Abstract
Heteropolyacids (HPAs) are well known for their application as catalysts in organic synthesis owing to their oxidizing capabilities and strong Brönsted acidity. However, a notable drawback of HPAs is their solubility in various reaction media, which has prompted the development of strategies to convert them into heterogeneous catalysts through immobilization on diverse materials. This review aims to describe recent advancements in the utilization of alumina as a support for HPAs and their applications as heterogeneous catalysts in the synthesis of organic compounds. Various strategies, methodologies, loading techniques, and the nature of HPAs, along with their acid and redox properties, are analyzed and compared. Several organic reactions, including oxidation processes, esterification, and the condensation synthesis of heterocycles, such as chromones and pyrroles, are explored.
1 Introduction
It is indisputable that the chemical industry is extremely important for today’s society. Wherever we look there are chemicals: medicines, electronics, food, clothing, pesticides, cleaning products, vehicles, etc. The demand that this industry has from society is enormous, and to this demand is added the pressure for using quick, safe, economical, and environmentally friendly methods (Clark 2005). To try to reach these “ideal conditions” with which a chemical process should comply, it must be adapted as best as possible according to the principles of green chemistry and considering a circular economy, so that the products are recycled and the waste is removed to protect the environment and natural resources (Kümmerer et al. 2020).
Catalysis is arguably the most important tool that green chemistry has. New catalytic methodologies are constantly being developed to produce various materials, which thanks to the use of catalysts can be carried out more efficiently and economically, or in environmentally friendly conditions instead of using toxic solvents, strong oxidizing agents, etc. In catalysis, heteropolyacids (HPAs) are a family of compounds of great relevance. Polymeric oxo anions (polyoxometalates) are polynuclear compounds made up of a close packing of oxides of a transition metal, or of several different metals. Homopolyoxometalates (isopolyoxometalates) have a single type of metal atom in their structure, for example, [Nb6O19]8− with the Lindqvist structure; on the other hand, heteropolyoxometalates are formed by at least two different metal atoms, for example, phosphomolybdate of the Keggin structure [PMo12O40]3−. The acids derived from the latter are called heteropolyacids, such as phosphomolybdic acid H3PMo12O40 (Canca Ruiz 2006; Jeannin 1998).
The structure of the Keggin anion is formed by a central XO4 tetrahedron surrounded by four M3O13 groups, composed of three MO6 octahedra sharing edges (Canca Ruiz 2006). The structure of Keggin HPA is complex, and this complexity allows a high movement of electrons, which makes these compounds interesting with numerous applications as catalysts, for example, in redox reactions (Supplementary Figure S1 shows a spatial representation of the phosphomolybdate anion).
If one or more of the octahedra are subtracted, lacunar species are formed, which constitute a very important class of heteropolyanions that can act as multidentate ligands for both inorganic and organic compounds (Liang et al. 2020; Kato et al. 2012; Wang et al. 2008).
The Wells–Dawson heteropolyanion [(Xn+)2M18O62](16−2n)− has an ellipsoidal structure formed by two identical “half units” XM9O31, which are a close-packed framework of MO6 octahedra surrounding a P atom, linked through the oxygen atoms (Heravi et al. 2007a). While the Preyssler anion [NaP5W30O110]14− consists of a cyclic assembly of five PW6O22 units derived from the Keggin anion that has been modified by the elimination of two sets of three WO6 octahedra from the shared corners. The structure has a cavity which encrypts a Na+ cation (Portilla-Zúñiga et al. 2018).
Other heteropolyanions mentioned in this manuscript are the Anderson-type [XM6O24Hx]n−, which possess a heteroatom (X) in a central octahedral cavity of the crown by edge-sharing six octahedral MO6 (Nomiya et al. 1987); and Dexter–Silverton [XM12O42]n− which structure involves face sharing between MO6 octahedra and the polyhedron formed by the M atoms results an icosahedron (Dexter and Silverton 1968).
HPAs are used as catalysts in numerous reactions such as hydration, dehydration, condensation, reduction, oxidation, heterocycle synthesis, etc. (Escobar et al. 2021; Li et al. 2007; Palermo et al. 2011), due to their great acid strength and redox properties. Their catalytic activity is determined by the primary structure of the heteropolyanion; the three-dimensional arrangement of the heteropolyanion, with cations and crystallization waters (secondary structure); and by the pore sizes, particle sizes, etc. (tertiary structure) (Deutschmann et al. 2009; Mothé-Esteves et al. 2010; Wu et al. 1996). HPAs are stable over a wide range of pressures and temperatures, are noncorrosive and economical, making them an excellent replacement for the traditional use of harmful acids such as sulfuric or hydrofluoric acid. However, HPAs have low surface area and are very soluble in polar solvents, which makes it necessary to support or include them in some porous materials so that they can be used as heterogeneous catalysts in liquid phase reactions.
HPAs are commercially not expensive and are employed in environmentally benign protocols for various organic transformations. It is known that in organic solvents, the molar catalytic activity of HPAs is higher than that of H2SO4 (100–1,000 times), making it possible to carry out catalytic processes at low concentration. The solid HPA has a surface area of 2 m2/g, but on the supports it is highly dispersed and increases the catalytically active surface. Supported HPAs are more active than typical solid acids. The acidic or neutral solid substances, such as silica gel, alumina, ion exchange resin, and active carbon, are suitable supports (Ramesh Kumar and Leelavathi 2007).
The support generally provides high porosity to favor contact between the substrates and the active phase, and gives stability in the reaction medium. In some cases, the support can also act as a catalyst, and the active phase and support can complement each other to generate a much more active material. There is a wide variety of materials that are commonly used as supports for catalysis, such as metal oxides (Da Conceição et al. 2017; Datka 1992; Freitas et al. 2017; Romanelli et al. 2004). For example, in its γ phase alumina is a microcrystalline solid that usually has a small particle size and a high surface area. It should be noted that not only is the metal oxide itself important, but also how it is structured. α-Alumina, in contrast to γ-alumina, is a dense material with a very low surface area (Atkins et al. 2010). Boehmite is a hydrated form of alumina (Supplementary Figure S2) and is used as a precursor to other species of this oxide (Dubey et al. 2017). In general, the boehmite structure is obtained when the synthesis temperature exceeds 80 °C (Brinker and Scherer 1990) and, for example, if it is then calcined at 600 °C, γ-alumina can be obtained (Zambrano et al. 2016).
For its part, the θ phase can be prepared by dehydration of boehmite since θ-alumina is usually the dominant phase (and in some cases the only type of phase present) before arriving into the more stable α phase (Kovarik et al. 2021).
Usually, the formation of θ-alumina is reported to start around 1,000 °C, and if it was given enough time to equilibrate, the formed microstructure contains about 43 %–46 % of tetrahedral sites, which accounts for the characteristic disorder of this phase. In contrast, the more stable and ordered α phase does not support any tetrahedral sites and have a rhombohedral structure with oxygen arranged on hexagonal closed-packed stacking (ABABAB stacking) (Kovarik et al. 2021).
Alumina is a commonly used support for commercial catalysts due to its high surface area, easily controlled pore structure, high packing density, thermal stability, physical strength, and recoverability (Escobar et al. 2021; Li et al. 2007; Palermo et al. 2011; Ramesh Kumar and Leelavathi 2007). Supports containing alumina are very useful in reactions that require acid or basic catalysis, as these may contain both Brönsted and Lewis acid sites. At high temperatures, the adjacent hydroxyl groups can condense, releasing a water molecule, which generates Al3+ acidic and O2− basic Lewis sites (Deutschmann et al. 2009). A large number of surface acidic and basic sites are then generated, which coexist thanks to the rigidity of the alumina surface. Furthermore, the combination of alumina with other oxides (TiO2/Al2O3 or SiO2/Al2O3) generates various surface acid sites in the final material, which makes it suitable for other types of reactions (He et al. 2019; Himmel et al. 1995; Trueba and Trasatti 2005). On the other hand, these highly reactive surface sites, whether acidic or basic, allow other catalytic centers to be deposited, especially metallic compounds. The metal particles deposited on the support are highly available to react with the substrate because only a part of the atoms that compose it is found inside the support, and the rest are on the surface and ready to react with other reagents in the medium since, being anchored to the support, they do not fuse with each other (Deutschmann et al. 2009).
Also, neutral alumina was conveniently used as support for different catalysts of many organic reactions such as oxidation of alcohols to carbonyl compounds (Varma et al. 1997), cleavage of acetals and ketals (Subhas Bose et al. 2000), and synthesis of nitriles from aldehydes (Subhas Bose and Venkat Narsaiah 1998).
In this review we report the advances associated with the use of heteropolyacid-alumina composites as heterogeneous catalysts in different organic transformations, and give some insights into the chemical structure of the catalyst that causes the catalytic activity (Figure 1).
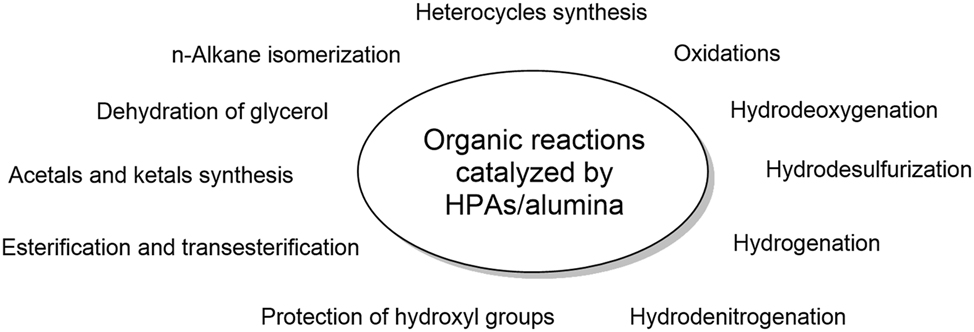
Applications of heteropolyacid-alumina composites in organic transformations.
2 Structure of the catalysts
The predominant application of catalytic aluminas is as supports (Knozinger 1985). Aluminas are commonly used to disperse a variety of active components, including oxides, sulfides, and metals. A common method for the preparation of alumina-supported metal catalysts involves impregnating the alumina with aqueous solutions that contain complex ions of the metal, whether anionic or cationic. The characteristics of the complex ion in solution-such as its size, charge, and ligand sphere-are crucial factors affecting the strength of its adsorption. Additionally, the presence of foreign ions on oxide surfaces can alter the isoelectric point (e.g., anions tend to lower the isoelectric point), thereby influencing the optimal pH conditions for the adsorption of complex noble metal ions.
The interaction of alumina oxides with aqueous solutions is primarily governed by the dissociation of δ-OH species on the surface. The equilibria involved can be represented as: Al–OH2+ ↔ Al–OH ↔ Al–OH− + H+. As the pH of the solution decreases, the equilibrium shifts to the left, which in turn increases the pH value. The isoelectric point, or point of zero charge, for aluminas has been reported to be between pH 8 and 9. Therefore, for effective anion adsorption, the pH should be below 8, whereas cations are best adsorbed at pH values above 9. Experimental data on the equilibrium loadings of molybdate and Na+ ions on alumina, as a function of the final pH of the solution, confirm this prediction. Specifically, molybdate anions exhibit high loadings at low pH values, but these loadings decrease to lower levels between pH 6 and 8 as the isoelectric point is approached (7 MoO42− + 8 H+ ↔ Mo7O246− + 4 H2O). Conversely, the equilibrium loading of Na+ cations increases as the pH rises above the isoelectric point (pH > 8). Additionally, the pH of the solution not only affects the number of molybdate anions adsorbed but also the nature of the molybdate species present in the solution. As the pH decreases, the equilibrium shifts further to the right (Knozinger 1985).
Therefore, polymolybdate anions should be adsorbed under low pH conditions, which favor anion adsorption. Raman and UV spectroscopies have confirmed the presence of polyanions on the γ-alumina surface after equilibrium adsorption at pH values below 6. During subsequent drying and calcination, these electrostatically adsorbed polyanions interact with the alumina surface, replacing the reactive δ-OH groups. These δ-OH groups are the most basic hydroxyl groups, while the less basic groups remain on the surface. The basic δ-OH species, which are exchangeable hydroxide-like surface anions, bind to the support surface in clusters of three-dimensional structures, likely resembling the original polymolybdate anion structures (Knozinger 1985).
Vázquez and Quaranta (2006) studied the behavior of phosphomolybdic acid (PMA) with Keggin structure, supported by incipient wetness impregnation method on different silica-alumina mixtures. Different PMA concentrations and calcined temperatures were evaluated. The synthesized supports exhibited varying textural properties depending on the preparation method, such as simultaneous hydrolysis of both silica and alumina precursors, prehydrolysis of the silica precursor or the use of preformed alumina. These differences in support properties had a notable impact on the characteristics of the final catalysts. The phosphomolybdic acid catalysts retained the defining characteristics of the Keggin primary structure; however, their acidic properties differed based on the support used. This variation led to differences in phosphomolybdic acid-support interactions and dispersion, so the authors believe that the materials may have promising catalytic properties for their use as heterogeneous catalysts for organic synthesis.
Vázquez et al. (1999) also studied the catalyst preparation by equilibrium adsorption of PMA in water–ethanol solutions onto Spheralite alumina. The authors confirmed the presence of the PMo12O403− species by DRS analysis of the PMA-impregnated alumina catalyst and concluded that the HPA keeps its Keggin-type structure when supported at room temperature on the alumina, and that the supported-catalyst remains stable at temperatures below 250 °C. The catalyst was successfully used in the dehydration reaction of isopropanol.
On the other hand, Castillo et al. (1996) found that the PMo12O403− species remains stable in solutions acidified with HCl or in ethanol-water, but depolymerizes to PMo11O397− in water.
When the support is put in contact with the HCl solution, the surface hydroxyl groups of the alumina become protonated, which leads to an increase of the pH of the solutions with respect to their initial values. With this pH increase, a depolymerization of the HPA to PMo11O397− occurs and consequently, protons are released. Proton release will be more intense as the HPA concentration increases, and so the pH will decrease.
On the other hand, when the support is put in contact with the ethanol-water solution, at different concentrations of the final solution, no significant variation of pH is observed. According to 31P NMR and DRS studies, it was proposed that the anion PMo12O403− was the species adsorbed from ethanol-water solution, and the species PMo11O397− from aqueous or hydrochloric solutions.
Tarlani et al. (2005) supported a Wells–Dawson tungsten HPA on sol–gel and non-sol–gel γ-alumina. Their results showed that sol–gel alumina had a greater tendency to adsorb Wells–Dawson tungsten HPA than the non-sol–gel, and in fact the interaction between the HPA and the sol–gel alumina was higher than with the non-sol–gel, with thermal stability of HPA up to 650 °C when it was supported on the sol–gel alumina. The greater tendency of sol–gel alumina to adsorb HPAs compared to commercial alumina is largely due to its hydroxyl-rich surface rather than to its larger surface area. This strong interaction between HPA and the surface OH groups can be explained by the transfer of acidic protons from the highly acidic Wells–Dawson HPA to the OH groups on the alumina surface. In conclusion, supporting the HPA on alumina significantly increases the total acidity of sol–gel alumina, with a lesser effect observed for commercial alumina. The enhanced total acidity of sol–gel alumina with supported HPA is evident from the improved esterification yield of propanoic acid with hexanol, demonstrating that the acidic sites of the supported HPA remain highly active.
In their work, Pizzio et al. (1998) supported phosphotungstic acid (PTA) by impregnation method, using solutions of PTA in ethanol-water and two types of γ-alumina: Akzo and Spheralite. The 31P NMR analysis for the PTA solution after contact with the γ-Al2O3 Akzo support showed a sign at −15.1 ppm that has been assigned to the PW12O403− species, while another sign at −13.1 ppm appeared in the spectrum for γ-Al2O3 Spheralite support, which was assigned to P2W21O716−. So, the contact of PTA with γ-Al2O3 Spheralite produced a partial transformation of the PTA anion.
On the other hand, 31P MAS-NMR analysis for the solid samples of supported PTA was carried out, where the chemical shift corresponding to the tungstophosphate anion PW12O403− was not observed. The spectrum of Akzo-supported PTA showed signs at −12.9 and −11.3 ppm, which were assigned to the dimeric species, whereas for the Spheralite-supported PTA the spectrum showed a sign at −9.8 ppm that would be attributed to the lacunar PW11O397− anion. Also, a DRS analysis was performed, which showed the presence of WO42− species, and so the transformation reactions of the PTA anion into the aforementioned species include the formation of tungstate ions.
It was proposed that when the hydroxyl concentration is increased, the following species transformation occurs: [PW12O40]3− ↔ [P2W21O71]6− ↔ [PW11O39]7−. So, PTA in ethanol-water solution in contact with slightly basic Akzo alumina leads to the transformation towards the dimeric heteropolyanion, and when it is in contact with more basic Spheralite, the transformation shifts further to the right, obtaining the lacunar species. Apparently, this transformation leads to a low acidity of the heterogeneous catalyst prepared and thus, to a decrease in isopropanol conversion in comparison to the bulk PTA.
Forster et al. (2022) analyzed the effect on γ-, θ- and α-alumina when:
it was grafted with 5 wt% zirconia,
it was doped with 30 wt% silicotungstic acid (H4SiW12O40, STA),
both zirconia and STA were used.
XRD analysis revealed a strong interaction between STA and both γ- and θ-Al2O3 (including zirconia-grafted and non-grafted forms). In contrast, the presence of bulk STA on the surface of α-Al2O3 indicated minimal to no interaction between STA and this alumina support. The absence of diffraction peaks characteristic of bulk zirconia in all diffraction patterns confirmed that zirconia was well-dispersed across the alumina surfaces.
The use of heteropolyacids as catalysts, for example, in the dehydration of glycerol to acrolein, is usually limited due to their relatively poor thermal stability. Scheme 1 shows the different steps of thermal decomposition of STA.
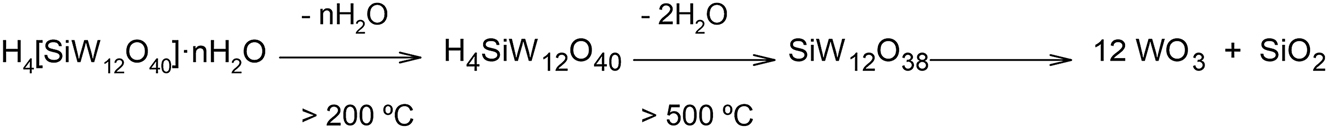
Thermal decomposition of STA with increasing temperature.
In most reactions, such as heterocyclic synthesis, dehydration, and esterification, HPAs act as acid catalysts thank to their Brönsted acidity, by a mechanism in which a proton of HPA is transferred to the organic substrate, mainly to the more electronegative atom. In gas and liquid phase heterogeneous catalysis with HPAs, the reactions can take place on the pore walls or on the outer surface. The activity of the catalysts depends on the nature of the support, the acidity of the HPA, and their load. Basic support, as alumina, strongly interacts with the active compounds. Atia et al. (2008) explained that when the HPA solution and the support are in contact, the surface hydroxyl groups of alumina become protonated acquiring a positive charge on the surface, that can strongly interact with heteropolyanion causing distortion and deactivation. On the other hand, too high loading produces strong interactions with the support, and the acidic properties of the HPA are lost through transformation of the HPA structure into less catalytically active one. PTA is more acid than PMA, therefore PTA is more active, giving the kinetic product, and PMA is more selective towards the product (Llewelyn 2011). The possible adsorption mechanism of HPA includes dehydroxylation of surface alumina, which creates a pair of basic (electron-donating, Al–O−) and acidic (electron acceptor, Al+) sites, which probably anchoring sites by chelate adsorption mechanism and creates an excess negative charge at the HPA. That causes an additional electrostatic binding by the Lewis acid site and also explains the distortion of the structure (Madhusudhan Rao et al. 2005).
The general oxidation mechanism with molecular oxygen or peroxides assisted by HPA follows an electron transfer mechanism. With molecular oxygen, it is included the oxygen atom transfer abstracted from the HPA lattice together with the reduction of the Mo or W, to the substrate. Then, the reduced catalyst can be regenerated by molecular oxygen with the formation of water (Neumann 2010). Meanwhile, when hydrogen peroxide is used as oxidant, the disintegration of HPA to active peroxo species was proposed, followed by the loss of active oxygen and re-polymerization to the original HPA structure (Chen et al. 2021). For example, in the oxidation of sulfides the peroxo species, with electrophilic characteristics, suffers a nucleophilic attack by the S atom of the sulfide to generate sulfoxide. Subsequently, sulfoxide is associated with HPA, through oxygen, and the electrophilic S of the sulfoxide suffers a nucleophilic attack by a new peroxide molecule (Colombo Migliorero et al. 2023, Maciuca et al. 2008). Besides, some oxidation reactions, like limonene epoxidation, are proposed to follow a free radical mechanism (Casuscelli et al. 2004).
3 Heterocycle synthesis
The following section reports the different advances associated with the synthesis of heterocyclic compounds using composites that are formed by the interaction of a support (in this case, alumina) and HPAs of varied structure. Our research group has made significant contributions to the development of HPAs as sustainable catalysts, utilizing a variety of supports such as silica, zirconia, titania, and a mixture of them. Furthermore, we have previously published several review articles on the synthesis of heterocycles, biomass valorization, and oxidation processes using bulk and composites based on HPAs (Escobar et al. 2021; Romanelli and Autino 2009; Ruiz et al. 2022).
This review aims to focus specifically on the use of alumina as a support for these catalysts. Clear differences in conversion rates and selectivity have been observed when HPAs are supported on alumina. The most relevant contributions will be provided regarding the use of compounds formed by alumina and HPAs in the synthesis of heterocycles corresponding to the families of 1,4-dihydropyridines, imidazoles, pyrroles, azlactones, quinoxalines, pyrazoles, chromones, and chromanones (Figure 2).
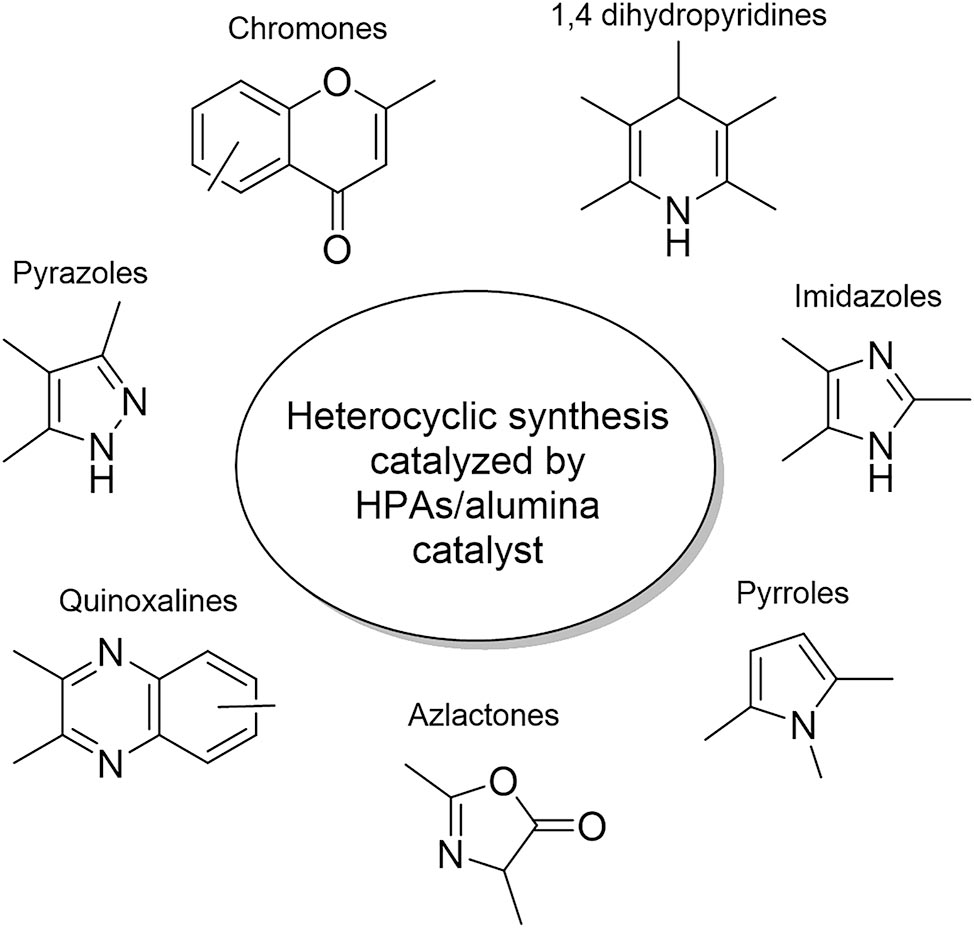
Heterocyclic synthesis catalyzed by heteropolyacid-alumina.
3.1 Dihydropyridines
The derivatives associated with the 1,4-dihydropyridine skeleton are molecules of great importance at biological level and in the pharmaceutical industry. In particular, nifedipine is the most representative compound of this group. It is used as a medication to lower vascular resistance and blood pressure, primarily due to its ability to block calcium channels (Sanchez et al. 2012; Shaldam et al. 2014).
