The effects of bushfire smoke exposure on oxidative stress and inflammatory biomarkers: a systematic review
-
Siti Nurshahida Nazli
, Gaurav Langan
Abstract
Introduction
Bushfire smoke (BFS) is an escalating global health concern, with increasing bushfire frequency due to climate change. Exposure to BFS significantly impacts public health due to worsening respiratory and cardiovascular conditions, causing increased of hospitalizations and mortality. While BFS exposure is linked to morbidity of these conditions, the underlying biological mechanisms, particularly oxidative stress and inflammation, remain unclear.
Content
This systematic review (PROSPERO ID: CRD42024554409) synthesized evidence on oxidative stress and inflammatory biomarkers from BFS exposure. Comprehensive searches of PubMed, Embase, Web of Science, Scopus, and Cochrane Library were conducted. Fourteen studies met inclusion criteria, encompassing occupational and non-occupational populations. Risk of bias was assessed using NIH tools, and findings were synthesized narratively due to study heterogeneity. Commonly examined biomarkers included IL-8, IL-6, TNF-α, 8-isoprostane, malondialdehyde (MDA), and 8-hydroxy-2′-deoxyguanosine (8-OHdG).
Summary
IL-8 emerged as the most consistent inflammatory biomarker, with a pooled random-effects analysis of three firefighter studies showing an acute post-shift increase in blood IL-8 (mean difference 9.76 pg/mL, 95 % CI –8.26 to 27.79), though with substantial heterogeneity. Other inflammatory and oxidative stress biomarkers showed mixed or inconsistent associations with BFS exposure. Small sample sizes, heterogeneous exposure assessment, inconsistent exposure metrics, and unadjusted confounding limited generalizability.
Outlook
This review identifies IL-8 as the most consistent short-term biomarker of BFS exposure in occupational settings but highlights inconsistent evidence for other biomarkers. Future research should use standardized biomarker protocols, larger sample size, longitudinal designs, and include vulnerable populations to clarify biomarker responses to BFS and strengthen the evidence base for public health and occupational safety measures.
Introduction
Bushfire smoke as a global health challenge
Bushfire smoke (BFS) has emerged as a significant public health concern, particularly as climate change intensifies the frequency and severity of bushfires worldwide [1], [2], [3], [4]. These fires release a complex mixture of gases and particles, including particulate matter (PM), carbon monoxide (CO), carbon dioxide (CO2), nitrogen oxides (NOx), volatile organic compounds (VOCs), water vapor, organic debris, and minerals from incomplete combustion [5], 6].
The public health burden of BFS is substantial. During the 2019–2020 Black Summer bushfires in Australia, BFS has been estimated to be responsible for more than 417 excess deaths, over 2000 hospitalizations for respiratory and cardiovascular diseases, and more than 1,300 emergency departments visits for asthma [4]. Similar patterns have been observed worldwide. In the western United States (U.S), BFS events caused spikes in respiratory morbidity, cardiovascular admissions, and premature deaths [7], [8], [9]. Transboundary event further worsened the problem. For example, the 2023 Canadian wildfires caused significantly elevated levels of PM2.5 in the U.S, exposing 354 million population in North America and Europe to hazardous air quality [10].
The burden is particularly severe in low-and middle-income countries (LMICs), where povert and poor health infrastructure exacerbate vulnerability [11]. Long-term exposure to PM and NO2 has been associated with reduced lung function and higher prevalence of chronic obstructive pulmonary disease (COPD) [12]. Emergency visits for respiratory infections are more common in low-income populations [11], and climate-driven increases in bushfires are projected to further raise PM2.5-related mortality in LMICs [13].
Known health effects of bushfire smoke exposure
BFS contribute a substantial proportion of ambient PM2.5, which is considered more toxic than PM2.5 from other sources [13]. Global average exposure to PM2.5 from BFS increased by 65 % between year 2000 and 2021, exposing a greater proportion of the world’s population to elevated health risks [13]. Acute exposure has been linked asthma exacerbations, and COPD flare-ups, reduced attention in adults, and higher risk of hospitalizations and mortality [1], 3], [6], [7], [8], [9, [12], [13], [14], [15], [16], [17], [18].
Long-term exposure to BFS is also increasingly recognized. Prolonged BFS exposure has been associated with cognitive decline, increased risk of stroke, and cardio-respiratory diseases, cancer, and neurodegenerative diseases such as dementia and Parkinson’s diseases [15], [18], [19], [20], [21]. Pregnant women exposed to BFS have higher risks of low birth weight and preterm birth [22], [23], [24], [25]. Elderly, people with pre-existing conditions, and socioeconomically disadvantaged groups are particularly vulnerable [12], 15], 26], 27]. Outdoor workers, especially firefighters, are likely to be exposed to higher levels which increases the risks of adverse health effects [3], 6], 28], 29]. Children and adolescents are also vulnerable, with respiratory-related morbidities being a major concern in this population [13], 30], 31].
These findings demonstrate that BFS exposure either in short-term or long-term cause significant health effects to human, especially among vulnerable populations. This broad spectrum of health effects underscores the importance of investigating the biological pathways, particularly oxidative stress and inflammation that may explain these outcomes.
Biological mechanism: oxidative stress and inflammation
While BFS has drawn attention due to its substantial risks to the human health, research is increasingly focusing on the biological mechanisms underlying the health effects caused by exposure to BFS, particularly oxidative stress and inflammation [32], [33], [34], [35], [36], [37]. Oxidative stress and inflammatory responses occurs when there is an imbalance between the production and buildup of reactive oxygen species (ROS) in cells and tissues and the capacity to detoxify or neutralize these reactive molecules [14], 38]. These processes are fundamental to the pathophysiology of many health conditions linked to air pollution exposure, including respiratory, cardiovascular, cerebrovascular damage, and neurological diseases [17], 21], 37], 39].
Particulate matter from BFS has been found to be more toxic to lung cells than PM from vehicle emissions, inducing greater oxidative stress, triggering systemic inflammation, alterations to the immune system, and increased susceptibility to various diseases and infections [1], 6], 14], 15], 37], 39]. BFS also contains immune-toxic components including VOCs, and polyaromatic hydrocarbons (PAHs) which deplete antioxidants like ascorbate and glutathione and elevates levels of inflammatory cytokines [37], 40], 41]. These processes generate airway and systemic inflammation, reinforcing oxidative stress as a key driver of health impacts.
Importance of biomarkers in environmental health research
Biomarkers of oxidative stress and inflammation provide measurable indicators of early physiological disruption and offer mechanistic evidence linking BFS exposure to health outcomes [14], 19], 37], 42]. They allow comparisons across populations and exposure settings, and help identify vulnerable groups. For example, studies on biomass smoke exposure have reported elevated levels of inflammatory cytokines such as interleukin-6 (IL-6), interleukin-8 (IL-8), and tumor necrosis factor-alpha (TNF-α), as well as lipid peroxidation markers such as 8-isoprostane and malondialdehyde (MDA), and DNA damage markers such as 8-hydroxy-2′-deoxyguanosine (8-OHdG) [37], 41], 43], 44]. These biomarkers provide insight into distinct pathways of oxidative injury, which are implicated in the onset of respiratory and cardiovascular diseases [30], 38], 45], 46].
Understanding the effects of BFS on the biomarkers could help to protect the health and safety of population at risks to the acute or chronic health effects of to BFS exposure. These biomarkers provide mechanistic evidence linking BFS exposure to disease outcomes and highlight the biological plausibility of observed epidemiological associations. Although studies have demonstrated the effects of BFS on health, the existing literature mediated through oxidative stress and inflammatory biomarkers are limited. Many studies specifically address other than BFS such as biomass smoke and fuel, making it unclear if the findings can be generalized to BFS contexts. To our knowledge, there is no comprehensive review to examine the impact of BFS on biomarkers of oxidative stress and inflammation across diverse populations. Thus, this review aims to address this gap by synthesizing current knowledge on this topic. By integrating epidemiological and mechanistic evidence, this review provides a novel and timely synthesis that advances understanding of the biological effects of BFS exposure and offers a roadmap for future studies and health interventions.
Methods
Search strategy and selection criteria
A total of 734 documents were identified from the search, in which 373 duplicates were removed by Covidence or manually. We screened 361 titles and abstracts for inclusion and retrieved 45 documents for full-text review. Fourteen studies met the inclusion criteria and were reviewed. This process is shown in Figure 1.
Identification of studies via databases using PRISMA flow diagram.
This systematic review adopted Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2020 statement that includes new reporting guidance in methods to identify, select, appraise, and synthesize studies [47]. A review protocol was developed and registered with PROSPERO (ID: CRD42024554409). Population, exposure, comparator, and outcomes (PECO) elements were followed for the inclusion criteria: (i) population: studies conducted in humans including children, adults, firefighters, and pregnant women. (ii) exposure: studies that evaluated the effects of BFS. (iii) comparator: a comparison population exposed to lower level, high level or pre and post exposures. (iv) outcome: oxidative stress or inflammatory biomarkers. The review only selected documents conducted in human subjects, without limitation to year of publication, with no limit on publication language. Reviews, commentary, letter, responses and editorials, duplicated publications, and conference abstract were excluded from the review.
Online database search was performed from five databases including PubMed, Embase, Web of Science, Scopus and Cochrane Library on 7th July 2024. Terms for BFS and biomarkers of interest were searched in title and abstracts. Example of search strategy performed in PubMed is below with full search available in Supplementary Table 1.
(“bushfire smoke(”[tiab] OR “bush fire smoke”[tiab] OR “wildfire smoke”[tiab] OR “forest fire smoke”[tiab] OR “vegetation fire smoke”[tiab]) AND (“oxidative stress”[tiab] OR “Reactive oxygen species”[tiab] OR “Oxidative damage”[tiab] OR “Oxidative injury”[tiab] OR “Oxidative markers”[tiab] OR “Inflammatory Biomarkers”[tiab] OR “Inflammation markers”[tiab] OR “inflammatory factors”[tiab] OR “inflammatory response”[tiab] OR “inflammatory mediators”[tiab]).
Study selection and data extraction
Document selection was managed using Covidence. Title and abstract screening were independently conducted by four reviewers (LR, IA, DR, SNN). Full-text review was conducted by three reviewers (GL, LR, SNN). Five reviewers (SNN, GL, LR, IA, DR) independently extracted data using extraction template provided in the Covidence. Any conflicts or disagreements throughout the three processes were solved through discussion and involvement of the expert (DV).
Risk of bias
A quality assessment tool for observational cohort and cross-sectional studies from National Heart, Lung, and Blood Institute (NIH) was used to assess the risk of bias in this review. The domain-based tool consists of 14 criteria that address research question clarity, study population selection, exposure and outcome assessments, timeframe sufficiency, control of confounding, and bias sources like selection, measurement, and attrition which evaluate the internal validity and risk of bias. The criteria were assessed as “Yes”, “No”, “Cannot Determine”, “Not Applicable”, and “Not Reported”. After considering each criterion, the overall quality of the study was reported as “good”, “fair” or “poor” based on fulfillment of ≥ 10, 7–9, or ≤6 criteria rating (Supplementary Table 2). We rated 10 studies as good quality, one fair, and three were poor quality.
Data analysis
A full meta-analysis was not feasible in this review due to substantial heterogeneity in study designs, exposure assessment metrics (e.g., PM2.5, CO, shift duration), biological matrices (e.g., blood, urine, EBC), and timing of biomarker sampling (e.g., pre-shift, post-shift, seasonal follow-up). These factors precluded direct comparability and risked introducing bias if pooled indiscriminately. Therefore, findings were synthesized narratively. However, to strengthen interpretation, we conducted a focused quantitative synthesis for the most consistent biomarker, IL-8, where sufficient homogeneity existed. Three studies examining IL-8 levels in firefighter blood samples were included in a forest plot analysis. Two studies used cross-shift comparisons (defined as pre- vs. post-shift biomarker levels), while one study assessed seasonal differences between baseline and post exposure periods. Effect sizes and 95 % confidence intervals were extracted or calculated, and a random-effects model was used to pool estimates. Two studies were excluded due to methodological differences but are discuss narratively. Analysis was conducted using R (version 2024.04.1) using the metafor package.
Results
Study characteristics
The systematic review included 14 studies that examined the effects of BFS exposure on oxidative stress and inflammatory biomarkers. Table 1 shows the detailed characteristics of the included studies. Most were conducted in the United States (n=8), with others from Australia (n=2), Canada (n=2), Thailand (n=1), and Greece (n=1). The study populations varied, encompassing firefighters (11/14), adults (2/14), and elderly (1/14). Firefighters were the most frequently studied population, reflecting their high-risk exposure to BFS.
