation, and solvent heat treatment are physical or chemical procedures used in the “top-down” approach to segment massive carbonaceous materials into CDs. Based on their structures, properties, and synthesis methods, CDs are classified into three types: CQDs, GQDs, and carbonised polymer dots (CPDs). 39 To customize the production of targeted carbon dots (CDs), optimization of synthesis parameters such as voltage, time, and temperature is imperative. Following synthesis, subsequent purification steps are typically employed to obtain separate, purified, and uniform-sized CDs. These purification techniques range from straightforward methods like centrifugation, dialysis, and filtration to more intricate processes such as electrophoresis, silica gel column chromatography, and high-performance liquid chromatography. 40 , 41 , 42 Among the various synthesis methods, hydrothermal/solvothermal synthesis stands out as the most prevalent approach for CD production. 43 , 44 Schematic synthesis of nanomaterials through solvothermal/hydrothermal is shown below in Figure 2.

Schematic illustration of hydrothermal/solvothermal technique. Reproduced from source. 32
This technology has several benefits, such as being easy to use, producing carbon dots (CDs) consistently at a high rate, and being flexible enough to incorporate additional components and modify CD variety. It is also simple and convenient to use. Su et al. conducted a study where multifunctional CDs were synthesized through one-step hydrothermal synthesis of nanotubes. 45 , 46 The multi-functional CDs showed a wide range of functions, including temperature sensing (which increases fluorescence over a linear range of 25–95 °C), specific fluorescence quenching for Ni(II) detection in the 80–6,000 mM range, and a visually discernible fluorescence transition from blue to green for doxycycline detection in the 10–1,000 mM range, all achieved through three independent pathways. 45
2.2 Electrochemical carbonization synthesis of carbon quantum dots
However, there is a lack of scientific research on electrochemical carbonisation to create CQDs. According to Zhang and colleagues, 47 , 48 CQDs were produced by electrochemical carbonisation of low molecular weight alcohols. A reference electrode was a calomel electrode embedded in a freely adjustable Luggin capillary, and two platinum plates were used as working and auxiliary electrodes. Utilizing electrochemical carbonisation, alcohols were transformed into CQDs, and their size and graphitization improved in proportion to the applied voltage. 48 CDs can remove pollutants with impressive efficiency with their substantial specific surface area, availability of functional groups, and remarkable stability. This quality has been recognised in the area of environmental pollution control. 40 , 49
2.3 Chemical vapor deposition and electrospinning process
In the 1960s, carbon nanofibers (CNFs) became essential in the scientific and technological industries. Their production involved carbon precursors in melt spinning processes, most notably polyacrylonitrile (PAN), modified with additives. They underwent low-temperature oxidation stabilization and were stretched during the stabilization and carbonization phases. Vapour-grown carbon fibers (VGCFs) were created using a catalytic chemical vapour deposition (CVD) method, revealing CNTs within the VGCFs. Carbon nanotubes, essential for modern nanotechnology progress, were seen to be created using arc-discharging. A simple CVD synthetic procedure is shown in Figure 3. There are two main techniques for producing carbon nanofibers: catalytic decomposition of carbon precursors and the carbonization process involving the electrospinning of polymers. 50 The choice of the most suitable technology relies on the specific characteristics sought for synthetic carbon nanofibers (CNFs). The CNFs are commonly produced by CVD, in which carbon-containing gases, including acetylene, ethylene, methane, and propylene, are decomposed with the help of metal particles. Fe, Co, and Ni are often utilised as metal catalysts, but research also investigates the potential of Cr, V, and Mo. 51 , 52 The surface area of CNFs is increased from 300–400 to 1,700 m2 g⁻1 after treatment with a KOH solution, a 4- to 7-fold increase, 53 as shown in Figure 3. Mass-producing CNFs are more cost-effective than creating single-walled CNTs, offering a clear benefit. 53 , 54 , 55 , 56 Electrospinning is an efficient method for producing polymer nanofibers in a range of sizes from tens of nanometers to several microns. This procedure entails subjecting a capillary attached to a polymer solution reservoir to a powerful electrostatic field. 56 The electrospinning process variables that significantly impact the final polymer fiber shape are the amount of the polymer solution, the spinning voltage, the working distance, and the feeding speed. The size of the fibers also decreases with increasing carbonization temperature. 57 , 58 , 59

Overview of CVD method.
Three main techniques for producing single-walled carbon nanotubes (SWCNTs) include arc discharge, laser evaporation, and CVD. The arc discharge method involves a sealed reaction chamber containing gases like H2. The anode comprises metal catalysts such as Fe, Co, Ni, and a thick graphite rod cathode. 60 The formation of highly pure, single-walled carbon nanotubes on the inside surface of the reaction vessel is induced by the electricity generated by the graphite electrodes. Arc discharge-produced SWCNTs demonstrate good yield, quick preparation time and crystallinity. The layer count of CNTs may be controlled by choosing the catalyst 61 , 62 (Table 1).
Shows the catalytic CVD synthesis of CNTs.
| Catalyst | Carbon source | Temperature | Product | References |
|---|---|---|---|---|
| Co, Ni, Fe/MgO | CH4/H2 | 1,000 | SWCNTs | 63] |
| Fe/Al2O3 | C2H4/N2, H2 | 650 | MWCNTs | 64] |
| Fe/Al2O3 | C2H4/N2, H2 | 500–700 | MWCNTs | 65] |
| Fe/Al2O3 | Ethylene/N2, H2 | 550 | CNTs | 66] |
| Fe/Al2O3, SiO2, | TiO or ZrO2 CH4/H2 | 650–800 | MWCNTs | 67] |
| Ni/Fe/CO/HZSM-5 zeolite | Hydrogen, polyethylene terephthalate (PET), polypropylene (PP), polyvinyl chloride (PVC), and PET/arsenate (PV/Ar) | 400–900 | MWCNTs | 68] |
| NiO/HZSM-5 zeolite | Polypropylene (PP)/H2 | 500–800 | MWCNTs | 69] |
Laser ablation method used for the synthesis of NMs.
| Nanomaterials | Laser light source | Ablation duration | Pulse width | Wavelength (nm) | Frequency (Hz) | Target material | Particle size | Fluence (J/cm2) | References |
|---|---|---|---|---|---|---|---|---|---|
| Carbon-based NMs | Nd:YAG laser | 10 | 6 ns | 532 | 10 | Graphite | 200 | 0.4 | 75] |
| TiO2 and ZnO | Nd:YAG laser | 30 | 2.0 ms | 1,064 | 5 | Ti and Zn plates | 5–65 | – | 76] |
| TiO2 | Nd:YAG | 5 | 10 ns | 1,067 and 532 | 10 | Ti rod | 1–40 | 49.9 and 27.6 | 77] |
| ZnO | Nd:YAG | 30 | 7 ns | 1,067 | 10 | Zn | 18 | 12.7 | 78] |
| FeO MNPs | Nd:YAG | 5 | 7 ns | 1,064 | 20 | Fe target | 2–80 | 150 | 79] |
2.4 Laser ablation method
Similar to arc discharge, laser evaporation is a process. Using a laser, a high-temperature quartz tube vaporises graphite composite rods and catalysts made of transition metals. 70 , 71 Inert gas flow moves the product onto a copper column cooled by water, forming CNTs. However, due to its high energy consumption, preliminary expenditures, and difficulties in widespread application, this technology is not appropriate for mass manufacturing. 53
Carbon dioxide (CO2) nanotubes are produced via CVD by the high-temperature breakdown of organic compounds, including ethylene, ethanol, and ethane. Figure 4 shows that several CVD methods can be employed for the bulk production of SWCNTs. These methods include fluidized bed CVD, fixed bed CVD, plasma-enhanced CVD, and aerosol-assisted CVD (Table 2). 73 , 74

Laser ablation method for the synthesis of SWCNTs redraw from the source. 72
2.5 Pyrolysis
Pyrolysis is an essential method used in different fields such as the production of carbon nanomaterials, 80 , 81 , 82 bulk carbon, 83 , 84 carbon-based devices, 85 , 86 , 87 the production of fuel from organic waste, and the use of gas chromatography-mass spectrometry for the study of molecules. 88 , 89 , 90 Noteworthy carbon materials synthesized through pyrolysis encompass graphene, 72 , 91 , 92 , 93 , 94 , 95 , 96 CNTs, 97 carbon fibers (CFs), 94 , 95 , 98 , 99 diamond-like carbon (DLC) coatings, 100 , 101 as well as other industrial carbons like glass-like carbon (GC) and graphite. 83 , 102 , 103 Unlike metals, carbon manufacturing mostly depends on synthetic methods. Some carbon allotropes, such as GC, are entirely man-made. Most carbon production methods are based on pyrolysis; when an organic substance is subjected to high temperatures in an oxygen-depleted environment, occasionally in the presence of a catalyst, it undergoes thermal decomposition. 104 This process results in the breakdown of the original material and the release of non-carbon atoms in diverse forms. Solid carbon is usually produced as a byproduct or smoke because carbon is thermally stable even when exposed to no oxidizing chemicals. Precursor type, breakdown properties, applied pressure (if any), creation of more giant carbon molecules, and thermodynamic stability of pyrolysis byproducts are some of the variables that could affect the structure of the resulting solid carbon. 101 , 105 This technique has been crucial in creating crystalline and disordered carbons for decades. Graphene, CNTs, VGCFs, vapour-deposited diamonds, and diamond-like carbon films produced by CVD based on pyrolysis are all examples of carbon nanomaterials. 106 As are vapour-deposited diamonds. This method was the framework for producing crystalline and disordered carbons for a long time. Examples of carbon nanomaterials are graphene, CNTs, and VGCFs, which are formed by vapour growth of carbon, vapour-deposited (VD) diamonds, and diamond-like carbon (DLC) films are produced using CVD built on the principles of pyrolysis, 107 , 108 where gaseous hydrocarbons decompose. Carbon manufacturing has been achieved using CVD and comparable methods since the 19th century; 109 , 110 pyrolytic carbon compounds were viewed as secondary products, with the main emphasis on synthesizing graphite. Carbon nanomaterials have been recognized for their application in recent decades and have been researched as separate entities. Research on single-layer (2D) graphite crystals was conducted in the 1960s after Wallace’s theoretical analysis of their electrical characteristics in 1947. 111 , 112 Graphene oxide, derived from single-crystal graphite, was first reported in 1859 113 for diverse applications. The term ‘Graphene’ was officially recognized in the IUPAC database in 1994 following its experimental synthesis, which was documented in 1962. Graphene oxide, a derivative of single-crystal graphite, was initially reported in 1859. In 2004, a method for mechanically exfoliating graphene from highly-oriented pyrolytic graphite (HOPG) was invented by Novoselov et al., who were awarded the Nobel Prize in 2010 for their work. 114 , 115 Graphene has become a vital substance of the 21st century due to discoveries in nano-scale characterisation methods and worldwide research endeavours. Beyond graphene, other carbon nanomaterials such as CNTs, 116 VGCFs, 117 , 118 , 119 and DLC 120 hold significant technological promise. They can be produced by heating gaseous or light liquid hydrocarbons by pyrolysis, resulting in different morphologies depending on the specific conditions and catalytic substrates used. Standard parameters for producing different carbon nanomaterials using CVD. 121
3 Types of carbon-based nanomaterials
Carbon-based nanomaterials encompass diverse structures, each with unique properties and applications. Carbon nanotubes, 72 cylindrical nanostructures with exceptional strength and electrical conductivity, find utility in electronics, materials science, and biomedical fields. Graphene, a single layer of carbon atoms arranged in a hexagonal lattice, boasts remarkable mechanical, thermal, and electrical properties, driving innovations in flexible electronics, energy storage, and composite materials. Fullerenes, spherical carbon molecules resembling soccer balls, exhibit intriguing properties such as high electron affinity, leading to drug delivery, catalysis, and superconductor applications. The tunable optical characteristics and low toxicity of carbon dots, which are nanometer-sized carbon nanoparticles, are also contributing to their rising profile in biomedicine, sensing, and imaging. 122 , 123 These carbon-based nanomaterials inspire breakthroughs across various disciplines, promising revolutionary advancements in technology and science.
Since their discovery by Iijima et al. in 1991, various technologies have been developed to produce CNTs in large quantities. 124 , 125 Notably, the production method can significantly influence the structure and properties of CNTs. 126 , 127 , 128 , 129 These materials possess exceptional strength, heat resistance, and electrical conductivity, 130 making them highly desirable in numerous fields, especially energy storage devices, 131 , 132 marking significant advancements in science and technology. Carbon nanotubes are cylindrical molecules of carbon atoms arranged in a hexagonal pattern with diameters varying from 0.7 to 50 nm. They come in two main types: single-walled CNTs, which consist of a single layer of rolled graphene sheets, and multi-walled CNTs, which comprise multiple layers.

Types of carbon-based nanomaterials: CDs, CNTs, CNFs and graphene reproduced from source. 133
Carbon-based nanomaterials such as CNTs have been synthesized early in 1958. 134 They are employed in energy storage systems like supercapacitors (SCs), rechargeable batteries, and fuel cells, where they act as additives to enhance the electrical conductivity of cathode and anode materials (Figure 5). 135 , 136 , 137
Because of their complex geometric structures, SWCNTs display various electrical properties, from metallic to semiconducting, making them useful in many fields of study. Carbon nanotubes’ remarkable electrical properties are enhanced by their one-dimensional nature, which enables electron conduction in the absence of scattering. Their exceptional mechanical, electrical, and thermal conductivity, ultrahigh aspect ratio, and unique covalent atomic tube structure make them perfect for reinforcing fibers in hybrid composites. Carbon nanotubes have long attracted interest for their potential use in composites, prompting substantial study into their synthesis and chemical modification. Chemical vapour deposition on a substrate is a standard method for directly synthesizing reasonably pure individual single-walled carbon nanotubes (SWCNTs). Individual SWCNTs with precise diameters may be efficiently guided to grow at temperatures surpassing 600 °C by applying catalyst materials to a solid substrate. According to previous research 138 the carbon source is usually acetylene or methane vapour. 139 Carbon nanotube varieties are seen in Figure 6 above.

Different types of carbon nanotube.
Graphene is described as a flawless single layer of graphite. In 2004, A. K. Geim and K. S. Novoslov conducted a groundbreaking experiment at Manchester University using scotch tape technology, creating monolayer graphene. 114 , 115 This achievement has significantly expanded the exploration of two-dimensional (2D) materials and introduced a novel technology with numerous applications. In 2010 Geim and Novoselov were awarded the Nobel Prize in Physics for their pioneering research on the physics and properties of graphene. 140 , 141 , 142 Graphene is a two-dimensional material composed of a single layer of carbon atoms linked by sp2 bonds, forming a densely packed honeycomb crystal lattice in a flat sheet just one atom thick. In contrast, graphene oxide (GO) is a yellow solid featuring a hexagonal lattice structure, representing an oxidized version of graphene that contains varying ratios of carbon, oxygen, and hydrogen atoms. 143 , 144 Integrating oxygen-containing groups in GO boosts its energy storage capabilities, rendering it a promising option for enhancing electrode performance. While GO is a single-layer material readily dispersible in water, its conductivity is constrained by the presence of oxygen groups. Introducing functional groups to modify these oxygen groups can modify its electrical, mechanical, and thermal characteristics, expanding its adaptability and facilitating its application in various energy storage devices, including supercapacitors (SCs), 145 , 146 batteries 147 and fuel cells 148 (Figure 7).
![Figure 7:
Structural representation of graphene, GO, and rGO are reproduced by 149].](/document/doi/10.1515/revic-2024-0017/asset/graphic/j_revic-2024-0017_fig_007.jpg)
Structural representation of graphene, GO, and rGO are reproduced by 149].

Carbon dots candidate in clean energy, reproduced. 156
Carbon nanodots (CNDs) are microcarbon nanoparticles with a quasi-spherical structure in zero dimensions. The finding was made in 2004 while refining SWCNTs, or single-walled carbon nanotubes. Carbon nanodots are chemically and physically identical to graphene oxide. 150 , 151 , 152 Furthermore, they are economical and easily accessible, which has led to their increasing favour among carbon nanoparticles. 153 Carbon nanodots are rich in oxygen-containing functional groups such as –NH2, OH–, and –COOH groups on their surface (Figure 8). These groups offer passivation effects and enable the adjustment of physicochemical properties. 154 , 155
Carbon nanofibers (CNFs) represent cutting-edge materials utilized across various domains, including energy conversion, composite reinforcement, and self-sensing devices. 157 , 158 Notably, they differ from traditional CFs in several aspects, with the diameter being the most prominent distinction. CNFs typically exhibit diameters ranging from 50 to 200 nm, whereas conventional CFs span several micrometres, as shown in Figure 9. Functionally, CNFs serve as one-dimensional nanomaterials with diverse applications such as selective adsorption, polymer reinforcement, electrochemical catalysis, and hydrogen recovery. Carbon nanofibers made of sp2-based discontinuous linear filaments with aspect ratios more than 100:1 are mechanically very sensitive to the direction of the carbon layers. 159 , 160 CNFs manifest in various forms Figure 9, 161 , 162 including platelet, ribbon, and fishbone configurations.

Structure of CNFs and superficial carbon fibers (CFs). Regenerated from source. 162
4 Application of carbon-based nanomaterials in fuel energy
Findings from our study suggest that modified carbon nanomaterials with specific shapes, improved attraction, and solubility might fulfil most requirements for solar energy fuels, 163 lithium-batteries 164 and supercapacitors 165 under various conditions. This review aims to give readers a thorough understanding of the basic principles before delving into specific carbon nanomaterials and their functions. Some fundamentals reviewed include an overview of carbon nanomaterials, what they are, and how to make or alter them chemically. Graphene stands out as a potential material for developing energy storage devices, particularly when combined with metal oxide to prevent sheet stacking. 166 Its highly conductive linked networks make it attractive for energy storage applications. Graphene has a porous microstructure, electrochemical stability, and mechanical toughness, making it highly useful in energy storage applications. 167 , 168 These applications cover a range of technologies, including fuel cells, solar cells, batteries, and supercapacitors. Graphene finds application in proton exchange membrane (PEM) fuel cell supports and serves as both anode and cathode material in lithium batteries. Additionally, graphene is employed as an electrode material in both pseudo-capacitors and double-layer capacitors within supercapacitors. Graphene is also utilized as dye-sensitizers in fuel cells. 169 , 170
4.1 Fuel cell
Fuel cells efficiently convert fuel’s chemical energy into electricity for various functions. 171 , 172 , 173 Graphene is a common material for catalyst support structures in fuel cells that use proton exchange membranes (PEMs). 174 , 175 The possibility of using metal oxides, non-precious metals, or metals coordinated with nitrogen as catalysts instead of platinum is an area of active investigation. Their stability and activity levels in comparison to platinum catalysts are still an issue, though. 176 , 177
While active carbons possess high surface areas, their instability necessitates coupling with other materials to address concerns, as shown in Figure 10. Graphene’s technological progression has altered this landscape, positioning active carbons as robust alternatives to platinum due to their conductivity, expansive surface areas, and strong adhesion to catalyst particles. 178 , 179 Moreover, with its abundant functional groups, graphene oxide facilitates nucleation and attachment of catalyst nanoparticles onto its surface. Graphene finds extensive application in fuel cells, primarily as support material for anode catalysts, cathode catalyst replacements, composite materials, standalone electrolyte membranes, and even bipolar plates. 180 , 181 Recent advancements underscore the significance of graphene in each component of fuel cells. Traditional active catalysts in fuel cell electrodes, such as Pt and Pt alloys, are common, particularly in low-temperature fuel cells fueled by hydrogen, methanol, and ethanol. However, Pt’s high cost, limited availability, and susceptibility to intermediates generated during fuel oxidation pose challenges. Numerous approaches have been explored to mitigate these issues, including reducing catalyst loading and replacing Pt catalysts with non-precious alternatives in fuel cells’ anodes and cathodes. 182

Schematic mechanism of fuel cell device.
