Abstract
The imbalance between supply and demand for bauxite resources, coupled with inadequate refining process compatibility, poses significant challenges in fulfilling the raw material requirements for the Bayer process, thereby severely hindering the sustainable development of the aluminum industry in China. Consequently, low alumina-to-silica ratio bauxite necessitates pretreatment to enhance its quality, ensuring compliance with the feedstock specifications of the Bayer process. Flotation technology, emerging as an efficacious desilication pretreatment approach, has garnered considerable attention and demonstrated substantial application potential in bauxite desilication. This study comprehensively analyses the chemical composition and mineralogical characteristics of bauxite, systematically elucidating and contrasting the advantages of direct and reverse flotation collectors and auxiliary reagents. Furthermore, it delves into the distinct mechanisms of action these reagents exhibit with diaspore and aluminosilicate minerals. Building upon this foundation, the study offers insights and projections for future research endeavours in bauxite flotation desilication, which holds profound theoretical significance in addressing the trend of depleting bauxite resources in China.
1 Introduction
Aluminum is an essential raw material for national economic development and advancements in science and technology. Renowned for its lightweight properties, ductility, exceptional corrosion resistance, and electrical conductivity, aluminum has emerged as a critical metal, second only to steel and iron, finding widespread applications across aerospace, construction, electronics and electrical engineering, the chemical industry, transportation, packaging, machinery manufacturing, shipbuilding (specifically in aluminum alloys), medical equipment, and other sectors (Guo et al. 2023; Sun 2023; Zeng et al. 2025; Zhang et al. 2024b). Estimates suggest that to achieve substantial modernization by 2035, China will necessitate a staggering 495 million tons of primary aluminum as foundational support (Zhang et al. 2022d). Alumina, the primary precursor for metallic aluminum, witnessed a remarkable production volume of 82.38 million tons in China by the end of 2023, accounting for an impressive 58.05 % of the global total (Figure 1a) (Liu et al. 2024). However, China’s reliance on imported alumina remains alarmingly high, surpassing 60 % (Zhang et al. 2024a). Consequently, amidst the swift economic growth in China, the escalating consumption of aluminium is poised to continually widen the demand-supply gap for alumina.
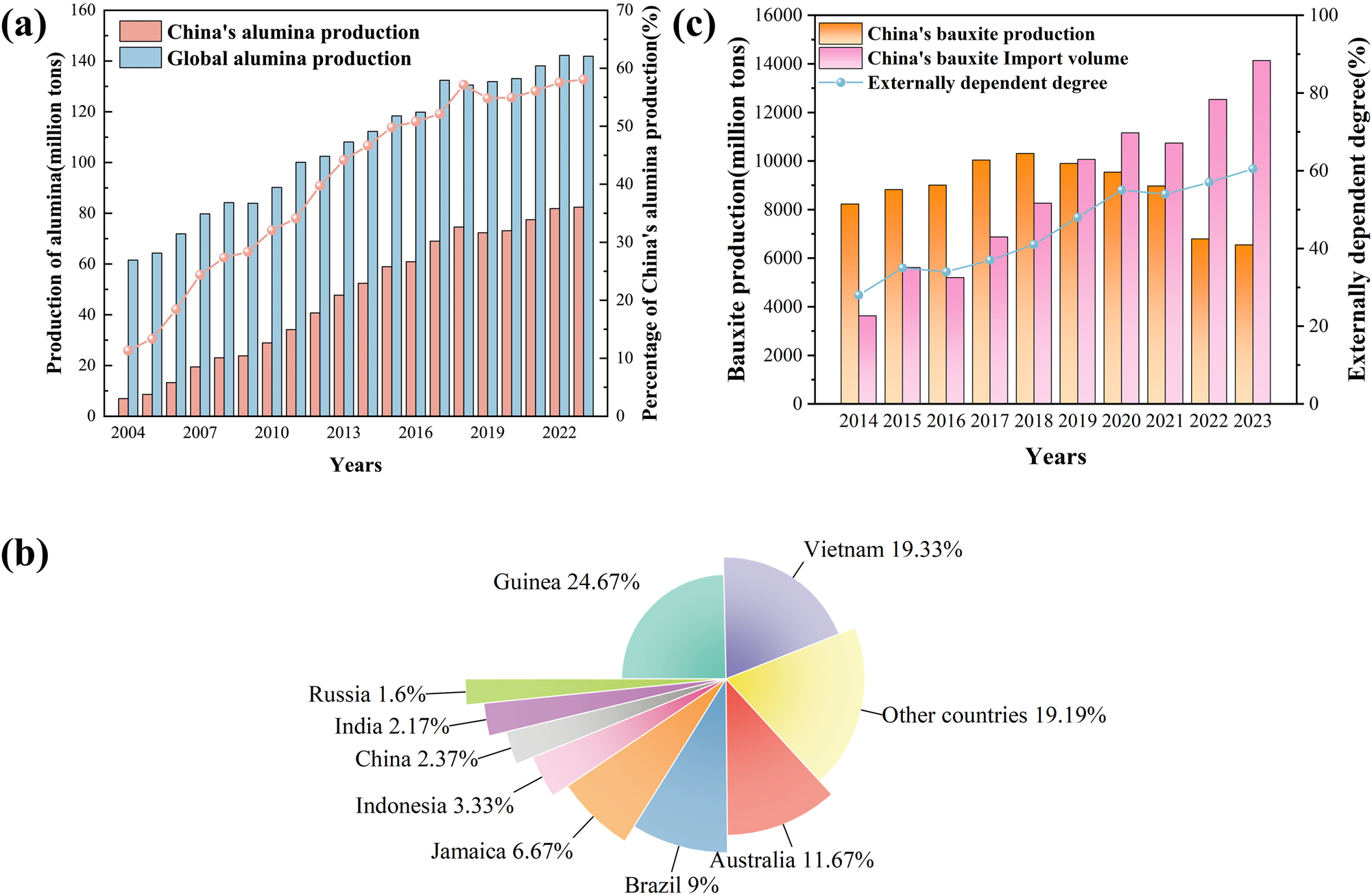
Bauxite resource situation. (a) The alumina production trends in China and globally spanning from 2004 to 2023. Reprinted with permission from Elsevier (copyright 2024) (Liu et al. 2024). (b) The reserves of bauxite in key countries and regions across the globe. Reprinted with permission from China Mining Association (copyright 2024) (Chen 2024). (c) The dynamics of bauxite production, imports, and the degree of external dependency in China between 2014 and 2023. Reprinted with permission from INFO (copyright 2024) (INFO 2024).
Bauxite is the cornerstone raw material for alumina production and is pivotal among China’s scarce and strategically significant minerals. In 2023 the global bauxite reserves totaled 30 billion tons, marking an abundant resource base (Survey 2024). According to the China Mineral Resources Report, by the end of 2023, China’s bauxite reserves amounted to 707.52 million tons, accounting for a mere 2.37 % of the world’s total reserves (Figure 1b) (Chen 2024). Additionally, China is grappling with increasingly acute supply-demand mismatches for bauxite, with production falling far short of domestic demand. Consequently, China is the world’s largest importer and consumer of bauxite (Chen et al. 2025), characterized by a significant external dependency rate of 65 %. Notably, imports of bauxite have surpassed 100 million tons annually for five consecutive years since 2019 (Figure 1c), with a persistent upward trend (INFO 2024). Hence, the inherent supply-demand imbalance in China’s bauxite resources poses a significant challenge to the sustainable development of the aluminum industry.
The Bayer process constitutes the predominant methodology for the industrial synthesis of alumina from bauxite (Zhang et al. 2017b). It primarily involves leaching alumina from bauxite with caustic soda solution to obtain sodium aluminate solution, which is then processed through a series of treatments, such as seed addition and calcination, to produce alumina products (Peng et al. 2018). This method is noted for its simple process, low energy consumption, and high product quality (Wang et al. 2025). Typically, the Bayer process necessitates raw materials exhibiting an alumina-to-silica ratio (A/S ratio) exceeding 7 (Wu et al. 2024a). Notably, elevated sulfur content within bauxite can induce various detrimental consequences, including augmented alkali consumption, compromised alumina product quality, accelerated corrosion of steel equipment, and heightened evaporation energy consumption (Hao et al. 2024). Unfortunately, the overall quality of China’s bauxite is suboptimal, characterized by a preponderance of difficult-to-process diaspore with a relatively low A/S ratio (ranging from 4 to 6) and significant impurities such as iron and sulfur (Han et al. 2019). Consequently, to fulfill the material prerequisites of the Bayer process, beneficiation techniques must be implemented for the desilication pretreatment of bauxite. Among these, flotation desilication is the most prevalent technology in bauxite pretreatment processes, distinguished by its straightforward process flow, low energy consumption, and well-established technology base. Consequently, the research has increasingly shifted towards flotation for silicon removal from bauxite (Wu et al. 2024b). In most investigative endeavours, flotation desilication is broadly categorized into direct and reverse flotation (Zhang et al. 2017a). Flotation represents a multifaceted interfacial separation process, with many factors being examined thus far, including the classification and dosage of flotation reagents, pulp pH, and mineral particle size, which collectively influence mineral flotation outcomes. Notably, adopting an appropriate flotation reagent regimen can substantially elevate the flotation indices of bauxite. Consequently, a comprehensive understanding of the flotation reagents employed in bauxite flotation processes is indispensable.
In light of the unique characteristics of China’s bauxite resources, this study delves into a concise analysis of bauxite beneficiation technology. It provides a comprehensive overview of the types and mechanisms of flotation reagents utilized in the flotation desilication process of bauxite, encompassing collectors, depressants, and ancillary reagents. This provides a reference perspective for researchers on how to choose suitable flotation reagents. It has important theoretical and practical significance for promoting the efficient development and utilization of bauxite in China and improving resource security capabilities.
2 Chemical composition and mineral composition of bauxite
Bauxite is broadly defined as a class of ore that can be industrially utilized and primarily consists of minerals such as gibbsite and diaspore (Zhang et al. 2021a). Depending on its primary mineral composition, bauxite can be categorized into three types: diaspore, boehmite, and gibbsite (Peng 2024). The majority of China’s bauxite resources belong to the diaspore type, accounting for over 90 % of the country’s total bauxite reserves and being the most extensively researched type domestically. However, the processing of diaspore-type bauxite in China poses significant challenges, requiring substantial energy consumption and often involving ore of low quality (Zhang et al. 2023). It is primarily due to the compact arrangement of diaspore crystal grains, which acts as a barrier to the penetration of alkaline solutions into the interior of the mineral grains for effective reaction (Sun 2024).
The primary constituents of bauxite include alumina (Al2O3), silicon dioxide (SiO2), iron oxide (Fe2O3), and titanium dioxide (TiO2), along with minor amounts of calcium oxide (CaO), magnesium oxide (MgO), sulfides, and trace quantities of Ga, V, P, Cr, and other elements. Table 1 provides an overview of the primary chemical compositions of bauxite sourced from various regions in China. The chemical composition of bauxite exhibits substantial variation across different regions, with the content of Al2O3 fluctuating between approximately 50 % and 65 %, SiO2 content exceeding 10 %, and TiO2 content exceeding 2 %. Iron in bauxite primarily exists in iron oxide and iron sulfide, and the removal of iron and enrichment of aluminum can be effectively achieved through combined magnetic separation techniques. For instance, Wang et al. utilized a suspension magnetization roasting-magnetic separation process to treat high-iron bauxite, achieving an impressive iron removal rate of 65.63 % (Wang et al. 2021).
Primary chemical composition of bauxite from various regions in China (wt/%).
| Region | Primary chemical composition | A/S ratio | ||||||
|---|---|---|---|---|---|---|---|---|
| Al2O3 | SiO2 | Fe2O3 | TiO2 | CaO | MgO | S | ||
| Guizhou (Peng 2024) | 55.69 | 14.27 | 5.68 | 2.13 | 2.00 | 1.69 | 1.71 | 3.90 |
| Guizhou (Li 2024) | 60.97 | 11.54 | 6.46 | 3.10 | 0.90 | 0.51 | 3.35 | 5.28 |
| Guizhou (Xiong 2021a) | 53.73 | 10.99 | 19.95 | 8.21 | – | – | 5.08 | 4.89 |
| Henan (Qiu 2020) | 55.72 | 24.65 | 1.78 | 2.43 | 0.82 | – | – | 2.26 |
| Henan (Li 2016) | 55.20 | 11.89 | 7.47 | 2.88 | 0.42 | – | 1.61 | 4.64 |
| Henan (Yan 2021) | 57.33 | 13.27 | 10.64 | 3.76 | 1.46 | 0.42 | 0.94 | 4.32 |
| Shanxi (Sun 2024) | 62.92 | 16.72 | 3.55 | 2.37 | 0.91 | 0.15 | – | 3.76 |
| Shanxi (Du et al. 2024) | 64.11 | 11.12 | 4.32 | 2.66 | 0.85 | 0.32 | 1.27 | 5.77 |
| Shanxi (Su et al. 2023) | 50.69 | 14.29 | 8.86 | 1.99 | 2.27 | 1.02 | 2.65 | 3.55 |
| Yunnan (Xiong 2021b) | 51.72 | 11.69 | 18.16 (TFe) | 4.68 | – | – | – | 4.42 |
| Guangxi (Li 2022b) | 48.30 | 10.01 | 24.23 | 3.12 | – | – | – | 4.83 |
| Guangxi (Zhang et al. 2024c) | 50.47 | 5.98 | 13.66 (TFe) | 3.24 | 0.09 | 0.04 | 16.24 | 8.44 |
| Guangxi (Wu et al. 2025) | 34.31 | 4.05 | 42.41 | 3.03 | – | – | – | 8.47 |
-
Reproduced with permission from the references listed in the Table.
The mineralogical composition of China’s bauxite deposits exhibits considerable complexity, with diaspore predominating as the primary mineral constituent, frequently accompanied by an assortment of accessory minerals such as kaolinite, illite, montmorillonite, chlorite, rutile, anatase, and pyrite (Liu and Cheng 2022). A detailed overview of the mineralogical compositions of prominent bauxite mining areas in China is provided in Table 2. Notably, China’s bauxite resources are characterized by their high alumina and silica contents, relatively low A/S ratio, and frequent occurrence of iron and sulfur impurities. During the production of alumina, the presence of impurities such as silicon, iron, and sulfur can exert a significant influence on the quality of the final product while also augmenting the challenges associated with beneficiation and smelting processes, thereby escalating energy consumption and production costs. Specifically, silicon reacts with sodium aluminate to form insoluble sodium silicate slag, which not only results in aluminum loss but also diminishes aluminum products’ strength and elasticity (Zhong 2024). Additionally, sulfur reacts with alkaline solutions to generate sodium silicate, elevating alkaline consumption. The accumulation of sulfur during the production process accelerates the corrosion of steel equipment, adversely affects evaporation and red mud settlement, and impedes the progression of numerous production procedures (Wang et al. 2024). Furthermore, the complexity of iron-containing phases, particularly when iron exists in the form of goethite, exacerbates the difficulties associated with the separation and settlement of red mud, increases the yield of red mud, and diminishes the production efficiency of alumina (Li et al. 2024). Hence, the A/S ratio and impurity content of the feedstock utilized in alumina production are pivotal factors that determine the feasibility and economic viability of the production process.