Characteristics of included studies.
| Study ID | Country | Study population | Median/Mean age ± (SD) | Study design | Diagnostic method/Sample matrix | Date of exposure | Length of exposure | Measured air pollutant/Exposure level | Analzsed biomarkers |
|---|---|---|---|---|---|---|---|---|---|
| Gaughan et al. [48] | USA | Firefighter (n=38 male) | 29±4.34 | Cross-sectional study | Urine | May 2011 | Pike Interagency Hotshot Crew had been exposed to smoke for 2 days (12.5 h each day) at the Sand Gulch fire 4 days before the testing | Levoglucosan | 8-OHDG 8-isoprostane |
| Main et al. [33] | Australia | Firefighter (n=38 male) | 38.5 | Observational cohort study | Blood | Weekend following “Black Saturday” wildfire event February 7, 2009 |
12 h | NA | IL-β, IL-2, IL-6, IL-8, IL-12P70, GM-CSF, TNF-α, IFNγ, IL-4, IL-5, IL-10, IL-13 |
| Niyatiwatchanchai et al. [49] | Thailand | Firefighter (n=38 male) | 29.6±5.3 | Observational cohort study | Blood | Low pollution (September - October 2020) High pollution (March 2021) |
48 h | PM10, PM2.5, Ozone, NO2, SO2 | hsCRP |
| Wu [32] | USA | Firefighter (n=12 (9 male, 3 female)) | 33.0 | Observational cohort study | Exhaled breath condensate (EBC) | Between January and July 2015 | 4.4 h | NA | 8-isoprostane, IL-6, IL-8, CRP, and sICAM-1 |
| Wu [50] | USA | Firefighter (n=19) | 35±7.2 | Observational cohort study | Urine | Between 2015 and 2018 | 4.98±1.34 | PM2.5 Black carbon |
8-isoprostane MDA Ox-GS |
| Adetona et al. [51] | USA | Firefighter (n=12 (9 men and 3 female)) | 33±5.4 | Observational cohort study | Urine | January to July 2015 | On burn days, the average work shift was 4.5 h (ranging from 1.9 to 9.4 h). | PM2.5 and CO | MDA 8-isoprostane |
| Adetona [52] | USA | Firefighter (n=19 (17 men and 2 female)) | 29.8±6.1 | Observational cohort study | Urine | January to March 2004 | Each work shift during which exposure occurred typically lasted around 11 h on the days when prescribed burns were conducted. The study monitored exposures over a period of 16 days, during which 10 prescribed burns were carried out. This means that the firefighters were exposed to woodsmoke during these burn days within the 16-day study period. |
PM2.5 and CO | 8-Oxo-dG MDA |
| Cherry [53] | Canada | Firefighter (n=68) | 35.4±9.4 | Observational cohort study | Blood | May 2016 (The Fort McMurray fire) | 2–3 days | PM2.5 | Eotaxin-1, G-CSF, GM-CSF, Fractalkine, IFNa2, IFN-γ, GROalpha, IL-10, MCP-3, IL-12P40, MDC, IL-12P70, IL-13, IL-15, sCD40L, IL-17A, IL-1RA, IL-1a, IL-9, IL-1β, IL-2, IL-3, IL-4, IL-5, IL-6, IL-8, IP-10, MCP-1, MIP-1a, MIP-1B, RANTES, TNF-α, TNFB, VEGF-A, and IL-18. |
| Gianniou et al. [54] | Greece | Firefighter (n=60 male) | 32.4±5.7 | Observational cohort study | Blood, sputum | 2008 | >10 h (38±22 h) and <10 h (6±3 h) | NA | IL-8, IL-4, IL-13, TNF-α, and ECP |
| Swiston [16] | Canada | Firefighter (n=52 (49 male, 3 female)) | 30.4±1.1 | Observational cohort study | Blood | During 2004 and 2005 fire seasons, specifically between May and August. | 6 h | CO as a surrogate for PM2.5 | IL-6, IL-8, MCP-1, CRP |
| Gaughan et al. [55] | USA | Firefighter (n=58 (52 male, 6 female)) | 26.0 | Observational cohort study | Induced sputum and nasal lavage analyses | 2004–2006 | 14 h average shift length/average 4 days firefighting | CO | MPO ECP |
| O’Dwyer et al. [27] | Australia | Elderly and adult 18+ (n=207 (110 female, 73 male)) | 63.5±12.2 | Observational cohort study | Airway inflammation test Blood |
Autumn 2013 to Autumn 2016 2013: Planned burns in Warburton. 2014: Wildfire smoke in Warburton, coal mine fire smoke in Traralgon, no smoke in Maffra. 2015: Planned burns in Warburton. 2016: Planned burns in Warburton. |
4-h, 12-h, 24-h and 48-h | PM2.5 | FeNO CRP |
| Ferguson [34] | USA | Adult (n=10 male) | NA | Randomized cross over design | Blood and exhaled breath condensate (EBC) | NA | 1.5 h with one session per week for three weeks | PM2.5 CO |
8-isoprostane PTX3 SP-D MPO H2O2 |
| Peters et al. [35] | USA | Adult (n=10) (gender not mentioned) | 26.4±3.5 | Randomized cross over design | Blood | NA | 1.5 h (only 1 trial) | PM2.5 | TEAC UA 8-Isoprostanes LOOH PC 3-NT MPO |
-
CRP, C-reactive protein; ECP, Eosinophil Cationic Protein; FeNO, Fractional Exhaled Nitric Oxide; G-CSF, Granulocyte Colony-Stimulating Factor; GM-CSF, granulocyte macrophage-colony stimulating factor; GROalpha, Growth-Regulated Oncogene-Alpha; H2O2, hydrogen peroxide; hsCRP, high-sensitivity C-reactive protein; IFNa2, Interferon Alpha 2; IFNγ, Interferon Gamma; IL-1RA, Interleukin-1 Receptor Antagonist; IL-1a, Interleukin-1 Alpha; IL-1β, Interleukin-1 Beta; IL-2, Interleukin-2; IL-3, Interleukin-3; IL-4, Interleukin-4; IL-5, Interleukin-5; IL-6, Interleukin-6; IL-8, Interleukin-8; IL-9, Interleukin-9; IL-10, Interleukin-10; IL-12P40, Interleukin-12 p40; IL-12P70, Interleukin-12 p70; IL-13, Interleukin-13; IL-15, Interleukin-15; IL-17A, Interleukin-17A; IL-18, Interleukin-18; IP-10, Interferon-γ-Inducible Protein 10; LOOH, Lipid Hydroperoxide; MCP-1, Monocyte Chemoattractant Protein-1; MCP-3, Monocyte Chemoattractant Protein-3; MDA, Malondialdehyde; MDCs, Macrophage-derived chemokine; MIP-1a, Macrophage Inflammatory Protein-1 Alpha; MIP-1B, Macrophage Inflammatory Protein-1 Beta; MPO, Myeloperoxidase; 3-NT, 3-Nitrotyrosine; Ox-GS, Oxidized guanine species; 8-OHDG, 8-hydroxy-2′-deoxyguanosine; OH-Pyrene, 1-hydroxypyrene; 8-Oxo-dG, 8-oxo-7,8-dihydro-2′-deoxyguanosine; PC, Protein carbonyls; PTX3, Pentraxin 3; RANTES, Regulated upon Activation, Normal T Cell Expressed and Secreted; sCD40L, soluble CD40 Ligand; sICAM-1, soluble Intercellular Adhesion Molecule-1; SP-D, Surfactant Protein D; TEAC, Trolox Equivalent Antioxidant Capacity; TNF-α, tumor necrosis factor-alpha; TNFB, Tumor Necrosis Factor Beta; UA, Uric acid; VEGF-A, Vascular Endothelial Growth Factor A.
Eleven studies employed an observational cohort design, one study was cross-sectional, and two studies were randomized cross-over design. The biological matrices varied across studies included urine, blood, sputum, and exhaled breath condensate (EBC) to measure various oxidative stress and inflammatory biomarkers. Exposure durations ranged from short-term exposures lasting a few hours during firefighting shifts to longer durations extending over several days during bushfire events. Exposure from different time points within a work shift was observed in some studies. This exposure, usually referred to as “cross-shift”, specifically involved measuring biomarkers levels at pre-shift, post-shift, the next morning after burn days, and during regular non-burn shifts. Across the studies, exposure to BFS was commonly assessed using PM2.5, CO, and black carbon either through personal monitoring or ambient measurements. However, the specific exposure assessment methods varied, with some studies using proxies such as work duration, fire event timing, or location-specific pollution data to estimate exposure.
Outcomes of oxidative stress and inflammatory biomarkers in associated studies
Oxidative stress
8- Isoprostane
8-Isoprostane was examined in 6/14 studies as an indicator for oxidative damage from BFS exposure, showing mixed results (Table 2). Most studies conducted in firefighters reported no significant cross-shift changes in EBC or urine levels despite 4–5 h prescribed burn exposure [32], 50], 51]. However, a study confirmed urinary 8-isoprostane increased 2.6-fold on burns days compared to non-burn days [50]. While a longer exposure duration (25 h) observed a small positive association with urinary levoglucosan (a biomarker of BFS exposure), the authors observed confounders from dietary effects, small sample size and self-reported nature among participants that may have played a part in the findings [48].
Association between bush fire smoke exposure to oxidative stress and inflammatory biomarkers.
| Study IDx | Exposure | Outcome | Outcome measurementy | Effectt,u,v,w |
|---|---|---|---|---|
| Gaughan et al. [48] | Levoglucosana | 8-OHdGf | Levoglucosan Concentration (Log 10)r | 0.41 (95 % CI: 0.04, 0.79) |
| 8-Isoprostanei | Levoglucosan Concentration (Log 10)r, regression coefficient | 0.52 (95 % CI: 0.06, 0.97) | ||
| Main et al. [33] | NA | IL-1βm | 12Hr (T2) – Beginning of shift (T1)z | Increase, NS |
| IL-2m | No change | |||
| IL-6m | Increase, p=0.003 | |||
| IL-8m | Increase, p=0.017 | |||
| IL-12P70m | Increase, NS | |||
| GM-CSFm | Increase, NS | |||
| TNF-αm | Decrease, NS | |||
| IFNγm | Increase, NS | |||
| IL-4m | Increase, NS | |||
| IL-5\m | No change | |||
| IL-10m | Decrease, p=0.021 | |||
| IL-13m | Increase, NS | |||
| Niyatiwatchanchai et al. [49] | PM10b, PM2.5b, Ozonee, NO2e, SO2e | hsCRPk (mg/L) | Pollution period in wildland firefighters and healthy controls | NS |
| Wu [32] | PMb | IL-8m | MA/Pre, cross shift ratios | 0.97 (95 % CI: 0.89–1.05) p=0.05 |
| Post/Pre, cross shift ratios Morning-after/Post, cross shift ratios |
NS | |||
| CRPk | Post/Pre, cross shift ratios Morning-after/Pre, cross shift ratios Morning-after/Post, cross shift ratios |
NS | ||
| sICAM-1m | Post/Pre, cross shift ratios Morning-after/Pre, cross shift ratios Morning-after/Post, cross shift ratios |
NS | ||
| 8-isoprostanei | Post/Pre, cross shift ratios Morning-after/Pre, cross shift ratios Morning-after/Post, cross shift ratios Post/Pre, cross shift ratios, Linear Mixed Effect Model Morning-after/Pre, cross shift ratios, Linear Mixed Effect Model Morning-after/Post, cross shift ratios, Linear Mixed Effect Model |
NS | ||
| Wu [50] | PM2.5b Black Carbonc |
8-isoprostanei | Pre-shift to post-shift, creatinine-adjusted value on burn days to non-burn dayss | 2.64 (95 % CI: 1.13–6.16) p=0.03 |
| Pre-shift to post-shift, Prescribed burn, creatinine-adjusted valuess Pre-shift to post-shift, Regular work, creatinine-adjusted valuess Pre-morning to next-morning, Prescribed burn, creatinine-adjusted valuess Pre-morning to next-morning, Regular work, creatinine-adjusted valuess Pre-shift to post-shift, creatinine-adjusted valuess Pre-shift to next-morning, creatinine-adjusted valuess Pre-shift to post-shift, different work taskss Pre-morning to next-morning, due to different work taskss Pre-shift to post-shift, association with PM2.5 (mg/m3)s Pre-shift to post-shift, association with black carbon (ug/m3)s Pre-morning to next-morning, association with PM2.5 (mg/m3)s Pre-morning to next-morning, association with black carbon (ug/m3)s Comparison of cross-shift changes (pre-shift to next morning) creatinine-adjusted value on prescribed burn day to workdayss |
NS | |||
| MDAh | Pre-shift to post-shift, association with black carbon (ug/m3)s | 0.36 (95 % CI: 0.10–0.63) p=0.01 | ||
| Pre-shift to post-shift, Prescribed burn, creatinine-adjusted valuess Pre-shift to post-shift, Regular work, creatinine-adjusted valuess Pre-morning to next-morning, Prescribed burn, creatinine-adjusted valuess Pre-morning to next-morning, Regular work, creatinine-adjusted valuess Pre-shift to post-shift, creatinine-adjusted valuess Pre-shift to next-morning, creatinine-adjusted valuess Pre-shift to post-shift, due to different work taskss Pre-morning to next-morning, due to different work taskss Pre-shift to post-shift, association with PM2.5 (mg/m3)s Pre-morning to next-morning, association with PM2.5 (mg/m3)s Pre-morning to next-morning, association with black carbon (ug/m3)s |
NS | |||
| Ox-GSg | Pre-morning to next-morning, Prescribed burn, creatinine-adjusted values on burn dayss | 1.62 (95 % CI: 1.04–2.53) p=0.03 | ||
| Pre-shift to next-morning, creatinine-adjusted values on burn days to non-burn dayss | 3.00 (95 % CI: 1.19–7.57) p=0.02 | |||
| Pre-shift to post-shift, Prescribed burn, corrected valuess Pre-shift to post-shift, Regular work, corrected valuess Pre-morning to next-morning, Regular work, corrected valuess Pre-shift to post-shift, creatinine-adjusted valuess Pre-shift to post-shift, due to different work taskss Pre-morning to next-morning, due to different work taskss Pre-shift to post-shift, association with PM2.5 (mg/m3)s Pre-shift to post-shift, association with black carbon (ug/m3)s Pre-morning to next-morning, association with PM2.5 (mg/m3)s Pre-morning to next-morning, association with black carbon (ug/m3)s |
NS | |||
| Adetona et al. [51] | PM2.5b and COd | MDAh | Cross-work shift changes on burn day and non-burn days | NS |
| Pre -to post-work shift changes (creatinine adjusted) day typess Pre to morning-after (MA) work shift changes (creatinine adjusted) day typess |
NS | |||
| Pre -to post-work shift changes (creatinine adjusted) work tasks Pre to morning-after (MA) work shift changes (creatinine adjusted) work tasks |