1,4-Dihydropyridine substructure is a constituent of numerous heterocyclic compounds of greater structural complexity that have anti-inflammatory (Idhayadhulla et al. 2015), antitubercular, antibacterial, and anticancer (Sirisha et al. 2010) properties, among others.
The traditional method for the synthesis of pyridines is the Hantzsch procedure, described in 1881 by Arthur R. Hantzsch. In this multicomponent process, an aldehyde, two equivalents of 1,3-dicarbonyl compound, and a nitrogen-containing molecule such as ammonia or ammonium acetate are combined to give 1,4-dihydropyridine. This precursor, also known as the Hantzsch intermediate, is transformed into the aromatic product by a subsequent oxidation step (Scheme 2) (Hantzsch 1881).

Pyridine synthesis by Hantzsch methodology.
The literature reports different processes under low environmental impact conditions for the production of 1,4-dihydropyridines by Hantzsch methodology, using HPAs. Sathicq et al. reported the catalytic activity of HPAs of Wells–Dawson-type structure and concluded that they can be used as efficient and reusable catalysts in the synthesis of dihydropyridines by Hantzsch methodology. On the other hand, Palermo et al. reported the synthesis and characterization of micellar Keggin HPA catalysts using PMA and H4PMo11VO4 (PMVA), and quaternary ammonium compounds such as hexadecyltrimethylammonium bromide. They were used as catalysts in the synthesis of several bioactive 1,4-dihydropyridine derivatives, including nemadipine B and nifedipine (Palermo et al. 2016; Sathicq et al. 2010).
The great versatility of the Hantzsch reaction allows obtaining different heterocycles by modifying the intervening substrates. For example, Gharib’s group reported the synthesis of 1,8-dioxodecahydroacridines by multicomponent synthesis of β-dicarbonyl compounds, dimedone, and ammonium acetate as starting materials (Supplementary Scheme S1). In this procedure, Preyssler-type HPAs (H14[NaP5W30O110]) were used as catalysts (Gharib et al. 2012). Similarly, Baradaran-Sirjani et al. reported the preparation of 1,8-dioxodecahydroacridines also using two Preyssler HPAs, H14[NaP5W29MoO110], (P5W30) and H14[NaP5W30O110] (P5W29Mo) (Baradaran-Sirjani et al. 2018).
An important advantage of supporting HPAs on materials with diverse properties is the enhancement of the composite’s surface characteristics. Since bulk HPAs have a very low specific surface area, this improvement makes the supported materials more effective catalysts for heterogeneous catalysis. On the other hand, it allows modulating their acid-base and redox properties to change the selectivity of some specific reactions.
A very interesting work by Bosica and collaborators shows how the association of HPAs with Al2O3 supports substantially modifies the products obtained from the Hantzsch multicomponent reaction (Bosica et al. 2020) (Scheme 3).
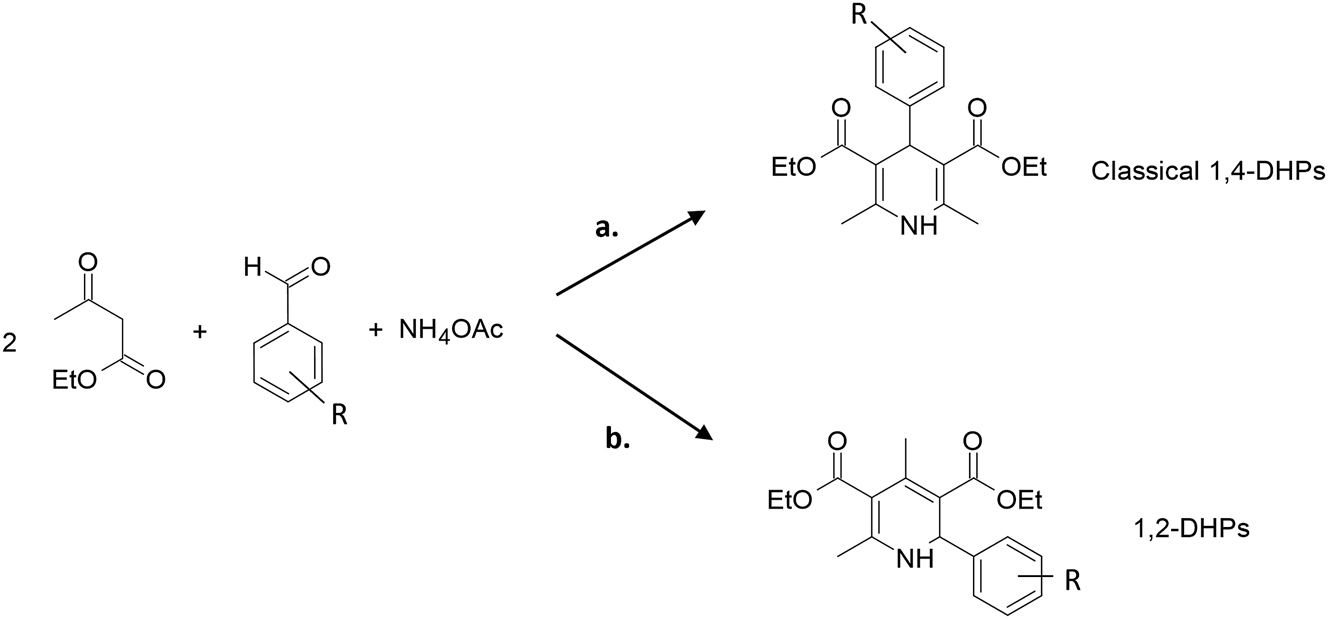
Hantzsch protocol to obtain the classical 1,4-DHPs (a) or 1,2-DHPs (b).
The researchers reported a very efficient route for the preparation of unsymmetrical 1,2-dihydropyridines (1,2-DHPs) as opposed to 1,4-dihydropyridines (1,4-DHPs) via Hantzsch multicomponent reaction (Scheme 3). In this case, a suitable procedure in solvent-free conditions was explored using PTA on alumina support (40 wt%). By means of this methodology, very good yields greater than 75 % were obtained in most cases, in reaction times lower than 4 h. The most active catalyst showed no appreciable loss of catalytic activity after 8 consecutive cycles (just 13 % yield loss but required more reaction time). So it may be concluded that working with a material of lower acidity, high surface, and conventionally lower temperatures close to ambient (20 °C) favors the formation of the kinetic reaction product, that is, 1,2-dihydropyridine. The protocol using HPAs supported on Al2O3 as catalyst represents an environmentally friendly alternative. High atom economy, mild reaction conditions (room temperature), absence of solvent, and high atom economy and selectivity (with high yields) are the great advantages of the methodology (Scheme 3b).
3.2 Imidazoles
Imidazoles are among the most crucial azoles that are well known for their valuable therapeutic properties. These compounds are particularly noted for their antiviral, antitumor, antibacterial, and antiallergenic effects. It is important to note that the antihypertensive compounds Losartan and Olmesartan, which incorporate the imidazole nucleus, are commercially available, and their worldwide consumption is notable (Leurs et al. 2011; Timmerman and van der Goot 2009).
The preparation of imidazole derivatives generally involves the use of multicomponent reactions with high atom economy. Thus, depending on the choice of substrates, highly substituted imidazoles can be obtained, for example, tri- and tetrasubstituted imidazoles when ammonium acetate or a specific amine is used as a source of nitrogen (Scheme 4).

Trisubstituted and tetrasubstituted imidazole synthesis.
Some studies can be found in the literature related to the synthesis of tri- and tetrasubstituted imidazole derivatives using materials based on HPAs (reaction between a 1,2-dicarbonyl compound, a substituted aldehyde, ammonium acetate, and primary amines). For example, Heravi´s group reported the multicomponent reaction of benzaldehyde derivatives, benzyl, primary amines, and ammonium acetate catalyzed by bulk Keggin HPAs (PTA, STA, PMVA, and Na2HPMo12O40) as an efficient and facile one-pot synthesis of tetrasubstituted imidazoles (Heravi et al. 2007b). Das et al. (2013) used aluminum-dopped Keggin-type (H6AlMo11O40) as catalyst for the synthesis of 1,2,4,5-tetrasubstituted imidazoles assisted by microwave (the presence of Lewis acid sites improves catalytic performance), and Yan et al. (2017) reported a microwave-assisted solvent-free catalyzed synthesis of 1,2,4,5-tetrasubstituted imidazoles bearing a 4-aminophenyl substituent. In this case, PTA supported on silica was used as a catalyst. Our research group reported the synthesis of new materials based on phosphotungstic acid supported on core–shell polystyrene-silica microspheres or hollow silica spheres to be used as catalyst in this transformation (Gorsd et al. 2016).
Although there are several reports on the utilization of HPAs in the synthesis of densely substituted imizadoles, only one work by Raffiee’s group described the use of alumina as a support for HPAs (Rafiee et al. 2011). The research is extensive and focuses on the synthesis of catalysts derived from HPAs, which are supported on various matrices. The synthesis of catalysts based on PTA and their support on materials with different characteristics, such as silica, alumina, titania, clay, and carbon, was addressed. They were tested in the multicomponent synthesis of imidazoles in solvent-free conditions.
Specifically, a catalyst based on PTA supported on γ-Al2O3 was prepared. It was made as follows: a specific amount of HPAs was dissolved in 25 mL of water and 25 mL of methanol. Then 5.0 g of γ-Al2O3 was dropped into the solution under vigorous stirring to be impregnated for 24 h. The catalyst was tested in the reaction between benzyl, benzaldehyde, benzylamine, and ammonium acetate (reactant ratio 1:1:1:1), with excellent yields (91 %–98 %) (Supplementary Scheme S2).
The reaction test was conducted using 200 mg of supported catalysts under solvent-free conditions at 140 °C. The catalysts demonstrated excellent activity and achieved 100 % selectivity towards the desired imidazole. The effectiveness of the catalyst in facilitating the transformation was found to correlate strongly with the high acidity of the supported HPAs. The reuse of this catalyst was not informed.
Although the researchers established that the reaction in silica was the fastest and the one that gave an excellent yield (97 %, 2 h), it was observed that using alumina the yield results were similar (97 %, 4 h). The favorable interaction between Al2O3 and HPAs renders alumina an ideal candidate for support materials. Unlike silica, which can adversely affect many organic compounds, alumina-based catalysts sidestep these compatibility challenges. The impressive yields obtained with PTA supported on Al2O3 not only highlight its effectiveness but also open the door to exploring new reactions in organic synthesis. These reactions require a specific and well-tuned acidity, which can be adjusted through the interaction between the support and the HPAs.
3.3 Pyrroles
Pyrroles, while highly versatile molecules in various chemical transformations, are characterized by a remarkable instability under certain conditions. Their excessive π aromatic structure (five-membered cycle, featuring a nitrogen atom integrated into the conjugated π system) contributes to their chemical reactivity. Pyrroles are very sensitive to oxidation, which often leads to the formation of polymeric products. In addition, strong acids can protonate the nitrogen, destabilizing the ring and potentially causing decomposition, while strong bases can deprotonate it, further compromising stability. They are sensitive to light, and elevated temperatures can also induce decomposition, producing a variety of by-products. These factors highlight the need for careful handling and control in reactions involving pyrroles to avoid unwanted decomposition and ensure desired results in synthetic processes (Trofimov et al. 2016).
Recent studies by our research group have shown that when Preyssler-structured HPAs are supported on silica-based materials, these materials easily induce the decomposition of pyrroles, which led us to search for more efficient materials that can inhibit such decomposition. For this reason, we have worked with Preyssler-structured HPAs supported on commercial aluminas with different acid-base properties (Portilla Zúñiga et al. 2020).
Pyrrole and its derivatives are significant nitrogenous heterocyclic compounds due to their presence in a variety of naturally occurring substances, including porphyrins, bacteriochlorins, chlorophyll, vitamin B12, bile pigments such as bilirubin and biliverdin, as well as alkaloids from marine sources (Azad et al. 2018). They are notable substrates for generating bioactive compounds valuable in medicinal chemistry and pharmacology. Various bioactivities have been reported, including HIV inhibition, antibacterial and antifungal effects, antioxidant properties, antitumor and anticancer activities, as well as antimalarial and anticonvulsant effects (Bhardwaj et al. 2015; Lu et al. 2016; Teixeira et al. 2008).
There are numerous reports on methods for the synthesis of pyrrole derivatives, including Michael additions, Friedel-Crafts alkylation and acylation, Knorr synthesis, Heck coupling, Paal-Knorr condensation, Hantzsch synthesis, carbenoid insertions and cyclization (Tzankova et al. 2018).
In recent years, our research group has reported a suitable procedure for the synthesis of pyrroles using Paal–Knorr methodology (Portilla-Zúñiga et al. 2018). For this purpose, catalysts based on Preyssler-type HPAs (H14[NaP5W30O110] and H14[NaP5W29MoO110]) supported on commercial mesoporous Al2O3 (CATALOX SBa-90®) were synthesized (bifunctional catalysts with acidic and basic properties).
The activity of the bulk and alumina-supported catalysts was tested, and 12 compounds were obtained in solvent-free reactions, short reaction times (0.5–3 h), and temperatures between 60 °C and 100 °C (Scheme 5). There were no significant differences between the yields achieved with the supported and bulk catalysts (53–98 %). However, the amount of active phase used in the supported catalysts was significantly lower. This is important because it allows the cost of the catalyst to be reduced on a large scale. The catalyst supported on alumina could be easily recovered and used for four cycles without appreciable loss of activity.

Paal-Knorr methodology for pyrrole synthesis.
3.4 Azlactones
Oxazol-5-(4H)-ones (azlactones) are molecules featuring a highly substituted five-membered ring with various reactive sites. This makes them a crucial building block for synthesizing various compounds of pharmacological and industrial significance. This family of compounds has been studied as starting substrates in the synthesis of a wide variety of reactions as precursors for the synthesis of biologically active peptides (Reed and Kingston 1986), and agrochemical intermediates (Kaushik and Verma 2011). Various azlactones are active as antihypertensives, antitumor, and as inhibitors of the central nervous system (Gelmi et al. 1997). They have been used in active site titrations of enzymes (Baese and Havsteen 1989) and in the asymmetric synthesis of amino acids (Chandrasekhar and Karri 2006).
The Erlenmeyer synthesis is the oldest and most efficient method for producing azlactones. It involves the condensation of benzaldehydes with hippuric acid, using sodium acetate as a catalyst and acetic anhydride as a dehydrating agent. The method’s significant versatility has led to the research into eco-efficient processes, including studies on the use of heterogeneous catalysts and solvent-free conditions (Cleary et al. 2010; Paul et al. 2004; Yu et al. 2006). Even more recently, alternative techniques have been tested to improve the efficiency of their synthesis, which involves the use of microwaves or ultrasound (Chandrasekhar and Karri 2007; Conway et al. 2009; Rostami et al. 2011b).
Two previous studies describe the use of HPAs in the synthesis of azlactones. Ruiz et al. (2012b) reported the synthesis of Well-Dawson (H6P2W18O62·24H2O) catalysts supported on silica for azlactone synthesis. Nine azlactones were obtained with excellent yields (70 %–93 %). The catalyst was reused several times without loss of catalytic activity.
Likewise, and under solvent-free conditions at 80 °C, Rostami et al. (2011a) evaluated organic-inorganic hybrid materials based on Keggin-type polyoxometalate PTA and Na2WO4 salts with 1-butyl-3-methylimidazolium. The authors reported 15 compounds obtained in 5–15 min with yields between 89 % and 95 %.
In both cases the materials were recovered and recycled without appreciable loss of catalytic activity (Rostami et al. 2011a). As previously mentioned, the preparation of mixed materials, for example, mixtures of silica and alumina (by the sol-gel method), generates materials with specific properties. In particular, the incorporation of aluminum into the matrix enables the formation of new acid and base centers of Lewis type. Depending on the preparation procedure, these materials with specific characteristics can interact with HPAs to form catalysts with different strengths and a different number of acid sites. Some reports have shown that adsorption on SiO2–Al2O3 mixtures is not as strong as in the case of pure SiO2, and even the case of reversible adsorption of HPA on a mixed silica-alumina support has been mentioned (Deutschmann et al. 2009; He et al. 2019; Sebulsky and Henke 1971).
Blanco and co-workers prepared and carried out a full characterization of new materials based on phosphomolybdic and phosphotungstic acid included in silica-alumina mixtures. Three different methods were employed, thus generating supports with different properties (Romanelli et al. 2009). The materials activity was tested in the synthesis of 4-benzylidene-2-phenyloxazolin-5-ones and 4-alkylidene-2-phenyloxazolin-5-ones (azlactones) by the Erlenmeyer methodology. In a standard experiment, the aldehyde, hippuric acid (ratio 1:1), and the catalyst were added to 2 mL of toluene and heated to 110 °C for 5 min. Then, a stoichiometric amount of acetic anhydride was added, and the reaction mixture was refluxed for 1 h (110 °C). Eleven compounds were obtained with high conversion and selectivity (87 %–96 %), with the exception of the azlactones synthesized from 2-nitrobenzaldehyde and cyclohexanone, which gave 70 % and 80 % yields respectively. The catalytic materials were tested three consecutive times without significant loss of catalytic activity (Scheme 6).

Synthesis of 4-benzylidene-2-phenyloxazolin-5-ones and 4-alkylidene-2-phenyloxazolin-5-ones by Erlenmeyer method.
3.5 Quinoxalines
Quinoxalines are nitrogen-containing heterocycles characterized by the fusion of a benzene ring with a pyrazine. They are of great relevance as they are very useful intermediates in organic synthesis. This substructure is considered as a relevant skeleton for the design of numerous heterocycles, which can present a few bioactivities. Many of these compounds are characterized by their great utility for technological applications such as electroluminescent materials, semiconductors, and dyes (Dailey et al. 2001; Jaung 2006; Justin Thomas et al. 2005). In addition, they are very useful for the pharmaceutical industry due to their biological properties, including antioxidant, antimalarial, antibacterial, antitumor, anticonvulsant activity, and many others (Burguete et al. 2007; Corona et al. 2009; Vicente et al. 2008; Wagle et al. 2009). Quinoxalines are very relevant in the pharmaceutical drug industry, being found as a substructure in some antibiotics such as echinomycin, levomycin, and actinoleutin (Raw et al. 2004).
The classical method for quinoxaline preparation is the condensation of 1,2-diamino compounds with 1,2-dicarbonylic compounds. Several catalysts were tested in these reactions such as acetic acid (Islami and Hassani 2008), nickel nanoparticles (A. Kumar et al. 2008), montmorillonite K10 (Huang et al. 2008), nano-TiO2 (Mirjalili and Akbari 2011), Al2O3 (Jafarpour et al. 2011), silica-bonded S-sulfonic acid (Niknam et al. 2009), sulfamic acid/MeOH (Darabi et al. 2007), and others.
In particular, the HPAs of Keggin, Wells-Dawson, and Preyssler structures have been used both in bulk and supported forms in the synthesis of quinoxalines from 1,2-dicarbonyl compounds and 1,2-diamines. Tai Kun Huang and co-workers reported a suitable method for quinoxaline synthesis using Keggin-type HPAs in bulk form in water as solvent (Huang et al. 2009). Hakimia et al. reported a suitable synthesis of quinoxaline using the reaction between α-diketones and o-phenylenediamines in the presence of Keggin-type HPAs with high yields, in short reaction times, and at room temperature (Hakimia and Mirjalili 2013). In this regard, Heravi et al. (2007a) reported the use of Wells-Dawson HPAs that catalyzed the synthesis of different quinoxaline derivatives at room temperature, and Venkateswara Rao et al. (2009) reported the use of iron-exchanged phosphomolybdic acid as a heterogeneous catalyst for the synthesis of these compounds.
To enhance the activity and reusability of HPAs, they have been supported on various materials. For instance, our research group developed a heterogeneous catalyst by supporting phosphotungstic acid on zirconia oxide. This catalyst was tested for synthesizing quinoxaline derivatives through the condensation of 1,2-diamines with 1,2-dicarbonyl compounds, using solvent-free conditions and conventional heating (Sosa et al. 2013, 2022).
Due to the properties of alumina and the way it is associated with HPAs, we explored the use of HPAs containing Fe and Cu (FeHPMo11VO40 and CuH2PMo11VCuO40), in a simple and efficient method for the preparation of quinoxaline derivatives (Ruiz et al. 2012a). The quinoxaline synthesis involving the reaction of substituted o-phenylenediamines and 1, 2-diketones is illustrated in reaction Scheme 7.

Synthesis of quinoxaline derivatives catalyzed by heteropolyacids on alumina (Me = Fe, Cu).
Initially, experiments were carried out to verify the influence of the metals (Cu and Fe) present in the HPAs, which were supported on the alumina matrix (commercial alumina Akzo). The AlCuMoVP and AlFeMoVP catalysts were tested.
The experimental conditions were: 100 mg of catalyst, 1 mmol of o-phenylenediamine, 1 mmol of benzyl, and 7 mL of toluene; temperature, 25 °C; reaction time, 2 h. Under these conditions, a quinoxaline yield of 92 % and 80 % was obtained with AlCuMoVP and AlFeMoVP, respectively. Encouraged by the remarkable results obtained with the previous reaction and to demonstrate the generality and scope of this new protocol, we used several substituted 1,2-phenylenediamines and two diketones under the optimized conditions at room temperature with AlCuMoVA. All reactions were carried out under very clean conditions at room temperature, and no undesirable side reactions were observed, although the reaction time for 100 % conversion of the substrates and the reaction yields of the products were highly dependent on the substituent. The results show that electron-donating groups on the phenyl ring of 1,2-diamine favored product formation. In contrast, electron-withdrawing groups, such as chlorine and bromine, slightly reduced yields at longer reaction times. 4-Nitro-2,3-diaminobenzene and 2,3-diaminopyridine also gave moderate yields. Nine examples were obtained with a yield between 68 % and 92 %. The catalyst was filtered and reused four times, without no appreciable loss of activity.
3.6 Pyrazoles
In general, both pyrazole and pyran molecules exhibit a number of bioactivities. Particularly, pyranopyrazoles have been studied for their therapeutic properties in medical applications (El-Gazzar et al. 2008; Fayed et al. 2009; Sondhi et al. 2005), including antibacterial, antifungal (Fathalla et al. 2009; Sayed et al. 2010) analgesic, anti-inflammatory (Hafez et al. 2008), ulcerogenic (El-Assiery et al. 2004), antituberculosis (Vaghasiya et al. 2008), antioxidant (Abu-Hashem et al. 2010), and antihypertensive (Svetlik et al. 2009) activity. Some pyrazole carbohydrazide derivatives have been found to have moderate anticancer activity (Xia et al. 2007).
Sukale et al. (2024) reported the synthesis of a series of heterogeneous catalysts formed by silicomolybdic acid impregnated in an alumina support (10 %–40 % by weight of SMA/Al2O3), which were tested for the multicomponent synthesis between aldehydes of different structural nature, hydrazine hydrate, ethyl acetoacetate, and malononitrile, in solvent-free conditions at 80 °C (Scheme 8).

Synthesis of pyranopyrazole using 30 % SMA/Al2O3 catalyst.
In a typical experiment, a mixture of aromatic aldehydes (1 mmol), ethyl acetoacetate (1 mmol), hydrazine hydrate (1 mmol), and malononitrile (1 mmol) was prepared and stirred at 80 °C by using SMA/Al2O3 (0.2 g) catalyst under solvent-free conditions. The reaction was monitored by TLC. At the end point of the reaction, 10 mL of ethanol was added to the reaction mixture, the system was heated to reflux and filtered while hot. The crude product was recovered by evaporation of the solvent. It was purified by recrystallization from ethanol and identified by appropriate spectroscopic techniques. The catalyst could be recovered to be used in the following tests by washing with ethanol at 20 °C and drying for 24 h in vacuum at 20 °C.
As can be seen, the methodology is general and in a simple way allows obtaining 12 pyrazole derivatives with excellent yields between 85 % and 95 %, in very short reaction times (8–15 min). The protocol is simple, in solvent-free conditions, and the catalyst can be recovered without appreciable loss of catalytic activity. No influence of the substituents in the aromatic ring on the reaction rate and yields is observed. Neither are steric hindrances recorded when working with bulky aldehydes such as 2,4,6-tribetoxybenzaldehyde or 2,6-dimethylbenzaldehyde.