An overview of the primary mineral composition of bauxite in different regions of China (wt%).
| Region | Primary mineral composition |
|---|---|
| Guizhou (Peng 2024) | Diaspore (54.00), kaolinite (6.50), muscovite (22.26), pyrite (3.64), anatase (2.20) |
| Guizhou (Li 2024) | Diaspore (72.88), illite (7.10), sericite (5.62), kaolinite (1.84), pyrite (5.37), anatase (1.16) |
| Guizhou (Zhai 2024) | Diaspore (60.6), kaolinite (30.2), quartz (1.5), siderite (1.9), anatase (2.4) |
| Henan (Li 2016) | Diaspore (53.88), kaolinite (23.78), quartz (2.50), siderite (8.52), anatase (2.88), pyrite (2.08) |
| Henan (Yan 2021) | Diaspore (48.2), boehmite (6.1), chlorite (12.1), kaolinite (16.3), siderite (3.1), anatase (2.7), pyrite (2.3) |
| Shanxi (Sun 2024) | Diaspore (63.4), kaolinite (31.2), anatase (2.3), hematite (0.9), calcite (2.2) |
| Shanxi (Du et al. 2024) | Diaspore (64.50), kaolinite (15.50), chlorite (5.10), illite (4.50), anatase (2.20), pyrite (2.40) |
| Shanxi (Su et al. 2023) | Diaspore (43.6), kaolinite (22.13), illite (10.87), siderite (8.6), anatase (1.50), pyrite (4.4) |
| Guangxi (Li 2022b) | Diaspore (47), kaolinite (22), anatase (3), hematite (24) |
| Guangxi (Yu 2020) | Diaspore (53.36), kaolinite (1.88), limonite (23.44), goethite (5.95), quartz (1.25), chlorite (0.64) |
| Guangxi (Wei et al. 2023) | Diaspore (50), kaolinite (2), chlorite (33), rutile (4), pyrite (4) |
-
Reproduced with permission from the references listed in the Table.
3 Bauxite direct flotation reagents
Direct flotation techniques are widely applied to the desilication of bauxite, achieved through the strategic addition of flotation reagents. These reagents augment the hydrophobicity of diaspore and concurrently mitigate the flotation tendencies of aluminosilicate minerals, ultimately resulting in an elevated A/S ratio within the bauxite. A pivotal determinant of the desilication efficacy via flotation, as observed in numerous studies, is the choice of the flotation reagent system, with a particular emphasis on the type of reagent employed. This emphasis arises from the considerable variability in mineralogical compositions exhibited by bauxite across diverse geographical locations. The judicious selection of flotation reagents can amplify the differences in flotability between diaspore and gangue minerals, thereby substantially enhancing the desilication efficiency of bauxite. Extensive research endeavours have been undertaken to ascertain the most efficacious set of conditions for optimizing flotation performance, encompassing a range of reagents such as collectors, depressants, and auxiliary reagents.
3.1 Collectors
In the process of direct flotation for bauxite, a variety of collectors are employed, among which prominent are anionic, chelating, and combined collectors.
3.1.1 Anionic collectors
Anionic collectors have been extensively utilized, including fatty acids and their soaps, sulfonates, and phosphates.
In fatty acids and their soaps, oleic acid, sodium oleate, oxidized paraffin soap, and tall oil are commonly used. Sodium oleate, in particular, stands out as the most prevalent collector in direct flotation of bauxite due to its cost-effectiveness and robust collecting properties. Its structural formula is depicted in Figure 2a. However, oleic acid collectors are less selective because oleate ions are mainly adsorbed on the mineral surface through hydrogen bonding (Wang 2023; Wang et al. 2023). Recent advancements in research have revealed that oleic acid collectors with varying iodine values exhibit different collecting efficiencies and selectivities. This correlation was attributed to the content of linoleic acid, which had a smaller frontier molecular orbital energy difference compared to oleic acid, imparting it with higher chemical reactivity and more stable chemical adsorption capabilities (Wang et al. 2017). As a result, linoleic acid demonstrated enhanced collecting ability and selectivity.
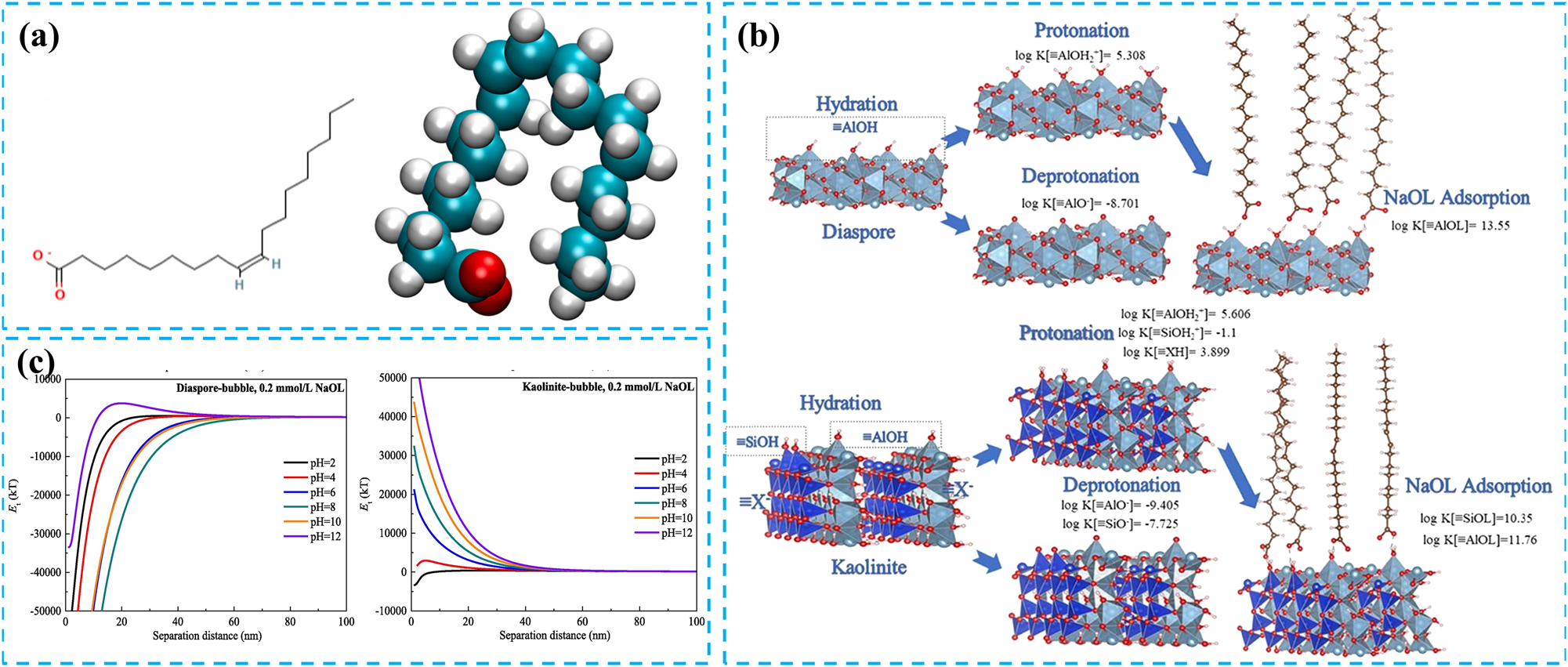
The influence of different conditions on mineral surface adsorption. (a) Planar and three-dimensional structural representations of OLL. Reprinted with permission from Elsevier (copyright 2023) (Wang et al. 2023). (b) Adsorption capacity of sodium oleate on mineral surfaces, as calculated using a surface complexation model. Reprinted with permission from Elsevier (copyright 2022) (Gao et al. 2022). (c) Influence of pulp pH on the DLVO interaction energy between diaspore and kaolinite particles and bubbles. Reprinted with permission from Elsevier (copyright 2022) (Zhang et al. 2022a).
The collecting capacity of oleic acid-based collectors tends to augment with an increase in temperature; however, their significant chemical adsorption on the surface of diaspore is confined to higher temperatures. Notably, the Al sites serve as the primary chemical adsorption points for oleate ions on diaspore and aluminosilicate mineral surfaces. Consequently, the differential abundance of active Al sites on these mineral surfaces dictates the collecting capacity of sodium oleate towards them (Xu et al. 2014). To quantify the adsorption reactions of sodium oleate on the active sites of diaspore and kaolinite, a surface complexation model (SCM) has been utilized for simulating complexation adsorption (Figure 2b). The adsorption equilibrium constants for ≡AlOL on diaspore and ≡AlOL and ≡SiOL on kaolinite are determined to be 1013.55, 1011.76, and 1010.35, respectively, with a discrepancy spanning nearly two orders of magnitude (Gao et al. 2022). Hence, sodium oleate demonstrated a superior collecting capacity for diaspore compared to kaolinite. Moreover, during the direct flotation of bauxite utilizing sodium oleate as a collector, the separation efficiency between diaspore and kaolinite in alkaline environments typically surpasses that in acidic environments (Zhang et al. 2022a). This was attributed to the significantly more significant difference in interaction forces between diaspore-bubble and kaolinite-bubble pairs in alkaline conditions (Figure 2c).
The limitations in selectivity and solubility associated with fatty acid-based collectors can be effectively mitigated through modification treatments. Li et al. conducted flotation tests on bauxite with a low A/S ratio using modified fatty acid soap YZ-3 as the collector, in conjunction with sodium hexametaphosphate and sodium silicate as depressants (Li 2022a). The A/S ratio of the bauxite was elevated from 3.62 to 9.20, accompanied by an Al2O3 concentrate recovery rate of 70.35 %, indicating good flotation performance. Yang et al. employed a modified combination of fatty acids KYB for flotation experiments on low-grade bauxite, ultimately achieving remarkable results with an A/S ratio of 9.79 and an Al2O3 concentrate recovery rate of 81.62 % (Yang et al. 2022).
Compared to fatty acid-based collectors, sulfonate-based collectors exhibit superior foaming properties, water solubility, and resistance to hard water conditions, albeit with slightly weaker collecting ability. Based on the variation in the number of carbon atoms within their structure, sulfonate-based collectors can be categorized into water-soluble and oil-soluble types. Notably, sulfonate-based collectors with fewer than 20 carbon atoms possess good water solubility but weaker collecting ability. In contrast, those with more carbon atoms demonstrate stronger collecting capacity. However, recent research on bauxite flotation using sulfonate-based collectors has been scarce. This scarcity may be attributed to the reduced electrostatic reactivity of sulfonate-based collectors in aqueous environments and their challenges in effectively separating diaspore and kaolinite (Chen 2007). Table 3 presents a compilation of recent anionic collectors employed in the direct flotation of bauxite.
Flotation indexes for anionic collectors in direct flotation of bauxite.
| Collectors | Collector dosage (g/t) | Flotation pH | Al2O3 concentrate recovery (%) | A/S ratio | |
|---|---|---|---|---|---|
| Raw ore | Concentrate | ||||
| 131OL (Wang et al. 2017) | 800 | 10.5 | 92.65 | 3.39 | 4.03 |
| 121OL (Wang et al. 2017) | 800 | 10.5 | 87.92 | 3.39 | 3.82 |
| 65OL (Wang et al. 2017) | 800 | 10.5 | 83.80 | 3.39 | 3.48 |
| Linoleic acid (Yang et al. 2022) | 1,000 | – | 53.19 | 4.21 | 7.06 |
| Tall oil (Yang et al. 2022) | 1,000 | – | 56.60 | 4.21 | 6.34 |
| NaOL (Sun et al. 2022) | 2,000 | 9 | 70.00 | 4.75 | 10.68 |
| NaOL (Jiang et al. 2011) | 800 | 7 | 80.30 | 4.18 | 6.71 |
| OL (Deng et al. 2016) | 1,000 | 10.07 | 80.93 | 5.55 | 11.94 |
| YZ-3 (Li 2022a) | 1,200 | 9 | 70.35 | 3.62 | 9.20 |
| KYB (Yang et al. 2022) | 1,000 | – | 81.62 | 4.21 | 9.79 |
| HXC (Huo et al. 2018) | 700 | – | 75.96 | 4.14 | 7.02 |
-
Reproduced with permission from the references listed in the Table.
3.1.2 Chelating collectors
In addition to anionic collectors, chelating collectors stand out as viable options for directly flotation bauxite. Table 4 presents a comprehensive overview of the research on chelating collectors employed in this context. Predominantly, chelating collectors are centered around hydroxamic acids (Liu et al. 2013). For example, 2-[tris(hyroxycarbamoyl)methyl] decanoic acid (THDA) (Jiang and Li 2013), 2-[tris(hyroxycarbamoyl)methyl] dodecanoic acid (THCA), and 2-[tris(hyroxycarbamoyl)methyl] tetradecanoic acid (THTA), pival hydroxamic acid (Liu et al. 2018), 3,3-bis(hydroxycarbamoyl) undecanoic acid (BHUA). Compared to anionic collectors, chelating collectors can form chelates with active sites on the mineral surface, enhancing their selectivity and collecting efficiency. Meanwhile, it has a poor trapping ability for aluminosilicate minerals (Sun et al. 2023). Jiang et al. undertook flotation separation experiments utilizing synthetic novel alkyl bis(hydroxycarbamoyl) propionic acids as collectors for bauxite (Jiang et al. 2012). Their findings revealed that these hydroxamic acid collectors exhibited superior collecting performance for diaspore compared to aluminosilicate minerals. Consequently, hydroxamic acid collectors have gained widespread adoption in the direct flotation of bauxite.
Flotation indexes for chelating collectors in direct flotation of bauxite.
| Collectors | Collector dosage (g/t) | Flotation pH | Al2O3 concentrate recovery (%) | A/S ratio | |
|---|---|---|---|---|---|
| Raw ore | Concentrate | ||||
| AHA-10 (Deng et al. 2016) | 400 | 10 | 86.42 | 5.55 | 12.51 |
| BHUA (Jiang et al. 2012) | 800 | 7 | 80.71 | 4.18 | 8.02 |
| HCDA (Jiang et al. 2011) | 800 | 81.12 | 4.18 | 8.01 | |
| Benzohydroxamic acid (Li et al. 2019) | 1,000 | – | 79.12 | 3.34 | 5.27 |
| Alkyl hydroxamic acid (Li et al. 2019) | 1,000 | – | 79.65 | 3.34 | 4.81 |
| NHOD (Deng et al. 2015) | 400 | 7.15 | 88.81 | 5.55 | 11.10 |
| PG (Lyu et al. 2019) | 60 mg/L | 8 | 64 % | 2.51 | 29.7 |
-
Reproduced with permission from the references listed in the Table.
Hydroxamic acid collectors primarily adsorb onto the surface of diaspore through chemical adsorption, while they mainly undergo physical adsorption with aluminosilicate minerals (Liu 2012, Yu-ren et al. 2010). Jiang et al. studied the bauxite flotation process using novel hydroxamic acid collectors OCB, OHB, and OTB (Jiang et al. 2010). Their research revealed that the polar groups –COOH and –NHOH of OCB possess strong coordination abilities, forming ternary chelate rings through covalent bonds with aluminum atoms on the surface of diaspore, which constitutes chemical adsorption. Simultaneously, the hydrogen atoms in the polar groups of OCB form hydrogen bonds (N–H⋯O and O–H⋯O) with oxygen atoms on the surface of aluminosilicate minerals, representing physical adsorption (Figure 3a). Consequently, hydroxamic acid collectors exhibit superior collecting ability for diaspore compared to aluminosilicate minerals. Similarly, Deng et al. demonstrated that the novel hydroxamic acid collector AHA-10 demonstrates excellent collecting ability for diaspore and selectivity towards aluminosilicate minerals (Figure 3b) (Deng et al. 2016). They investigated the FTIR spectra of diaspore, kaolinite, and illite before and after treatment with AHA-10 (Figure 3c). Their findings indicated that characteristic peaks of AHA-10 appeared in diaspore. In contrast, almost no such peaks were observed in kaolinite and illite, suggesting that the adsorption amount of AHA-10 on diaspore is greater than that on kaolinite and illite. Notably, new peaks have appeared on the FTIR spectrum of diaspore. These indicate that AHA-10 is adsorbed on the surface of the diaspore through chemical adsorption, as shown in the adsorption model diagram. During the flotation process of bauxite, the pH of the flotation environment is essential, as it affects the collecting performance of hydroxamic acid collectors. For example, as shown in Figure 3d, the NHOD collector chelated with aluminum atoms on the surface of diaspore through C(O)NHOH or C(O)NH groups to form Al–O coordination bonds at pH 7, resulting in chemical adsorption on the surface of diaspore (Deng et al. 2015). However, when pH > 9, the chelate structure between NHOD and diaspore gradually transformed into a hydrogen bond structure, decreasing the adsorption amount of NHOD on the surface of the diaspore and reducing its collecting performance.