Fundamental components of the fuel cell’s operation were covered, along with elements influencing its performance such as cathode and anode performance deterioration and stack performance degradation. To conduct current and catalyse electrode processes, the cathode mostly moves air. The whole fuel cell structure is supported by the cathode in a cathode-supported fuel cell. To lower the activation loss of the cell and enhance cell performance, the cathode material must possess a strong electrochemical catalytic capacity for oxygen reduction. To lower ohmic losses, the cathode material also must have a high electrical conductivity. Meanwhile, the cathode material has to have strong chemical stability in the oxidising environment because of the high working temperature of the fuel cell. It is also incapable of having a chemical reaction with nearby materials. The primary functions of the anode include the current conductor, electrode reaction catalyst, and fuel gas transportation. The anode supports the whole fuel cell structure in a fuel cell that is anode-supported. A nickel-doped yttrium oxide stabilised zirconium oxide (YSZ) composite electrode is the most widely used material for fuel cell anodes (Ni-YSZ). As a metallic substance, nickel has an extremely high electrical and thermal conductivity. Ni, meantime, oxidises fuels like carbon monoxide and hydrogen by acting as a catalyst.
It can directly reform hydrocarbons like methane within. Furthermore, nickel is less costly than precious metals like platinum (Pt) and can lower anode costs. The Ni-YSZ composite electrode’s thermal expansion coefficient may satisfy thermal stress requirements and is compatible with the YSZ electrolyte. The main factors causing fuel cell anode performance deterioration are carbon deposition, gas impurity poisoning, coarsening of Ni particles, and Ni particle redox. 183 , 184 The mechanism of the fuel cell can be seen in Figure 10.
4.1.1 Replacement of the catalyst at anode
Graphene serves two primary purposes:
4.1.1.1 Reducing the loading of the Pt and/or Pt alloy catalyst
To reduce catalyst loading or find non-platinum alternatives to Pt, graphene, which has a much larger surface area than other carbon materials, is a good candidate for Pt catalysts. Graphene in Pt catalysts enhances electrical conductivity and promotes effective Pt dispersion, thereby boosting activity. This heightened activity reduces Pt catalyst loading in fuel cell anodes and enhances stability. Zhao et al.’s research demonstrated that Pt/graphene exhibits five times the methanol oxidation activity of Pt/C, as shown in Figure 6a. Similarly, PtRu/graphene shows a 170 % increase in activity compared to PtRu/C, with additional enhancement observed upon Ni addition. 185 This enhancement is attributed to improved electrical properties and enhanced wettability achieved through heteroatom doping, such as sulfur, boron or nitrogen. Doping enhances conductivity and improves the adsorption of fuel molecules, thereby increasing active sites. 186 , 187 , 188 Moreover, doping with boron enhances resistance to CO poisoning and facilitates better catalyst dispersion. Pt activity increases with graphene usage and is further enhanced through boron doping. 189 , 190 , 191
4.1.1.2 Replacing Pt and Pt alloys with a non-precious catalyst
Graphene serves as a catalyst support for nickel, recognized as one of the most effective non-precious catalysts for low-temperature fuel cell anodes, including methanol, ethanol and urea fuel cells, particularly under alkaline conditions. 192 , 193 The activity of nickel supported by graphene is much higher than that of carbon nanoparticles or sheets of carbon. An increase in activity is observed when the Ni loading is increased up to 6 wt%. 194 , 195 The enhanced activity is caused by the incorporation and even distribution of Ni nanoparticles throughout the graphene surface. A combination of graphene’s structural imperfections, the uniform distribution of the catalyst, and functional groups throughout its surfaces and edges is thought to be responsible for the enhanced efficiency of non-precious catalysts on graphene. 196 , 197
4.1.2 Replacement of catalyst at the cathode
4.1.2.1 For supporting the Pt catalyst
Extensive research focuses on graphene as a catalyst support for Pt catalysts in oxygen reduction reactions. 198 , 199 , 200 In addition to lowering the size of Pt nanoparticles and maintaining their uniform distribution on the graphene surface, imperfections in graphene are discovered to lower from 0.37 to 0.16 eV, the activation energy needed to dissociate oxygen molecules drops. 201 , 202 , 203 This decrease amplifies the instability of HO*, a critical stage in the oxygen reduction cycle. Doping graphene with boron or nitrogen enhances Pt dispersion and significantly boosts activity in methanol oxidation. 193 , 204 , 205
4.1.3 Standalone catalyst
Combining graphene with carbon nanotubes improves its oxygen reduction reaction (ORR) efficiency by inhibiting the reaggregation of graphene layers, a common issue in graphene produced using Hammers’ process. 206 , 207 , 208 , 209 Figures 11(a and b) demonstrate that the combination of graphene and carbon nanotubes exhibits a remarkable efficiency in the oxygen reduction process (ORR) in both acidic and alkaline conditions, with an overpotential below 100 mV, 210 has led to a notable increase in ORR activity. 211 , 212 , 213 By making it easier for graphene structures to conjugate with nitrogen’s lone pairs of electrons, nitrogen doping enhances the activity of the oxygen reduction process (ORR). This enhancement is due to the activation of carbon atoms by nitrogen atoms, which accept electrons. In the oxygen reduction reaction (ORR), graphitic nitrogen plays a crucial role. 214 , 215 By using transition metals like Co and/or Fe, the activity of graphene can be substantially enhanced. 216 The presence of M–N x active sites, linked to the improved activity of M–N–C (where M is the transition metal), is a common finding on graphene’s edges. 217 , 218
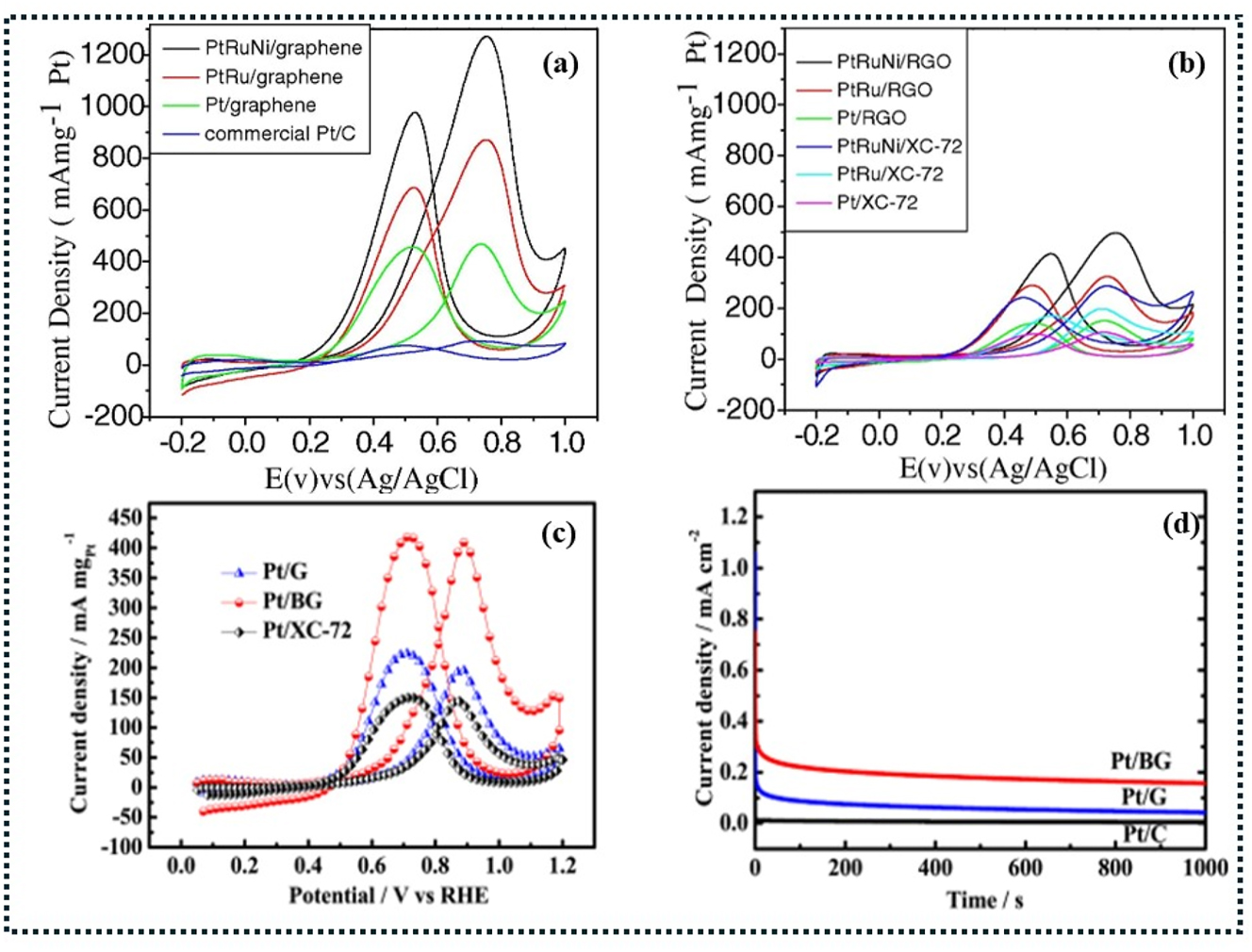
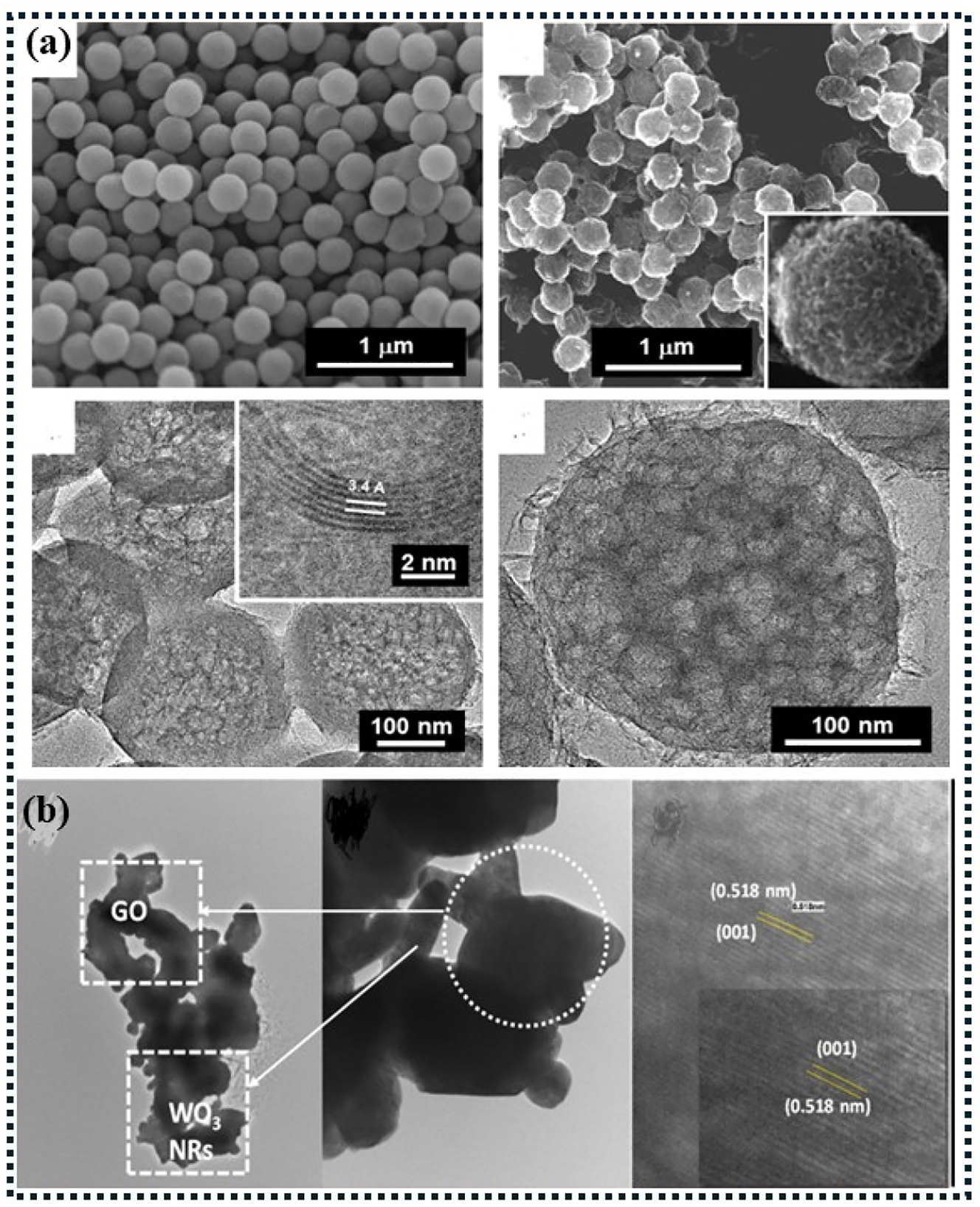
Oxidative reduction reactions. (a) Some cyclic voltammograms of Pt/C, Pt/graphene, PtRu/graphene, and PtRuNi/graphene Pt, PtRu, and PtRu cyclic voltammograms on XC-72 carbon nanoparticles and rGO. (b) A mixture of 1 M methanol and 0.5 M H2SO4 was used for all experiments, with a scan rate of 20 mV s−1. 185 (c and d) The cyclic voltammograms of Pt/C commercial (Pt/XC-72), Pt/graphene (Pt/G), and Pt/graphene loaded with boron (Pt/BG). 219
4.2 Supercapacitors
Due to their unique ability to store energy, supercapacitors are essential in many electronic systems and play a key role in energy storage. Supercapacitors form a double layer of electrolyte ions using a non-insulating electrode material. This allows them to store energy. Carbon nanoparticles are perfect for making high-efficiency electrodes because of their significant potential specific surface area and high electrical conductivity, which decreases effective series resistance and increases charge storage capacity. A longer operational lifetime and a better power density are the outcomes of this. 220 , 221 Figure 12 below illustrates an essential supercapacitor device. Combining CDs with various carbon materials and polymers 204 , 205 or incorporating carbon nanomaterials or metal oxides has been demonstrated to improve the electrochemical performance of supercapacitors. 222 , 223 Various functional groups, core–shell topologies, and crosslinked networks are all part of carbonized polymer dots (CPDs). These features combine to create a three-dimensional carbon network that is both conductive and porous, with a high specific surface area and good wettability. 224 , 225 Because of this, robust and adaptable multilayer energy storage devices are more accessible to construct. Kaner’s transformation of carbon-based materials co-doped with nitrogen and oxygen into open porous three-dimensional turbostratic graphene structures facilitated the invention of supercapacitors. They outperformed traditional aluminium-based capacitors in terms of charging speed, relaxation time (3.44 ms), and energy density (17.7 Wh kg−1). 225 , 226 This opens the door to creating layered energy storage devices that are both long-lasting and adaptable. Kaner transformed carbon-based materials co-doped with nitrogen and oxygen into open-pored, three-dimensional turbostratic graphene structures to make supercapacitors. 137 , 227 , 228
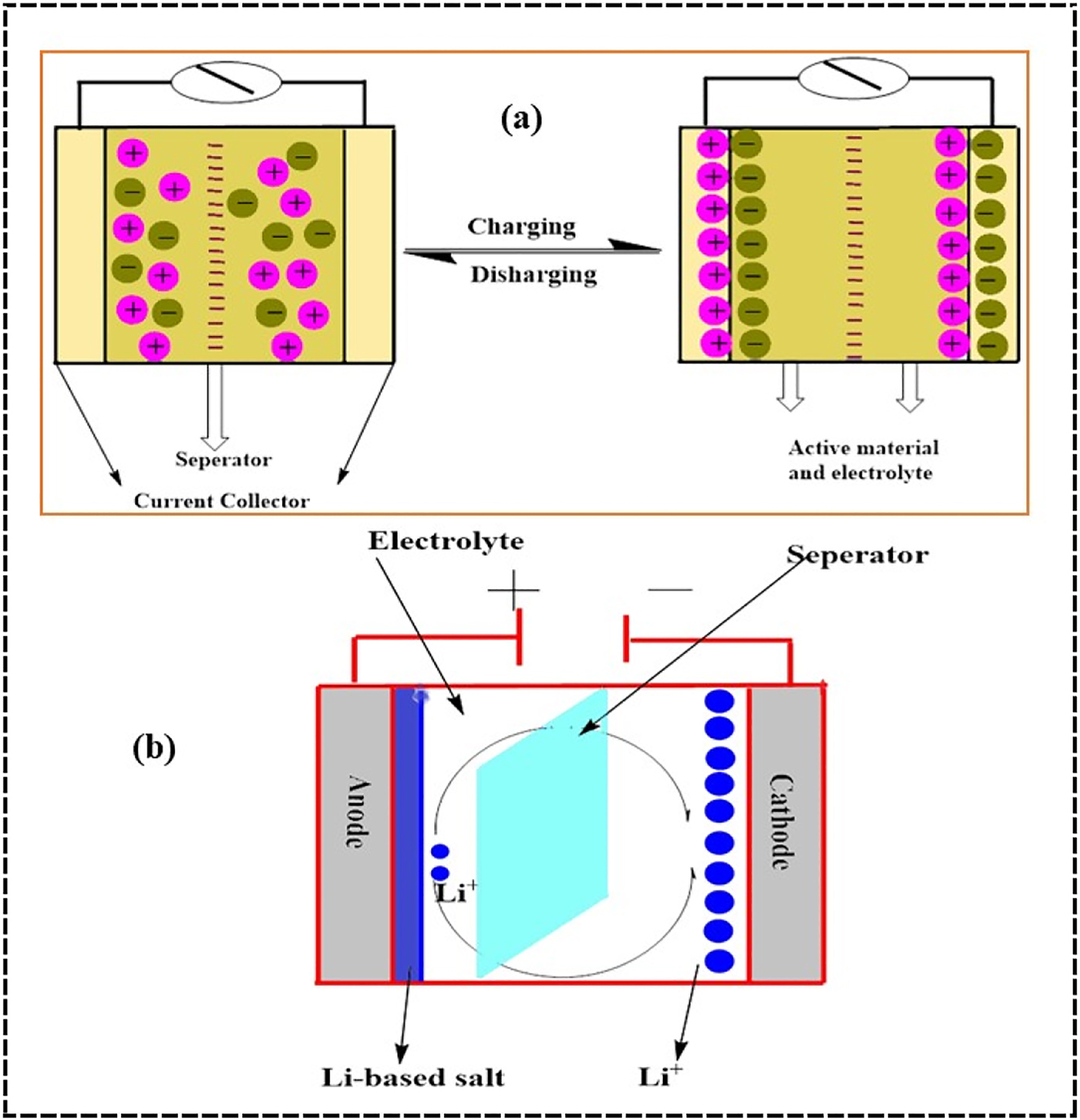
Illustration of supercapcitor device. (a) Simple supercapacitor and (b) hybrid supercapacitor redraw from source. 229
4.2.1 Supercapacitor electrodes are composed of graphene dots and powders
Chemical exfoliation of graphite to graphene oxide (GO) and controlled GO reduction with a reducing agent like hydrazine hydrate are the steps in synthesising graphene dots and particles. Graphene particles clump together quickly, and reduced graphene oxide (rGO) becomes water-repellent after reduction, impacting its processing in water or aquatic environments. GO stands out as an amphiphile due to its hydrophobic base plane and negatively charged hydrophilic edges. The ability of GO to interact selectively with certain surfactants allows for the control of rGO assembly and manipulation of GO’s amphiphilicity. Surfactants such as sodium dodecylbenzene sulfonate, cetyltrimethylammonium bromide, and tetrabutylammonium hydroxide (TBAOH) were studied by Zhang and colleagues as potential stabilizers for graphene-based materials. 230 , 231 , 232 The study revealed that reduced graphene oxide sheets can be intercalated between layers of surfactants. At a current density of 1 A g−1, an electrode of graphene stabilised with TBAOH displayed a specific capacitance of 194 F g−1. This was partly due to reduced rGO re-stacking and improved wettability from surfactant intercalation. Furthermore, surfactants can evenly distribute rGO into individual or a small number of layers in water-based solutions. rGO’s characteristics enable chemical interactions with second phases in aqueous solvents. Commercially accessible block copolymers PEO106–PPO70–PEO106 (F127) have been extensively studied among various surfactants for their proven chemical properties. Block copolymers can self-assemble in aqueous environments via hydrogen bonding and hydrophobic–hydrophilic interactions, forming cysts. These cysts can be flexible templates for synthesising mesoporous carbon materials. 233 , 234 Graphene oxide (GO) was introduced using a triblock copolymer called Pluronic F127 by Ke and colleagues using a hydrothermal technique. The structure was then restored by thermal annealing. The surface area of the surfactant-modified graphene is 696 m2 g−1, three times higher than the surface area of pure graphene, which is 200 m2 g−1. In a 6 M KOH electrolyte, the most stable cycle was seen at a scanning rate of 1 mV s−1, with a specific capacitance of 210 F g−1. 235 The large surface area and excellent electrical conductivity of graphene particles allow supercapacitor electrodes made of them to transfer charges quickly and exhibit a high power density. 236 The low capacitance value of about 200 F g−1 limits their capability. Consequently, the device’s energy density and overall performance are usually inadequate. In contrast, reversible faradaic redox reactions at the electrode surface cause pseudocapacitance resulting from certain transition metal oxides or hydroxides, electrically conductive polymers, and certain transition metals (e.g., MnO2, RuO2, NiO, Co3O4, and Fe3O4) to have a higher specific capacitance. The main electrode material used in electrolyte droplet chromatography (EDLCs) is graphene, a carbon-based substance. 237 , 238 , 239
Extensive research has been conducted on graphene–MnO2 composites among various metal oxide composites. 240 A straightforward method for producing graphene–MnO2 composites by hydrothermal reduction was developed by Yang and coworkers. At a potential scan rate of 2 mV s−1, the graphene–MnO2 composite powders display a specific capacitance of 211.5 F g−1, according to the experimental data. In an electrolyte with 1 M Na2SO4, around 75 % of the capacitance remains after 1,000 cycles of charging and discharging. Hydrothermally reduced graphene–MnO2 composites were made easily by Yang et al. The specific capacitance of the graphene–MnO2 composite powders is 211.5 F g−1 at a potential scan rate of 2 mV s−1, as shown by the experimental results. After 1,000 cycles of charging and discharging in a 1 M Na2SO4 electrolyte, around 75 % of the capacitance is still there. 241 Dai and colleagues created Ni(OH)2 nanoplates on graphene sheets. High power density and energy density are also displayed by the hybrid electrode material, which has a specific capacitance of approximately 1,335 F g−1 at a charge and discharge current density of 2.8 A g−1 and around 953 F g−1 at 45.7 A g−1. 242 Nanocomposite powders of Fe3O4 and rGO are produced via electrostatic contact. At a current density of 1 A g−1, this powder has a specific capacitance of 169 F g−1, much more significant than Fe3O4’s (68 F g−1) in a 6 M KOH electrolyte. 243 As shown in Figure 13(a), Lee and colleagues created a mesoporous graphene nanoball (MGB) that can be mass-produced using a CVD process that uses precursors. This nanoball will be used in supercapacitors 244 Csp and Cg; the specific capacitance and gravimetric capacitance of the GO/WO3 are maximized at 10 % GO. A capacitance of 123 mAh g−1 and a capacitance of 738 F g−1 are documented correspondingly. At a current density of 5 mA cm−2, the energy density (Ed) reaches 37 Wh kg−1, and the power density (Pd) reaches 500 W kg−1. 245
4.3 Rechargeable batteries
An urgent demand is for electrochemical storage devices capable of carrying high energy and power densities, such as electrochemical capacitors and lithium batteries, fueled by advancements in portable electronics and low-emission vehicles. The choice of electrode materials and their fabrication methods play a vital role in electrochemical energy storage nanotechnology applications. The properties of electrode materials significantly impact the effectiveness of nearly all energy storage applications. The charge storage capacity is consistently influenced by the internal structure, chemical composition, macroscopic form, surface morphology, and size of the electrodes. Carbon-based functional nanomaterials exhibit distinctive morphological, electrical, optical, and mechanical features as compared to conventional energy resources, potentially enhancing energy conversion efficiency and cycle performance. 246 , 247