3.7 Chromones and chromanones
The heterocycle 1-benzopyran-4-one, commonly called chromone, is the core of a very abundant family of natural compounds, such as flavones, flavonols, and isoflavones. Many of the compounds derived from this ring have been relevant to the discovery and design of compounds with therapeutic properties, such as anticancer, anti-inflammatory, and anti-HIV. The different substituent of the chromone ring determines their different biological activities. Among the compounds containing the chromone ring in their structure, the most important are 2-aryl substituted chromones, particularly flavones. They are present in different food sources (fruits, infusions, wines, and oils) (Ravisankar et al. 2020; Tawfik et al. 2015). Molecules present in cereals belonging to the flavone families are associated with properties such as improved insulin sensitivity and increased interaction with intestinal membrane transporters. Neuropharmacological properties have been found in flavonoids such as chrysin present in honey, and there are many studies on the anticancer activity of natural flavonoid compounds. Other properties identified in compounds based on the chromone skeleton make them appropriate for optical applications and as chelating agents, reflecting their innumerable possibilities of being used in the design of new organic materials (Awika et al. 2018; German-Ponciano et al. 2020; Menezes et al. 2014; Raffa et al. 2017).
Among the numerous methods to synthesize flavones and their derivatives, such as carbonylative cyclization, oxa-Michael-oxidative annulation, oxidative coupling/CeH activation, and oxidative dehydrogenation of flavanones, the cyclodehydration of 1-(2-hydroxyphenyl)-1,3-diketones, using different catalysts, is the most important. It includes acetic acid, hydrogenchloride in acetic acid, and sulfuric acid in acetic acid (Bennardi et al. 2007; Hirao et al. 1984; Ollis and Weight 1952; Ravishankar et al. 2016; Sagrera and Seoane 2005).
The literature reports some experiments to synthesize chromones, particularly flavones using HPA-based materials. Gharib et al. (2010) reported the use of Keggin and Preyssler HPAs supported on silica as catalysts for obtaining substituted chromones and flavones by direct cyclization-dehydration of 1-(2-hydroxyphenyl)-3-aryl-1,3-propanediones. Our research group developed new materials for the synthesis of these compounds such as mesoporous compounds of titania and phosphotungstic acid (Pérez et al. 2013), and Wells–Dawson HPAs supported on silica (Bennardi et al. 2011). On the other hand, Colombo Migliorero et al. (2018) reported a similar procedure to prepare flavones and chromones using phosphomolybdic acid and glycerol as suitable solvent.
Similarly, the chromanone core is present in different heterocycles, among which the flavanones stand out, which have different biological activities such as antitumor, antifungal, and antibacterial (Sagrera and Seoane 2005). They are prepared by an intramolecular conjugated addition of 2-hydroxychalcones using a wide variety of catalysts. Among them, the following stand out: polyethylene glycol 400 (D. Kumar et al. 2008), sodium acetate in ethanol (Ganguly et al. 2013), and methanesulfonic acid (Kulkarni et al. 2012).
Our research group performed a systematic study on the use of a hybrid material based on the combination of a silica-alumina mixture (prepared by sol-gel) and a Keggin HPA (H3PMo12O40) as a catalyst in the cyclization of 1-(2-hydroxyphenyl)-3-(2-furyl)-1,3-propanedione under solvent-free conditions (Scheme 9a). Furthermore, 1-(2-hydroxyphenyl)-3-(2-furyl)-2-propene-1-one was cyclized to 2-(2-furyl)-2-chromanone (Scheme 9b) (Palermo et al. 2022). In previous research we found that the preparation of a hybrid material based on a support and a Keggin HPA improves the catalytic activity in alcohol pyranylation reactions. The obtained hybrid material also facilitates a better separation of the reaction medium and its subsequent reuse. Substrates containing the furan ring were chosen, since furfural is a building block present in biomass, whose valorization is highly desirable.

Synthesis of 2-(2-furyl) chromone (a) and 2-(2-furyl) chromanone (b).
Different reaction conditions were investigated for optimizing the cyclization of 1-(2-hydroxyphenyl)-3-(2-furyl)-1,3-propanedione. The work was carried out in solvent-free conditions at 110 °C. The optimum catalyst was selected after several experiments with different materials that were prepared by varying the support preparation method, SiO2/Al2O3 ratio in the support, the content of active phase (HPAs), and the calcination temperature of the composite. The most active catalyst was the one containing 20 % PMA and calcined at 250 °C for a period of 24 h. The details of its preparation can be found in the corresponding reported bibliography. The best reaction yields correspond to the materials that presented the highest strength and number of acid sites. It was also observed that the calcination temperature up to 250 °C favored the transformation. The yields using dehydrated HPAs were higher than those using HPAs without dehydration. This is associated with the dehydration of HPAs, which decreases the number of water molecules in the secondary structure, so the acid strength and, consequently, the catalytic activity increase.
The optimal conditions established for 2-(2-furyl)-chromone synthesis were: substrate (0.5 mmol), catalyst (1 mmol%), solvent-free, 100 °C, 1 h, stirring. In these conditions chromone was obtained with a yield of 90 %. The catalyst was reused five times and maintained its activity (90 %, 89 %, 89 %, 87 %, 86 %, and 86 % respectively), without considerable weight loss after each consecutive cycle.
In a similar way, the synthesis of chromanones from chalcones was studied under standard conditions (without solvent, the same catalyst, and a temperature of 110 °C). Under these conditions the reaction did not take place, and the starting material was quantitatively recovered. However, when the reaction temperature was raised to 140 °C (sealed tube), a good yield of 2-(2-furyl)-chromanone was obtained without secondary product formation. Under the optimized conditions for both procedures (110 °C for chromones and 140 °C for chromanones), three 2-(2-furyl)-chromones and three 2-(2-furyl)-chromanones were synthesized. The results are given in Scheme 10.

Synthesized 2-(2-furyl)-chromones and 2-(2-furyl)-chromanones.
In all the examples, the expected products were obtained with very good yields and excellent selectivity without appreciable amounts of secondary products. It can be observed in Scheme 10 that the proposed methodologies give very good yields of 2-furyl-2-chromones (71 %–90 %) and 2-(2-furyl)-chromanones (65 %–72 %). In all the cases, the products were obtained with excellent selectivity and practically without formation of secondary products. In addition, unreacted starting material could be recovered.
4 Oxidation reactions
This section summarizes the use of heteropolyacid-alumina composites in oxidation processes such as oxidation of sulfides into sulfoxides and sulfones, oxidation and ammoxidation of alkanes, and epoxidation of alkenes (Figure 3).
Oxidation reaction catalyzed by heteropolyacid-alumina.
4.1 Selective sulfoxidation
The sulfoxidation converts sulfides into sulfoxides or sulfones, which are important intermediates in organic synthesis, especially for bioactive compounds. For example, chiral cyclic sulfones play a crucial role as scaffolds in several pharmaceutically significant compounds and natural products, which demonstrate a wide range of biological activities, including the inhibition of hepatitis C virus, HIV-1 protease, human carbonic anhydrase II and influenza neuraminidase (Alam et al. 2018). Also, some sulfone-bis-compounds were formulated and showed as potential anticancer agents. These compounds, which have a biologically active 1,2-dihydropyridine-2-one, acrylamide, chromene and chromenopyridine moieties, were investigated for Al-Said and collaborators and have been found to exhibit strong anticancer activity against the human breast cancer cell line (Al-Said et al. 2012). Legros et al. (2005) summarized various sulfoxides used in the treatment of diverse diseases. For example, the compound named Sulindac was probed to be an efficient anti-inflammatory drug which is mostly used in the treatment of pain and various types of arthritis. Also, a racemic sulfoxide (YM-38336) was found to be a potent neurokinin receptor agonist-induced. For his part, Hassan (2014) studied the antibacterial activities of dimethylsulfoxide against a series of Gram positive and Gram negative pathogenic organisms. The antibacterial activity of the sulfoxide was tested in vitro with and without of some ligands complex of the transitional metal ions of ethyl coumarin, and found that dimethylsulfoxide is effective against the proliferation of the bacteria.
This transformation could be done using traditional oxidants or environmentally friendly oxidants, such as hydrogen peroxide in the presence of an appropriate catalyst (Kupwade 2019).
Vázquez’s group reported the selective oxidation of diphenyl sulfide for obtaining diphenyl sulfoxide in green conditions (Scheme 11), using niobium-doped PMA, included in alumina and silica-alumina matrices (Colombo Migliorero et al. 2021).
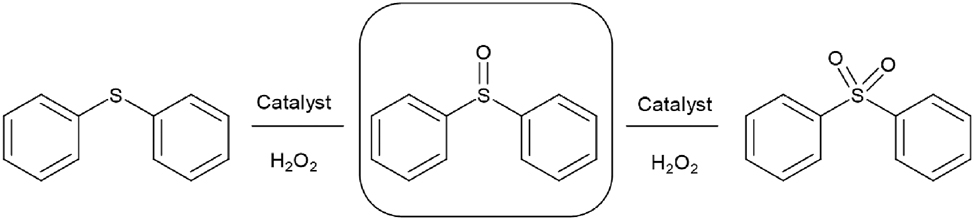
Selective oxidation of diphenyl sulfide.
Catalysts were prepared by sol-gel method from aluminum tri-sec-butoxide and tetraethoxysilane precursors, adding the HPA solubilized in ethanol, which also acts as an acid catalyst for the sol-gel synthesis, so that the matrix is formed with the HPA included in it. Thus, catalysts containing Nb-PMA incorporated in matrices of alumina, silica, alumina:silica 1:1, and alumina:silica 4:1 were prepared. For comparative purposes, PMA included in the mixed matrix was also synthesized: PMA-SiAl-1:1 and PMA-SiAl-4:1.
All the catalysts prepared were tested in the selective oxidation of diphenyl sulfide, using hydrogen peroxide as oxidizing agent, and the matrices without HPA inclusion were tested under the same reaction conditions.
The Nb-PMA catalysts showed higher values in diphenyl sulfide conversion than PMA catalysts. Regarding supports without active phase, no conversion was observed using SiO2 after 24 h, but when Al2O3 was used, 82 % conversion and 65 % selectivity were obtained. The mixed matrices produced lower conversion and high selectivity (up to 95 %). On the other hand, high diphenyl sulfide conversion (near 100 %) was reached after 5 h for all Nb-PMA catalysts. However, at this time, the selectivity toward diphenyl sulfoxide was moderate due to the subsequent oxidation of diphenyl sulfoxide to diphenyl sulfone. Taking into account the conversion and selectivity, the best results were obtained at 4 h with Nb-PMA in the silica matrix (92 % conversion and 94 % selectivity), and at 3 h with Nb-PMA in the silica:alumina 4:1 matrix (92 % conversion and 95 % selectivity).
The reuse of these two catalysts was also tested. The results showed a slight decrease in the catalytic activity for both catalysts, but a bigger decrease was obtained with Nb-PMA in the silica matrix. It was concluded that both matrices were efficient for the inclusion of HPA, allowing their use as recyclable catalyst in the selective sulfoxidation of diphenyl sulfide. Moreover, mixed framework the silica:alumina 4:1 matrix presented better performance in its reuse.
4.2 Oxidative desulfurization
Apart from hydrodesulfurization (see below), which has limited efficiency for highly substituted thiophenic and dibenzothiophenic compounds, oxidative desulfurization, biodesulfurization, adsorption, and extraction were employed for sulfur removal. In oxidative desulfurization, sulfur compounds are oxidized to the corresponding sulfone, which then can be removed by extraction. 4,6-Dimethyldibenzothiophene (4,6-DMDBT) is the main organosulfur compound present in diesel fuel.
For instance, García-Gutierrez et al. (2014) used tungsten-supported catalysts for the oxidative desulfurization of diesel with hydrogen peroxide as oxidant agent. Among the tungsten catalysts (tungsten oxide, tungstic acid, and ammonium tungstate) PTA on alumina was the most effective. Other supports, such as silica, titania, and titania-alumina, were also employed. Supported catalysts were prepared by equilibrium adsorption technique. The reaction test was carried out using diesel fuel and H2O2 (30 wt%) in acetonitrile at 60 °C and atmospheric pressure (Scheme 12). After the end of reaction (1 h), sulfone was separated by extraction with acetonitrile. Sulfur content of the diesel phase was measured. Almost 71 % sulfur removal was achieved using PTA/titania, 69 % with PTA/titania-alumina, and 55 % with PTA/alumina (in concordance with the isoelectric point of the supports, which allow the adsorption of heteropolyanions). In alumina PTA catalyst, the formation of Dawson anion was detected by Raman spectroscopy due to the pH value in the impregnation. The reuse of catalysts was evaluated, observing a partial leaching of active phase after six cycles and a light decrease in sulfur elimination.
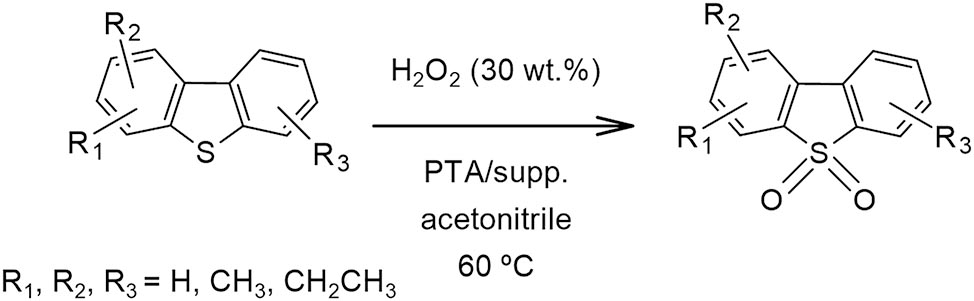
Oxidative desulfurization of dibenzothiophenes.
De Angelis et al. (2007) used in situ produced peroxides to convert sulfides into sulfones, which can be removed by adsorption chromatography. This methodology reduces the sulfur content to less than 10 ppm. In de Angelis’ work, many HPAs with different structures, Keggin (PMA, PTA, SMA, H5PMo10V2O40, H3PMo10W2O40, and H4PMo9W2VO40); Anderson [(NH4)3TeMo6O24, (NH4)3Co(OH)6Mo6O18]; Dawson [(NH4)6P2Mo18O62]; and Dexter–Silverton [(NH4)8CeMo12O42], were supported on alumina pretreated with HNO3 (to remove basic sites that can modify the HPA structure) by wetness imbibition with aqueous solution of HPA. The solids were dried at 100 °C for 5 h. The Mo, W, or V content was 12 wt%. Catalysts were evaluated in the conversion of 4,6-dimethyl-dibenzothiophene to sulfone in a batch reactor and in a fixed-bed reactor (Supplementary Scheme S3).
In a batch reactor 200 g of synthesized diesel (80 % n-hexadecane and 20 % tetralin), 90 mmol of 4,6-DMDBT, 600 mg of catalysts, and 10.6 mmol of TBHP as oxidant were maintained at 5 bar, and 80 °C for 2 h. Meanwhile, in the fixed-bed reactor 30 mmol/kg real diesel was treated with 3 g of catalyst and TBHP at 110 °C. Keggin HPA, particularly H5PMo10V2O40, was the most active and selective catalyst in the batch experiment (complete sulfur conversion in 2 h) since Keggin HPAs are able to form the peroxo-HPA complex, and Dexter-Silverton HPA cannot (despite the redox potential E° = 1.6 V vs. 0.6 V). Also, PMo and PMo10V2 were active in the fixed-bed reactor using real diesel. PMo10V2 catalyzed TBHP decomposition, increasing the process costs, whereas PMo prevented oxidant decomposition, so the latter was more active (more than 2,000 h on stream), and the residual sulfur content was lower than 10 ppm. Besides, both catalysts can be regenerated by treatment at 250 °C under air flow.
Bryzhin et al. (2019) studied oxidative desulfurization using supported ionic liquid (IL) (4-(3′-ethylimidazolium)-butanesulfonate protonated with PTA and PMA) as heterogeneous catalysts. The supports, silica and granulated γ-alumina, were impregnated with aqueous solutions of IL. It was observed that during the functionalization, the heteropolyanions partly decomposed on alumina, due to the strong interaction between the heteropolyanion and the alumina surface. However, compared with bare HPA on alumina, stabilization from the imidazolium cation was observed. On the other hand, no deterioration of HPA occurred on the silica surface.
Thiophene, thioanisole, and DBT in isooctane were used as model sulfur compounds. Special interest presents the oxidation of DBT, which is difficult to remove by hydrodesulfurization. The most active catalyst was based on PMA on silica, associated with the high oxidative potential of PMA and its stability on this support. The reaction tests were performed using hydrogen peroxide (50 wt%) in a refluxed glass reactor at 60 °C. Thiophene was oxidized to sulfuric acid and carbon dioxide (no organic product was detected), while thioanisole and DBT produce the corresponding sulfones. The authors proposed that the acidity of catalysts played an important role: the substrates were adsorbed by the imidazolium cation on the surface by a hydrogen bond, activating the thiophene. Also, peroxo species formed in the anionic part acted on the thiophene with a distorted aromatic structure. Using fuel, the residual amount of S was less than 10 ppm. For evaluated the recycling of catalyst, the reaction solution was decanted and the catalyst was washed with isooctane. The catalyst on silica remains stable throughout five operation cycles, meanwhile the reuse of the catalyst on alumina was not informed.
Manríquez et al. (2022) employed PTA supported on alumina, V2O5, and ZrO2 (prepared by impregnation) in the oxidative desulfurization of 4,6-DMDBT using hydrogen peroxide as oxidant, in acetonitrile, at room temperature and atmospheric pressure, as reaction model to reduce the sulfur content in diesel.
Two load amounts of PTA (3 and 3.5 mol %) were evaluated. Among the characterization techniques, potentiometric titration with pyridine was done to determine the acidity of catalysts. Among the oxides used as support, alumina produced the catalysts with the highest surface area and acidity.
4.3 Ammoxidation
Guerrero-Pérez et al. (2008) reported the use of different HPAs supported on alumina as catalyst for the ammoxidation of propane to acrylonitrile at 400 °C (Supplementary Scheme S4). Acrylonitrile is principally used as monomer for the manufacture of useful acrylic fibers, resins, and nylons. Also is precursor in the synthesis of acrylamides, polyacrylamides, and acrylic acid. The supported catalysts were synthesized using a single-step incipient wetness impregnation method with Al2O3 support. The metal precursors included ammonium metavanadate (NH4VO3), ammonium molybdate tetrahydrate ((NH4)2MoO4·4H2O), and/or TeO2, with tartaric acid used as a complexing agent. The aqueous solution was allowed to stabilize for at least 12 h before the impregnation process. Following this, the materials were dried for 12 h at 100 °C in air, and subsequently calcined at 600 °C for 2 h under a nitrogen atmosphere.
Heteropolyacid-like structures appear to be dominant in all the synthesized materials, according to the Raman spectra, and it was found that Mo–V–W–O based catalysts are not selective to propane ammoxidation. When tellurium is added to Mo–V–O based bulk catalyst, the catalytic activity is hindered, but the selectivity to acrylonitrile is enhanced. According to the XRD patterns, the incorporation of Te to Al2O3-supported catalysts does not drastically change the structure of catalysts, but the Raman spectra showed that the presence of Te modifies the V5+/V4+ surface ratio, changing the environmental sites responsible for propane activation and thus affecting the final activity.
The authors demonstrated the presence of V2O5, which reduces the selectivity towards acrylonitrile while increasing the selectivity towards COx. In the context of propane ammoxidation over the Sb–V–O system, it seems that a VSbO4-rutile structure, where vanadium is reduced to V3+, is responsible for the increased selectivity to acrylonitrile. The reusability of the catalysts was not evaluated.
4.4 Epoxidation
Some terpenes and related oxygenated compounds derived from them are important for the pharmaceutical and other industries. Limonene has multiple uses in different industries: in the paint and coatings industry, it serves as a solvent for various formulations, including paints, resins, inks, and pigments, and it is also used in the production of adhesives; it is a valuable component in the formulation of fragrances for its distinctive citrus aroma; and in the food industry, limonene is utilized for its aromatic properties and is incorporated into flavoring agents. Epoxidation is one of the most widely used reactions to prepare some fragrances and flavors (Casuscelli et al. 2004).
Limonene epoxidation with H2O2, using Keggin PTA and PMA supported on alumina, was reported by Blanco’s group (Casuscelli et al. 2004). Catalysts were prepared by pore filling impregnation on commercial alumina (Spheralite) using a water-ethanol HPA solution. Also, the lacunar phase of PTA was supported on commercial activated carbon by equilibrium adsorption impregnation. Solids were washed with acetonitrile, dried and calcined at 150 °C for 2 h. DRS analysis showed that HPAs are partially degraded when they are supported on alumina. The reaction tests were conducted in a batch reactor using 8.34 mmol limonene, 100 mg catalyst, 2.58 mmol H2O2 (35 wt%), and acetonitrile as solvent at 70 °C, under ambient or nitrogen atmosphere. Previously, the authors found that HPA-alumina led to higher conversion and selectivity to epoxide than HPA-carbon, and the lacunar phase of HPA-carbon gave the highest conversion and selectivity to epoxide, but only 11 % limonene conversion was achieved with 31 %–37 % limonene selectivity at 60 °C after 7 h.
HPA-alumina, which presented weakly acidity according to potentiometric titration, decomposed the hydrogen peroxide. Light influence was also evaluated, and an increase in conversion was observed in the absence of light. The main products were limonene oxide, carvone, carveol, diols, and limonene hydroperoxide (Scheme 13). The authors proposed a free radical competitive mechanism for reactions using HPA-alumina as catalyst (2,6-ditertbutyl-4-methylphenol was used as radical scavenger). The bests results using PTA/alumina were achieved under dark conditions (19.7 % conversion, 33 % epoxide selectivity, 51 % ketone selectivity). Using lacunary PTA-carbon, the conversion was higher and the selectivity toward epoxide was enhanced, and no differences were observed in the absence of light or with the addition of a radical scavenger. The lacunar structure, deficient in a WO6 octahedron, allowed the formation of peroxometal species. The reuse of the catalysts was not reported.
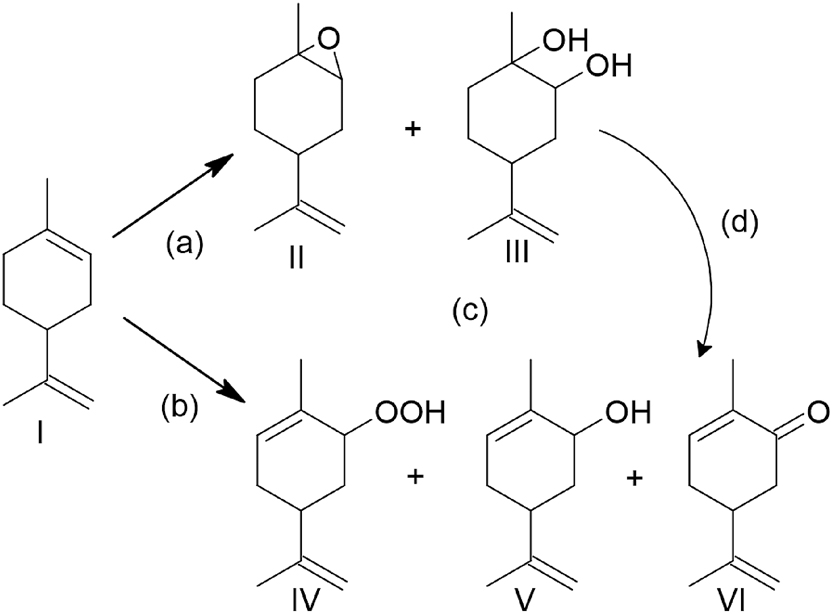
Reaction pathway in the epoxidation of limonene with hydrogen peroxide: concerted process (a); free radical process (b); rearrangement processes (c, d). Limonene (I); 1,2-epoxilimonene (II); diol (III); hydroperoxides (IV); carveol (V); carvone (VI).
4.5 Oxidation of ethane
Sopa and co-workers (Sopa et al. 2005) reported the oxidation of ethane to acetic acid using Keggin HPA (HPMoVx) supported on SiO2, TiO2, and γ-Al2O3 prepared by incipient wetness and calcined at 350 °C. Acetic acid is an industrial commodity chemical used for the manufacture of various industrial precursors such as vinyl acetate, acetic anhydride, terephthalic acid, and ethyl acetate among others. The γ-alumina was prepared from aluminum i-propoxide and calcined at 600 °C for 6 h. The catalytic test was conducted using 2 g of catalysts, atmospheric pressure, in the range of reaction temperature from 250 to 400 °C. The authors found that vanadium atoms introduced into Keggin structure improved the oxidative activity, while vanadyl groups exchanged into cationic position diminished ethane conversion. The nature of support, either acidic or base, can influence ethane conversion and the distribution of products. In HPMoVx supported on silica and titania, ethane oxidation was attributed to the presence of regular or defected Keggin structure, while low catalytic performance on alumina-supported samples was due to Mo–V–P mixed oxide species formed from HPMoVn decomposition. The presence of water vapors in the reaction mixture was required for both catalysts’ surface modification and for acetic acid desorption. Under water vapors, the catalysts showed stable activity for about 20 h.