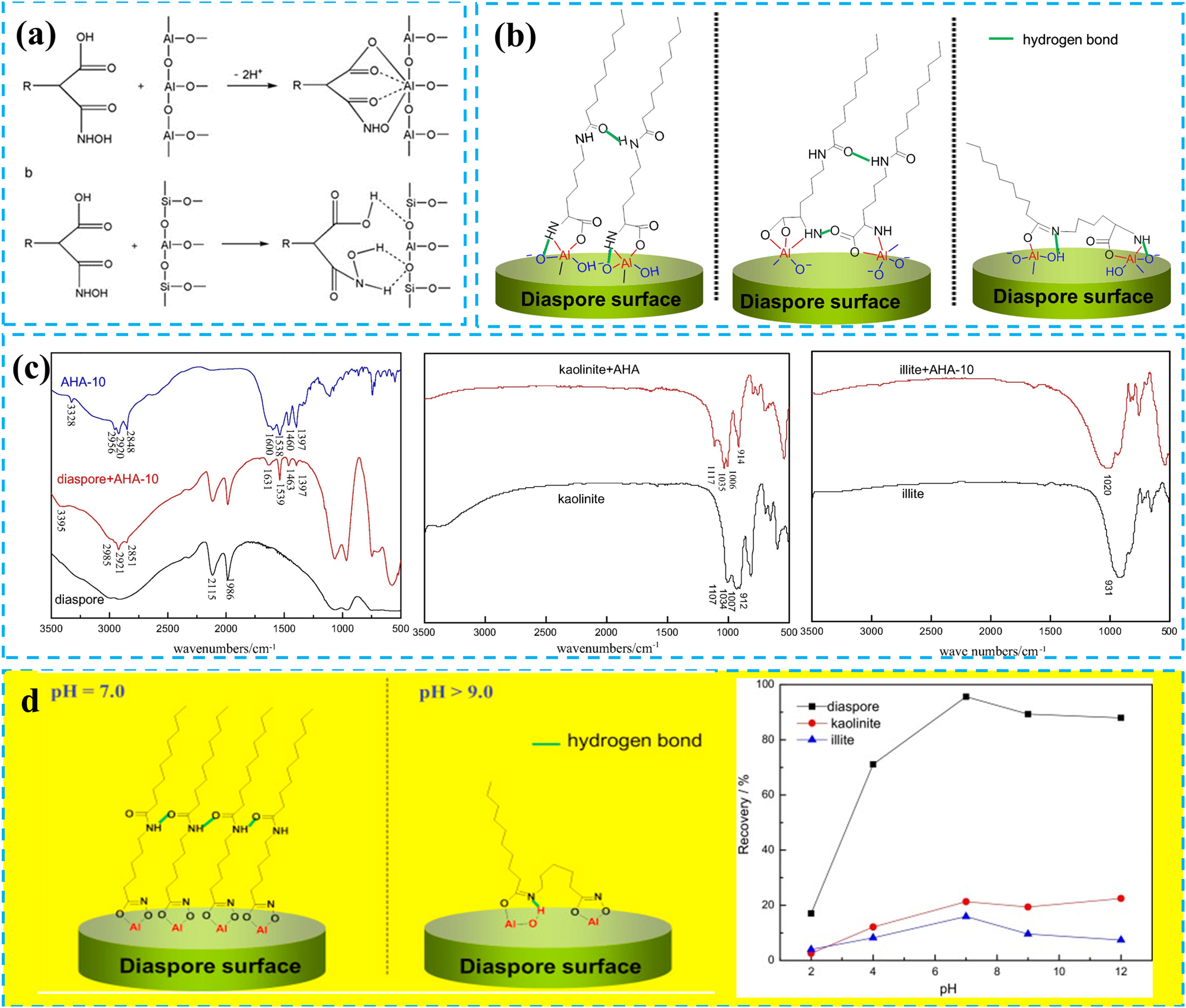
The interaction between hydroxamic acid collectors and minerals. (a) Action mode of carboxyl hydroxamic acid on mineral surfaces. Reprinted with permission from Elsevier (copyright 2010) (Jiang et al. 2010). (b) Schematic diagram of possible adsorption model of AHA-10 on diaspore surface. Reprinted with permission from Elsevier (copyright 2016) (Deng et al. 2016). (c) FTIT spectra of diaspore and aluminosilicate minerals before and after interaction with AHA-10. Reprinted with permission from Elsevier (copyright 2016) (Deng et al. 2016). (d) Effect of pH on the collecting performance of NHOD. Reprinted with permission from Elsevier (copyright 2015) (Deng et al. 2015).
In addition to hydroxamic acid collectors, propyl gallate is a novel chelating collector. Lyu et al. investigated the action mechanism of propyl gallate as a collector on the surface of minerals (Lyu et al. 2019). The study showed that propyl gallate chelates with Al on the surface of the diaspore to form a five-membered ring, adsorbing onto the diaspore surface through chemical adsorption. Meanwhile, propyl gallate barely adsorbed on the surface of kaolinite, exhibiting excellent selectivity.
3.1.3 Combined collectors
Compared to single collectors, combined collectors exhibit some satisfactory properties, including excellent collecting ability and selectivity (Man et al. 2022). This improvement is attributed to the complementary functions achieved through the synergistic effects of various flotation reagents, which enhance the collecting ability and selectivity of the collectors. Consequently, as bauxite quality continues to decline, more researchers are focusing on combined collectors for the flotation of bauxite. Table 5 presents the combined collectors employed in the direct flotation process of bauxite.
Flotation indexes for combined collectors in direct flotation of bauxite.
| Collectors | Collector dosage (g/t) | Flotation pH | Al2O3 concentrate recovery (%) | A/S ratio | |
|---|---|---|---|---|---|
| Raw ore | Concentrate | ||||
| KMY (Yang et al. 2023) | 1,000 | 9 | 70.30 | 2.80 | 7.69 |
| NaOL+BHA (Sun et al. 2022) | 2,000 | 9 | 70.81 | 4.75 | 15.27 |
| KMY (Yang et al. 2023) | 1,000 | 9 | 70.30 | 2.80 | 7.69 |
| NaOL+D-phe (Li et al. 2021) | 1.2 × 10−4 mol/L | 9 | 80.26 | 3.76 | 10.67 |
| NaOL+SLS (Lan et al. 2025) | 2,000 | 9 | – | 4.75 | 9.69 |
| NaOL+SDS (Lan et al. 2025) | 2,000 | 9 | – | 4.75 | 10.33 |
| NaOL+BHA (Lan et al. 2025) | 2,000 | 9 | – | 4.75 | 13.56 |
| VOA+NA+AEP12 (Liu et al. 2016) | 800 | – | 90.22 | 4.99 | 7.08 |
| VOA+NA+AEP12 (Liu et al. 2016) | 800 | – | 92.16 | 4.50 | 7.04 |
| NaOL+TDM (Man et al. 2019) | 0.1 mM | 9 | 83.6 | 3.49 | 11.9 |
| EMB-506 (Dou 2022) | 1,200 | – | 86.66 | 4.35 | 8.53 |
-
Reproduced with permission from the references listed in the Table.
In most studies, enhancing the chemical adsorption of the traditional collector sodium oleate on diaspore has further expanded the wettability difference between diaspore and aluminosilicate minerals. This enhancement significantly improves the alumina-silica ratio of bauxite (Lan 2022; Man et al. 2019). For instance, Sun et al. conducted flotation experiments on bauxite using sodium oleate (NaOL) and benzohydroxamic acid (BHA) as combined collectors (Sun et al. 2022). The results indicated that compared to NaOL, the combined collector demonstrated enhanced collecting performance and selectivity. The improvement increased the alumina-silica ratio and the recovery rate of the final concentrate by 4.59 % and 4.43 %, respectively. Zeta analysis (Figure 4a) revealed that the enhancement was attributed to BHA’s promotion of the chemical adsorption of NaOL on the diaspore surface while concurrently inhibiting its chemical adsorption on the kaolinite surface. Similarly, Li et al. found that compared to flotation of bauxite using a single NaOL collector, the combined collector of d-phenylalanine (D-phe) and NaOL improved the alumina-silica ratio (9.73 → 10.68) and the recovery rate of diaspore (77.13 % → 91.07 %), while reducing the recovery of kaolinite (Li et al. 2021). Mechanism analysis indicated that the synergistic collecting mechanism of the two was primarily through the bonding of the –COO– groups of D-phe and NaOL with the –AlO2 2− or –Al(OH)O– groups on the diaspore surface, as well as the chelation of –CO and –NH2/NH3 + groups with Al (Figure 4b). More importantly, D-phe forms associated molecules with NaOL through hydrogen bonding, which enables NaOL to be indirectly adsorbed on the diaspore surface through D-phe, thereby enhancing the hydrophobic difference between diaspore and aluminosilicate minerals.
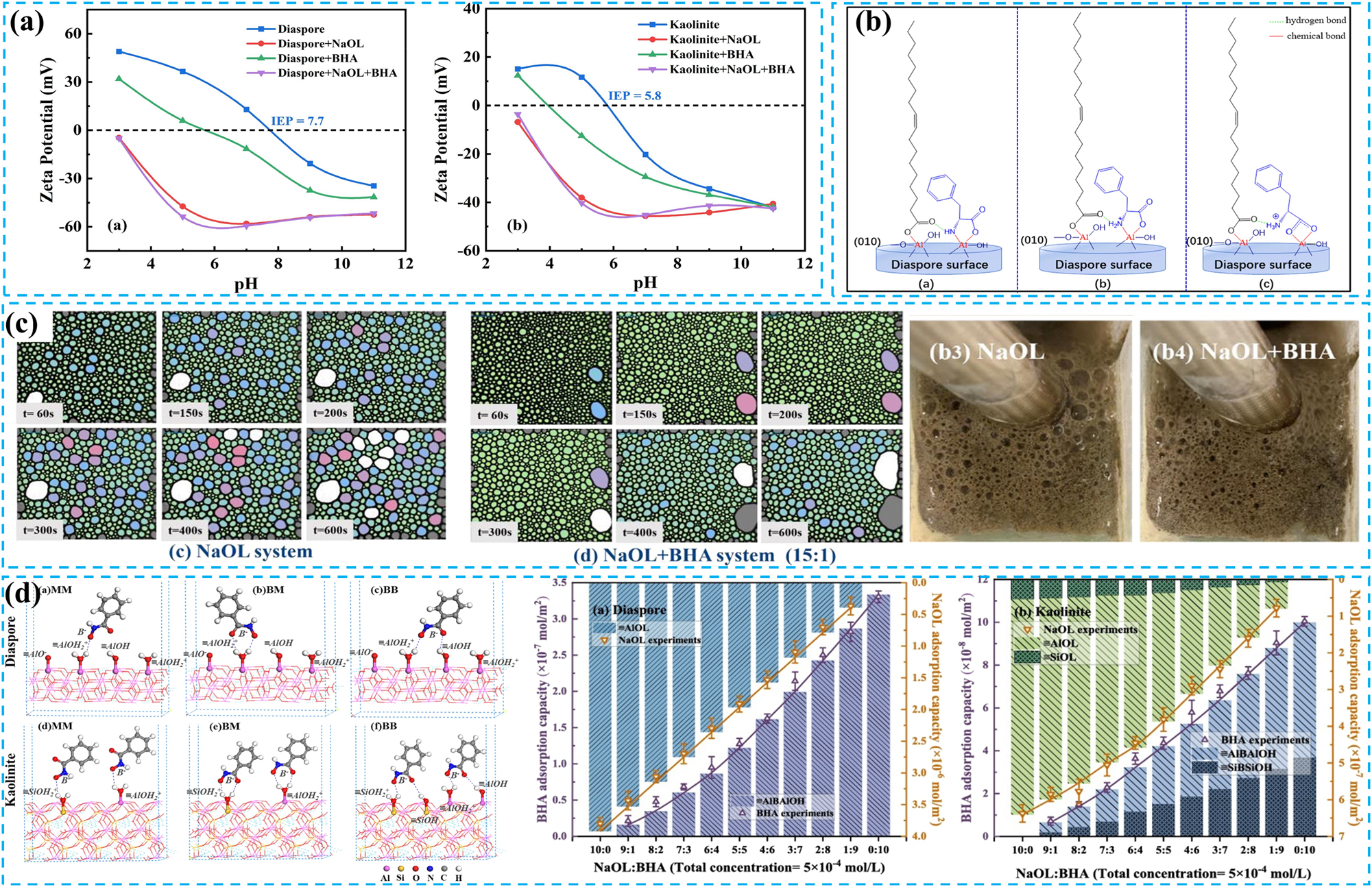
Strengthening the collection effect of sodium oleate. (a) Effect of pH on the ζ-potential of stibnite diaspore and kaolinite in the presence of NaOL/BHA. Reprinted with permission from Elsevier (copyright 2022) (Sun et al. 2022). (b) D Co-adsorption model of D-phe and NaOL on diaspore surface. Reprinted with permission from Elsevier (copyright 2021) (Li et al. 2021). (c) Foam structure of NaOL and NaOL+BHA. Reprinted with permission from Elsevier (copyright 2025) (Lan et al. 2025). (d) The three complex configurations of BHA adsorbed on diaspore and kaolinite and the verification of BB configuration. Reprinted with permission from Elsevier (copyright 2024) (Gao et al. 2024).
Combined collectors have been shown to enhance traditional anionic collectors’ foam performance and bubble quality. The traditional collector NaOL solution has high viscoelasticity, producing thick and durable foam, which is not conducive to bubble formation and flotation. However, adding BHA can significantly reduce the viscoelasticity of the NaOL solution and effectively improve the foam structure (Lan et al. 2025). Notably, the NaOL and BHA system exhibits excellent foam uniformity and smaller bubbles (Figure 4c), improving flotation efficiency.
The synergistic effect and selectivity mechanism of combined collectors can often be explained by changes in the active sites on mineral surfaces, consistent with what is mentioned for anionic collectors. Based on this conclusion, Gao et al. proposed an algorithm to simulate the inherent adsorption constants of BHA on diaspore and kaolinite and introduced three adsorption models (Figure 4d) (Gao et al. 2024). The results indicated that the bidentate binuclear mode aligned well with experimental findings, accurately and fully quantifying the active sites on minerals, thus providing a quantitative perspective for explaining combined collectors’ synergistic effect and selectivity.
3.2 Depressant
To date, in the positive flotation desilication of bauxite, commonly used depressants include sodium hexametaphosphate (SHMP), sodium silicate, and sodium carbonate, which effectively achieve a dispersing effect. Among these, sodium carbonate is primarily utilized to adjust pH levels and can also function as a grinding aid during the grinding stage. Additionally, other depressants such as phosphates, carboxylates, and sodium humate are employed in the direct flotation of bauxite; however, their depressing effects are generally inferior to those of the aforementioned three depressants.