Carbon-doped materials have garnered considerable attention as traditional anodes for Li and Na-ion batteries due to their cost-effectiveness, abundant sources, and impressive cycling durability. 248 , 249 Nevertheless, their low capacitance and sluggish kinetics severely restrict their further application in these batteries. To address these challenges, significant efforts have been devoted to offering various methods, such as composing nanocomposites, introducing diverse surfaces, creating multiple porosities, and incorporating heteroatoms. 250 Rechargeable batteries are highly efficient energy storage solutions that balance renewable energy production and consumption. 251 , 252 , 253 Carbon-doped substances (CDs) facilitate intercalations between electrodes and electrolytes through surface engineering lithium, sodium, or potassium ion batteries. 254 , 255 , 256 The supplementary active sites provided for ion insertion and extraction, thus improving stability, electron/ion transfer, diffusion, and overall electrochemical characteristics. To reduce Na+ diffusion pathways and enhance ensemble conductivity, carbon-doped materials have been employed as “custom additives” alongside graphene-rich petal-like materials such as TiO2. Sodium storage with increased capacity and long-term cycle stability improved. 252 , 257 Metal cations attracted by the oxygen-containing functional groups originating from carbon dots (CDs) facilitate the creation of a consistent solid electrolyte interphase and maintain the integrity of electrode structures, enhancing electrochemical performance. Xiong and collaborators engineered polyethyleneimine-functionalized carbon-doped materials (PEI-CPDs) for modified cathodes to address the challenges posed by the polysulfide shuttle, increase sulfur loading and improve the operational current density in lithium–sulfur (Li–S) batteries. The compact size, an abundance of amine groups in PEI-CPDs and outstanding dispersibility offered sample absorption sites capable of effectively suppressing polysulfide dissolution and enhancing Li+ conductivity around the solid electrolyte interface. 258
For example, He et al. 259 conducted research into synthesizing a nanocomposite comprising Fe3O4/CNTs. They further investigated the electrochemical characteristics of this nanocomposite, revealing a charge capacity of 656 mA h g−1, which remained stable for 145 cycles. The exceptional electrochemical performance of these materials stems from the establishment of a conductive pathway within the composite structure. In a separate research endeavour, Wei and his team developed composite materials incorporating multi-walled carbon nanotubes (MWCNT) and sulfur (S) for battery applications. Their findings demonstrated that these materials retained 96.5 % of their capacity after 100 cycles, indicating their suitability for practical use in batteries. 260 , 261 , 262
The Li–air battery exhibits a specific capacity exceeding 11,000 mAh g−1 and can achieve a high-energy density of approximately 3,500 Wh kg−1, nearly 10 times greater than conventional LIBs and comparable to gasoline fuel. 263 , 264 , 265 The Li–O2 battery represents an up-and-coming power source because it offers high-energy density for applications in electric vehicles, aircraft technology and industrial electrification. At the cathode, the primary discharge product is lithium peroxide with a small amount of lithium oxide. 266 , 267 , 268
Anode:
Cathode: 269
Lithium oxide is often not preferred for an utterly reversible reaction to produce O2 and Li, while lithium peroxide is the optimal discharge product for a cathode. Lithium–air batteries change considerably from standard polymer electrolyte-based Li batteries, as shown in Figure 14. Researchers are enhancing the discharge capacity of non-aqueous rechargeable Li–O2 batteries. Nevertheless, there are still weaknesses to the advancement of Li–O2 batteries. A nonaqueous electrolyte rechargeable Li–O2 battery with specific energy ranging from 250 to 350 W h kg−1 was 1st published by Abraham and Jian. 270 The MnO@NHCNT-1 hybrid shows unusual cycling stability, maintaining a specific discharge capacity of 966.7 mAh g−1 at 0.2 A g−1 after 300 cycles and a rate capability of 510.3 mAh g−1 even at a high current density of 1.6 A g−1 when utilized as the anode in lithium-ion batteries as showing in the Figure 15. 271

Description of lithium-air batteries. (a) Proposed schematic design for the rechargeable aprotic Li–air battery. (b) Comparison of gravimetric energy densities (Wh kg−1) for different types of rechargeable batteries with gasoline.

Description of MnO@NHCNT-1 hybrid in lithium-air batteries. (a–g) SEM images of a MnO@NHCNT and (h–k) CV, charge/discharge profile, cycling performance of different electrodes and performance. 271
5 Challenges and future perspectives
Carbon-based nanomaterials hold tremendous promise in various fields, yet they also face significant challenges that must be addressed for their widespread application. Carbon-based nanomaterials (CBNMs) hold much promise for improving energy storage and generating systems. Carbon nanotubes, graphene, and other CBNMs can provide next-generation batteries with improved energy densities, faster charge/discharge rates, and longer cycle lifetimes. In that case, the industry will be in for a significant shakeup. Hydrogen generation and storage systems, fuel cells, and supercapacitors can all benefit from these materials, leading to more efficient and environmentally friendly energy systems. Combining CBNMs with solar cells may improve photovoltaic stability and efficiency. However, several obstacles must be overcome before these resources can be fully utilised. Significant challenges include high production costs, problems with scalability, and maintaining constant material quality. Questions of material compatibility and thermodynamics make integration with current technologies challenging. Comprehensive research is required to address environmental and health problems, such as the toxicity and long-term viability of CBNMs. There are also significant obstacles to overcome in the form of regulations and the market, which include things like creating industry standards and winning over consumers. It is crucial to address these difficulties through continuous research and development to adopt CBNMs successfully in the energy sector. In summary, while carbon-based nanomaterials hold great promise for revolutionizing various fields like energy storage devices, medicine, environment and industries, addressing existing challenges and exploring new avenues of research are essential for realizing their full potential in the years to come. Collaborative efforts across disciplines and sustained investment in research and development will play a critical role in shaping the future of carbon-based nanomaterials.
6 Conclusions
Carbon and its allotropes have demonstrated tremendous potential in meeting the increasing energy demands caused by population growth and the diminishing supply of fossil fuels, because of their remarkable characteristics and ease of manufacturing. Materials like graphene, fullerenes, and carbon nanotubes have gained attention as possible energy storage and production options in the last 20 years. This review emphasises the significance of learning about the structural features and synthetic pathways of different carbon-based nanomaterials (CBNMs) to use them in fuel energy systems. Thermodynamic compatibility and accumulating problems are two of the few obstacles carbon-based materials like expanded graphite, carbon nanotubes, carbon fiber, and carbon fiber face. However, their realization hinges upon effectively addressing the formidable challenges they confront. Nevertheless, these materials provide substantial increases in energy device performance. The significance of CBNMs in improving the performance of fuel cells, energy storage devices, and catalytic applications is highlighted by the review’s examination of several synthetic procedures and their pros and cons, highlighting the necessity for ongoing research and development. Improving fuel energy systems and addressing future demands requires thoroughly understanding these materials’ specific properties and fabrication techniques. As we navigate the path forward, we must foster collaborative endeavours across disciplines, supported by sustained investment in research and development. By doing so, we can unlock the full spectrum of possibilities carbon-based nanomaterials offer, paving the way for groundbreaking innovations and advancements that benefit society. With concerted efforts and a commitment to exploration, we are poised to shape a future where carbon-based nanomaterials are pivotal in addressing pressing global challenges and driving progress towards a more sustainable and technologically advanced world.
-
Research ethics: Not applicable.
-
Author contributions: The authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Competing interests: The authors state no conflict of interests.
-
Research funding: None declared.
-
Data availability: Not applicable.
References
1. Zhang, Y.; Yin, Q.-Z. Carbon and Other Light Element Contents in the Earth’s Core Based on First-Principles Molecular Dynamics. Proc. Natl. Acad. Sci. USA 2012, 109 (48), 19579–19583; https://doi.org/10.1073/pnas.1203826109.Search in Google Scholar PubMed PubMed Central
2. Pace, N. R. The Universal Nature of Biochemistry. Proc. Natl. Acad. Sci. USA 2001, 98 (3), 805–808; https://doi.org/10.1073/pnas.98.3.805.Search in Google Scholar PubMed PubMed Central
3. Lin, H.-J.; Lu, Y. S.; Zhang, L. T.; Liu, H. Z.; Edalati, K.; Révész, Á. Recent Advances in Metastable Alloys for Hydrogen Storage: A Review. Rare Met. 2022, 41 (6), 1797–1817; https://doi.org/10.1007/s12598-021-01917-8.Search in Google Scholar
4. Marty, B.; Alexander, C. M. O. D.; Raymond, S. N. Primordial Origins of Earth’s Carbon. Rev. Mineral. Geochem. 2013, 75 (1), 149–181; https://doi.org/10.2138/rmg.2013.75.6.Search in Google Scholar
5. Zhao, C.; Kang, J.; Li, Y.; Wang, Y.; Tang, X.; Jiang, Z. Carbon-based Stimuli-Responsive Nanomaterials: Classification and Application. Cyborg Bionic Syst. 2023, 4, 0022; https://doi.org/10.34133/cbsystems.0022.Search in Google Scholar PubMed PubMed Central
6. Long, C. M.; Nascarella, M. A.; Valberg, P. A. Carbon Black Vs. Black Carbon and Other Airborne Materials Containing Elemental Carbon: Physical and Chemical Distinctions. Environ. Pollut. 2013, 181, 271–286; https://doi.org/10.1016/j.envpol.2013.06.009.Search in Google Scholar PubMed
7. Georgakilas, V.; Perman, J. A.; Tucek, J.; Zboril, R. Broad Family of Carbon Nanoallotropes: Classification, Chemistry, and Applications of Fullerenes, Carbon Dots, Nanotubes, Graphene, Nanodiamonds, and Combined Superstructures. Chem. Rev. 2015, 115 (11), 4744–4822; https://doi.org/10.1021/cr500304f.Search in Google Scholar PubMed
8. Clancy, A. J.; Bayazit, M. K.; Hodge, S. A.; Skipper, N. T.; Howard, C. A.; Shaffer, M. S. P. Charged Carbon Nanomaterials: Redox Chemistries of Fullerenes, Carbon Nanotubes, and Graphenes. Chem. Rev. 2018, 118 (16), 7363–7408; https://doi.org/10.1021/acs.chemrev.8b00128.Search in Google Scholar PubMed
9. Hassan, M.; Abbas, G.; Afzal, A.; Haider, Z.; Ahmed, S.; Xu, X.; Pan, C.; Peng, Z. Significance of Flexible Substrates for Wearable and Implantable Devices: Recent Advances and Perspectives. Adv. Mater. Technol. 2022, 7 (3), 2100773; https://doi.org/10.1002/admt.202100773.Search in Google Scholar
10. Vlad, A.; Singh, N.; Galande, C.; Ajayan, P. M. Design Considerations for Unconventional Electrochemical Energy Storage Architectures. Adv. Energy Mater. 2015, 5 (19), 1402115; https://doi.org/10.1002/aenm.201402115.Search in Google Scholar
11. Rauti, R.; Musto, M.; Bosi, S.; Prato, M.; Ballerini, L. Properties and Behavior of Carbon Nanomaterials When Interfacing Neuronal Cells: How Far Have We Come? Carbon 2019, 143, 430–446; https://doi.org/10.1016/j.carbon.2018.11.026.Search in Google Scholar
12. Novoselov, K. S.; Fal’ko, V. I.; Colombo, L.; Gellert, P. R.; Schwab, M. G.; Kim, K. A Roadmap for Graphene. Nature 2012, 490 (7419), 192–200; https://doi.org/10.1038/nature11458.Search in Google Scholar PubMed
13. Díez-Pascual, A. M. Carbon-Based Nanomaterials. Int. J. Mol. Sci. 2021, 22 (14), 7726. https://doi.org/10.3390/ijms22147726.Search in Google Scholar PubMed PubMed Central
14. Sahu, S.; Rathore, G. S.; Tiwari, S. Carbon Allotropes in Waste Decomposition and Management. Carbon Allotropes Compos.: Mater. Environ. Prot. Rem. 2023, 229–255; https://doi.org/10.1002/9781394167913.ch11.Search in Google Scholar
15. Stankovich, S.; Dikin, D. A.; Piner, R. D.; Kohlhaas, K. A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S. T.; Ruoff, R. S. Synthesis of Graphene-Based Nanosheets via Chemical Reduction of Exfoliated Graphite Oxide. Carbon 2007, 45 (7), 1558–1565; https://doi.org/10.1016/j.carbon.2007.02.034.Search in Google Scholar
16. Chen, Y.; Fu, K.; Zhu, S.; Luo, W.; Wang, Y.; Hitz, E.; Yao, Y.; Dai, J.; Wan, J.; Danner, V. A.; Li, T. Reduced Graphene Oxide Films with Ultrahigh Conductivity as Li-Ion Battery Current Collectors. Nano Lett. 2016, 16 (6), 3616–3623; https://doi.org/10.1021/acs.nanolett.6b00743.Search in Google Scholar PubMed
17. Georgakilas, V.; Tiwari, J. N.; Kemp, K. C.; Perman, J. A.; Bourlinos, A. B.; Kim, K. S.; Zboril, R. Noncovalent Functionalization of Graphene and Graphene Oxide for Energy Materials, Biosensing, Catalytic, and Biomedical Applications. Chem. Rev. 2016, 116 (9), 5464–5519; https://doi.org/10.1021/acs.chemrev.5b00620.Search in Google Scholar PubMed
18. Liu, J.; Cui, L.; Losic, D. Graphene and Graphene Oxide as New Nanocarriers for Drug Delivery Applications. Acta Biomater. 2013, 9 (12), 9243–9257; https://doi.org/10.1016/j.actbio.2013.08.016.Search in Google Scholar PubMed
19. Deng, J.; Li, M.; Wang, Y. Biomass-Derived Carbon: Synthesis and Applications in Energy Storage and Conversion. Green Chem. 2016, 18 (18), 4824–4854; https://doi.org/10.1039/c6gc01172a.Search in Google Scholar
20. Tarascon, J.-M., Key Challenges in Future Li-Battery Research. Phil. Trans. Math. Phys. Eng. Sci. 2010, 368 (1923), 3227–3241; https://doi.org/10.1098/rsta.2010.0112.Search in Google Scholar PubMed
21. Liu, L.; Wu, Y.; Wang, Y.; Wu, J.; Fu, S. Exploration of Environmentally Friendly Marine Power Technology-Ammonia/Diesel Stratified Injection. J. Clean. Prod. 2022, 380, 135014; https://doi.org/10.1016/j.jclepro.2022.135014.Search in Google Scholar