NS | |||
| 8-Isoprostanei | Cross-work shift changes on burn day and non-burn days | NS | ||
| Pre -to post-work shift changes (creatinine adjusted) day typess Pre to morning-after (MA) work shift changes (creatinine adjusted) day typess |
NS | |||
| Pre -to post-work shift changes (creatinine adjusted) work tasks Pre to morning-after (MA) work shift changes (creatinine adjusted) work tasks |
NS | |||
| Adetona [52] | PM2.5b and COd | MDAh | Cross-shift difference (creatinine adjusted)s: Study days, Ages Age, adjusteds Study days, adjusted for firefighting yearss Firefighting years, adjusteds Firefighting years (0–2 years), adjusteds Firefighting years (3–5 years), adjusteds Firefighting years (6–9 years), adjusteds Study days, adjusted for PM2.5 exposures PM2.5 exposure, adjusteds Study days, adjusted for firefighting years and ages Age, adjusteds Firefighting years, adjusted for ages Firefighting years (0–2 years), adjusted for ages Firefighting years (3–5 years), adjusted for ages Firefighting years (6–9 years), adjusted for ages Study days, adjusted for age and PM2.5 exposures PM2.5 exposure, adjusteds Age, adjusted for PM2.5 exposures Study days, adjusted for firefighting years and PM2.5 exposures PM2.5 exposure, adjusteds Firefighting years, adjusted for PM2.5 exposures Firefighting years (0–2 years), adjusted for PM2.5 exposures Firefighting years (3–5 years), adjusted for PM2.5 exposures Firefighting years (6–9 years), adjusted for PM2.5 exposures Study days, adjusted for all variabless Age, adjusted for all variabless PM2.5 exposure, adjusted for all variabless Firefighting years, adjusted all variabless Firefighting years (0–2 years), adjusted for all variabless Firefighting years (3–5 years), adjusted for all variabless Firefighting years (6–9 years), adjusted for all variabless |
NS |
| 8-Oxo-dGf | Cross-shift difference (creatinine adjusted) Ages | −0.108 (p=0.01) | ||
| Cross-shift difference (creatinine adjusted) Firefighting years (overall)s | p=0.03 | |||
| Cross-shift difference (creatinine adjusted) Firefighting years (0–2 years)s | 1.78 (p=0.004) | |||
| Cross-shift difference (creatinine adjusted) Age, adjusted for PM2.5 exposures | −0.131 (p=0.008) | |||
| Cross-shift difference (creatinine adjusted) Firefighting years (overall), adjusted for PM2.5 exposures | p=0.05 | |||
| Cross-shift difference (creatinine adjusted) Firefighting years (0–2 years), adjusted for PM2.5 exposures | 1.1916 (p=0.008) | |||
| Cross-shift difference (creatinine adjusted) Firefighting years (3–5 years), adjusted for PM2.5 exposures | 1.752 (p=0.02) | |||
| Cross-shift difference (creatinine adjusted) Firefighting years (6–9 years), adjusted for PM2.5 exposures | 1.1316 (p=0.05) | |||
| Cross-shift difference (creatinine adjusted): Study days, adjusted for ages Study days, adjusted for firefighting yearss Firefighting years (3–5 years), adjusteds Firefighting years (6–9 years), adjusteds Study days, adjusted for PM2.5 exposures PM2.5 exposure, adjusteds Study days, adjusted for firefighting years and ages Age, adjusteds Study days, adjusted for firefighting years and PM2.5 exposures Firefighting years, adjusted for ages Firefighting years (0–2 years), adjusted for ages Firefighting years (3–5 years), adjusted for ages Firefighting years (6–9 years), adjusted for ages Study days, adjusted for age and PM2.5 exposures PM2.5 exposure, adjusteds Study days, adjusted for all variabless Age, adjusted for all variabless PM2.5 exposure, adjusted for all variabless Firefighting years, adjusted all variabless Firefighting years (0–2 years), adjusted for all variabless Firefighting years (3–5 years), adjusted for all variabless Firefighting years (6–9 years), adjusted for all variabless |
NS | |||
| Cherry [53] | PM2.5b | GM-CSFm | Mean difference between May and Aug/Septs | 0.607 (95 % CI: −1.086–0.127), p=0.013 |
| IFNa2m | 0.825 (95 % CI: −1.388–0.261), p=0.004 | |||
| IFNym | 0.715 (95 % CI: −1.316 to −0.113), p=0.020 | |||
| GRO alpham | 0.343 (95 % CI: −0.636 to −0.050), p=0.022 | |||
| MCP-3m | 0.541 (95 % CI: −1.042 to −0.039), p=0.035 | |||
| IL-12P40m | 1.142 (95 % CI: −2.200 to −0.085), p=0.034 | |||
| IL-12P70m | 0.950 (95 % CI: −1.796 to −0.103), p=0.028 | |||
| IL-13m | 0.776 (95 % CI: −1.538 to −0.014), p=0.046 | |||
| IL-10m | 0.760 (95 % CI = −1.449 to −0.072), p=0.03 | |||
| IL-15m | 0.758 (95 % CI: −1.353 to −0.162), p=0.013 | |||
| sCD40Lm | 0.359 (95 % CI: −0.653 to −0.065), p=0.017 | |||
| IL-17Am | 0.916 (95 % CI: −1.646 to −0.187), p=0.014 | |||
| IL-1RAm | 1.059 (95 % CI: −2.085 to −0.034), p=0.043 | |||
| IL-1am | 0.700 (95 % CI: −1.369 to −0.032), p=0.040 | |||
| IL-1βm | 0.955 (95 % CI: −1.541 to −0.368), p=0.001 | |||
| IL-3m | 0.232 (95 % CI: −0.439 to −0.025), p=0.028 | |||
| IL-6m | 1.067 (95 % CI: −1.714 –0.419), p=0.001) | |||
| MIP-1am | 0.617 (95 % CI: −1.163 to −0.072), p=0.027) | |||
| MIP-1Bm | 1.149 (95 % CI: −1.823 to −0.475), p=0.001) | |||
| TNFam | 0.306 (95 % CI: −0.597 to −0.014), p=0.040 | |||
| Eotaxin-1m, G-CSFm, Fractalkinem, MDCm, IL-9m, IL-2m, IL-4m, IL-5m, IL-8 m , IP-10m, MCP-1m, RANTESm, TNFBm, IL-18m | NS | |||
| Gianniou et al. [54] | Smoke exposure during firefighting | Sputum IL-8m Serum IL-8m |
Comparison between off-season and post exposure | p=0.03 p=0.03 |
| Sputum IL-4m Serum IL-4m |
Increase, NS | |||
| Sputum IL-13m Serum IL-13m |
Increase, NS | |||
| Sputum ECPk Serum ECPk |
Increase, NS | |||
| Sputum TNF-αm Serum TNF-αm |
p=0.04 p=0.03 |
|||
| Swiston [16] | COd surrogate for PM2.5 | IL-6m | Cross-shifts differences | Increase, p<0.02 |
| IL-8m | Increase, p<0.001 | |||
| MCP-1m | Increase, p<0.02 | |||
| CRPk | NS | |||
| Gaughan et al. [55] | COd | Sputum: ECPk | Postfire Upper Respiratory Symptom Score Postfire lower Respiratory Symptom Score |
Increase (p<0.01) Increase (p<0.001) |
| Sputum: MPOj | Increase (p<0.01) Increase (p<0.001) |
|||
| Nasal lavage fluid: ECPk | Increase (p<0.01) Increase (p<0.001) |
|||
| Nasal lavage fluid: MPOj | Increase (NS) Increase (p<0.05) |
|||
| O’Dwyer et al. [27] | PM2.5b | FeNOk | All types of smoke, Lag period 4 Planned burns smoke Lag period 4 h Wildfire smoke, Lag period 4 h Coal mine fire, Lag period 4 h |
0.500 (95 % CI: 0.192–0.808) p<0.001 0.335 (95 % CI: −0.012 -0.681) p=0.058 0.644 (95 % CI: 0.447–0.842) p<0.001 1.533 (95 % CI: 0.461–2.605) p=0.005 |
| All types of smoke, Lag period 12 h Coal mine fire, Lag period 12 h |
0.308 (95 % CI: 0.028–0.588) p=0.031 1.027 (95 % CI: 0.816–1.239) p<0.001 |
|||
| Wildfire smoke, Lag period 24 h | 1.073 (95 % CI: 0.846–1.299) p<0.001 | |||
| Wildfire smoke, Lag period 48 h | 0.789 (95 % CI: 0.539–1.040) p<0.001 | |||
| Planned burns smoke Lag period 12 h Coal mine fire Lag period 12 h All types of smoke Lag period 24 h Planned burns smoke Lag period 24 h Coal mine fire Lag period 24 h All types of smoke Lag period 48 h Planned burns smoke Lag period 48 h Coal mine fire Lag period 48 h |
NS | |||
| CRPk | All types of burn | NS | ||
| Ferguson [34] | PM2.5b A: Filtered air B: 250 μg/m3 C: 500 μg/m3 |
8-isoprostanei (pg/mL) | EBC, Pre – Post (Combined Exposure vs Filtered air)s | Decrease, p<0.05 |
| EBC, Pre –1Hr (Combined Exposure vs Filtered air)s | Increase, p<0.05 | |||
| EBC, Pre-Immediate Post Exposures EBC, Pre-1Hr Post Exposures |
NS | |||
| PTX3n (ng/mL) | Plasma, Post-Pre Means Plasma, 1Hr Post-Pre Means |
A: 9.0, B: 33.2, C: 57.5 p=0.048 A: 25.7, B: 49.5, C: 207.1 p=0.012 |
||
| EBC, Post-Pre Mean differences EBC, 1Hr Post-Pre Mean differences |
NS | |||
| SP-Do (ng/mL) | Plasma, 1Hr Post-Pre Means | A: −3.1, B: 14.5, C: 1.1 p=0.022 | ||
| EBC, Post-Pre Mean differences EBC, 1Hr Post-Pre Mean differences Plasma, Post-Pre Mean differences |
NS | |||
| MPOj | EBC, Post-Pre Mean differences EBC, 1Hr Post-Pre Mean differences |
NS | ||
| H2O2j | EBC, Post-Pre Mean differences EBC, 1Hr Post-Pre Mean differences Plasma, Post-Pre Means Plasma, 1Hr Post-Pre Means |
NS | ||
| Peters et al. [35] | PM2.5b Clean air: 0 μg/m3 Low exposure: 250 μg/m3 High exposure: 500 μg/m3 |
Uric acidp | Pre – Post (Low exposure)s | Decrease, p=0.042 |
| Pre – Post (Combined exposure)s | Decrease, p=0.032 | |||
| Pre – Post (High exposure)s Pre – Post (Clean Air)s Pre-1Hr (Clean air)s Pre-1Hr (High exposure)s Pre-1Hr (Combined exposure)s |
NS | |||
| Plasma TEACp | Pre – Post (Clean Air)s | Increase, p=0.015 | ||
| Pre – Post (High exposure)s | Increase, p=0.001 | |||
| Pre-1Hr (Clean air)s | Increase, p=0.001 | |||
| Pre-1Hr (High exposure)s | Increase, p=0.031 | |||
| Pre-1Hr (Combined exposure)s Pre – Post (Low exposure)s Pre – Post (Combined exposure)s |
NS | |||
| Lipid Hydroperoxideh | Pre-1Hr (High exposure)s | Decrease, p=0.036 | ||
| Pre-1Hr (Combined exposure)s | Decrease, p=0.011 | |||
| Other trialss | NS | |||
| 8-Isoprostanei | Pre – Post (Low exposure)s | Increase, p=0.004 | ||
| Pre – Post (High exposure)s | Increase, p=0.009 | |||
| Pre – Post (Combined exposure)s | Increase, p=0.002 | |||
| Protein carbonylsj | Pre – Post (Combined exposure)s | Decrease, p=0.053 | ||
| Pre – Post (Low exposure)s Pre – Post (High exposure)s Pre – Post (Clean Air)s Pre-1Hr (Clean air)s Pre-1Hr (High exposure)s Pre-1Hr (Combined exposure)s |
NS | |||
| Plasma 3NTq | Pre – Post (Clean Air-High exposure)s | Increase, p=0.014 | ||
| Pre – Post (Combined exposure)s | Increase, p=0.049 | |||
| Pre – Post (Clean Air)s | Increase, p=0.012 | |||
| Pre – Post (Low exposure)s Pre – Post (High exposure)s Pre-1Hr (Clean air)s Pre-1Hr (High exposure)s Pre-1Hr (Combined exposure)s |
NS | |||
| MPOj | Pre – Post (Low Exposure)s | Increase, p=0.035 | ||
| Pre – Post (High Exposure)s | Increase, p=0.019 | |||
| Pre – Post (Combined exposure)s | Increase, p=0.005 | |||
| Pre – Post (Clean Air)s Pre-1Hr (Clean air)s Pre-1Hr (High exposure)s Pre-1Hr (Combined exposure)s |
NS |
-
aLevoglucosan: tracer of biomass/bushfire smoke exposure, measured in air samples; bPM10 and PM2.5: particulate matter with aerodynamic diameter ≤10 μm and ≤2.5 μm, respectively; cBlack Carbon (BC): marker of combustion-related particulate exposure; dCO: carbon monoxide, a combustion by-product; eOzone (O3), nitrogen dioxide (NO2), and sulfur dioxide (SO2): gaseous pollutants; f8-OHdG: 8-hydroxy-2′-deoxyguanosine ; 8-Oxo-dG: 8-hydroxy-2′-deoxyguanosine or 8-oxo-2′-deoxyguanosine, biomarker of oxidative DNA damage; gOx-GS: oxidized guanine species, biomarker of oxidative DNA damage; hMalondialdehyde (MDA) ; Lipid hydroperoxide: : a biomarker of lipid peroxidation; i8-Isoprostane: a lipid peroxidation marker of oxidative stress; j MPO: myeloperoxidase, an oxidative enzyme biomarker; H₂O₂:(hydrogen peroxide), a reactive oxygen species and substrate for MPO, Protein carbonyls: biomarkers of oxidative protein damage formed by ROS-mediated oxidation of amino acid residues; khsCRP/CRP: (high-sensitivity) C-reactive protein, inflammatory biomarker, ECP: eosinophil cationic protein, biomarker of eosinophilic airway inflammation, FeNO: fractional exhaled nitric oxide, non-invasive biomarker of airway inflammation; lICAM-1, VCAM-1: soluble adhesion molecules, biomarkers of endothelial inflammation; mIL: interleukin; TNF-α: tumor necrosis factor-alpha; IFNγ: interferon-gamma; GM-CSF: granulocyte-macrophage colony-stimulating factor; CRP: C-reactive protein; sICAM-1: soluble intercellular adhesion molecule-1, MCP-1: monocyte chemoattractant protein-1; nPTX3: pentraxin-3, biomarker of vascular inflammation; oSP-D: surfactant protein D, biomarker of lung injury; pTEAC: Trolox equivalent antioxidant capacity, marker of plasma antioxidant status; q3NT: 3-nitrotyrosine, oxidative stress biomarker of protein nitration; rRegression coefficients: reported per log10 increase in levoglucosan concentration; sCross-shift ratio: ratio of post-exposure to pre-exposure biomarker levels; tLinear mixed effect models: account for repeated measures across participants; uValues in parentheses=95 % confidence intervals (CI); vNS: non-significant; wWhere p-values are given, p<0.05 is considered statistically significant; xSome studies include specific populations: firefighters (Gaughan et al. [48], 55]; Main,et al.[33]; Niyatiwatchanchai et al. [49]; Wu [32], 50]; Adetona et al. [51], Adetona [52]; Cherry [53]; Gianniou et al. [54]; Swiston [16];) and general or experimental populations (Ferguson [34]; Peters et al. [35]; O’Dwyer et al. [27]); yPre-shift, post-shift, and morning-after refer to biomarker sampling relative to firefighting or exposure periods; yT1/T2: T1 = beginning of shift; zT2 = 12 h after start of shift.