So far, a series of studies have been conducted on using SHMP and water glass as depressants in the direct flotation of bauxite. SHMP exhibits both inhibitory and dispersing effects. Its dispersing effect is achieved by adsorbing onto the active Al sites on the mineral surface, thereby enhancing the negative charge of the mineral surface and causing repulsion between minerals, thus achieving dispersion. SHMP interacts with active Al sites through its PO2 groups and adsorbs onto the mineral surface, thereby inhibiting the adsorption of collectors on the mineral surface and achieving an inhibitory effect (Wang et al. 2007). Although SHMP exhibits a more substantial adsorption capacity on diaspore than kaolinite, the density of active Al sites on the diaspore surface surpasses that of kaolinite. Furthermore, only sure edges of kaolinite possess active Al sites, leading to a complete coverage of the kaolinite surface by a limited amount of SHMP (Figure 5a) (Choi et al. 1993; Zhang et al. 2022b). Therefore, a low dosage of SHMP exhibits a more substantial inhibitory effect on kaolinite than on diaspore. It is worth noting that both SHMP and NaOL adsorb onto the active Al sites on the mineral surface, and SHMP hinders the adsorption of NaOL on the mineral surface, indicating competitive adsorption between the two (Zhang et al. 2001). Therefore, to ensure the alumina–silica ratio and concentrate recovery rate, the dosage of SHMP must be strictly controlled.
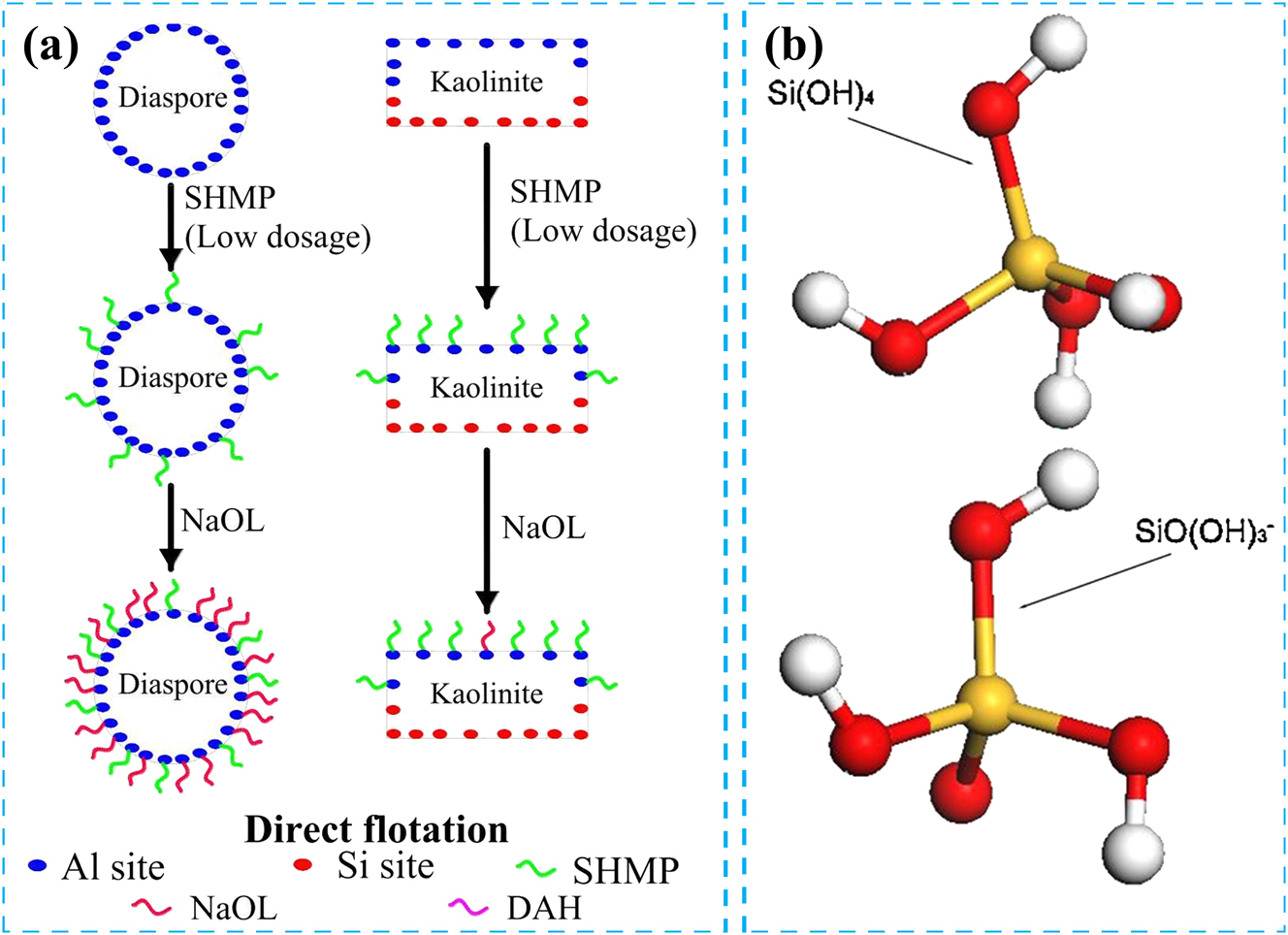
The role of depressant in flotation process. (a) Schematic of the mechanism of action of SHMP on bauxite direct flotation system. Reprinted with permission from Elsevier (copyright 2022) (Zhang et al. 2022b). (b) Hydrolysates of water glass in aqueous solution. Reprinted with permission from Elsevier (copyright 2016) (Han et al. 2016).
Water glass is an effective depressant for the positive flotation of bauxite, exhibiting a notable dispersing effect. This dispersing effect results from the hydrolysis of water glass, which generates negatively charged colloidal particles (Figure 5b) (Han et al. 2016). Negatively charged colloidal particles adsorb onto the surface of aluminosilicate minerals due to silicate groups, increasing the mineral surface’s negative potential and enhancing the mineral surface’s repulsive force, thus achieving a dispersing effect. On the other hand, the silicic acid component is strongly hydrophilic, and water glass forms a layer of silica colloidal polymer on the mineral surface, enhancing its hydrophilicity and achieving a depressing effect. In addition, to augment the depressing effect of water glass, high-valent metal ions are often incorporated into the water glass to increase the proportion of its effective components (Guo 2010).
3.3 Other auxiliary reagents
In the flotation process of bauxite, the pH of the pulp is a critical factor, as it influences the surface electrical properties of minerals and the dissociation degree of collectors. Sodium carbonate, the most commonly used pH adjuster, not only regulates the pulp’s pH but also enhances the negative charge on the surface of kaolinite. This increase in negative charge results in electrostatic repulsion between mineral particles, thereby improving the dispersion of aluminosilicate minerals. Yang et al. utilized sodium carbonate as a pH adjuster and KMY as a collector for the flotation desilication of bauxite characterized by a low alumina-to-silica ratio. The difference in Al2O3 content between the concentrate produced with sodium carbonate and that without its addition was approximately 40 % (Yang et al. 2023). Additionally, sodium carbonate can inhibit the influence of slime on flotation. Generally, slime particles smaller than 0.010 mm can significantly hinder the effectiveness of collectors during flotation. Wang et al. demonstrated that by introducing sodium carbonate prior to the flotation process for bauxite desilication, the recovery rate of Al2O3 in the concentrate increased from 81.93 % to 96.25 % while simultaneously reducing the number of flotation reagents required (Wang et al. 2004). It is worth noting that sodium carbonate can eliminate the negative effects of Ca2+ and Mg2+ during the flotation process. On the one hand, Ca2+ and Mg2+ can influence the dispersion of individual minerals, such as diaspore and kaolinite (Yuhua et al. 2011). On the other hand, Ca2+ and Mg2+ can modify the surface properties of the diaspore, thereby altering the interaction between the mineral and the collector (Figure 6a), affecting the diaspore’s flotation (Fang et al. 2019).
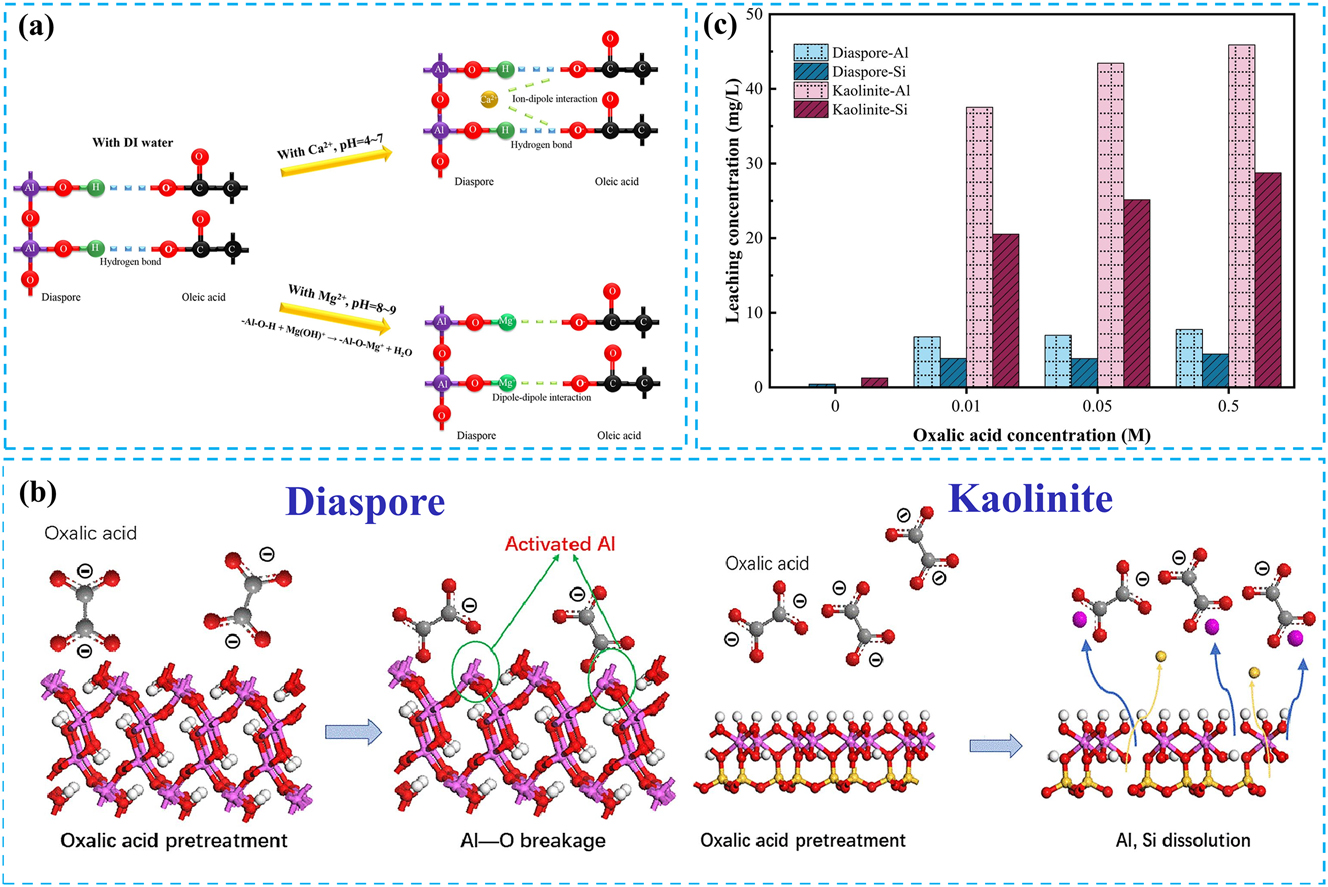
The effect of calcium magnesium ions and oxalic acid pretreatment on mineral floatability. (a) Cationic effect model of the adsorption of collectors in diaspore flotation. Reprinted with permission from Elsevier (copyright 2019) (Fang et al. 2019). (b) Mechanical model of oxalic acid pretreatment of diaspore and kaolinite. Reprinted with permission from Elsevier (copyright 2022) (Li et al. 2022). (c) Effect of oxalic acid on Al and Si dissolution of diaspore and kaolinite. Reprinted with permission from Elsevier (copyright 2022) (Li et al. 2022).
Acid treatment of the bauxite surface before flotation can effectively enhance flotation performance. For instance, Li et al. pretreated bauxite with oxalic acid, leading to an 8.50 % increase in the adsorption capacity of sodium oleate on diaspore and an 11.72 % decrease on kaolinite (Li et al. 2022). Figure 6b illustrates the mechanistic model of oxalic acid treatment on diaspore and kaolinite. This phenomenon occurs because oxalic acid can dissolve Al on the mineral surface, exposing more fractured Al–O bonds on the diaspore surface. In contrast, the amount of dissolved Al on the kaolinite surface is more significant than that on the diaspore (Figure 6c), which enhances the flotation difference between the two minerals.
Anionic polyacrylamide can function as an adjuster in the positive flotation process of bauxite. A minimal quantity of anionic polyacrylamide effectively enhances flotation indicators. This improvement is attributed to its bridging effect, which increases the apparent particle size of minerals, thereby facilitating their flotation (Huo et al. 2018). However, when the concentration of anionic polyacrylamide reaches a certain value, it can diminish the selectivity of collectors and inhibit the flotation of minerals. Zhang et al. corroborated this finding and suggested that utilizing an appropriate amount of sodium hexametaphosphate is essential to enhance the dispersibility of mineral particles, thereby improving flotation performance (Chang et al. 2021).
Lead acetate can activate in the positive flotation process of bauxite. Lead ions can activate the flotation of bauxite to a certain extent. Jiang et al. conducted flotation experiments on high-silica bauxite using lead acetate as an activator and sodium oleate as a collector (Jiang et al. 2015). The results indicated that increasing the dosage of lead acetate to a certain extent could enhance the alumina-silica ratio and improve the concentrate recovery rate. Similarly, Ding and Xie conducted bauxite flotation experiments utilizing lead acetate as an activator, achieving favourable flotation indicators (Ding et al. 2017, Xie et al. 2018).
4 Bauxite reverse flotation reagents
The reverse flotation desilication of bauxite is achieved by adding flotation reagents to promote the flotability of aluminosilicate minerals and inhibit the floatation of diaspore target minerals. The method adheres to the flotation principle of ‘floating less and inhibiting more’, offering advantages such as less foam, reduced reagent usage, and minimal impact on subsequent alumina production. To achieve effective reverse flotation desilication of bauxite, it is essential to inhibit the floatation of diaspore and enhance the adsorption of collectors on aluminosilicate minerals. Therefore, the selection of appropriate flotation reagents is crucial.
4.1 Collectors
The reverse flotation of cationic collectors primarily relies on the difference in surface zeta potential between diaspore and aluminosilicate minerals. Since the surface of aluminosilicate minerals is negatively charged, it is prone to adsorption by cationic collectors, thereby increasing the hydrophobicity of the aluminosilicate minerals to achieve separation. In reverse flotation, the target minerals that float out are the aluminosilicate minerals in bauxite. The primary collectors used for desilication in reverse flotation are cationic collectors, primarily amine collectors, including linear alkyl amines, quaternary ammonium salts, and polyamines.
4.1.1 Cationic collectors
The linear alkyl amines primarily include dodecylamine and tetradecylamine. These amines exhibit low solubility and relatively weak collecting capabilities for aluminosilicate minerals, which complicates the practical separation of diaspore from silicate minerals during the reverse flotation process. Typically, these amines are combined with acetic acid, hydrochloric acid, and other reagents to react and form salt products, with the effective component being the cation that is ionized by the salt. Zhong et al. employed cationic collectors such as dodecyl ammonium chloride (DDAC), dodecyl guanidinium sulfate (DDGS), and dodecyl trimethyl ammonium chloride (DTAC) in the reverse flotation of bauxite (Zhong et al. 2008). The results indicated that all three cationic collectors demonstrated excellent selectivity for diaspore. Furthermore, during the reverse flotation process of bauxite (pH = 4), the introduction of the cationic collector dodecylamine hydrochloride (DAH) enhanced the adsorption force between diaspore and kaolinite, with the latter consistently being more significant than the former (Zhang et al. 2022a).