22. Liu, L.; Wu, J.; Liu, H.; Wu, Y.; Wang, Y. Investigation of Combustion and Emissions Characteristics in a Low-Speed Marine Engine Using Ammonia under Thermal and Reactive Atmospheres. Int. J. Hydrogen Energy 2024, 63, 1237–1247; https://doi.org/10.1016/j.ijhydene.2024.02.308.Search in Google Scholar
23. Muraru, S.; Ionita, M. Towards Performant Design of Carbon-Based Nanomotors for Hydrogen Separation through Molecular Dynamics Simulations. Int. J. Mol. Sci. 2020, 21 (24), 9588; https://doi.org/10.3390/ijms21249588.Search in Google Scholar PubMed PubMed Central
24. Liu, L.; Mei, Q.; Jia, W. A Flexible Diesel Spray Model for Advanced Injection Strategy. Fuel 2022, 314, 122784; https://doi.org/10.1016/j.fuel.2021.122784.Search in Google Scholar
25. Seoane, B.; Coronas, J.; Gascon, I.; Benavides, M. E.; Karvan, O.; Caro, J.; Kapteijn, F.; Gascon, J. Metal–organic Framework Based Mixed Matrix Membranes: A Solution for Highly Efficient CO2 Capture? Chem. Soc. Rev. 2015, 44 (8), 2421–2454; https://doi.org/10.1039/c4cs00437j.Search in Google Scholar PubMed PubMed Central
26. Zheng, S.; Hai, Q.; Zhou, X.; Stanford, R. J. A Novel Multi-Generation System for Sustainable Power, Heating, Cooling, Freshwater, and Methane Production: Thermodynamic, Economic, and Environmental Analysis. Energy 2024, 290, 130084; https://doi.org/10.1016/j.energy.2023.130084.Search in Google Scholar
27. Zhu, C.; Wang, M.; Guo, M.; Deng, J.; Du, Q.; Wei, W.; Zhang, Y.; Ashraf Talesh, S. S. Optimizing Solar-Driven Multi-Generation Systems: A Cascade Heat Recovery Approach for Power, Cooling, and Freshwater Production. Appl. Therm. Eng. 2024, 240, 122214; https://doi.org/10.1016/j.applthermaleng.2023.122214.Search in Google Scholar
28. Doung, T. 2002 Annual Progress Report for Energy Storage Research and Development, Freedom Car & Vehicle Technologies Program; 2003.Search in Google Scholar
29. Ohkubo, K.; Kohno, N.; Yamada, Y.; Fukuzumi, S. Laser-induced Pinpoint Hydrogen Evolution from Benzene and Water Using Metal Free Single-Walled Carbon Nanotubes with High Quantum Yields. Chem. Sci. 2015, 6 (1), 666–674; https://doi.org/10.1039/c4sc02269f.Search in Google Scholar PubMed PubMed Central
30. Kamat, P. V. Graphene-Based Nanoassemblies for Energy Conversion. J. Phys. Chem. Lett. 2011, 2 (3), 242–251; https://doi.org/10.1021/jz101639v.Search in Google Scholar
31. Huang, X.; Yin, Z.; Wu, S.; Qi, X.; He, Q.; Zhang, Q.; Yan, Q.; Boey, F.; Zhang, H. Graphene-Based Materials: Synthesis, Characterization, Properties, and Applications. Small 2011, 7 (14), 1876–1902; https://doi.org/10.1002/smll.201002009.Search in Google Scholar PubMed
32. Abid, N.; Khan, A. M.; Shujait, S.; Chaudhary, K.; Ikram, M.; Imran, M.; Haider, J.; Khan, M.; Khan, Q.; Maqbool, M. Synthesis of Nanomaterials Using Various Top-Down and Bottom-Up Approaches, Influencing Factors, Advantages, and Disadvantages: A Review. Adv. Colloid Interface Sci. 2022, 300, 102597; https://doi.org/10.1016/j.cis.2021.102597.Search in Google Scholar PubMed
33. Lu, W.-C.; Tseng, L.-C.; Chang, K.-S. Fabrication of TiO2-Reduced Graphene Oxide Nanorod Composition Spreads Using Combinatorial Hydrothermal Synthesis and Their Photocatalytic and Photoelectrochemical Applications. ACS Comb. Sci. 2017, 19 (9), 585–593; https://doi.org/10.1021/acscombsci.7b00077.Search in Google Scholar PubMed
34. Gonçalves, B. S.; Palhares, H. G.; Souza, T. C. D.; Castro, V. G. D.; Silva, G. G.; Silva, B. C.; Krambrock, K.; Soares, R. B.; Lins, V. F.; Houmard, M.; Nunes, E. H. Effect of the Carbon Loading on the Structural and Photocatalytic Properties of Reduced Graphene Oxide-TiO2 Nanocomposites Prepared by Hydrothermal Synthesis. J. Mater. Res. Technol. 2019, 8 (6), 6262–6274; https://doi.org/10.1016/j.jmrt.2019.10.020.Search in Google Scholar
35. He, H., Zhang, W., Ye, S., Li, S., Nie, Z., Zhang, Y., Xiong, M., Chen, W. T., Hu, G. Magnetical Multi-Walled Carbon Nanotubes with Lewis Acid-Base Imprinted Sites for Efficient Ni(II) Recovery with High Selectivity. Surf Interfaces 2024, 48, 104383; https://doi.org/10.1016/j.surfin.2024.104383.Search in Google Scholar
36. Anzar, N.; Hasan, R.; Tyagi, M.; Yadav, N.; Narang, J. Carbon Nanotube – A Review on Synthesis, Properties and Plethora of Applications in the Field of Biomedical Science. Sens. Int. 2020, 1, 100003; https://doi.org/10.1016/j.sintl.2020.100003.Search in Google Scholar
37. Rathinavel, S.; Priyadharshini, K.; Panda, D. A Review on Carbon Nanotube: An Overview of Synthesis, Properties, Functionalization, Characterization, and the Application. Mater. Sci. Eng., B 2021, 268, 115095; https://doi.org/10.1016/j.mseb.2021.115095.Search in Google Scholar
38. Wang, Z.; Zhang, L.; Zhang, K.; Lu, Y.; Chen, J.; Wang, S.; Hu, B.; Wang, X. Application of Carbon Dots and Their Composite Materials for the Detection and Removal of Radioactive Ions: A Review. Chemosphere 2022, 287, 132313; https://doi.org/10.1016/j.chemosphere.2021.132313.Search in Google Scholar PubMed
39. Sun, H.; Yang, G.; Yang, B. Synthesis, Structure Control and Applications of Carbon Dots. Chem. J. Chinese Univ.–Chinese 2021, 42 (2), 349–365.Search in Google Scholar
40. He, C.; Xu, P.; Zhang, X.; Long, W. The Synthetic Strategies, Photoluminescence Mechanisms and Promising Applications of Carbon Dots: Current State and Future Perspective. Carbon 2022, 186, 91–127; https://doi.org/10.1016/j.carbon.2021.10.002.Search in Google Scholar
41. Liu, H.; Zhong, X.; Pan, Q.; Zhang, Y.; Deng, W.; Zou, G.; Hou, H.; Ji, X. A Review of Carbon Dots in Synthesis Strategy. Coord. Chem. Rev. 2024, 498, 215468; https://doi.org/10.1016/j.ccr.2023.215468.Search in Google Scholar
42. Vinoth Kumar, J.; Kavitha, G.; Arulmozhi, R.; Arul, V.; Singaravadivel, S.; Abirami, N. Green Sources Derived Carbon Dots for Multifaceted Applications. J. Fluoresc. 2021, 31, 915–932; https://doi.org/10.1007/s10895-021-02721-4.Search in Google Scholar PubMed
43. Li, H.; Han, S.; Lyu, B.; Hong, T.; Zhi, S.; Xu, L.; Xue, F.; Sai, L.; Yang, J.; Wang, X.; He, B. Tunable Light Emission From Carbon Dots by Controlling Surface Defects. Chin. Chem. Lett. 2021, 32 (9), 2887–2892; https://doi.org/10.1016/j.cclet.2021.03.051.Search in Google Scholar
44. Jiao, Y.; Liu, Y.; Meng, Y.; Gao, Y.; Lu, W.; Liu, Y.; Gong, X.; Shuang, S.; Dong, C. Novel Processing for Color-Tunable Luminescence Carbon Dots and Their Advantages in Biological Systems. ACS Sustain. Chem. Eng. 2020, 8 (23), 8585–8592; https://doi.org/10.1021/acssuschemeng.0c01016.Search in Google Scholar
45. Korah, B. K.; Chacko, A. R.; Mathew, S.; John, B. K.; Abraham, T.; Mathew, B. Biomass-Derived Carbon Dots as a Sensitive and Selective Dual Detection Platform for Fluoroquinolones and Tetracyclines. Anal. Bioanal. Chem. 2022, 414 (17), 4935–4951; https://doi.org/10.1007/s00216-022-04119-y.Search in Google Scholar PubMed
46. Annamalai, K.; Annamalai, A.; Ravichandran, R.; Jeevarathinam, A.; Annamalai, P.; Valdes, H.; Elumalai, S. Simple Devising of N-Doped Carbon Dots (N-CDs) as a Low-Cost Probe for Selective Environmental Toxin Detection and Security Applications. New J. Chem. 2024, 48 (1), 216–227; https://doi.org/10.1039/d3nj04571d.Search in Google Scholar
47. Wang, F.-T.; Wang, L. N.; Xu, J.; Huang, K. J.; Wu, X. Synthesis and Modification of Carbon Dots for Advanced Biosensing Application. Analyst 2021, 146 (14), 4418–4435; https://doi.org/10.1039/d1an00466b.Search in Google Scholar PubMed
48. Nallayagari, A., Sgreccia, E., Pizzoferrato, R., Cabibbo, M., Kaciulis, S., Bolli, E., Pasquini, L., Knauth, P., Di Vona, M. L. Tuneable Properties of Carbon Quantum Dots by Different Synthetic Methods. J. Nanostruct. Chem. 2021, 12, 1–16; https://doi.org/10.1007/s40097-021-00431-8.Search in Google Scholar
49. de Boëver, R.; Town, J. R.; Li, X.; Claverie, J. P. Carbon Dots for Carbon Dummies: The Quantum and the Molecular Questions Among Some Others. Chem.–Eur. J. 2022, 28 (47), e202200748; https://doi.org/10.1002/chem.202200748.Search in Google Scholar PubMed
50. Yadav, D.; Amini, F.; Ehrmann, A. Recent Advances in Carbon Nanofibers and Their Applications – A Review. Eur. Polym. J. 2020, 138, 109963; https://doi.org/10.1016/j.eurpolymj.2020.109963.Search in Google Scholar
51. Ghulam, M. Highly-Efficient Ni@CuS/SGCN Nanocomposite with Superior Bifunctional Electrocatalytic Activity for Water Splitting. J. Electrochem. Soc. 2023, 170 (11), 116506; https://doi.org/10.1149/1945-7111/ad0ebc.Search in Google Scholar
52. Qamar, M. A.; Javed, M.; Shahid, S. Designing and Investigation of Enhanced Photocatalytic and Antibacterial Properties of 3d (Fe, Co, Ni, Mn and Cr) Metal-Doped Zinc Oxide Nanoparticles. Opt. Mater. 2022, 126, 112211; https://doi.org/10.1016/j.optmat.2022.112211.Search in Google Scholar
53. Ruiz-Cornejo, J. C.; Sebastián, D.; Lázaro, M. J. Synthesis and Applications of Carbon Nanofibers: A Review. Rev. Chem. Eng. 2020, 36 (4), 493–511; https://doi.org/10.1515/revce-2018-0021.Search in Google Scholar
54. Nguyen, T. D.; Lee, J. S. Electrospinning-Based Carbon Nanofibers for Energy and Sensor Applications. Appl. Sci. 2022, 12 (12), 6048; https://doi.org/10.3390/app12126048.Search in Google Scholar
55. Moulefera, I.; Trabelsi, M.; Mamun, A.; Sabantina, L. Electrospun Carbon Nanofibers from Biomass and Biomass Blends – Current Trends. Polymers 2021, 13 (7), 1071; https://doi.org/10.3390/polym13071071.Search in Google Scholar PubMed PubMed Central
56. Heckova, M.; Streckova, M.; Orinakova, R.; Hovancova, J.; Guboova, A.; Sopcak, T.; Kovalcikova, A.; Plesingerova, B.; Medved, D.; Szabo, J.; Dusza, J. Porous Carbon Fibers for Effective Hydrogen Evolution. Appl. Surf. Sci. 2020, 506, 144955; https://doi.org/10.1016/j.apsusc.2019.144955.Search in Google Scholar
57. Streckova, M.; Orinakova, R.; Hovancova, J.; Heckova, M.; A Guboova; Girman, V.; Mudra, E.; Dankova, Z.; Bekenyiova, A.; Dusza, J. Novel Electrocatalysts for Hydrogen Evolution Based on Carbon Fibers Modified by Cobalt Phosphides. Appl. Surf. Sci. 2020, 507, 144927; https://doi.org/10.1016/j.apsusc.2019.144927.Search in Google Scholar
58. Chen, X.; Yu, B.; Dong, Y.; Zhu, X.; Zhang, W.; Ramakrishna, S.; Liu, Z. Electrospun Porous Carbon Nanofibers Decorated with Iron-Doped Cobalt Phosphide Nanoparticles for Hydrogen Evolution. J. Alloys Compd. 2022, 918, 165733; https://doi.org/10.1016/j.jallcom.2022.165733.Search in Google Scholar
59. Thaweelap, N.; Plerdsranoy, P.; Poo-arporn, Y.; Khajondetchairit, P.; Suthirakun, S.; Fongkaew, I.; Hirunsit, P.; Chanlek, N.; Utke, O.; Pangon, A.; Utke, R. Ni-Doped Activated Carbon Nanofibers for Storing Hydrogen at Ambient Temperature: Experiments and Computations. Fuel 2021, 288, 119608; https://doi.org/10.1016/j.fuel.2020.119608.Search in Google Scholar
60. Wang, X.; Luo, Z.; Huang, J.; Chen, Z.; Xiang, T.; Feng, Z.; Wang, J.; Wang, S.; Ma, Y.; Yang, H. S/N-co-Doped Graphite Nanosheets Exfoliated via Three-Roll Milling for High-Performance Sodium/potassium Ion Batteries. J. Mater. Sci. Technol. 2023, 147, 47–55; https://doi.org/10.1016/j.jmst.2022.11.015.Search in Google Scholar
61. Ali, Z.; Mehmood, M.; Ghazi, Z. A. Herring Bone Graphitic Nanofibers Grown on NiFe-Silica Nanocomposites by CVD Method for HER Activity in Alkaline Media. Mater. Lett. 2021, 305, 130838; https://doi.org/10.1016/j.matlet.2021.130838.Search in Google Scholar
62. Wu, L.; Wu, H.; Wang, X.; Zhong, H.; Wang, Z.; Cai, G.; Jiang, C.; Ren, F. A General Method for Large-Scale Fabrication of Metal Nanoparticles Embedded N-Doped Carbon Fiber Cloth with Highly Efficient Hydrogen Production in All pH Range. Electrochim. Acta 2020, 353, 136475; https://doi.org/10.1016/j.electacta.2020.136475.Search in Google Scholar
63. Colomer, J.-F.; Stephan, C.; Lefrant, S.; Van Tendeloo, G.; Willems, I.; Kónya, Z.; Fonseca, A.; Laurent, C.; Nagy, J. Large-Scale Synthesis of Single-Wall Carbon Nanotubes by Catalytic Chemical Vapor Deposition (CCVD) Method. Chem. Phys. Lett. 2000, 317 (1–2), 83–89; https://doi.org/10.1016/s0009-2614(99)01338-x.Search in Google Scholar
64. Corrias, M.; Caussat, B.; Ayral, A.; Durand, J.; Kihn, Y.; Kalck, P.; Serp, P. Carbon Nanotubes Produced by Fluidized Bed Catalytic CVD: First Approach of the Process. Chem. Eng. Sci. 2003, 58 (19), 4475–4482; https://doi.org/10.1016/s0009-2509(03)00265-3.Search in Google Scholar
65. Wang, Y.; Wei, F.; Luo, G.; Yu, H.; Gu, G. The Large-Scale Production of Carbon Nanotubes in a Nano-Agglomerate Fluidized-Bed Reactor. Chem. Phys. Lett. 2002, 364 (5–6), 568–572; https://doi.org/10.1016/s0009-2614(02)01384-2.Search in Google Scholar
66. Weizhong, Q.; Fei, W.; Zhanwen, W.; Tang, L.; Hao, Y.; Guohua, L.; Lan, X.; Xiangyi, D. Production of Carbon Nanotubes in a Packed Bed and a Fluidized Bed. AIChE J. 2003, 49 (3), 619–625; https://doi.org/10.1002/aic.690490308.Search in Google Scholar
67. Ermakova, M. A.; Ermakov, D. Y.; Chuvilin, A. L.; Kuvshinov, G. G. Decomposition of Methane over Iron Catalysts at the Range of Moderate Temperatures: The Influence of Structure of the Catalytic Systems and the Reaction Conditions on the Yield of Carbon and Morphology of Carbon Filaments. J. Catal. 2001, 201 (2), 183–197; https://doi.org/10.1006/jcat.2001.3243.Search in Google Scholar
68. Bazargan, A.; McKay, G. A Review – Synthesis of Carbon Nanotubes from Plastic Wastes. Chem. Eng. J. 2012, 195, 377–391; https://doi.org/10.1016/j.cej.2012.03.077.Search in Google Scholar
69. Liu, J.; Jiang, Z.; Yu, H.; Tang, T. Catalytic Pyrolysis of Polypropylene to Synthesize Carbon Nanotubes and Hydrogen through a Two-Stage Process. Polym. Degrad. Stabil. 2011, 96 (10), 1711–1719; https://doi.org/10.1016/j.polymdegradstab.2011.08.008.Search in Google Scholar
70. Qamar, M. A.; Javed, M.; Shahid, S.; Iqbal, S.; Abubshait, S. A.; Abubshait, H. A.; Ramay, S. M.; Mahmood, A.; Ghaithan, H. M. Designing of Highly Active G-C3N4/Co@ZnO Ternary Nanocomposites for the Disinfection of Pathogens and Degradation of the Organic Pollutants from Wastewater under Visible Light. J. Environ. Chem. Eng. 2021, 9 (4), 105534; https://doi.org/10.1016/j.jece.2021.105534.Search in Google Scholar
71. Qamar, M. A.; Javed, M.; Shahid, S.; Sher, M. Fabrication of G-C3N4/Transition Metal (Fe, Co, Ni, Mn and Cr)-Doped ZnO Ternary Composites: Excellent Visible Light Active Photocatalysts for the Degradation of Organic Pollutants From Wastewater. Mater. Res. Bull. 2022, 147, 111630; https://doi.org/10.1016/j.materresbull.2021.111630.Search in Google Scholar
72. Rao, N.; Singh, R.; Bashambu, L. Carbon-Based Nanomaterials: Synthesis and Prospective Applications. Mater. Today: Proc. 2021, 44, 608–614; https://doi.org/10.1016/j.matpr.2020.10.593.Search in Google Scholar