In controlled experiments mimicking firefighter exposure, two studies used similar protocols where 10 healthy adult participants inhaled PM2.5 from woodsmoke during three trials (Clean Air or Filtered Air: 0 μg/m3, Low Exposure: 250 μg/m3, and High Exposure: 500 μg/m3) for 1.5 h [34], 35]. These studies provided evidence of acute oxidative responses, with blood 8-isoprostane increased significantly after low and high exposure trials as well as in combined exposure analysis [35], with delayed or null changes in EBC [34]. These findings suggest that systemic measurements might be more effective to capture early oxidative stress than airway condensates.
Malondialdehyde (MDA)
Three studies (3/14) examined the relationship between exposure to PM2.5 and black carbon (BC) with MDA, a marker of lipid peroxidation levels, in firefighters [50], [51], [52]. Only one study reported a small significant association between creatinine-corrected urinary MDA and BC exposure [50], while others showed no cross-shift changes despite longer exposures [51], 52]. These discrepancies may be due to differences in exposure intensity, biomarker adjustment methods, or inter-individual variability in oxidative stress responses.
8-Oxo-2′-deoxyguanosine (8-Oxo-dG) or 8-hydroxy-2′-deoxyguanosine(8-OHdG)
Two studies (2/14) have assessed an indicator of oxidative DNA damage in firefighters using 8-Oxo-dG and 8-OHdG [48], 52]. This marker is the same molecule denoted by a modified guanine base in DNA that results from oxidative damage caused by reactive oxygen species (ROS). Although there was no significant cross-shift change observed, 8-Oxo-dG levels were correlated with shorter firefighting experience and younger age, suggesting that less experienced firefighters may be more susceptible to early oxidative DNA damage, potentially due to limited occupational adaptation or lower cumulative antioxidant defense mechanisms [52]. However, this interpretation needs further investigation. The directions in cross-shift changes of creatinine adjusted for various factors were not consistent as can be seen in Table 2.
In a cross-sectional study among firefighters, urinary 8-OHdG levels also increased significantly with levoglucosan [48]. Although the authors noted that more than 50 % of participants consumed fried foods and 84–89 % consumed bacon or candy, levoglucosan is a recognized tracer of biomass combustion and is not typically derived from dietary sources. Therefore, the elevated urinary levoglucosan levels are more plausibly attributed to BFS exposure. However, further research is needed to confirm this interpretation.
Other measured oxidative stress biomarkers
Several other oxidative stress biomarkers were sporadically reported across studies which limiting cross-study comparisons. Urinary oxidized guanine species (Ox-GS) level was reported significantly increased among firefighters during prescribed burn shifts compared to regular workdays, due to the exposure to PM2.5 and BC [50]. Controlled experiments revealed complex systemic responses, including decreases in uric acid (UA) and lipid hydroperoxide (LOOH), alongside increases in Trolox equivalent antioxidant capacity (TEAC) and 3-nitrotyrosine (3NT) [35], while no significant change was found in hydrogen peroxide (H2O2) levels [34]. These findings emphasize the complexity of oxidative stress responses which need further explanation.
Inflammatory biomarkers
Cytokines and chemokines
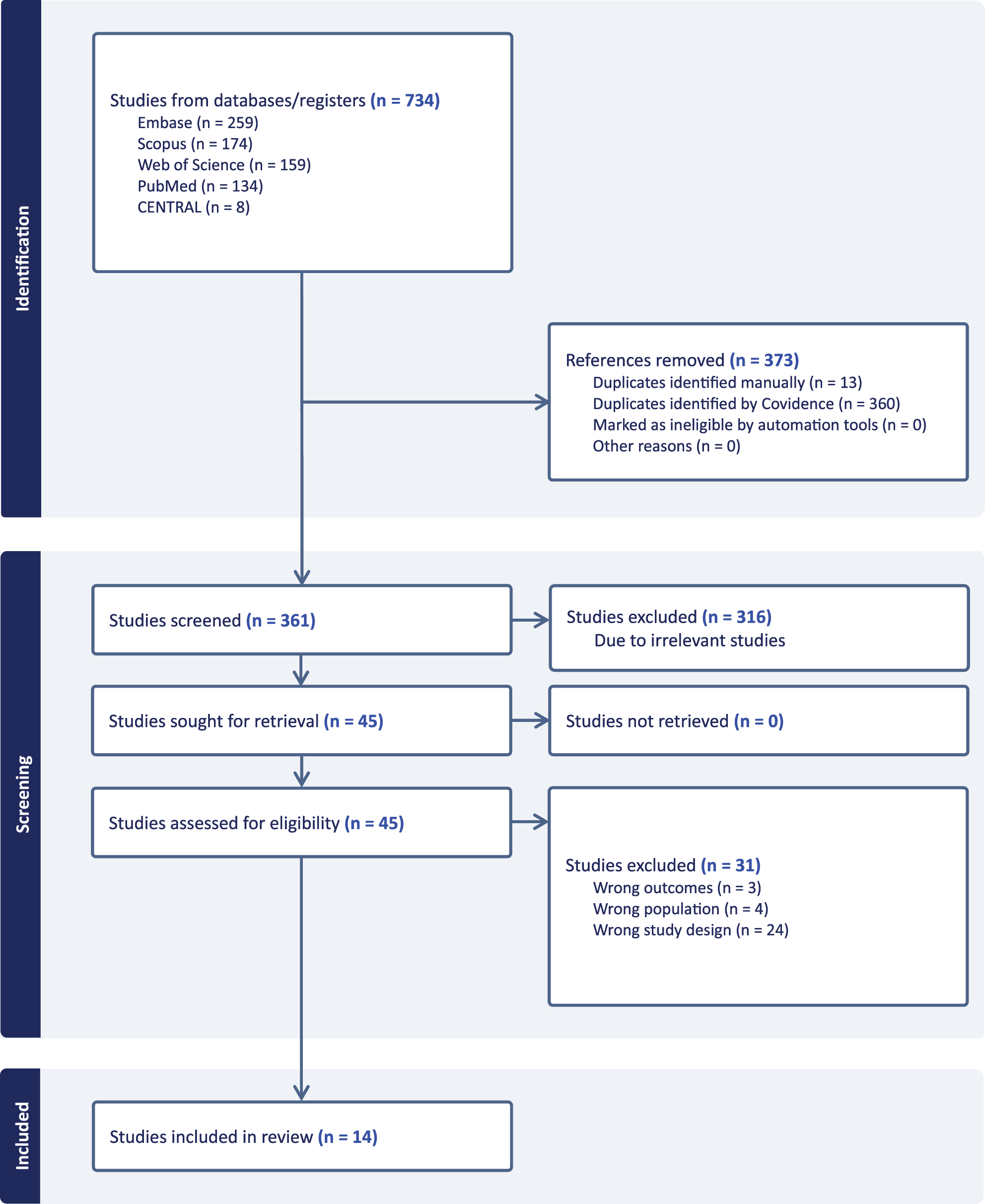
Pro-inflammatory IL-8 showed the most consistent acute response to BFS in firefighters. Two studies reported significant post-shift increases in blood IL-8, while a seasonal follow-up study found IL-8 levels returned to baseline after three months, suggesting potential adaptation or changes in immune response over time [53]. To further synthesize evidence, three firefighter studies that measured IL-8 in blood samples with comparable pre- and post-shift [16], 33] or seasonal designs [53] were included in a forest plot analysis (Figure 2). The pooled estimate indicated an overall elevation in IL-8 levels following BFS exposure (mean difference 9.76 pg/mL, 95 % CI –8.26, 27.79), although heterogeneity across studies was substantial. By contrast, a study that assessed EBC reported null findings [32], while another study observed increases in sputum and serum IL-8 [54]. Together, these findings suggest that blood-based measures more consistently capture IL-8 changes in firefighters, whereas results vary across matrices and exposure durations.
![Figure 2:
Forest plot of mean differences in IL-8 (pg/mL) pre- vs. post-exposure among firefighters. Three blood-based studies were included (Swiston [16]; Main et al. [33]; Cherry [53]). Effect sizes are shown as mean differences with 95% confidence intervals. A random-effects model was applied to account for between-study heterogeneity. Swiston and Main reported significant post-shift increases in IL-8, whereas Cherry’s seasonal comparison showed no significant change.](/document/doi/10.1515/reveh-2025-0109/asset/graphic/j_reveh-2025-0109_fig_002.jpg)
Forest plot of mean differences in IL-8 (pg/mL) pre- vs. post-exposure among firefighters. Three blood-based studies were included (Swiston [16]; Main et al. [33]; Cherry [53]). Effect sizes are shown as mean differences with 95% confidence intervals. A random-effects model was applied to account for between-study heterogeneity. Swiston and Main reported significant post-shift increases in IL-8, whereas Cherry’s seasonal comparison showed no significant change.
The same findings were observed with IL-6 [16], 33], 53]. A study did not include IL-6 analysis due to limited detection in EBC (only three of 142 samples detectable), possibly due to lower exposure intensity and use of a less sensitive matrix [32]. IL-10 consistently decreased across work-shift and seasonal analysis [33], 53]. These variations suggest that both the timing of biomarker measurement and magnitude and duration of exposure may influence these markers detectability and direction of response. Besides that, factors such as strenuous physical activities, and thermal strain may play important role in modulating the inflammatory response [33].