Compared to linear alkyl amines, quaternary ammonium salt collectors undergo complete water cationization, enhancing their selectivity and adsorption capabilities, particularly towards aluminosilicate minerals. Quaternary ammonium salt collectors typically undergo electrostatic adsorption on the surface of the diaspore, as well as electrostatic adsorption and hydrogen bonding on the kaolinite surface. Yue et al. investigated the mechanism of action of dodecyltrimethylammonium chloride (DTAC) and tetrabutylammonium chloride (TBAC) in the reverse flotation of bauxite (Yue et al. 2014). The results indicated that TBAC, with its unique bulky head and short hydrocarbon chain structure, possessed a contact area 3.4 times greater than that of DTAC, resulting in enhanced hydrogen bonding with the kaolinite surface (Figure 7a). Notably, TBAC demonstrated superior selectivity, a broader pH range of application, and a more effective dosage compared to DTAC. Studies have demonstrated that during the reverse flotation of bauxite, the flotation recovery rate of kaolinite generally decreases as the pH increases. Xu et al. (2014) examined the flotation behaviour of kaolinite utilizing dodecyltrimethylammonium chloride (DTAC) and cetyltrimethylammonium chloride (CTAC), both of which are quaternary ammonium salt collectors (Xu et al. 2015). The results indicated that the flotation recovery rate of kaolinite decreases as pH increases, which contradicts the findings from the Zeta potential measurements. Quantum chemical calculations suggest that this discrepancy arises from the structural and property differences between the (001) silicon face and the (001) aluminum face of kaolinite. In acidic environments, kaolinite self-aggregates through electrostatic interactions, while hydrophobic DTAC adsorbs onto the (001) silicon face, thereby enhancing the flotability of kaolinite (Figure 7b).
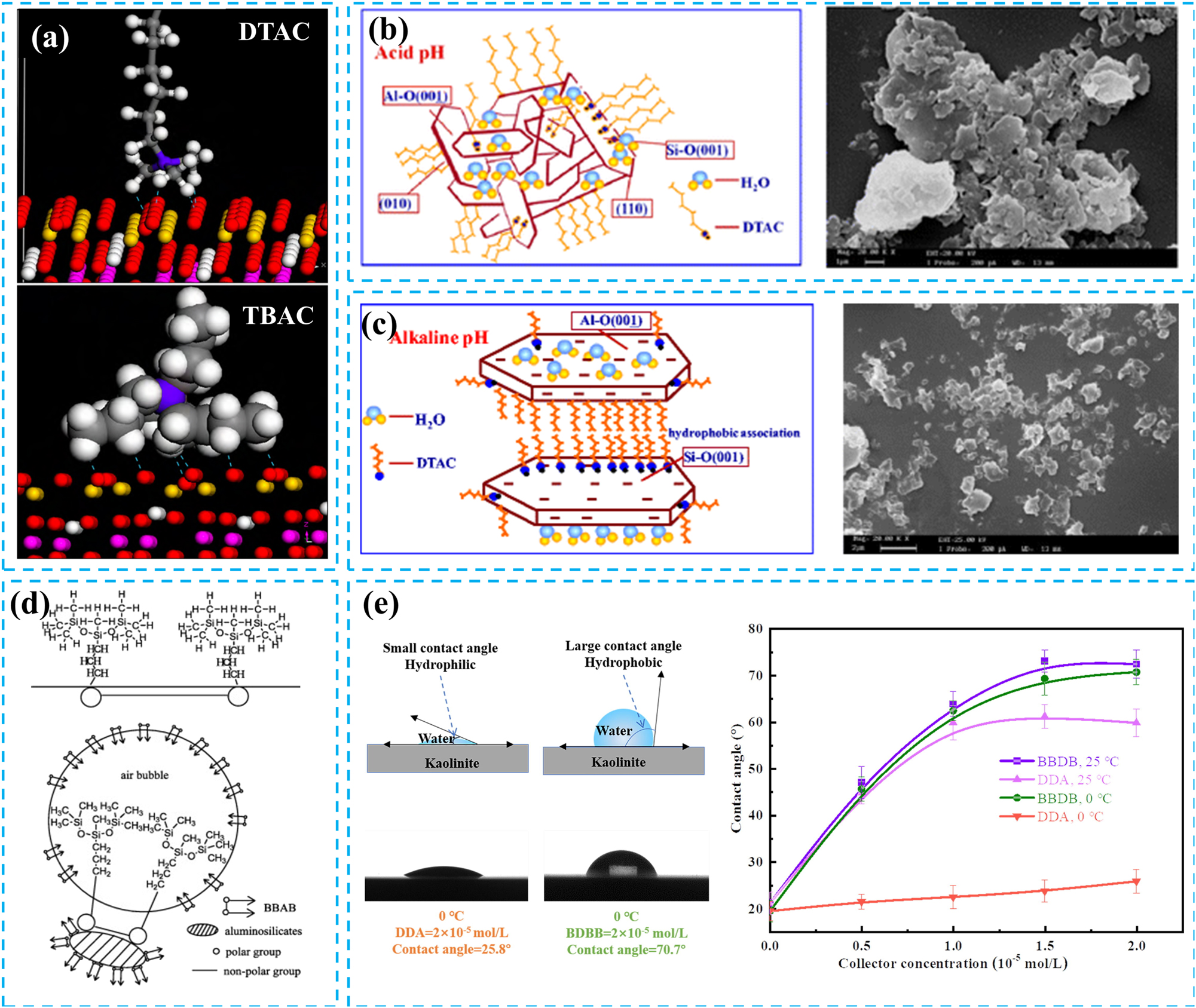
Forms of interaction between cationic collectors and mineral surfaces. (a) Hydrogen bonding between DTAC and TBAC formed on the kaolinite (001) surface. Reprinted with permission from The Nonferrous Metals Society of China (copyright 2014) (Yue et al. 2014). (b) Hydrophobic structural modelling and SEM of kaolinite particles adsorbed with DTAC in acidic solution. Reprinted with permission from Springer (copyright 2015) (Xu et al. 2015). (c) Hydrophilic structural modelling and SEM of kaolinite particles adsorbed with DTAC in alkaline solution. Reprinted with permission from Springer (copyright 2015) (Xu et al. 2015). (d) The ‘parachute’ shape structure of BBAB trap and the flotation mechanism of aluminosilicate minerals. Reprinted with permission from Elsevier (copyright 2014) (Huang et al. 2014). (e) The effect of BBDB and DDA concentrations on the contact angle of kaolinite surface at 25 °C and 0 °C. Reprinted with permission from Elsevier (copyright 2021) (Zhang et al. 2021b).
In alkaline environments, kaolinite disperses due to electrostatic repulsion. In contrast, DTAC molecules aggregate through hydrophobic interactions, resulting in decreased flotability of kaolinite (Figure 7c). Beyond pH, both the particle size of kaolinite and the carbon chain length of quaternary ammonium salts significantly influence the recovery rate of kaolinite. Generally, the flotation recovery rate of coarse kaolinite particles increases with the increasing carbon chain length of quaternary ammonium salts. In contrast, fine kaolinite particles’ flotation recovery rate decreases with quaternary ammonium salts’ longer carbon chain lengths (Jiang et al. 2013). Jiang et al. investigated diaspore and kaolinite flotation behaviour using two quaternary ammonium salt collectors, DTAC and CTAC (Jiang et al. 2014). In this study, CTAC, which has a longer carbon chain and stronger collecting ability, resulted in a lower flotation recovery rate than DTAC for fine kaolinite particles.
In addition to the conventional cationic collectors, Gemini quaternary ammonium salt collectors have also been utilized in reverse bauxite flotation. These collectors are composed of two identical amphiphilic moieties, which are covalently linked by a spacer group positioned at or near the hydrophilic head (Zhang et al. 2018b). They exhibit superior capabilities, including reducing surface tension in solutions and enhancing foam stability. Huang et al. synthesized an amino-trisiloxane Gemini cationic surfactant, butane-1,4-bis(dimethyl-(3-(3-aminopropyl trisiloxane-3-yl)-propyl)-ammonium bromide) (BBAB), for application in the reverse flotation process of bauxite (Huang et al. 2014). Compared to conventional cationic collectors such as DTAB and DDA, the pH of the slurry does not significantly affect the collecting ability of BBAB. It demonstrates a strong collecting ability for aluminosilicate minerals even at high pH levels. Notably, BBAB interacts primarily with silicate minerals through electrostatic attraction. At the same time, the unique ‘parachute’ structure of BBAB’s hydrophobic siloxane group enhances its collecting ability in strongly alkaline environments (Figure 7d). Zhang et al. prepared a novel Gemini collector butanediyl-1,4-bis (dimethyldodecylammonium bromide) (BBDB), as a collector for kaolinite (Zhang et al. 2021b). In this study, BBDB significantly enhanced the hydrophobicity of kaolinite even at low temperatures (0 °C) (Figure 7e), demonstrating excellent flotation performance. Similarly, BBDB is also adsorbed on the surface of kaolinite through electrostatic attraction.
In addition to linear alkyl amine collectors and quaternary ammonium salt collectors, there are tertiary amine collectors, ether amine collectors, amide collectors, and polyamine collectors. For instance, Tan et al. performed a reverse flotation desilication test on bauxite utilizing the polyamine collector DN12 (Tan et al. 2013). DN12 exhibited a significantly superior collecting ability and a broader flotation pH range compared to DDA amine collectors. DN12 adsorbed onto the surface of aluminosilicate minerals through electrostatic and hydrogen bonding interactions. Similarly, Zhou et al. conducted a reverse flotation simultaneous desilication test on low-grade high-sulfur bauxite using a self-developed polyamine collector, ultimately achieving commendable flotation indicators with an Al–Si ratio of 6.63 and a concentrate Al2O3 recovery rate of 83.47 % (Zhou et al. 2018).
Electrostatic forces primarily drive the adsorption of cationic collectors on the surface of aluminosilicate minerals. Additionally, hydrogen bonding forces may also be present. Generally, the electrostatic attraction between cationic collectors and aluminosilicate surfaces is significantly stronger than that observed with diasporic minerals. Table 6 presents the cationic collectors utilized in the reverse flotation process of bauxite.
Flotation indexes for cationic collectors in reverse flotation of bauxite.
| Collectors | Collector dosage (g/t) | Flotation pH | Al2O3 concentrate recovery (%) | A/S ratio | |
|---|---|---|---|---|---|
| Raw ore | Concentrate | ||||
| Dodecyltrimethylammonium chloride (Su et al. 2023) | 150 | 5.5 | 72.07 | 3.55 | 4.40 |
| Lauramide (Su et al. 2023) | 150 | 5.5 | 58.97 | 3.55 | 4.52 |
| Dodecylamine (Su et al. 2023) | 150 | 5.5 | 57.81 | 3.55 | 4.31 |
| Cetyltrimethylammonium chloride (Su et al. 2023) | 150 | 5.5 | 58.27 | 3.55 | 4.58 |
| DDGS (Zhong et al. 2008) | 0.2 mM | 9.5–11 | >72 | 5.85 | >10 |
| Gemini (Xia et al. 2010) | 200 | 10 | 71.73 | 5.70 | 9.66 |
| Cetyldimethylbenzylammonium chloride (Hu et al. 2008) | 20 | 5.5 | 43.07 | 5.0 | 15.2 |
| Polyamine collectors (Zhou et al. 2018) | 80 | 8.5 | 83.47 | 4.58 | 6.63 |
-
Reproduced with permission from the references listed in the Table.
4.1.2 Combined collectors
The number of studies conducted on using combined collectors for reverse flotation desilication of bauxite is currently quite scarce. Recent research has shown that combined collectors exhibit superior collecting ability to aluminosilicate minerals compared to single cationic collectors. Zhang et al. employed a combined collector consisting of dodecylamine (DDAA) and cetyltrimethylammonium bromide (CTAB) to reverse bauxite flotation (Zhang et al. 2022c). The results indicated that, compared to flotation with CTAB alone, the addition of DDAA increased the alumina-silica ratio from 7.49 to 8.55 and the Al2O3 recovery rate from 71.11 % to 75.53 %, demonstrating excellent flotation separation performance. This is attributed to the preferential adsorption of DDAA and CTAB on the surfaces of diaspore and kaolinite, respectively, which enhances the adsorption difference between the two minerals.
In addition to the above anionic and cationic collectors, amphoteric collectors are also applied in the flotation of bauxite. Their molecular structures contain both anionic and cationic groups, exhibiting strong interference resistance and good surface activity within a wide pH range. Additionally, they possess excellent water solubility, foaming properties, and low-temperature resistance. These characteristics determine that amphoteric collectors perform better than conventional collectors in the beneficiation of bauxite. For instance, Zhu et al. investigated the collecting performance of amphoteric collector DJL-2 on diaspore and kaolinite (Zhu et al. 2018). The results indicated that DJL-2 forms bonding and hydrogen bonding interactions with the surface of the diaspore, whereas no hydrogen bonding or bonding interactions occur with kaolinite. Consequently, the adsorption amount of this amphoteric collector on the diaspore surface is significantly higher than that on kaolinite due to this interaction mechanism.
4.2 Depressants
Compared to direct floatation modifying reagents, reverse flotation modifying reagents exhibit significant differences. They primarily inhibit the flotation of diaspore or activate aluminosilicate minerals, thereby enhancing the floatability disparity between the two minerals and facilitating efficient separation of bauxite. Current industrial applications predominantly utilize polymeric organic depressants, including starch and polyacrylamide, for desilication in bauxite reverse flotation processes (Elhaei et al. 2021).
Starch, a widely utilized depressant in bauxite reverse flotation, has been relatively mature and systematic with related studies. The starch structure contains abundant hydroxyl groups, conferring hydrophilic properties. Simultaneously, starch adsorbs onto the mineral surface, inhibiting mineral flotation (Li et al. 2010b). For instance, Yu et al. conducted reverse bauxite flotation using TAS101 as a collector and starch as a depressant (Yu et al. 2016). Under optimized conditions, the process achieved superior flotation metrics, including an Al/Si ratio of 9.58 and an Al2O3 recovery rate of 83.34 % in the concentrate. This study revealed that starch exhibited pronounced inhibitory efficacy on diaspore flotation under alkaline pH conditions while concurrently demonstrating no discernible interference with the floatability of aluminosilicate minerals. Xia et al. investigated the selective inhibitory effects of corn starch on diaspore (Xia et al. 2009). The study demonstrated that corn starch chemically adsorbed onto mineral surfaces through the formation of a five-membered starch-alumina complex with Al–OH sites (Figure 8a). The more Al sites exposed on the mineral surface, the stronger the adsorption of starch. Since the Al sites on the kaolinite surface were significantly fewer than those on the diaspore, corn starch selectively inhibited the diaspore.