73. Gamal, A.; Eid, K.; El-Naas, M. H.; Kumar, D.; Kumar, A. Catalytic Methane Decomposition to Carbon Nanostructures and COx-free Hydrogen: A Mini-Review. Nanomaterials 2021, 11 (5), 1226; https://doi.org/10.3390/nano11051226.Search in Google Scholar PubMed PubMed Central
74. Pham, C. Q.; Siang, T. J.; Kumar, P. S.; Ahmad, Z.; Xiao, L.; Bahari, M. B.; Cao, A. N. T.; Rajamohan, N.; Qazaq, A. S.; Kumar, A.; Show, P. L.; Vo, D. V. N. Production of Hydrogen and Value-Added Carbon Materials by Catalytic Methane Decomposition: A Review. Environ. Chem. Lett. 2022, 20 (4), 2339–2359; https://doi.org/10.1007/s10311-022-01449-2.Search in Google Scholar
75. Ganash, E. A.; Al-Jabarti, G. A.; Altuwirqi, R. M. The Synthesis of Carbon-Based Nanomaterials by Pulsed Laser Ablation in Water. Mater. Res. Express 2019, 7 (1), 015002; https://doi.org/10.1088/2053-1591/ab572b.Search in Google Scholar
76. Mintcheva, N.; Yamaguchi, S.; Kulinich, S. A. Hybrid TiO2-ZnO Nanomaterials Prepared Using Laser Ablation in Liquid. Materials 2020, 13 (3), 719; https://doi.org/10.3390/ma13030719.Search in Google Scholar PubMed PubMed Central
77. Guillén, G. G.; Shaji, S.; Palma, M. I. M.; Avellaneda, D.; Castillo, G.; Roy, T. D.; Gutiérrez, D. G.; Krishnan, B. Effects of Ablation Energy and Post-Irradiation on the Structure and Properties of Titanium Dioxide Nanomaterials. Appl. Surf. Sci. 2017, 405, 183–194; https://doi.org/10.1016/j.apsusc.2017.01.282.Search in Google Scholar
78. Guo, Y.; Fu, X.; Peng, Z. Controllable Synthesis of MoS2 Nanostructures From Monolayer Flakes, Few-Layer Pyramids to Multilayer Blocks by Catalyst-Assisted Thermal Evaporation. J. Mater. Sci. 2018, 53 (11), 8098–8107; https://doi.org/10.1007/s10853-018-2103-0.Search in Google Scholar
79. Svetlichnyi, V. A.; Shabalina, A. V.; Lapin, I. N.; Goncharova, D. A.; Kharlamova, T. S.; Stadnichenko, A. I. Comparative Study of Magnetite Nanoparticles Obtained by Pulsed Laser Ablation in Water and Air. Appl. Surf. Sci. 2019, 467, 402–410; https://doi.org/10.1016/j.apsusc.2018.10.189.Search in Google Scholar
80. Manawi, Y. M.; Ihsanullah; Samara, A.; Al-Ansari, T.; Atieh, M. A. A Review of Carbon Nanomaterials’ Synthesis via the Chemical Vapor Deposition (CVD) Method. Materials 2018, 11 (5), 822; https://doi.org/10.3390/ma11050822.Search in Google Scholar PubMed PubMed Central
81. Wang, H.; Zhu, H.; Wang, S.; Qi, D.; Shen, K. Dicarbonyl-Tuned Microstructures of Hierarchical Porous Carbons Derived From Coal-Tar Pitch for Supercapacitor Electrodes. RSC Adv. 2019, 9 (35), 20019–20028; https://doi.org/10.1039/c9ra03813b.Search in Google Scholar PubMed PubMed Central
82. Yang, X.; Wang, F.; Li, X.; Zhang, Z.; Wang, C.; Yang, C.; Zhen, Y.; Wang, D.; Fu, F. Facile and Low-Cost Fabrication of Interconnected Hierarchically Porous Carbon for High-Performance Supercapacitors. J. Alloys Compd. 2022, 921, 166127; https://doi.org/10.1016/j.jallcom.2022.166127.Search in Google Scholar
83. de Souza Vieira, L. A Review on the Use of Glassy Carbon in Advanced Technological Applications. Carbon 2022, 186, 282–302; https://doi.org/10.1016/j.carbon.2021.10.022.Search in Google Scholar
84. Duan, Z., Yuan, Z., Jiang, Y., Yuan, L., Tai, H. Amorphous Carbon Material of Daily Carbon Ink: Emerging Applications in Pressure, Strain, and Humidity Sensors. J. Mater. Chem. C 2023, 11 (17), 5585–6000; https://doi.org/10.1039/d3tc00016h.Search in Google Scholar
85. Walker, P. L. Chemistry & Physics of Carbon: Volume 16; CRC Press: USA, 16, 2021.Search in Google Scholar
86. Abdulhafez, M.; Tomaraei, G. N.; Bedewy, M. Fluence-dependent Morphological Transitions in Laser-Induced Graphene Electrodes on Polyimide Substrates for Flexible Devices. ACS Appl. Nano Mater. 2021, 4 (3), 2973–2986; https://doi.org/10.1021/acsanm.1c00101.Search in Google Scholar
87. Houeix, Y.; Romero, F. J.; Moraila, C. L.; Rivadeneyra, A.; Rodriguez, N.; Morales, D. P.; Salinas-Castillo, A. Laser-Synthesis of Conductive Carbon-Based Materials From Two Flexible Commercial Substrates: A Comparison. Appl. Surf. Sci. 2023, 634, 157629; https://doi.org/10.1016/j.apsusc.2023.157629.Search in Google Scholar
88. Craddock, P. R.; Haecker, A.; Bake, K. D.; Pomerantz, A. E. Universal Curves Describing the Chemical and Physical Evolution of Type II Kerogen during Thermal Maturation. Energy Fuels 2020, 34 (12), 15217–15233; https://doi.org/10.1021/acs.energyfuels.0c02376.Search in Google Scholar
89. Guo, F.; Qin, S.; Xu, L.; Bai, Y.; Xing, B. Thermal Degradation Features of Soil Humic Acid Sub-Fractions in Pyrolytic Treatment and Their Relation to Molecular Signatures. Sci. Total Environ. 2020, 749, 142318; https://doi.org/10.1016/j.scitotenv.2020.142318.Search in Google Scholar PubMed
90. Lv, J.; Huang, Z.; Luo, L.; Zhang, S.; Wang, Y. Advances in Molecular and Microscale Characterization of Soil Organic Matter: Current Limitations and Future Prospects. Environ. Sci. Technol. 2022, 56 (18), 12793–12810; https://doi.org/10.1021/acs.est.2c00421.Search in Google Scholar PubMed
91. Gacem, A.; Modi, S.; Yadav, V. K.; Islam, S.; Patel, A.; Dawane, V.; Jameel, M.; Inwati, G. K.; Piplode, S.; Solanki, V. S.; Basnet, A. Recent Advances in Methods for Synthesis of Carbon Nanotubes and Carbon Nanocomposite and Their Emerging Applications: A Descriptive Review. J. Nanomater. 2022, 2022, 1–16; https://doi.org/10.1155/2022/7238602.Search in Google Scholar
92. Mbayachi, V. B.; Ndayiragije, E.; Sammani, T.; Taj, S.; Mbuta, E. R.; Khan, A. U. Graphene Synthesis, Characterization and its Applications: A Review. Results Chem. 2021, 3, 100163; https://doi.org/10.1016/j.rechem.2021.100163.Search in Google Scholar
93. Ashok Kumar, S. S.; Bashir, S.; Ramesh, K.; Ramesh, S. A Review on Graphene and its Derivatives as the Forerunner of the Two-Dimensional Material Family for the Future. J. Mater. Sci. 2022, 57 (26), 12236–12278; https://doi.org/10.1007/s10853-022-07346-x.Search in Google Scholar
94. Ahmad, M.; Silva, S. R. P. Low Temperature Growth of Carbon Nanotubes – A Review. Carbon 2020, 158, 24–44; https://doi.org/10.1016/j.carbon.2019.11.061.Search in Google Scholar
95. Choudhary, M.; Sharma, A.; Aravind Raj, S.; Sultan, M. T. H.; Hui, D.; Shah, A. U. M. Contemporary Review on Carbon Nanotube (CNT) Composites and Their Impact on Multifarious Applications. Nanotechnol. Rev. 2022, 11 (1), 2632–2660; https://doi.org/10.1515/ntrev-2022-0146.Search in Google Scholar
96. Xu, S., Zhang, L., Wang, B., Ruoff, R. S. Chemical Vapor Deposition of Graphene on Thin-Metal Films. Cell Rep. Phys. Sci. 2021, 2 (3), 1–38; https://doi.org/10.1016/j.xcrp.2021.100410.Search in Google Scholar
97. Gupta, N.; Gupta, S. M.; Sharma, S. Carbon Nanotubes: Synthesis, Properties and Engineering Applications. Carbon Lett. 2019, 29, 419–447; https://doi.org/10.1007/s42823-019-00068-2.Search in Google Scholar
98. Sanchez-Valencia, J. R.; Dienel, T.; Gröning, O.; Shorubalko, I.; Mueller, A.; Jansen, M.; Amsharov, K.; Ruffieux, P.; Fasel, R. Controlled Synthesis of Single-Chirality Carbon Nanotubes. Nature 2014, 512 (7512), 61–64; https://doi.org/10.1038/nature13607.Search in Google Scholar PubMed
99. Peebles, L. H. Carbon Fibers: Formation, Structure, and Properties; CRC Press: USA, 2018.10.1201/9781351070423Search in Google Scholar
100. Januś, M. DLC Layers Created Using CVD Techniques and Their Application. Chem. Vap. Depos. Nanotechnol. 2018, https://doi.org/10.5772/intechopen.79526.Search in Google Scholar
101. Sanchette, F.; El Garah, M.; Achache, S.; Schuster, F.; Chouquet, C.; Ducros, C.; Billard, A. DLC-Based Coatings Obtained by Low-Frequency Plasma-Enhanced Chemical Vapor Deposition (LFPECVD) in Cyclohexane, Principle and Examples. Coatings 2021, 11 (10), 1225; https://doi.org/10.3390/coatings11101225.Search in Google Scholar
102. Sharma, S. Glassy Carbon: A Promising Material for Micro-and Nanomanufacturing. Materials 2018, 11 (10), 1857; https://doi.org/10.3390/ma11101857.Search in Google Scholar PubMed PubMed Central
103. Uskoković, V. A Historical Review of Glassy Carbon: Synthesis, Structure, Properties and Applications. Carbon Trends 2021, 5, 100116; https://doi.org/10.1016/j.cartre.2021.100116.Search in Google Scholar
104. He, X.; Jiang, Z.; Akakuru, O. U.; Li, J.; Wu, A. Nanoscale Covalent Organic Frameworks: From Controlled Synthesis to Cancer Therapy. Chem. Commun. 2021, 57 (93), 12417–12435; https://doi.org/10.1039/d1cc04846e.Search in Google Scholar PubMed
105. Gholizadeh, M.; Li, C.; Zhang, S.; Wang, Y.; Niu, S.; Li, Y.; Hu, X. Progress of the Development of Reactors for Pyrolysis of Municipal Waste. Sustain. Energy Fuels 2020, 4 (12), 5885–5915; https://doi.org/10.1039/d0se01122c.Search in Google Scholar
106. Wang, X.; Xia, Y.; Huang, J.; Su, Y.; Chen, Z.; Chen, L.; Wu, Z.; Feng, Z.; Yang, H. A Facile Strategy for Large-Scale Production of 2D Nanosheets Exfoliated by Three-Roll Milling. J. Adv. Ceram. 2024, 13 (1); https://doi.org/10.26599/jac.2024.9220831.Search in Google Scholar
107. Morosanu, C. E. Thin Films by Chemical Vapour Deposition; Elsevier: Amsterdam, 7, 2016.Search in Google Scholar
108. Barroso Bogeat, A. Understanding and Tuning the Electrical Conductivity of Activated Carbon: A State-of-the-Art Review. Crit. Rev. Solid State Mater. Sci. 2021, 46 (1), 1–37; https://doi.org/10.1080/10408436.2019.1671800.Search in Google Scholar
109. Ishii, T.; Okuhara, D.; Kobayashi, R.; Ozaki, J. I. Modulation of the Electronic State of Carbon Thin Films by Inorganic Substrates. Carbon 2022, 196, 313–319; https://doi.org/10.1016/j.carbon.2022.04.079.Search in Google Scholar
110. Krungleviciute, V.; Ziegler, C. A.; Banjara, S. R.; Yudasaka, M.; Iijima, S.; Migone, A. D. Neon and CO2 Adsorption on Open Carbon Nanohorns. Langmuir 2013, 29 (30), 9388–9397; https://doi.org/10.1021/la401033u.Search in Google Scholar PubMed
111. Chen, C.; Zhu, Y.; Tian, M.; Chen, Y.; Yang, Y.; Jiang, K.; Gao, S. Sustainable Self-Powered Electro-Fenton Degradation Using N, S Co-doped Porous Carbon Catalyst Fabricated with Adsorption-Pyrolysis-Doping Strategy. Nano Energy 2021, 81, 105623; https://doi.org/10.1016/j.nanoen.2020.105623.Search in Google Scholar
112. AlShammari, A.; Halim, M.; Yam, F.; Kaus, N. M. Synthesis of Titanium Dioxide (TiO2)/Reduced Graphene Oxide (rGO) Thin Film Composite by Spray Pyrolysis Technique and its Physical Properties. Mater. Sci. Semicond. Process. 2020, 116, 105140; https://doi.org/10.1016/j.mssp.2020.105140.Search in Google Scholar
113. Gnanasekar, T.; Valanarasu, S.; Ubaidullah, M.; Alam, M.; Nafady, A.; Mohanraj, P.; Poul Raj, I. L.; Ahmad, T.; Shahazad, M.; Pandit, B. Fabrication of Er, Tb Doped CuO Thin Films Using Nebulizer Spray Pyrolysis Technique for Photosensing Applications. Opt. Mater. 2022, 123, 111954; https://doi.org/10.1016/j.optmat.2021.111954.Search in Google Scholar
114. Toh, C.-T.; Zhang, H.; Lin, J.; Mayorov, A. S.; Wang, Y. P.; Orofeo, C. M.; Ferry, D. B.; Andersen, H.; Kakenov, N.; Guo, Z.; Abidi, I. H.; Sims, H.; Suenaga, K.; Pantelides, S. T.; Özyilmaz, B. Synthesis and Properties of Free-Standing Monolayer Amorphous Carbon. Nature 2020, 577 (7789), 199–203; https://doi.org/10.1038/s41586-019-1871-2.Search in Google Scholar PubMed
115. Shi, Y.; Xu, S.; Yang, Y.; Slizovskiy, S.; Morozov, S. V.; Son, S. K.; Ozdemir, S.; Mullan, C.; Barrier, J.; Yin, J.; Berdyugin, A. I.; Piot, B. A.; Taniguchi, T.; Watanabe, K.; Fal’ko, V. I.; Novoselov, K. S.; Geim, A. K.; Mishchenko, A. Electronic Phase Separation in Multilayer Rhombohedral Graphite. Nature 2020, 584 (7820), 210–214; https://doi.org/10.1038/s41586-020-2568-2.Search in Google Scholar PubMed
116. Li, M., Liu, X., Zhao, X., Yang, F., Wang, X., Li, Y. Metallic Catalysts for Structure-Controlled Growth of Single-Walled Carbon Nanotubes. In Single-Walled Carbon Nanotubes: Preparation, Properties and Applications; Springer: New York, 2019; pp. 25–67.10.1007/978-3-030-12700-8_2Search in Google Scholar
117. Zhao, G.; Zhang, C.; Lv, L.; Liu, J.; Guo, S. Research on Dynamic Microwave Low-Temperature Carbonization of High Performance Carbon Fiber. Diam. Relat. Mater. 2022, 125, 108989; https://doi.org/10.1016/j.diamond.2022.108989.Search in Google Scholar
118. Rife, J. L.; Kung, P.; Hooper, R. J.; Allen, J.; Thompson, G. B. Structural and Mechanical Characterization of Carbon Fibers Grown by Laser Induced Chemical Vapor Deposition at Hyperbaric Pressures. Carbon 2020, 162, 95–105; https://doi.org/10.1016/j.carbon.2020.02.018.Search in Google Scholar
119. Yue, Z.; Economy, J. Carbonization and Activation for Production of Activated Carbon Fibers. Act. Carbon Fiber Text. 2017, 61–139.10.1016/B978-0-08-100660-3.00004-3Search in Google Scholar
120. Mandal, S. Nucleation of Diamond Films on Heterogeneous Substrates: A Review. RSC Adv. 2021, 11 (17), 10159–10182; https://doi.org/10.1039/d1ra00397f.Search in Google Scholar PubMed PubMed Central
121. Devi, M.; Rawat, S.; Sharma, S. A Comprehensive Review of the Pyrolysis Process: From Carbon Nanomaterial Synthesis to Waste Treatment. Oxf. Open Mater. Sci. 2021, 1 (1), itab014; https://doi.org/10.1093/oxfmat/itab014.Search in Google Scholar
122. Raju, K. S., Das, G. S., Tripathi, K. M. Nitrogen-Doped Carbon Quantum Dots From Biomass as a FRET-Based Sensing Platform for the Selective Detection of H2O2 and Aspartic Acid. RSC Sustain. 2024, 2 (1), 223–232; https://doi.org/10.1039/d3su00343d.Search in Google Scholar
123. Jiang, Z.; Han, X.; Zhao, C.; Wang, S.; Tang, X. Recent Advance in Biological Responsive Nanomaterials for Biosensing and Molecular Imaging Application. Int. J. Mol. Sci. 2022, 23 (3), 1923; https://doi.org/10.3390/ijms23031923.Search in Google Scholar PubMed PubMed Central
124. Turnbull, T. Energy, History, and the Humanities: Against a New Determinism. Hist. Technol. 2021, 37 (2), 247–292; https://doi.org/10.1080/07341512.2021.1891394.Search in Google Scholar
125. Melsted, O.; Pallua, I. The Historical Transition From Coal to Hydrocarbons: Previous Explanations and the Need for an Integrative Perspective. Can. J. Hist. 2018, 53 (3), 395–422; https://doi.org/10.3138/cjh.ach.53.3.03.Search in Google Scholar
126. Pan, F.; Ni, K.; Xu, T.; Chen, H.; Wang, Y.; Gong, K.; Liu, C.; Li, X.; Lin, M. L.; Li, S.; Wang, X.; Yan, W.; Yin, W.; Tan, P. H.; Sun, L.; Yu, D.; Ruoff, R. S.; Zhu, Y. Long-Range Ordered Porous Carbons Produced From C60. Nature 2023, 614 (7946), 95–101; https://doi.org/10.1038/s41586-022-05532-0.Search in Google Scholar PubMed
