Abstract
N-heterocyclic carbenes (NHCs) are an eminent class of carbenes having a heterocyclic ring in which a divalent carbon atom is attached directly to a nitrogen atom. In the NHCs, the donation of lone pair is another important research in the dative bonding and not only in NHCs the dative bond plays a functionalized role in the other classes of complex formation like ylidones L → E ← L and carbones L → C ← L. M–NHC bond is L-M sigma-dative bond and NHCs are considered as strong sigma-donor ligands. The clear picture of the M–NHC bond can be better understood by M–NHC pi-interaction. M-L pi interaction is comprised of two steps. One is L → M sigma-donation and M → L π* back bonding. This dative donor nature of NHC and also its behavior in organoselenium is studied through DFT in which it’s optimized structure, bond lengths, molecular vibrations are calculated.
Introduction
Understanding the nature of the chemical bonding is of great importance especially the bonding of carbon atoms with the other elements. Throughout the entire existence of the atomic coordination chemistry, the dative bonding is considered as the essential idea and consigned as a coordinate-covalent bond, dipolar, donor-acceptor, or semi-polar which have been not the same as the classical “covalent bond” (Klein et al., 2018). So, the dative ligand is defined as the coordinate bond formed by the interactions of different molecular species in which one will act as a donor while the other will be considered as an acceptor in the electron pair sharing during complex formation (Lepetit et al., 2016). For example, a compound A‒B shows a covalent bond and share two electrons from each side like one electron is sharing by A while the other electron from B but in case of A → B shows a coordinate covalent bond and both electrons come from A but physically the strength of the A → B dative bond in sharing of the electron is always less than that of the A‒B between the same atoms (Burford et al., 2004).
The direction of the flow of charge is always happening from donor to acceptor site which is important in a molecule for electronic charge distribution so, an appropriation that might be inverse to what one would anticipate from the electronegativities of the atom (Lepetit et al., 2016). The dative bond has to dominate characteristics to determine the reactivity and structural properties of the metal complexes, as well as the dative ligand, also functioned as the other class of metal complexes like carbones and ylidones (Frenking and Tonner, 2009).
Like the chemical bond mostly term covalent bond is used despite electron sharing which is not correct because the covalent bond is formed from the low energy by the resonating mixing of the fragments wave functions A and B which give a molecule AB that has the two different forms of the bond-like in case of the molecule A‒B the molecule shared unpaired electrons shown in Figure 1a while in the form of A → B both the electrons contribute as a donor shown in Figure 1b (Das et al., 2007). The distinction between these two bonds is due to the induced polarization where two different fragments attract towards each other through static coulombic interaction of the molecules having opposite charges (Zhao et al., 2017).

Types of possible covalent bonding.
Selenium was first discovered by Jons Jacob Berzelius, Swedish chemist in 1817. Diethyl selenide was the pioneer organoselenium compound which was discovered in 1836 by a renowned scientist named as Lowing. He isolated it in its purest form after some years in 1869. At earlier stage the development of selenium based compounds was very slow because of difficulties in their synthesis. In 1847 an organoselenium compound Ethyl selenol was synthesized by Wohler and Siemens, then in 1973 a series of organoselenium were synthesized and it was a milestone (Li et al., 2020).
Later on in 1990s due to efforts of many research groups who invented some easy ways such as green synthesis of organoselenium it was an easy and interesting job to synthesize such compounds (Arora et al., 2021). Organoselenium compounds were recorded as a good anticancer agent by different research groups such as (Selamoglu, 2017).
NHC as a dative donor and its behavior in organoselenium compounds
N-heterocyclic carbenes (NHCs) are an eminent class of carbene having a heterocyclic ring in which a divalent carbon atom is attached directly to a nitrogen atom (Kuwata and Hahn, 2018; Lopes and Royo, 2017; Smith et al., 2019; Yaqoob et al., 2020). The work on carbene was gained more consideration when Wanzlick and Ofele freely synthesized the primary NHC-metal complex 1,3-bis(adamantyl)imidazol-2-ylidene bearing mercury(II) and chromium(0) in 1968. It has been investigated that the NHCs exhibit property to stabilize elements having low coordinates in various oxidation states (Nesterov et al., 2018). Deprotonation of imidazolium salt results in the formation of free carbene or may result due to the reduction of thione with molten potassium as shown in Scheme 1 (Suzuki, 2011).