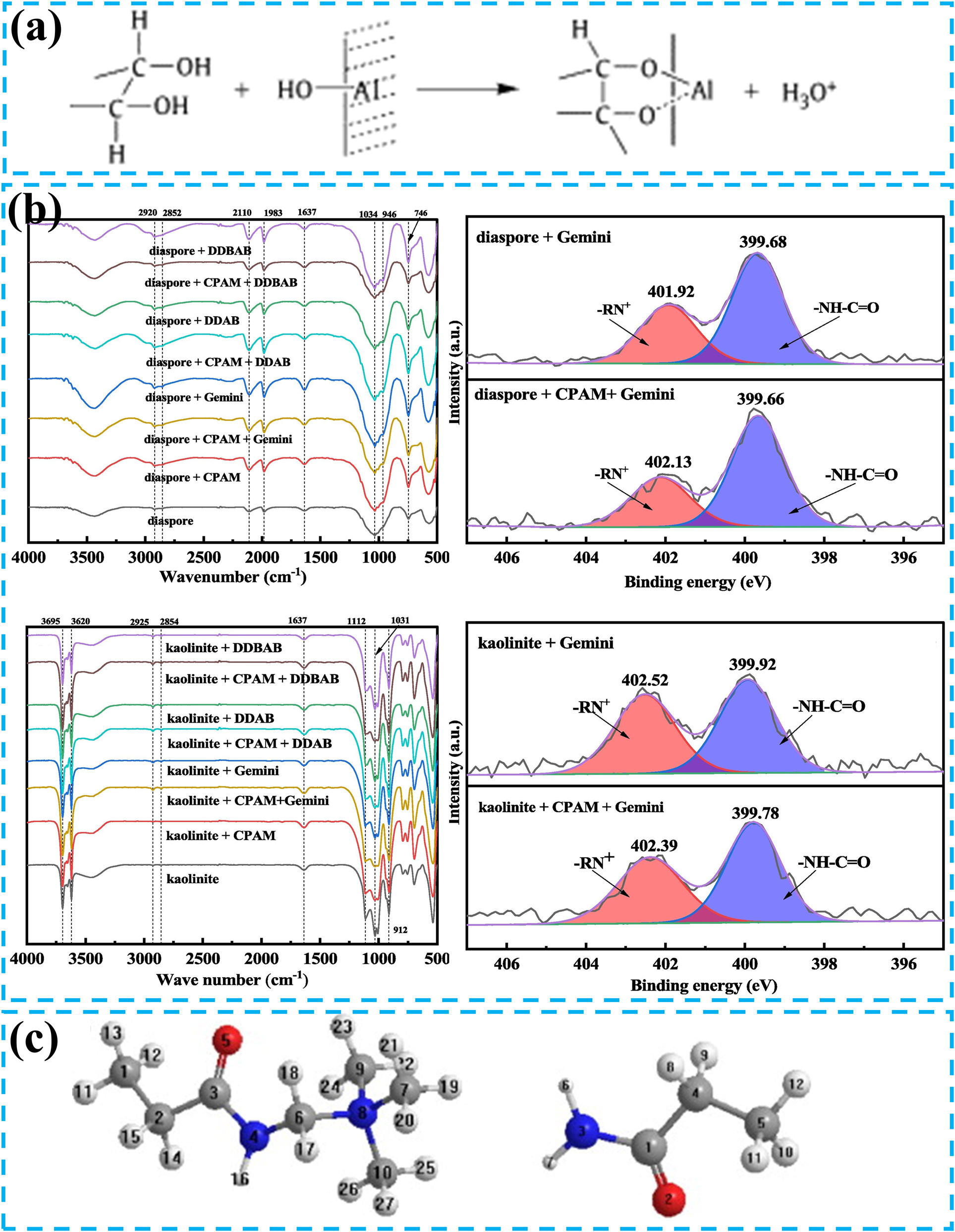
Common inhibitors and mineral interaction forms for reverse flotation desilication. (a) Equation of interaction between starch and metal hydroxylated substances. Reprinted with permission from Elsevier (copyright 2009) (Xia et al. 2009). (b) FTIR and XPS spectra of diaspore and kaolinite before and after CPAM adsorption. Reprinted with permission from Elsevier (copyright 2025) (Zhang et al. 2025). (c) Cationic groups (left) and amide groups (right) of CPAM. Reprinted with permission from Elsevier (copyright 2007) (Liu et al. 2007).
However, modified starch has been proposed with the accelerating depletion of high-grade bauxite reserves and the concomitant deterioration of ore grade. Modified starch breaks its molecular structure through depolymerization, rearrangement, oxidation and chemical modification. These processes incorporate additional polar groups, effectively addressing the shortcomings of natural starch. Li et al. investigated the inhibitory effects of cationic starch (CAS), carboxymethyl starch (CMS), amphoteric starch (AMS), and soluble starch (SS) on diaspore during reverse flotation tests, utilizing dodecylamine as a collector (Li et al. 2010a). The results demonstrated that positively charged CAS and AMS demonstrated superior adsorption and depression capacities compared to non-ionic SS and cationic CNS. This phenomenon was attributed to the electrostatic adsorption interactions between CAS, AMS and the diaspore. In contrast, non-ionic SS achieved mineral surface adherence predominantly through hydrogen bonding interactions. Modified starch slightly activates kaolinite during bauxite reverse flotation processes (Li et al. 2011).
CPAM serves as another commonly used depressant in bauxite reverse flotation processes. Similarly, FTIR and XPS analyses (Figure 8b) confirmed its strong depressive efficacy toward diaspore but a negligible impact on kaolinite during the reverse flotation process. This selectivity was primarily attributed to the RN+ groups of CPAM, which primarily interacted with aluminum atoms on mineral surfaces (Zhang et al. 2025). Notably, diaspore possessed substantially more reactive Al sites compared to kaolinite. Liu et al. investigated the role of CPAM in bauxite reverse flotation (Liu et al. 2007). The results demonstrated that CPAM adsorbs across all crystallographic planes of diaspore, thereby preventing the adsorption of most DDA cationic collectors and effectively suppressing diaspore flotation. Concurrently, the adsorption of CPAM on kaolinite was attenuated due to the inductive effects of methyl groups and steric hindrance (Figure 8c). The behaviour resulted in minimal influence of CPAM on the flotability of kaolinite.
Polymeric sodium silicate has been implemented in the flotation of aluminosilicate minerals. Polymeric sodium silicate amplifies the hydrophobicity difference between diaspore and aluminosilicate minerals through the selective depression of diaspore and activation of kaolinite. This phenomenon is attributed to the differences in the surface properties of the two minerals. Polymeric sodium silicate predominantly interacts with Al sites on mineral surfaces. In contrast, the surface of the diaspore contains only Al sites, and the kaolinite surface comprises both Al and Si sites. The probability of interaction between the Al sites of the two minerals and polymeric sodium silicate is equivalent (Yan 2021). Following interaction with polymeric sodium silicate, the Si sites on kaolinite surfaces will be adsorbed with the cationic collectors, thereby amplifying the hydrophobicity disparity between the two minerals. Liu et al. conducted reverse flotation experiments on bauxite using polymeric sodium silicate as a depressant and amine-based composite collectors as desilication reagents. The process achieved an optimized flotation index, including an Al/Si ratio of 7.22 and an Al2O3 recovery rate of 84.92 % in the concentrate (Liu et al. 2020).
4.3 Other auxiliary reagents
In bauxite reverse flotation processes, sodium carbonate is conventionally employed as a pH modifier and dispersant, analogous to its application in direct flotation. Notably, acid pretreatment of bauxite before reverse flotation operations has enhanced the flotation performance index. Zhang et al. conducted reverse flotation experiments on sulfuric acid-treated bauxite using sodium hexametaphosphate as the depressant and dodecylamine hydrochloride as the collector (Zhang et al. 2018a). The results indicated that acid pretreatment facilitated the separation of diaspore and kaolinite, thereby enhancing the reverse flotation recovery rate of bauxite. The phenomenon was attributed to the acid-treated reduction in Al site density on both mineral surfaces, which diminished sodium hexametaphosphate adsorption density. However, kaolinite exhibited increased Si site post-treatment, promoting dodecylamine hydrochloride adsorption. These surface modifications collectively enhanced the hydrophobicity difference between the two minerals (Figure 9a). It is noteworthy that acid treatment alters the surface properties of diaspore and kaolinite, which is a fundamental factor influencing the adsorption behaviour of flotation reagents. Specifically, the surface of acid-treated diaspore develops ‘unsaturated Al’ (Figure 9b), thereby modifying the adsorption density of the flotation reagent (Zhang et al. 2019). However, acid treatment usually requires alkali to adjust the pH, significantly increasing the cost of bauxite production. At the same time, acid treatment produces acidic waste streams that corrode equipment.
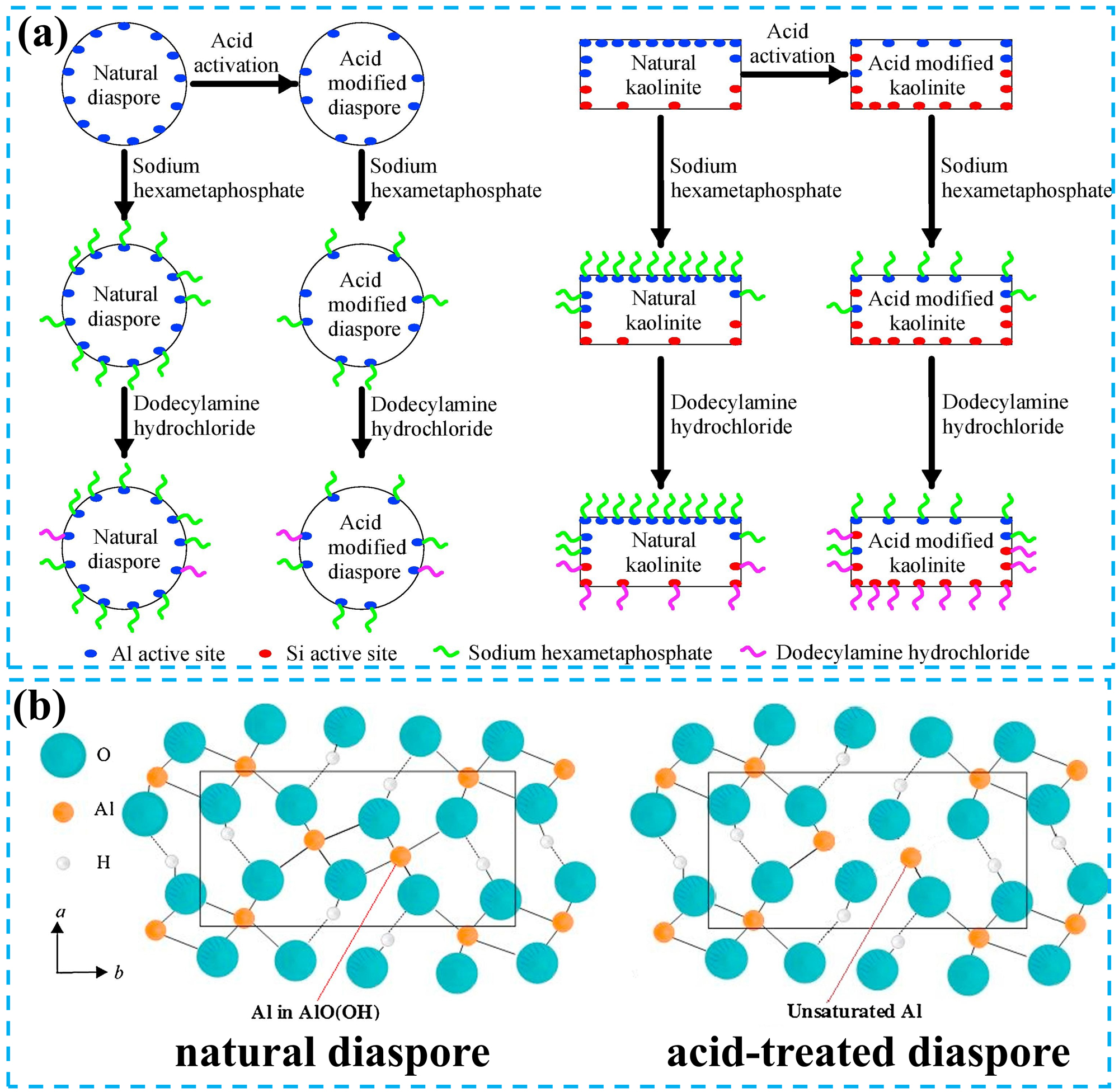
The effect of acid treatment on the surface active sites of minerals. (a) Schematic diagram of the effect of acid treatment on mineral surface active sites and the adsorption of depressants and collectors on diaspore and kaolinite. Reprinted with permission from Elsevier (copyright 2018) (Zhang et al. 2018a). (b) Crystal structure projection of the surface of diaspore before and after acid treatment. Reprinted with permission from Elsevier (copyright 2019) (Zhang et al. 2019).
In bauxite reverse flotation, sodium fluoride and sodium chloride are activators, predominantly enhancing the floatability of aluminosilicate minerals while exerting minimal impact on diaspore. Fluoride and chloride ions significantly depress the mineral surface’s Zeta potential, enhancing cationic collectors’ adsorption. Fluorine ions diffused into the layered aluminosilicate mineral crystal layers through intercalation; however, they were only adsorbed on the surface of the diaspore. The behaviour resulted in a concentration gradient of fluorine ions between the two materials, activating aluminosilicate minerals’ flotation. Similarly, chloride ions not only diffused into the interlayer structures of pyrophyllite crystals via intercalation but also substantially lowered the critical micelle concentration of cationic collectors, thereby enhancing the capture performance of the collector (Chen et al. 2004).
Although bauxite flotation desilication technology has been widely used in actual production, the flotation reagents used have the following problems: The dosage of reagent required for bauxite direct flotation is large, resulting in the residual reagent of beneficiation products affecting the subsequent dissolution of alumina, and it is difficult to filter. In addition, the commonly used fatty acid collectors have poor selectivity, hard water resistance and poor dispersion. The selectivity of cationic collectors commonly used in bauxite reverse flotation is poor, and the flotation effect is not ideal. At the same time, the foaming ability is too strong, and the separation effect of fine particles is poor.
5 Conclusions and prospects
The vigorous development of the aluminum industry and the exhaustion of high-quality bauxite resources have made the development of low Al–Si ratio bauxites an unavoidable issue. Flotation desilication of bauxite resources is a promising approach to enhance the Al–Si ratio and alleviate the demand for alumina supply. Therefore, gaining a deep understanding of the mechanisms of flotation reagents and developing a collector with strong capturing ability and good selectivity, along with related reagent systems, is crucial to address the depletion of China’s bauxite resources. In recent years, various novel flotation reagents have emerged in the field of bauxite flotation desilication. This study discusses the differences in chemical composition and mineralogy of bauxites from different regions, focusing on bauxite’s reverse and direct flotation processes. It examines the differences in reagents and their mechanisms, including collectors, depressants, and other auxiliary reagents. While significant achievements have been made in studying novel flotation reagents, the increasing depletion and complexity of bauxite resources in China present numerous challenges that must be overcome.
Based on the differences in mineral composition and surface physicochemical properties across different regions, combined with the molecular design theory of flotation reagents, it is reasonable to design flotation desilication collectors and depressants. The structural characteristics of new collectors and depressants should enhance the selectivity of the collector and the adsorption active sites by redesigning the polar and non-polar groups of collectors and depressants and introducing suitable groups, such as –COOH and –OH groups in the collector structure, which can form chelates with the mineral surface and thus enhance the collecting performance. Additionally, the design of depressants should pay attention to the synergistic effect with collectors to enhance the inhibition of gangue minerals.
In-depth study of the synergistic action mechanisms among flotation reagents, combined with molecular dynamics simulation, to evaluate the collecting and selective properties of flotation reagents and comprehensively investigate the relationship between the structure of flotation reagents and their performance, thereby reducing the reagent design cycle and experimental costs.
Flotation reagents need to be matched with reasonable and effective desilication process, which can not only improve the effect of bauxite flotation desilication, but also reduce the production cost. At the same time, with advanced flotation technology and equipment, it can effectively improve the flotation effect of micro fine minerals, such as nano bubble technology.
Funding source: National Natural Science Foundation of China
Award Identifier / Grant number: 52474287
-
Research ethics: Not applicable.
-
Informed consent: Not applicable.
-
Author contributions: The authors have accepted responsibility for the entire content of this manuscript and approved its submission. Jinyu Zhang: conceptualization, data curation, formal analysis, writing – original draft. Yanjun Li: investigation, methodology, resources. Peng Gao: funding acquisition, supervision. Shuai Yuan: project administration, resources, supervision. Wentao Zhou: supervision, validation.