127. Pal, M., Subhedar, K. M. CNT Yarn Based Solid State Linear Supercapacitor With Multi-Featured Capabilities for Wearable and Implantable Devices. Energy Storage Mater. 2023, 57, 136–172; https://doi.org/10.1016/j.ensm.2023.01.051.Search in Google Scholar
128. Zhao, X.; Zhang, J.; Lv, K.; Kong, N.; Shao, Y.; Tao, J. Carbon Nanotubes Boosts the Toughness and Conductivity of Wet-Spun MXene Fibers for Fiber-Shaped Super Capacitors. Carbon 2022, 200, 38–46; https://doi.org/10.1016/j.carbon.2022.08.045.Search in Google Scholar
129. Centi, G.; Perathoner, S. Carbon Nanotubes for Sustainable Energy Applications. Chem. Sus. Chem. 2011, 4 (7), 913–925; https://doi.org/10.1002/cssc.201100084.Search in Google Scholar PubMed
130. Zhu, S.; Sheng, J.; Chen, Y.; Ni, J.; Li, Y. Carbon Nanotubes for Flexible Batteries: Recent Progress and Future Perspective. Natl. Sci. Rev. 2021, 8 (5), nwaa261; https://doi.org/10.1093/nsr/nwaa261.Search in Google Scholar PubMed PubMed Central
131. Ding, R.; Huang, Y.; Li, G.; Liao, Q.; Wei, T.; Liu, Y.; Huang, Y.; He, H. Carbon Anode Materials for Rechargeable Alkali Metal Ion Batteries and In-Situ Characterization Techniques. Front. Chem. 2020, 8, 607504; https://doi.org/10.3389/fchem.2020.607504.Search in Google Scholar PubMed PubMed Central
132. Guo, Z.; Xu, Z.; Xie, F.; Jiang, J.; Zheng, K.; Alabidun, S.; Crespo-Ribadeneyra, M.; Hu, Y.; Au, H.; Titirici, M. Investigating the Superior Performance of Hard Carbon Anodes in Sodium-Ion Compared with Lithium-and Potassium-Ion Batteries. Adv. Mater. 2023, 35 (42), 2304091; https://doi.org/10.1002/adma.202304091.Search in Google Scholar PubMed
133. Liu, Z.; Ling, Q.; Cai, Y.; Xu, L.; Su, J.; Yu, K.; Wu, X.; Xu, J.; Hu, B.; Wang, X. Synthesis of Carbon-Based Nanomaterials and Their Application in Pollution Management. Nanoscale Adv. 2022, 4 (5), 1246–1262; https://doi.org/10.1039/d1na00843a.Search in Google Scholar PubMed PubMed Central
134. Mubarik, S.; Qureshi, N.; Sattar, Z.; Shaheen, A.; Kalsoom, A.; Imran, M.; Hanif, F. Synthetic Approach to Rice Waste-Derived Carbon-Based Nanomaterials and Their Applications. Nanomanufacturing 2021, 1 (3), 109–159; https://doi.org/10.3390/nanomanufacturing1030010.Search in Google Scholar
135. Anil Kumar, Y.; Koyyada, G.; Ramachandran, T.; Kim, J. H.; Sajid, S.; Moniruzzaman, M.; Alzahmi, S.; Obaidat, I. M. Carbon Materials as a Conductive Skeleton for Supercapacitor Electrode Applications: A Review. Nanomaterials 2023, 13 (6), 1049; https://doi.org/10.3390/nano13061049.Search in Google Scholar PubMed PubMed Central
136. Zhong, M.; Zhang, M.; Li, X. Carbon Nanomaterials and Their Composites for Supercapacitors. Carbon Energy 2022, 4 (5), 950–985; https://doi.org/10.1002/cey2.219.Search in Google Scholar
137. Cheng, F.; Yang, X.; Zhang, S.; Lu, W. Boosting the Supercapacitor Performances of Activated Carbon With Carbon Nanomaterials. J. Power Sources 2020, 450, 227678; https://doi.org/10.1016/j.jpowsour.2019.227678.Search in Google Scholar
138. Balasubramanian, K.; Burghard, M. Chemically Functionalized Carbon Nanotubes. Small 2005, 1 (2), 180–192; https://doi.org/10.1002/smll.200400118.Search in Google Scholar PubMed
139. Karousis, N.; Tagmatarchis, N.; Tasis, D. Current Progress on the Chemical Modification of Carbon Nanotubes. Chem. Rev. 2010, 110 (9), 5366–5397; https://doi.org/10.1021/cr100018g.Search in Google Scholar PubMed
140. Anwer, A. H., Khan, M. Z. Graphene Quantum Dots for Clean Energy Solutions. In Graphene Quantum Dots; Elsevier: Amsterdam, 2023; pp. 183–209.10.1016/B978-0-323-85721-5.00004-2Search in Google Scholar
141. Ansari, S. Graphene Quantum Dots: Novel Properties and Their Applications for Energy Storage Devices. Nanomaterials 2022, 12, 3814, 2022, s Note: MDPI stays neutral with regard to jurisdictional claims in published; https://doi.org/10.3390/nano12213814.Search in Google Scholar PubMed PubMed Central
142. Prabhu, S. A.; Kavithayeni, V.; Suganthy, R.; Geetha, K. Graphene Quantum Dots Synthesis and Energy Application: A Review. Carbon Lett. 2021, 31, 1–12; https://doi.org/10.1007/s42823-020-00154-w.Search in Google Scholar
143. Anwer, A. H.; Shoeb, M.; Mashkoor, F.; Ali, A.; Kareem, S.; Ansari, M. Z.; Park, J. M.; Jeong, C. Simultaneous Reduction of Carbon Dioxide and Energy Harvesting Using RGO-Based SiO2-TiO2 Nanocomposite for Supercapacitor and Microbial Electrosynthesis. Appl. Catal. B Environ. 2023, 339, 123091; https://doi.org/10.1016/j.apcatb.2023.123091.Search in Google Scholar
144. Guardia, L.; Suárez, L.; Querejeta, N.; Vretenár, V.; Kotrusz, P.; Skákalová, V.; Centeno, T. A. Biomass Waste-Carbon/Reduced Graphene Oxide Composite Electrodes for Enhanced Supercapacitors. Electrochim. Acta 2019, 298, 910–917; https://doi.org/10.1016/j.electacta.2018.12.160.Search in Google Scholar
145. El Nady, J.; Shokry, A.; Khalil, M.; Ebrahim, S.; Elshaer, A. M.; Anas, M. One-Step Electrodeposition of a polypyrrole/NiO Nanocomposite as a Supercapacitor Electrode. Sci. Rep. 2022, 12 (1), 3611; https://doi.org/10.1038/s41598-022-07483-y.Search in Google Scholar PubMed PubMed Central
146. Abdah, M. A. A. M.; Mohd Razali, N. S.; Lim, P. T.; Kulandaivalu, S.; Sulaiman, Y. One-Step Potentiostatic Electrodeposition of Polypyrrole/Graphene Oxide/Multi-Walled Carbon Nanotubes Ternary Nanocomposite for Supercapacitor. Mater. Chem. Phys. 2018, 219, 120–128; https://doi.org/10.1016/j.matchemphys.2018.08.018.Search in Google Scholar
147. Tian, X.; Wang, T.; Ma, H.; Tang, K.; Hou, S.; Jin, H.; Cao, G. A Universal Strategy Towards 3D Printable Nanomaterial Inks for Superior Cellular High-Loading Battery Electrodes. J. Mater. Chem. A 2021, 9 (29), 16086–16092; https://doi.org/10.1039/d1ta03236d.Search in Google Scholar
148. Dhamodharan, D.; Ghoderao, P. P.; Dhinakaran, V.; Mubarak, S.; Divakaran, N.; Byun, H. S. A Review on Graphene Oxide Effect in Energy Storage Devices. J. Ind. Eng. Chem. 2022, 106, 20–36; https://doi.org/10.1016/j.jiec.2021.10.033.Search in Google Scholar
149. Ławkowska, K.; Pokrywczyńska, M.; Koper, K.; Kluth, L. A.; Drewa, T.; Adamowicz, J. Application of Graphene in Tissue Engineering of the Nervous System. Int. J. Mol. Sci. 2021, 23 (1), 33; https://doi.org/10.3390/ijms23010033.Search in Google Scholar PubMed PubMed Central
150. Liu, Q.; Sun, J.; Gao, K.; Chen, N.; Sun, X.; Ti, D.; Bai, C.; Cui, R.; Qu, L. Graphene Quantum Dots for Energy Storage and Conversion: From Fabrication to Applications. Mater. Chem. Front. 2020, 4 (2), 421–436; https://doi.org/10.1039/c9qm00553f.Search in Google Scholar
151. Cui, L.; Ren, X.; Sun, M.; Liu, H.; Xia, L. Carbon Dots: Synthesis, Properties and Applications. Nanomaterials 2021, 11 (12), 3419; https://doi.org/10.3390/nano11123419.Search in Google Scholar PubMed PubMed Central
152. Nair, A.; Haponiuk, J. T.; Thomas, S.; Gopi, S. Natural Carbon-Based Quantum Dots and Their Applications in Drug Delivery: A Review. Biomed. Pharmacother. 2020, 132, 110834; https://doi.org/10.1016/j.biopha.2020.110834.Search in Google Scholar PubMed PubMed Central
153. Kalashgrani, M. Y.; Nejad, F. F.; Rahmanian, V. Carbon Quantum Dots Platforms: As Nano Therapeutic for Biomedical Applications. Adv. Appl. NanoBio-Technol. 2022, 3 (2), 38–42.Search in Google Scholar
154. El-Shabasy, R. M.; Farouk Elsadek, M.; Mohamed Ahmed, B.; Fawzy Farahat, M.; Mosleh, K. N.; Taher, M. M. Recent Developments in Carbon Quantum Dots: Properties, Fabrication Techniques, and Bio-Applications. Processes 2021, 9 (2), 388; https://doi.org/10.3390/pr9020388.Search in Google Scholar
155. Sivasankarapillai, V. S.; Vishnu Kirthi, A.; Akksadha, M.; Indu, S.; Dhiviya Dharshini, U.; Pushpamalar, J.; Karthik, L. Recent Advancements in the Applications of Carbon Nanodots: Exploring the Rising Star of Nanotechnology. Nanoscale Adv. 2020, 2 (5), 1760–1773; https://doi.org/10.1039/c9na00794f.Search in Google Scholar PubMed PubMed Central
156. Gaurav, A.; Jain, A.; Tripathi, S. K. Review on Fluorescent Carbon/Graphene Quantum Dots: Promising Material for Energy Storage and Next-Generation Light-Emitting Diodes. Materials 2022, 15 (22), 7888; https://doi.org/10.3390/ma15227888.Search in Google Scholar PubMed PubMed Central
157. Zhou, Y.; Raghu, S. N.; Kumar, V.; Okada, T.; Yokozeki, T. Simulated Lightning Strike Investigation of CFRP Comprising a Novel Polyaniline/Phenol Based Electrically Conductive Resin Matrix. Compos. Sci. Technol. 2021, 214, 108971; https://doi.org/10.1016/j.compscitech.2021.108971.Search in Google Scholar
158. Zhao, Q.; Zhang, K.; Zhu, S.; Xu, H.; Cao, D.; Zhao, L.; Zhang, R.; Yin, W. Review on the Electrical Resistance/Conductivity of Carbon Fiber Reinforced Polymer. Appl. Sci. 2019, 9 (11), 2390; https://doi.org/10.3390/app9112390.Search in Google Scholar
159. Guo, S.; Zhang, Y.; Chen, J.; Wu, Y.; Cao, J.; Tang, S.; Ji, G. The Excellent Electromagnetic Wave Absorbing Properties of Carbon Fiber Composites: The Effect of Metal Content. Inorg. Chem. Front. 2022, 9 (13), 3244–3250; https://doi.org/10.1039/d2qi00854h.Search in Google Scholar
160. Zhou, X.; Wang, Y.; Gong, C.; Liu, B.; Wei, G. Production, Structural Design, Functional Control, and Broad Applications of Carbon Nanofiber-Based Nanomaterials: A Comprehensive Review. Chem. Eng. J. 2020, 402, 126189; https://doi.org/10.1016/j.cej.2020.126189.Search in Google Scholar
161. Yu, Y.; Wang, L.; Ni, S.; Li, D.; Liu, J.; Chu, H. Y.; Zhang, N.; Sun, M.; Ren, Q.; Zhuo, Z.; Zhong, C.; Xie, D.; Li, Y.; Zhang, Z. K.; Zhang, H.; Li, M.; Zhang, Z.; Chen, L.; Pan, X.; Xia, W.; Zhang, S.; Lu, A.; Zhang, B. T.; Zhang, G. Targeting Loop3 of Sclerostin Preserves its Cardiovascular Protective Action and Promotes Bone Formation. Nat. Commun. 2022, 13 (1), 4241; https://doi.org/10.1038/s41467-022-31997-8.Search in Google Scholar PubMed PubMed Central
162. Wilberforce, T.; Abdelkareem, M. A.; Elsaid, K.; Olabi, A.; Sayed, E. T. Role of Carbon-Based Nanomaterials in Improving the Performance of Microbial Fuel Cells. Energy 2022, 240, 122478; https://doi.org/10.1016/j.energy.2021.122478.Search in Google Scholar
163. Dong, L.; Feng, Y.; Wang, L.; Feng, W. Azobenzene-Based Solar Thermal Fuels: Design, Properties, and Applications. Chem. Soc. Rev. 2018, 47 (19), 7339–7368; https://doi.org/10.1039/c8cs00470f.Search in Google Scholar PubMed
164. Wang, W.; Li, Y.; Feng, Y.; Han, J.; Zhang, F.; Long, P.; Peng, C.; Cao, C.; Cao, Y.; Yang, H.; Feng, W. Asymmetric Self-Supporting Hybrid Fluorinated Carbon Nanotubes/Carbon Nanotubes Sponge Electrode for High-Performance Lithium-Polysulfide Battery. Chem. Eng. J. 2018, 349, 756–765; https://doi.org/10.1016/j.cej.2018.05.088.Search in Google Scholar
165. Lv, P.; Zhang, P.; Feng, Y.; Li, Y.; Feng, W. High-Performance Electrochemical Capacitors Using Electrodeposited MnO2 on Carbon Nanotube Array Grown on Carbon Fabric. Electrochim. Acta 2012, 78, 515–523; https://doi.org/10.1016/j.electacta.2012.06.085.Search in Google Scholar
166. Jiang, K.; Xiong, P.; Ji, J.; Zhu, J.; Ma, R.; Sasaki, T.; Geng, F. Two-Dimensional Molecular Sheets of Transition Metal Oxides Toward Wearable Energy Storage. Acc. Chem. Res. 2020, 53 (10), 2443–2455; https://doi.org/10.1021/acs.accounts.0c00483.Search in Google Scholar PubMed
167. Razaq, A.; Bibi, F.; Zheng, X.; Papadakis, R.; Jafri, S. H. M.; Li, H. Review on Graphene-Graphene Oxide-Reduced Graphene Oxide-Based Flexible Composites: From Fabrication to Applications. Materials 2022, 15 (3), 1012; https://doi.org/10.3390/ma15031012.Search in Google Scholar PubMed PubMed Central
168. Tiwari, S. K.; Sahoo, S.; Wang, N.; Huczko, A. Graphene Research and Their Outputs: Status and Prospect. J. Sci.: Adv. Mater. Devices 2020, 5 (1), 10–29; https://doi.org/10.1016/j.jsamd.2020.01.006.Search in Google Scholar
169. Wang, H.; Tran, D.; Qian, J.; Ding, F.; Losic, D. MoS2/Graphene Composites as Promising Materials for Energy Storage and Conversion Applications. Adv. Mater. Interfaces 2019, 6 (20), 1900915; https://doi.org/10.1002/admi.201900915.Search in Google Scholar
170. Khan, K.; Tareen, A. K.; Aslam, M.; Mahmood, A.; Khan, Q.; Zhang, Y.; Ouyang, Z.; Guo, Z.; Zhang, H. Going Green With Batteries and Supercapacitor: Two Dimensional Materials and Their Nanocomposites Based Energy Storage Applications. Prog. Solid State Chem. 2020, 58, 100254; https://doi.org/10.1016/j.progsolidstchem.2019.100254.Search in Google Scholar
171. Nassef, A. M.; Fathy, A.; Sayed, E. T.; Abdelkareem, M. A.; Rezk, H.; Tanveer, W. H.; Olabi, A. Maximizing SOFC Performance Through Optimal Parameters Identification by Modern Optimization Algorithms. Renew. Energy 2019, 138, 458–464; https://doi.org/10.1016/j.renene.2019.01.072.Search in Google Scholar
172. Xiong, G.; Zhang, J.; Shi, D.; Zhu, L.; Yuan, X. Optimal Identification of Solid Oxide Fuel Cell Parameters Using a Competitive Hybrid Differential Evolution and Jaya Algorithm. Int. J. Hydrogen Energy 2021, 46 (9), 6720–6733; https://doi.org/10.1016/j.ijhydene.2020.11.119.Search in Google Scholar
173. Abdelkareem, M. A.; Sayed, E. T.; Nakagawa, N. Significance of Diffusion Layers on the Performance of Liquid and Vapor Feed Passive Direct Methanol Fuel Cells. Energy 2020, 209, 118492; https://doi.org/10.1016/j.energy.2020.118492.Search in Google Scholar
174. Wang, X.; Ma, Y.; Gao, J.; Li, T.; Jiang, G.; Sun, Z. Review on Water Management Methods for Proton Exchange Membrane Fuel Cells. Int. J. Hydrogen Energy 2021, 46 (22), 12206–12229; https://doi.org/10.1016/j.ijhydene.2020.06.211.Search in Google Scholar
175. Siracusano, S.; Trocino, S.; Briguglio, N.; Pantò, F.; Aricò, A. S. Analysis of Performance Degradation during Steady-State and Load-Thermal Cycles of Proton Exchange Membrane Water Electrolysis Cells. J. Power Sources 2020, 468, 228390; https://doi.org/10.1016/j.jpowsour.2020.228390.Search in Google Scholar
176. Osman, S. H.; Kamarudin, S. K.; Karim, N. A.; Basri, S. Application of Graphene in Low-Temperature Fuel Cell Technology: An Overview. Int. J. Energy Res. 2021, 45 (13), 18318–18336; https://doi.org/10.1002/er.6969.Search in Google Scholar
177. Iqbal, M. Z.; Siddique, S.; Khan, A.; Haider, S. S.; Khalid, M. Recent Developments in Graphene Based Novel Structures for Efficient and Durable Fuel Cells. Mater. Res. Bull. 2020, 122, 110674; https://doi.org/10.1016/j.materresbull.2019.110674.Search in Google Scholar
178. Su, H.; Hu, Y. H. Recent Advances in Graphene-Based Materials for Fuel Cell Applications. Energy Sci. Eng. 2021, 9 (7), 958–983; https://doi.org/10.1002/ese3.833.Search in Google Scholar
179. Vashistha, V. K.; Kumar, A. Design and Synthesis of MnN4 Macrocyclic Complex for Efficient Oxygen Reduction Reaction Electrocatalysis. Inorg. Chem. Commun. 2020, 112, 107700; https://doi.org/10.1016/j.inoche.2019.107700.Search in Google Scholar