The formation of free NHCs.
M–NHC bond is an L-M sigma-dative bond and NHCs are strong sigma donor ligands. The clear picture of the M–NHC bond can be better understood by M–NHC pi-interaction. M-L pi interaction is comprised of two steps. One is L → M pi-donation and M → L π* backbonding. The overlap between the HOMO orbital of the metal center and π* of the carbene result in π* backbonding. The overlap between d-orbital and the filled NHC pi orbital lead to the L → M pi-donation. M–NHC bond electronically can be explained by three focal components as depicted in Figure 2 (Suzuki, 2011).

The various type of bonding pattern of NHCs.
Depicted in Figure 2 that the imidazole ring is directed away from the metal center and the groups attached to N in such a way that they are present in the coordination sphere. NHCs are bent carbenes which means that the frontier orbitals are an sp 2-hybridized orbital and a p orbital is orthogonal to the sp 2 plane. NHCs are represented probably by four electronic configurations shown in Figure 3. It has been reported that the stability of NHCs based on the singlet-triplet -p gap, ES–T. It has been concluded by Apeloig and co-workers that electron delocalization is present in imidazolin-2-ylidene NHC ring that enables the pπ donation to carbene C atom from N atoms (Jacobsen et al., 2009).

The electronic configuration of the NHC carbenes.
As it has been depicted in Scheme 1 that the deprotonation leads to the formation of 1, 3-imidazole-2-ylidenes. These are known as classical NHCs because they coordinate with the metal center at carbon atom (C2), the carbon atom that is situated between two nitrogen atoms that provide it remarkable stability owing to the mesomeric effect. The deprotonation of imidazolium salt at position 2 occurs mainly due to the higher acidic nature of hydrogen atoms as compared to C4-H or C5-H. It has been reported that NHC complexes may also be originated by the binding of the imidazole ring to the metal center through C4 and C5 carbon atoms. Studies showed that the binding at C4 leads to a strong donor character of NHCs as compared to classical NHCs depicted in Figure 4 (Ghadwal, 2016).

The classification of NHCs.
It has been questioned that why carbon atom acts as an electron-pair donor in NHC it must participate merely in covalent bonds. The reason behind this was the trick that conjugate system maintains the lone pair on carbon attributed to the pi framework that is provided by adjacent electronegative N atoms. This factor enables the carbene to make strong sigma-dative bonds as a donor. The electron-donating or electron-withdrawing groups at C4 and C5 positions in classical carbenes contribute to the higher sigma-donor capability of NHCs (Merschel et al., 2020). In this way the dative bonds formation take place between carbenes and metal center.
NHCs complexes auxiliary ligand which strongly binds and stabilizes the transitions, attribute more attention to form strong M–C σ-bonds, leading to an inclusive variation of the well-defined catalytic system. Particularly, the late transition metal chemistry of functionalized NHC has concerned incredible interest that offers superfluous prospects for the development and design of the complexes having mixture ligands containing at least one NHC ligand (Doddi et al., 2019). It has been reported that 1,3-dimethylimidazoline-2-ylidene NHC ligand coordinates with three different transition metals (Hg, Cr, and Be) through M−C single bond to form three NHC complexes (1–3) shown in Figure 5, NHC imidazolium salt form a single bond with Pd to form compound (4) (Herrmann et al., 1996).