-
Use of Large Language Models, AI and Machine Learning Tools: None declared.
-
Conflict of interest: The authors state no conflict of interest.
-
Research funding: This work was supported by the National Natural Science Foundation of China (Grant no. 52474287).
-
Data availability: Not applicable.
References
Chang, Z., Sun, C., Kou, J., Fu, G., and Qi, X. (2021). Experimental and molecular dynamics simulation study on the effect of polyacrylamide on bauxite flotation. Miner. Eng. 164: 106810, https://doi.org/10.1016/j.mineng.2021.106810.Search in Google Scholar
Chen, Y. (2007). Research and application of high efficiency bauxite flotation collector, Ph.D. thesis.Search in Google Scholar
Chen, X. (2024). Distribution characteristics and exploration & development pattern of global bauxite resources and its prospect. Chin. Min. Mag. 33: 59–68, (in Chinese).Search in Google Scholar
Chen, X., Hu, Y., and Wang, Y. (2004). Effects of sodium chloride on flotation of alumina-silicates minerals and its mechanism. Chin. J. Nonferrous Met. 14: 1770–1775, https://doi.org/10.19476/j.ysxb.1004.0609.2004.10.026.Search in Google Scholar
Chen, T., Ma, H., Pang, K., Wang, Y., and Zhang, Y. (2025). Mechanism and kinetics of alkaline leaching for low-grade bauxite activated by pre-roasting. Chin. J. Process Eng. 25: 1–8, (in Chinese).Search in Google Scholar
Choi, I.K., Wen, W.W., and Smith, R.W. (1993). The effect of a long chain phosphate on the adsorption of collectors on kaolinite. Miner. Eng. 6: 1191–1197, https://doi.org/10.1016/0892-6875(93)90096-6.Search in Google Scholar
Deng, L., Wang, S., Zhong, H., and Liu, G. (2015). N-(6-(hydroxyamino)-6-oxohexyl) decanamide collector: flotation performance and adsorption mechanism to diaspore. Appl. Surf. Sci. 347: 79–87, https://doi.org/10.1016/j.apsusc.2015.03.138.Search in Google Scholar
Deng, L., Wang, S., Zhong, H., and Liu, G. (2016). A novel surfactant 2-amino-6-decanamidohexanoic acid: flotation performance and adsorption mechanism to diaspore. Miner. Eng. 93: 16–23, https://doi.org/10.1016/j.mineng.2016.04.002.Search in Google Scholar
Ding, C., Xie, H., Zhang, P., and Liu, R. (2017). Study on distributing desilication and upgrading of high-alumina and high-silica bauxite. Light Met. 6: 5–8, (in Chinese).Search in Google Scholar
Dou, Z. (2022). Experimental study on beneficiation of a high mud accumulation type bauxite in southwestern district. Metal Mine 7: 193–197, (in Chinese).Search in Google Scholar
Du, W., Zhang, J., Ma, J., Wei, Z., and Li, S. (2024). Optimization of flotation quality improvement experiment of bauxite under a certain coal mine in Shanxi Province using response surface methodology. Nonferrous Met. Eng. 14: 83–91.Search in Google Scholar
Elhaei, R., Kharrat, R., and Madani, M. (2021). Stability, flocculation, and rheological behavior of silica suspension-augmented polyacrylamide and the possibility to improve polymer flooding functionality. J. Mol. Liq. 322: 114572, https://doi.org/10.1016/j.molliq.2020.114572.Search in Google Scholar
Fang, C., Yu, S., Wei, X., Peng, H., Ou, L., Zhang, G., and Wang, J. (2019). The cation effect on adsorption of surfactant in the froth flotation of low-grade diasporic bauxite. Miner. Eng. 144: 106051, https://doi.org/10.1016/j.mineng.2019.106051.Search in Google Scholar
Gao, Y., Fu, X., Khoso, S.A., Pan, Z., Han, H., Sun, W., and Yue, T. (2024). A quantitative innovation perspective on synergism and selectivity mechanism of mixed collectors in flotation. Miner. Eng. 206: 108474, https://doi.org/10.1016/j.mineng.2023.108474.Search in Google Scholar
Gao, Y., Fu, X., Yue, T., Han, H., Gao, Z., Wang, L., and Sun, W. (2022). Quantitative analysis of surface adsorption reactivity during flotation process by surface complexation model: diaspore and kaolinite. Miner. Eng. 183: 107623, https://doi.org/10.1016/j.mineng.2022.107623.Search in Google Scholar
Guo, J. (2010). A novel depressor useful for flotation separation of diaspore and kaolinite. Min. Sci. Technol. 20: 292–295, https://doi.org/10.1016/S1674-5264(09)60200-3.Search in Google Scholar
Guo, M., Feng, D., Tong, X., Xiong, Y., Luo, H., Luo, X., and Dong, M. (2023). Research progress on flotation desilication for bauxite. Conserv. Util. Miner. Resour. 43: 140–157, (in Chinese).Search in Google Scholar
Han, Y., Liu, W., and Chen, J. (2016). DFT simulation of the adsorption of sodium silicate species on kaolinite surfaces. Appl. Surf. Sci. 370: 403–409, https://doi.org/10.1016/j.apsusc.2016.02.179.Search in Google Scholar
Han, Y., Liu, X., He, F., Gao, P., and Ma, S. (2019). Current situation of bauxite resource and its beneficiation technology in China. Conserv. Util. Miner. Resour. 39: 151–158, (in Chinese).Search in Google Scholar
Hao, J., Li, B., Dai, J., Fu, D., Chang, Y., Chen, J., Xu, N., Liu, K., and Han, Q. (2024). Degradation of thiosulfate ions in sodium aluminate solution photocatalyzed by Co-TiO2@ZIF-8. Miner. Eng. 218: 109038, https://doi.org/10.1016/j.mineng.2024.109038.Search in Google Scholar
Hu, Y.-h., Ouyang, K., Cao, X.-f., and Zhang, L.-m. (2008). Flotation of kaolinite and diaspore with hexadecyl dimethyl benzyl ammonium chloride. J. Cent. South Univ. Technol. 15: 378–381, https://doi.org/10.1007/s11771-008-0071-2.Search in Google Scholar
Huang, Z., Zhong, H., Wang, S., Xia, L., Zhao, G., and Liu, G. (2014). Gemini trisiloxane surfactant: synthesis and flotation of aluminosilicate minerals. Miner. Eng. 56: 145–154, https://doi.org/10.1016/j.mineng.2013.11.006.Search in Google Scholar
Huo, Q., Liu, X., Xie, J., Liu, X., and Qiu, G. (2018). Desilication of low-grade high-iron bauxite by flotation. Min. Metall. Eng. 38: 51–54, (in Chinese).Search in Google Scholar
INFO, I.A. (2024). Analysis of the current situation of China’s bauxite industry, Available at https://www.chinabaogao.com/detail/730336.html.Search in Google Scholar
Jiang, Y., Huang, L., Zhu, K., Jiang, Z., and Yang, T. (2015). Study of separation silicon from a high silicon bauxite by direct flotation with two stage. Nonferrous Met. 2: 49–53+63, (in Chinese).Search in Google Scholar
Jiang, Y.-R. and Li, X.-X. (2013). The theoretical study on the efficiencies of 2-[tris(hyroxycarbamoyl)methyl] carboxylic acids as chelating agents in flotation separation of diaspore and aluminosilicates. Sep. Purif. Technol. 104: 114–120, https://doi.org/10.1016/j.seppur.2012.11.006.Search in Google Scholar
Jiang, Y.-R., Li, W., and Feng, R. (2011). Preparation and performance of 4-alkyl-4,4-bis(hydroxycarbamoyl) carboxylic acid for flotation separation of diaspore against aluminosilicates. Miner. Eng. 24: 1571–1579, https://doi.org/10.1016/j.mineng.2011.08.008.Search in Google Scholar
Jiang, Y.-R., Li, X.-X., Feng, R., Chen, D., and Li, J.-C. (2012). Novel alkyl bis(hydroxycarbamoyl) propionic acids for flotation separation of diaspore against aluminosilicate minerals. Sep. Purif. Technol. 87: 135–141, https://doi.org/10.1016/j.seppur.2011.11.036.Search in Google Scholar
Jiang, H., Liu, G., Hu, Y., Xu, L., Yu, Y., Xie, Z., and Chen, H. (2013). Flotation and adsorption of quaternary ammonium salts collectors on kaolinite of different particle size. Int. J. Min. Sci. Technol. 23: 249–253, https://doi.org/10.1016/j.ijmst.2013.04.011.Search in Google Scholar
Jiang, H., Sun, Z., Xu, L., Hu, Y., Huang, K., and Zhu, S. (2014). A comparison study of the flotation and adsorption behaviors of diaspore and kaolinite with quaternary ammonium collectors. Miner. Eng. 65: 124–129, https://doi.org/10.1016/j.mineng.2014.05.023.Search in Google Scholar
Jiang, Y.-R., Zhao, B.-N., Zhou, X.-H., and Zhou, L.-Y. (2010). Flotation of diaspore and aluminosilicate minerals applying novel carboxyl hydroxamic acids as collector. Hydrometallurgy 104: 112–118, https://doi.org/10.1016/j.hydromet.2010.05.006.Search in Google Scholar
Lan, L. (2022). Study on the application and mechanism of anionic combination collector in silica removal in bauxite flotation, M.D. thesis.Search in Google Scholar
Lan, L., Sun, W., Yang, Y., Jiang, F., and Wang, L. (2025). Auxiliary collector optimizing foam characteristic and adsorption behavior towards efficient flotation desilication of bauxite. Sep. Purif. Technol. 353: 128322, https://doi.org/10.1016/j.seppur.2024.128322.Search in Google Scholar
Li, J. (2016). Experimental research on desulfurization roasting of high sulfur bauxite in suspended state, M.D. thesis.Search in Google Scholar
Li, M. (2022a). A study on process mineralogy and aluminum recovery technology of ores from an accumulative bauxite deposit in Yunnan Province, China. Acta Mineral. Sin. 42: 785–792, (in Chinese).Search in Google Scholar
Li, Y. (2022b). Research on roasting and activation technology of high silicon bauxite, M.D. thesis.Search in Google Scholar
Li, H. (2024). Study on mineral characteristics and flotation desulfurization of a high sulfur bauxite, M.D. thesis.10.37190/ppmp/204689Search in Google Scholar
Li, H., Chai, W., Cao, Y., Wu, Y., and Yang, S. (2021). Synergistic collection mechanism of D-phenylalanine and sodium oleate in flotation of diaspore from kaolinite. Appl. Surf. Sci. 538: 147937, https://doi.org/10.1016/j.apsusc.2020.147937.Search in Google Scholar
Li, H., Chai, W., Cao, Y., and Yang, S. (2022). Flotation enhancement of low-grade bauxite using oxalic acid as surface pretreatment agent. Appl. Surf. Sci. 577: 151964, https://doi.org/10.1016/j.apsusc.2021.151964.Search in Google Scholar
Li, X., Duan, Y., Zhao, B., and Yuan, S. (2024). Research progress on desulfurization and iron removal of high sulfur and high-iron bauxite. Nonferrous Met.: 1–13+23, (in Chinese).Search in Google Scholar
Li, Z., Wang, X., Wan, B., Gao, P., Dang, Y., Li, S., and Zhang, S. (2019). Beneficiation test of a low grade iron-bearing bauxite. Nonferrous Met. 1: 62–66+83, (in Chinese).Search in Google Scholar
Li, H., Zhang, S., Jiang, H., Hu, Y., and Wang, D. (2010b). Selective depression of diaspore with waxy maize starch. Miner. Eng. 23: 1192–1197, https://doi.org/10.1016/j.mineng.2010.08.002.Search in Google Scholar
Li, H.-p., Zhang, S.-s., Jiang, H., and Li, B. (2010a). Effect of modified starches on depression of diaspore. Nonferrous Met. Soc. China, Trans. 20: 1494–1499, https://doi.org/10.1016/S1003-6326(09)60327-7.Search in Google Scholar
Li, H.-p., Zhang, S.-s., Jiang, H., Li, B., and Li, X. (2011). Effect of degree of substitution of carboxymethyl starch on diaspore depression in reverse flotation. Nonferrous Met. Soc. China, Trans. 21: 1868–1873, https://doi.org/10.1016/S1003-6326(11)60943-6.Search in Google Scholar
Liu, S. (2012). Study on the interaction and flotation of hydroxamic acid reagents with bauxite aluminum silicate minerals, Ph.D. thesis.Search in Google Scholar
Liu, Y. and Cheng, H. (2022). Research progress on characteristics and comprehensive utilization of bauxite resource in China. Conserv. Util. Miner. Resour. 42: 106–114, (in Chinese).Search in Google Scholar
Liu, C.-m., Feng, A., and Guo, Z. (2016). Investigation and optimization of use of anionic collectors in direct flotation of bauxite ores. Physicochem. Probl. Miner. Process. 52: 932–942, (in Chinese).Search in Google Scholar
Liu, Y., Liu, Y., Zhang, T.-a., and Xu, J. (2024). Summary of sulfur hazards in high-sulfur bauxite and desulfurization methods. Sci. Total Environ. 948: 174631, https://doi.org/10.1016/j.scitotenv.2024.174631.Search in Google Scholar PubMed
Liu, A., Peng, W., Liu, H., and Wang, Z. (2020). Experimental study on flotation desulfurization and desilication of a bauxite in Guizhou. Metal Mine 8: 102–106, (in Chinese).Search in Google Scholar
Liu, S., Qin, W., Peng, T., and Hu, Y. (2013). Flotation mechanism of octylic hydroxamic acid on diaspore. Chin. J. Geochem. 32: 191–194, https://doi.org/10.1007/s11631-013-0621-7.Search in Google Scholar
Liu, G., Zhong, H., Hu, Y., Zhao, S., and Xia, L. (2007). The role of cationic polyacrylamide in the reverse flotation of diasporic bauxite. Miner. Eng. 20: 1191–1199, https://doi.org/10.1016/j.mineng.2007.05.003.Search in Google Scholar
Liu, W., Zhong, H., and Wang, S. (2018). Synthesis of pival hydroxamic acid and its flotation properties on aluminosilicate minerals. Min. Metall. Eng. 38: 53–57, (in Chinese).Search in Google Scholar
Lyu, F., Gao, J., Sun, N., Liu, R., Sun, X., Cao, X., Wang, L., and Sun, W. (2019). Utilisation of propyl gallate as a novel selective collector for diaspore flotation. Miner. Eng. 131: 66–72, https://doi.org/10.1016/j.mineng.2018.11.002.Search in Google Scholar
Man, X., Ou, L., Wang, C., Jin, S., and Ma, X. (2019). Flotation separation of diaspore and kaolinite by using a mixed collector of sodium oleate-tert dodecyl mercaptan. Front. Chem. 7: 813, https://doi.org/10.3389/fchem.2019.00813.Search in Google Scholar PubMed PubMed Central
Man, X., Wang, C., Yu, S., Yang, X., Liu, J., Fu, Y., Dong, Z., Zhi, H., and Ou, L. (2022). Low-temperature flotation separation of diaspore from kaolinite by using a mixed collector. Minerals 12: 891, (in Chinese), https://doi.org/10.3390/min12070891.Search in Google Scholar
Peng, L. (2024). Research on flotation desulfurization and desilication of low-quality bauxite, M.D. thesis.Search in Google Scholar
Peng, H., Vaughan, J., and Vogrin, J. (2018). The effect of thermal activation of kaolinite on its dissolution and re-precipitation as zeolites in alkaline aluminate solution. Appl. Clay Sci. 157: 189–197, https://doi.org/10.1016/j.clay.2018.03.002.Search in Google Scholar
Qiu, Y. (2020). Chemical pre-desilication of the low-grade bauxite and the resource utilization of silicon, M.D. thesis.Search in Google Scholar