Other interleukins showed various associations with BFS exposure. These include IL-13, IL-1β, IP-12P70, and GM-CSF which increased during cross-shift [33], 54] and decreased during seasonal follow-up [53]. Conversely, there were significant decrease in IL-15, IL-17A, IL-12P40, IL-1RA, IL-1a, IL-3, IFN-γ, IFN-α2, and TGF- β in some studies [53], 54]. These findings highlight the critical influence of sample timing and biological matrices on cytokine interpretation but require more studies to confirm the associations.
Other inflammatory biomarkers
Other markers of respiratory inflammation such as MPO and ECP were also examined in sputum, nasal lavage fluid, and blood samples, with mixed findings. Significant associations were reported for MPO levels and ECP in sputum and nasal lavage fluid [55] as well as blood [35], whereas others observed no significant changes [34], 54]. PTX3 and SP-D demonstrated significant increases in plasma and EBC following controlled BFS exposure [34]. However, post-exposure effects were observed in blood samples, suggesting that systemic markers may be more sensitive to PM exposure than EBC markers. Fractional exhaled nitric oxide (FeNO) showed positive associations with BFS across all lag periods and coal mine fire smoke at 4hr lag [27], suggesting its utility as a short-term exposure indicator. By contrast, CRP showed no significant associations in most studies [27], 32], 49].
Discussion
IL-8 as a consistent inflammatory biomarker
Our review, supported by a forest plot analysis of three firefighter studies [16], 33], 53], identifies IL-8 as the most consistent inflammatory biomarker associated of BFS exposure. Blood-based measures showed reproducible post-shift increases, while results from EBC, sputum and seasonal assessments were more variable. IL-8 is a pro-inflammatory chemokine involved in the recruitment and activation of neutrophils, airway inflammation and immune responses [56]. The findings indicate that IL-8 levels typically rose within 6–12 h post-exposure [16], 33], but declined after 24 h or over months [53], 54]. These findings suggest that while acute inflammation occurs shortly after exposure, IL-8 gradually return to baseline over time as supported by several studies that found inverse associations between IL-8 and long-term exposure to air pollution [57], 58].
Our findings also suggest that IL-8 responses could depends on the biological matrix used. However, more studies are needed especially among firefighters who are at risk to smoke inhalation, heat exposure and strenuous physical activities. Biological matrix from blood shows promising result. While a study observed a significant increase of IL-8 in nasal lavage fluid from PM2.5 exposure in ambient air pollution [59], we could not find other literature to support this finding. This highlights the robustness of IL-8 across different biological matrices. Experimental evidence in animal models further supports the role of IL-8 in the initiation and progression of lung inflammation after smoke inhalation, indicating it‘s importance as a mechanistic mediator of airway injury [60].
Although the subsequent decline over time suggests inflammatory resolution, repeated exposures could contribute to cumulative immune activation, which may have long-term health implications particularly for firefighters. However, there is limited evidence to support this hypothesis, highlighting the need for further research on the long-term effects of repeated BFS exposure among this group.
Other inflammatory cytokines (TNF-α, IL-6, GM-CSF, IFN-γ)
Other inflammatory biomarkers, like IL-6 [16], 33], 53], TNF-α [33], 53], 54], GM-CSF [33], 53], and IFN-γ [33], 53] were also reported, suggesting that BFS exposure triggers an acute immune response. While these findings indicate their potential as informative biomarkers, interpretation of their levels requires an understanding of their role and kinetics in the inflammatory cascade. For example, Main et al. [33] and Swiston [16] reported acute increases in IL-6 within hours of exposure, while Cherry [53] found IL-6 increases between off season and post exposure periods. These findings are biologically plausible, as IL-6 plays a key role in the acute phase reaction [61] and has been implicated as a predictor of COPD exacerbations [62]. Evidence from experimental study also demonstrate that prolonged smoke exposure (six months) can elevate IL-6 alongside TNF-α and GM-CSF [63], supporting observations from BFS studies.
Evidence shown that stimulation of IL-6 produced CRP [64]. Our reviewed identified only two studies measured CRP together with IL-6 [16], 32], with only one observed significant association with BFS exposure. While CRP typically peaks 36–50 h after an inflammatory trigger [64], short hours exposure might explain why CRP result in another study is null. This likely explains why cytokines such as IL-8, which respond rapidly within hours, consistently demonstrated acute changes, whereas slower-responding systemic markers like C-reactive protein (CRP) showed null results. These observations indicate that BFS exposure may similarly trigger a cascade of cytokine production in the respiratory system [36], although comparisons are limited by the scarcity of BFS specific studies.
The timing of cytokine release is critical for interpretation. TNF-α, for example, is rapidly released by epithelial cells in response to irritants and serves to stimulate IL-8 production from both epithelial cells and macrophages, leading to neutrophil recruitment. Most BFS studies measured outcomes within short exposure (1–5 h), which aligns with these early cytokine responses [16], 32], 33], 53], 54].
Studies among firefighters also explored other inflammatory biomarkers such as ICAM-1, VCAM-1, IL-1β, IL-13, ECP, serum amyloid A (SAA), immunoglobulin G, and leukocyte count [19]. However, the evidence base remains relatively small. These findings emphasize the importance of investigating not only which biomarkers change, but also when they change and why, to better understand the immune response to BFS exposure.
Oxidative stress biomarkers (8-isoprostane, MDA, 8-OHdG)
In BFS exposure studies, 8-isoprostane, MDA and 8-OHdG were commonly assessed oxidative stress biomarkers. 8-isoprostane and MDA reflect damage to lipids in cell membranes while 8-OHdG signifies damage to DNA [65], [66], [67]. These biomarkers provide complementary insight into different pathways of oxidative injury. However, there were mixed results that complicates these biomarkers interpretation as reliable indicators of oxidative damage from BFS exposure.
8-isoprostane was the most frequently examined biomarker, but findings were inconsistent. Few studies among firefighters reported no significant cross-shift changes in urinary or EBC concentrations [32], 50], 51], while a longer exposure study found only a modest associations with levoglucosan [48]. Urinary levoglucosan however is not suitable to be used as a biomarker to assess BFS exposure due to influence from demographic, dietary intake, and behavioral factors [68], 69]. Controlled exposure experiments further demonstrated significant increases of 8-isoprostane after short-term duration of woodsmoke at varying PM2.5 concentrations (0, 250, 500 μg/m3) [34], 35]. This contrast suggests that systemic biomarkers measured under standardized exposure conditions may be more sensitive to short-term oxidative stress than in field-based assays. Variability in findings may be due to differences in sampling matrices, timing of measurements, and exposure intensities [45], 70], 71], reinforcing the need for harmonized biomarker protocol. Despite its limitations, 8-isoprostane remains the most commonly used biomarker for oxidative stress assessment in human [45], but studies are needed specifically in occupational BFS contexts.
Our review also indicates inconsistent results for MDA as biomarker of oxidative stress due to BFS exposure. Most studies observed no significant associations between MDA and BFS exposure, with only one study reporting increased of urinary MDA with black carbon concentrations [50], 51]. These inconsistencies were likely due to small sample sizes (12–19 subjects) and dietary influences from high-lipid content food [50], 51] which reinforce the need for bigger sample size and the use of diverse biological matrix, and refined methods to validate MDA as a biomarker of BFS induced outcome [72]. Although urine is the most commonly used matrix due to convenience and non-invasive nature [73], other matrices like EBC, serum, and nasal fluid may offer greater sensitivity [74], 75]. Supporting this, studies on traffic-related air pollution (TRAP) also consistently show stronger associations with MDA levels when assessed in EBC, serum or plasma in traffic-exposed populations including residence, urban workers, and taxi drivers [76], [77], [78], [79]. This suggests that BFS studies may benefit from adopting similar approaches to improve reproducibility across populations.
While 8-OHdG provided valuable insights into the effects of BFS exposure on oxidative damage biomarkers among firefighters, it still remains limited and scarce. In broader literature, 8-OHdG has been widely used a biomarker of oxidative DNA damage from PM2.5 exposure, with levels shown to increase in proportion to the PM concentration [72], 80]. However, its application in BFS studies is still underdeveloped. Existing studies are constrained by small sample size, reliance on convenience sampling, and confounders such as dietary factors that weakened the reliability of 8-OHdG as a robust biomarker of BFS exposure [48], 51], 52].
Taken together, evidence of oxidative stress biomarkers in BFS exposure remains inconsistent. While chamber experiments demonstrate clear oxidative responses, field studies often show null or variable results, highlighting the influence of methodological heterogeneity. Standardized protocols, use of multiple biological matrices, and adequately powered longitudinal cohorts are critical to clarify the role of 8-isoprostane, MDA, and 8-OHdG in capturing oxidative stress responses to BFS.
Impact of study populations and exposure settings
Most included studies were observational cohort designs comparing the levels of biomarker levels across firefighting work-shifts (pre-and post-shift) during bushfire events and two randomized controlled trials mimicking firefighter exposure. This reflects their occupational exposure risk, including repeated deployments over consecutive weeks, prolonged work shifts up to 16 h, and exposure to extreme levels of smoke increase susceptibility to acute cardiovascular effects and long-term risks such as lung cancer mortality [6], 28], 29]. Fire-related exposures are known to alter inflammatory cytokines as well as of vascular damage and tissue injury in firefighters [19].
Importantly, physiological adaptation may also influence biomarker outcomes. Newer firefighters (≤2 years of service) showed higher oxidative DNA damage, while those with longer service may develop adaptation to the upregulation of antioxidant enzymes [52]. One possible explanation for these findings is that newer firefighters may not yet have developed adaptive physiological responses to repeated smoke exposure. Occupational adaptation could involve the upregulation of endogenous antioxidant defense systems such as superoxide dismutase, glutathione peroxidase, and catalase, which help mitigate reactive oxygen species generated during intense exposure [81], [82], [83]. Gianniou et al. [54] reported higher oxidative DNA damage in newer firefighters, possibly reflecting an underdeveloped antioxidant response to repeated exposures. Broader literature supports that repeated exposures may strengthen antioxidant defense systems [84], 85], partly explaining lower biomarker levels among experienced firefighters.
Another plausible mechanism is epigenetic modification. Prolonged occupational exposure to PM and combustion products has been linked to DNA methylation changes in genes regulating oxidative stress and inflammatory pathways [86], [87], [88]. These modifications may alter individual susceptibility over time, suggesting that the differences between newer and experienced firefighters reflects not only years of service but also complex biological interactions. However, this pattern may be confounded by the healthy worker effect, a form of selection bias wherein individuals who remain employed in high-stress occupations like firefighting tend to be healthier and more resilient that those who leave early [89]. Thus, lower biomarker levels in long-serving firefighters may reflect selective retention rather than true physiological resilience. Despite potential adaptation, long-serving firefighters remain at risk of chronic inflammation and cardiovascular disease [19], suggesting adaptation may reduce acute effects but not prevent cumulative damage.
These observations highlight the importance of occupational health surveillance. Early-career firefighters may benefit from biomarker-based monitoring, antioxidant or nutrition support, and physiological screening to identify those at greatest risk, while longitudinal monitoring of experienced workers is needed to assess whether adaptation offers protection or masks cumulative long-term risks [19], 90].
While valuable for characterizing acute and high-level exposures, the included studies may not reflect the effects of BFS on the general population. Only one study examined elderly people [27], reporting increased FeNO after short-term exposure to PM2.5 at levels as low as 10 μg/m3. This aligns with epidemiological evidence showing that BFS PM2.5 exposure>80 μg/m3 increases respiratory hospitalizations, while even lower levels (>25 μg/m3) can elevate cardiovascular mortality in adults above 65 years old [9], 91]. This is because aging renders the cardiovascular system more susceptible to the damaging effects of BFS [29].
Other vulnerable groups remain underrepresented. Studies observed significant health effects from air pollution on lung function and COPD among individuals from lower-income households [12], and maternal BFS exposure has been associated with pre-term birth during the second trimester and decreased birth weight during the first trimester [92]. Despite this findings, few biomarker-based studies have targeted pregnant women, children, or disadvantaged groups, limiting mechanistic understanding in these populations.
Overall, current evidence is dominated by firefighter cohorts, limiting generalizability. While epidemiological research consistently demonstrates that PM2.5 from BFS contributes to respiratory disease [13], biomarker studies among broader vulnerable populations remain limited. Expanding biomarker research to elderly individuals, pregnant women, children, and socioeconomically disadvantaged communities is critical to improve health risk assessments and guide protective interventions across diverse populations. This underscores the importance to improve the reliability of health assessments of PM2.5 from bushfire through assessment of oxidative stress and inflammation biomarkers accross diverse populations.
Methodological heterogeneity and challenges
A key limitation of the existing literature on BFS biomarkers is the substantial heterogeneity in study design, exposure assessment, and outcome measurement. Most included studies were observational cohort studies that compared biomarker levels pre- and post-shift in firefighters. While such designs are valuable for capturing acute occupational exposures, they lack the standardization needed to facilitate comparisons across populations and settings. Only two studies employed controlled exposure experiments simulating firefighter conditions [34], 35], which allowed for tighter control over exposure levels but may not accurately reflect real-world variability.