The synthesis of different transition metals through NHC ligands.
The 1-naphthyl-2-pyridine-2-yl-2H-imidazole [1,5]pyridin-4ylium hexafluorophosphate NHC proligand coordinates single bond with Ag and Au metals to form other NHC complexes (5–7) with different NHC ligands which were reported below in Figure 5 (Samanta et al., 2015), a similar type of gold and silver complexes (5, 6) were also reported with ligand 1,3-Dicyclohexylbenzimidazolium chloride (Kumar and Cisarova, 2013), another silver complex was also reported through M−C single bond with nitrile functionalized benzimidazole-2-ylidenes (Haque et al., 2015). From the all above-mentioned shreds of evidence it has been proven that the transition metals only coordinates through single bonding with different NHCs ligands.
Dative nature of organoselenium by DFT studies
NHC is considered as a strong sigma donor ligand (Ali et al., 2019). The prominent sigma donation capability of NHCs enhance their catalytic activity. So due to a very demanding class of synthetic chemistry, there were studies theoretically (Zaccaria et al., 2016). Computational studies are an important and reliable way to find out the feasibility of its synthesis and results as well (de Carvalho et al., 2017). This all happens through the DFT studies. It performs all the calculations from the energy and optimization of the structure until its results and even biological potential can also be checked. This theory is based on quantum calculations and brought phenomenal change to the structural chemistry for helping the researchers (Bhat et al., 2018; Yaqoob et al., 2020).
Different functional such as B3LYP, CAMB3LYP, WB97XD, and MPWiPW91 and the basis set of 6-31G are used for these calculations (Ali et al., 2019). In the calculation of the dipole moment of selected molecules, the distribution of charges on the atoms is checked and it also explains the solvent effect, because the dipole moment has a great influence on the solubility of organic solvents. When it was compared by changing the linkers with carbene ring which bring change in its nature, theoretical studies proved that it increases with an increase in dipole moment due to different types of attached linkers, such as o-xylene and 1, 2-dibromoethane. There was a difference in hybridization in both structures and it was proved theoretically that by an increase in the S character, electronegativity increases, as a result of this increase in electronegativity the dipole moment of the compound also increases.
To elucidate the antioxidant potential of NHC compounds, bond dissociation energy (BDE) can be calculated and B3LYP with the basis set of 6-31G is the most suitable functional for it. As cited from the literature, the BDE of carbene carbon and its acidic proton can be calculated to check the radical scavenging capability of respective compounds. In this process, Ereactant as well as Eproduct is being calculated. Those compounds which have a higher value of BDE are poor antioxidant compounds because it is very difficult to remove their proton (Yaqoob et al., 2020).
The DFT studies of compounds of pyridylselenium are recorded in the literature (Sharma et al., 2017), which are the best source of signals in the semiconductor material. A Quantum molecular model was developed to study the structure property relationship of the compounds. Mulliken charges of the compounds were also calculated that explain the vibrational properties of the atoms. From NBO analysis the stability of C–C, C–N, C–Se, etc., was done to check the stability of their bonds which can explain the dative nature of the NHC ligand with the selenium metal (Zaccaria et al., 2016).
It has been reported in the literature that the bonding of selenium with NHCs exist in two canonical forms, in one form it shows Se–C single bond i.e., coordinate covalent (dative bond), and in the second form double bond between selenium-NHC has been observed as shown in Figure 6 (Shaves, 2019). The reason behind these confirmations is also explained. When molecular orbitals of selones were analyzed they indicated that HOMOs of this molecule are having a stronger influence in the p-character of selenium while the LUMOs are strongly associated with its pi-bonding in the C–Se bond. The MOs involved in the sigma bonding of C–Se are inner energy levels and outer highly occupying MOs are responsible for pi-bonding in C–Se (Yadav et al., 2019).

The two canonical forms of organoselenium compounds.
The bond distance in dative bond C–Se is 1.831 Å which is shorter than the typical single bond length (1.937 Å) and larger than the double bond length (1.795 Å). So that the DFT calculation is suggesting that there is a partial double bond between carbon and selenium possibly a π-back donation. In the comparison of theoretical calculation, there are experimental evidences cited from the literature, that the bond length of the C–Se bond is 1.884 Å which is significantly larger than the isolated double bond (1.698 Å) that is in CSe2, but closer to the single bond length (1.94 Å) (Yadav et al., 2019). The bond lengths of C–Se bond and chemical shifts values reported from X-ray crystallographic data and 13CNMR are enlisted in Table 1.
The bond lengths of the C–Se bond, reported from X-ray crystallographic data and 13CNMR shifts values.
| No of evidence | Structure of compounds | C–Se bond lengths (Å) |
|---|---|---|
| 1 |

|
1.849 (Shaves, 2019) |

C–Se with pi-character |
1.78 (Shaves, 2019) | |

C–Se with sigma-character |
1.89 (Shaves, 2019) | |
| 2 |

|
1.96 (Sharma et al., 2018) |
| 3 |

|
1.884 (Iqbal et al., 2016) |
| 4 |

|
1.827 (Iqbal et al., 2016) |
| 5 |

|
1.834 (Iqbal et al., 2016) |
| 6 |

|
1.826 (Iqbal et al., 2016) |
| 7 |

|
1.820 (Engl, 2017) |
| 8 |

|
1.815 (Engl, 2017) |
| 9 |

|
1.803 (Engl, 2017) |
| 10 |

|
1.8197 (Engl, 2017) |
| Sr. no | Structure of compounds | C13NMR (CDCl3) | References |
|---|---|---|---|
| 1 |

