Abstract
Communities living in proximity to coal-fired power plants (CFPPs) may be at greater risk of negative health impacts from exposure to air pollution than communities living further away. The aim of this scoping review was to provide an update on the evidence of the health risks of air pollution exposure associated with living in proximity to CFPPs and to evaluate the relationship between residential proximity and the extent of the health burden. We followed the PRISMA-ScR guidelines and searched Google Scholar, PubMed, ScienceDirect, Scopus and Web of Science for relevant studies from inception up to 31 January 2024. Fifty-six studies were included with most articles published from 2016 to 2023 (n=33, 59 %) and 35 were in high income countries (63 %). Living close to CFPPs was frequently associated with increased odds or likelihood of respiratory disorders, adverse birth outcomes and child developmental issues. Interventions such as emission control systems or total shutdown of CFPPs led to improved health among communities living near CFPPs. The review highlights the health impacts from air pollution associated with living in proximity to CFPPs and the need for policy measures to reduce air pollution by installing emission control technologies or transitioning to cleaner energy sources.
Introduction
Coal is a cheap and large fuel source for power generation worldwide, accounting for 35 % of the global electricity mix in 2023 Energy Institute [1]. Over the years, the use of coal has negatively impacted the environment and human health. Coal-fired power plants (CFPPs) burn coal to produce electricity and are significant sources of air pollution. Coal combustion releases air pollutants: carbon dioxide (CO2), sulphur dioxide (SO2), nitrogen oxides (NOx), particulate matter (PM), potentially toxic elements (arsenic [As], mercury [Hg], and lead [Pb]), organic hydrocarbons polycyclic aromatic hydrocarbons (PAHs), volatile organic compounds and fly ash as a residue into the atmosphere [2].
According to the International Energy Agency (IEA), China, United State of America (USA) and India are among the countries with the highest coal consumption in 2023 [3]. On the other hand, countries with the highest percentage of electricity produced from coal include Botswana, Kosovo, Mongolia and South Africa have (96 , 88, 84 and 84 %, respectively) [1]. Reports show that there has been an increase in operating coal capacity (45.5 GW in 2022 and 48.6 GW in 2023) with new coal plants coming online in 2023 [4], 5]. Additionally, the number of coal-fired units that are scheduled to retire has lowered in 2023 compared to 2022.
Both long-term and short-term exposure to air pollution affects lung and heart functioning. Several studies link exposure to particulate matter with a diameter of 2.5 µm or less (PM2.5) and respiratory diseases [6], [7], [8], [9]. Exposure to PM2.5, particularly in children, has been associated with increases in the risks of poor lung, neurological and brain development [10], 11]. Studies have also shown that pregnant women who live in areas with high levels of air pollution are at greater risk of adverse birth outcomes compared to those living in areas with low levels [12], [13], [14], [15]. Air pollution can trigger asthma attacks, cause shortness of breath, coughing, suffocation and headaches in individuals living in areas near CFPPs. Increases in PM2.5 concentrations have been associated with increases in lung cancer mortality rates [16]. Air pollution from CFPPs has been linked to nearly 500,000 premature deaths of individuals 65 years and older in the USA [17].
In addition to air pollution that emanates from CFPPs, coal ash also poses a major health risk. Coal ash is often disposed of in storage ponds or landfills near CFPPs. Also known as coal combustion residuals, coal ash is the byproduct of burning coal to generate electricity. Many coal ash landfills are neither capped nor lined, which allows fugitive dust to be blown into the air and leachate to contaminate surface, and groundwater. Even after a CFPP is closed, the coal ash landfills may remain for a long time, posing a persistent environmental and health risk to nearby communities as emphasized recently by Zhang and Zierold [18].
Communities that live near pollution sources are at higher risk of exposure to air pollution leading to adverse health effects compared to communities living far from pollution sources. Communities far from pollution sources can also be vulnerable to air pollution as it can travel to neighbouring states or provinces located downwind [19]. Prevailing wind patterns contribute to the long-range transportation of air pollutants. Considering this, several modelling studies have projected health and cost benefits of reducing emissions associated with CFPPs [20], 21]. As per the Paris Agreement, all regions should have phased out coal which includes electricity production by between 2030 and 2040 [22]. Few studies investigated the extent of health impacts of exposure to air pollution in individuals living near CFPPs. Only Amster [23] has evaluated literature on the impacts of emissions from CFPPs on mortality and morbidity. Therefore, this review aims to provide an update of the evidence of the health impacts associated with air pollution exposure among communities living in proximity to CFPPs. We also evaluated the relationship between the distance from the CFPPs and the risk of negative health outcomes. We did not isolate emissions from CFPPs’ stacks vs. coal ash hence our review considers all air pollution around a CFPP.
Methods and materials
This scoping review followed the guidelines by the Joanna Briggs Institute [24]. The reporting of the scoping review was guided by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Extension for Scoping Reviews (PRISMA-ScR) guidelines and the PRISMA-ScR checklist (Table S1) [25]. We developed and registered the review protocol with the Open Science Framework [26]. More details are presented in the published review protocol [27].
Information sources
In summary, we searched five databases, i.e., Google Scholar, PubMed, ScienceDirect, Scopus and Web of Science, for relevant studies. We also searched grey literature for reports from federal agencies, commercial and non-profit organizations. The search was conducted on 5 March 2024. Additional records were identified by screening the reference lists of included articles.
Search strategy
The medical subject headings (MeSH) were used to develop the search strategy. The search terms used were (“air pollutants” OR “air pollution”) AND (“coal-fired power plant*” OR “coal-fired power station*”) AND health. The search was restricted to English language published up to and including 31 January 2024. The full search strategy is shown in the supplementary material (Table S2).
Eligibility criteria
The Population, Exposure, Context, Outcome and Study design (PECOS) framework was followed to identify relevant articles. The inclusion criteria consisted of articles that focused on humans of all ages, gender and geographical regions including infants and pregnant women exposed to air pollution from CFPPs. The proximity of the population to CFPPs was considered. Articles that measured at least one health outcome were included. Studies focussing on health risk assessment, exposure risk assessment, health impact assessment and health modelling only were excluded. Studies focussing on in vivo, animal, and environmental samples only were excluded. Occupational exposure was not considered. Reviews, conference abstracts, editorials, and dissertations were excluded.
Screening
The retrieved articles were uploaded on Endnote reference management software and duplicates were removed. The articles were then exported to Rayyan online tool for the screening process [28]. The remaining duplicates were removed. Four reviewers (CW, CYW, NM and TK) independently screened the title and abstracts of articles according to the inclusion and exclusion criteria. This was followed by the full-text screening of the relevant articles. Discrepancies were resolved through discussion until consensus was reached.
Data extraction
A data extraction tool was developed and piloted against 10 studies. Data was independently extracted from eligible studies by five reviewers (CH-D, CW, CYW, NM and TK). The data extracted from the studies included the following: author(s), year of publication, study design, distance from CFPP, type of pollutant, health outcomes, and key findings.
Quality assessment
The methodological quality of the included studies was assessed by one reviewer using the Critical Appraisal Skills Programme (CASP) checklists for cohort, case control and qualitative studies (Table S3A and S3B) [29], 30]. We used the National Heart, Lung and Blood Institute (NHLBI) Quality Assessment Tool for Observational Cohort and Cross-sectional studies checklist for the cross-sectional studies and Before-After (Pre-Post) studies checklist for controlled intervention studies (Table S3B and S3C) [31]. We used the Authority, Accuracy, Coverage, Objectivity, Date, Significance (AACODS) checklist for grey literature (Table S3D) [32]. The CASP tool consists of questions that examine the study validity, results, and relevance. There were three options to each question: yes, no, cannot tell. For the NHLBI tool, the options were yes, no, cannot determine or not applicable (NA). We calculated the percentage of “yes” for each article. Questions that were answered with “can’t tell” or “cannot determine” or “NA” were excluded from the calculation. No studies were excluded based on the CASP, NHLBI and AACODS results.
Impact of CFPPs closure
While we focused on finding studies that presented data on the health impacts associated with living near CFPPs (i.e., our search terms did not include the impact of CFPPs closures on health) we also noted that some of these studies included mention of CFPPs closure, hence we note those findings here too.
Data synthesis and analysis
Descriptive tables were created to summarize the characteristics of the studies and synthesized the studies by the main themes from extracted data. The tables consisted of (a) sample characteristics, (b) distance from the CFPP, (c) pollutant studied, (d) health outcomes and (e) key findings.
Results
Descriptive findings
The initial literature search identified 3 449 records from the five databases, and 30 records from the grey literature with 10 additional records found through the reference lists (Figure 1). For the database search, duplicates were removed, and 2,435 records were screened by title and abstract, and 90 studies were selected for a full-text review. For the grey literature search, 40 records (30 reports and 10 additional studies) were retrieved and assessed for eligibility. After the full-text assessment of the 90 studies and 40 records, 56 studies (including two reports) met the inclusion criteria and were included in the review. Multiple studies (presented in more than one study) of the same cohort were only considered if the results of the health outcomes were different. The same cohorts of pupils [33], 34], non-smoking mothers and newborns [35], [36], [37], [38], [39], [40], [41], [42], and 6–14-year-old children [43], [44], [45] with different health outcomes were included.
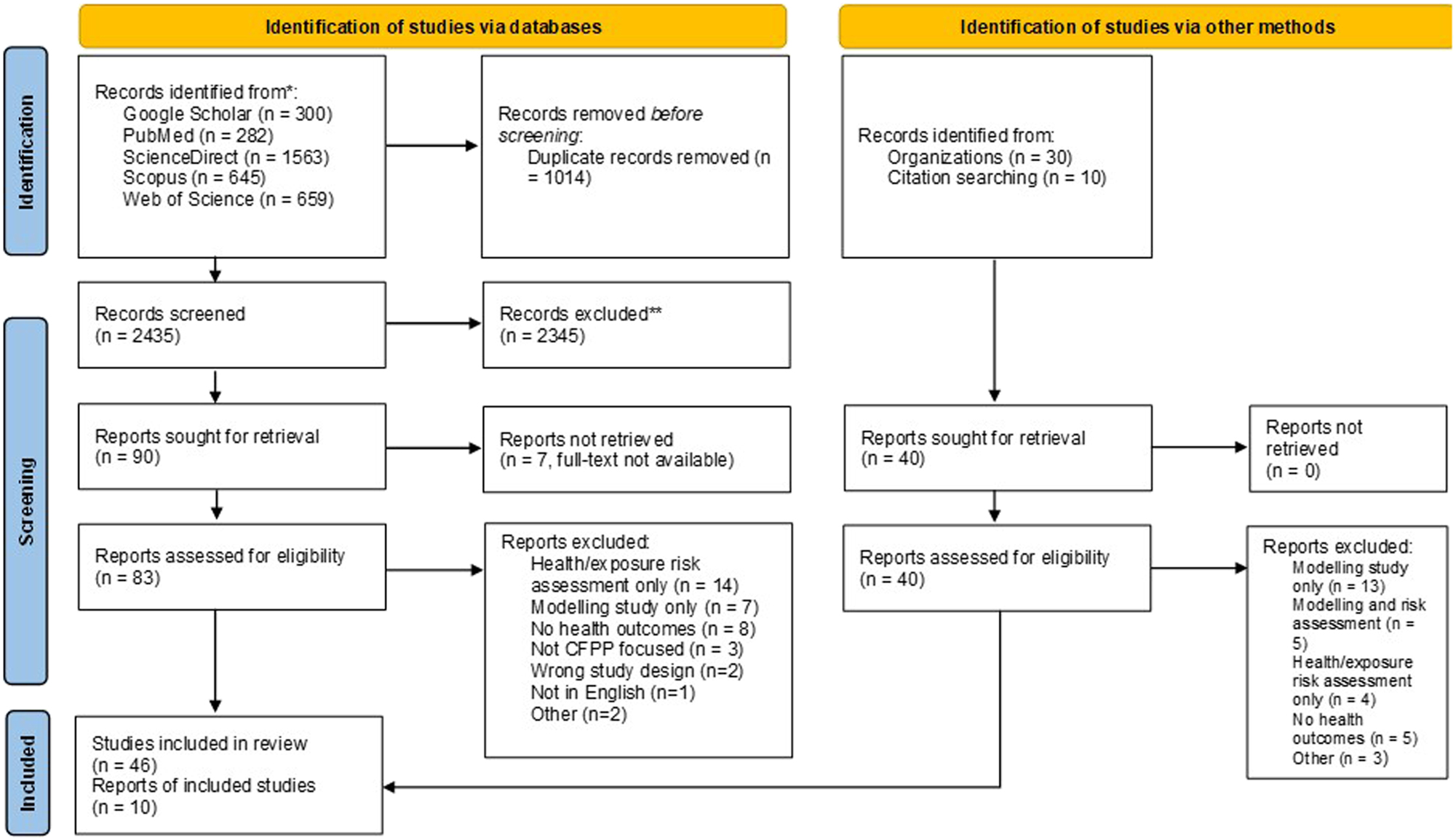
PRISMA flow diagram of the study selection.