5 Hydrodeoxygenation
Aromatic hydrocarbons, such as benzene, toluene, and xylene, are extensively used as solvent in many industries for the manufacture of detergents, dyes, additives for gasoline, etc. Since aromatic hydrocarbons are mostly produced from petroleum, alternative ways of obtaining them are being developed by various research groups. One attractive alternative is biomass, but, unfortunately, biomass-based fuels obtained by pyrolysis have a large number of oxygen-containing compounds, which contribute to high viscosity, corrosiveness, poor heating value, bioliquid immiscibility with hydrocarbon fuels, and formation of carbon deposits in parts of automotive engines upon combustion. To solve these issues, it is necessary to reduce the oxygen content in the bio-oil, for example, by catalytic hydrodeoxygenation (HDO). HDO consists in removing O atoms as water from phenolic compounds by H2 at 200–400 °C and high pressure. Two pathways are followed: direct deoxygenation (DDO), which produces the C–O bond cleavage, generating aromatic hydrocarbons; and hydrogenation (HYD) of aromatic ring to produce naphthenes (Scheme 14) (Farah et al. 2023). Two of the catalysts studied for HDO are sulfide CoMo/Al2O3 and NiMo/Al2O3 systems, in which the coordinatively unsaturated sites, or sulfur anion vacancies located at the edges of MoS2 slabs, are the active sites. However, in the absence of a sulfiding agent, these catalysts are deactivated, and the selectivity to different hydrocarbons changes due to oxidation of the active phase. Moreover, the presence of weak Lewis acidic sites on the support surface, such as alumina, leads to large coke formation. For this reason, activated carbon (AC), which is less active than alumina, was employed to support PTA and STA together with Ni. A very low coke formation on the surface of AC, with respect to the classical alumina support, was observed in the HDO of phenol (Echeandia et al. 2010).

Hydrodeoxygenation of phenol.
As MoO3 on different supports is extensively used as catalyst for HDO, Farah et al. (2023) studied MoO3 supported on commercial ZrO2, γ-Al2O3, TiO2, and mesoporous silica (COK12) for the HDO of m-cresol. Catalysts were prepared by incipient wetness impregnation using an aqueous solution of (NH4)6Mo7O24·4H2O, and calcined at 500 °C for 4 h, to allow the decomposition to the salt to form MoO3. The reaction test was carried out at 340 °C under 30 bar for 6 h on stream. H2-TPR analysis revealed that the reducibility of Mo depended on the nature of the support: reduction was complete on ZrO2, 92 % on TiO2, 72 % on Al2O3, and 44 % on COK12. The activity, corresponding to Mo5+ species, was Mo/ZrO2 > Mo/TiO2 > Mo/Al2O3 ∼ Mo/COK12, according to the reducibility. No deactivation was observed. The conversion was 60 %, and toluene was the main product, meanwhile only traces of fully hydrogenated product methylcyclohexane were detected. The reusability of the catalysts was not studied. Then, the authors studied Mo/W mixed oxides prepared from H4SiMoxW12-xO40 (x = 0, 3, 6, 9, and 12), supported on ZrO2.
Since HDO reaction requires bifunctional catalysts with both metal and acid sites, Pt–Al2O3 (1 wt% Pt) and Ni–Al2O3 (28 wt% Ni) catalysts containing PTA and Cs2.5H0.5PW12O40 (∼20 wt%) were prepared by Nunes et al. (2023). The authors focused on the interaction between the HPA and Al2O3 using two methodologies for the preparation of the catalysts: impregnation of PTA aqueous solution onto a Ni-modified Al2O3, and mechanical mixture of Al2O3 support with Cs2.5H0.5PW12O40. The solids were then heated in a nitrogen atmosphere at 350 °C for 2 h. HDO catalysts have a metal function, Pt or Ni, for hydrogenation and C–C bond cleavage; and an acidic function, HPA, for C–O activation. The catalysts were evaluated in the HDO of guaiacol, in n-heptane in a vertical fixed-bed glass reactor at 300 °C, under H2 and atmospheric pressure.
All XRD patterns resembled the support ones, and no peaks of bulk PTA were observed, which was attributed to good dispersion. However, for the physically mixed catalysts (Pt–Al2O3/Cs2.5 and Ni–Al2O3/Cs2.5), the diffraction pattern of Cs2.5 salt prevailed over that of the support, due to salt crystallinity and difference in absorptivity. In Raman spectra, the characteristic bands of the Keggin anion could be observed for Pt–Al2O3/Cs2.5, Ni–Al2O3/Cs2.5, and PTA/Ni–Al2O3. However, PTA/Pt–Al2O3 presented a very poorly resolved peak because of the strong interaction between the PTA and Pt–Al2O3 (Pt content was lower than that of Ni). There is a strong interaction between an HPA and a basic support, such as alumina, when the amount of HPA is less than 40 wt%, and the support surface is not completely covered. In these conditions, the HPA does not show Brönsted acidity, but Lewis acid sites instead, due to the formation of aluminum salts of PTA. XPS analysis showed the presence of two different HPA species dispersed onto the alumina support, probably with distinct acidity properties.
In the hydrodeoxygenation of guaiacol, the addition of PTA led to an increase in the initial conversion, compared to the bare support, although the deactivation was more pronounced, especially for Pt–Al2O3-based series, attributed to the formation of carbonaceous deposits on the surface, and the decomposition of PTA by the basic (OH) sites in Al2O3, thus the conversion of PTA/Pt–Al2O3 catalyst was lower. In the case of Ni–Al2O3 support, the higher metal content could attenuate this effect. The main products identified and quantified were phenol, cyclohexanone, anisole, and benzene (Supplementary Scheme S5). As the main product was phenol (except for Ni–Al2O3 and PTA/Ni–Al2O3 where benzene was the main product at 10 min) it can be assumed that the main reaction pathway is direct deoxygenation rather than hydrogenation, due to the steric effect of the methoxy group, which makes guaiacol co-planar adsorption onto the support a more difficult process and leads to a direct deoxygenation pathway.
Ni-based catalysts led to higher conversion and selectivity to deoxygenated compounds, such as benzene, due to their higher metal and acidic contents, but underwent a more severe deactivation with time-on-stream. The formation of cyclohexanone suggested that phenol was suffering secondary reactions. For the samples with higher acidity, Ni–Al2O3 and PTA/Ni–Al2O3, a significant amount of benzene was detected, but the selectivity decreased while phenol selectivity increased with time, meaning that there was a loss in hydrodeoxygenation capacity as some deactivation occurred, attributed to a synergetic effect between Ni and acid sites in the presence of H2. The spent catalyst maintained its structure (confirmed by XRD). However, in IR spectra two bands characteristic of guaiacol were observed. The adsorption of these compounds on the alumina surface causes the deactivation of the catalyst.
6 Hydrodesulfurization, hydrogenation, and hydrodenitrogenation
The hydrodesulfurization (HDS), hydrogenation, and hydrodenitrogenation of fuels are investigated to reduce the sulfur content, the emission of nitrogen oxides (NOx), and residual hydrocarbons. Among the catalysts employed for these reactions, NiMoP/Al2O3 and CoMoP/Al2O3 stand out. They are traditionally prepared by co-impregnation of (NH4)6Mo7O24, Ni(NO3)2 (or Co(NO3)2), and H3PO4 solutions.
Blanco’s group prepared Ni-promoted catalysts by impregnation of PTA and PMA on different supports for the hydrodesulfurization and hydrodenitrogenation of a cyclohexane-pyridine-thiophene mixture. Silica and γ-alumina were impregnated by pore filling method using dimethylformamide solutions of HPA. This solvent allows the preservation of Keggin structure (Rives et al. 2001), while ethanol/water solutions were employed for γ-alumina, zirconia, and titania (Pizzio et al. 1999). After drying, the solids were impregnated with Ni(NO3)2 solution and calcined at 350 °C for 1 h. Catalysts were presulfurized in situ using H2S/H2 at 280 °C and 1.8 MPa. FTIR and DRS analyses showed that Keggin HPAs were degraded during drying and calcination if the support was commercial alumina, whereas HPAs were stable on zirconia and titania. However, catalysts supported on alumina presented the highest activity attributed to the greatest surface area. TPR spectra of sulfide samples showed that HPA-alumina presented more intense peaks and reducible sites than HPA on silica, titania, or zirconia. On the other hand, a better sulfurization was observed for silica-supported HPA. A stronger interaction between HPA and alumina allowed better dispersion, and the formation of bulk oxides was observed on silica (Rives et al. 2001). Reaction tests were performed in a fixed-bed reactor at 3 MPa, 280 °C, H2/hydrocarbon (molar ratio = 5), using mixtures of cyclohexane-thiophene (Supplementary Scheme S6), cyclohexane-pyridine (Supplementary Scheme S7), and cyclohexane-pyridine-thiophene as liquid feed. Silica-supported catalysts presented higher activity than alumina ones, due to the strong interaction between HPA and alumina (Rives et al. 2001). The reuse of the catalysts was not investigated.
Romero Galarza et al. (2011) prepared CoMoP/Al2O3 catalyst using Co7/2PMo12O40 as precursor. Before the reaction test, the catalyst was activated with H2S/H2 at 400 °C for 4 h. This material presented a high number of Co-promoted molybdenum sites that led to higher HDS of 4,6-DMDBT than conventionally prepared catalysts. The Co/Co+Mo ratio was 0.23, while that of traditional catalysts was 0.11. The reaction was performed in a batch reactor using 4,6-DMDBT in decane at 320 °C and 8.3 MPa (Supplementary Scheme S8). The authors found that the calcination temperature affected the catalytic activity, the uncalcined material being the most active, which reduced the hydrogenation pathway (selectivity to methylcyclohexyltoluene and dimethylcyclohexyl) and enhanced the direct desulfurization pathway, producing dimethyldiphenyl as the main product. The reuse of the catalysts was not reported.
A series of bimetallic and trimetallic catalysts, WnMomS and Ni(Co)WnMomS, prepared from HPA/alumina was developed by Nikulshin’s group (Kokliukhin et al. 2021a,b; Nikulshina et al. 2018b, 2019). This research group studied the W/Mo molar ratio (Kokliukhin et al. 2021b; Nikulshina et al. 2018a, 2019), the addition of Co, Ni, and also carbon to the composites (Nikulshin et al. 2015). The catalytic materials were evaluated in the dibenzothiophene (DBT) and 4,6-DMDBT hydrodesulfurization (Supplementary Scheme S9), naphthalene HYD (Supplementary Scheme S10), and quinoline hydrodenitrogenation (HDN) (Supplementary Scheme S11) in a fixed-bed microreactor featuring a high-pressure flow system, using a mixture of DBT, naphthalene, quinoline (as nitrogen-containing inhibitor), hexadecane (as an internal standard), and toluene (as solvent) at 260–320 °C, 3.0 MPa of hydrogen.
Previously, Ninh et al. (2014) studied the effect of 4,6-DMDBT HDS using different supports for Co(Ni)MoS: γ-alumina, silica, zirconia, and titania. It was found that silica had fewer promoted sites (MoS2), and that the catalysts supported on alumina allowed a higher DDS/HYD ratio. The HDS of DBT produced biphenyl (BP) via the direct desulfurization pathway (Supplementary Scheme S9). The absence of hydrogenated tetrahydro- and perhydrodibenzothiophenes, cyclohexylbenzene, and bicyclohexyl (BCH) via the HYD pathway of DBT HDS was caused by the strong inhibition effect of quinoline. Tetralin, c- and t-decalins were the main products of naphthalene HYD reaction (Supplementary Scheme S10). Moreover, 90 %–95 % quinoline rapidly turned into tetrahydroquinoline (Supplementary Scheme S11).
Catalysts (MonWmS/Al2O3) were prepared by impregnation of γ-alumina by incipient wetness with aqueous solutions of mixed HPAs (H4XMonW12-nO40 (X = Si, P; n = 1, 3, 6, 9)) (Nikulshina et al. 2019) or mixtures of PTA, PMA, STA, and SMA (Nikulshina et al. 2018b). For trimetallic composites (Ni (Co)MonWmS/Al2O3), Ni or Co was incorporated using nickel or cobalt, citrate or carbonate (Nikulshin et al. 2015). Then, they were activated by gas phase sulfidation, using H2S in H2 at 400 °C or by liquid sulfidation with a mixture of dimethylsulfide in decane (or toluene) in H2 atmosphere (3–3.5 MPa) at 240 °C (8–10 h) and 340 °C (8–10 h). The species present in the catalysts were revealed by XPS: NiMo(W)S, NiS, Ni2+, MoS2, WS2, MoSxOy, WSxOy, Mo6+, and W6+.
The structure of the mixed NiMoW active phase depends on the sulfidation conditions: ‘‘core–shell” (Mo atoms are surrounded by W atoms) was obtained by gas phase activation; and ‘‘random” (Mo and W atoms are disordered in the crystallite) was obtained by liquid phase activation (Van Haandel et al. 2015). In a previous work, Tomina et al. (2015) prepared NiMoW–S catalysts from mixtures of PTA and PMA, and found that Ni–Mo6W6 catalyst was the most active in the HDS of fuel. It was observed that W sulfidation degree increased with Mo loading. The highest activity was achieved with Mo3W9/Al2O3, i.e., DBT conversion varied in a range of 42 %–75 %, and naphthalene conversion in a range of 32 %–49 %, observing an increment with Mo content in the catalysts (Kokliukhin et al. 2021a). The Mo/W atomic ratio in the HPA precursor directly affects the ratio of the hydrogenation and hydrodesulfurization performance. A synergistic effect occurs only when MoW/Al2O3 is promoted by Ni, while in Co-promoted samples the activity depends only on the Mo content, since Co prevents the formation of the highly active NiMoS phase. Trimetallic NiMonW12−n/Al2O3 catalysts based on mixed HPAs are more active than their Ni(Mon + W12−n)/Al2O3 counterparts, the synergistic effect is enhanced due to the closer interaction between Mo and W, which increases the sulfidation degree of metals and contributes to the formation of highly active mixed NiMoWS sites. Researchers attributed the catalytic synergism of Ni(Co)Mo(W)/Al2O3 to the formation of CoMoS or NiMo(W)S phases where highly dispersed Mo(W)S2 crystallites are decorated with Ni or Co, which act as promoter. Therefore, the catalytic improvement is not only due to the simultaneous presence of Mo and W, but also to the close interaction in the mixed oxide precursor (Kokliukhin et al. 2021b; Nikulshina et al. 2018a). To investigate the nature of heteroatoms in the Keggin structure, catalysts based on SMA and PMA were compared. The incorporation of phosphorus weakened metal–support interactions and facilitated the formation of more synergetic NiW–MoS phases with higher stacks. However, these changes had a positive effect on catalytic cracking diesel only in the case of WMoNi/Al2O3 catalyst prepared using a W/Mo-hybrid nanocrystal-assisted hydrothermal deposition method (Nikulshina et al. 2018b, 2019). The reuse of the catalysts was not reported.
Since alumina supports strongly interact with metal oxide precursors and hinder their sulfidation, carbon was deposited on this support, which resulted in a higher average stacking number of Co(Ni)MoS and NiWS active phases (Nikulshin et al. 2015). TPR measurements showed that carbon-coated alumina can accumulate in gas phase hydrogenation and could be a source of hydrogen for HDS and HYD reactions. It was found that the support altered the morphology and structure of the NiWS active phase, and the catalytic activities depended on the carbon content in the support. The increase of carbon content up to 5 wt% reduced reactivity, sulfidation degree, average length, and the stacking number of WS2 crystallites in the catalysts increased. The conversion of all substrates varied from 8.5 % to 33 %.
Continuing with this study, the same group (Kokliukhin et al. 2021c) found that MonW12−nS2 are the active species for simultaneous hydrodesulfurization of DBT (Supplementary Scheme S9) and hydrogenation of naphthalene (Supplementary Scheme S10). For that reason, they prepared a new catalyst by removing alumina from HPA/Al2O3 by HF etching, resulting in an increase of total HDS and HYD activity, which can be attributed to the absence of metal-support interaction and the increase of metal sulfidation degree. Researchers found that the substitution of one or three tungsten atoms by molybdenum in the catalyst resulted in an increase in HDS and HYD activities compared to monometallic MoS2 and WS2. Moreover, WS2 sample based on STA had improved catalytic properties compared to the monometallic MoS2 one. Catalysts were also tested in the hydrotreating of straight-run gas oil (0.815 wt% S and 156 ppm N). The catalytic activity was evaluated by the residual sulfur content in the hydrogenated product. The catalysts exhibited stable performance for 48 h. The yield of hydrogenated diesel fraction was close to 96 %–97 %. The highest residual sulfur content was observed over the NiW12/Al2O3 catalyst. Replacement of even one tungsten atom with molybdenum led to a decrease in the content of sulfur and nitrogen (Kokliukhin et al. 2021a).
The conversions varied from 20.6 % to 61.3 % for HDS of DBT and from 23.8 % to 57.3 % for HYD of naphthalene over all prepared catalysts. It was noted that with an increase in the Mo/W ratio of 0.33 or more, the catalytic activity increased in both HDS and HYD reactions (Kokliukhin et al. 2021b).
Other heteropolyacids were also reported for the preparation of alumina/sulfide composites: H6[Co2Mo10O38H4] (Mozhaev et al. 2016) and H3+xPMo12−xVxO40 (x = 1–3) (Timoshkina et al. 2022). Vinogradov et al. (2023) studied CoMo–S catalysts supported on γ-Al2O3 and halloysite nanotubes (HNTs). HPAs containing phosphorus are precursors of active oxide catalysts (for oxidative dehydrogenation) and sulfide catalysts (for hydrotreating). Moreover, the catalytic activity is enhanced by the addition of vanadium to the heteropolyanion (H4PMo11V1O40), and the addition of aluminosilicate HNTs (Al2Si2O5[OH]4) to an oxide support improves the strength and textural properties (the external and internal surfaces of halloysite consist of a negatively charged silica and a positively charged alumina, respectively). CoPMoV catalysts were prepared by incipient wetness impregnation of the support with a combined solution of H4PMo11V1O40, CoCO3·mCo(OH)2·nH2O, and citric acid. The samples were dried at 60 and 80 °C for 2 h each and at 110 °C for 6 h. The content of MoO3 was 18 wt%. The catalytic tests, HDS and HYD, were performed using a fixed-bed flow-type reactor setup. The catalysts were sulfided in the gas phase (H2S/H2 atmosphere, 1:9) at 400 °C and 1 MPa for 2.5 h. The reaction mixture, 0.86 wt% DBT and 3.0 wt% naphthalene in toluene, with n-hexadecane as an internal standard, was brought to 300–340 °C, under 3.0 MPa H2, for more than 8 h. No toluene derivatives were detected during the catalytic test. For HYD (direct hydrogenation) the products were tetrahydrodibenzothiophene, dicyclohexyl, and cyclohexylbenzene. Meanwhile, biphenyl (BP) was obtained by the direct desulfurization of DBT (Supplementary Scheme S9).
Raman spectroscopy revealed that the Keggin structure was partially retained in the impregnation solution. TPR analysis indicated that the reduction of sulfided CoMo catalysts caused Mo–S bond breakage, which made it possible to evaluate the bond strength and the potential for the generation of coordinatively unsaturated sites. CoMoS/HNT and CoMoS/Al2O3 exhibited hydrogen uptake between 150 °C and 300 °C, and the reduction of the HNT-supported sample started at lower temperature than the alumina-supported one, indicating a lower Mo–S binding energy due to the greater mobility of sulfur atoms. This is associated with a weak interaction between the sulfided active phase and the support, because of the presence of silica in HNTs. The high catalytic activity of the HNT-supported catalyst is associated with its better-developed pore system. The DBT conversion was from 23.3 % to 96.2 % for CoMoS/HNT and from 11.4 % to 82.3 % for CoMoS/Al2O3. The highest conversion values were achieved at 340 °C for CoMoS/HNT (85 %). Meanwhile, naphthalene conversion was 7.9 %–45.2 % for CoMoS/HNT and 2.3 %–25.8 % for CoMoS/Al2O3. Tetralin was the only naphthalene hydrogenation product (Supplementary Scheme S10). The reuse of the catalysts was not reported.
7 Protection of hydroxyl groups
Protective group techniques are essential for the development of organic synthesis, for example, in the oligosaccharides (Vidal 2019) and peptides (Stewart 1981) synthesis. Several methods for the protection of hydroxyl functional groups have been reported, some of which involve the use of supported heteropolyacid catalysts.
Phosphomolybdic and phosphotungstic acids were supported on a silica-alumina material obtained by sol-gel methods. Various techniques were employed to synthesize the supports. In one method, commercial alumina was introduced into a tetraethyl orthosilicate (TEOS) solution, which was subsequently hydrolyzed. In alternative preparations, TEOS and aluminum tri-sec-butoxide were hydrolyzed either simultaneously or following the prehydrolysis of TEOS. Comprehensive characterization was conducted, including diffuse reflectance spectroscopy and X-ray diffraction techniques. The structural properties of the support and the acidic strength of the prepared materials influenced their catalytic activity in the tetrahydropyranylation of phenol.
Optimal reaction parameters were achieved with the catalyst exhibiting the highest acidity (Romanelli et al. 2004). Vázquez and co-workers reported that various alcohols and phenols were protected through the formation of trimethylsilyl ethers (Villabrille et al. 2010). This reaction was catalyzed by molybdophosphovanadates supported on commercial alumina cylinders, prepared via the incipient wetness impregnation method. The solids were thoroughly characterized. Phenol trimethylsilyl ethers were successfully obtained, even after multiple uses of the catalyst. The H2PMo11VCuO40 supported on alumina gave higher yields and conversions.
8 Esterification and transesterification reactions
One of the most important reactions in organic synthesis is the esterification of alcohols and carboxylic acids (Supplementary Scheme S12). Traditionally, this reaction has been catalyzed using conventional mineral acids. In recent decades, various supported HPAs have been investigated for the esterification of different substrates. Notably, alumina-supported HPAs have been evaluated in this context.
The use of PTA and PMA supported on various carriers as catalysts was investigated by Blanco and collaborators (Pizzio et al. 2003). The catalysts were characterized and tested in the gas-solid esterification of isoamyl alcohol and acetic acid, using a conventional flow fixed-bed reactor operating at atmospheric pressure. The results obtained were unsatisfactory compared to those achieved with other catalysts reported in the same paper. Specifically, HPAs supported on silica materials demonstrated higher catalytic performance than those supported on alumina and inert carbon. In 2004, Sharma et al. (2004) employed HPAs supported on neutral alumina for the esterification of n-butanol and cyclohexanol with three carboxylic acids: formic, acetic, and propionic acids. They investigated the structure of the amorphous solid acid catalysts prepared and their influence on the reaction of primary and secondary alcohols with these aliphatic acids. After analyzing several correlations, they concluded that a Keggin-type structure catalyst (30 wt% PTA) supported on neutral alumina and calcined at 500 °C was the most effective option. Moreover, it was regenerated with only a 5 % yield decrease. Finally, it is noteworthy to mention the work of Tarlani et al. (2005). The Wells-Dawson heteropolyacid H6P2W18O62 supported on both sol–gel and non-sol-gel alumina was structurally studied and fully characterized. A comprehensive set of these catalysts was investigated for the esterification of hexanol and propanoic acid. The results revealed limited conversions and yields, indicating that while the new catalyst had an acidic character and showed some activity in the reaction, its overall performance was suboptimal.
The acetylation of glycerol to produce triacetin (Supplementary Scheme S13) is of industrial interest, as glycerol is a by-product of biodiesel production, and acetylated glycerol has applications in the food and leather industries. Several HPAs were supported on silica, alumina, and silica-alumina to develop heterogeneous catalysts for this reaction. The reaction course was extensively investigated, demonstrating that the conversion to the desired product depended on both the nature of the HPA and the choice of support. However, only the silica-supported catalyst exhibited remarkable performance, achieving high yields of triacetin, while the alumina- and silica-alumina-supported catalysts gave poor results (Kale et al. 2016).
The transesterification reaction has emerged as a strategic approach for producing renewable fuels such as biodiesel. Various catalysts have been investigated to achieve a sustainable process for this economically significant reaction. The transesterification of crude Jatropha oil to fatty acid methyl esters was performed under ultrasonic irradiation using heteropolyacid-based catalysts. PTA supported on γ-alumina was prepared and structurally characterized. The data obtained from the tests of three heteropolyacid-based catalysts were analyzed using an artificial neural network to model the reaction parameters. The mathematical models developed were capable of accurately predicting the reaction yields (Badday et al. 2014). Cesium-doped heteropoly tungstate (CsHPW) supported on γ-alumina (20 wt%) was prepared and evaluated as a catalyst for biodiesel production from 10 % oleic acid in soybean oil (Sheikh et al. 2013). The prepared catalyst exhibited good performance, although leaching was observed. The heterogeneous material showed reduced activity compared to the bulk CsHPW. In another reported study, different PTA catalysts supported on γ-alumina were prepared, characterized, and used for the transesterification of canola oil with methanol. Biodiesel production was successfully achieved, with conversions exceeding 90 % when 10 wt% of the catalyst was used at 200 °C for 10 h under 4 MPa. However, the reuse of the catalysts was not reported (Kurhade and Dalai 2018). Methyl and ethyl esters from macaw palm oil were successfully produced using PMA/Al2O3 heterogeneous catalysts (Da Conceição et al. 2019). Optimization of the reaction conditions led to a robust methodology, achieving over 98 % conversion. Furthermore, the catalyst demonstrated good stability, with minimal loss of activity over up to four cycles. Patiño et al. (2024) prepared PTA and STA supported on high surface area materials such as graphite, montmorillonite, and alumina using a wet impregnation method. The transesterification of sewage sludge lipids with methanol was tested and analyzed. In some cases, minimal leaching of the active phase was observed.