|
ƍ = 183 ppm | Verlinden et al. (2015) |
| 2 |

|
ƍ = 191 ppm | Verlinden et al. (2015) |
| 3 |

|
153.8 ppm | Steiner et al. (2005) |
| 4 |

|
ƍ = 162.3 ppm | Liske et al. (2013) |
| 5 |

|
ƍ = 164.9 | Liske et al. (2013) |
| 6 |

|
ƍ = 157.8 ppm | Jia et al. (2008) |
| 7 |

|
ƍ = 168.6 ppm | Jamil and Endot (2020) |
| 8 |

|
ƍ = 163.3 ppm | Jamil (2019) |
| 9 |

|
ƍ = 190 ppm | Yaqoob et al. (2020) |
| 10 |

|
ƍ = 194 ppm | Sharma et al. (2018) |
| 11 |

|
ƍ = 167.1 ppm | Iqbal et al. (2016) |
| 12 |
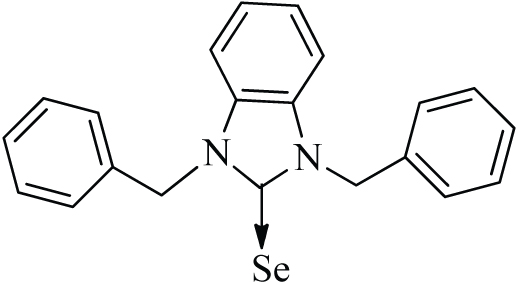
|
ƍ = 168.6 ppm | Iqbal et al. (2016) |
| 13 |

|
ƍ = 167.1 ppm | Iqbal et al. (2016) |
-
Evidence by NMR spectroscopy.
The tabulated results conclude that there might be a double or single (dative) bond between C–Se, depending upon the environment. There is much evidence that in many cases the bond length lies between the length of a typical C–Se single bond and isolated double bond C=Se, which indicates that there might be a coordinate covalent bond (dative bond) with a partial π-back donation in some cases as shown in Figure 7 (Yaqoob et al., 2020). It has been observed from the data of 13CNMR that the C–Se bond (might be dative bond) show peaks in the range of 190–195 ppm, whereas the C=Se bond show peak in the range of 150–185 ppm that vary due to the attachment of various groups. It can be concluded that dative character shifts the chemical shift towards downfield as compared to back donation that contributes to C=Se bond.

The chemical structure of organoselenium compound (Chemical Formula: C30H50N4Se2) having partial π-back donation by selenium.
Theoretical studies through DFT calculations of the compound, its chemical structure is shown in Figure 7, are also supporting the behavior in the carbon-selenium single bond that is a dative bond with a bond length of 1.84 Å as its optimized structure is shown in Figure 8 (Iqbal et al., 2016; Yaqoob et al., 2020).