The four countries with the highest number of studies were USA (n=21), China (n=10), Israel (n=6) and India (n=5) (Figure 2). There were no studies observed for Africa and South America. Table 1 shows the descriptive characteristics of the included studies. A detailed description of the characteristics of the included studies is shown in Table 2. About 59 % of the studies were published between 2016 and 2023. Cross-sectional (55 %) and cohort (33 %) were the most common study designs followed by intervention (4 %) and qualitative studies (4 %). Commonly investigated air pollutants included SO2, PM2.5, PAHs and NOx. Six studies compared the health outcomes in exposed and control groups [51], 65], 67], 70], 75], 86]. Twenty-three studies included children (3–14 years) [33], 34], [43], [44], [45], [46, [51], [52], [53, 55], 58], 59], 61], 67], [71], [72], [73, [77], [78], [79, 81], 84], 85], nine studies included mothers and infants or neonates [35], [36], [37], [38], [39], [40], [41], [42, 60] and four studies considered births as the study population [50], 64], 82], 83].
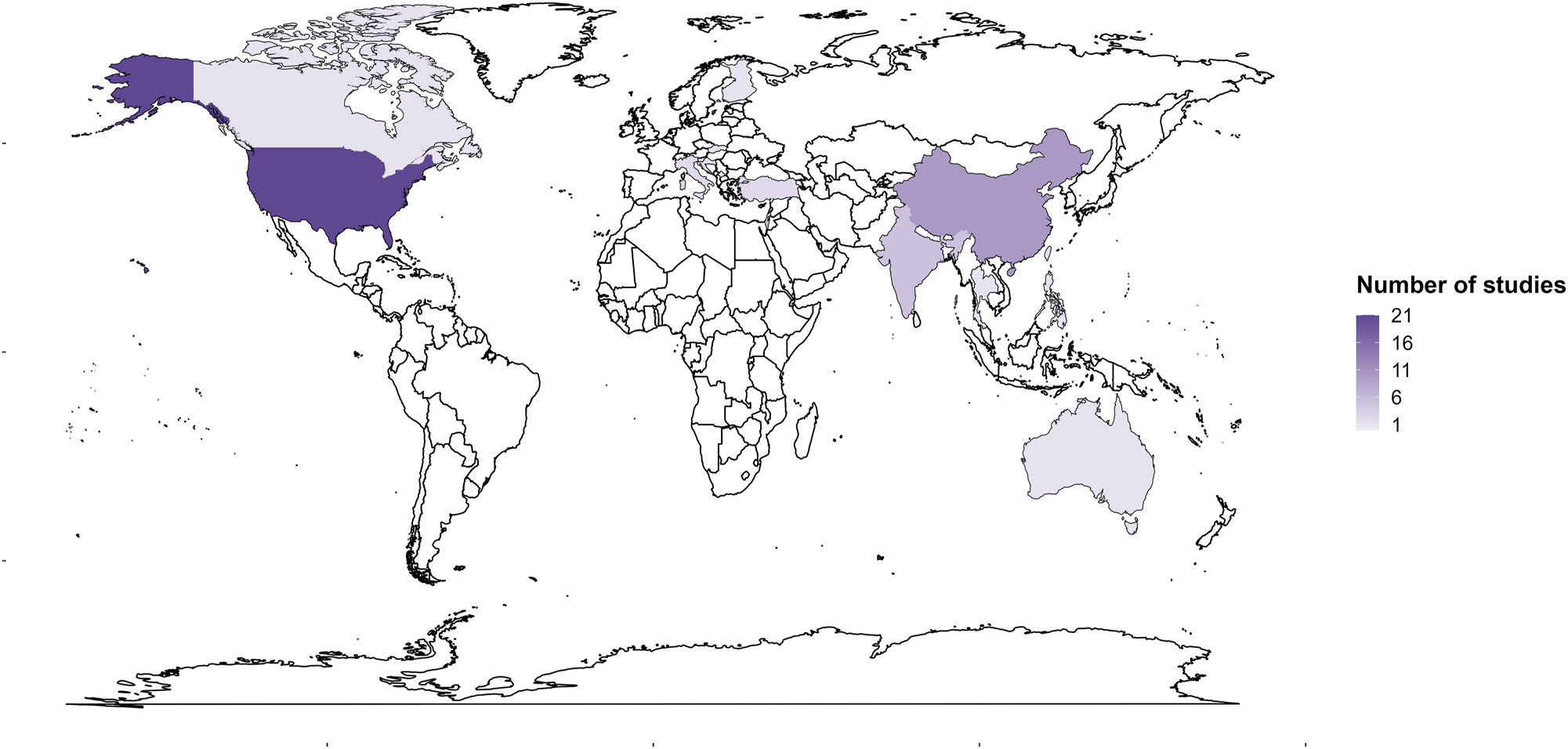
Map showing the distribution of included studies.
Descriptive characteristics of included articles (n=56).
| Items | Number of studies n, % |
|---|---|
| Income group/level | |
| High income | 35 (63) |
| Upper middle income | 14 (25) |
| Lower middle income | 6 (11) |
| Other (multi-country) | 1 (1) |
| Publication year | |
| ≤2000 | 6 (11) |
| 2001–2005 | 3 (5) |
| 2006–2010 | 5 (9) |
| 2011–2015 | 9 (16) |
| 2016–2020 | 18 (32) |
| 2021–2023 | 15 (27) |
| Study design | |
| Cross-sectional | 31 (55) |
| Cohort | 19 (33) |
| Intervention | 2 (4) |
| Qualitative | 2 (4) |
| Other | 2 (4) |
| Pollutant(s) (some articles included more than one pollutant) | |
| PM2.5 | 13 |
| SO2 | 22 |
| PAHs | 9 |
| NOx | 8 |
| Hg | 6 |
| PM10 | 6 |
| NO2 | 4 |
| O3 | 3 |
| Pb | 3 |
| As | 2 |
| CO | 2 |
| Fly ash | 2 |
| SO2/PM2.5 | 2 |
| PM1 | 1 |
| Other | 4 |
| NR | 7 |
| Health outcomes (some studies included more than one pollutant) | |
| Respiratory disorders | 31 |
| Adverse birth outcomes | 11 |
| Neurodevelopment disorders | 9 |
| Cause-specific and all-cause mortality | 8 |
| Foetal and child development | 4 |
| Heart conditions | 4 |
| Cancer | 3 |
| Internalizing disorders | 2 |
| Other | 7 |
Detailed characteristics of the included studies.
| Study ID | Country | Income level | Study design | Sample characteristics | Distance from CFPP | Pollutant(s) studied | Health outcomes | Key findings |
|---|---|---|---|---|---|---|---|---|
| Aekplakorn et al. [46] | Thailand | UMI | Cross-sectional | n=175 children (6–14 years) (n=83 asthmatic, n=92 non-asthmatics) | 7–8 km | SO2 & PM10 | Pulmonary function | A weak association was observed between reduced pulmonary function and changes in the ambient concentration of SO2. An association was observed between increased daily PM10 levels and lower pulmonary function in asthmatic children. |
| Amster et al. [47] | Israel | HI | Cross-sectional | n=2,244 adults (18–75 years) | 20–30 km | NOx & SO2 | Respiratory symptoms | Data did not support an association between CFPPs emissions and prevalence of chronic obstructive pulmonary disease or asthma. Nearly all the respiratory symptoms studied were however associated with NOx power plant emissions. |
| Barbhaya et al. [48] | India | LMI | Cross-sectional | n=NR, individuals in 597 districts (15–69 years) | NR | PM2.5 | All-cause mortality | 47,000 premature adult deaths in India were attributable to CFPPs in 2014 and were concentrated in the 10 % of Indian districts that housed these CFPPs. |
| Barik et al. [49] | India | LMI | Cross-sectional | NR | ≤20 km | PM1, PM2.5, PM10 | URTI | Increased average annual prevalence of URTI was observed in the central Indian population residing near coal-fired TPPs. |
| Barrows et al. [50] | India | LMI | Cross-sectional | n=NR, live births and infant deaths | 0–50 km | PM2.5, NO2, SO2 | Infant mortality | 1 GW increase in coal-fired capacity increased infant mortality rates by roughly 15 % relative to other districts in the same state. Impact of infant mortality rate was reported to be larger for older plants, located in districts with higher baseline pollution levels and burning domestic rather than imported coal. Urban areas within districts were more exposed to coal-powered capacity than rural areas as CFPPs were located in urban areas |
| Bencko et al. [51] | Slovakia | HI | Cross-sectional | n=107 children (9.5–11 years) (n=56 exposed, n=51 control) | NR | As | Hearing loss | Higher rate of enlarged and large tonsils and adenoids and concomitant phlegm-pus flow in the posterior nasopharynx was found in the exposed group compared to the control group. Higher incidence of repeated rhinitis was observed in the exposed group. |
| Blanchard et al. [52] | USA | HI | Cross-sectional | n=NR, children (3–5 years) | NR | Hg | Autism | Higher levels of ambient mercury were geographically associated with point sources of mercury emission, such as CFPPs and cement plants with coal-fired kilns. School districts in closest proximity to these areas had the highest autism rates in the county. |
| Casey et al. [53] | USA | HI | Cohort | n=57,005 births | ≤20 km | PM2.5 | Preterm birth | The prevalence of preterm birth decreased near CFPPs after retirement, with larger reductions closer to CFPPs. |
| Casey et al. [54] | USA | HI | Intervention | n=207 asthmatic individuals (average age=45 years) | NR | SO2 | ER asthma visits and hospitalization | The reductions in CFPP air pollution exposure after retirements and SO2 control installations translated to fewer asthma-related ERVs and hospitalizations, as well as fewer average daily short-acting beta-agonists (SABA) uses. |
| Chen et al. [55] | Taiwan | HI | Cross-sectional | n=252 participants (children, 9–15 years; elderly, >55 years) (n=111 high exposure group, n=141 low exposure group) | 7–13 km (high exposure), 16–27 km (low exposure) | PAHs & heavy metals | Urinary metabolomic biomarkers linked to oxidative stress | Ambient concentration of vanadium and PAHs were significantly higher in high exposure groups that lived closer to the CFPP when compared to the low exposure group, for both children and elderly participants. |
| Chen et al. [56] | China | UMI | Cross-sectional | n=NR, individuals in 161 counties | ≤50 km | SO2 | Respiratory and cardiovascular diseases mortality | The results reveal that air pollution from neighbouring power plants has significant negative effects on local public health, and the resulting treatment costs are enormous. |
| Chen et al. [57] | China | UMI | Time-series | n=NR | NR | SO2 | Respiratory disease and lung cancer mortality | SO2 emissions have significantly negative effects on public health. SO2 emissions resulted in 230,000 extra deaths every year and the related economic costs over the study period amount to RMB 8.179 billion |
| Collarile et al. [58] | Italy | HI | Descriptive cross-sectional | n=1,726 lung and bladder cancer cases (all ages) | NR | PM10, C6H6, NO2, SO2 | Lung and bladder cancer | An excess risk of lung and bladder cancer was associated with high residential exposure to benzene and NO2 in women aged ≥75 years old. |
| Daouda et al. [59] | USA | HI | Cross-sectional | n=NR, neonates born to non-Hispanic Black and non-Hispanic White in 289 counties | NR | SO2 converted to PM2.5 | Preterm birth | A positive non-linear relationship between coal PM2.5 and PTB rate was observed and plateaued at higher levels of pollution. Differential associations by maternal race were observed; the association was stronger for White women, especially at higher levels of coal PM2.5 (>2.0 μg m−3). |
| Datt et al. [60] | India | LMI | Cross-sectional | n=176,583 women (18–49 years) and children (6–60 months) (n=39,356 children, n=137,227 women) | NR | PM2.5 | Anaemia | The increase of coal units closer to residential areas had a stronger effect on the likelihood that the child was anaemic. A negative correlation was observed between distance from the coal primary sampling unit and the magnitude of the harmful impact of coal on women’s health. |
| Dubnov et al. [61] | Israel | HI | Cohort | n=1,492 children (7–14 years) | ≤10 km | NOx & SO2 | Pulmonary function | A negative association was found between changes in the pulmonary function results and the estimated individual levels of air pollution. The estimated adverse effect of NOx & SO2 on children’s pulmonary function growth was found to range from 4 % for the mean level of air pollution to 10 % for the maximum concentration of these pollutants. |
| Fan and Wang [62] | USA | HI | Cross-sectional | n=NR, U.S residents (≥65 years) | ≤50 km | PM2.5 | Mortality | Instrumental variable and difference-in-differences approaches found that power plant retirements lead to reductions in PM2.5 levels and consequently decreased monthly mortality among older adults. The mortality effects were higher among males than females and its impact was the greatest among people >75 years. |
| Goren and Hellmann [33] | Israel | HI | Cohort | n=NR, 2nd, 5th and 8th grade pupils (8, 11 and 14 years) (1980: n=737 pupils, 1983: n=698 pupils, 1986: n=993 pupils, 1989: n=963 pupils) | ≤19 km | SO2, NOx, O3, CO, total hydrocarbons | Asthma and related respiratory conditions | The rise in prevalence of asthma was not related to any residential area. Air pollution levels were very low and previous health studies in the area around the CFPP did not find any negative health effects. |
| Goren et al. [63] | Israel | HI | Cohort | n=30,000 patients | ≤10 km | SO2, NOx, O3, CO, total hydrocarbons | Respiratory diseases | The air pollution levels in both 1980 (before CFPP operation) and 1983 (when CFPP was in operation) were highest in the community located in the city (closer to CFPP). Air pollution levels measured around the CFPP were low and did not seem to cause adverse health effects. |
| Goren et al. [34] | Israel | HI | Cohort | n=NR, 2nd and 5th grade pupils (1980: 2nd graders n=991 and 5th graders n=999, 1983: 2nd graders n=785 and 5th graders n=693) | ≤19 km | NR | Pulmonary function | No negative health effects among children residing in areas expected to be affected by the operation of CFPP as compared to the expected low-pollution areas. The respiratory symptoms among children were linked to age, epidemics and background variables rather than air pollution levels. |
| Ha et al. [64] | USA | HI | Cohort | n=423,719 births | <5 to ≥20 km | PM2.5 | Preterm delivery, very preterm delivery, and low birth weight | Women who lived closer to coal and solid waste power plants were exposed to higher levels of PM2.5. When exposure was changed to the number of plants within 20 km, CFPPs had the highest association with all adverse birth outcomes. About 1.8 % increased odds for PTD, 2.2 % for VPTD, and 1.1 % for term LBW was observed for each 5 km closer to any power plant. |
| Hagemeyer et al. [65] | USA | HI | Qualitative | n=401 adults (≥18 years) (n=231 exposed population, n=170 non-exposed population) | <1 km (exposed), ∼97 km (non-exposed) | NR | Respiratory symptoms | Adults residing near a CFPP with a coal ash facility (exposed) were more likely to report respiratory symptoms than the non-exposed population. Participants living near coal ash storage who spent more time outside were more likely to report having a respiratory infection. |