9 Synthesis of acetals and ketals
Ketals and acetals are used as starting material in organic synthesis and in the perfume industry. Gao et al. (2007) used PTA supported onto commercial neutral alumina, prepared by equilibrium impregnation technique from an ethanol:water solution, for the synthesis of acetals and ketals. The catalytic test was carried out at reflux using 2-butanone, butyraldehyde, and iso-butyraldehyde as carbonylic compounds, and ethylene glycol and 1,2-propyleneglycol as alcohol (Supplementary Scheme S14). Cyclohexane was used as water-stripped reagent. Catalysts containing 30 wt% PTA calcined at 500 °C showed the highest activity in carbonylic/glycol 1:1.5 in 1 h (with 61 %–87 % yields). Neutral alumina was used instead of basic one in order to avoid the destruction of Keggin structure. The Keggin structure in the supported catalysts was confirmed by FTIR. No leaching of PTA was detected, indicating a chemical interaction between PTA and the support. The increment in the PTA stability in calcined catalysts was observed by TGA, suggesting the formation of an intermolecular hydrogen bonding. The recovered catalyst was washed with water, dried at 100 °C, and reused with only a 5 % yield loss after three runs.
Finally, acetal and ketal formation was accomplished using a catalyst prepared by impregnating PTA onto Al2O3. The solid containing 30 % active acid was the most effective for hydroxyl protection via acetal synthesis. The catalyst was recovered and used four times without changing the activity (Gao et al. 2007).
10 Dehydration of glycerol
Biodiesel production has surged rapidly in recent decades. The transesterification of vegetable oils with methanol, catalyzed by an acid, generates substantial amounts of glycerol as a by-product. Consequently, the conversion of glycerol into valuable chemical products has become a significant area of research. Among the various alternatives, the dehydration of glycerol to produce acrolein has been identified as a particularly promising commercial opportunity (Scheme 15).

Preparation of acrolein from glycerol.
Several studies have investigated HPAs supported on alumina for this transformation, yielding a range of results. Initially, we will describe the work of Brückner (Erfle et al. 2011) in which Keggin-structured acids were supported on SiO2–Al2O3 materials with varying Si/Al ratios. The structure of the obtained solids was analyzed, revealing that the Keggin anions degrade into various ion fragments. This degradation results in reduced product formation and lower selectivity towards acrolein compared to processes using bulk acid as catalyst. In another study, various phosphomolybdic, phosphotungstic, and silicotungstic acids were supported on silica, alumina, and aluminosilicate materials to produce heterogeneous catalysts. These solids were tested for the gas-phase dehydration of glycerol. Among the materials tested, alumina-supported catalysts demonstrated greater activity, conversion, and stability for the acrolein formation process: conversion was maintained above 70 % after 300 h on-stream. Moreover, catalysts were regenerated by in situ oxidation (Atia et al. 2008).
Moreover, silica-alumina has been identified as an excellent support for PTA catalysts. The structurally characterized materials were evaluated for acrolein production, with PTA/SA-85 exhibiting the highest acidity and demonstrating the greatest activity for the desired product formation. Additionally, the results indicated a synergistic effect between the acid and the support. The catalyst’s reusability was also assessed, with satisfactory performance being reported upon several reuses (Kang et al. 2015).
At the end of this section, we mention the work of Kraleva and Atia in which glycerol dehydration to acrolein was conducted at high temperatures using Keggin-type heteropolyacids supported on sol–gel-prepared alumina, titania, zirconia, and a three-component mixed oxide (AlTiZr). Tungsten-based heteropolyacids exhibited exceptional performance and stability. Acrolein consistently emerged as the predominant product. However, alumina-derived materials showed lower results compared to those supported on zirconia and titania. The reuse of the catalysts was not reported (Kraleva and Atia 2019).
11 n-alkane isomerization
The interest in converting low octane paraffins into high octane iso-paraffins is due to their use for additive suppression and benzene reduction in gasoline. Two catalysts are mainly used in the industry for this transformation: Pt on chlorinated alumina and Pt on mordenite, but they are sensitive to poisons, especially to sulfur (Travers et al. 2001). To solve this problem, many research groups have tried to develop new catalysts for isomerization of n-alkanes. Here, we list the advances related to the use of HPA supported on alumina.
Travers et al. (2001) used Cs2HPW12O40 promoted on Pt/alumina for n-hexane isomerization. The catalyst was prepared by mechanical mixing of HPA and pre-reduced Pt on alumina. The catalytic test was performed in a fixed-bed reactor at 4 bar, 180–220 °C, and H2:n-hexane 10:1. The presence of Pt increased the conversion of Cs-HPA, since Pt sites can dissociate the hydrogen molecule and allow the migration of hydride. Similar conversion and selectivity to industrial Pt/zeolite were achieved. Cs salt HPA developed the highest microporosity and Brönsted/Lewis ratio. The reuse of the catalysts was not mentioned.
Ivanov et al. (2000), based on previous studies that found that PTA supported on silica increased the selectivity for the isomerization of n-hexane by Pt/Al2O3 addition (Kuang et al. 2003), prepared Pt/PTA/Al2O3 and Pt/PTA/Al2O3–F by impregnation of 20 wt% PTA on γ-Al2O3 and γ-Al2O3–F, 0.5 wt% Pt, which were finally calcined at 350 °C. These catalysts were evaluated in n-pentane isomerization under atmospheric pressure, at 170–440 °C, using the flow mixture H2:n-heptane 3:1. The addition of 3.5 % F had the objective to prevent the partial decomposition of the HPA by the basic support and salt formation. The acidic properties of the fluorinated alumina were evaluated by DRIFT, and the replacement of basic terminal groups by fluorine anions, according to the decrease in the signal of OH, was proposed. The yield of isopentane on Pt/PTA/Al2O3 did not exceed 7 %, meanwhile with Pt/PTA/Al2O3–F the conversion was 64 % at 310 °C (97 % selectivity to isopentane (64 % yield) over the whole temperature range). The reuse of the catalysts was not investigated.
Yang et al. (2009) studied the hydroisomerization of n-heptane using Pt/PTA/WSAn, where WSAn represents Al-MCM-41, n = Si/Al molar ratio = 50, 30, and 10. WSAn was prepared using fumed silica, aluminum sulfate, and cetyltrimethylammonium bromide (as surfactant template), and calcined at 600 °C for 6 h in the last step. The solid presented M−OH groups on the surface (observed in the FTIR spectra), which can associate with PTA, inducing high dispersion. PTA was grafted from a methanolic solution and, after solvent evaporation, 25 wt% PTA/WSAn was calcined at 350 °C for 2 h, and finally impregnated with H2PtCl6 aqueous solution (to obtain 1 wt% Pt in the catalyst). Before the reaction, the catalysts were treated at 350 °C for 2 h using H2 to reduce the platinum oxide to metallic Pt. The authors suggested that the pore system of WSA10 had partially collapsed due to the high aluminum content. The Pt/PTA/WSAn surface area was 609, 625, and 202 m2/g for n = 50, 30, and 10, respectively. PTA was found to retain its primary structure after dispersion but underwent partial distortion by the interaction with the support (FTIR, P-NMR, and UV–vis analyses). Since Al-MCM-41 materials possess surface acidity, the HPA-support interaction is not as strong as if dispersed on a basic support, such as γ-alumina, and the Keggin unit responsible for the strong Brönsted acidity can be preserved.
Reaction tests, hydroisomerization of n-heptane (Scheme 16), were carried out in a down-flow fixed-bed reactor in the presence of hydrogen at atmospheric conditions, in a temperature range from 200 °C to 440 °C. The main product was isoheptane, whose selectivity decreased with the increment of reaction temperature. Pt/PTA/WSA30 showed the highest activity and selectivity, associated with the large number of Brönsted acid sites (measured by Py-FTIR). The authors found a correlation between the Brönsted acidity and the aluminum population. The WSA30 sample contained the biggest fraction of Al ions with pentahedral and tetrahedral coordination. When compared with the bare WSAn support, the number of Brönsted acid sites on the Pt/PTA/WSAn catalysts increased by approximately four times. However, the smaller number of Lewis acid sites was attributed to deposition of PTA. As a result, the number of total acid sites decreased.
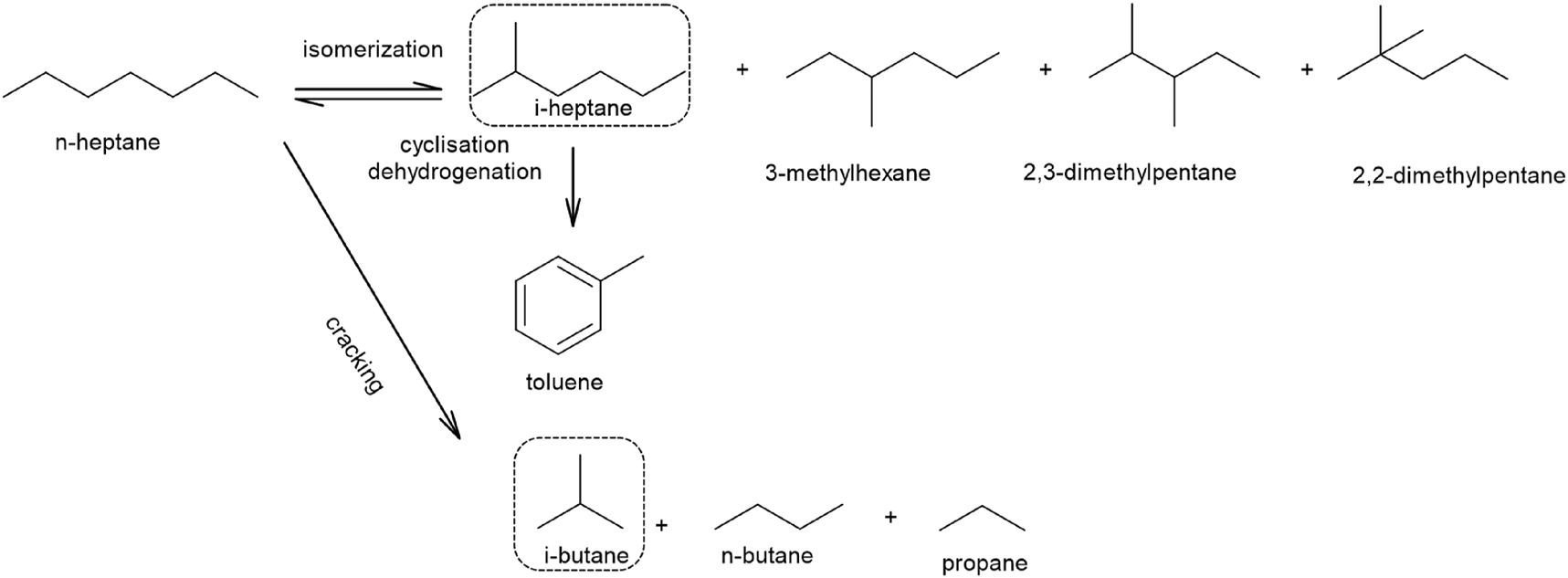
Isomerization and cracking of n-heptane.
Pt/PTA/WSA30 was the most active catalyst with the highest n-heptane conversion and selectivity to C7 isomers, while the Pt/PTA/WSA10 catalyst exhibited the lowest catalytic activity and isomerization selectivity. It is believed that the selectivity of C7 isomers over the Pt/PTA/WSAn catalysts is controlled by the surface acidity, aluminum ion coordination as well as the structural regularity of the catalyst. The product distribution was similar to that obtained from the Pt/PTA/Zr-MCM-41 catalysts: 2-methylhexane, 3-methylhexane, 2,2-dimethylpentane, 2,3-dimethylpentane, 2,4-dimethylpentane, 3,3-dimethylpentane, and traces of 2,2,3-trimethylbutane. In the monobranched isomers, 2-methylhexane predominates, and in the multibranched isomers, 2,3-dimethylpentane is the major component.
The structural ordering of the WSA30 support was the best, which highly favored both the diffusion of the reactants into the active sites and the outward diffusion of branched isomers formed inside the channels. On the contrary, due to thermal instability at high aluminum content, the structure of the WSA10 solid was rather disordered after calcination, and its pore system almost collapsed. Moreover, the WSA30 support had a high content of Al ions in tetrahedral positions, whereas the WSA10 support contained many extra-framework Al ions, which may catalyze cracking reactions, reducing the isomerization selectivity.
For the Pt/PTA/WSAn catalysts, at a reaction temperature above 360 °C, less than 3.6 % of toluene was formed. Toluene formation may follow two plausible pathways: in the initial stage n-heptane can be dissociatively adsorbed on the metal active clusters to form an alkene-like intermediate that may be transformed to toluene via cyclization–dehydrogenation or isomerization–cyclization–dehydrogenation. It is believed that toluene formation requires a relatively longer residence time for the corresponding intermediates at the active centers than that required for C7 isomer formation. If the structure of the catalysts is well ordered, the reactants and the products rapidly reach or leave the active sites, with a short residence time that is unfavorable to the formation of toluene.
It was observed that the yield to cracking products linearly increased with increasing reaction temperature. Only propane, isobutane, and n-butane were detected as the cracking products. The isobutane yield is usually higher than that of n-butane. More cracking products were formed on the Pt/PTA/WSA10 catalyst, associated with its disordered structure and poor textural properties as well as with a high fraction of aluminum ions at octahedral positions in the support. The reusability of the catalysts was not reported.
12 Miscellanea
Although we have presented different examples of transformations assisted by heteropolyacids supported on alumina, such as simple one-step transformations, synthesis of heterocycles, redox processes, to conclude this review we present other interesting examples.
12.1 Synthesis of β-amino alcohols
Ramesh Kumar and Leelavathi (2007) employed PMA on alumina as heterogenous catalyst in the regioselective reaction of oxiranes with amines, obtaining beta-amino alcohols (Scheme 17). The catalyst was prepared from a mixture of PMA (1 g) and neutral alumina (10 g) in dichloromethane. Reactions were performed with equimolar amounts of epoxide, an aryl or alkyl amine, and the catalyst (200 mg) in dichloromethane at room temperature, 0.5–2.5 h. 2-Amino alcohols are compounds of interest in the synthesis on amino acids, oxazolines, and natural products, among others. Excellent yields (84 %–93 %) and high regioselectivity were achieved (12 examples). The catalysts can be reused at least three times without loss of activity.
Regioselective reaction of oxiranes with amines to obtain beta-amino alcohols.
12.2 Tert-butanol synthesis
Lim et al. (1999) reported the use of PMA-polymer composite on alumina as heterogeneous catalyst in the liquid-phase tert-butanol synthesis from isobutene and water at 70 °C (Supplementary Scheme S15). PMA-PPO, with polyphenylene oxide as blending polymer, coated on commercial γ-Al2O3, was prepared by impregnation method and thermally treated at 170 °C. It was found that the composite film catalyst was stable, only a small amount of PMA leaching was detected (2.7 wt% after 30 h) and had higher catalytic activity than homogeneous PMA. It is expected that this catalyst could recover and reuse.
12.3 Azaarene substituted 3-hydroxy-2-oxindole derivatives
The 3-hydroxy-2-oxindole substructure is present in several molecules with biological activity such as donaxaridine, welwitindolinone, dioxibrasinine, maremycin-B (Niu et al. 2012).
Undoubtedly, the most representative and efficient method for obtaining these compounds is the direct functionalization with C(sp3)-H of the azaarene substituted with the alkyl group, and subsequent addition to the corresponding isatin molecule. For this purpose, different catalysts have been used, such as transition metals, Brönsted acids, and even the reactions were accelerated by the action of microwaves.
Hao et al. (2015) reported an efficient system using a Keggin-type heteropolyacid (PTA) supported on alumina for the synthesis of 3-hydroxy-2-oxindole derivatives from 2-methyl azaarenes and isatins in dioxane as solvent (Scheme 18), with good to excellent yields (14 examples, yield 62 %–95 %).
Alumina-supported heteropolyacid as catalyst for the synthesis of azaarene substituted 3-hydroxy-2-oxindole derivatives via C(sp3) H bond functionalization.
In a representative experiment, 2,6-lutidine (0.75 mmol, 3 equiv.), 1-methyl isatin (0.25 mmol), dioxane (0.5 mL), and PTA/Al2O3 (0.03 equiv.) were added and mixed with magnetic stirring in a sealed tube at 120 °C for 24 h. After completion of the reaction, the catalysts were filtered, and the reaction mixture was extracted with ether (3 × 10 mL). The organic layer was dried with anhydrous Na2SO4, and the solvent evaporated. The residue was chromatographed on a silica gel column eluted with a mixture of petroleum ether and ethyl acetate to give pure products.
In conclusion, the authors described an efficient catalytic system (HPAs supported on alumina) for the suitable synthesis of potentially active 3-hydroxy-2-oxindole derivatives in very good to excellent yields. The alumina-supported HPA could be recovered and recycled by a simple filtration of the reaction solution, and reused for six consecutive runs without significant loss of activity.
12.4 Diels alder reaction
The Diels-Alder-type cycloaddition reaction represents an interesting transformation to be used in the valorization of building blocks present in biomass. Particularly furan derivatives such as 2,5-dimethylfuran can be transformed into p-xymene by said transformation (cyclization followed by dehydration, Scheme 19).

Synthesis of p-xylene from 2,5-dimethylfurane assisted by HPA catalyst.
In a very recent work, Wijaya et al. (2017) reported the catalytic performance of different catalysts based on phosphotungstic and silicotungstic acids supported on materials of different nature such as SiO2, Al2O3, TiO2, and ZrO2. They studied the relationship between the structure of the material and the conversion performance of 2,5-dimethylfuran. The results of the TPD studies with ammonia and n-propylamine showed that the different supported HPA catalysts have Brönsted acid sites, the order of decreasing acidity being: HPA/SiO2 > HPA/Al2O3 > HPA/ZrO2 > HPA/TiO2.
To evaluate the catalytic activity of the different synthesized catalysts, the reaction between 2,5-dimethylfurane and ethylene was explored. The conditions used in the test reaction were: P ethylene = 20 bar, T = 250 °C, and t = 1 h. The conversion of DMF and the initial rate of P production generally increase with an increase in total acidity.
Bulk PTA and STA were the most active catalysts (PTA: 69 % conv. and 53 % selectivity; STA: 72 % conv. and 46 % selectivity). However, the inclusion of a support favored separation in recyclability and reuse. Both PTA and STA supported on TiO2 and ZrO2 gave very low conversions and selectivities of less than 10 %. The most active supported catalysts were those included in silica and alumina. Particularly, a material containing 15 % of STA on Al2O3 gave 44 % conversion and 50 % selectivity towards p-xylene, while PTA containing the same amount of active phase gave a similar conversion of 42 %, with the highest selectivity (72 %).
This study confirms the efficiency of materials based on HPAs supported on SiO2 and alumina as efficient catalysts for the transformation of building blocks present in biomass, as part of different initiatives to reduce dependence on petroleum-based chemical derivatives. The reusability of STA on silica was evaluated, observing lower conversion and selectivity than fresh catalysts due to coke deposit on the surface.
12.5 Synthesis of α-aminonitriles
α-Aminonitriles are used as intermediates for the synthesis of α-aminoacids, imidazoles, thiadiazoles, and biologically active molecules. Rafiee et al. (2008) prepared α-aminotitriles by Strecker reaction of benzaldehyde and aniline with trimethylsilyl cyanide, using STA, PMA, and PTA supported on γ-alumina (by impregnation from aqueous solutions, and calcined at 200 °C) as catalysts in acetonitrile at room temperature (Supplementary Scheme S16). High yields (more than 92 %) in short reaction times (1–5 min) were achieved with supported catalysts. Moreover, the best result (98 % yield in 1 min) was obtained with 40 wt% STA-AL2O3 and PMA-Al2O3. Then the rection was extended, with excellent yields in short reaction times (1–30 min), to other aliphatic and aromatic aldehydes and amines (19 different compounds) using STA-Al2O3. This catalyst was recovered and reused with a slight loss of activity.
12.6 Synthesis of enaminones
Rafiee et al. (2008) also prepared enamines using PTA, STA, and PMA supported on silica and alumina. The reaction test was performed with ethylacetoacetate and aniline in acetonitrile, and solvent-free conditions, at room temperature (Scheme 20). A high yield (70 %–98 %) in short reaction times (5–15 min) was obtained, with 40 wt% PTA-silica and STA-alumina. The reaction was extended to other β-ketoesters and alkyl/arylamines (22 β-enaminones were synthesized). The reuse showed that PTA-silica activity suffered just a little decrement in both, solvent free and acetonitrile media, after four runs. On the other hand, the activity of STA-alumina is only maintained in solvent free medium, since HPA leaching took place when acetonitrile was used as solvent.
Synthesis of β-enaminone.
12.7 Cumene cracking and toluene disproportionation
Nowinska et al. (1991) used PTA and STA impregnated on different supports, including alumina, silica, magnesia, and amorphous aluminosilicate (13 wt% Al2O3), as acid catalysts for cumene cracking (Supplementary Scheme S17) at 150 °C (90 % conversion), toluene disproportionation at 250 °C, and isomerization of n-hexane at 30 and 75 °C (120 h), Reactions were performed in a pulse microreactor. Acid sites are required to absorb ammonia (ammonium salt of HPA was used). PTA/γ-alumina was the most active catalyst for cumene cracking. The catalytic activity increased with PTA content, the maximum being in the range 38–50 wt% PTA, 80 %–95 % cumene conversion. For toluene disproportionation at 400 °C, low loadings of PTA (<28 wt%), alumina-supported catalysts were more active, but with 38–44 wt% PTA content silica catalysts showed higher activity. Very small conversion (less than 3 %) of n-hexane was observed. Alumina was prepared by hydrolysis of aluminum isopropoxide. HPA aqueous solutions were used for support impregnation and calcined at 400 °C for 2 h (10–66 wt% HPA). The authors suggested the formation of aluminum salts. The reuse of the catalysts was not reported.
12.8 Conversion of methanol to hydrocarbons
Conversion of methanol to hydrocarbons was studied by Ehwald et al. (1987) using STA supported on alumina as acid catalyst. Commercial γ-Al2O3 (Degussa) was washed, annealed at 600 °C, and impregnated with aqueous solution of STA. The effect on STA loading (5–40 wt%) was investigated, finding that an increase in the concentration of STA weakened the intensity of γ-alumina lines in the X-ray diffraction pattern, and that also SBET decreased. By Raman spectroscopy it was determined that STA anion was unchanged, but the presence of Al4(SiW)3 was detected on the catalyst surface for 40 wt% STA content. The presence of Al salt was confirmed by NH3-TPD analysis. Thus, a neutralization between the HPA and the support occurred during the impregnation. An increase in the Brönsted centers was observed when the STA content increased, whereas the distribution of acidity was almost unchanged.
The reaction test was done in a stainless-steel reactor, using a flow of methanol and nitrogen mixture at 300 °C. The amount of C1–C5 hydrocarbons was 60 % of the total hydrocarbons formed.
The increase in the initial activity was attributed to hydrolysis of Al–O–W bonds on the surface, with an increase in the acidity. However, the increase in the surface density of acid centers favored the formation of coke, blocking part of the catalyst surface. Thus, the best results regarding the stability and yields to C2–C3 olefins were obtained with the catalysts containing 20 wt% STA.
12.9 Alkylation
The interest in alkylation with C10–C14 linear alkenes is due the use of the products in the manufacture of detergents. Hu et al. (1999) studied the alkylation of phenol with 1-octene and nonene, using PTA and STA supported on silica, γ-alumina, and diatomaceous earth. HPA supported on silica showed the best activity at 80–120 °C; 90 % para-isomer was achieved with (20 wt%) STA/SiO2. A similar reaction was reported by Hernandez-Cortez et al. (2010), who supported PTA (20 wt%) on different materials (zirconia, silica, boehmite-alumina, and activated carbon) for using it as catalyst in liquid phase benzene alkylation. The highest activity was achieved with PTA/SiO2 for alkylation of benzene with 1-decene at 80 °C in 3 h (70 % 1-decene conversion and 40 % selectivity) On the other hand, no activity was detected with PTA/alumina. The higher activity of HPA on silica was related to the presence of Brönsted and Lewis acid sites in this catalyst, meanwhile the other catalysts presented Lewis sites mainly.