180. Zhao, J.; Fu, N.; Liu, R. Graphite-Wrapped Fe Core–Shell Nanoparticles Anchored on Graphene as pH-Universal Electrocatalyst for Oxygen Reduction Reaction. ACS Appl. Mater. Interfaces 2018, 10 (34), 28509–28516; https://doi.org/10.1021/acsami.8b06153.Search in Google Scholar PubMed
181. Kumar, A.; Yasin, G.; Vashistha, V. K.; Das, D. K.; Rehman, M. U.; Iqbal, R.; Mo, Z.; Nguyen, T. A.; Slimani, Y.; Nazir, M. T.; Zhao, W. Enhancing Oxygen Reduction Reaction Performance via CNTs/graphene Supported Iron Protoporphyrin IX: A Hybrid Nanoarchitecture Electrocatalyst. Diam. Relat. Mater. 2021, 113, 108272; https://doi.org/10.1016/j.diamond.2021.108272.Search in Google Scholar
182. Niu, H.-J.; Zhang, L.; Feng, J. J.; Zhang, Q. L.; Huang, H.; Wang, A. J. Graphene-Encapsulated Cobalt Nanoparticles Embedded in Porous Nitrogen-Doped Graphitic Carbon Nanosheets as Efficient Electrocatalysts for Oxygen Reduction Reaction. J. Colloid Interface Sci. 2019, 552, 744–751; https://doi.org/10.1016/j.jcis.2019.05.099.Search in Google Scholar PubMed
183. Wilson, J. R.; Cronin, J. S.; Duong, A. T.; Rukes, S.; Chen, H. Y.; Thornton, K.; Mumm, D. R.; Barnett, S. Effect of Composition of (La0.8Sr0.2MnO3–Y2O3-Stabilized ZrO2) Cathodes: Correlating Three-Dimensional Microstructure and Polarization Resistance. J. Power Sources 2010, 195 (7), 1829–1840; https://doi.org/10.1016/j.jpowsour.2009.09.074.Search in Google Scholar
184. Khan, M. Z.; Song, R. H.; Lee, S. B.; Lim, T. H. Lifetime Prediction of Anode-Supported Solid Oxide Fuel Cell on the Basis of Individual Components Degradation. ECS Trans. 2019, 91 (1), 621; https://doi.org/10.1149/09101.0621ecst.Search in Google Scholar
185. Zhang, X.; Zhang, J.; Huang, H.; Jiang, Q.; Wu, Y. Platinum Nanoparticles Anchored on Graphene Oxide-Dispersed Pristine Carbon Nanotube Supports: High-Performance Electrocatalysts Toward Methanol Electrooxidation. Electrochim. Acta 2017, 258, 919–926; https://doi.org/10.1016/j.electacta.2017.11.142.Search in Google Scholar
186. Long, G.-F.; Li, X. H.; Wan, K.; Liang, Z. X.; Piao, J. H.; Tsiakaras, P. Pt/CN-Doped Electrocatalysts: Superior Electrocatalytic Activity for Methanol Oxidation Reaction and Mechanistic Insight into Interfacial Enhancement. Appl. Catal. B Environ. 2017, 203, 541–548; https://doi.org/10.1016/j.apcatb.2016.10.055.Search in Google Scholar
187. Askari, M. B.; Salarizadeh, P.; Seifi, M.; Rozati, S. M. Ni/NiO Coated on Multi-Walled Carbon Nanotubes as a Promising Electrode for Methanol Electro-Oxidation Reaction in Direct Methanol Fuel Cell. Solid State Sci. 2019, 97, 106012; https://doi.org/10.1016/j.solidstatesciences.2019.106012.Search in Google Scholar
188. An, M.; Du, L.; Du, C.; Sun, Y.; Wang, Y.; Yin, G.; Gao, Y. Pt Nanoparticles Supported by Sulfur and Phosphorus Co-doped Graphene as Highly Active Catalyst for Acidic Methanol Electrooxidation. Electrochim. Acta 2018, 285, 202–213; https://doi.org/10.1016/j.electacta.2018.07.237.Search in Google Scholar
189. Damte, J. Y.; Lyu, S. L.; Leggesse, E. G.; Jiang, J. C. Methanol Decomposition Reactions Over a Boron-Doped Graphene Supported Ru–Pt Catalyst. Phys. Chem. Chem. Phys. 2018, 20 (14), 9355–9363; https://doi.org/10.1039/c7cp07618e.Search in Google Scholar
190. Chang, C.-C.; Liu, C.-Y.; Sun, Y.-C. Effective Methane Conversion to Methanol on Bi-functional Graphene-Oxide-Supported Platinum Nanoclusters (Pt 5) – A DFT Study. Phys. Chem. Chem. Phys. 2020, 22 (9), 4967–4973; https://doi.org/10.1039/c9cp06002b.Search in Google Scholar PubMed
191. Lu, H.; Zhong, Y.; Jie, Y.; Yin, P.; Zhao, X. J.; Feng, Y. L.; Shen, T. Y.; Guo, J. Y.; Zhang, W.; Pu, M.; Yan, H. A DFT Study on Methanol Decomposition Over Single Atom Pt/CeO2 Catalysts: The Effect of the Position of Pt. Phys. Chem. Chem. Phys. 2023, 25 (20), 14232–14244; https://doi.org/10.1039/d3cp01066j.Search in Google Scholar PubMed
192. Barakat, N. A.; Ali Abdelkareem, M.; Abdelghani, E. A. Influence of Sn Content, Nanostructural Morphology, and Synthesis Temperature on the Electrochemical Active Area of Ni-Sn/C Nanocomposite: Verification of Methanol and Urea Electrooxidation. Catalysts 2019, 9 (4), 330; https://doi.org/10.3390/catal9040330.Search in Google Scholar
193. Bastos, T. L., Gelamo, R. V., Colmati, F. Carbon-graphene Hybrid Supporting Platinum–Tin Electrocatalyst to Enhance Ethanol Oxidation Reaction. J. Appl. Electrochem. 2023, 54 (6), 1–13; https://doi.org/10.1007/s10800-023-02027-2.Search in Google Scholar
194. Wang, H.; Lu, L.; Subramanian, P.; Ji, S.; Kannan, P. Co, Fe-Ions Intercalated Ni(OH)2 Network-like Nanosheet Arrays as Highly Efficient Non-noble Catalyst for Electro-Oxidation of Urea. Int. J. Hydrogen Energy 2021, 46 (69), 34318–34332; https://doi.org/10.1016/j.ijhydene.2021.08.022.Search in Google Scholar
195. Li, B.; Song, C.; Yin, J.; Yan, J.; Ye, K.; Cheng, K.; Zhu, K.; Cao, D.; Wang, G. Effect of Graphene on the Performance of Nickel Foam-Based CoNi Nanosheet Anode Catalyzed Direct Urea-Hydrogen Peroxide Fuel Cell. Int. J. Hydrogen Energy 2020, 45 (17), 10569–10579; https://doi.org/10.1016/j.ijhydene.2019.05.158.Search in Google Scholar
196. Abdelkareem, M. A.; Sayed, E. T.; Mohamed, H. O.; Obaid, M.; Rezk, H.; Chae, K. J. Nonprecious Anodic Catalysts for Low-Molecular-Hydrocarbon Fuel Cells: Theoretical Consideration and Current Progress. Prog. Energy Combust. Sci. 2020, 77, 100805; https://doi.org/10.1016/j.pecs.2019.100805.Search in Google Scholar
197. Zhang, S.; Chen, M.; Zhao, X.; Cai, J.; Yan, W.; Yen, J. C.; Chen, S.; Yu, Y.; Zhang, J. Advanced Noncarbon Materials as Catalyst Supports and Non-Noble Electrocatalysts for Fuel Cells and Metal–Air Batteries. Electrochem. Energy Rev. 2021, 4, 336–381; https://doi.org/10.1007/s41918-020-00085-0.Search in Google Scholar
198. Pushkareva, I. V.; Kalinichenko, V. N.; Chumakov, R. G.; Soloviev, M. A.; Liang, Y.; Millet, P.; Grigoriev, S. A. Reduced Graphene Oxide-Supported Pt-Based Catalysts for PEM Fuel Cells With Enhanced Activity and Stability. Catalysts 2021, 11 (2), 256; https://doi.org/10.3390/catal11020256.Search in Google Scholar
199. Garapati, M. S.; Sundara, R. Highly Efficient and ORR Active Platinum-Scandium Alloy-Partially Exfoliated Carbon Nanotubes Electrocatalyst for Proton Exchange Membrane Fuel Cell. Int. J. Hydrogen Energy 2019, 44 (21), 10951–10963; https://doi.org/10.1016/j.ijhydene.2019.02.161.Search in Google Scholar
200. Xu, J.; Elangovan, A.; Li, J.; Liu, B. Graphene-Based Dual-Metal Sites for Oxygen Reduction Reaction: A Theoretical Study. J. Phys. Chem. C 2021, 125 (4), 2334–2344; https://doi.org/10.1021/acs.jpcc.0c10617.Search in Google Scholar
201. Han, C.; Chen, Z. The Mechanism Study of Oxygen Reduction Reaction (ORR) on Non-Equivalent P, N Co-doped Graphene. Appl. Surf. Sci. 2020, 511, 145382; https://doi.org/10.1016/j.apsusc.2020.145382.Search in Google Scholar
202. Qi, M.-Y.; Zhang, Y.; Li, Q.; Wu, L.; Zhou, B.; Wang, Z.; Huang, Z. H.; Wang, M. X. Experimental and Theoretical Insights into Metal-Free Catalytic Reduction of Nitrophenols Over Porous Nitrogen-Doped Reduced Graphene Oxide. Chem. Eng. J. 2023, 474, 145823; https://doi.org/10.1016/j.cej.2023.145823.Search in Google Scholar
203. Chalani, M.; Daneshvari-Esfahlan, V.; Hosseini, M. G. Synthesis of Nitrogen and Phosphorus Co-Doped Graphene Quantum Dots as Metal-Free Electrocatalysts for Ethanol Electrooxidation. Fullerenes, Nanotub. Carbon Nanostruct. 2022, 30 (8), 853–862; https://doi.org/10.1080/1536383x.2022.2030315.Search in Google Scholar
204. Zhiani, M.; Gholamian, M.; Barzi, S. Pd Electrodeposition on a Novel Substrate of Reduced Graphene Oxide/poly (Melem-Formaldehyde) Nanocomposite as an Active and Stable Catalyst for Ethanol Electrooxidation in Alkaline Media. Int. J. Hydrogen Energy 2022, 47 (6), 3801–3813; https://doi.org/10.1016/j.ijhydene.2021.11.033.Search in Google Scholar
205. Ehsani, A.; Heidari, A.; Asgari, R. Electrocatalytic Oxidation of Ethanol on the Surface of Graphene Based Nanocomposites: An Introduction and Review to it in Recent Studies. Chem. Rec. 2019, 19 (11), 2341–2360; https://doi.org/10.1002/tcr.201800176.Search in Google Scholar PubMed
206. Li, J. C.; Hou, P. X.; Liu, C. Heteroatom-Doped Carbon Nanotube and Graphene-Based Electrocatalysts for Oxygen Reduction Reaction. Small 2017, 13 (45), 1702002; https://doi.org/10.1002/smll.201702002.Search in Google Scholar PubMed
207. Murdachaew, G.; Laasonen, K. Oxygen Evolution Reaction on Nitrogen-Doped Defective Carbon Nanotubes and Graphene. J. Phys. Chem. C 2018, 122 (45), 25882–25892; https://doi.org/10.1021/acs.jpcc.8b08519.Search in Google Scholar PubMed PubMed Central
208. Tan, H.; Tang, J.; Henzie, J.; Li, Y.; Xu, X.; Chen, T.; Wang, Z.; Wang, J.; Ide, Y.; Bando, Y.; Yamauchi, Y. Assembly of Hollow Carbon Nanospheres on Graphene Nanosheets and Creation of Iron–Nitrogen-Doped Porous Carbon for Oxygen Reduction. ACS Nano 2018, 12 (6), 5674–5683; https://doi.org/10.1021/acsnano.8b01502.Search in Google Scholar PubMed
209. Hou, Y.; Lv, S.; Liu, L.; Liu, X. High-Quality Preparation of Graphene Oxide via the Hummers’ Method: Understanding the Roles of the Intercalator, Oxidant, and Graphite Particle Size. Ceram. Int. 2020, 46 (2), 2392–2402; https://doi.org/10.1016/j.ceramint.2019.09.231.Search in Google Scholar
210. Yang, J.; Cao, C.; Qiao, W.; Qiao, J.; Tang, C.; Xue, Y. B/N Co-doping rGO/BNNSs Heterostructure With Synergistic Adsorption-Electrocatalysis Function Enabling Enhanced Electrochemical Performance of Lithium-Sulfur Batteries. Chem. Eng. J. 2023, 467, 143377; https://doi.org/10.2139/ssrn.4393896.Search in Google Scholar
211. Tavakkoli, M.; Flahaut, E.; Peljo, P.; Sainio, J.; Davodi, F.; Lobiak, E. V.; Mustonen, K.; Kauppinen, E. I. Mesoporous Single-Atom-Doped Graphene–Carbon Nanotube Hybrid: Synthesis and Tunable Electrocatalytic Activity for Oxygen Evolution and Reduction Reactions. ACS Catal. 2020, 10 (8), 4647–4658; https://doi.org/10.1021/acscatal.0c00352.Search in Google Scholar
212. Xia, D.; Yang, X.; Xie, L.; Wei, Y.; Jiang, W.; Dou, M.; Li, X.; Li, J.; Gan, L.; Kang, F. Direct Growth of Carbon Nanotubes Doped With Single Atomic Fe–N4 Active Sites and Neighboring Graphitic Nitrogen for Efficient and Stable Oxygen Reduction Electrocatalysis. Adv. Funct. Mater. 2019, 29 (49), 1906174; https://doi.org/10.1002/adfm.201906174.Search in Google Scholar
213. Kaushal, S.; Kaur, M.; Kaur, N.; Kumari, V.; Singh, P. P. Heteroatom-Doped Graphene as Sensing Materials: A Mini Review. RSC Adv. 2020, 10 (48), 28608–28629; https://doi.org/10.1039/d0ra04432f.Search in Google Scholar PubMed PubMed Central
214. Sohal, N.; Maity, B.; Basu, S. Recent Advances in Heteroatom-Doped Graphene Quantum Dots for Sensing Applications. RSC Adv. 2021, 11 (41), 25586–25615; https://doi.org/10.1039/d1ra04248c.Search in Google Scholar PubMed PubMed Central
215. Ubhi, M. K.; Kaur, M.; Grewal, J. K.; Sharma, V. K. Phosphorous-and Boron-Doped Graphene-Based Nanomaterials for Energy-Related Applications. Materials 2023, 16 (3), 1155; https://doi.org/10.3390/ma16031155.Search in Google Scholar PubMed PubMed Central
216. Qamar, M. A.; Shahid, S.; Javed, M.; Shariq, M.; Fadhali, M. M.; Madkhali, O.; Ali, S. K.; Syed, I. S.; Awaji, M. Y.; Shakir Khan, M.; Hassan, D. A.; Al Nasir, M. H. Accelerated Decoloration of Organic Dyes From Wastewater Using Ternary Metal/g-C3N4/ZnO Nanocomposites: An Investigation of Impact of g-C3N4 Concentration and Ni and Mn Doping. Catalysts 2022, 12 (11), 1388; https://doi.org/10.3390/catal12111388.Search in Google Scholar
217. Matsoso, J. B.; Journet, C.; Coville, N. J.; Sofer, Z. Co-Doping Graphene With B and N Heteroatoms for Application in Energy Conversion and Storage Devices. Chem. Nano. Mat. 2022, 8 (7), e202200134; https://doi.org/10.1002/cnma.202200134.Search in Google Scholar
218. Ngidi, N. P.; Ollengo, M. A.; Nyamori, V. O. Heteroatom-Doped Graphene and its Application as a Counter Electrode in Dye-Sensitized Solar Cells. Int. J. Energy Res. 2019, 43 (5), 1702–1734; https://doi.org/10.1002/er.4326.Search in Google Scholar
219. Olabi, A. G.; Abdelkareem, M. A.; Wilberforce, T.; Sayed, E. T. Application of Graphene in Energy Storage Device – A Review. Renew. Sustain. Energy Rev. 2021, 135, 110026; https://doi.org/10.1016/j.rser.2020.110026.Search in Google Scholar
220. Augustyn, V.; Simon, P.; Dunn, B. Pseudocapacitive Oxide Materials for High-Rate Electrochemical Energy Storage. Energy Environ. Sci. 2014, 7 (5), 1597–1614; https://doi.org/10.1039/c3ee44164d.Search in Google Scholar
221. Cao, X.; Yin, Z.; Zhang, H. Three-Dimensional Graphene Materials: Preparation, Structures and Application in Supercapacitors. Energy Environ. Sci. 2014, 7 (6), 1850–1865; https://doi.org/10.1039/c4ee00050a.Search in Google Scholar
222. Li, Z.; Liu, X.; Wang, L.; Bu, F.; Wei, J.; Pan, D.; Wu, M. Hierarchical 3D All-Carbon Composite Structure Modified with N-Doped Graphene Quantum Dots for High-Performance Flexible Supercapacitors. Small 2018, 14 (39), 1801498; https://doi.org/10.1002/smll.201801498.Search in Google Scholar PubMed
223. Qing, Y.; Jiang, Y.; Lin, H.; Wang, L.; Liu, A.; Cao, Y.; Sheng, R.; Guo, Y.; Fan, C.; Zhang, S.; Jia, D.; Fan, Z. Boosting the Supercapacitor Performance of Activated Carbon by Constructing Overall Conductive Networks Using Graphene Quantum Dots. J. Mater. Chem. A 2019, 7 (11), 6021–6027; https://doi.org/10.1039/c8ta11620b.Search in Google Scholar
224. Yang, Y.-X.; Ge, K. K.; ur Rehman, S.; Bi, H. Nanocarbon-Based Electrode Materials Applied for Supercapacitors. Rare Met. 2022, 41 (12), 3957–3975; https://doi.org/10.1007/s12598-022-02091-1.Search in Google Scholar
225. Dong, Y.; Zhu, J.; Li, Q.; Zhang, S.; Song, H.; Jia, D. Carbon Materials for High Mass-Loading Supercapacitors: Filling the Gap between New Materials and Practical Applications. J. Mater. Chem. A 2020, 8 (42), 21930–21946; https://doi.org/10.1039/d0ta08265a.Search in Google Scholar
226. Liu, T.; Zhang, F.; Song, Y.; Li, Y. Revitalizing Carbon Supercapacitor Electrodes With Hierarchical Porous Structures. J. Mater. Chem. A 2017, 5 (34), 17705–17733; https://doi.org/10.1039/c7ta05646j.Search in Google Scholar
227. Pan, Y.; Xu, K.; Wu, C. Recent Progress in Supercapacitors Based on the Advanced Carbon Electrodes. Nanotechnol. Rev. 2019, 8 (1), 299–314; https://doi.org/10.1515/ntrev-2019-0029.Search in Google Scholar
228. Niu, Y.; Guo, M.; Zhang, Y.; Yang, J.; Zhang, X.; Gao, Y.; Wang, X.; Sheng, L.; Shi, J. Heteroatom-Doped Layered Hierarchical Porous Carbon Electrodes With High Mass Loadings for High-Performance Supercapacitors. J. Energy Storage 2023, 73, 109064; https://doi.org/10.1016/j.est.2023.109064.Search in Google Scholar