| Henneman et al. [66] | USA | HI | Cross-sectional | n=NR | NR | SO2 converted to PM2.5 | Cardiac, respiratory health and all-cause mortality | Rates reductions in six cardiac and respiratory health outcomes decreased with decreases in PM2.5 and coal exposure. A secondary analysis found that nonlinearities in relationships between changing health outcome rates and coal exposure may explain differences in their associations. |
| Henry et al. [67] | Australia | HI | Cohort | n=99 primary schoolchildren with history of wheezing (n=49 in exposed area Lake Munmorah (LM), n=50 in control area Nelson Bay (NB)) | ≤5 km (exposed area), ∼80 km (control area) | NOx & SO2 | Asthma | The asthma symptoms were similar in exposed and control areas and the frequency of asthma was low on both areas. SO2 and NOx concentrations were within recommended guidelines, air quality had no effect on asthmatic symptoms |
| Hii et al. [68] | USA | HI | Cross-sectional | n=2,327 adults (21–74 years old) | ≤35 km (within), >35 km (away) | NR | Pulmonary function | Adults living near 1 of 11 CFPPs may have worse pulmonary function. The odds ratio of FEV1/FVC values below 80 % for those living within 35 km of a CFPP was 1.24 (95 % CI, 0.90–1.70) when compared to those living greater than 35 km from a plant. There was a statistically significant higher percentages of Black and Hispanic survey respondents living near CFPPs. |
| Kamath et al. [69] | India | LMI | Cross-sectional | n=3,533 participants from 5 villages | >5 km to<10 km | PM2.5, Hg, Pb, other heavy metals | Respiratory function | There was significant association between abnormalities in pulmonary function tests and those living in the vicinity of the CFPP (p<0.05). Those living near the CFPP had higher abnormal lung function test compared to those residing far. |
| Karavuş et al. [70] | Turkey | UMI | Cross-sectional | n=502 individuals (≥15 years) (n=277 people in exposed villages, n=225 people in control villages) | ≤5 km (exposed villages), >30 km (control villages) | NR | Respiratory complaints and function | Individuals over 35 years in villages around CFPP had more frequent complaints of chest tightness than those in control villages (p=0.0001). The FEV1, FVC, and FEF25–75 % of the nonsmokers living in the villages around power plant were statistically significantly reduced compared to nonsmokers living in the control villages. |
| Komisarow and Pakhtigian [71] | USA | HI | Cross-sectional | n=NR, children (0–4 years) | ≤10 km | PM2.5 | Asthma-related conditions | After closure of CFPPs in 2012, ZIP codes in close proximity to the three CFPPs experienced reductions in emergency department visits for asthma-related conditions among children. An increasingly negative pattern of estimated effects for the years 2012 and later was observed, which suggests that the effects of CFPP closures increase over time. |
| Komisarow and Pakhtigian [72] | USA | HI | Cross-sectional | n=NR, students in public elementary schools | ≤10 km (treatment group), >10 km (control group) | NO2, SO2, PM2.5 | Asthma-related conditions | After closure of CFPPs, school-level absence rates decreased by around 6 % in schools located near the CFPPs. School absence reductions were larger for boys than for girls. Evidence suggests that children’s respiratory health improved following the closure of the 3 CFPPs. A decline in rates of emergency department visits for asthma-related conditions was observed among school-age children in ZIP codes near the CFPPs compared to ZIP codes farther away. |
| Lee et al. [35] | China | UMI | Cohort | N=NR, mothers (≥20 years) and newborns (2002 cohort: n=110 women and newborns, 2005 cohort: n=107 women and newborns) | ≤2.5 km | Hg, Pb, PAHs | Neurodevelopment outcomes | An inverse association between prenatal exposure to PAH, measured by PAH-DNA adducts in cord blood, and LINE1 methylation status in both cohorts combined; and a direct correlation between LINE1 methylation status and child IQ scores at 5 years of age in the 2002 cohort was observed. |
| Minichilli et al. [73] | Italy | HI | Population-based cohort | n=144,019, all ages | NR | NOx & SO2 | Natural and all-cause mortality | Exposure to SO2 from CFPP and ISDI were significantly correlated (p<0.001). An association was also found in both males and females between increasing exposure to SO2 and diseases of the nervous system, and sense organ circulatory and respiratory system. The hospitalization data supported the mortality results. |
| Mohorovic [74] | Croatia | HI | Cohort | n=704, pregnant women | 3.5–12 km | SO2 | Preterm delivery and low birth weight | A greater and longer exposure to SO2 emissions during the initial 2 months of pregnancy resulted in a significantly shorter gestation at the end of the 1st and 2nd month of pregnancy (p=0.008, p=0.016, respectively) and in lower birthweight of newborns (p=0.016, p=0.026, respectively). |
| Pala et al. [75] | Turkey | UMI | Cross-sectional | n=2,819 individuals (≥15 years) (n=2,350 study group, n=469 control group) | 1.5–12 km (study group), ∼22 km (control group) | NR | Respiratory function | FEV1 and FVC averages for the study group villages were significantly lower than those for the control group and residents directly wind of the power plant’s smokestack showed greater impairment of respiratory functions compared with residents upwind |
| Perera et al. [36] | China | UMI | Cohort | n=100 nonsmoking women (≥20 years) and newborns | ≤2.5 km | PAHs | Child IQ | CFPP was the major source of environmental PAHs. After adjusting for potential confounders, neither PAH-DNA adducts nor exposure to ETS had significant main effects on IQ. |
| Perera et al. [37] | China | UMI | Cohort | n=NR, nonsmoking women (≥20 years) and their newborns (2002 before CFPP shutdown: n=110, 2005 after CFPP shutdown: n=107) | ≤2.0 km | PAHs | Child neurodevelopment | PAH–DNA adducts in cord blood were significantly associated with DQ decrements in the motor area and in the average DQ among children who were in utero during the operation of the CFPP (2002 cohort), however these significant associations were not seen among children who were in utero after the CFPP had been shut down (2005 cohort). In the 2002 cohort, PHA-DNA adducts were associated with an ∼2-fold increased odds of developmental delay in the motor area. |
| Perera et al. [38] | China | UMI | Cohort | n=255, nonsmoking women (≥20 years) and their newborns (2002 before CFPP shutdown: n=122, 2005 after CFPP shutdown: n=133) | ≤2.5 km | PAHs | Neurodevelopment outcomes | Mean telomere length (TL) was significantly higher in the 2005 cohort compared to the 2002 cohort. PAH-DNA adducts were significantly and inversely correlated with TL (p=0.018). A significant association between adducts and TL after adjusting for key covariates and cord Hg was observed (p=0.001). Longer telomeres in the 2005 cohort and the observed association between increased TL and higher levels of BDNF indicated benefits for health and development of children due to CFPP closure. |
| Pershagen et al. [76] | Finland | HI | Cross-sectional | n=8,762 individuals (15–64 years) | NR | SO2 & dust | Respiratory symptoms | Plant A had the largest dust emissions. In area A there was a greater proportion of respondents reporting annoyance due to soot, dust, or fly ash in the subareas less than 2 km from plant A than in the subareas further away. Respiratory symptoms and diseases were more prevalent in areas with CFPPs (with more industries and roads) than in the reference areas. |
| Quizon et al. [77] | Philippines | LMI | Cross-sectional | n=370 households with children (6–10 years) | NR | NO2, SO2, PM10 | Respiratory symptoms and pulmonary function | The SO2 and NO2 levels are below the ambient standards. No significant association on the 8-h average for PM10 between the ‘near’ barangays (near CFPPs) and the ‘far’ barangays (far from CFPPs). PM10 and cigarette smoking were significant risk factors for wheezing and the predicted FEV1, respectively, reflecting the environmental exposure of children inside homes. |
| Rodriguez-Villamizar et al. [78] | Canada | HI | Cross-sectional | n=10,421 ED visits of asthmatic children (2–14 years) | NR | NR | ED asthma visits | There was an inverse association of the distance to the power plant (coefficient=−0.01 per km) with asthma visits. |
| Sears et al. [79] | USA | HI | Cross-sectional | n=221 children (6–14 years) | ≤16 km | PM10 | Cognitive control | Among females, higher PM10 concentration was associated with a higher risk of commission errors, but the association between PM10 concentration and CPT commission errors was attenuated among males. |
| Severnini [80] | USA | HI | Cross-sectional | n=56,000 observations | NR | SO2 | Low birth weight | The shutdown of the TVA nuclear power plants in 1985 induced increases in coal-fired power generation and air pollution. Average birth weight declined ∼134 g, or 5.4 log points, after the nuclear shutdown |
| Shabani Isenaj et al. [81] | Kosovo | UMI | Cross-sectional | n=NR, children hospitalized for respiratory diseases (0–18 years) (n=1,838 hospital admissions records, n=7,372 ambulatory visits) | NR | PM2.5 | Respiratory diseases | An increase in PM2.5 led to significant increases in ambulatory visits on the first and second day after the pollution episode. Stationary hospital admissions only increased on the day following the pollution episode |
| Tang et al. [39] | China | UMI | Cohort | n=NR, nonsmoking women (≥20 years) and their newborns (2002 before CFPP shutdown: n=110, 2005 after CFPP shutdown: n=107) | ≤2.5 km | PAHs | Neurodevelopment outcomes | In the two cohorts combined, PAH-DNA adducts were inversely associated with mBDNF as well as scores for motor (p=0.05), adaptive (p=0.022), and average DQ (p=0.014). BDNF levels were positively associated with DQ scores. The findings indicate that the closure of a coal-burning plant resulted in the reduction of PAH-DNA adducts in newborns and increased mBDNF levels that in turn, were positively associated with neurocognitive development. |
| Tang et al. [40] | China | UMI | Cohort | n=NR, nonsmoking women (≥20 years) and their newborns (2002 before CFPP shutdown: n=150, 2005 after CFPP shutdown: n=158) | ≤2.5 km | PAHs | Foetal and child development | A decrease in detectable PAH-DNA adducts in the umbilical cord was observed in the 2005 cohort compared to the 2002 cohort. The percentage of infants categorized as delayed for the motor area was significantly lower in 2005 than in 2002. Initial birth weight, height and head circumference for infants in the 2002 cohort were lower or reduced than for those in the 2005 cohort. |
| Tang et al. [41] | China | UMI | Cohort | n=150 nonsmoking women (≥20 years) and their newborns | ≤2.5 km | PAHs | Foetal and child development | Among females, high cord blood adduct level was significantly associated with smaller birth head circumference, as well as lower weight and shorter length. There was a significant association between longer duration of exposure and shorter length at birth and height. |
| Tang et al. [42] | China | UMI | Cohort | n=110 nonsmoking women (≥20 years) and their newborns | ≤2.5 km | PAHs, Pb, Hg | Child neurodevelopment | Increased adduct levels were associated with decreased motor area development quotients (DQ), language area DQ and average DQ. Decrements in one or more DQs were significantly associated with cord blood levels of PAH–DNA adducts and lead, but not mercury. |
| Wilkie et al. [82] | USA | HI | Intervention | n=42,231 singleton births (CFPP-scrubber: n=42,231, CFPP-retired: n=41,218) | ≤24 km | SO2 | Preterm birth | SO2 emission reduction interventions were associated with a decrease in preterm birth for gestational parents living within 4–<10 miles (6.4–<16 km) compared with 10–<15 miles (16–<24 km) away, especially among those living near CFPPs that installed scrubbers on coal electricity generating units. |