We found that exposure assessment methods varied widely across studies. Most studies conducted direct measurement of pollutants such as PM2.5, black carbon, and CO or surrogate markers like levoglucosan, while some studies conducted controlled exposure experiments. These approaches can introduce misclassification and make it difficult to directly compare the results, particularly when individual-level exposures are not measured. In our opinion, there is potential recall bias when some studies supplemented self-reported information on work tasks, firefighting years, or shift activities. Similarly, biological matrix assessment in the studies is differed across studies. The use of blood, urine, EBC, or nasal lavage fluid could influence both sensitivity and specificity of biomarker detection. For example, measurement of 8-isoprostane in blood showed stronger responses than EBC and urine [32], 34], 35], 48], 50], 51]. Thus, it is important to select appropriate biological matrix for analysis which requires more studies.
Finally, many cohort studies were limited by small sample sizes, with some had less than 20 participants [32], [50], [51], [52]. Such underpowered designs reduce the ability to detect meaningful associations and increase susceptibility to random error. Potential confounders such as diet, concurrent air pollution exposures, or individual differences in antioxidant capacity were often unmeasured or insufficiently controlled, weakening the reliability of findings.
Together, these methodological limitations highlight the challenges of synthesizing evidence across studies and underscore the need for standardized protocols in biomarker research on BFS. Harmonizing exposure metrics, expanding sampling windows, and adopting multi-matrix biomarker approaches could significantly enhance comparability and strengthen causal inference in future studies. These sources of heterogeneity also explain why only IL-8 across three firefighter studies was pooled quantitatively using a forest plot, while all other biomarkers were narratively synthesized. The differences in biomarker matrices, exposure characterization, and timing of outcome assessment would have made quantitative pooling misleading, reinforcing the importance of cautious interpretation.
Implications for public health and occupational safety
The findings of this review carry important implications for both public health and occupational safety. The consistent observation of acute inflammatory responses, particularly IL-8 shows that BFS exposure can trigger immediate immune activation. For firefighters, who often experience repeated and prolonged exposures, these short-term responses may accumulate into long-term health risks, including chronic respiratory disease, cardiovascular dysfunction, and even cancer risk. This highlights the need for strengthened occupational health surveillance and protective measures, such as optimized respiratory protection, rotational deployment schedules, and regular biomarker-based health monitoring.
From a broader public health perspective, the limited evidence among elderly individuals, pregnant women, and lower-income populations points to significant gaps in current preparedness strategies. Vulnerable groups are more likely to experience adverse outcomes, such as pre-term birth, impaired lung function, and cardiovascular events, even at relatively modest levels of PM2.5 exposure. Developing targeted public health interventions during bushfire events, including real-time air quality warnings, accessible indoor clean-air shelters, and community-level education campaigns on exposure mitigation are essential.
Moreover, the inconsistent results for oxidative stress and inflammatory biomarkers highlight methodological gaps that impede translation into practice. Standardized biomarker protocols across diverse populations would improve the reliability of health assessments and provide stronger evidence for risk communication and policymaking. Integrating biomarker data with epidemiological outcomes could also support the development of exposure thresholds that are specific to BFS rather than generalized from urban or traffic-related air pollution studies.
These findings emphasize that protecting both firefighters and the general population from BFS exposure requires a dual approach. This includes targeted occupational safeguards for high-risk workers and inclusive public health strategies for vulnerable groups. Strengthening biomarker research and surveillance will be critical to mitigating the long-term health burden of increasingly frequent and severe bushfire events.
Limitations and potential biases
This review has several limitations that should be considered when interpreting the findings. First, most studies had small sample sizes, often fewer than 50 participants which increases the risk of random error, limits statistical power, and may reduce the representativeness of the study population. No study provided sample size justifications or power calculations, further constraining confidence in the robustness of their results. As emphasized by Sadiq [93], adequate sample sizes are essential to ensure robust and applicable observed associations to broader populations [93].
Second, substantial heterogeneity in study design, exposure measurement methods, and biomarker matrices complicates direct comparisons or meta-analytic synthesis. For example, exposure metrics ranged from PM2.5 and black carbon to gaseous pollutants, while biomarkers were measured in diverse matrices such as urine, blood, exhaled breath condensate, and nasal lavage fluid. Inconsistent timing of sample collection from pre-shift to post-shift or days or months later likely contributing to inconsistent results.
Third, most studies did not adequately adjust for confounding variables such as diet, physical exertion, and baseline health status, all of which can influence oxidative and inflammatory biomarkers. The absence of proper adjustment might introduce potential bias in estimating the effects of BFS.
Fourth, most studies were derived from firefighter cohorts, reflecting occupational risk but limiting generalizability of findings to other vulnerable groups such as children, older adults, pregnant individuals, and those with pre-existing conditions.
Lastly, few studies examined long-term outcomes, with most investigations restricted to short-term or cross-shift exposures. This prevents firm conclusions about the cumulative effects of repeated BFS exposure or potential progression to chronic diseases such as COPD, cardiovascular disease, or adverse birth outcomes. Taken together, these limitations constrain the interpretability and generalizability of current findings.
Research gaps and future directions
Building on the limitations above, several research gaps warrant urgent attention. First, while IL-8 emerged as the most consistent short-term inflammatory biomarker, the variability in its measurements across biological matrices other than blood as well as inconsistencies in other biomarkers including IL-6, TNF-α, GM-CSF, IFN-γ, 8-isoprostane, MDA, and 8-OHdG, requires standardized biomarker protocols and harmonized sampling strategies. These would improve reproducibility and allow meta-analysis across studies.
Second, future studies should prioritize community-based populations, particularly pregnant women, the elderly, children, and socioeconomically disadvantaged populations who seems to be neglected. Longitudinal studies on the effects of BFS exposure are needed to better capture chronic and population-level health impacts.
Third, the phenomenon of physiological adaptation in firefighters, whereby oxidative stress responses may decline with years of service, requires deeper investigation. It remains unclear whether adaptation lowers long-term disease risk or simply delays the onset of chronic conditions. Longitudinal biomarker monitoring alongside clinical outcomes could help clarify these effects.
Finally, integration of biomarker data with epidemiological and exposure modeling studies is needed to establish exposure-response relationships specific to BFS, rather than extrapolating from urban or traffic-related air pollution. Such evidence would support the development of health-protective air quality guidelines tailored to BFS events.
To our knowledge, this is the first systematic review to comprehensively synthesize evidence on oxidative stress and inflammatory biomarkers associated with bushfire smoke (BFS) exposure across occupational and community populations. The study was conducted with methodological rigor, following the PRISMA 2020 guidelines, registered in PROSPERO, and supported by a formal risk-of-bias assessment to ensure transparency and reproducibility. By integrating findings from multiple biomarkers, biological matrices, and exposure contexts, this review provides a broad and mechanistic understanding of BFS-related health effects. Importantly, the synthesis identifies key research gaps and proposes a structured roadmap for future studies, offering practical value for both scientific inquiry and public health policy development. Future research should expand beyond occupational cohorts, adopt standardized biomarker protocols, and employ longitudinal and multidisciplinary approaches. Addressing these gaps will strengthen the evidence base, improve public health preparedness, and inform occupational safety standards in the face of increasingly frequent and severe bushfires.
Conclusions
This systematic review provides the first comprehensive synthesis of evidence on the effects of BFS exposure on oxidative stress and inflammatory biomarkers. Across 14 studies, we found consistent short-term elevations in blood IL-8, supporting its role as the most reliable biomarker of acute inflammatory response to BFS, but more research is needed for other biological matrices. Other cytokines (IL-6, TNF-α, GM-CSF, IFN-γ) and oxidative stress markers (8-isoprostane, MDA, 8-OHdG) showed variable associations, reflecting heterogeneity in study designs, exposure assessment methods, and sampling matrices. These inconsistencies highlight the need for standardized protocols to improve comparability and reproducibility.
Importantly, most of the existing evidence derives from firefighter cohorts, whose repeated, high-intensity exposures differ from those experienced by the general population. Vulnerable groups such as children, pregnant women, older adults, and individuals from lower-income households remain underrepresented, despite strong epidemiological evidence of their heightened susceptibility to BFS-related morbidity and mortality. This gap limits generalizability and underscores the need for research that integrates biomarker monitoring with community-based and longitudinal studies.
The novelty of this review lies in its dual focus on both oxidative stress and inflammatory biomarkers, offering mechanistic insights that link BFS exposure to downstream health outcomes. By consolidating evidence across occupational and environmental contexts, this study provides a roadmap for future investigations to validate biomarkers, clarify exposure-response relationships, and expand to diverse populations.
In the broader context, understanding biomarker responses to BFS is critical for developing targeted interventions, guiding occupational safety protocols for high-risk groups such as firefighters, and informing public health preparedness strategies during increasingly frequent and severe bushfire events. Strengthening this evidence base will ultimately support the development of health-protective policies and improve resilience against the growing global burden of bushfire smoke exposure.
Acknowledgments
We wish to acknowledge the essential contributions of the colleagues and our academic institutions, whose resources and encouragement were instrumental in its completion. We would like to extend our heartfelt thanks to everyone who offered their support during the research and writing process, especially our family and friends, for their unwavering encouragement and patience.
-
Research ethics: Not applicable.
-
Informed consent: Not applicable.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Use of Large Language Models, AI and Machine Learning Tools: None declared.
-
Conflict of interest: The authors state no conflict of interest.
-
Research funding: None declared.
-
Data availability: Not applicable.
References
1. Wilgus, M-L, Merchant, M. Clearing the air: understanding the impact of wildfire smoke on asthma and COPD. Healthcare 2024;12:307. https://doi.org/10.3390/healthcare12030307.Search in Google Scholar PubMed PubMed Central