The optimized structure of the organoselenium compound.
NHCs form a stable adduct with main group elements and rare earth metals, such as aluminum, magnesium, and thallium. These metals receive sigma electrons from NHC and are not able to donate π electrons back to the NHC because NHC is having a pure sigma donor character. There is also evidence that in the case of beryllium there is no back donation. As cited from the literature that in 1998, Boehme and Frenking had been recorded the first negligible interaction of π back donation from metal to ligand. Lee and Hu also reported some evidences by using density functional theory (DFT ) that π-back donation is almost negligible as compared to sigma-donation. In silylene and germylene, the π-back donation is stronger than other carbenes it means that the strength and dominance of sigma and π donation vary according to the chemical environment (Ezugwu et al., 2016). In the NHC-Ag 15–20% π-back donation is observed (Nolan, 2014).
Applications of organoselenium compounds
Selenium based NHC compounds show the efficient oxygen resistance and good corrosion inhibition capability and they replaced the sulfur and tellurium as well. Because of high sigma donation ability of selenium, it cause some chemical interactions within the bond as compared to sulfur and oxygen. As cited from the literature that organoselenium compounds are very good anticancer and therapeutic agents. They also act as anti-inflammatory agents (Arora et al., 2021; Kamal et al., 2018; Moreno-Martin et al., 2021).
Due to desired electronic properties organoselenium compounds are efficiently used in the various catalytic reactions (Arora et al., 2021; Iqbal et al., 2016). In the field of biochemistry, materials science and medicinal chemistry, organoselenium compounds play the vital role. They act as both electrophile specie and nucleophile as well and act as a ligand and catalyst in the organic synthesis (Wang et al., 2020). The chemistry of organoselenium compounds presents the strong sources of key intermediates in the different type of organic transformations and also in many synthetic reactions (Kostić et al., 2020; Naz et al., 2020).
Conclusions
This review is about the critical analysis of the dative donor property of the NHC its behavior in the organoselenium compounds. All the properties of carbene carbon are studied experimentally as well as theoretically. It has been investigated that the strong donor behavior is exhibited by carbene (imidazolium salt) at position 4, it has been also reported that most of the NHC complexes result due to bonding at C2. It proved by the DFT calculation that carbene carbon in NHC forms the dative bond with the selenium to form the organoselenium compounds which are very significant and versatile in many fields.
Funding source: Higher Education Commission Pakistan https://www.hec.gov.pk/english/services/universities/nrpu/Pages/Introduction.aspx
Award Identifier / Grant number: NRPU-8198
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Research funding: This study was supported by Higher Education Commission Pakistan (grant no. NRPU-8198).
-
Conflict of interest statement: The authors declare no conflicts of interest regarding this article.
References
Ali, U.; Ans, M.; Iqbal, J.; Iqbal, M. A.; Shoaib, M. Benchmark study of benzamide derivatives and four novel theoretically designed (L1, L2, L3, and L4) ligands and evaluation of their biological properties by DFT approaches. J. Mol. Model. 2019, 25(8), 223; https://doi.org/10.1007/s00894-019-4115-3.Search in Google Scholar PubMed
Arora, A.; Oswal, P.; Rao, G. K.; Kumar, S.; Kumar, A. Organoselenium ligands for heterogeneous and nanocatalytic systems: development and applications. Dalton Trans. 2021, 50, 8628–8656; https://doi.org/10.1039/d1dt00082a.Search in Google Scholar PubMed
Bhat, M. A.; Lone, S. H.; Ali, S.; Srivastava, S. K. Synthesis, spectral characterization, reactivity and DFT studies of novel ligand phenylseleno benzylacetate (L) and its complexes with group 12 metal chlorides. J. Mol. Struct. 2018, 1171, 233–242; https://doi.org/10.1016/j.molstruc.2018.05.091.Search in Google Scholar