| Yang et al. [83] | USA | HI | Cohort | n=1,676,798 observations of singleton births | 32–48 km | SO2 | Low birth weight and very low birth weight | Babies born to mothers who live as far as 20–30 miles (32–48 km) away downwind from the power plant during the final stage of pregnancy were at greater risks of low birth weight and very low birth weight. Also, an increase of 1,000 tons of SO2 monthly emissions that come from upwind directions during the final stage of pregnancy could increase the likelihood of low birth weight. |
| Yogev-Baggio et al. [84] | Israel | HI | Cohort | n=1,181 school children (n=537 healthy subgroup, n=357 chest symptoms subgroup, n=287 pulmonary disease subgroup) | NR | NOx & SO2 | Pulmonary function | There was substantial decrease in the number of heathy children in the cohort in the most air polluted areas. Analysis of variance also confirmed that the interaction between air pollution levels and the children’s health status was associated significantly with the children’s PFT change. |
| Zhang et al. [85] | USA | HI | Cross-sectional | n=235 participants (6–14 years) | ≤16 km | PM10 | Neurobehavioral problems | Significant and inverse associations were observed between distance to the nearest power plant and the four CBCL diagnoses (i.e., affective problems, anxiety problems, ADHD, and social problems). Statistically significant hot spots of participants who had elevated levels of attention deficit hyperactivity disorder, anxiety, and social problems were observed in the vicinity of the two CFPPs. |
| Zierold et al. [86] | USA | HI | Qualitative | n=401 residents (37–65 years) (n=231 exposed group, n=170 non-exposed group) | 97 km (non-exposed group) | NR | Health symptoms | Adults who lived near the CFPP were significantly more likely to suffer from respiratory, gingiva, and skin symptoms. Adults living near the CFPP were significantly more likely to report having lung symptoms. |
| Zierold et al. [43] | USA | HI | Cross-sectional | n=261 children (6–14 years) | ≤16 km | Fly ash | School and social competency | Children with fly ash in their homes scored an average 2.63 points lower on school competency compared with children who did not have fly ash in their homes. |
| Zierold et al. [44] | USA | HI | Cross-sectional | n=260 children (6–14 years) (n=62 children with internalizing behaviour, n=198 children without internalizing behaviour) | ≤16 km | As, other heavy metals | Internalizing behaviour disorders | Exposure to zinc and imputed zirconium were associated with internalizing behaviours in children. |
| Zierold et al. [45] | USA | HI | Cross-sectional | n=266 children (6–14 years) | ≤16 km | Fly (coal) ash | Depression | Children with fly ash indoors had more depressive problems compared to non-exposed children. |
| American Lung Association [2] | USA | HI | Report | NR | NR | PM2.5, Hg, SO2 | CVD, asthma and other lung diseases | The EPA proposed a new requirement that all coal and oil-fired power plants that produce 25 MW of power for sale will be required to install cleanup technology such as scrubbers, as required by the Clean Air Act. This was done to reduce harmful air pollutants that make breathing difficult and causes asthma attacks and increase the risk of emergency room or hospital visits. |
| Health and Environmental Alliance [87] | Multi-country | NA | Report | NR | NR | CO2, NOx, PM2.5, Hg | CVDs, respiratory symptoms and diseases, heart disease, and cancer | Significant evidence exists on how long-term exposure to these air pollutants affects the lungs and the heart. Recent research suggests that air pollution may also result in low birth weight and pre-term delivery because of maternal exposure during pregnancy. |
-
World Bank income group/level: HI, high income; LMI, lower middle income; UMI, upper middle income; BDNF, brain-derived neurotrophic factor; CVD, cardiovascular diseases; DQ, developmental quotient; EDV/ERV, emergency department visits/emergency room visits; ETS, environmental tobacco smoke; FVC, forced vital capacity; FEV1, forced expiratory volume at 1 s; ISDI, Individual socioeconomic deprivation index; NA, not applicable; NR, Not reported; PAH, polycyclic aromatic hydrocarbons; PFT, pulmonary function test; URTI, upper respiratory tract infection.
Studies that measured emissions from CFPPs’ stacks
Four studies reported on the air pollution data near CFPPs [40], 41], 46], 49]. A study by Aekplakorn et al. [46] used daily outdoor air pollution data from air monitoring stations around the CFPP in Thailand. The study found low levels of SO2 and PM10, below Thai daily 24-h mean SO2 and PM10 standards (300 μg/m3 SO2 and 120 μg/m3 PM10). This was attributed to the recent installation of scrubbers in the CFPP and use of low-sulphur coal. Lower pulmonary function in asthmatic children in the study villages were associated with increases in daily PM10 concentrations. Barik et al. [49] carried out real-time air quality monitoring to determine the levels of air pollutants in seven locations in the study area around two CFPPs in central India. The findings show that the PM concentrations were higher in areas near the two CFPPs. PM (PM10, PM2.5 and PM1) concentrations were measured in the morning, afternoon and evenings. Most locations had PM concentrations exceeding regulatory limits set by National Ambient Air Quality Standards (NAAQS) and WHO. The highest concentrations of PM10, PM2.5 and PM1 were reported in two locations that are separated by a distance of 4 km.
Tang et al. [40] and Tang et al. [41] reported on a previous study that measured PM2.5 and PAH concentrations in the same study area in Tongliang, China, near the CFPP. The air quality monitoring was done before the shutdown of the CFPP. The higher PAH concentrations and seasonal variations in air pollution in the area were largely attributed to the CFPP emissions. PAHs and PM2.5 concentrations were reported to be higher in winter and low in summer. PAHs of higher molecular weight were 1.5–3.5 times higher during the CFPP operational period. The air monitoring data showed that the CFPP was a major contributor to PAHs in the air.
Association between distance between community and CFPP and health/risks impacts
Ten studies found a significant association between distance from CFPPs and the risk of negative health outcomes [41], 64], 68], 70], 75], 76], 78], 83], 85], 86]. Individuals of age ≥35 living in villages around the Seyitömer CFPP in Turkey had statistically significant more frequent complaint of chest tightness and repeated coughs for more than a year than individuals of age ≥35 in control villages (chest tightness: Fisher’s exact chi-square p=0.0006 and chi-square=14.774, p=0.0001; coughs: chi-square=5.08, p=0.024) [70]. A significant increased prevalence rate of throat clearing was reported for individuals living near CFPP in Finland compared to its reference area (further away from CFPP) (p<0.001) [76]. Using negative binomial multivariable models Rodriguez-Villamizar et al. [78] found a significant inverse association between distance from CFPP and direction of emergency department visits in Alberta, Canada (coefficient=−0.001, 95 % CI: −0.01, −0.01, p=0.000). A weaker negative coefficient was found for the latitude function (coefficient=−0.72, 95 % CI: −1.05, −0.39, p=0.000) and a strong positive coefficient for the longitude function (coefficient=0.93, 95 % CI: 0.37, 1.49, p=0.001) for directional effects indicating that children with acute asthma at the east and southeast of the CFPP were at high risk of emergency department visits. A significant difference between perception of health of exposed population (Kentucky, USA) to non-exposed comparison group (Indiana, USA) was observed (p<0.0001). Adults living near the CFPP were significantly more likely to report having lung symptoms (96 vs. 82 %, p<0.0001), muscular symptoms (94 vs. 85 %, p=0.004), gingiva symptoms (34 vs. 19 %, p=0.009) and skin symptoms (60 vs. 33 %, p<0.0001) compared to the non-exposed comparison group. Adults who lived near the CFPP were significantly more likely to suffer from respiratory symptoms (AOR=5.27, 95 % CI: 2.16, 12), gingiva symptoms (AOR=2.46, 95 % CI: 1.46, 4.15), and skin symptoms (AOR=3.37, 95 % CI: 2.09, 5.43) [86].
Karavuş et al. [70] observed that the mean FEV1 value and FEF25-75 % was significantly lower for individuals in villages around the Seyitömer CFPP in Turkey compared to individuals of the control villages (p=0.0001 and p=0.0001, respectively). Similarly, Hii et al. [68] found lower FEV1/FVC ratio for those living within 35 km from one of the 11 CFPPs (OR=1.24; 95 % CI: 0.90, 1.70) compared to those living farther than 35 km away from a CFPP. In a study to evaluate the respiratory function of residents around the Orhaneli CFPP in Turkey, the study (exposed) group was observed to have increased odds of lower FEV1 (OR=1.60, 95 % CI: 1.29, 1.99, p=0.000) and FVC (OR=2.69, 95 % CI: 2.14, 3.39, p=0.000) values compared to the control group [75].
A cohort study investigating the association between residential proximity to CFPPs and risk of adverse birth outcomes in Florida, USA, found that pregnant women living near two or more CFPPs within a 20 km radius had a 12 % increased odds of term low birth weight (OR=1.12, 95 % CI: 1.03, 1.22), 20 % increased odds of preterm delivery (OR=1.20, 95 % CI: 1.14, 1.25), and 23 % increased odds of very preterm delivery (OR=1.23, 95 % CI: 1.10, 1.36) [64]. Tang et al. [41] evaluated the relationship between prenatal PAH and foetal and child development in Tangliang, China. There was a significant positive association between longer distance from CFPP and birth length (p=0.03).
Yang et al. [83] evaluated estimated effects of living downwind from CFPP and low birth weight and very low birth weight. Among mothers living in the four counties in USA, downwind of the CFPP during the last month of pregnancy, the low-birth-weight likelihood significantly increases by about 6.5 % (p<0.01), and the very low birth weight likelihood significantly increases by about 17.1 % (p<0.01). The effect of being downwind of the CFPP on low birth weight was also evaluated. The results showed that male maternal exposure to CFPP emissions during the last month of pregnancy could significantly increase the likelihood of low birth weight by 0.59 percentage points (p<0.01) and for female maternal exposure the likelihood of low birth weight could significantly increase by 0.45 percentage points (p<0.10) two months prior to the birth month.
Zhang et al. [85] examined the relationship between neurobehavioral symptoms in children and proximity to CFPPs in Louisville, USA. The findings showed that the nearest distance to a CFPP had a significant and negative regression coefficient with four neurobehavioral symptoms: affective problems (−0.395, p<0.10), anxiety problems (−0.609, p<0.05), ADHD (−0.531, p<0.05), and social problems (−0.934, p<0.01). Clustering analyses spatial showed that nearly all the hot spots (28 out of 30) for social problems were found in near two CFPPs (Mill Creek CFPP and Cane Run CFPP, <2 miles). A total of 29 statistically significant hots spots at the 95 and 90 % confidence levels were observed for ADHD problems, and these were clustered around the two CFPPs. Most hot spots for anxiety were identified near the Mill Creek CFPP (significant at 95 % confidence level) and three hot spots (significant at 99 % confidence level) near the Cane Run CFPP.