2. Ebi, KL, Vanos, J, Baldwin, JW, Bell, JE, Hondula, DM, Errett, NA, et al.. Extreme weather and climate change: population health and health system implications. Annu Rev Publ Health 2021;42:293–315. https://doi.org/10.1146/annurev-publhealth-012420-105026.Search in Google Scholar PubMed PubMed Central
3. Weheba, A, Vertigan, A, Abdelsayad, A, Tarlo, SM. Respiratory diseases associated with wildfire exposure in outdoor workers. J Allergy Clin Immunol Pract 2024;12:1989–96. https://doi.org/10.1016/j.jaip.2024.03.033.Search in Google Scholar PubMed
4. Borchers, AN, Palmer, AJ, Bowman, DMJS, Morgan, GG, Jalaludin, BB, Johnston, FH. Unprecedented smoke-related health burden associated with the 2019–20 bushfires in eastern Australia. Med J Aust 2020;213:282–3. https://doi.org/10.5694/mja2.50545.Search in Google Scholar PubMed
5. Sambodo, NP, Pradhan, M, Sparrow, R, van Doorslaer, E. When the smoke gets in your lungs: short-term effects of Indonesia’s 2015 forest fires on health care use. Environ Health 2024;23:44. https://doi.org/10.1186/s12940-024-01079-x.Search in Google Scholar PubMed PubMed Central
6. Chen, H, Samet, JM, Bromberg, PA, Tong, H. Cardiovascular health impacts of wildfire smoke exposure. Part Fibre Toxicol 2021;18:2. https://doi.org/10.1186/s12989-020-00394-8.Search in Google Scholar PubMed PubMed Central
7. Cleland, SE, Serre, ML, Rappold, AG, West, JJ. Estimating the acute health impacts of fire-originated PM(2.5) exposure during the 2017 California wildfires: sensitivity to choices of inputs. Geohealth 2021;5:e2021GH000414. https://doi.org/10.1029/2021gh000414.Search in Google Scholar
8. Vega, SL, Childs, ML, Aggarwal, S, Nethery, RC. Wildfire smoke exposure and cause-specific hospitalization in older adults. JAMA Netw Open 2025;8:e257956. https://doi.org/10.1001/jamanetworkopen.2025.7956.Search in Google Scholar PubMed PubMed Central
9. Heaney, A, Stowell, JD, Liu, JC, Basu, R, Marlier, M, Kinney, P. Impacts of fine particulate matter from wildfire smoke on respiratory and cardiovascular health in California. Geohealth 2022;6:e2021GH000578. https://doi.org/10.1029/2021gh000578.Search in Google Scholar
10. Zhang, Q, Wang, Y, Xiao, Q, Geng, G, Davis, SJ, Liu, X, et al.. Long-range PM2.5 pollution and health impacts from the 2023 Canadian wildfires. Nature 2025;645:672–8. https://doi.org/10.1038/s41586-025-09482-1.Search in Google Scholar PubMed PubMed Central
11. Madani, NA, Jones, LE, Carpenter, DO. Different volatile organic compounds in local point source air pollution pose distinctive elevated risks for respiratory disease-associated emergency room visits. Chemosphere 2023;344:140403. https://doi.org/10.1016/j.chemosphere.2023.140403.Search in Google Scholar PubMed
12. Doiron, D, de Hoogh, K, Probst-Hensch, N, Fortier, I, Cai, Y, De Matteis, S, et al.. Air pollution, lung function and COPD: results from the population-based UK Biobank study. Eur Respir J 2019;54:1802140. https://doi.org/10.1183/13993003.02140-2018.Search in Google Scholar PubMed
13. Zhong, Q, Shen, G, Wang, B, Ma, J, Tao, S. Climate-driven escalation of global PM2.5 health burden from wildland fires. Environ Sci Technol 2025;59:3131–42. https://doi.org/10.1021/acs.est.4c10320.Search in Google Scholar PubMed
14. Lim, EY, Kim, G-D. Particulate matter-induced emerging health effects associated with oxidative stress and inflammation. Antioxidants 2024;13:1256. https://doi.org/10.3390/antiox13101256. [Internet].Search in Google Scholar PubMed PubMed Central
15. Milton, LA, White, AR. The potential impact of bushfire smoke on brain health. Neurochem Int 2020;139:104796. https://doi.org/10.1016/j.neuint.2020.104796.Search in Google Scholar PubMed
16. Swiston, JR, Davidson, W, Attridge, S, Li, GT, Brauer, M, van Eeden, SF. Wood smoke exposure induces a pulmonary and systemic inflammatory response in firefighters. Eur Respir J 2008;32:129–38. https://doi.org/10.1183/09031936.00097707.Search in Google Scholar PubMed
17. Sangkham, S, Phairuang, W, Sherchan, SP, Pansakun, N, Munkong, N, Sarndhong, K, et al.. An update on adverse health effects from exposure to PM2.5. Environ Adv 2024;18:100603. https://doi.org/10.1016/j.envadv.2024.100603.Search in Google Scholar
18. Cleland, SE, Wyatt, LH, Wei, L, Paul, N, Serre, ML, West, JJ, et al.. Short-term exposure to wildfire smoke and PM2.5 and cognitive performance in a brain-training game: a longitudinal study of U.S. adults. Environ Health Perspect 2022;130:1–12. https://doi.org/10.1289/ehp10498.Search in Google Scholar
19. Barros, B, Oliveira, M, Morais, S. Firefighters’ occupational exposure: contribution from biomarkers of effect to assess health risks. Environ Int 2021;156:106704. https://doi.org/10.1016/j.envint.2021.106704.Search in Google Scholar PubMed
20. Elser, H, Frankland, TB, Chen, C, Tartof, SY, Mayeda, ER, Lee, GS, et al.. Wildfire smoke exposure and incident dementia. JAMA Neurol 2025;82:40–8. https://doi.org/10.1001/jamaneurol.2024.4058.Search in Google Scholar PubMed PubMed Central
21. Kwon, D, Paul, KC, O’Sharkey, K, Paik, S-A, Yu, Y, Bronstein, JM, et al.. Challenges in studying air pollution to neurodegenerative diseases. Environ Res 2025;278:121597. https://doi.org/10.1016/j.envres.2025.121597.Search in Google Scholar PubMed
22. Amjad, S, Chojecki, D, Osornio-Vargas, A, Ospina, M. Wildfire exposure during pregnancy and the risk of adverse birth outcomes: a systematic review. Environ Int 2021;156:106644. https://doi.org/10.1016/j.envint.2021.106644.Search in Google Scholar PubMed
23. Zhang, Y, Ye, T, Yu, P, Xu, R, Chen, G, Yu, W, et al.. Preterm birth and term low birth weight associated with wildfire-specific PM2.5: a cohort study in New South Wales, Australia during 2016–2019. Environ Int 2023;174:107879. https://doi.org/10.1016/j.envint.2023.107879.Search in Google Scholar PubMed
24. Khalili, R, Liu, Y, Xu, Y, O’Sharkey, K, Pavlovic, N, McClure, C, et al.. Adverse birth outcomes associated with heat stress and wildfire smoke exposure during preconception and pregnancy. Environ Sci Technol 2025;59:12458–71. https://doi.org/10.1021/acs.est.4c10194.Search in Google Scholar PubMed PubMed Central
25. Basilio, E, Ozarslan, N, Buarpung, S, Benmarhnia, T, Padula, AM, Robinson, JF, et al.. Gestational age-dependent decrease in fetal Hofbauer cells in placentas from pregnancies exposed to wildfire smoke in California. medRxiv 2023. https://doi.org/10.1101/2023.01.11.23284125.Search in Google Scholar PubMed PubMed Central
26. MacIntyre, CR, Nguyen, PY, Trent, M, Seale, H, Chughtai, AA, Shah, S, et al.. Adverse health effects in people with and without preexisting respiratory conditions during bushfire smoke exposure in the 2019/2020 Australian summer. Am J Respir Crit Care Med 2021;204:368–71. https://doi.org/10.1164/rccm.202012-4471le.Search in Google Scholar
27. O’Dwyer, T, Abramson, MJ, Straney, L, Salimi, F, Johnston, F, Wheeler, AJ, et al.. Sub-clinical effects of outdoor smoke in affected communities. Int J Environ Res Public Health 2021;18:1131. https://doi.org/10.3390/ijerph18031131.Search in Google Scholar PubMed PubMed Central
28. Wah, W, Gelaw, A, Glass, DC, Sim, MR, Hoy, RF, Berecki-Gisolf, J, et al.. Systematic review of impacts of occupational exposure to wildfire smoke on respiratory function, symptoms, measures and diseases. Int J Hyg Environ Health 2025;263:114463. https://doi.org/10.1016/j.ijheh.2024.114463.Search in Google Scholar PubMed
29. Williams, VA, Perreault, LR, Yazbeck, CT, Micovic, NA, Oakes, JM, Bellini, C. Impact of wildfires on cardiovascular health. Circ Res 2024;134:1061–82. https://doi.org/10.1161/circresaha.124.323614.Search in Google Scholar PubMed PubMed Central
30. Zhang, Y, Tingting, Y, Huang, W, Yu, P, Chen, G, Xu, R, et al.. Health impacts of wildfire smoke on children and adolescents: a systematic review and meta-analysis. Curr Environ Health Rep 2024;11:46–60. https://doi.org/10.1007/s40572-023-00420-9.Search in Google Scholar PubMed
31. Balasooriya, NN, Bandara, JS, Rohde, N. Air pollution and health outcomes: evidence from black Saturday bushfires in Australia. Soc Sci Med 2022;306:115165. https://doi.org/10.1016/j.socscimed.2022.115165.Search in Google Scholar PubMed
32. Wu, CM, Adetona, A, Song, CC, Naeher, L, Adetona, O. Measuring acute pulmonary responses to occupational wildland fire smoke exposure using exhaled breath condensate. Arch Environ Occup Health 2020;75:65–9. https://doi.org/10.1080/19338244.2018.1562413.Search in Google Scholar PubMed PubMed Central
33. Main, LC, Wolkow, AP, Tait, JL, Della Gatta, P, Raines, J, Snow, R, et al.. Firefighter’s acute inflammatory response to wildfire suppression. J Occup Environ Med 2020;62:145–8. https://doi.org/10.1097/jom.0000000000001775.Search in Google Scholar
34. Ferguson, MD, Semmens, EO, Dumke, C, Quindry, JC, Ward, TJ. Measured pulmonary and systemic markers of inflammation and oxidative stress following wildland firefighter simulations. J Occup Environ Med 2016;58:407–13. https://doi.org/10.1097/jom.0000000000000688.Search in Google Scholar PubMed PubMed Central
35. Peters, B, Ballmann, C, Quindry, T, Zehner, EG, McCroskey, J, Ferguson, M, et al.. Experimental woodsmoke exposure during exercise and blood oxidative stress. J Occup Environ Med 2018;60:1073–81. https://doi.org/10.1097/jom.0000000000001437.Search in Google Scholar
36. Hamon, R, Tran, HB, Roscioli, E, Ween, M, Jersmann, H, Hodge, S. Bushfire smoke is pro-inflammatory and suppresses macrophage phagocytic function. Sci Rep 2018;8:13424. https://doi.org/10.1038/s41598-018-31459-6.Search in Google Scholar PubMed PubMed Central
37. Frumento, D, Țãlu, Ș. Effects of wildfire exposure on the human immune system. Fire [Internet] 2024;7:469. https://doi.org/10.3390/fire7120469.Search in Google Scholar
38. Pizzino, G, Irrera, N, Cucinotta, M, Pallio, G, Mannino, F, Arcoraci, V, et al.. Oxidative stress: harms and benefits for human health. Oxid Med Cell Longev 2017;2017:8416763. https://doi.org/10.1155/2017/8416763.Search in Google Scholar PubMed PubMed Central
39. Pardo, M, Li, C, Zimmermann, R, Rudich, Y. Health impacts of biomass burning aerosols: relation to oxidative stress and inflammation. Aerosol Sci Technol 2024;58:1093–113. https://doi.org/10.1080/02786826.2024.2379551.Search in Google Scholar
40. Kurmi, O, Dunster, C, Ayres, J, Kelly, F. Oxidative potential of smoke from burning wood and mixed biomass fuels. Free Radic Res 2013;47:829–35. https://doi.org/10.3109/10715762.2013.832831.Search in Google Scholar PubMed
41. Dutta, A, Roychoudhury, S, Chowdhury, S, Ray, M. Changes in sputum cytology, airway inflammation and oxidative stress due to chronic inhalation of biomass smoke during cooking in premenopausal rural Indian women. Int J Hyg Environ Health 2013;216:301–8. https://doi.org/10.1016/j.ijheh.2012.05.005.Search in Google Scholar PubMed
42. Akdis, CA, Nadeau, KC. Human and planetary health on fire. Nat Rev Immunol 2022;22:651–2. https://doi.org/10.1038/s41577-022-00776-3.Search in Google Scholar PubMed PubMed Central
43. Caravedo, M, Herrera, P, Mongilardi, N, Ferrari, A, Dávila-Román, V, Gilman, R, et al.. Chronic exposure to biomass fuel smoke and markers of endothelial inflammation. Indoor Air 2016;26:768–75. https://doi.org/10.1111/ina.12259.Search in Google Scholar PubMed PubMed Central
44. Tochukwu, U, Chukwuemeka, M, Emmanuel, D, Nnaemeka, I, Desmond, CO, O’Brien, C, et al.. Short and long-term exposure to biomass fuel (wood smoke) and its effects on cardiovascular risk markers and lipid peroxidation of Male albino rats. Asian J Res Cardiovasc Dis 2021;3:1–10.Search in Google Scholar
45. Graille, M, Wild, P, Sauvain, JJ, Hemmendinger, M, Guseva Canu, I, Hopf, NB. Urinary 8-isoprostane as a biomarker for oxidative stress. A systematic review and meta-analysis. Toxicol Lett 2020;328:19–27. https://doi.org/10.1016/j.toxlet.2020.04.006.Search in Google Scholar PubMed
46. Martinez-Moral, M-P, Kannan, K. How stable is oxidative stress level? An observational study of intra- and inter-individual variability in urinary oxidative stress biomarkers of DNA, proteins, and lipids in healthy individuals. Environ Int 2019;123:382–9. https://doi.org/10.1016/j.envint.2018.12.009.Search in Google Scholar PubMed PubMed Central
47. Page, MJ, McKenzie, JE, Bossuyt, PM, Boutron, I, Hoffmann, TC, Mulrow, CD, et al.. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. https://doi.org/10.1136/bmj.n71.Search in Google Scholar PubMed PubMed Central
48. Gaughan, DM, Siegel, PD, Hughes, MD, Chang, CY, Law, BF, Campbell, CR, et al.. Arterial stiffness, oxidative stress, and smoke exposure in wildland firefighters. Am J Ind Med 2014;57:748–56. https://doi.org/10.1002/ajim.22331.Search in Google Scholar PubMed PubMed Central