Burford, N.; Herbert, D. E.; Ragogna, P. J.; McDonald, R.; Ferguson, M. J. Diphosphine− phosphenium coordination complexes representing monocations with pendant donors and ligand tethered dications. J. Am. Chem. Soc. 2004, 126(51), 17067–17073; https://doi.org/10.1021/ja0452121.Search in Google Scholar PubMed
Das, U.; Raghavachari, K.; Woo, R. L.; Hicks, R. F. Phosphine adsorption on the In-rich InP (001) surface: evidence of surface dative bonds at room temperature. Langmuir 2007, 23(20), 10109–10115; https://doi.org/10.1021/la700790h.Search in Google Scholar PubMed
de Carvalho, L. P.; Silva, K. F.; dos Santos, L. L.; dos Santos, M. V.; da Silva, J. A.; Longo, R. L. Chemoselective oxidation of unsaturated organosulfur, selenium and phosphorus compounds by molybdenum oxodiperoxo complexes: a computational investigation. Inorg. Chim. Acta. 2017, 467, 351–357; https://doi.org/10.1016/j.ica.2017.08.021.Search in Google Scholar
Doddi, A.; Peters, M.; Tamm, M. N-heterocyclic carbene adducts of main group elements and their use as ligands in transition metal chemistry. Chem. Rev. 2019, 119(12), 6994–7112; https://doi.org/10.1021/acs.chemrev.8b00791.Search in Google Scholar PubMed
Engl, P. S. Development and Application of N-Trifluoromethyl NHC Ligands for Transition-Metal Catalysis; Doctoral dissertation, ETH Zurich, 2017.Search in Google Scholar
Ezugwu, C. I.; Kabir, N. A.; Yusubov, M.; Verpoort, F. Metal–organic frameworks containing N-heterocyclic carbenes and their precursors. Coord. Chem. Rev. 2016, 307, 188–210; https://doi.org/10.1016/j.ccr.2015.06.012.Search in Google Scholar
Frenking, G.; Tonner, R. Divalent carbon (0) compounds. Pure Appl. Chem. 2009, 81(4), 597–614; https://doi.org/10.1351/pac-con-08-11-03.Search in Google Scholar
Ghadwal, R. S. Carbon-based two electron σ-donor ligands beyond classical N-heterocyclic carbenes. Dalton Trans. 2016, 45(41), 16081–16095; https://doi.org/10.1039/c6dt02158a.Search in Google Scholar PubMed
Haque, R. A.; Choo, S. Y.; Budagumpi, S.; Abdullah, A. A.-A.; Ahamed, M. B. K.; Majid, A. M. A. Synthesis, crystal structures, characterization and biological studies of nitrile-functionalized silver (I) N-heterocyclic carbene complexes. Inorg. Chim. Acta. 2015, 433, 35–44; https://doi.org/10.1016/j.ica.2015.04.023.Search in Google Scholar
Herrmann, W. A.; Elison, M.; Fischer, J.; Köcher, C.; Artus, G. R. N‐Heterocyclic carbenes: generation under mild conditions and formation of group 8–10 transition metal complexes relevant to catalysis. Chem. Eur J. 1996, 2(7), 772–780; https://doi.org/10.1002/chem.19960020708.Search in Google Scholar
Iqbal, M. A.; Haque, R. A.; Ng, W. C.; Hassan, L. E.; Majid, A. M.; Razali, M. R. Green synthesis of mono-and di-selenium-N-heterocyclic carbene adducts: characterizations, crystal structures and pro-apoptotic activities against human colorectal cancer. J. Organomet. Chem. 2016, 801, 130–138; https://doi.org/10.1016/j.jorganchem.2015.10.023.Search in Google Scholar
Jacobsen, H.; Correa, A.; Poater, A.; Costabile, C.; Cavallo, L. Understanding the M (NHC)(NHC= N-heterocyclic carbene) bond. Coord. Chem. Rev. 2009, 253(5–6), 687–703; https://doi.org/10.1016/j.ccr.2008.06.006.Search in Google Scholar
Jamil, M. S. S. Synthesis, Characterisation and Catalytic Activity of Gold, Rhodium and Palladium Complexes Featuring Fluorinated N-Heterocyclic Carbene Ligands; The University of Manchester: United Kingdom, 2019.Search in Google Scholar
Jamil, M. S. S.; Endot, N. A. Influence of fluorine substituents on the electronic properties of selenium-N-heterocyclic carbene compounds. Molecules 2020, 25(21), 5161; https://doi.org/10.3390/molecules25215161.Search in Google Scholar PubMed PubMed Central
Jia, W.-G.; Huang, Y.-B.; Lin, Y.-J.; Jin, G.-X. Syntheses and structures of half-sandwich iridium (III) and rhodium (III) complexes with organochalcogen (S, Se) ligands bearing N-methylimidazole and their use as catalysts for norbornene polymerization. Dalton Trans. 2008, 41, 5612–5620; https://doi.org/10.1039/b801862f.Search in Google Scholar PubMed