Eleven studies reported on benefits of interventions such as CFPP retirement and installation of emission control technologies and subsequent health outcomes [35], [37], [38], [39], [40, 53], 54], 62], 71], 72], 82]. Studies by Lee et al. [35], Perera et al. [37], Perera et al. [38], Tang et al. [39] and Tang et al. [40] investigated the health benefits of shutting down the Tongliang CFPP in China in May 2004. The mother and infant cohorts were recruited in 2002 (before CFPP shutdown) and 2005 (after CFPP shutdown). The authors observed that the mean birth head circumference of the 2005 infants was significantly greater than that of the 2002 cohort (p<0.05). The mean PAH-DNA cord adduct level were significantly higher in the 2002 cohort than the 2005 cohort (p<0.05). Casey et al. [53] observed a significant association between CFPP retirements and decreases in the proportion of moderate to late preterm birth at 0–5 km (β=−0.020, 95 % CI: −0.031, −0.009) and 5–10 km (β=−0.016, 95 % CI: −0.025, −0.008) of the CFPP in California, USA. Another study by Casey et al. [54] observed a large reduction in risk asthma-related hospitalization and emergency room visits after the second quarter of the 2015 power plant energy transitions (one natural gas and three installed SO2 scrubbers) in Louisville, USA (rate ratio [RR]=0.81, 95 % CI: 0.70, 0.92). The installation of the SO2 scrubber was associated with a 17 % reduction in monthly average daily short-acting beta-agonists (SABA) use (RR=0.83, 95 % CI: 0.69, 1.00) and a 2 % reduction (95 % CI: −5 %, 1 %) for each month thereafter. Fan and Wang [62] observed that CFPP retirement reduced mortality of U.S adults older than 65 years by 3.6 % in treated counties (within 50 km downwind of CFPPs) between 1999 and 2013 (p<0.01), where retirement occurred between 2011 and 2013.
Komisarow and Pakhtigian [71] estimated the effect of coal-fired power plant closures on emergency department visits for asthma-related conditions among 0- to 4-year-old children in Chicago, USA. Emergency department visits for asthma-related conditions among 0- to 4-year-old children decreased by 12.1 % (95 % CI=−0.24, −0.02) near the three CFPPs following their closures relative to rates in zip codes farther away. In another study, Komisarow and Pakhtigian [72] observed that school-level rates of absences decreased by 6.14 % in schools located near the CFPPs (within 10 km) relative to those farther away following the closures in Chicago, USA (difference=1.01, p<0.01). Emergency department visits for asthma-related conditions after the closure of three CFPPs decreased by around 9 % (p<0.05) in school-aged children in zip codes near CFPPs compared to zip codes farther way.
Wilkie et al. [82] investigated the relationship between SO2 emission reduction strategies and preterm birth in North Carolina, USA. Among births within 4–<10 miles (∼6–16 km) of CFPPs, the prevalence of preterm birth decreased from 9.9 to 8.5 % after SO2 scrubbers were installed and from 9.0 to 7.6 % after CFPPs were retired. Using difference-in-difference approach; for gestational parents within 4–<10 miles from a CFPP, the absolute prevalence of preterm birth was estimated to decrease by −1.5 % (95 % CI: −2.6, −0.4) associated with the installation of scrubbers and decrease by −0.5 % (95 % CI: −1.6, 0.6) associated with CFPP retirements and decreased by −1.0 % (95 % CI: −1.8, −0.2) with both SO2 reduction intervention strategies.
Quality assessment in included studies
About 96.4 % (n=54) of the studies were classified as moderate to high quality and 3.6 % (n=2) were of low quality (supplementary material, Table S3A–D). Of the two studies with the lowest quality, one study did not clearly define the study population and inclusion criteria, and the other study did not clearly define the exposure measure and did not account for confounding factors [50], 51]. Most studies did not report on power estimations. Ten studies had not identified or adjusted for potential confounders [33], 34], [49], [50], [51, 58], 70], 74], 77], 81]. Smoking habits, indoor air pollution, proximity to roads, socio-demographic factors were some of the confounders identified and adjusted for in the study design or data analysis in most studies. Most of the studies used statistical models to measure the association between exposure and health outcomes.
Discussion
In this review, we observed mixed results. In some studies, there was an association between exposure to air pollutants, namely NOx, SO2, and PM2.5 from CFPPs and increases in the prevalence of respiratory symptoms and diseases and reduced pulmonary function among people living in proximity to CFPPs [46], 47], 61], 69], 76], 78], 81], 84], 86]. On the other hand, Goren et al. [63] and Quizon et al. [77] reported low levels of air pollutants in the study area near CFPPs and that respiratory symptoms were not linked to air pollution from CFPPs. Interestingly, recent studies show that low PM2.5 and NO2 levels (<12 μg/m3 and <53 part per billion, respectively) may also have negative health effects [88], 89]. Inhaled NO2 interacts and damages the lung lining fluid and epithelial cell membrane; PM10 deposits mainly on the tracheobronchitis region while PM2.5 settles on the pulmonary region, penetrates the alveoli, and enters the bloodstream [90], [91], [92]. The findings suggest that long-term exposure to air pollution from CFPPs reduced lung function and increases the risk of developing chronic respiratory diseases. Maternal exposure to PM2.5 and SO2 near CFPPs was associated with preterm birth and low birth weight of their neonates [59], 64], 74]. Higher exposure to PM2.5 and SO2 was reported in the first trimester. Furthermore, exposure to SO2 was linked to shorter gestation times [74]. Inhaled PM2.5 and SO2 can move through the mother’s lungs into bloodstream and enter the placenta. This can induce oxidative stress and inflammatory response in mothers and restrict foetal growth [93].
Several studies have shown an association between air pollution and infant mortality [94], [95], [96], [97]. Infants and children are particularly vulnerable to air pollution as their lungs are smaller and still developing. A study on the effects of CFPPs on infant mortality rates reported that a 1 GW increase in coal-fired capacity increased infant mortality by 19.3 % in urban areas (p<0.05) [50]. In other studies, exposure to PAHs, Hg, Pb, PM10 and other air pollutants was linked to neurodevelopmental disorders such as reduced cognitive control, developmental delays and intellectual quotient (IQ) test scores [36], 42], 79]. Exposure to PAHs, Hg, Pb, PM10 and other air pollutants was also linked to behavioural issues such as affective problems, anxiety problems, attention deficit hyperactivity disorder, depressive symptoms and social problems [44], 45], 85]. Prenatal exposure to PM was associated with reduced composite cognitive scores in children where PM was thought to induce neuroinflammation and oxidative stress processes [98]. Previous studies also show a link between heavy metals, cognitive abilities, IQ and infant development [99], 100]. The blood-brain barrier develops in utero, therefore heavy metals can freely enter the brain and stunt development [99]. Blanchard et al. [52] observed that the prevalence rate of autism was greater in geographic areas of higher Hg levels. Evidence shows that high levels of toxic metals are associated with autism [101]. The number of CFPPs in an area increases the chances of incidence of anaemia in young children [60].
A few studies investigated the association between the prevalence of disease and proximity of communities to CFPPs. CFPPs release air pollutants through smokestacks which are vertical pipes or chimneys. The air pollutant dispersion is dependent upon the wind speed and direction. The air pollutants can travel from an upwind emission source to a downwind location [102]. If the wind is blowing from the west, then as the emissions come off the smokestacks, they will blow and spread towards the east (downwind). Communities living downwind of the CFPP will experience higher exposure to air pollution compared to those located ‘upwind’. The United States Environmental Protection Agency (U.S. EPA) recently issued the “Good Neighbor” rule to restrict emissions from CFPP that burdens downwind areas with air pollution [103]. Also, another U.S. EPA report indicates that millions of Americans live within a 3-mile (∼5 km) radius of CFPPs thus are vulnerable to health burdens [104]. Our findings show that women who lived closer to CFPPs or downwind from CFPPs at the first and third trimester of pregnancy had increased odds of adverse birth outcomes compared to those who were further away [64], 83]. The most common distance of exposure to air pollution studied was between 0 and 20 km from the CFPP [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47, 53], 55], 61], [63], [64], [65, 67], [69], [70], [71], [72, 74], 75], 79], 82], 85] with control sites located at a distance above 20 km from CFPP [65], 67], 68], 70], 75].
Several studies evaluated the health benefits of the closure or retirement of CFPPs. A significant reduction in asthma-related emergency department visit, preterm births, improvement in child neurodevelopment and physical development was reported [35], [37], [38], [39], [40, 53], 54], 71], 72], 82]. Previous studies have estimated the health benefits of decommissioning or closure of CFPPs [105], 106]. A study in China showed that the reduced operation of CFPPs during the COVID-19 pandemic lockdown resulted in lower levels of NO2, PM10 and PM2.5 (lower by 1.54 μg/m3, 3.73 μg/m3 and 2.22 μg/m3, respectively) [107]. Another study found that elimination of emissions from CFPPs could prevent more than 53,200 premature deaths each year in USA [108]. Similarly, Fan and Wang [62] report that 1 μg/m3 reduction in PM2.5 led to a 3.6 % decrease in mortality in adults older than 65 years after the retirement of CFPPs in USA. The delays in the phasing out of coal for electricity generation and transitioning to cleaner energy sources have been due to concerns such as possible power shortages, increases in electricity price and economic impact [109]. The Just Energy Transition addresses these challenges, particularly in countries where there is a high coal dependency for power generation. A just energy transition ensures that there is fair distribution costs and benefits of the shift of the energy sector from coal to renewables [110]. Local communities will have cleaner air to breathe, improve health and this will in turn reduce the burden on the healthcare system.
Retrofitting the CFPPs with emission control technologies have been to be effective in reducing emissions [111], 112]. Reduction in exposure to air pollution from CFPPs has been reported after the installation of SO2 emission control systems [53], 82]. In the U.S., the Clean Air Act Amendments require CFPPs to install flue-gas desulfurization units or scrubbers to reduce SO2 emissions. The scrubbers (wet and dry) are reported to have additional benefits of reducing Hg and particulate matter. Similarly, the European Union adopted the Best Available Technique standards for the energy sector which refer to the use of most economically and technically viable techniques to reduce emissions and impact on the environment [113]. Previous studies show that the implementation of the air quality law and standards, and installation of clean technologies led to improved air quality [114], 115]. This can be one of the strategies to reduce CFPP emissions and health impacts.
It is important to recognize that there is some difficulty and uncertainty in defining proximity and estimating exposure to air pollutants from CFPPs could bias the investigation of the health impacts of CFPPs. The spatial dispersion of air pollutants is not only affected by proximity but also by wind speed, direction, and other meteorological factors [116]. Moreover, population exposures does not necessarily reflect variations in individual exposures to environmental risk factors. Therefore, it is important for future research to improve more precise exposure measures, such as wearable mobile devices for individual participants, and to collect data on long-term exposure, if not lifetime exposome, to environmental toxicants.
We found that most studies used statistical models to measure the association between exposure and health outcomes. While a variety of regression models were used, we recommend that spatial statistical methods (such as spatial regression or geographically weighted regression) should also be used in the future. The existence of spatial dependence or autocorrelation or spatial heterogeneity could bias the estimates of regression parameters when examining the impacts of proximity/exposure to CFPPs.
This scoping review strictly followed the guidelines for conducting such a review and therefore, the findings are deemed reliable. A limitation of the study is that in our quality assessment, we did not exclude studies that were of low quality. However, we highlighted key issues or problems in the study design and other aspects of the studies. Confounders distort the exposure and health outcome association. This review did not explore the impact of confounders, but reported on studies that did not adjust for confounders in the study design.
Conclusions
The findings from this scoping review highlight the evidence showing the health impacts associated with living in proximity to CFPPs. There are limited epidemiological studies in low- and middle-income countries which may warrant attention. Children and pregnant women were among the most studied population groups. There was a statistically significant association between distance from CFPPs and increased odds of respiratory disorders, preterm birth and low birth weight, increased risk of foetal or child development and neurodevelopment problems. It is important to note that other sources of air pollution in the areas near CFPPs may have also contributed to air pollution-related health impacts. More cohort studies covering a larger geographical area to fully display the health impacts of air pollution from CFPPs. Emissions control and the closure or retirement of CFPPs can reduce exposure to air pollution and negative health impacts. Policies that seek to reduce air pollution from CFPPs need to be implemented as we move towards phasing out coal from electricity generation.