49. Niyatiwatchanchai, N, Pothirat, C, Chaiwong, W, Liwsrisakun, C, Phetsuk, N, Duangjit, P, et al.. Short-term effects of air pollutant exposure on small airway dysfunction, spirometry, health-related quality of life, and inflammatory biomarkers in wildland firefighters: a pilot study. Int J Environ Health Res 2023;33:850–63. https://doi.org/10.1080/09603123.2022.2063263.Search in Google Scholar PubMed
50. Wu, CM, Warren, SH, Demarini, DM, Song, CC, Adetona, O. Urinary mutagenicity and oxidative status of wildland firefighters working at prescribed burns in a Midwestern US forest. Occup Environ Med 2021;78:315–22. https://doi.org/10.1136/oemed-2020-106612.Search in Google Scholar PubMed PubMed Central
51. Adetona, AM, Martin, WK, Warren, SH, Hanley, NM, Adetona, O, Zhang, JJ, et al.. Urinary mutagenicity and other biomarkers of occupational smoke exposure of wildland firefighters and oxidative stress. Inhal Toxicol 2019;31:73–87. https://doi.org/10.1080/08958378.2019.1600079.Search in Google Scholar PubMed PubMed Central
52. Adetona, O, Zhang, JJ, Hall, DB, Wang, JS, Vena, JE, Naeher, LP. Occupational exposure to woodsmoke and oxidative stress in wildland firefighters. Sci Total Environ 2013;449:269–75. https://doi.org/10.1016/j.scitotenv.2013.01.075.Search in Google Scholar PubMed
53. Cherry, N, Beach, J, Galarneau, JM. Are inflammatory markers an indicator of exposure or effect in firefighters fighting a devastating wildfire? Follow-up of a cohort in Alberta, Canada. Ann Work Expo Health 2021;65:635–48. https://doi.org/10.1093/annweh/wxaa142.Search in Google Scholar PubMed PubMed Central
54. Gianniou, N, Giannakopoulou, C, Dima, E, Kardara, M, Katsaounou, P, Tsakatikas, A, et al.. Acute effects of smoke exposure on airway and systemic inflammation in forest firefighters. J Asthma Allergy 2018;11:81–8. https://doi.org/10.2147/jaa.s136417.Search in Google Scholar
55. Gaughan, DM, Cox-Ganser, JM, Enright, PL, Castellan, RM, Wagner, GR, Hobbs, GR, et al.. Acute upper and lower respiratory effects in wildland firefighters. J Occup Environ Med 2008;50:1019–28. https://doi.org/10.1097/jom.0b013e3181754161.Search in Google Scholar PubMed
56. Zarogoulidis, P, Katsikogianni, F, Tsiouda, T, Sakkas, A, Katsikogiannis, N, Zarogoulidis, K. Interleukin-8 and Interleukin-17 for cancer. Cancer Invest 2014;32:197–205. https://doi.org/10.3109/07357907.2014.898156.Search in Google Scholar PubMed
57. Mostafavi, N, Vlaanderen, J, Chadeau-Hyam, M, Beelen, R, Modig, L, Palli, D, et al.. Inflammatory markers in relation to long-term air pollution. Environ Int 2015;81:1–7. https://doi.org/10.1016/j.envint.2015.04.003.Search in Google Scholar PubMed
58. Fiorito, G, Vlaanderen, J, Polidoro, S, Gulliver, J, Galassi, C, Ranzi, A, et al.. Oxidative stress and inflammation mediate the effect of air pollution on cardio- and cerebrovascular disease: a prospective study in nonsmokers. Environ Mol Mutagen 2018;59:234–46. https://doi.org/10.1002/em.22153.Search in Google Scholar PubMed
59. Chen, B-Y, Chan, C-C, Lee, C-T, Cheng, T-J, Huang, W-C, Jhou, J-C, et al.. The association of ambient air pollution with airway inflammation in schoolchildren. Am J Epidemiol 2012;175:764–74. https://doi.org/10.1093/aje/kwr380.Search in Google Scholar PubMed
60. Perng, D-W, Chang, T-M, Wang, J-Y, Lee, C-C, Lu, S-H, Shyue, S-K, et al.. Inflammatory role of AMP-activated protein kinase signaling in an experimental model of toxic smoke inhalation injury. Crit Care Med 2013;41:120–32. https://doi.org/10.1097/ccm.0b013e318265f653.Search in Google Scholar
61. Gabay, C. Interleukin-6 and chronic inflammation. Arthritis Res Ther. 2006;8:S3, https://doi.org/10.1186/ar1917.Search in Google Scholar PubMed PubMed Central
62. Fahyim, SMM, AbdelHalim, HA, Hassan, ESSM. Association between serum interleukin- 6 concentrations, chronic obstructive pulmonary disease, and its relation to severity, acute exacerbation, and its effect on pulmonary function test. Egypt J Bronchol 2025;19:31. https://doi.org/10.1186/s43168-025-00388-0.Search in Google Scholar
63. van der Deen, M, Timens, W, Timmer-Bosscha, H, van der Strate, BW, Scheper, RJ, Postma, DS, et al.. Reduced inflammatory response in cigarette smoke exposed Mrp1/Mdr1a/1b deficient mice. Respir Res 2007;8:49. https://doi.org/10.1186/1465-9921-8-49.Search in Google Scholar PubMed PubMed Central
64. Piagnerelli, M, Biston, P, Zouaoui Boudjeltia, K, Anselin, S, Lelubre, C, Gerlach, A. Interpretation of C-Reactive protein concentrations in critically ill patients. BioMed Res Int. 2013;2013(2013):1-11, https://doi.org/10.1155/2013/124021.Search in Google Scholar PubMed PubMed Central
65. Kinnula, VL, Ilumets, H, Myllärniemi, M, Sovijärvi, A, Rytilä, P. 8-Isoprostane as a marker of oxidative stress in nonsymptomatic cigarette smokers and COPD. Eur Respir J 2007;29:51–5. https://doi.org/10.1183/09031936.00023606.Search in Google Scholar PubMed
66. Tugasworo, D, Prasetyo, A, Kurnianto, A, Retnaningsih, R, Andhitara, Y, Ardhini, R, et al.. Malondialdehyde (MDA) and 8-hydroxy-2′-deoxyguanosine (8-OHdG) in ischemic stroke: a systematic review. Egypt J Neurol, Psychiatry and Neurosurg 2023;59:87. https://doi.org/10.1186/s41983-023-00688-6.Search in Google Scholar
67. Sürmelioğlu, D, Gündoğar, H, Taysi, S, Bağiş, Y. Effect of different bleaching techniques on DNA damage biomarkers in serum, saliva, and GCF. Hum Exp Toxicol 2021;40:1332–41. https://doi.org/10.1177/0960327121996030.Search in Google Scholar PubMed
68. Balogh, B, Csákó, Z, Nyiri, Z, Szabados, M, Kakucs, R, Erdélyi, N, et al.. Levoglucosan and its isomers as markers and biomarkers of exposure to wood burning. Toxics 2025;13:742. https://doi.org/10.3390/toxics13090742.Search in Google Scholar PubMed PubMed Central
69. Naeher, LP, Barr, DB, Adetona, O, Simpson, CD. Urinary levoglucosan as a biomarker for woodsmoke exposure in wildland firefighters. Int J Occup Environ Health 2013;19:304–10. https://doi.org/10.1179/2049396713y.0000000037.Search in Google Scholar PubMed
70. Peel, AM, Crossman-Barnes, C-J, Tang, J, Fowler, SJ, Davies, GA, Wilson, AM, et al.. Biomarkers in adult asthma: a systematic review of 8-isoprostane in exhaled breath condensate. J Breath Res 2017;11:016011. https://doi.org/10.1088/1752-7163/aa5a8a.Search in Google Scholar PubMed
71. Murgia, N, Barregard, L, Sällsten, G, Almstrand, A-C, Montuschi, P, Ciabattoni, G, et al.. 8-isoprostane in exhaled breath condensate after experimental exposure to wood smoke in humans. J Biol Regul Homeost Agents 2016;30:263–70.Search in Google Scholar
72. Hu, W, Wang, Y, Wang, T, Ji, Q, Jia, Q, Meng, T, et al.. Ambient particulate matter compositions and increased oxidative stress: exposure-response analysis among high-level exposed population. Environ Int 2021;147:106341. https://doi.org/10.1016/j.envint.2020.106341.Search in Google Scholar PubMed
73. Toto, A, Wild, P, Graille, M, Turcu, V, Crézé, C, Hemmendinger, M, et al.. Urinary malondialdehyde (MDA) concentrations in the general population—a systematic literature review and meta-analysis. Toxics 2022;10:160. https://doi.org/10.3390/toxics10040160. [Internet].Search in Google Scholar PubMed PubMed Central
74. He, L, Cui, X, Li, Z, Teng, Y, Barkjohn, KK, Norris, C, et al.. Malondialdehyde in nasal fluid: a biomarker for monitoring asthma control in relation to air pollution exposure. Environ Sci Technol 2020;54:11405–13. https://doi.org/10.1021/acs.est.0c02558.Search in Google Scholar PubMed
75. Cui, X, Gong, J, Han, H, He, L, Teng, Y, Tetley, T, et al.. Relationship between free and total malondialdehyde, a well-established marker of oxidative stress, in various types of human biospecimens. J Thorac Dis 2018;10:3088–97. https://doi.org/10.21037/jtd.2018.05.92.Search in Google Scholar PubMed PubMed Central
76. Romieu, I, Castro-Giner, F, Kunzli, N, Sunyer, J. Air pollution, oxidative stress and dietary supplementation: a review. Eur Respir J 2008;31:179–97. https://doi.org/10.1183/09031936.00128106.Search in Google Scholar PubMed
77. Brucker, N, Moro, AM, Charão, MF, Durgante, J, Freitas, F, Baierle, M, et al.. Biomarkers of occupational exposure to air pollution, inflammation and oxidative damage in taxi drivers. Sci Total Environ 2013;463-464:884–93. https://doi.org/10.1016/j.scitotenv.2013.06.098.Search in Google Scholar PubMed
78. Laumbach, RJ, Kipen, HM. Acute effects of motor vehicle traffic-related air pollution exposures on measures of oxidative stress in human airways. Ann N Y Acad Sci 2010;1203:107–12. https://doi.org/10.1111/j.1749-6632.2010.05604.x.Search in Google Scholar PubMed PubMed Central
79. Park, N-Y, Song, G, Lee, K, Kho, Y. Levels of OH-PAHs and markers of oxidative stress in urine of taxi drivers and controls. Environ Anal Health Toxicol 2024;39:e2024027-0. https://doi.org/10.5620/eaht.2024027.Search in Google Scholar PubMed PubMed Central
80. Park, S, Kwon, E, Lee, G, You, Y-A, Kim, SM, Hur, YM, et al.. Correction: park et al. Effect of Particulate Matter 2.5 on Fetal Growth in Male and Preterm Infants through Oxidative Stress. Antioxidants 2023, 12 , 1916. Antioxidants 2024;13:135. https://doi.org/10.3390/antiox13020135.Search in Google Scholar PubMed PubMed Central
81. Poljsak, B, Šuput, D, Milisav, I. Achieving the balance between ROS and antioxidants: when to use the synthetic antioxidants. Oxid Med Cell Longev 2013;2013:956792. https://doi.org/10.1155/2013/956792.Search in Google Scholar PubMed PubMed Central
82. Kurutas, EB. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: current state. Nutr J 2016;15:71. https://doi.org/10.1186/s12937-016-0186-5.Search in Google Scholar PubMed PubMed Central
83. Korczowska-Łącka, I, Słowikowski, B, Piekut, T, Hurła, M, Banaszek, N, Szymanowicz, O, et al.. Disorders of endogenous and exogenous antioxidants in neurological diseases. Antioxidants 2023;12:1811. https://doi.org/10.3390/antiox12101811.Search in Google Scholar PubMed PubMed Central
84. Radak, Z, Chung, HY, Goto, S. Systemic adaptation to oxidative challenge induced by regular exercise. Free Radic Biol Med 2008;44:153–9. https://doi.org/10.1016/j.freeradbiomed.2007.01.029.Search in Google Scholar PubMed
85. Valko, M, Leibfritz, D, Moncol, J, Cronin, MTD, Mazur, M, Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int J Biochem Cell Biol 2007;39:44–84. https://doi.org/10.1016/j.biocel.2006.07.001.Search in Google Scholar PubMed
86. Liang, Y, Hu, L, Li, J, Liu, F, Jones, KC, Li, D, et al.. Short-term personal PM2.5 exposure and change in DNA methylation of imprinted genes: panel study of healthy young adults in Guangzhou city, China. Environ Pollut 2021;275:116601. https://doi.org/10.1016/j.envpol.2021.116601.Search in Google Scholar PubMed
87. Jung, KH, Torrone, D, Lovinsky-Desir, S, Perzanowski, M, Bautista, J, Jezioro, JR, et al.. Short-term exposure to PM(2.5) and vanadium and changes in asthma gene DNA methylation and lung function decrements among urban children. Respir Res 2017;18:63. https://doi.org/10.1186/s12931-017-0550-9.Search in Google Scholar PubMed PubMed Central
88. Esteves, F, Madureira, J, Costa, C, Pires, J, Barros, B, Alves, S, et al.. Occupational exposure to wildland firefighting and its effects on systemic DNA damage. Int J Hyg Environ Health 2025;266:114576. https://doi.org/10.1016/j.ijheh.2025.114576.Search in Google Scholar PubMed
89. Humans IWGotIoCHt. Occupational exposure as a firefighter IARC monographs on the identification of carcinogenic hazards to humans. Lyon (FR): International Agency for Research on Cancer; 2023. For more information contact publications@iarc.fr.Search in Google Scholar
90. Park, E, Lee, YJ, Lee, SW, Bang, CH, Lee, G, Lee, JK, et al.. Changes of oxidative/antioxidative parameters and DNA damage in firefighters wearing personal protective equipment during treadmill walking training. J Phys Ther Sci 2016;28:3173–7. https://doi.org/10.1589/jpts.28.3173.Search in Google Scholar PubMed PubMed Central
91. Nunes, KV, Ignotti, E, Hacon Sde, S. Circulatory disease mortality rates in the elderly and exposure to PM(2.5) generated by biomass burning in the Brazilian Amazon in 2005. Cad Saude Publica 2013;29:589–98. https://doi.org/10.1590/s0102-311x2013000300016.Search in Google Scholar PubMed
92. Abdo, M, Ward, I, O’Dell, K, Ford, B, Pierce, JR, Fischer, EV, et al.. Impact of wildfire smoke on adverse pregnancy outcomes in Colorado, 2007-2015. Int J Environ Res Public Health 2019;16:3720. https://doi.org/10.3390/ijerph16193720.Search in Google Scholar PubMed PubMed Central
93. Sadiq, IZ, Usman, A, Muhammad, A, Ahmad, KH. Sample size calculation in biomedical, clinical and biological sciences research. J Umm Al-Qura Univ Appl Sci 2024;11:133–41. https://doi.org/10.1007/s43994-024-00153-x.Search in Google Scholar
Supplementary Material
This article contains supplementary material (https://doi.org/10.1515/reveh-2025-0109).
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.