Su, H., Kang, Z., Yan, K., Ma, J., and Zhang, J. (2023). Experimental study on recovery of silicate minerals from bauxite by flotation. Light Met. 5: 1–5+10 (in Chinese).Search in Google Scholar
Sun, Y. (2023). The use of aluminum alloys in structures: review and outlook. Structures 57: 105290, https://doi.org/10.1016/j.istruc.2023.105290.Search in Google Scholar
Sun, Y. (2024). Basic and applied research on the two-component sintering process for low-grade bauxite, Ph.D. thesis.Search in Google Scholar
Sun, Q., Chen, J., Zhao, Z., Yang, D., Xiao, Y., Zhang, H., Ma, X., Zhong, H., and Zeng, H. (2023). Tailored pH-triggered surfactant for stepwise separation of a three-component mineral system. Sep. Purif. Technol. 316: 123753, https://doi.org/10.1016/j.seppur.2023.123753.Search in Google Scholar
Sun, W., Lan, L., Zeng, H., Zhou, J., Ahmed Khoso, S., and Wang, L. (2022). Study on the flotation separation mechanism of diaspore from kaolinite using mixed NaOL/BHA collector. Miner. Eng. 186: 107719, https://doi.org/10.1016/j.mineng.2022.107719.Search in Google Scholar
Survey, U.S.G. (2024). Mineral commodity summaries.Search in Google Scholar
Tan, K., Cao, Y., Wang, Q., and Cao, X. (2013). Design and synthesis of collectors for flotation of aluminosilicate minerals and flotation mechanism. J. Univ. South China 27: 10–15, (in Chinese).Search in Google Scholar
Wang, Y., Hu, Y., He, P., and Gu, G. (2004). Reverse flotation for removal of silicates from diasporic-bauxite. Miner. Eng. 17: 63–68, https://doi.org/10.1016/j.mineng.2003.09.010.Search in Google Scholar
Wang, J. (2023). Multi-scale interfacial interaction mechanism between unsaturated fatty acids and alumina silica minerals in bauxite, Ph.D. thesis.Search in Google Scholar
Wang, Y., Feng, Y., Zhang, Q., Lu, D., and Hu, Y. (2017). Flotation separation of diaspore from aluminosilicates using commercial oleic acids of different iodine values. Int. J. Miner. Process. 168: 95–101, https://doi.org/10.1016/j.minpro.2017.09.013.Search in Google Scholar
Wang, Y.H., Hu, Y.H., and Chen, X.Q. (2007). Effect of phosphate and fluoride on the flotation of aluminum-silicates with cationic collector. Can. Metall. Q. 46: 221–226, https://doi.org/10.1179/cmq.2007.46.3.221.Search in Google Scholar
Wang, Y., Liu, W., Liu, W., Chen, M., and Hu, L. (2025). Two-step acid leaching extraction of Fe&Al from karst bauxite and optimization experimental study. Chem. Eng. Process. 208: 110131, https://doi.org/10.1016/j.cep.2024.110131.Search in Google Scholar
Wang, H., Liu, Y., Zhang, T., and Li, X. (2024). Research progress on desulfurization methods for high sulfur bauxite. China Nonferrous Metall. 53: 13–23, (in Chinese).Search in Google Scholar
Wang, C., Man, X., and Ou, L. (2023). Molecular dynamics study of the effect of temperature on the flotation behavior of sodium oleate on the surface of diaspore. Miner. Eng. 192: 108004, https://doi.org/10.1016/j.mineng.2023.108004.Search in Google Scholar
Wang, R., Yuan, S., Gao, P., Li, Y., and Sun, Y. (2021). Study on new technology for separation of iron and aluminum minerals from high-iron bauxite by suspension magnetization roasting. Metal Mine 4: 113–118, (in Chinese).Search in Google Scholar
Wei, L., Chen, Y., Lei, M., and Huang, Q. (2023). Mineralogy characteristics of sedimentary bauxite in western Guangxi. Rock Miner. Anal. 42: 1220–1229, (in Chinese).Search in Google Scholar
Wu, G., Ma, J., Du, W., and Zhang, Z. (2024a). Current status and progress of desilication and desulfurization technology in bauxite beneficiation. Miner. Explor. 15: 1–7, (in Chinese).Search in Google Scholar
Wu, H., Ma, S., and Ou, Y. (2025). Dissolution and separation of alumina from Guangxi high-iron bauxite. Chin. J. Process Eng. 25: 2–9+1, (in Chinese).Search in Google Scholar
Wu, Y., Yang, S., Chai, W., Zhang, Y., and Cao, Y. (2024b). Probing the selective interaction mechanisms of propyl gallate with diaspore and kaolinite from a microscopic force view. Colloids Surf., A 687: 133480, https://doi.org/10.1016/j.colsurfa.2024.133480.Search in Google Scholar
Xia, L.-y., Zhong, H., and Liu, G.-y. (2010). Flotation techniques for separation of diaspore from bauxite using Gemini collector and starch depressant. Nonferrous Met. Soc. China, Trans. 20: 495–501, https://doi.org/10.1016/S1003-6326(09)60168-0.Search in Google Scholar
Xia, L., Zhong, H., Liu, G., Huang, Z., and Chang, Q. (2009). Flotation separation of the aluminosilicates from diaspore by a Gemini cationic collector. Int. J. Miner. Process. 92: 74–83, https://doi.org/10.1016/j.minpro.2009.02.012.Search in Google Scholar
Xie, H., Liu, R., Tian, X., Xiao, W., Wu, J., and Tong, X. (2018). Gravity-flotation combined desilication for high-aluminum and high-silicon bauxite Ore. Nonferrous Met. 5: 53–57, (in Chinese).Search in Google Scholar
Xiong, P. (2021a). Study on the synergistic roasting and separation of iron and aluminum from high sulfur bauxite and Bayer process red mud, M.D. thesis.Search in Google Scholar
Xiong, Y. (2021b). The influence of magnetization roasting on the mineral phase structure and leaching properties of Wenshan high-speed railway bauxite, M.D. thesis.Search in Google Scholar
Xu, L., Hu, Y., and Dong, F (2015). Effects of particle size and chain length on flotation of quaternary ammonium salts onto kaolinite. Mineral. Petrol. 109: 309–316, https://doi.org/10.1007/s00710-014-0332-8.Search in Google Scholar
Xu, L., Hu, Y., Dong, F., Gao, Z., Wu, H., and Wang, Z. (2014). Anisotropic adsorption of oleate on diaspore and kaolinite crystals: implications for their flotation separation. Appl. Surf. Sci. 321: 331–338, https://doi.org/10.1016/j.apsusc.2014.10.042.Search in Google Scholar
Yan, Z. (2021). Study on a new combined collector for simultaneous desulfurization and desilication of low-grade high-sulfur, M.D. thesis.Search in Google Scholar
Yang, L., Zhang, J., Kan, S., and Lv, C. (2023). Separation and recovering of aluminum, silicon, and iron in a stacked high-speed bauxite mine in Yunnan Province. Min. Metall. Eng. 43: 75–78+83, (in Chinese).Search in Google Scholar
Yang, L., Zhang, J., Lv, C., and Ma, Y. (2022). Experimental study on flotation separation of aluminum and silicon for a low grade bauxite in Yunnan. Metal Mine 51: 124–128, (in Chinese).Search in Google Scholar
Yu, T. (2020). Investigation on iron removal technology of high speed bauxite based on suspension magnetization roasting, M.D. thesis.Search in Google Scholar
Yu, X.-y., Wang, H.-l., Wang, Q.-q., Feng, B., and Zhong, H. (2016). Flotation of low-grade bauxite using organosilicon cationic collector and starch depressant. Nonferrous Met. Soc. China, Trans. 26: 1112–1117, https://doi.org/10.1016/S1003-6326(16)64209-7.Search in Google Scholar
Yu-ren, J., Zhi-gang, Y., Yun-lai, Y., and Xiao-hong, Z. (2010). Synthesis and collecting properties of novel carboxyl hydroxamic acids for diaspore and aluminosilicate minerals. Miner. Eng. 23: 830–832, https://doi.org/10.1016/j.mineng.2010.05.016.Search in Google Scholar
Yue, T., Sun, W., and Chen, P. (2014). Mechanism of reverse flotation desilication for bauxite by quaternary ammonium salt collector. Chin. J. Nonferrous Met. 24: 2872–2878, https://doi.org/10.19476/j.ysxb.1004.0609.2014.11.024.Search in Google Scholar
Yuhua, W., Daxiang, S., Liguang, W., and Yulin, Z. (2011). Effects of sodium tripolyphosphate and sodium carbonate on the selective flocculation of diasporic-bauxite in the presence of calcium and magnesium ions. Miner. Eng. 24: 1031–1037, https://doi.org/10.1016/j.mineng.2011.04.027.Search in Google Scholar
Zeng, B., Chen, C., Zhao, H., Zhang, C., Liu, P., Wang, G., He, A., and Liu, F. (2025). A systematic review of the production of high-purity aluminum: applications, preparation, and mechanisms. J. Mater. Res. Technol. 34: 987–1009, https://doi.org/10.1016/j.jmrt.2024.12.085.Search in Google Scholar
Zhai, Y. (2024). Study on enhanced flotation of high silica bauxite, M.D. thesis.Search in Google Scholar
Zhang, N., Ejtemaei, M., Nguyen, A.V., and Zhou, C. (2019). XPS analysis of the surface chemistry of sulfuric acid-treated kaolinite and diaspore minerals with flotation reagents. Miner. Eng. 136: 1–7, https://doi.org/10.1016/j.mineng.2019.03.002.Search in Google Scholar
Zhang, G., Feng, Q., Lu, Y., Liu, G., and Ou, L. (2001). The role of sodium hexametaphosphate in bauxite flotation. J. Cent. South Univ. Technol. 32: 127–130, (in Chinese).Search in Google Scholar
Zhang, H., Hu, P., Jiang, J., Cheng, X., Wang, J., Liu, J., and Xiang, P. (2021a). Distribution, genetic types and current situation of exploration and development of bauxite resources. Geol. China. 48: 68–81, (in Chinese).Search in Google Scholar
Zhang, S., Huang, Z., Wang, H., Liu, R., Cheng, C., Shuai, S., Hu, Y., Guo, Z., Yu, X., He, G., et al.. (2021b). Flotation performance of a novel Gemini collector for kaolinite at low temperature. Int. J. Min. Sci. Technol. 31: 1145–1152, https://doi.org/10.1016/j.ijmst.2021.09.001.Search in Google Scholar
Zhang, X., Le, Q., Zhao, D., Jiang, Y., Wang, Y., Wang, T., and Liao, Q. (2024b). Research status and prospect of grain refinement in aluminum alloy. J. Mater. Res. Technol. 34: 1880–1893, https://doi.org/10.1016/j.jmrt.2024.12.205.Search in Google Scholar
Zhang, S., Ma, Y., Wang, K., Shao, X., and Ding, J. (2025). Cooperative effects of quaternary ammonium salt collectors and cationic polyacrylamide on flotation separation of diaspore and kaolinite: experimental study and simulation. J. Mol. Liq. 417: 126605, https://doi.org/10.1016/j.molliq.2024.126605.Search in Google Scholar
Zhang, N., Nguyen, A.V., and Zhou, C. (2018a). Impact of interfacial Al- and Si-active sites on the electrokinetic properties, surfactant adsorption and floatability of diaspore and kaolinite minerals. Miner. Eng. 122: 258–266, https://doi.org/10.1016/j.mineng.2018.04.002.Search in Google Scholar
Zhang, N., Nguyen, A.V., and Zhou, C. (2018b). A review of the surface features and properties, surfactant adsorption and floatability of four key minerals of diasporic bauxite resources. Adv. Colloid Interface Sci. 254: 56–75, https://doi.org/10.1016/j.cis.2018.03.005.Search in Google Scholar PubMed
Zhang, N., Pang, T., Han, R., Chen, S., Li, Z., Yu, Y., Shi, Z., Liu, L., Qu, J., and Zhou, A. (2022a). Interactions between bubble and particles of key minerals of diasporic bauxite through the extended DLVO theory. Int. J. Min. Sci. Technol. 32: 201–214, https://doi.org/10.1016/j.ijmst.2021.11.002.Search in Google Scholar
Zhang, S., Ren, J., Wen, Y., and Shao, X. (2022c). Flotation separation of diaspore and kaolinite by using dodecanamide and cetyltrimethylammonium bromide as an effective mixed collector. Miner. Eng. 179: 107434, https://doi.org/10.1016/j.mineng.2022.107434.Search in Google Scholar
Zhang, N., Shi, Z., Han, R., Li, Z., Chen, S., Yu, Y., Zhu, Z., Chang, J., and Zhou, A. (2022b). New insights into flotation mechanism of diasporic bauxite from a perspective of liquid film drainage. Colloids Surf., A 636: 128178, https://doi.org/10.1016/j.colsurfa.2021.128178.Search in Google Scholar
Zhang, S., Wang, Z., Li, Y., Mo, X., and Dong, Q. (2022d). List, application and global pattern of critical minerals of China. Conserv. Util. Miner. Resour. 42: 138–168, (in Chinese).Search in Google Scholar
Zhang, W., Wang, X., Wu, W., and Zhao, K. (2024a). Research progress on desulfurization of high-sulfur bauxite and comprehensive utilization of associated cobalt resources in China. Metal Mine: 75–83, (in Chinese).Search in Google Scholar
Zhang, Z., Wang, H., Yang, W., Yang, Y., Wei, Q., Peng, Y., and Wang, W. (2024c). Research on oxygen pressure upgrading, desulfurization and iron reduction of high-speed railway and high sulfur bauxite. Nonferrous Met.: 119–127, (in Chinese).Search in Google Scholar
Zhang, S., Wang, G., Ye, T., and Xue, Q. (2023). Research status and development trend of bauxite desulfurization and iron removal in China. Mod. Min. 39: 102–106, (in Chinese).Search in Google Scholar
Zhang, N.-N., Zhou, C.-C., Liu, C., Pan, J.-H., Tang, M.-C., Cao, S.-S., Ouyang, C.-H., and Peng, C.-B. (2017a). Effects of particle size on flotation parameters in the separation of diaspore and kaolinite. Powder Technol. 317: 253–263, https://doi.org/10.1016/j.powtec.2017.04.049.Search in Google Scholar
Zhang, N.-N., Zhou, C.-C., Pan, J.-H., Xia, W., Liu, C., Tang, M.-C., and Cao, S.-S. (2017b). The response of diasporic-bauxite flotation to particle size based on flotation kinetic study and neural network simulation. Powder Technol. 318: 272–281, https://doi.org/10.1016/j.powtec.2017.06.010.Search in Google Scholar
Zhong, B. (2024). Research on the characteristics of bauxite resources and their beneficiation and desilication technologies in the new era. World Nonferrous Met. 19: 100–102, (in Chinese).Search in Google Scholar
Zhong, H., Liu, G., Xia, L., Lu, Y., Hu, Y., Zhao, S., and Yu, X. (2008). Flotation separation of diaspore from kaolinite, pyrophyllite and illite using three cationic collectors. Miner. Eng. 21: 1055–1061, https://doi.org/10.1016/j.mineng.2008.05.007.Search in Google Scholar
Zhou, J., Mei, G., Yu, M., and Cheng, Q. (2018). Research on synchronous desulphurization and desilication of low grade high-sulfur bauxite by reverse flotation. Metal Mine 7: 123–126, (in Chinese).Search in Google Scholar
Zhu, Y., Zhang, L., Chen, J., Li, Y., and Han, Y. (2018). Flotation performance and mechanism of new bauxite flotation collectors. Chin. Min. Mag. 27: 121–126+137, (in Chinese).Search in Google Scholar
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.