Kamal, A.; Iqbal, M. A.; Bhatti, H. N. Therapeutic applications of selenium-derived compounds. Rev. Inorg. Chem. 2018, 38(2), 49–76; https://doi.org/10.1515/revic-2018-0008.Search in Google Scholar
Klein, J. E.; Havenith, R. W.; Knizia, G. The pentagonal‐pyramidal hexamethylbenzene dication: many shades of coordination chemistry at carbon. Chemistry 2018, 24(47), 12340; https://doi.org/10.1002/chem.201705812.Search in Google Scholar PubMed PubMed Central
Kostić, M. D.; Mihajlović, K.; Divac, V. M. Kinetic study of the pyridine-catalyzed selenolactonization of 4-pentenoic acid. Catal. Lett. 2020, 150(7), 2076–2081.10.1007/s10562-020-03107-0Search in Google Scholar
Kumar, P.; Cisarova, I. Synthesis and characterization of silver and gold NHC complexes: crystal structures and mass spectral studies. J. Organomet. Chem. 2013, 735, 32–37; https://doi.org/10.1016/j.jorganchem.2013.03.017.Search in Google Scholar
Kuwata, S.; Hahn, F. E. Complexes bearing protic N-heterocyclic carbene ligands. Chem. Rev. 2018, 118(19), 9642–9677; https://doi.org/10.1021/acs.chemrev.8b00176.Search in Google Scholar PubMed
Lepetit, C.; Maraval, V.; Canac, Y.; Chauvin, R. On the nature of the dative bond: coordination to metals and beyond. The carbon case. Coord. Chem. Rev. 2016, 308, 59–75; https://doi.org/10.1016/j.ccr.2015.07.018.Search in Google Scholar
Li, Q.; Zhang, Y.; Chen, Z.; Pan, X.; Zhang, Z.; Zhu, J.; Zhu, X. Organoselenium chemistry-based polymer synthesis. Org. Chem. Front. 2020, 7(18), 2815–2841; https://doi.org/10.1039/d0qo00640h.Search in Google Scholar
Liske, A.; Verlinden, K.; Buhl, H.; Schaper, K.; Ganter, C. Determining the π-acceptor properties of N-heterocyclic carbenes by measuring the 77Se NMR chemical shifts of their selenium adducts. Organometallics 2013, 32(19), 5269–5272; https://doi.org/10.1021/om400858y.Search in Google Scholar
Lopes, R.; Royo, B. Iron N‐heterocyclic carbenes in reduction reactions. Isr. J. Chem. 2017, 57(12), 1151–1159; https://doi.org/10.1002/ijch.201700055.Search in Google Scholar
Merschel, A.; Rottschäfer, D.; Neumann, B.; Stammler, H.-G.; Ghadwal, R. S. Quantifying the electronic and steric properties of 1, 3-imidazole-based mesoionic carbenes (iMICs). Organometallics 2020, 39(10), 1719–1729; https://doi.org/10.1021/acs.organomet.0c00045.Search in Google Scholar
Moreno-Martin, G.; Sanz-Landaluze, J.; León-González, M. E.; Madrid, Y. In vivo quantification of volatile organoselenium compounds released by bacteria exposed to selenium with HS-SPME-GC-MS. Effect of selenite and selenium nanoparticles. Talanta 2021, 224, 121907; https://doi.org/10.1016/j.talanta.2020.121907.Search in Google Scholar PubMed
Naz, N.; Saqib, S.; Ashraf, R.; Majeed, M. I.; Iqbal, M. A. Synthesis of new organoselenium compounds: characterization and biological studies. Macedonian J. Chem. Chem. Eng. 2020, 39(1), 1–10; https://doi.org/10.20450/mjcce.2020.1912.Search in Google Scholar
Nesterov, V.; Reiter, D.; Bag, P.; Frisch, P.; Holzner, R.; Porzelt, A.; Inoue, S. NHCs in main group chemistry. Chem. Rev. 2018, 118(19), 9678–9842; https://doi.org/10.1021/acs.chemrev.8b00079.Search in Google Scholar PubMed
Nolan, S. P. N-Heterocyclic Carbenes: Effective Tools for Organometallic Synthesis; John Wiley & Sons: Weinheim, Germany, 2014.Search in Google Scholar
Samanta, T.; Munda, R. N.; Roymahapatra, G.; Nandy, A.; Saha, K. D.; Al-Deyab, S. S.; Dinda, J. Silver (I), Gold (I) and Gold (III)-N-Heterocyclic carbene complexes of naphthyl substituted annelated ligand: synthesis, structure and cytotoxicity. J. Organomet. Chem. 2015, 791, 183–191; https://doi.org/10.1016/j.jorganchem.2015.05.049.Search in Google Scholar
Selamoglu, Z. Organoselenium compounds in cancer: a review. J. Med. Genet. 2017, 1, 2–3; https://doi.org/10.31031/madd.2017.01.000510.Search in Google Scholar