Madhusudhan Rao et al. (2004) carried out the alkylation of catechol with t-butyl alcohol (Supplementary Scheme S18), in the presence of nitrobenzene, at 100 °C, using PTA on silica, alumina, and alumina-grafted silica. Complete leaching of PTA on silica was observed after washing with methanol. The strong interaction between PTA and alumina, due to its basicity, caused partially structural damage of HPA, which produced low catalytic activity. Thus, alumina-grafted silica was the best support for PTA. Reuse essays were not reported.
13 Conclusions
This compilation explores various organic transformations catalyzed by the HPA-alumina system, as documented in the literature. The reported procedures leverage the bifunctional character of HPAs, which possess both acidic and redox properties. Among the different alumina structures, the γ-phase, which contains both Brönsted and Lewis basic sites, is the most extensively studied due to its ability to provide high dispersion of HPAs. Additionally, combined alumina-silica materials have been investigated to reduce the strong interactions between HPAs and the alumina surface. Notably, Keggin-type phosphotungstic acid (PTA) and phosphomolybdic acid (PMA) are the most commonly utilized HPAs in these studies.
In most reported processes, which include the synthesis of heterocycles, esterification, oxidation, and hydrogenation, the strong acidity of heteropolyacids is typically reduced when supported on alumina. While this effect can be a disadvantage in some reactions, it may enhance catalytic stability, dispersion, and reusability in others. Regarding the applications of HPA-alumina catalysts, the synthesis of heterocycles is particularly noteworthy. A variety of heterocyclic compounds, known for their bioactivity, such as dihydropyridines, pyrroles, imidazoles, and chromones, can be synthesized using HPA-alumina catalysts as an eco-friendly alternative to traditional methods.
The redox capacity of the HPA-alumina system was evaluated in oxidative processes, including the epoxidation of alkenes and the production of acetic acid from ethane. The oxidation of sulfides to sulfoxides and sulfones is particularly interesting for its application in the desulfurization of diesel fuels. Hydrodesulfurization, hydrogenation, hydrodenitrogenation, and hydrodeoxygenation of aromatic hydrocarbons were studied for the production of organic solvents and to reduce sulfur and oxygen content in biofuels, thereby decreasing NOx emissions. For these transformations, HPA/alumina materials containing platinum (Pt) or nickel (Ni) were evaluated, yielding promising results.
Esterification and transesterification reactions, including biodiesel synthesis, were conducted using HPA-alumina catalysts; however, the results showed no improvement compared to HPA-silica catalysts. The utilization of HPA-alumina composites also allows the valorization of biomass; in this regard, the production of acrolein via the dehydration of glycerol was investigated. Additionally, the catalytic activity of HPA-alumina was tested for the production of both saturated and unsaturated C1–C5 hydrocarbons from methanol, as well as for the isomerization of alkanes and the protection of hydroxyl (OH) groups. Other applications included the synthesis of ketals and acetals.
As demonstrated throughout this review, HPA-alumina catalysts can be employed in a wide variety of reactions. Although their performance is not always greater than that of HPA-silica or HPA-titania, the strong interaction between the active phase and the basic sites of alumina encourages research groups to continue developing catalytic systems composed of HPAs supported on alumina.
Abbreviations
| Abbreviation | Full name | Chemical formula |
|
|
||
| AC | Activated carbon | – |
| AlCuMoVP | – | CuH2PMo11VCuO40/Al2O3 |
| AlFeMoVP | – | FeHPMo11VO40/Al2O3 |
| BCH | Bicyclohexyl | C12H22 |
| BP | Biphenyl | C12H10 |
| CsHPW | Cesium-doped heteropoly tungstate | CsxHyPW12O40 |
| DBT | Dibenzothiophene | C12H8S |
| DDO | Direct deoxygenation | – |
| DHP | Dihydropyridine | – |
| 4,6-DMDBT | 4,6-dimethyl-dibenzothiophene | C14H12S |
| DRIFTS | Diffuse reflectance infrared spectroscopy | – |
| DRS | Diffuse reflectance spectroscopy | – |
| FTIR | Fourier transform infrared | – |
| HDO | Hydrodeoxygenation | – |
| HDS | Hydrodesulfurization | – |
| HIV | Human immunodeficiency virus | – |
| HNT | Halloysite nanotube | – |
| HPA | Heteropolyacid | – |
| HYD | Hydrogenation | – |
| HYN | Hydrodenitrogenation | – |
| IL | Ionic liquid | – |
| IR | Infrared | – |
| NMR | Nuclear magnetic resonance | – |
| PMA | Phosphomolybdic acid | H3PMo12O40 |
| PMVA | – | H4PMo11VO40 |
| PMo10V2 | – | H5PMo10V2O40 |
| PTA | Phosphotungstic acid | H3PW12O40 |
| P5W30 | – | H14[NaP5W30O110] |
| P5W29Mo | – | H14[NaP5W30O110] |
| SBET | Brunauer-Emmett-Teller surface area | – |
| SMA | Silicomolybdic acid | H4SiMo12O40 |
| STA | Silicotungstic acid | H4SiW12O40 |
| TBHP | Tert-butyl hydroperoxide | C4H10O2 |
| TEOS | Tetraethyl orthosilicate | SiC8H20O4 |
| TGA | Thermogravimetric analysis | – |
| TLC | Thin layer chromatography | – |
| TPD | Temperature programmed desorption | – |
| TPR | Temperature programmed reduction | – |
| UV | Ultraviolet | – |
| XPS | X-ray photoelectron spectroscopy | – |
| XRD | X-ray diffraction | – |
Funding source: Agencia Nacional de Promoción Científica y Tecnológica
Award Identifier / Grant number: 2021/00438
Funding source: Consejo Nacional de Investigaciones Científicas y Técnicas
Award Identifier / Grant number: PIP0111
Funding source: Comisión de Investigaciones Científicas de la provincia de Buenos Aires
Funding source: Facultad de Ciencias Exactas, Universidad Nacional de La Plata
Award Identifier / Grant number: X941
Award Identifier / Grant number: X988
Funding source: Fundación Williams
Award Identifier / Grant number: Nº 101
Acknowledgments
The authors thank CONICET (PIP0111), CIC-PBA, ANPCyP (2021/00438), Fundación Williams (Nº 101), and UNLP (X941 and X988) for the financial support.
-
Research ethics: Not applicable.
-
Informed consent: Not applicable.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Use of Large Language Models, AI and Machine Learning Tools: None declared.
-
Conflict of interest: The authors state no conflict of interest.
-
Research funding: CONICET (PIP0111), CIC-PBA, ANPCyP (2021/00438), Fundación Williams (Nº 101), and UNLP (X941 and X988).
-
Data availability: Not applicable.
References
Abu-Hashem, A., El-Shehry, M., and Badria, F. (2010). Design and synthesis of novel thiophenecarbohydrazide, thienopyrazole and thienopyrimidine derivatives as antioxidant and antitumor agents. Acta Pharm. 60: 311–323, https://doi.org/10.2478/v10007-010-0027-6.Search in Google Scholar PubMed
Al-Said, M.S., Ghorab, M.M., and Nissan, Y.M. (2012). Dapson in heterocyclic chemistry, part VIII: synthesis, molecular docking and anticancer activity of some novel sulfonylbiscompounds carrying biologically active 1, 3dihydropyridine, chromene and chromenopyridine moieties. Chem. Cent. J. 6: 64, https://doi.org/10.1186/1752-153x-6-64.Search in Google Scholar PubMed PubMed Central
Alam, Md. A, Shimada, K., Jahan, A., Wahab Khan, Md. Md. M.H., Bhuiyan, Alam, M.S., and Matin, M.M. (2018). Synthesis, reactions and medicinal importance of cyclic sulfone derivatives: a review. Nat. Prod. Chem. Res. 6: 4–11.Search in Google Scholar
Atia, H., Armbruster, U., and Martin, A. (2008). Dehydration of glycerol in gas phase using heteropolyacid catalysts as active compounds. J. Catal. 258: 71–82, https://doi.org/10.1016/j.jcat.2008.05.027.Search in Google Scholar
Atkins, P., Overton, T., Rourke, J., Weller, M., Armstrong, F., and Hagerman, M. (2010). Shriver & Atkins’ inorganic chemistry, 5th ed. Oxford University Press: Great Britain.Search in Google Scholar
Awika, J.M., Rose, D.J., and Simsek, S. (2018). Complementary effects of cereal and pulse polyphenols and dietary fiber on chronic inflammation and gut health. Food Funct. 9: 1389–1409, https://doi.org/10.1039/c7fo02011b.Search in Google Scholar PubMed
Azad, I., Hassan, F., Saquib, M., Ahmad, N., Rahman Khan, A., Al-Sehemi, A.G., and Nasibullah, M. (2018). A critical review on advances in the multicomponent synthesis of pyrroles. Orient. J. Chem. 34: 1670–1700, https://doi.org/10.13005/ojc/340401.Search in Google Scholar
Badday, A.S., Abdullah, A.Z., and Lee, K.-T. (2014). Artificial neural network approach for modeling of ultrasound-assisted transesterification process of crude Jatropha oil catalyzed by heteropolyacid based catalyst. Chem. Eng. Process. 75: 31–37, https://doi.org/10.1016/j.cep.2013.10.008.Search in Google Scholar
Baese, H.-J. and Havsteen, B. (1989). Oxazolones as substrates in active site titrations of hydrolases. Anal. Biochem. 181: 321–330, https://doi.org/10.1016/0003-2697(89)90251-0.Search in Google Scholar PubMed
Baradaran-Sirjani, Z., Roshani, M., Khashi, M., and Beyramabadi, S.A. (2018). Catalytic performance of Preyssler heteropolyacids and synthesis, experimental, theoretical characterizations, fluorescence properties of 1,8-dioxodecahydroacridine derivative. Indian J. Chem. 57B: 715–721.Search in Google Scholar
Bennardi, D.O., Romanelli, G.P., Jios, J.L., Vázquez, P.G., Cáceres, C.V., and Autino, C. (2007). Synthesis of substituted flavones and arylchromones using ρ and si keggin heteropolyacids as catalysts. Heterocycl. Commun. 13: 77–82, https://doi.org/10.1515/hc.2007.13.1.77.Search in Google Scholar
Bennardi, D.O., Romanelli, G.P., Sathicq, Á.G., Autino, J.C., Baronetti, G.T., and Thomas, H.J. (2011). Wells-Dawson heteropolyacid as reusable catalyst for sustainable synthesis of flavones. Appl. Catal. A: Gen. 404: 68–73.10.1016/j.apcata.2011.07.011Search in Google Scholar
Bhardwaj, V., Gumber, D., Abbot, V., Dhiman, S., and Sharma, P. (2015). Pyrrole: a resourceful small molecule in key medicinal hetero-aromatics. RSC Adv. 5: 15233–15266, https://doi.org/10.1039/c4ra15710a.Search in Google Scholar
Bosica, G., Demanuele, K., Padrón, J.M., and Puerta, A. (2020). One-pot multicomponent green Hantzsch synthesis of 1,2-dihydropyridine derivatives with antiproliferative activity. Beilstein J. Org. Chem. 16: 2862–2869, https://doi.org/10.3762/bjoc.16.235.Search in Google Scholar PubMed PubMed Central
Brinker, J.C. and Scherer, G.W. (1990). Sol–gel science. The physics and chemistry of sol-gel processing. Academic Press, Inc, Oxford, UK.Search in Google Scholar
Bryzhin, A.A., Gantman, M.G., Buryak, A.K., and Tarkhanova, I.G. (2019). Brønsted acidic SILP-based catalysts with H3PMo12O40 or H3PW12O40 in the oxidative desulfurization of fuels. Appl. Catal. B: Environ. 257: 117938, https://doi.org/10.1016/j.apcatb.2019.117938.Search in Google Scholar
Burguete, A., Pontiki, E., Hadjipavlou-Litina, D., Villar, R., Vicente, E., Solano, B., Ancizu, S., Pérez-Silanes, S., Aldana, I., and Monge, A. (2007). Synthesis and anti-inflammatory/antioxidant activities of some new ring substituted 3-phenyl-1-(1,4-di-N-oxide quinoxalin-2-yl)-2-propen-1-one derivatives and of their 4,5-dihydro-(1H)-pyrazole analogues. Bioorg. Med. Chem. Lett. 17: 6439–6443, https://doi.org/10.1016/j.bmcl.2007.10.002.Search in Google Scholar PubMed
Canca Ruiz, J. (2006). Química de los polioxometalatos estudio termodifractométrico de la deshidratación del compuesto [Ni(C6H5NO2)3]2[GeW12O40]·18H2O. Universidad del País Vasco, Leioa, Spain.Search in Google Scholar
Castillo, M.A., Vazquez, P.G., Blanco, M.N., and Caceres, C.V. (1996). Adsorption studies and species characterizationin catalysts obtained from aqueous solutions or phosphomolybdic acid and γ-alumina. J. Chem. Soc., Faraday Trans. 92: 3239–3246, https://doi.org/10.1039/ft9969203239.Search in Google Scholar
Casuscelli, S.G., Crivello, M.E., Perez, C.F., Ghione, G., Herrero, E.R., Pizzio, L.R., Vázquez, P.G., Cáceres, C.V., and Blanco, M.N. (2004). Effect of reaction conditions on limonene epoxidation with H2O2 catalyzed by supported Keggin heteropoly compounds. Appl. Catal. A: Gen. 274: 115–122, https://doi.org/10.1016/j.apcata.2004.05.043.Search in Google Scholar
Chandrasekhar, S. and Karri, P. (2006). Aromaticity in azlactone anions and its significance for the Erlenmeyer synthesis. Tetrahedron Lett. 47: 5763–5766, https://doi.org/10.1016/j.tetlet.2006.06.006.Search in Google Scholar
Chandrasekhar, S. and Karri, P. (2007). Erlenmeyer azlactone synthesis with aliphatic aldehydes under solvent-free microwave conditions. Tetrahedron Lett. 48: 785–786, https://doi.org/10.1016/j.tetlet.2006.11.174.Search in Google Scholar
Chen, W., Tan, C.H., Wang, H., and Ye, X. (2021). Molybdenum/tungsten-based heteropoly salts in oxidations. Chem.–Asian J. 16: 2753–2772, https://doi.org/10.1002/asia.202100686.Search in Google Scholar PubMed
Clark, J. (2005). Handbook of green chemistry and technology. Blackwell Science, Repr. Oxford.Search in Google Scholar
Cleary, T., Rawalpally, T., Kennedy, N., and Chavez, F. (2010). Catalyzing the Erlenmeyer Plöchl reaction: organic bases versus sodium acetate. Tetrahedron Lett. 51: 1533–1536, https://doi.org/10.1016/j.tetlet.2009.11.125.Search in Google Scholar
Colombo Migliorero, M.B., Palermo, V., Alarcon Durango, E.A., Villa Holguin, A.L., Vazquez, P.G., Sathicq, A.G., and Romanelli, G.P. (2018). Green and efficient synthesis of flavones and chromones using heteropolyacids as catalyst in glycerol. Lett. Org. Chem. 15: 826–832.10.2174/1570178615666180509150014Search in Google Scholar
Colombo Migliorero, M.B., Palermo, V., Romanelli, G.P., and Vázquez, P.G. (2021). New niobium heteropolyacid included in a silica/alumina matrix: application in selective sulfoxidation. Catal. Today 372: 89–97, https://doi.org/10.1016/j.cattod.2020.10.034.Search in Google Scholar
Colombo Migliorero, M.B., Palermo, V., Romanelli, G.P., and Vázquez, P.G. (2023). Niobium-doped phosphomolybdic acid catalyst included in silica matrix: study of stability and reactivity using different loads. Lat. Am. Appl. Res. 53: 49–54, https://doi.org/10.52292/j.laar.2023.1129.Search in Google Scholar
Conway, P.A., Devine, K., and Paradisi, F. (2009). A simple and efficient method for the synthesis of Erlenmeyer azlactones. Tetrahedron 65: 2935–2938, https://doi.org/10.1016/j.tet.2009.02.011.Search in Google Scholar
Corona, P., Carta, A., Loriga, M., Vitale, G., and Paglietti, G. (2009). Synthesis and in vitro antitumor activity of new quinoxaline derivatives. Eur. J. Med. Chem. 44: 1579–1591, https://doi.org/10.1016/j.ejmech.2008.07.025.Search in Google Scholar PubMed
Da Conceição, L.R.V., Carneiro, L.M., Giordani, D.S., and De Castro, H.F. (2017). Synthesis of biodiesel from macaw palm oil using mesoporous solid catalyst comprising 12-molybdophosphoric acid and niobia. Renew. Energy 113: 119–128, https://doi.org/10.1016/j.renene.2017.05.080.Search in Google Scholar
Da Conceição, L.R.V., Reis, C.E.R., De Lima, R., Cortez, D.V., and De Castro, H.F. (2019). Keggin-structure heteropolyacid supported on alumina to be used in trans/esterification of high-acid feedstocks. RSC Adv. 9: 23450–23458, https://doi.org/10.1039/c9ra04300d.Search in Google Scholar PubMed PubMed Central
Dailey, S., Feast, W.J., Peace, R.J., Sage, I.C., Till, S., and Wood, E.L. (2001). Synthesis and device characterisation of side-chain polymer electron transport materials for organic semiconductor applications. J. Mater. Chem. 11: 2238–2243, https://doi.org/10.1039/b104674h.Search in Google Scholar
Darabi, H.R., Mohandessi, S., Aghapoor, K., and Mohsenzadeh, F. (2007). A recyclable and highly effective sulfamic acid/MeOH catalytic system for the synthesis of quinoxalines at room temperature. Catal. Commun. 8: 389–392, https://doi.org/10.1016/j.catcom.2006.06.033.Search in Google Scholar
Das, P.J., Das, J., Ghosh, M., and Sultana, S. (2013). Solvent free one-pot synthesis of 1,2,4,5-tetrasubstituted imidazoles catalyzed by secondary amine based ionic liquid and defective Keggin heteropoly acid. Green Sustain. Chem. 03: 6–13.10.4236/gsc.2013.34A002Search in Google Scholar
Datka, J. (1992). Acidic properties of supported niobium oxide catalysts: an infrared spectroscopy investigation. J. Catal. 135: 186–199, https://doi.org/10.1016/0021-9517(92)90279-q.Search in Google Scholar
De Angelis, A., Pollesel, P., Molinari, D., O’Neal, Parker Jr., W., Frattini, A., Cavani, F., Martins, S., and Perego, C. (2007). Heteropolyacids as effective catalysts to obtain zero sulfur diesel. Pure Appl. Chem. 79: 1887–1894, https://doi.org/10.1351/pac200779111887.Search in Google Scholar
Deutschmann, O., Knözinger, H., Kochloefl, K., and Turek, T. (2009) Heterogeneous catalysis and solid catalysts. In: Ullmann’s encyclopedia of industrial chemistry. Wiley-VCH, Weinheim, Germany.10.1002/14356007.a05_313.pub2Search in Google Scholar
Dexter, D.D. and Silverton, J.V. (1968). A new structural type for heteropoly anions. The crystal structure of (NH4)2H6(CeMo12O42).12H2O. J. Am. Chem. Soc. 90: 3589–3590, https://doi.org/10.1021/ja01015a067.Search in Google Scholar
Dubey, S.P., Dwivedi, A.D., Sillanpää, M., Lee, H., Kwon, Y.-N., and Lee, C. (2017). Adsorption of As(V) by boehmite and alumina of different morphologies prepared under hydrothermal conditions. Chemosphere 169: 99–106, https://doi.org/10.1016/j.chemosphere.2016.11.052.Search in Google Scholar PubMed
Echeandia, S., Arias, P.L., Barrio, V.L., Pawelec, B., and Fierro, J.L.G. (2010). Synergy effect in the HDO of phenol over Ni–W catalysts supported on active carbon: effect of tungsten precursors. Appl. Catal. B: Environ. 101: 1–12, https://doi.org/10.1016/j.apcatb.2010.08.018.Search in Google Scholar
Ehwald, H., Fiebig, W., Jerschkewitz, H.G., Lischke, G., Parlitz, B., Reich, P., and Öhlmann, G. (1987). Synthesis of olefins from methanol on alumina-supported H4[SiW12040] catalysts. Appl. Catal. 34: 23–38, https://doi.org/10.1016/s0166-9834(00)82443-4.Search in Google Scholar
El-Assiery, S.A., Sayed, G.H., and Fouda, A. (2004). Synthesis of some new annulated pyrazolo-pyrido (or pyrano) pyrimidine, pyra-zolopyridine and pyranopyrazole derivatives. Acta Pharm 54: 143–150.Search in Google Scholar
El-Gazzar, A.R.B.A., El-Enany, M.M., and Mahmoud, M.N. (2008). Synthesis, analgesic, anti-inflammatory, and antimicrobial activity of some novel pyrimido[4,5-b]quinolin-4-ones. Bioorg. Med. Chem. 16: 3261–3273, https://doi.org/10.1016/j.bmc.2007.12.012.Search in Google Scholar PubMed
Erfle, S., Armbruster, U., Bentrup, U., Martin, A., and Brückner, A. (2011). Impact of redox properties on dehydration of glycerol to acrolein over heteropolyacids assessed by operando-EPR spectroscopy. Appl. Catal. A: Gen. 391: 102–109, https://doi.org/10.1016/j.apcata.2010.04.042.Search in Google Scholar
Escobar, A.M., Blustein, G., Luque, R., and Romanelli, G.P. (2021). Recent applications of heteropolyacids and related compounds in heterocycle synthesis. Contributions between 2010 and 2020. Catalysts 11: 291, https://doi.org/10.3390/catal11020291.Search in Google Scholar
Farah, B., Lancelot, C., Blanchard, P., Richard, F., and Lamonier, C. (2023). Beneficial effect of W incorporation in supported Mo‐based catalysts for the HDO of m‐cresol. ChemCatChem 15: e202201409, https://doi.org/10.1002/cctc.202201409.Search in Google Scholar
Fathalla, O.A., Zeid, I.F., Haiba, M.E., Soliman, A.M., Abd-Elmoez, Sh.I., and El-Serwy, W.S. (2009). Synthesis, antibacterial and anticancer evaluation of some pyrimidine derivatives. World J. Chem. 4: 127–132.Search in Google Scholar
Fayed, A.A., Hosni, H.M., Flefel, E.M., and Amr, A.E.E. (2009). Synthesis and pharmacological activity of some new thieno[2,3-d]pyrimidine and pyrimidopyrazolothienopyrimidine derivatives. World J. Chem. 4: 58–65.Search in Google Scholar
Forster, L., Qie, Z., Hu, M., Mavridis, A., Price, C., Parlett, C.M.A., Fan, X., and D’Agostino, C. (2022). Heteropolyacids supported on zirconia-doped γ, θ and α alumina: a physicochemical assessment and characterisation of supported solid acids. Appl. Surf. Sci. 605: 154696, https://doi.org/10.1016/j.apsusc.2022.154696.Search in Google Scholar
Freitas, E.F., Paiva, M.F., Dias, S.C.L., and Dias, J.A. (2017). Generation and characterization of catalytically active sites of heteropolyacids on zeolite Y for liquid-phase esterification. Catal. Today 289: 70–77, https://doi.org/10.1016/j.cattod.2016.08.010.Search in Google Scholar
Ganguly, N.C., Chandra, S., and Barik, S.K. (2013). Sodium perborate tetrahydrate–mediated transformations of 2′-hydroxychalcones to flavanones, flavones, and 3′,5′-diiodoflavone under mild, environmentally friendly conditions. Synth. Commun. 43: 1351–1361, https://doi.org/10.1080/00397911.2011.633734.Search in Google Scholar
Gao, J., Wei, Y., Wang, X., and Yang, W. (2007). Tungstophosphoric heteropolyacid supported onto neutral alumina: characterization and synthesis of acetals and ketals. Rare Met. 26: 152–157, https://doi.org/10.1016/s1001-0521(07)60176-4.Search in Google Scholar