-
Research ethics: Not applicable.
-
Informed consent: Not applicable.
-
Author contributions: Conceptualization: CYW; Investigation: NM, TK, CW, CH-D; Writing – original draft: NM; Writing – review & editing: NM, TK, CW, CH-D, CYW; Funding acquisition: CYW. All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Use of Large Language Models, AI and Machine Learning Tools: None declared.
-
Conflict of interest: The authors state no conflict of interest.
-
Research funding: This work was supported by the UK International Development from the UK government, grant/award number: 301523-404; however, the views expressed do not necessarily reflect the UK government’s official policies.
-
Data availability: Not applicable.
References
1. Energy Institute. Statistical review of world energy; 2024. Available from: https://www.energyinst.org/statistical-review [Accessed 25 May 2024].Search in Google Scholar
2. American Lung Association. Toxic air: the case for cleaning up coal-fired power plants. 2011.Search in Google Scholar
3. International Energy Agency. Coal; 2023. Available from: https://www.iea.org/energy-system/fossil-fuels/coal [Accessed 20 May 2024].Search in Google Scholar
4. Global Energy Monitor C; E3G, Reclaim Finance; Sierra Club; SFOC; Kiko Network; CAN Europe; Bangladesh Groups; ACJCE; Chile Sustentable. Boom and bust coal 2023: tracking the global coal plant pipeline. Covina: Global Energy Monitor; 2023.Search in Google Scholar
5. Global Energy Monitor C; E3G; Reclaim Finance; Sierra Club; SFOC; Kiko Network; CAN Europe; Bangladesh Groups; Trend Asia; ACJCE; Chile Sustentable; POLEN Transiciones Justas; Iniciativa Climatica de Mexico; Arayara. Boom and bust coal 2024: tracking the global coal plant pipeline. Covina: Global Energy Monitor; 2024.Search in Google Scholar
6. Glenn, BE, Espira, LM, Larson, MC, Larson, PS. Ambient air pollution and non-communicable respiratory illness in sub-Saharan Africa: a systematic review of the literature. Environ Health 2022;21:40. https://doi.org/10.1186/s12940-022-00852-0.Search in Google Scholar PubMed PubMed Central
7. Liu, Q, Xu, C, Ji, G, Liu, H, Shao, W, Zhang, C, et al.. Effect of exposure to ambient PM(2.5) pollution on the risk of respiratory tract diseases: a meta-analysis of cohort studies. J Biomed Res 2017;31:130–42. https://doi.org/10.7555/jbr.31.20160071.Search in Google Scholar
8. Pun, VC, Kazemiparkouhi, F, Manjourides, J, Suh, HH. Long-term PM2.5 exposure and respiratory, cancer, and cardiovascular mortality in older US adults. Am J Epidemiol 2017;186:961–9. https://doi.org/10.1093/aje/kwx166.Search in Google Scholar PubMed PubMed Central
9. Yan, M, Ge, H, Zhang, L, Chen, X, Yang, X, Liu, F, et al.. Long-term PM2.5 exposure in association with chronic respiratory diseases morbidity: a cohort study in Northern China. Ecotoxicol Environ Saf 2022;244:114025. https://doi.org/10.1016/j.ecoenv.2022.114025.Search in Google Scholar PubMed PubMed Central
10. Cserbik, D, Chen, J-C, McConnell, R, Berhane, K, Sowell, ER, Schwartz, J, et al.. Fine particulate matter exposure during childhood relates to hemispheric-specific differences in brain structure. Environ Int 2020;143:105933. https://doi.org/10.1016/j.envint.2020.105933.Search in Google Scholar PubMed PubMed Central
11. Kao, C-C, Chen, C-C, Avelino, JL, Cortez, M-sP, Tayo, LL, Lin, Y-H, et al.. Infants’ neurodevelopmental effects of PM2.5 and persistent organohalogen pollutants exposure in southern Taiwan. Aerosol Air Qual Res 2019;19:2793–803. https://doi.org/10.4209/aaqr.2019.10.0550.Search in Google Scholar
12. Institute of Medicine of the National Academies. Preterm birth: causes, consequences, and prevention. In: Behrman, R, Butler, AS, editors. The role of environmental toxicants in preterm birth. Washington DC: National Academies Press; 2007.Search in Google Scholar
13. Liu, Y, Xu, J, Chen, D, Sun, P, Ma, X. The association between air pollution and preterm birth and low birth weight in Guangdong, China. BMC Public Health 2019;19:3. https://doi.org/10.1186/s12889-018-6307-7.Search in Google Scholar PubMed PubMed Central
14. Mendola, P, Nobles, C, Williams, A, Sherman, S, Kanner, J, Seeni, I, et al.. Air pollution and preterm birth: do air pollution changes over time influence risk in consecutive pregnancies among low-risk women? Int J Environ Res Publ Health 2019;16. https://doi.org/10.3390/ijerph16183365.Search in Google Scholar PubMed PubMed Central
15. Stieb, DM, Chen, L, Eshoul, M, Judek, S. Ambient air pollution, birth weight and preterm birth: a systematic review and meta-analysis. Environ Res 2012;117:100–11. https://doi.org/10.1016/j.envres.2012.05.007.Search in Google Scholar PubMed
16. Zhang, T, Mao, W, Gao, J, Song, X, Li, L, Sun, X, et al.. The effects of PM2.5 on lung cancer-related mortality in different regions and races: a systematic review and meta-analysis of cohort studies. Air Qual Atmos Health 2022;15:1523–32. https://doi.org/10.1007/s11869-022-01193-0.Search in Google Scholar
17. Henneman, L, Choirat, C, Dedoussi, I, Dominici, F, Roberts, J, Zigler, C. Mortality risk from United States coal electricity generation. Science 2023;382:941–6. https://doi.org/10.1126/science.adf4915.Search in Google Scholar PubMed PubMed Central
18. Zhang, CH, Zierold, KM. Birth defects: spatial disparities and associations with proximity to coal-fired power plants in the United States. Exposure Health 2024. https://doi.org/10.1007/s12403-024-00673-1 [Epub ahead of print].10.1007/s12403-024-00673-1Search in Google Scholar
19. Morehouse, J, Rubin, E. Downwind and out: the strategic dispersion of power plants and their. Pollut SSRN Electron J 2021:1–46.10.2139/ssrn.3915247Search in Google Scholar
20. Milner, J, Turner, G, Ibbetson, A, Eustachio Colombo, P, Green, R, Dangour, AD, et al.. Impact on mortality of pathways to net zero greenhouse gas emissions in England and Wales: a multisectoral modelling study. Lancet Planet Health 2023;7:e128–36. https://doi.org/10.1016/s2542-5196(22)00310-2.Search in Google Scholar PubMed PubMed Central
21. Qian, H, Xu, S, Cao, J, Ren, F, Wei, W, Meng, J, et al.. Air pollution reduction and climate co-benefits in China’s industries. Nat Sustain 2021;4:417–25. https://doi.org/10.1038/s41893-020-00669-0.Search in Google Scholar
22. Paola, A. Global and regional coal phase-out requirements of the Paris Agreement: insights from the IPCC special report on 1.5°C. Berlin: Climate Analytics; 2019.Search in Google Scholar
23. Amster, E. Public health impact of coal-fired power plants: a critical systematic review of the epidemiological literature. Int J Environ Health Res 2021;31:558–80. https://doi.org/10.1080/09603123.2019.1674256.Search in Google Scholar PubMed
24. Aromataris, E, Munn, Z. JBI manual for evidence synthesis. JBI; 2020. https://synthesismanual.jbi.global [Accessed 25 May 2024].Search in Google Scholar
25. Tricco, AC, Lillie, E, Zarin, W, O’Brien, KK, Colquhoun, H, Levac, D, et al.. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med 2018;169:467–73. https://doi.org/10.7326/m18-0850.Search in Google Scholar
26. Mahlangeni, N, Kapwata, T, Laban, T, Wright, CY. Health risks among children from exposure to air pollution in areas where coal-fired power plants are located: a scoping review protocol. OSF 2023. https://osf.io/a8q3j [Accessed 20 May 2024].Search in Google Scholar
27. Mahlangeni, N, Kapwata, T, Laban, T, Wright, CY. Health risks of exposure to air pollution in areas where coal-fired power plants are located: protocol for a scoping review. BMJ Open 2024;14:e084074. https://doi.org/10.1136/bmjopen-2024-084074.Search in Google Scholar PubMed PubMed Central
28. Ouzzani, M, Hammady, H, Fedorowicz, Z, Elmagarmid, A. Rayyan—a web and mobile app for systematic reviews. Syst Rev 2016;5:210. https://doi.org/10.1186/s13643-016-0384-4.Search in Google Scholar PubMed PubMed Central
29. Critical Appraisal Skills Programme. CASP cohort study checklist; 2023. Available from: https://casp-uk.net/casp-tools-checklists/cohort-study-checklist/ [Accessed 25 May 2024].Search in Google Scholar
30. Critical Appraisal Skills Programme. CASP qualitative studies checklist; 2023. Available from: https://casp-uk.net/casp-tools-checklists/qualitative-studies-checklist/ [Accessed 25 May 2024].Search in Google Scholar
31. National Heart, Lung and Blood Institute (NHLBI). Study quality assessment tools; 2021. Available from: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools [Accessed 25 May 2024].Search in Google Scholar
32. Tyndall, J. AACODS checklist. Flinders University; 2010. http://dspace.flinders.edu.au/dspace/.Search in Google Scholar
33. Goren, AI, Hellmann, S. Changing prevalence of asthma among schoolchildren in Israel. Eur Respir J 1997;10:2279–84. https://doi.org/10.1183/09031936.97.10102279.Search in Google Scholar PubMed
34. Goren, AI, Helman, S, Goldsmith, JR. Longitudinal study of respiratory conditions among schoolchildren in Israel: interim report of an epidemiological monitoring program in the vicinity of a new coal-fired power plant. Arch Environ Health 1988;43:190–4. https://doi.org/10.1080/00039896.1988.9935852.Search in Google Scholar PubMed
35. Lee, J, Kalia, V, Perera, F, Herbstman, J, Li, T, Nie, J, et al.. Prenatal airborne polycyclic aromatic hydrocarbon exposure, LINE1 methylation and child development in a Chinese cohort. Environ Int 2017;99:315–20. https://doi.org/10.1016/j.envint.2016.12.009.Search in Google Scholar PubMed PubMed Central
36. Perera, F, Li, TY, Lin, C, Tang, D. Effects of prenatal polycyclic aromatic hydrocarbon exposure and environmental tobacco smoke on child IQ in a Chinese cohort. Environ Res 2012;114:40–6. https://doi.org/10.1016/j.envres.2011.12.011.Search in Google Scholar PubMed
37. Perera, F, Li, TY, Zhou, ZJ, Yuan, T, Chen, YH, Qu, L, et al.. Benefits of reducing prenatal exposure to coal-burning pollutants to children’s neurodevelopment in China. Environ Health Perspect 2008;116:1396–400. https://doi.org/10.1289/ehp.11480.Search in Google Scholar PubMed PubMed Central
38. Perera, F, Lin, CJ, Qu, L, Tang, D. Shorter telomere length in cord blood associated with prenatal air pollution exposure: benefits of intervention. Environ Int 2018;113:335–40. https://doi.org/10.1016/j.envint.2018.01.005.Search in Google Scholar PubMed
39. Tang, D, Lee, J, Muirhead, L, Li, TY, Qu, L, Yu, J, et al.. Molecular and neurodevelopmental benefits to children of closure of a coal burning power plant in China. PLoS One 2014;9:e91966. https://doi.org/10.1371/journal.pone.0091966.Search in Google Scholar PubMed PubMed Central
40. Tang, D, Li, TY, Chow, JC, Kulkarni, SU, Watson, JG, Ho, SS, et al.. Air pollution effects on fetal and child development: a cohort comparison in China. Environ Pollut 2014;185:90–6. https://doi.org/10.1016/j.envpol.2013.10.019.Search in Google Scholar PubMed
41. Tang, D, Li, TY, Liu, JJ, Chen, YH, Qu, L, Perera, F. PAH-DNA adducts in cord blood and fetal and child development in a Chinese cohort. Environ Health Perspect 2006;114:1297–300. https://doi.org/10.1289/ehp.8939.Search in Google Scholar PubMed PubMed Central
42. Tang, D, Li, TY, Liu, JJ, Zhou, ZJ, Yuan, T, Chen, YH, et al.. Effects of prenatal exposure to coal-burning pollutants on children’s development in China. Environ Health Perspect 2008;116:674–9. https://doi.org/10.1289/ehp.10471.Search in Google Scholar PubMed PubMed Central