229. Waris; Chaudhary, M. S.; Anwer, A. H.; Sultana, S.; Ingole, P. P.; Nami, S. A. A. A Review on Development of Carbon-Based Nanomaterials for Energy Storage Devices: Opportunities and Challenges. Energy Fuels 2023, 37 (24), 19433–19460; https://doi.org/10.1021/acs.energyfuels.3c03213.Search in Google Scholar
230. Byon, H. R.; Lee, S. W.; Chen, S.; Hammond, P. T.; Shao-Horn, Y. Thin Films of Carbon Nanotubes and Chemically Reduced Graphenes for Electrochemical Micro-capacitors. Carbon 2011, 49 (2), 457–467; https://doi.org/10.1016/j.carbon.2010.09.042.Search in Google Scholar
231. Zhang, K.; Mao, L.; Zhang, L. L.; On Chan, H. S.; Zhao, X. S.; Wu, J. Surfactant-Intercalated, Chemically Reduced Graphene Oxide for High Performance Supercapacitor Electrodes. J. Mater. Chem. 2011, 21 (20), 7302–7307; https://doi.org/10.1039/c1jm00007a.Search in Google Scholar
232. Mao, L.; Zhang, K.; On Chan, H. S.; Wu, J. Surfactant-Stabilized Graphene/Polyaniline Nanofiber Composites for High Performance Supercapacitor Electrode. J. Mater. Chem. 2012, 22 (1), 80–85; https://doi.org/10.1039/c1jm12869h.Search in Google Scholar
233. Liang, C.; Li, Z.; Dai, S. Mesoporous Carbon Materials: Synthesis and Modification. Angew. Chem. Int. Ed. 2008, 47 (20), 3696–3717; https://doi.org/10.1002/anie.200702046.Search in Google Scholar PubMed
234. Yang, Z.-C.; Tang, C. H.; Gong, H.; Li, X.; Wang, J. Hollow Spheres of Nanocarbon and Their Manganese Dioxide Hybrids Derived From Soft Template for Supercapacitor Application. J. Power Sources 2013, 240, 713–720; https://doi.org/10.1016/j.jpowsour.2013.05.034.Search in Google Scholar
235. Ke, Q.; Liu, Y.; Liu, H.; Zhang, Y.; Hu, Y.; Wang, J. Surfactant-Modified Chemically Reduced Graphene Oxide for Electrochemical Supercapacitors. RSC Adv. 2014, 4 (50), 26398–26406; https://doi.org/10.1039/c4ra03826f.Search in Google Scholar
236. Cai, Y.; Zhang, G.; Zhang, Y.-W. Polarity-Reversed Robust Carrier Mobility in Monolayer MoS2 Nanoribbons. J. Am. Chem. Soc. 2014, 136 (17), 6269–6275; https://doi.org/10.1021/ja4109787.Search in Google Scholar PubMed
237. Stenger-Smith, J. D.; Webber, C. K.; Anderson, N.; Chafin, A. P.; Zong, K.; Reynolds, J. R. Poly(3,4-Alkylenedioxythiophene)-Based Supercapacitors Using Ionic Liquids as Supporting Electrolytes. J. Electrochem. Soc. 2002, 149 (8), A973; https://doi.org/10.1149/1.1485773.Search in Google Scholar
238. Frackowiak, E.; Khomenko, V.; Jurewicz, K.; Lota, K.; Béguin, F. Supercapacitors Based on Conducting Polymers/Nanotubes Composites. J. Power Sources 2006, 153 (2), 413–418; https://doi.org/10.1016/j.jpowsour.2005.05.030.Search in Google Scholar
239. Du, X.; Wang, C.; Chen, M.; Jiao, Y.; Wang, J. Electrochemical Performances of Nanoparticle Fe3O4/Activated Carbon Supercapacitor Using KOH Electrolyte Solution. J. Phys. Chem. C 2009, 113 (6), 2643–2646; https://doi.org/10.1021/jp8088269.Search in Google Scholar
240. Chen, S.; Zhu, J.; Wu, X.; Han, Q.; Wang, X. Graphene Oxide−MnO2 Nanocomposites for Supercapacitors. ACS Nano 2010, 4 (5), 2822–2830; https://doi.org/10.1021/nn901311t.Search in Google Scholar PubMed
241. Li, Z.; Wang, J.; Liu, S.; Liu, X.; Yang, S. Synthesis of Hydrothermally Reduced graphene/MnO2 Composites and Their Electrochemical Properties as Supercapacitors. J. Power Sources 2011, 196 (19), 8160–8165; https://doi.org/10.1016/j.jpowsour.2011.05.036.Search in Google Scholar
242. Wang, H.; Casalongue, H. S.; Liang, Y.; Dai, H. Ni(OH)2 Nanoplates Grown on Graphene as Advanced Electrochemical Pseudocapacitor Materials. J. Am. Chem. Soc. 2010, 132 (21), 7472–7477; https://doi.org/10.1021/ja102267j.Search in Google Scholar PubMed
243. Ke, Q.; Tang, C.; Liu, Y.; Liu, H.; Wang, J. Intercalating Graphene With Clusters of Fe3O4 Nanocrystals for Electrochemical Supercapacitors. Mater. Res. Express 2014, 1 (2), 025015; https://doi.org/10.1088/2053-1591/1/2/025015.Search in Google Scholar
244. Lee, J.-S.; Kim, S. I.; Yoon, J. C.; Jang, J. H. Chemical Vapor Deposition of Mesoporous Graphene Nanoballs for Supercapacitor. ACS Nano 2013, 7 (7), 6047–6055; https://doi.org/10.1021/nn401850z.Search in Google Scholar PubMed
245. Shembade, U. V.; Gurav, S. R.; Gaikwad, M. A.; Wategaonkar, S. B.; Ghatage, S. R.; Sonkawade, R. G.; Kim, J. H.; Moholkar, A. V. Hydrothermal Synthesis of Graphene Oxide Interspersed in Non-Uniform Tungsten Oxide Nanorod and its Performance Towards Highly Efficient Hybrid Supercapacitor. Ceram. Int. 2024, 50 (1), 340–350; https://doi.org/10.1016/j.ceramint.2023.10.107.Search in Google Scholar
246. Liu, R.; Duay, J.; Lee, S. B. Heterogeneous Nanostructured Electrode Materials for Electrochemical Energy Storage. Chem. Commun. 2011, 47 (5), 1384–1404; https://doi.org/10.1039/c0cc03158e.Search in Google Scholar PubMed
247. Bulbula, S. T.; Lu, Y.; Dong, Y.; Yang, X. Y. Hierarchically Porous Graphene for Batteries and Supercapacitors. New J. Chem. 2018, 42 (8), 5634–5655; https://doi.org/10.1039/c8nj00652k.Search in Google Scholar
248. Soltani, N.; Bahrami, A.; Giebeler, L.; Gemming, T.; Mikhailova, D. Progress and Challenges in Using Sustainable Carbon Anodes in Rechargeable Metal-Ion Batteries. Prog. Energy Combust. Sci. 2021, 87, 100929; https://doi.org/10.1016/j.pecs.2021.100929.Search in Google Scholar
249. Thakur, A. K.; Kurtyka, K.; Majumder, M.; Yang, X.; Ta, H. Q.; Bachmatiuk, A.; Liu, L.; Trzebicka, B.; Rummeli, M. H. Recent Advances in Boron-and Nitrogen-Doped Carbon-Based Materials and Their Various Applications. Adv. Mater. Interfac. 2022, 9 (11), 2101964; https://doi.org/10.1002/admi.202101964.Search in Google Scholar
250. Ma, G.; Ning, G.; Wei, Q. S-Doped Carbon Materials: Synthesis, Properties and Applications. Carbon 2022, 195, 328–340; https://doi.org/10.1016/j.carbon.2022.03.043.Search in Google Scholar
251. Zhang, H.; Jiang, Y.; Qi, Z.; Zhong, X.; Yu, Y. Sulfur Doped Ultra-Thin Anatase TiO2 Nanosheets/Graphene Nanocomposite for High-Performance Pseudocapacitive Sodium Storage. Energy Storage Mater. 2018, 12, 37–43; https://doi.org/10.1016/j.ensm.2017.11.008.Search in Google Scholar
252. Maça, R. R.; Cíntora Juárez, D.; Castillo Rodríguez, M.; Etacheri, V. Nanointerface-Driven Pseudocapacitance Tuning of TiO2 Nanosheet Anodes for High-Rate, Ultralong-Life and Enhanced Capacity Sodium-Ion Batteries. Chem. Eng. J. 2020, 391, 123598; https://doi.org/10.1016/j.cej.2019.123598.Search in Google Scholar
253. Cha, G.; Mohajernia, S.; Nguyen, N. T.; Mazare, A.; Denisov, N.; Hwang, I.; Schmuki, P. Li+ Pre-Insertion Leads to Formation of Solid Electrolyte Interface on TiO2 Nanotubes That Enables High-Performance Anodes for Sodium Ion Batteries. Adv. Energy Mater. 2020, 10 (6), 1903448; https://doi.org/10.1002/aenm.201903448.Search in Google Scholar
254. Zheng, C.; Luo, N.; Huang, S.; Wu, W.; Huang, H.; Wei, M. Nanocomposite of Mo2N Quantum Dots@MoO3@Nitrogen-Doped Carbon as a High-Performance Anode for Lithium-Ion Batteries. ACS Sustain. Chem. Eng. 2019, 7 (12), 10198–10206; https://doi.org/10.1021/acssuschemeng.9b00080.Search in Google Scholar
255. Li, Z.; Wang, C.; Chen, X.; Wang, X.; Li, X.; Yamauchi, Y.; Xu, X.; Wang, J.; Lin, C.; Luo, D.; Wang, X.; Zhao, X. S. MoOx Nanoparticles Anchored on N-Doped Porous Carbon as Li-Ion Battery Electrode. Chem. Eng. J. 2020, 381, 122588; https://doi.org/10.1016/j.cej.2019.122588.Search in Google Scholar
256. Zhao, Q.; Yu, K.; Wang, S.; Liang, C. Nanoparticle MoO2 Composited with Biomass-Derived Carbon Micron Balls Used as Enhanced Bifunctional Cathode Materials. J. Alloys Compd. 2022, 927, 167004; https://doi.org/10.1016/j.jallcom.2022.167004.Search in Google Scholar
257. Zeng, C.; Xie, F.; Yang, X.; Jaroniec, M.; Zhang, L.; Qiao, S. Ultrathin Titanate Nanosheets/Graphene Films Derived From Confined Transformation for Excellent Na/K Ion Storage. Angew. Chem. 2018, 130 (28), 8676–8680; https://doi.org/10.1002/ange.201803511.Search in Google Scholar
258. Hu, Y.; Chen, W.; Lei, T.; Zhou, B.; Jiao, Y.; Yan, Y.; Du, X.; Huang, J.; Wu, C.; Wang, X.; Wang, Y.; Chen, B.; Xu, J.; Wang, C.; Xiong, J. Carbon Quantum Dots–Modified Interfacial Interactions and Ion Conductivity for Enhanced High Current Density Performance in Lithium–Sulfur Batteries. Adv. Energy Mater. 2019, 9 (7), 1802955; https://doi.org/10.1002/aenm.201802955.Search in Google Scholar
259. Xu, X.; Liu, J.; Liu, Z.; Wang, Z.; Hu, R.; Liu, J.; Ouyang, L.; Zhu, M. FeP@C Nanotube Arrays Grown on Carbon Fabric as a Low Potential and Freestanding Anode for High-Performance Li-Ion Batteries. Small 2018, 14 (30), 1800793; https://doi.org/10.1002/smll.201800793.Search in Google Scholar PubMed
260. Guo, L.; Sun, H.; Qin, C.; Li, W.; Wang, F.; Song, W.; Du, J.; Zhong, F.; Ding, Y. Flexible Fe3O4 Nanoparticles/N-Doped Carbon Nanofibers Hybrid Film as Binder-Free Anode Materials for Lithium-Ion Batteries. Appl. Surf. Sci. 2018, 459, 263–270; https://doi.org/10.1016/j.apsusc.2018.08.001.Search in Google Scholar
261. Park, C.; Samuel, E.; Joshi, B.; Kim, T.; Aldalbahi, A.; El-Newehy, M.; Yoon, W. Y.; Yoon, S. S. Supersonically Sprayed Fe2O3/C/CNT Composites for Highly Stable Li-Ion Battery Anodes. Chem. Eng. J. 2020, 395, 125018; https://doi.org/10.1016/j.cej.2020.125018.Search in Google Scholar
262. Lv, X.; Deng, J.; Wang, B.; Zhong, J.; Sham, T. K.; Sun, X.; Sun, X. γ-Fe2O3@CNTs Anode Materials for Lithium Ion Batteries Investigated by Electron Energy Loss Spectroscopy. Chem. Mater. 2017, 29 (8), 3499–3506; https://doi.org/10.1021/acs.chemmater.6b05356.Search in Google Scholar
263. Sun, M.-H.; Huang, S. Z.; Chen, L. H.; Li, Y.; Yang, X. Y.; Yuan, Z. Y.; Su, B. L. Applications of Hierarchically Structured Porous Materials from Energy Storage and Conversion, Catalysis, Photocatalysis, Adsorption, Separation, and Sensing to Biomedicine. Chem. Soc. Rev. 2016, 45 (12), 3479–3563; https://doi.org/10.1039/c6cs00135a.Search in Google Scholar PubMed
264. Zhang, J.-G.; Wang, D.; Xu, W.; Xiao, J.; Williford, R. Ambient Operation of Li/Air Batteries. J. Power Sources 2010, 195 (13), 4332–4337; https://doi.org/10.1016/j.jpowsour.2010.01.022.Search in Google Scholar
265. Sun, H.; Xu, G. L.; Xu, Y. F.; Sun, S. G.; Zhang, X.; Qiu, Y.; Yang, S. A Composite Material of Uniformly Dispersed Sulfur on Reduced Graphene Oxide: Aqueous One-Pot Synthesis, Characterization and Excellent Performance as the Cathode in Rechargeable Lithium-Sulfur Batteries. Nano Res. 2012, 5, 726–738; https://doi.org/10.1007/s12274-012-0257-7.Search in Google Scholar
266. Zhang, L.-S.; Jiang, L. Y.; Yan, H. J.; Wang, W. D.; Wang, W.; Song, W. G.; Guo, Y. G.; Wan, L. J. Mono Dispersed SnO2 Nanoparticles on Both Sides of Single Layer Graphene Sheets as Anode Materials in Li-Ion Batteries. J. Mater. Chem. 2010, 20 (26), 5462–5467; https://doi.org/10.1039/c0jm00672f.Search in Google Scholar
267. Ma, Z.; Yuan, X.; Ma, Z. F.; Wilkinson, D. P.; Zhang, L.; Zhang, J. A Review of Cathode Materials and Structures for Rechargeable Lithium–Air Batteries. Energy Environ. Sci. 2015, 8 (8), 2144–2198; https://doi.org/10.1039/c5ee00838g.Search in Google Scholar
268. Sun, C.; Ma, C.; Wang, Y.; Ren, Y.; Yang, W.; Ma, Z.; Li, J.; Chen, Y.; Kim, Y.; Chen, L. Graphene–Co3O4 Nanocomposite as an Efficient Bifunctional Catalyst for Lithium–Air Batteries. J. Mater. Chem. A 2014, 2 (20), 7188–7196; https://doi.org/10.1039/c4ta00802b.Search in Google Scholar
269. Wang, Z. L.; Xu, D.; Xu, J.; Zhang, L.; Zhang, X. Graphene Oxide Gel-Derived, Free-Standing, Hierarchically Porous Carbon for High-Capacity and High-Rate Rechargeable Li-O2 Batteries. Adv. Funct. Mater. 2012, 22 (17), 3699–3705; https://doi.org/10.1002/adfm.201200403.Search in Google Scholar
270. Abraham, K.; Jiang, Z. A Polymer Electrolyte-Based Rechargeable Lithium/Oxygen Battery. J. Electrochem. Soc. 1996, 143 (1), 1; https://doi.org/10.1149/1.1836378.Search in Google Scholar
271. Liu, Y.-P.; Xu, C. X.; Ren, W. Q.; Hu, L. Y.; Fu, W. B.; Wang, W.; Yin, H.; He, B. H.; Hou, Z. H.; Chen, L. Self-Template Synthesis of Peapod-Like MnO@N-Doped Hollow Carbon Nanotubes as an Advanced Anode for Lithium-Ion Batteries. Rare Met. 2023, 42 (3), 929–939; https://doi.org/10.1007/s12598-022-02203-x.Search in Google Scholar
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- A review of coordination compounds: structure, stability, and biological significance
- Effluent wastewater technologies for textile industry: a review
- Efficient removal of Cr(VI) ions from industrial wastewater using carbon-based adsorbents functionalized with boronic acid
- Exploring inorganic phosphors: basics, types, fabrications and their luminescence properties for LED/WLED/displays
- Recent advances on hydrogen generation based on inorganic metal oxide nano-catalyst using water electrolysis approach
- Antituberculosis, antimicrobial, antioxidant, cytotoxicity and anti-inflammatory activity of Schiff base derived from 2,3-diaminophenazine moiety and its metal(II) complexes: structural elucidation, computational aspects, and biological evaluation
- Carbon-based nanomaterials: synthesis, types and fuel applications: a mini-review
- Recent advances in carbon quantum dots for antibiotics detection
- Modern innovations in the provision and efficient application of 2D inorganic nanoscale materials
- Bioinorganic metal nanoparticles and their potential applications as antimicrobial, antioxidant and catalytic agents: a review
Articles in the same Issue
- Frontmatter
- A review of coordination compounds: structure, stability, and biological significance
- Effluent wastewater technologies for textile industry: a review
- Efficient removal of Cr(VI) ions from industrial wastewater using carbon-based adsorbents functionalized with boronic acid
- Exploring inorganic phosphors: basics, types, fabrications and their luminescence properties for LED/WLED/displays
- Recent advances on hydrogen generation based on inorganic metal oxide nano-catalyst using water electrolysis approach
- Antituberculosis, antimicrobial, antioxidant, cytotoxicity and anti-inflammatory activity of Schiff base derived from 2,3-diaminophenazine moiety and its metal(II) complexes: structural elucidation, computational aspects, and biological evaluation
- Carbon-based nanomaterials: synthesis, types and fuel applications: a mini-review
- Recent advances in carbon quantum dots for antibiotics detection
- Modern innovations in the provision and efficient application of 2D inorganic nanoscale materials
- Bioinorganic metal nanoparticles and their potential applications as antimicrobial, antioxidant and catalytic agents: a review