Sharma, N.; Dhau, J. S.; Singh, A.; Singh, A.; Malik, A. K. FT-IR, NMR, molecular structure, and HOMO-LUMO studies of 3, 5-dimethyl-2-pyridylselenium compounds by density functional theory. Phosphorus, Sulfur, Silicon Relat. Elem. 2017, 192(3), 368–375; https://doi.org/10.1080/10426507.2016.1244205.Search in Google Scholar
Sharma, N.; Kumar, S.; Kumar, S.; Mehta, S.; Bhasin, K. Synthesis and characterization of fused imidazole heterocyclic selenoesters and their application for chemical detoxification of HgCl 2. New J. Chem. 2018, 42(4), 2702–2710; https://doi.org/10.1039/c7nj03908e.Search in Google Scholar
Shaves, C. L. Rigid, Dianionic Carbene Scaffolds for Phosphenium Catalysis; University of Kent: UK, 2019.Search in Google Scholar
Smith, C. A.; Narouz, M. R.; Lummis, P. A.; Singh, I.; Nazemi, A.; Li, C.-H.; Crudden, C. M. N-Heterocyclic carbenes in materials chemistry. Chem. Rev. 2019, 119(8), 4986–5056; https://doi.org/10.1021/acs.chemrev.8b00514.Search in Google Scholar PubMed
Steiner, G.; Kopacka, H.; Ongania, K. H.; Wurst, K.; Preishuber‐Pflügl, P.; Bildstein, B. Heteroditopic imino N‐heterocyclic carbenes and their sulfur, selenium, and tungsten tetracarbonyl derivatives. Eur. J. Inorg. Chem. 2005, 2005(7), 1325–1333; https://doi.org/10.1002/ejic.200400868.Search in Google Scholar
Suzuki, A. Cross coupling reactions in organic synthesis themed issue. Chem. Soc. Rev. 2011, 40, 4912–4924; https://doi.org/10.1246/cl.2011.894.Search in Google Scholar
Verlinden, K.; Buhl, H.; Frank, W.; Ganter, C. Determining the ligand properties of N‐heterocyclic carbenes from 77Se NMR parameters. Eur. J. Inorg. Chem. 2015, 2015(14), 2416–2425; https://doi.org/10.1002/ejic.201500174.Search in Google Scholar
Wang, X.; Wang, Q.; Xue, Y.; Sun, K.; Wu, L.; Zhang, B. An organoselenium-catalyzed N 1-and N 2-selective aza-Wacker reaction of alkenes with benzotriazoles. Chem. Commun. 2020, 56(32), 4436–4439; https://doi.org/10.1039/d0cc01079k.Search in Google Scholar PubMed
Yadav, S.; Deka, R.; Singh, H. B. Recent developments in the chemistry of NHC-based selones: syntheses, applications and reactivity. Chem. Lett. 2019, 48(2), 65–79; https://doi.org/10.1246/cl.180748.Search in Google Scholar
Yaqoob, M.; Gul, S.; Zubair, N. F.; Iqbal, J.; Iqbal, M. A. Theoretical calculation of selenium N-heterocyclic carbene compounds through DFT studies: synthesis, characterization and biological potential. J. Mol. Struct. 2020, 1204, 127462; https://doi.org/10.1016/j.molstruc.2019.127462.Search in Google Scholar
Zaccaria, F.; Wolters, L. P.; Fonseca Guerra, C.; Orian, L. Insights on selenium and tellurium diaryldichalcogenides: a benchmark DFT study. J. Comput. Chem. 2016, 37(18), 1672–1680; https://doi.org/10.1002/jcc.24383.Search in Google Scholar PubMed
Zhao, L.; Hermann, M.; Holzmann, N.; Frenking, G. Dative bonding in main group compounds. Coord. Chem. Rev. 2017, 344, 163–204; https://doi.org/10.1016/j.ccr.2017.03.026.Search in Google Scholar
© 2021 Walter de Gruyter GmbH, Berlin/Boston
Articles in the same Issue
- Frontmatter
- Dative behavior of N-heterocyclic carbenes (NHCs) with selenium in Se-NHC compounds
- Dynamic green synthesis of iron oxide and manganese oxide nanoparticles and their cogent antimicrobial, environmental and electrical applications
- Modification of bentonite clay & its applications: a review
- Recent progress in decontamination system against chemical and biological materials: challenges and future perspectives
- Heterotridentate organodiphosphines in Pt(η3–P1X1P2)(Y) derivatives-structural aspects
Articles in the same Issue
- Frontmatter
- Dative behavior of N-heterocyclic carbenes (NHCs) with selenium in Se-NHC compounds
- Dynamic green synthesis of iron oxide and manganese oxide nanoparticles and their cogent antimicrobial, environmental and electrical applications
- Modification of bentonite clay & its applications: a review
- Recent progress in decontamination system against chemical and biological materials: challenges and future perspectives
- Heterotridentate organodiphosphines in Pt(η3–P1X1P2)(Y) derivatives-structural aspects