43. Zierold, KM, Myers, JV, Brock, GN, Sears, CG, Zhang, CH, Sears, L. Indoor coal ash and school and social competency among children aged 6-14 years. J Expo Sci Environ Epidemiol 2023;33:434–8. https://doi.org/10.1038/s41370-022-00500-2.Search in Google Scholar PubMed PubMed Central
44. Zierold, KM, Myers, JV, Brock, GN, Zhang, CH, Sears, CG, Sears, L. Heavy metal(loid) body burden in environmentally exposed children with and without internalizing behavior problems. Exposure Health 2022;14:903–14. https://doi.org/10.1007/s12403-022-00469-1.Search in Google Scholar PubMed PubMed Central
45. Zierold, KM, Sears, CG, Myers, JV, Brock, GN, Zhang, CH, Sears, L. Exposure to coal ash and depression in children aged 6-14 years old. Environ Res 2022;214:114005. https://doi.org/10.1016/j.envres.2022.114005.Search in Google Scholar PubMed PubMed Central
46. Aekplakorn, W, Loomis, D, Vichit-Vadakan, N, Shy, C, Wongtim, S, Vitayanon, P. Acute effect of sulphur dioxide from a power plant on pulmonary function of children, Thailand. Int J Epidemiol 2003;32:854–61. https://doi.org/10.1093/ije/dyg237.Search in Google Scholar PubMed
47. Amster, ED, Haim, M, Dubnov, J, Broday, DM. Contribution of nitrogen oxide and sulfur dioxide exposure from power plant emissions on respiratory symptom and disease prevalence. Environ Pollut 2014;186:20–8. https://doi.org/10.1016/j.envpol.2013.10.032.Search in Google Scholar PubMed
48. Barbhaya, D, Hejjaji, V, Vijayaprakash, A, Rahimian, A, Yamparala, A, Yakkali, S, et al.. The burden of premature mortality from coal-fired power plants in India is high and inequitable. Environ Res Lett 2022;17:104022. https://doi.org/10.1088/1748-9326/ac91e3.Search in Google Scholar
49. Barik, P, Naoghare, P, Sivanesan, S, Kannan, K, Middey, A. Increased average annual prevalence of upper respiratory tract infection (UTRI) in the central Indian population residing near the coal-fired thermal power plants. SN Appl Sci 2021;3:214. https://doi.org/10.1007/s42452-021-04222-2.Search in Google Scholar
50. Barrows, G, Garg, T, Jha, A. The health costs of coal-fired power plants in India. IZA Institute of Labor Economics; 2019.10.2139/ssrn.3510449Search in Google Scholar
51. Bencko, V, Symon, K, Chládek, V, Pihrt, J. Health aspects of burning coal with a high arsenic content: II. Hearing changes in exposed children. Environ Res 1977;13:386–95. https://doi.org/10.1016/0013-9351(77)90019-6.Search in Google Scholar PubMed
52. Blanchard, KS, Palmer, RF, Stein, Z. The value of ecologic studies: mercury concentration in ambient air and the risk of autism. Rev Environ Health 2011;26:111–8. https://doi.org/10.1515/reveh.2011.015.Search in Google Scholar PubMed
53. Casey, JA, Karasek, D, Ogburn, EL, Goin, DE, Dang, K, Braveman, PA, et al.. Retirements of coal and oil power plants in California: association with reduced preterm birth among populations nearby. Am J Epidemiol 2018;187:1586–94. https://doi.org/10.1093/aje/kwy110.Search in Google Scholar PubMed PubMed Central
54. Casey, JA, Su, JG, Henneman, LRF, Zigler, C, Neophytou, AM, Catalano, R, et al.. Improved asthma outcomes observed in the vicinity of coal power plant retirement, retrofit, and conversion to natural gas. Nat Energy 2020;5:398–408. https://doi.org/10.1038/s41560-020-0600-2.Search in Google Scholar PubMed PubMed Central
55. Chen, C-HS, Yuan, T-H, Shie, R-H, Wu, K-Y, Chan, C-C. Linking sources to early effects by profiling urine metabolome of residents living near oil refineries and coal-fired power plants. Environ Int 2017;102:87–96. https://doi.org/10.1016/j.envint.2017.02.003.Search in Google Scholar PubMed
56. Chen, S, Li, Y, Shi, G, Zhu, Z. Gone with the wind? Emissions of neighboring coal-fired power plants and local public health in China. China Econ Rev 2021;69:101660. https://doi.org/10.1016/j.chieco.2021.101660.Search in Google Scholar
57. Chen, S, Li, Y, Yao, Q. The health costs of the industrial leap forward in China: evidence from the sulfur dioxide emissions of coal-fired power stations. China Econ Rev 2018;49:68–83. https://doi.org/10.1016/j.chieco.2018.01.004.Search in Google Scholar
58. Collarile, P, Bidoli, E, Barbone, F, Zanier, L, Del Zotto, S, Fuser, S, et al.. Residence in proximity of a coal-oil-fired thermal power plant and risk of lung and bladder cancer in North-eastern Italy. A population-based study: 1995–2009. Int J Environ Res Publ Health 2017;14. https://doi.org/10.3390/ijerph14080860.Search in Google Scholar PubMed PubMed Central
59. Daouda, M, Henneman, L, Kioumourtzoglou, M-A, Gemmill, A, Zigler, C, Casey, JA. Association between county-level coal-fired power plant pollution and racial disparities in preterm births from 2000 to 2018. Environ Res Lett 2021;16:034055. https://doi.org/10.1088/1748-9326/abe4f7.Search in Google Scholar PubMed PubMed Central
60. Datt, G, Maitra, P, Menon, N, Ray, R. Coal plants, air pollution and anaemia: evidence from India. J Dev Stud 2023;59:533–51. https://doi.org/10.1080/00220388.2022.2132151.Search in Google Scholar
61. Dubnov, J, Barchana, M, Rishpon, S, Leventhal, A, Segal, I, Carel, R, et al.. Estimating the effect of air pollution from a coal-fired power station on the development of children’s pulmonary function. Environ Res 2007;103:87–98. https://doi.org/10.1016/j.envres.2006.02.009.Search in Google Scholar PubMed
62. Fan, M, Wang, Y. The impact of PM(2.5) on mortality in older adults: evidence from retirement of coal-fired power plants in the United States. Environ Health 2020;19:28. https://doi.org/10.1186/s12940-020-00573-2.Search in Google Scholar PubMed PubMed Central
63. Goren, AI, Hellmann, S, Glaser, ED. Use of outpatient clinics as a health indicator for communities around a coal-fired power plant. Environ Health Perspect 1995;103:1110–5. https://doi.org/10.2307/3432606.Search in Google Scholar
64. Ha, S, Hu, H, Roth, J, Kan, H, Xu, X. Associations between residential proximity to power plants and adverse birth outcomes. Am J Epidemiol 2015;182:215–24. https://doi.org/10.1093/aje/kwv042.Search in Google Scholar PubMed PubMed Central
65. Hagemeyer, AN, Sears, CG, Zierold, KM. Respiratory health in adults residing near a coal-burning power plant with coal ash storage facilities: a cross-sectional epidemiological study. Int J Environ Res Publ Health 2019;16:3642. https://doi.org/10.3390/ijerph16193642.Search in Google Scholar PubMed PubMed Central
66. Henneman, LRF, Choirat, C, Zigler, ACM. Accountability assessment of health improvements in the United States associated with reduced coal emissions between 2005 and 2012. Epidemiology 2019;30:477–85. https://doi.org/10.1097/ede.0000000000001024.Search in Google Scholar PubMed PubMed Central
67. Henry, RL, Bridgman, HA, Wlodarczyk, J, Abramson, R, Adler, JA, Hensley, MJ. Asthma in the vicinity of power stations: II. Outdoor air quality and symptoms. Pediatr Pulmonol 1991;11:134–40. https://doi.org/10.1002/ppul.1950110210.Search in Google Scholar PubMed
68. Hii, M, Beyer, K, Namin, S, Malecki, K, Schultz, A, Rublee, C. Respiratory diseases, racial disparities, and residential proximity to coal power plants in Wisconsin, USA: a cross-sectional study. Lancet Global Health 2021;9:S19–S. https://doi.org/10.1016/s2214-109x(21)00127-3.Search in Google Scholar
69. Kamath, R, Udayar, SE, Jagadish, G, Prabhakaran, P, Madhipatla, KK, Research Team. Assessment of health status and impact of pollution from thermal power plant on health of population and environment around the plant in Udupi District, Karnataka. Indian J Publ Health 2022;66:91–7. https://doi.org/10.4103/ijph.ijph_1422_20.Search in Google Scholar
70. Karavuş, M, Aker, A, Cebeci, D, Taşdemir, M, Bayram, N, Çali, Ş. Respiratory complaints and spirometric parameters of the villagers living around the seyitomer coal-fired thermal power plant in Kütahya, Turkey. Ecotoxicol Environ Saf 2002;52:214–20. https://doi.org/10.1006/eesa.2002.2158.Search in Google Scholar PubMed
71. Komisarow, S, Pakhtigian, EL. The effect of coal-fired power plant closures on emergency department visits for asthma-related conditions among 0- to 4-year-old children in Chicago, 2009–2017. Am J Publ Health 2021;111:881–9. https://doi.org/10.2105/ajph.2021.306155.Search in Google Scholar
72. Komisarow, S, Pakhtigian, EL. Are power plant closures a breath of fresh air? Local air quality and school absences. J Environ Econ Manag 2022;112:102569. https://doi.org/10.1016/j.jeem.2021.102569.Search in Google Scholar
73. Minichilli, F, Gorini, F, Bustaffa, E, Cori, L, Bianchi, F. Mortality and hospitalization associated to emissions of a coal power plant: a population-based cohort study. Sci Total Environ 2019;694:133757-. https://doi.org/10.1016/j.scitotenv.2019.133757.Search in Google Scholar PubMed
74. Mohorovic, L. First two months of pregnancy—critical time for preterm delivery and low birthweight caused by adverse effects of coal combustion toxics. Early Hum Dev 2004;80:115–23. https://doi.org/10.1016/j.earlhumdev.2004.06.001.Search in Google Scholar PubMed
75. Pala, K, Türkkan, A, Gerçek, H, Osman, E, Aytekin, H. Evaluation of respiratory functions of residents around the Orhaneli thermal power plant in Turkey. Asia Pac J Publ Health 2012;24:48–57. https://doi.org/10.1177/1010539510363622.Search in Google Scholar PubMed
76. Pershagen, G, Hammar, N, Vartiainen, E. Respiratory symptoms and annoyance in the vicinity of coal-fired plants. Environ Health Perspect 1986;70:239–45. https://doi.org/10.2307/3430360.Search in Google Scholar
77. Quizon, RR, Torres, EB, Torres-Briola, TY, Lomboy, MFTC. Indoor air quality monitoring of communities surrounding a coal-fired power plant in Pagbilao, Quezon. Acta Med Philipp 2016. https://doi.org/10.47895/amp.v50i3.814.Search in Google Scholar
78. Rodriguez-Villamizar, LA, Rosychuk, RJ, Osornio-Vargas, A, Villeneuve, PJ, Rowe, BH. Proximity to two main sources of industrial outdoor air pollution and emergency department visits for childhood asthma in Edmonton, Canada. Can J Public Health 2018;108:e523–9. https://doi.org/10.17269/cjph.108.6136.Search in Google Scholar PubMed PubMed Central
79. Sears, CG, Sears, L, Zierold, KM. Sex differences in the association between exposure to indoor particulate matter and cognitive control among children (age 6–14 years) living near coal-fired power plants. Neurotoxicol Teratol 2020;78:106855-. https://doi.org/10.1016/j.ntt.2020.106855.Search in Google Scholar PubMed PubMed Central
80. Severnini, E. Impacts of nuclear plant shutdown on coal-fired power generation and infant health in the Tennessee Valley in the 1980s. Nat Energy 2017;2:1–9. https://doi.org/10.1038/nenergy.2017.51.Search in Google Scholar
81. Shabani, IZ, Berisha, M, Gjorgjev, D, Dimovska, M, Moshammer, H, Ukëhaxhaj, A. Air pollution in Kosovo: short term effects on hospital visits of children due to respiratory health diagnoses. Int J Environ Res Publ Health 2022;19. https://doi.org/10.3390/ijerph191610141.Search in Google Scholar PubMed PubMed Central
82. Wilkie, AA, Richardson, DB, Luben, TJ, Serre, ML, Woods, CG, Daniels, JL. Sulfur dioxide reduction at coal-fired power plants in North Carolina and associations with preterm birth among surrounding residents. Environ Epidemiol 2023;7:e241. https://doi.org/10.1097/ee9.0000000000000241.Search in Google Scholar