García-Gutiérrez, J.L., Laredo, G.C., García-Gutiérrez, P., and Jiménez-Cruz, F. (2014). Oxidative desulfurization of diesel using promising heterogeneous tungsten catalysts and hydrogen peroxide. Fuel 138: 118–125, https://doi.org/10.1016/j.fuel.2014.07.049.Search in Google Scholar
Gelmi, M.L., Clerici, F., and Melis, A. (1997). 5(4H)-Oxazolones. Part X. Acid and base effects on the translactonization reaction of 4-(2-Oxa-alkylidene)-5(4H)-oxazolones: new synthesis of 5-alkylidene-3-benzoylamino-2(5H)-furanones. Tetrahedron 53: 1843–1854, https://doi.org/10.1016/s0040-4020(96)01101-5.Search in Google Scholar
German-Ponciano, L.J., Dutra Costa, B.P., Feitosa, L.M., Campos, K.D.S., Da Silva Chaves, S.N., Cueto-Escobedo, J., Lima-Maximino, M., Rodríguez-Landa, J.F., and Maximino, C. (2020). Chrysin, but not flavone backbone, decreases anxiety-like behavior in animal screens. Neurochem. Int. 140: 104850, https://doi.org/10.1016/j.neuint.2020.104850.Search in Google Scholar PubMed
Gharib, A., Jahangir, M., Roshani, M., and Scheeren, J. (Hans) W. (2010). Effective catalytic synthesis of substituted flavones and chromones using Preyssler and heteropolyacids (HPAs) as catalysts. Bulg. Chem. Commun. 42: 210–216.Search in Google Scholar
Gharib, A., Jahangir, M., Roshani, M., and Scheeren, J. (Hans) W. (2012). Catalytic synthesis of fused 1,4-dihydropyridines and 1,4-dihydropyridine derivatives using Preyssler heteropolyacids catalyst. Synth. Commun. 42: 3311–3320, https://doi.org/10.1080/00397911.2011.581405.Search in Google Scholar
Gorsd, M., Sathicq, G., Romanelli, G., Pizzio, L., and Blanco, M. (2016). Tungstophosphoric acid supported on core-shell polystyrene-silica microspheres or hollow silica spheres catalyzed trisubstituted imidazole synthesis by multicomponent reaction. J. Mol. Catal. A: Chem. 420: 294–302, https://doi.org/10.1016/j.molcata.2016.04.010.Search in Google Scholar
Guerrero-Pérez, M.O., Herrera, M.C., Malpartida, I., Larrubia, M.A., and Alemany, L.J. (2008). Effect of tellurium addition to supported Mo-V-O catalysts for the ammoxidation of propane to acrylonitrile. Catal. Today 133–135: 919–924, https://doi.org/10.1016/j.cattod.2007.12.101.Search in Google Scholar
Hafez, H., Abbas, H.-A., and El-Gazzar, A.-R. (2008). Synthesis and evaluation of analgesic, anti-inflammatory and ulcerogenic activities of some triazolo- and 2-pyrazolyl-pyrido[2,3-d]-pyrimidines. Acta Pharm. 58: 359–378, https://doi.org/10.2478/v10007-008-0024-1.Search in Google Scholar PubMed
Hakimia, F. and Mirjalili, B.B.F. (2013). Synthesis of quinoxalines in the presence of heteropoly acids. Curr. Chem. Lett. 2: 105–108, https://doi.org/10.5267/j.ccl.2013.01.001.Search in Google Scholar
Hantzsch, A. (1881). Condensationprodukte aus Aldehydammoniak und ketoniartigen Verbindungen. Ber. Dtsch. Chem. Ges. 14: 1637–1638, https://doi.org/10.1002/cber.18810140214.Search in Google Scholar
Hao, S.-H., Zhang, X.-Y., Dong, D.-Q., and Wang, Z.-L. (2015). Alumina-supported heteropoly acid: an efficient catalyst for the synthesis of azaarene substituted 3-hydroxy-2-oxindole derivatives via C(sp3)H bond functionalization. Chin. Chem. Lett. 26: 599–602, https://doi.org/10.1016/j.cclet.2014.12.018.Search in Google Scholar
Hassan, A.S. (2014). The antibacterial activity of dimethyl sulfoxide (DMSO) with and without of some ligand complexes of the transitional metal ions of ethyl coumarin against bacteria isolate from burn and wound infection. J. Nat. Sci. Res. 4: 106–111.Search in Google Scholar
He, E., Huang, G., Fan, H., Yang, C., Wang, H., Tian, Z., Wang, L., and Zhao, Y. (2019). Macroporous alumina- and titania-based catalyst for carbonyl sulfide hydrolysis at ambient temperature. Fuel 246: 277–284, https://doi.org/10.1016/j.fuel.2019.02.097.Search in Google Scholar
Heravi, M.M., Bakhtiari, K., Bamoharram, F.F., and Tehrani, M.H. (2007a). Wells-Dawson type heteropolyacid catalyzed synthesis of quinoxaline derivatives at room temperature. Monatsh. Chem. 138: 465–467, https://doi.org/10.1002/chin.200735159.Search in Google Scholar
Heravi, M.M., Derikvand, F., and Bamoharram, F.F. (2007b). Highly efficient, four-component one-pot synthesis of tetrasubstituted imidazoles using Keggin-type heteropolyacids as green and reusable catalysts. J. Mol. Catal. A: Chem. 263: 112–114, https://doi.org/10.1016/j.molcata.2006.08.048.Search in Google Scholar
Hernández-Cortez, J.G., Martinez, L., Soto, L., López, A., Navarrete, J., Manríquez, Ma., Lara, V.H., and López-Salinas, E. (2010). Liquid phase alkylation of benzene with dec-1-ene catalyzed on supported 12-tungstophosphoric acid. Catal. Today 150: 346–352, https://doi.org/10.1016/j.cattod.2009.12.003.Search in Google Scholar
Himmel, B., Gerber, Th., Burger, H., Holzhuter, G., and Olbertz, A. (1995). Structural characterization of SiO2-Al2O3 aerogels. J. Non-Cryst. Solids 186: 149–158, https://doi.org/10.1016/0022-3093(95)00045-3.Search in Google Scholar
Hirao, I., Yamaguchi, M., and Hamada, M. (1984). A convenient synthesis of 2- and 2,3-substituted 4H-chromen-4-ones. Synthesis 1984: 1076–1078, https://doi.org/10.1055/s-1984-31089.Search in Google Scholar
Hu, C., Zhang, Y., Xu, L., and Peng, G. (1999). Continuous syntheses of octyl phenol or nonane phenol on supported heteropoly acid catalysts. Appl. Catal. A: Gen. 177: 237–244, https://doi.org/10.1016/s0926-860x(98)00269-5.Search in Google Scholar
Huang, T., Wang, R., Shi, L., and Lu, X. (2008). Montmorillonite K-10: an efficient and reusable catalyst for the synthesis of quinoxaline derivatives in water. Catal. Commun. 9: 1143–1147, https://doi.org/10.1016/j.catcom.2007.10.024.Search in Google Scholar
Huang, T.K., Shi, L., Wang, R., Guo, X.Z., and Lu, X.X. (2009). Keggin type heteropolyacids-catalyzed synthesis of quinoxaline derivatives in water. Chin. Chem. Lett. 20: 161–164, https://doi.org/10.1016/j.cclet.2008.10.048.Search in Google Scholar
Idhayadhulla, A., Kumar, R.S., Nasser, A.J.A., Kavimani, S., and Indhumathy, S. (2015). Anti-inflammatory activity of new series of 1,4-dihydropyridine derivatives. Pharm. Chem. J. 49: 463–466, https://doi.org/10.1007/s11094-015-1305-x.Search in Google Scholar
Islami, M.R. and Hassani, Z. (2008). One-pot and efficient protocol for synthesis of quinoxaline derivatives. Arkivoc 2008: 280–287, https://doi.org/10.3998/ark.5550190.0009.f24.Search in Google Scholar
Ivanov, A.V., Vasina, T.V., Masloboishchikova, O.V., Khelkovskaya-Sergeeva, E.G., Kustov, L.M., and Houzvicka, J.I. (2000). n-Alkane isomerization on heteropolyacids. 1. The influence of acid-base properties of alumina systems on the state of supported 12-tungstophosphoric heteropolyacid in Pt-containing catalysts and their activity in n-pentane isomerization. Russ. Chem. Bull. 49: 1726–1731, https://doi.org/10.1007/bf02496342.Search in Google Scholar
Jafarpour, M., Rezaeifard, A., and Danehchin, M. (2011). Easy access to quinoxaline derivatives using alumina as an effective and reusable catalyst under solvent-free conditions. Appl. Catal. A: Gen. 394: 48–51, https://doi.org/10.1016/j.apcata.2010.12.022.Search in Google Scholar
Jaung, J. (2006). Synthesis and halochromism of new quinoxaline fluorescent dyes. Dyes Pigments 71: 245–250, https://doi.org/10.1016/j.dyepig.2005.07.008.Search in Google Scholar
Jeannin, Y.P. (1998). The nomenclature of polyoxometalates: how to connect a name and a structure. Chem. Rev. 98: 51–76, https://doi.org/10.1021/cr960397i.Search in Google Scholar PubMed
Justin Thomas, K.R., Velusamy, M., Lin, J.T., Chuen, C.-H., and Tao, Y.-T. (2005). Chromophore-labeled quinoxaline derivatives as efficient electroluminescent materials. Chem. Mater. 17: 1860–1866, https://doi.org/10.1021/cm047705a.Search in Google Scholar
Kale, S.S., Armbruster, U., Eckelt, R., Bentrup, U., Umbarkar, S.B., Dongare, M.K., and Martin, A. (2016). Understanding the role of Keggin type heteropolyacid catalysts for glycerol acetylation using toluene as an entrainer. Appl. Catal. A: Gen. 527: 9–18, https://doi.org/10.1016/j.apcata.2016.08.016.Search in Google Scholar
Kang, T.H., Choi, J.H., Bang, Y., Yoo, J., Song, J.H., Joe, W., Choi, J.S., and Song, I.K. (2015). Dehydration of glycerin to acrolein over H3PW12O40 heteropolyacid catalyst supported on silica-alumina. J. Mol. Catal. A: Chem. 396: 282–289, https://doi.org/10.1016/j.molcata.2014.10.015.Search in Google Scholar
Kato, C.N., Makino, Y., Yamasaki, M., Kataoka, Y., Kitagawa, Y., and Okumura, M. (2012). Synthesis and X-ray crystal structure of α-Keggin-type aluminum-substituted polyoxotungstate. In: Mastai, Y. (Ed.). Advances in crystallization processes. InTech, Rijeka, Croatia.Search in Google Scholar
Kaushik, D. and Verma, T. (2011). 5-[(5-Chloro-3-methyl-1-phenyl-1H-pyrazol-4-yl)methylene]-2-(4-nitrobenzoyl)-3-phenyl-2,5-dihydro-1,2,4-triazin-6(1H)-one. Molbank 2011: M733, https://doi.org/10.3390/m733.Search in Google Scholar
Knozinger, H. (1985). Acidic and basic properties of aluminas in relation to their properties as catalysts and supports. Stud. Surf. Sci. Catal. 20: 111–125, https://doi.org/10.1016/s0167-2991(09)60161-0.Search in Google Scholar
Kokliukhin, A., Nikulshina, M., Mozhaev, A., Lancelot, C., Blanchard, P., Briois, V., Marinova, M., Lamonier, C., and Nikulshin, P. (2021a). Study of hydrotreating performance of trimetallic NiMoW/Al2O3 catalysts prepared from mixed MoW Keggin heteropolyanions with various Mo/W ratios. J. Catal. 403: 141–159, https://doi.org/10.1016/j.jcat.2021.02.019.Search in Google Scholar
Kokliukhin, A., Nikulshina, M., Mozhaev, A., Lancelot, C., Blanchard, P., Mentré, O., Marinova, M., Lamonier, C., and Nikulshin, P. (2021b). The effect of the Mo/W ratio on the catalytic properties of alumina supported hydrotreating catalysts prepared from mixed SiMo6W6 and SiMo9W3 heteropolyacids. Catal. Today 377: 100–113, https://doi.org/10.1016/j.cattod.2020.07.050.Search in Google Scholar
Kokliukhin, A., Nikulshina, M., Mozhaev, A., Lancelot, C., Lamonier, C., Nuns, N., Blanchard, P., Bugaev, A., and Nikulshin, P. (2021c). Bulk hydrotreating MonW12-nS2 catalysts based on SiMonW12-n heteropolyacids prepared by alumina elimination method. Catal. Today 377: 26–37, https://doi.org/10.1016/j.cattod.2020.07.018.Search in Google Scholar
Kovarik, L., Bowden, M., and Szanyi, J. (2021). High temperature transition aluminas in δ-Al2O3/θ-Al2O3 stability range: review. J. Catal. 393: 357–368, https://doi.org/10.1016/j.jcat.2020.10.009.Search in Google Scholar
Kraleva, E. and Atia, H. (2019). Keggin-type heteropolyacids supported on sol–gel oxides as catalysts for the dehydration of glycerol to acrolein. React. Kinet. Mech. Catal. 126: 103–117, https://doi.org/10.1007/s11144-018-1471-4.Search in Google Scholar
Kuang, W., Rives, A., Fournier, M., and Hubaut, R. (2003). Structure and reactivity of silica-supported 12-tungstophosphoric acid. Appl. Catal. A: Gen. 250: 221–229, https://doi.org/10.1016/s0926-860x(03)00239-4.Search in Google Scholar
Kulkarni, P., Wagh, P., and Zubaidha, P. (2012). An improved and eco-friendly method for the synthesis of flavanone by the cyclization of 2’-hydroxy chalcone using methane sulphonic acid as catalyst. Chem. J. 02: 106–110.Search in Google Scholar
Kumar, A., Kumar, S., Saxena, A., De, A., and Mozumdar, S. (2008). Ni-nanoparticles: an efficient catalyst for the synthesis of quinoxalines. Catal. Commun. 9: 778–784, https://doi.org/10.1016/j.catcom.2007.08.021.Search in Google Scholar
Kumar, D., Patel, G., Mishra, B.G., and Varma, R.S. (2008). Eco-friendly polyethylene glycol promoted Michael addition reactions of α,β-unsaturated carbonyl compounds. Tetrahedron Lett. 49: 6974–6976, https://doi.org/10.1016/j.tetlet.2008.09.116.Search in Google Scholar
Kümmerer, K., Clark, J.H., and Zuin, V.G. (2020). Rethinking chemistry for a circular economy. Science 367: 369–370, https://doi.org/10.1126/science.aba4979.Search in Google Scholar PubMed
Kupwade, R.V. (2019). A concise review on synthesis of sulfoxides and sulfones with special reference to oxidation of sulfides. J. Chem. Rev. 1: 99–113.10.33945/SAMI/JCR.2019.1.99113Search in Google Scholar
Kurhade, A. and Dalai, A.K. (2018). Physiochemical characterization and support interaction of alumina‐supported heteropolyacid catalyst for biodiesel production. Asia-Pac. J. Chem. Eng. 13: e2249, https://doi.org/10.1002/apj.2249.Search in Google Scholar
Legros, J., Dehli, J.R., and Bolm, C. (2005). Applications of catalytic asymmetric sulfide oxidations to the syntheses of biologically active sulfoxides. Adv. Synth. Catal. 347: 19–31, https://doi.org/10.1002/adsc.200404206.Search in Google Scholar
Leurs, R., Vischer, H.F., Wijtmans, M., and De Esch, I.J.P. (2011). En route to new blockbuster anti-histamines: surveying the offspring of the expanding histamine receptor family. Trends Pharmacol. Sci. 32: 250–257, https://doi.org/10.1016/j.tips.2011.02.004.Search in Google Scholar PubMed
Li, G., Ding, Y., Wang, J., Wang, X., and Suo, J. (2007). New progress of Keggin and Wells–Dawson type polyoxometalates catalyze acid and oxidative reactions. J. Mol. Catal. A: Chem. 262: 67–76, https://doi.org/10.1016/j.molcata.2006.08.067.Search in Google Scholar
Liang, Z., Yu, J., Zhang, Y., Ma, P., Mao, Q., and Wang, J. (2020). A lacunary heteropolyniobate linked by [Co(H2O)6]2+ ions. Inorg. Chem. Commun. 111: 107612, https://doi.org/10.1016/j.inoche.2019.107612.Search in Google Scholar
Lim, S.S., Kim, Y.H., Park, G.I., Lee, W.Y., Song, I.K., and Youn, H.K. (1999). Heterogeneous liquid-phase hydration of isobutene by heteropoly acid–polymer composite film catalyst. Catal. Lett. 60: 199–204, https://doi.org/10.1023/a:1019031729493.10.1023/A:1019031729493Search in Google Scholar
Llewelyn, P. (2011). Supported heteropoly acids for acid catalysed reactions, PhD Thesis. Pro.Quest LLC, Ann. Arbor, U.S.Search in Google Scholar
Lu, N.-N., Weng, Z.-Y., Chen, Q.-Y., Boison, D., Xiao, X.-X., and Gao, J. (2016). Evaluation on the inhibition of pyrrol-2-yl ethanone derivatives to lactate dehydrogenase and anticancer activities. Spectrochim. Acta A: Mol. Biomol. Spectrosc. 165: 21–25, https://doi.org/10.1016/j.saa.2016.04.010.Search in Google Scholar PubMed
Maciuca, A.L., Ciocan, C.E., Dumitriu, E., Fajula, F., and Huela, V. (2008). V-Mo- and W-containing layered double hydroxides as effective catalysts for mild oxidation of thioethers and thiophenes with H2O2. Catal. Today 138: 33–37, https://doi.org/10.1016/j.cattod.2008.04.031.Search in Google Scholar
Madhusudhan Rao, P., Wolfson, A., Landau, M.V., and Herskowitz, M. (2004). Efficient immobilization of 12-tungstophosphoric acid catalyst at the surface of silica support grafted with alumina. Catal. Commun. 5: 327–331, https://doi.org/10.1016/j.catcom.2004.04.001.Search in Google Scholar
Madhusudhan Rao, P., Wolfson, A., Kababya, S., Vega, S., and Landau, M.V. (2005). Immobilization of molecular H3PW12O40 heteropolyacid catalyst in alumina-grafted silica-gel and mesostructured SBA-15 silica matrices. J. Catal. Commun. 232: 210–225.10.1016/j.jcat.2005.03.006Search in Google Scholar
Manríquez, Ma.E., Ortiz, A.L., Trejo-Valdez, M., Castro, L.V., and Ortiz-Islas, E. (2022). Catalytic oxidative desulfurization of 4,6-dimethyl dibenzothiophene by phosphotungstic acid loaded on Al2O3, V2O5, and ZrO2 oxides. Reac. Kinet. Mech. Cat. 135: 1523–1539, https://doi.org/10.1007/s11144-022-02201-1.Search in Google Scholar
Menezes, J., Vaz, L., De Abreu Vieira, P., Da Silva Fonseca, K., Carneiro, C., and Taylor, J. (2014). Synthesis and anti-trypanosoma cruzi activity of diaryldiazepines. Molecules 20: 43–51, https://doi.org/10.3390/molecules20010043.Search in Google Scholar PubMed PubMed Central
Mirjalili, B.B.F. and Akbari, A. (2011). Nano-TiO2: an eco-friendly alternative for the synthesis of quinoxalines. Chin. Chem. Lett. 22: 753–756, https://doi.org/10.1016/j.cclet.2010.12.016.Search in Google Scholar
Mothé-Esteves, P., Pereira, M.M., Arichi, J., and Louis, B. (2010). How Keggin-type polyoxometalates self-organize into crystals. Cryst. Growth Des. 10: 371–378, https://doi.org/10.1021/cg900984z.Search in Google Scholar
Mozhaev, A.V., Nikulshin, P.A., Pimerzin, Al.A., Maslakov, K.I., and Pimerzin, A.A. (2016). Investigation of co-promotion effect in NiCoMoS/Al2O3 catalysts based on Co2Mo10-heteropolyacid and nickel citrate. Catal. Today 271: 80–90, https://doi.org/10.1016/j.cattod.2015.11.002.Search in Google Scholar
Neumann, R. (2010). Activation of molecular oxygen, polyoxometalates, and liquid phase catalytic oxidation. Inorg. Chem. 49: 3594–3601, https://doi.org/10.1021/ic9015383.Search in Google Scholar PubMed
Niknam, K., Saberi, D., and Mohagheghnejad, M. (2009). Silica bonded S-sulfonic acid: a recyclable catalyst for the synthesis of quinoxalines at room temperature. Molecules 14: 1915–1926, https://doi.org/10.3390/molecules14051915.Search in Google Scholar PubMed PubMed Central
Nikulshin, P.A., Minaev, P.P., Mozhaev, A.V., Maslakov, K.I., Kulikova, M.S., and Pimerzin, A.A. (2015). Investigation of co-effect of 12-tungstophosphoric heteropolyacid, nickel citrate and carbon-coated alumina in preparation of NiW catalysts for HDS, HYD and HDN reactions. Appl. Catal. B: Environ. 176–177: 374–384, https://doi.org/10.1016/j.apcatb.2015.04.011.Search in Google Scholar
Nikulshina, M., Mozhaev, A., Lancelot, C., Marinova, M., Blanchard, P., Payen, E., Lamonier, C., and Nikulshin, P. (2018a). MoW synergetic effect supported by HAADF for alumina based catalysts prepared from mixed SiMonW12-n heteropolyacids. Appl. Catal. B: Environ. 224: 951–959, https://doi.org/10.1016/j.apcatb.2017.11.049.Search in Google Scholar
Nikulshina, M.S., Blanchard, P., Mozhaev, A., Lancelot, C., Griboval-Constant, A., Fournier, M., Payen, E., Mentré, O., Briois, V., Nikulshin, P.A., et al.. (2018b). Molecular approach to prepare mixed MoW alumina supported hydrotreatment catalysts using H4SiMonW12-nO40 heteropolyacids. Catal. Sci. Technol. 8: 5557–5572, https://doi.org/10.1039/c8cy00672e.Search in Google Scholar
Nikulshina, M., Mozhaev, A., Lancelot, C., Blanchard, P., Marinova, M., Lamonier, C., and Nikulshin, P. (2019). Enhancing the hydrodesulfurization of 4,6-dimethyldibenzothiophene through the use of mixed MoWS2 phase evidenced by HAADF. Catal. Today 329: 24–34, https://doi.org/10.1016/j.cattod.2018.11.051.Search in Google Scholar
Ninh, T.K.T., Laurenti, D., Leclerc, E., and Vrinat, M. (2014). Support effect for CoMoS and CoNiMoS hydrodesulfurization catalysts prepared by controlled method. Appl. Catal. A: Gen. 487: 210–218, https://doi.org/10.1016/j.apcata.2014.07.042.Search in Google Scholar
Niu, R., Xiao, J., Liang, T., and Li, X. (2012). Facile synthesis of azaarene-substituted 3-hydroxy-2-oxindoles via Brønsted acid catalyzed sp3 C–H functionalization. Org. Lett. 14: 676–679, https://doi.org/10.1021/ol2030982.Search in Google Scholar PubMed
Nomiya, K., Takahashi, T., Shirai, T., and Miwa, M. (1987). Anderson-type heteropolyanions of molybdenum(VI) and tungsten(VI). Polyhedron 6: 213–218, https://doi.org/10.1016/s0277-5387(00)80791-3.Search in Google Scholar
Nowińska, K., Fiedorow, R., and Adamiec, J. (1991). Catalytic activity of supported heteropoly acids for reactions requiring strong acid centres. J. Chem. Soc., Faraday Trans. 87: 749–753, https://doi.org/10.1039/ft9918700749.Search in Google Scholar
Nunes, R.F., Costa, D., Ferraria, A.M., Botelho Do Rego, A.M., Ribeiro, F., Martins, Â., and Fernandes, A. (2023). Heterogenization of heteropolyacid with metal-based alumina supports for the guaiacol gas-phase hydrodeoxygenation. Molecules 28: 2245, https://doi.org/10.3390/molecules28052245.Search in Google Scholar PubMed PubMed Central
Ollis, W.D. and Weight, D. (1952). The synthesis of 3-substituted chromones by rearrangement of o-acyloxyacetophenones. J. Chem. Soc. 1952: 3826–3830, https://doi.org/10.1039/jr9520003826.Search in Google Scholar
Palermo, V., Sathicq, Á.G., Vázquez, P.G., Thomas, H.J., and Romanelli, G.P. (2011). Doped Keggin heteropolyacids as catalysts in sulfide oxidation. React. Kinet. Mech. Catal. 104: 181–195.10.1007/s11144-011-0341-0Search in Google Scholar
Palermo, V., Sathicq, Á.G., Constantieux, T., Rodríguez, J., Vázquez, P.G., and Romanelli, G.P. (2016). First report about the use of micellar Keggin heteropolyacids as catalysts in the green multicomponent synthesis of nifedipine derivatives. Catal. Lett. 146: 1634–1647, https://doi.org/10.1007/s10562-016-1784-8.Search in Google Scholar
Palermo, V., Ruiz, D., Sathicq, A., Vázquez, P., and Romanelli, G. (2022). A hybrid system bases on silica-alumina and Keggin heteropolyacids as catalyst in the suitable 2-(2-furyl)-chromones and chromanones synthesis. Cienc. Desarr. 13: 93–102.10.19053/01217488.v13.n1.2022.14165Search in Google Scholar
Patiño, Y., Faba, L., Díaz, E., and Ordóñez, S. (2024). Biodiesel production from sewage sludge using supported heteropolyacid as heterogeneous acid catalyst. J. Environ.l Manag. 365: 121643, https://doi.org/10.1016/j.jenvman.2024.121643.Search in Google Scholar PubMed
Paul, S., Nanda, P., Gupta, R., and Loupy, A. (2004). Calcium acetate catalyzed synthesis of 4-arylidene-2-phenyl-5(4 H )-oxazolones under solvent-free conditions. Tetrahedron Lett. 45: 425–427, https://doi.org/10.1016/j.tetlet.2003.10.125.Search in Google Scholar
Pérez, M.E., Ruiz, D.M., Autino, J.C., Blanco, M.N., Pizzio, L.R., and Romanelli, G.P. (2013). Mesoporous titania/tungstophosphoric acid composites: suitable synthesis of flavones. J. Porous Mater. 20: 1433–1440, https://doi.org/10.1007/s10934-013-9729-8.Search in Google Scholar
Pizzio, L.R., Cáceres, C.V., and Blanco, M.N. (1998). Acid catalysts prepared by impregnation of tungstophosphoric acid solutions on different supports. Appl. Catal. A: Gen. 167: 283–294.10.1016/S0926-860X(97)00328-1Search in Google Scholar
Pizzio, L., Vázquez, P., Cáceres, C., and Blanco, M. (1999). Hydrotreating catalysts on alumina, titania or zirconia from ethanol/water solutions of heteropolyacids. Stud. Surf. Sci. Catal. 127: 413–420, https://doi.org/10.1016/s0167-2991(99)80443-1.Search in Google Scholar
Pizzio, L.R., Vázquez, P.G., Cáceres, C.V., and Blanco, M.N. (2003). Supported Keggin type heteropolycompounds for ecofriendly reactions. Appl. Catal. A: Gen. 256: 125–139.10.1016/S0926-860X(03)00394-6Search in Google Scholar
Portilla Zúñiga, O.M. (2020). Sólidos ácidos de estructura tipo Preyssler como catalizadores en la síntesis de pirroles y dihidropirimidinonas, Ph.D. Thesis. Universidad Nacional de La Plata, La Plata, Argentina.Search in Google Scholar
Portilla-Zúñiga, O., Sathicq, Á., Martínez, J., Rojas, H., De Geronimo, E., Luque, R., and Romanelli, G.P. (2018). Novel bifunctional mesoporous catalysts based on Preyssler heteropolyacids for green pyrrole derivative synthesis. Catalysts 8: 419, https://doi.org/10.3390/catal8100419.Search in Google Scholar
Raffa, D., Maggio, B., Raimondi, M.V., Plescia, F., and Daidone, G. (2017). Recent discoveries of anticancer flavonoids. Eur. J. Med. Chem. 142: 213–228, https://doi.org/10.1016/j.ejmech.2017.07.034.Search in Google Scholar PubMed
Rafiee, E., Joshaghani, M., Eavani, S., and Rashidzadeh, S. (2008). A revision for the synthesis of β-enaminones in solvent free conditions: efficacy of different supported heteropoly acids as active and reusable catalysts. Green Chem. 10: 982, https://doi.org/10.1039/b803249a.Search in Google Scholar
Rafiee, E., Mahdavi, H., and Joshaghani, M. (2011). Supported heteropoly acids offering strong option for efficient and cleaner processing for the synthesis of imidazole derivatives under solvent-free condition. Mol. Divers. 15: 125–134, https://doi.org/10.1007/s11030-009-9213-1.Search in Google Scholar PubMed
Ramesh Kumar, S. and Leelavathi, P. (2007). Phosphomolybdic acid-Al2O3: a mild, efficient, heterogeneous and reusable catalyst for regioselective opening of oxiranes with amines to β-amino alcohols. J. Mol. Catal. A: Chem. 266: 65–68, https://doi.org/10.1016/j.molcata.2006.10.045.Search in Google Scholar
Ravisankar, S., Queiroz, V.A.V., and Awika, J.M. (2020). Rye flavonoids – structural profile of the flavones in diverse varieties and effect of fermentation and heat on their structure and antioxidant properties. Food Chem. 324: 126871, https://doi.org/10.1016/j.foodchem.2020.126871.Search in Google Scholar PubMed
Ravishankar, D., Watson, K.A., Greco, F., and Osborn, H.M.I. (2016). Novel synthesised flavone derivatives provide significant insight into the structural features required for enhanced anti-proliferative activity. RSC Adv. 6: 64544–64556, https://doi.org/10.1039/c6ra11041j.Search in Google Scholar
Raw, S.A., Wilfred, C.D., and Taylor, R.J.K. (2004). Tandem oxidation processes for the preparation of nitrogen- containing heteroaromatic and heterocyclic compounds. Org. Biomol. Chem. 2: 788–796, https://doi.org/10.1039/b315689c.Search in Google Scholar PubMed
Reed, J.W. and Kingston, D.G.I. (1986). Biosynthesis of antibiotics of the virginiamycin family, 5. The conversion of phenylalanine to phenylglycine in the biosynthesis of virginiamycin S1. J. Nat. Prod. 49: 626–630, https://doi.org/10.1021/np50046a011.Search in Google Scholar PubMed
Rives, A., Payen, E., Hubaut, R., Vázquez, P., Pizzio, L., Cáceres, C., and Blanco, M. (2001). Silica and alumina impregnated with dimethylformamide solutions of molybdophosphoric or tungstophosphoric acids for hydrotreatment reactions. Catal. Lett. 71: 193–201, https://doi.org/10.1023/a:1009090718280.10.1023/A:1009090718280Search in Google Scholar
Romanelli, G. and Autino, J. (2009). Recent applications of heteropolyacids and related compounds in heterocycles synthesis. Mini-Rev. Org. Chem. 6: 359–366, https://doi.org/10.2174/157019309789371578.Search in Google Scholar
Romanelli, G., Vázquez, P., Pizzio, L., Quaranta, N., Autino, J., Blanco, M., and Cáceres, C. (2004). Phenol tetrahydropyranylation catalyzed by silica-alumina supported heteropolyacids with Keggin structure. Appl. Catal. A: Gen. 261: 163–170.10.1016/j.apcata.2003.10.043Search in Google Scholar
Romanelli, G., Autino, J.C., Vázquez, P., Pizzio, L., Blanco, M., and Cáceres, C. (2009). A suitable synthesis of azlactones (4-benzylidene-2-phenyloxazolin-5-ones and 4-alkylidene-2-phenyloxazolin-5-ones) catalyzed by silica–alumina supported heteropolyacids. Appl. Catal. A: Gen. 352: 208–213.10.1016/j.apcata.2008.10.003Search in Google Scholar
Romero-Galarza, A., Gutiérrez-Alejandre, A., and Ramírez, J. (2011). Analysis of the promotion of CoMoP/Al2O3 HDS catalysts prepared from a reduced H–P–Mo heteropolyacid Co salt. J. Catal. 280: 230–238.10.1016/j.jcat.2011.03.021Search in Google Scholar
Rostami, M., Khosropour, A., Mirkhani, V., Moghadam, M., Tangestaninejad, S., and Mohammadpoor-Baltork, I. (2011a). Organic–inorganic hybrid polyoxometalates: efficient, heterogeneous and reusable catalysts for solvent-free synthesis of azlactones. Appl. Catal. A: Gen. 397: 27–34, https://doi.org/10.1016/j.apcata.2011.02.004.Search in Google Scholar
Rostami, M., Khosropour, A.R., Mirkhani, V., Mohammadpoor-Baltork, I., Moghadam, M., and Tangestaninejad, S. (2011b). [C6(MIm)2]2W10O32.2H2O: a novel and powerful catalyst for the synthesis of 4-arylidene-2-phenyl-5(4)-oxazolones under ultrasonic condition. C. R. Chim. 14: 869–877, https://doi.org/10.1016/j.crci.2011.02.003.Search in Google Scholar
Ruiz, D.M., Autino, J.C., Quaranta, N., Vázquez, P.G., and Romanelli, G.P. (2012a). An efficient protocol for the synthesis of quinoxaline derivatives at room temperature using recyclable alumina-supported heteropolyoxometalates. Sci. World J. 2012: 1–8, https://doi.org/10.1100/2012/174784.Search in Google Scholar PubMed PubMed Central
Ruiz, D.M., Baronetti, G.T., Thomas, H.J., and Romanelli, G.P. (2012b). H6P2W12O62.24H2O supported on silica: a powerful and reusable catalyst for the synthesis of 4-arylidene-2-phenyl-5(4)-oxazolones (azlactones). Curr. Catal 1: 67–72.10.2174/2211545511201010067Search in Google Scholar
Ruiz, D.M., Pasquale, G.A., Martínez, J.J., and Romanelli, G.P. (2022). Advances in novel activation methods to perform green organic synthesis using recyclable heteropolyacid catalysis. Green Process. Synth. 11: 766–809.10.1515/gps-2022-0068Search in Google Scholar
Sagrera, G.J. and Seoane, G.A. (2005). Microwave accelerated solvent-free synthesis of flavanones. J. Braz. Chem. Soc. 16: 851–856, https://doi.org/10.1590/s0103-50532005000500026.Search in Google Scholar
Sanchez, L.M., Sathicq, A.G., Thomas, H.J., and Romanelli, G.P. (2012). Synthesis of dihydropyridines: patented catalysts and biological applications. Recent Pat. Catal 1: 119–128, https://doi.org/10.2174/2211548x11201020119.Search in Google Scholar
Sathicq, A.G., Romanelli, G.P., Ponzinibbio, A., Baronetti, G.T., and Thomas, H.J. (2010). An efficient one-step Hantzsch multicomponent synthesis of 1,4-dihydropyridines via a Wells-Dawson heteropolyacid catalyst under solvent-free conditions. Lett. Org. Chem. 7: 511–518.10.2174/157017810793362334Search in Google Scholar
Sayed, H., Abbas, H.-A., Morsi, E., Amr, A., and Abdelwahad, N. (2010). Antimicrobial activity of some synthesized glucopyranosyl-pyrimidine carbonitrile and fused pyrimidine systems. Acta Pharm. 60: 479–491, https://doi.org/10.2478/v10007-010-0033-8.Search in Google Scholar PubMed
Sebulsky, R.T. and Henke, A.M. (1971). Alkylation of benzene with dodecene-1 catalyzed by supported silicotungstic acid. Ind. Eng. Chem. Process Des. Dev. 10: 272–279, https://doi.org/10.1021/i260038a022.Search in Google Scholar
Shaldam, M.A., Elhamamsy, M.H., Esmat, E.A., and El-Moselhy, T.F. (2014). 1,4-Dihydropyridine calcium channel blockers: homology modeling of the receptor and assessment of structure activity relationship. ISRN Med. Chem. 2014: 1–14, https://doi.org/10.1155/2014/203518.Search in Google Scholar
Sharma, P., Vyas, S., and Patel, A. (2004). Heteropolyacid supported onto neutral alumina: characterization and esterification of 1° and 2° alcohol. J. Mol. Catal. A: Chem. 214: 281–286, https://doi.org/10.1016/j.molcata.2003.12.038.Search in Google Scholar
Sheikh, R., Choi, M.-S., Im, J.-S., and Park, Y.-H. (2013). Study on the solid acid catalysts in biodiesel production from high acid value oil. J. Ind. Eng. Chem. 19: 1413–1419, https://doi.org/10.1016/j.jiec.2013.01.005.Search in Google Scholar
Sirisha, K., Achaiah, G., and Reddy, V.M. (2010). Facile synthesis and antibacterial, antitubercular, and anticancer activities of novel 1,4‐dihydropyridines. Arch. Pharm. 343: 342–352, https://doi.org/10.1002/ardp.200900243.Search in Google Scholar PubMed
Sondhi, S.M., Singh, N., Johar, M., and Kumar, A. (2005). Synthesis, anti-inflammatory and analgesic activities evaluation of some mono, bi and tricyclic pyrimidine derivatives. Bioorg. Med. Chem. 13: 6158–6166, https://doi.org/10.1016/j.bmc.2005.06.063.Search in Google Scholar PubMed
Sopa, M., Wącław-Held, A., Grossy, M., Pijanka, J., and Nowińska, K. (2005). Ethane to acetic acid oxidation over supported heteropoly acids. Appl. Catal. A: Gen. 285: 119–125.10.1016/j.apcata.2005.02.013Search in Google Scholar
Sosa, A.A., Rivera, T.S., Blanco, M.N., Pizzio, L.R., and Romanelli, G.P. (2013). Tungstophosphoric acid supported on zirconia: a recyclable catalyst for the green synthesis on quinoxaline derivatives under solvent-free conditions. Phosphorus Sulfur Silicon Relat. Elem. 188: 1071–1079, https://doi.org/10.1080/10426507.2012.710678.Search in Google Scholar
Sosa, A.A., Palermo, V., Langer, P., Luque, R., Romanelli, G.P., and Pizzio, L.R. (2022). A tungstophosphoric acid/mesoporous silicas as suitable catalysts in quinoxaline synthesis. Mol. Catal. 517: 112046, https://doi.org/10.1016/j.mcat.2021.112046.Search in Google Scholar
Stewart, J.M.. (1981) Chapter: 4- Protection of the hydroxyl group in peptide synthesis. In: The peptides: analysis, synthesis, biology. Academic Press, Oxford, UK, pp. 169–201.10.1016/B978-0-12-304203-3.50011-6Search in Google Scholar
Subhas Bose, D. and Venkat Narsaiah, A. (1998). Efficient one pot synthesis of nitriles from aldehydes in solid state using peroxymonosulfate on alumina. Tetrahedron Lett. 39: 6533–6534, https://doi.org/10.1016/s0040-4039(98)01358-6.Search in Google Scholar
Subhas Bose, D., Jayalakshmi, B., and Venkat Narsaiah, A. (2000). Efficient method for selective cleavage of acetals and ketals using peroxymonosulfate on alumina under solvent-free conditions. Synthesis 2000: 67–68, https://doi.org/10.1055/s-2000-6239.Search in Google Scholar
Sukale, S.B., Aher, D.S., Chavan, L.D., Khillare, K.R., and Shankarwar, S.G. (2024). Synthesis and characterization of alumina supported molybdosilicic acid (SiMo12/Al2O3): efficient solid acid catalyst for the synthesis of pyranopyrazole derivatives. Synth. Commun. 54: 826–842, https://doi.org/10.1080/00397911.2024.2345380.Search in Google Scholar
Svetlik, J., Veizerova, L., Liptaj, T., and Kubist, J. (2009). Transformation of oxygen-bridged pyrimidines with nitrogen nucleophiles and characterization of resulting products. Arkivoc 2009: 79–86, https://doi.org/10.3998/ark.5550190.0010.a09.Search in Google Scholar
Tarlani, A., Abedini, M., Khabaz, M., and Amini, M.M. (2005). Adsorption of Wells–Dawson tungsten heteropolyacid on sol–gel alumina: structural features and thermal stability. J. Colloid Interface Sci. 292: 486–492, https://doi.org/10.1016/j.jcis.2005.05.090.Search in Google Scholar PubMed
Tawfik, H.A., Ewies, E.F., and El-Hamouly, W.S. (2015). ChemInform abstract: synthesis of chromones and their applications during the last ten years. ChemInform 46, https://doi.org/10.1002/chin.201513331.Search in Google Scholar
Teixeira, C., Barbault, F., Rebehmed, J., Liu, K., Xie, L., Lu, H., Jiang, S., Fan, B., and Maurel, F. (2008). Molecular modeling studies of N-substituted pyrrole derivatives: potential HIV-1 gp41 inhibitors. Bioorg. Med. Chem. 16: 3039–3048, https://doi.org/10.1016/j.bmc.2007.12.034.Search in Google Scholar PubMed
Timmerman, H. and van der Goot, H. (2009). Histamine receptors and their ligands: mechanisms and applications. In: Squire, L.R. (Ed.). Encyclopedia of neuroscience. Academic Press, San Diego, US., pp. 1149–1166.10.1016/B978-008045046-9.01149-9Search in Google Scholar
Timoshkina, V.V., Vinogradov, N.A., Pimerzin, A.A., Vutolkina, A.V., and Glotov, A.P. (2022). Vanadium-containing heteropoly acids of Keggin structure as precursors of CoPMoV sulfide catalysts for hydroconversion of dibenzothiophene and naphthalene. Pet. Chem. 62: 1343–1349, https://doi.org/10.1134/s0965544122110044.Search in Google Scholar
Tomina, N.N., Solmanov, P.C., Maksimov, N.M., and Pimerzin, A.A. (2015). Hydrotreatment of petroluem on Ni6-PMonW(12–n)(S)/Al2O3 catalysts. Catal. Ind. 7: 307–313, https://doi.org/10.1134/s2070050415040157.Search in Google Scholar
Travers, C., Essayem, N., Delage, M., and Quelen, S. (2001). Heteropolyanions based catalysts for paraffins isomerization. Catal. Today 65: 355–361, https://doi.org/10.1016/s0920-5861(00)00590-3.Search in Google Scholar
Trofimov, B.A., Mikhaleva, A.I., Schmidt, E.Y., and Sobenina, L.N. (2016). Chemistry of pyrroles, 1st ed. CRC Press, Boca Raton, US.Search in Google Scholar
Trueba, M. and Trasatti, S.P. (2005). γ‐Alumina as a support for catalysts: a review of fundamental aspects. Eur. J. Inorg. Chem. 2005: 3393–3403, https://doi.org/10.1002/ejic.200500348.Search in Google Scholar
Tzankova, D., Vladimirova, S., Peikova, L., and Georgieva, M. (2018). Synthesis of pyrrole and substituted pyrroles (review). J. Chem. Technol. Metall. 53: 451–464.Search in Google Scholar
Vaghasiya, S.J., Dodiya, D.K., Trivedi, A.R., Surani, J.J., and Shah, V.H. (2008). Synthesis and biological screening of some novel pyrazo-lo[3’,4’:4,5]thieno[2,3d]pyrimidin-8-ones via Gewald Reaction. Arkivoc 2008: 1–8, https://doi.org/10.3998/ark.5550190.0009.c01.Search in Google Scholar
Van Haandel, L., Bremmer, M., Kooyman, P.J., Van Veen, J.A.R., Weber, T., and Hensen, E.J.M. (2015). Structure–activity correlations in hydrodesulfurization reactions over Ni-promoted Mox W(1–x)S2/Al2O3 catalysts. ACS Catal. 5: 7276–7287, https://doi.org/10.1021/acscatal.5b01806.Search in Google Scholar
Varma, R.S., Dahiya, R., and Saini, R.K. (1997). Lodobenzene diacetate on alumina: rapid oxidation of alcohols to carbonyl compounds in solventless system using microwaves. Tetrahedron Lett. 38: 7029–7032, https://doi.org/10.1016/s0040-4039(97)01660-2.Search in Google Scholar
Vazquez, P. and Quaranta, N. (2006). Heteropolyacids with Keggin structure supported on silica-alumina. Int. J. Mater. Prod. Technol. 27: 101, https://doi.org/10.1504/ijmpt.2006.010676.Search in Google Scholar
Vazquez, P.G., Blanco, M.N., and Caceres, C.V. (1999). Catalysts based on supported 12-molybdophosphoric acid. Catal. Lett. 60: 205–215.10.1023/A:1019071410838Search in Google Scholar
Venkateswara Rao, K.T., Sai Prasad, P.S., and Lingaiah, N. (2009). Iron exchanged molybdophosphoric acid as an efficient heterogeneous catalyst for the synthesis of quinoxalines. J. Mol. Catal. A: Chem. 312: 65–69, https://doi.org/10.1016/j.molcata.2009.07.005.Search in Google Scholar
Vicente, E., Lima, L.M., Bongard, E., Charnaud, S., Villar, R., Solano, B., Burguete, A., Perez-Silanes, S., Aldana, I., Vivas, L., et al.. (2008). Synthesis and structure–activity relationship of 3-phenylquinoxaline 1,4-di-N-oxide derivatives as antimalarial agents. Eur. J. Med. Chem. 43: 1903–1910, https://doi.org/10.1016/j.ejmech.2007.11.024.Search in Google Scholar PubMed
Vidal, S. (2019). Protecting groups: strategies and applications in carbohydrate chemistry. Wiley-VCH, Weinheim, Germany.10.1002/9783527697014Search in Google Scholar
Villabrille, P., Romanelli, G., Quaranta, N., and Vázquez, P. (2010). An efficient catalytic route for the preparation of silyl ethers using alumina-supported heteropolyoxometalates. Appl. Catal. B: Environ. 96: 379–386, https://doi.org/10.1016/j.apcatb.2010.02.035.Search in Google Scholar
Vinogradov, N.A., Timoshkina, V.V., Tsilimbaeva, E.A., Zasypalov, G.O., Pimerzin, A.A., and Glotov, A.P. (2023). CoPMoV sulfide catalysts supported on natural halloysite nanotubes in hydrotreating of dibenzothiophene and naphthalene. Pet. Chem. 63: 931–938, https://doi.org/10.31857/s002824212304007x.Search in Google Scholar
Wagle, S., Adhikari, A.V., and Kumari, N.S. (2009). Synthesis of some new 4-styryltetrazolo[1,5-a]quinoxaline and 1-substituted-4-styryl[1,2,4]triazolo[4,3-a]quinoxaline derivatives as potent anticonvulsants. Eur. J. Med. Chem. 44: 1135–1143, https://doi.org/10.1016/j.ejmech.2008.06.006.Search in Google Scholar PubMed
Wang, J., Ma, P., Shen, Y., and Niu, J. (2008). Tetra-transition-metal substituted weakley-type sandwich germanotungstates and their derivatives decorated by transition-metal complexes. Cryst. Growth Des. 8: 3130–3133, https://doi.org/10.1021/cg701278b.Search in Google Scholar
Wijaya, Y.P., Winoto, H.P., Park, Y.-K., Suh, D.J., Lee, H., Ha, J.-M., and Jae, J. (2017). Heteropolyacid catalysts for Diels-Alder cycloaddition of 2,5-dimethylfuran and ethylene to renewable p-xylene. Catal. Today 293–294: 167–175, https://doi.org/10.1016/j.cattod.2016.12.032.Search in Google Scholar
Wu, Y., Ye, X., Yang, X., Wang, X., Chu, W., and Hu, Y. (1996). Heterogenization of heteropolyacids: a general discussion on the preparation of supported acid catalysts. Ind. Eng. Chem. Res. 35: 2546–2560, https://doi.org/10.1021/ie950473s.Search in Google Scholar
Xia, Y., Dong, Z.-W., Zhao, B.-X., Ge, X., Meng, N., Shin, D.-S., and Miao, J.-Y. (2007). Synthesis and structure–activity relationships of novel 1-arylmethyl-3-aryl-1H-pyrazole-5-carbohydrazide derivatives as potential agents against A549 lung cancer cells. Bioorg. Med. Chem. 15: 6893–6899, https://doi.org/10.1016/j.bmc.2007.08.021.Search in Google Scholar PubMed
Yan, L., Chen, Y., Sun, X., You, M., Chen, X., Gu, Q., and Zhang, Y. (2017). Microwave-assisted solvent-free catalyzed synthesis and luminescence properties of 1,2,4,5-tetrasubstituted imidazoles bearing a 4-aminophenyl substituent. Chem. Pap. 71: 627–637, https://doi.org/10.1007/s11696-016-0051-1.Search in Google Scholar
Yang, X.K., Chen, L.F., Wang, J.A., Noreña, L.E., and Novaro, O. (2009). Study of the Keggin structure and catalytic properties of Pt-promoted heteropoly compound/Al-MCM-41 hybrid catalysts. Catal. Today 148: 160–168, https://doi.org/10.1016/j.cattod.2009.03.022.Search in Google Scholar
Yu, C., Zhou, B., Su, W., and Xu, Z. (2006). Erlenmeyer synthesis for azlactones catalyzed by ytterbium(III) triflate under solvent‐free conditions. Synth. Commun. 36: 3447–3453, https://doi.org/10.1080/00397910600941521.Search in Google Scholar
Zambrano, D.N., Gosatti, M.O., Dufou, L.M., Serrano, D.A., Guraya, M.M., and Perez-Catán, S. (2016). Nanostructure of gamma-alumina prepared by a modified sol-gel technique. World Acad. Sci. Eng. Technol. Int. J. Chem. Molec. Nuclear Mater. Metall. Eng. 10: 655–660.Search in Google Scholar
Supplementary Material
This article contains supplementary material (https://doi.org/10.1515/revce-2024-0085).
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- Reviews
- Trends and perspectives in deterministic MINLP optimization for integrated planning, scheduling, control, and design of chemical processes
- Self-heat recuperation (SHR) theory: principle and applications
- Heteropolyacid-alumina composites as heterogeneous catalysts for the synthesis of organic compounds: a comprehensive review
- Mini Review
- Hybrid MOF-COF structures for advanced gas separation membranes: a short review of synthesis, performance analysis and application potential
Articles in the same Issue
- Frontmatter
- Reviews
- Trends and perspectives in deterministic MINLP optimization for integrated planning, scheduling, control, and design of chemical processes
- Self-heat recuperation (SHR) theory: principle and applications
- Heteropolyacid-alumina composites as heterogeneous catalysts for the synthesis of organic compounds: a comprehensive review
- Mini Review
- Hybrid MOF-COF structures for advanced gas separation membranes: a short review of synthesis, performance analysis and application potential