83. Yang, M, Bhatta, RA, Chou, S-Y, Hsieh, C-I. The impact of prenatal exposure to power plant emissions on birth weight: evidence from a Pennsylvania power plant located upwind of New Jersey. J Pol Anal Manag 2017;36:557–83. https://doi.org/10.1002/pam.21989.Search in Google Scholar PubMed
84. Yogev-Baggio, T, Bibi, H, Dubnov, J, Or-Hen, K, Carel, R, Portnov, BA. Who is affected more by air pollution—sick or healthy? Some evidence from a health survey of schoolchildren living in the vicinity of a coal-fired power plant in Northern Israel. Health Place 2010;16:399–408. https://doi.org/10.1016/j.healthplace.2009.11.013.Search in Google Scholar PubMed
85. Zhang, CH, Sears, L, Myers, JV, Brock, GN, Sears, CG, Zierold, KM. Proximity to coal-fired power plants and neurobehavioral symptoms in children. J Expo Sci Environ Epidemiol 2022;32:124–34. https://doi.org/10.1038/s41370-021-00369-7.Search in Google Scholar PubMed PubMed Central
86. Zierold, KM, Hagemeyer, AN, Sears, CG. Health symptoms among adults living near a coal-burning power plant. Arch Environ Occup Health 2020;75:289–96. https://doi.org/10.1080/19338244.2019.1633992.Search in Google Scholar PubMed
87. Health and Environmental Alliance. The unpaid health bill: how coal power plants makes us sick; 2013.Search in Google Scholar
88. Papadogeorgou, G, Kioumourtzoglou, MA, Braun, D, Zanobetti, A. Low levels of air pollution and health: effect estimates, methodological challenges, and future directions. Curr Environ Health Rep 2019;6:105–15. https://doi.org/10.1007/s40572-019-00235-7.Search in Google Scholar PubMed PubMed Central
89. Yazdi, MD, Wang, Y, Di, Q, Requia, WJ, Wei, Y, Shi, L, et al.. Long-term effect of exposure to lower concentrations of air pollution on mortality among US Medicare participants and vulnerable subgroups: a doubly-robust approach. Lancet Planet Health 2021;5:e689–7. https://doi.org/10.1016/s2542-5196(21)00204-7.Search in Google Scholar PubMed PubMed Central
90. Cipoli, YA, Furst, L, Feliciano, M, Alves, C. Respiratory deposition dose of PM2.5 and PM10 during night and day periods at an urban environment. Air Qual Atmos Health 2023;16:2269–83. https://doi.org/10.1007/s11869-023-01405-1.Search in Google Scholar
91. Deng, Q, Deng, L, Miao, Y, Guo, X, Li, Y. Particle deposition in the human lung: health implications of particulate matter from different sources. Environ Res 2019;169:237–45. https://doi.org/10.1016/j.envres.2018.11.014.Search in Google Scholar PubMed
92. Frampton, MW, Boscia, J, Roberts, NJ, Azadniv, M, Torres, A, Cox, C, et al.. Nitrogen dioxide exposure: effects on airway and blood cells. Am J Physiol Lung Cell Mol Physiol 2002;282:L155–65. https://doi.org/10.1152/ajplung.2002.282.1.l155.Search in Google Scholar PubMed
93. Sun, X, Luo, X, Zhao, C, Zhang, B, Tao, J, Yang, Z, et al.. The associations between birth weight and exposure to fine particulate matter (PM2.5) and its chemical constituents during pregnancy: a meta-analysis. Environ Pollut 2016;211:38–47. https://doi.org/10.1016/j.envpol.2015.12.022.Search in Google Scholar PubMed
94. Li, G, Li, L, Liu, D, Qin, J, Zhu, H. Effect of PM2.5 pollution on perinatal mortality in China. Sci Rep 2021;11:7596. https://doi.org/10.1038/s41598-021-87218-7.Search in Google Scholar PubMed PubMed Central
95. Luben, TJ, Wilkie, AA, Krajewski, AK, Njie, F, Park, K, Zelasky, S, et al.. Short-term exposure to air pollution and infant mortality: a systematic review and meta-analysis. Sci Total Environ 2023;898:165522. https://doi.org/10.1016/j.scitotenv.2023.165522.Search in Google Scholar PubMed PubMed Central
96. Nazarpour, S, Poursani, AS, Simbar, M, Yarandi, RB. The relationship between air pollution and infant mortality rate. Iran J Public Health 2023;52:1278–88. https://doi.org/10.18502/ijph.v52i6.12994.Search in Google Scholar PubMed PubMed Central
97. Woodruff, TJ, Darrow, LA, Parker, JD. Air pollution and postneonatal infant mortality in the United States, 1999-2002. Environ Health Perspect 2008;116:110–5. https://doi.org/10.1289/ehp.10370.Search in Google Scholar PubMed PubMed Central
98. Morgan, ZEM, Bailey, MJ, Trifonova, DI, Naik, NC, Patterson, WB, Lurmann, FW, et al.. Prenatal exposure to ambient air pollution is associated with neurodevelopmental outcomes at 2 years of age. Environ Health 2023;22:11. https://doi.org/10.1186/s12940-022-00951-y.Search in Google Scholar PubMed PubMed Central
99. Capelo, R, Rohlman, DS, Jara, R, García, T, Viñas, J, Lorca, JA, et al.. Residence in an area with environmental exposure to heavy metals and neurobehavioral performance in children 9-11 Years old: an explorative study. Int J Environ Res Publ Health 2022;19. https://doi.org/10.3390/ijerph19084732.Search in Google Scholar PubMed PubMed Central
100. Heng, YY, Asad, I, Coleman, B, Menard, L, Benki-Nugent, S, Hussein, WF, et al.. Heavy metals and neurodevelopment of children in low and middle-income countries: a systematic review. PLoS One 2022;17:e0265536. https://doi.org/10.1371/journal.pone.0265536.Search in Google Scholar PubMed PubMed Central
101. Amadi, CN, Orish, CN, Frazzoli, C, Orisakwe, OE. Association of autism with toxic metals: a systematic review of case-control studies. Pharmacol Biochem Behav 2022;212:173313. https://doi.org/10.1016/j.pbb.2021.173313.Search in Google Scholar PubMed
102. Zhou, S, Collier, S, Xu, J, Mei, F, Wang, J, Lee, Y-N, et al.. Influences of upwind emission sources and atmospheric processing on aerosol chemistry and properties at a rural location in the Northeastern U.S. J Geophys Res Atmos 2016;121:6049–65. https://doi.org/10.1002/2015jd024568.Search in Google Scholar
103. U.S. EPA. Good neighbor plan for 2015 ozone NAAQS; 2024. Available from: https://www.epa.gov/Cross-State-Air-Pollution/good-neighbor-plan-2015-ozone-naaqs [Accessed 19 September 2024].Search in Google Scholar
104. U.S. EPA. Power plants and neighboring communities; 2024. Available from: https://www.epa.gov/power-sector/power-plants-and-neighboring-communities [Accessed 31 July 2024].Search in Google Scholar
105. Burney, JA. The downstream air pollution impacts of the transition from coal to natural gas in the United States. Nat Sustain 2020;3:152–60. https://doi.org/10.1038/s41893-019-0453-5.Search in Google Scholar
106. Martenies, SE, Akherati, A, Jathar, S, Magzamen, S. Health and environmental justice implications of retiring two coal-fired power plants in the southern front range region of Colorado. GeoHealth 2019;3:266–83. https://doi.org/10.1029/2019gh000206.Search in Google Scholar
107. Weng, Z, Song, Y, Cheng, C, Tong, D, Xu, M, Wang, M, et al.. Possible underestimation of the coal-fired power plants to air pollution in China. Resour Conserv Recycl 2023;198:107208. https://doi.org/10.1016/j.resconrec.2023.107208.Search in Google Scholar
108. Mailloux, NA, Abel, DW, Holloway, T, Patz, JA. Nationwide and regional PM2.5-related air quality health benefits from the removal of energy-related emissions in the United States. GeoHealth 2022;6:e2022GH000603. https://doi.org/10.1029/2022gh000603.Search in Google Scholar PubMed PubMed Central
109. Do, TN, Burke, PJ. Phasing out coal power in a developing country context: insights from Vietnam. Energy Policy 2023;176:113512. https://doi.org/10.1016/j.enpol.2023.113512.Search in Google Scholar
110. European Commission. The Just Transition Mechanism: making sure no one is left behind; 2024. Available from: https://commission.europa.eu/strategy-and-policy/priorities-2019-2024/european-green-deal/finance-and-green-deal/just-transition-mechanism_en [Accessed 19 September 2024].Search in Google Scholar
111. Li, J, Zhou, S, Wei, W, Qi, J, Li, Y, Chen, B, et al.. China’s retrofitting measures in coal-fired power plants bring significant mercury-related health benefits. One Earth 2020;3:777–87. https://doi.org/10.1016/j.oneear.2020.11.012.Search in Google Scholar
112. Tsai, J-H, Chen, S-H, Chen, S-F, Chiang, H-L. Air pollutant emission abatement of the fossil-fuel power plants by multiple control strategies in Taiwan. Energies 2021;14. https://doi.org/10.3390/en14185716.Search in Google Scholar
113. EPA. BAT guidance note on best available techniques for the energy sector (large combustion plant sector), 1 ed. Wexford: Environmental Protection Agency; 2008.Search in Google Scholar
114. Harrington, W, Morgenstern, R, Shih, J-S, Bell, ML. Did the clean air Act Amendments of 1990 really improve air quality? Air Qual Atmos Health 2012;5:353–67. https://doi.org/10.1007/s11869-012-0176-5.Search in Google Scholar
115. U.S. EPA. The benefits and costs of the clean air Act from 1990 to 2020; 2024. Second Prospective Study: Available from: https://www.epa.gov/clean-air-act-overview/benefits-and-costs-clean-air-act-1990-2020-second-prospective-study [Accessed 19 September 2024].Search in Google Scholar
116. Liu, Y, Zhou, Y, Lu, J. Exploring the relationship between air pollution and meteorological conditions in China under environmental governance. Sci Rep 2020;10:14518. https://doi.org/10.1038/s41598-020-71338-7.Search in Google Scholar PubMed PubMed Central
Supplementary Material
This article contains supplementary material (https://doi.org/10.1515/reveh-2024-0173).
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- Editorials
- A changing of the guard at Reviews on Environmental Health
- Rachel Carson and Theo Colborn: two pioneering women of science opposing an epoch of toxic entanglement
- Reviews
- Impacts of PM2.5 on stillbirth and the potential mechanism: a narrative review
- Radiofrequency radiation-induced gene expression
- Prevalence of foodborne pathogens in ready-to-eat foods: systematic review, meta-analysis and meta-regression
- m6A methylation – a new target of metabolic diseases induced by environmental pollutants
- Genetic susceptibility to adverse arsenic-related cardiometabolic outcomes: a systematic review
- Potential effects of MPs and their co-pollutants on human intestinal tract
- Fine particulate matter exposure and cancer risk: a systematic review and meta-analysis of prospective cohort studies
- Prenatal exposure to bisphenol-A and neurocognitive changes in children aged 2 to 5 years: a systematic review
- Exposure to air pollution from coal-fired power plants and impacts on human health: a scoping review
Articles in the same Issue
- Frontmatter
- Editorials
- A changing of the guard at Reviews on Environmental Health
- Rachel Carson and Theo Colborn: two pioneering women of science opposing an epoch of toxic entanglement
- Reviews
- Impacts of PM2.5 on stillbirth and the potential mechanism: a narrative review
- Radiofrequency radiation-induced gene expression
- Prevalence of foodborne pathogens in ready-to-eat foods: systematic review, meta-analysis and meta-regression
- m6A methylation – a new target of metabolic diseases induced by environmental pollutants
- Genetic susceptibility to adverse arsenic-related cardiometabolic outcomes: a systematic review
- Potential effects of MPs and their co-pollutants on human intestinal tract
- Fine particulate matter exposure and cancer risk: a systematic review and meta-analysis of prospective cohort studies
- Prenatal exposure to bisphenol-A and neurocognitive changes in children aged 2 to 5 years: a systematic review
- Exposure to air pollution from coal-fired power plants and impacts on human health: a scoping review

