Abstract
To alleviate the global climate changes driven by the utilization of carbon-based fuel, various countries of the world have put in more efforts in developing corresponding technologies that can curb global warming and reduce the use of fossil fuels in recent years. Self-heat recuperation (SHR) is a new energy-saving technology that guides the development of energy-consuming systems. Based on the theory of exergy recovery, SHR can minimize energy requirements and optimize energy structure. According to this theory, the heat of the whole process could be recycled into its designed exergy recovery process and circulated within the process without requiring additional heat sources. Herein, first, we introduce three principal analyses (energy analysis, exergy analysis, and entransy analysis) of SHR theory and its applications in distillation, drying, absorption, and other areas. Then, the advantages and existing issues of these applications are summarized in details. Finally, it is proposed that the future development direction for SHR theory should involve the integration of SHR with other energy-saving technologies for optimizing the energy-saving design and improving its industrial applications. This approach can help to achieve cascade energy utilization at the system level.
1 Introduction
Energy crisis and the greenhouse effect have become two major issues affecting the sustainable development of the world today. Energy-saving technology is one of the significant approaches that can resolve these global issues. In the last century, Linnhoff et al. (1979), Linnhoff and Hindmarsh (1983); Linnhoff (1993), Linnhoff and Eastwood (1997) proposed an overall optimization design method (pinch technology) for system energy saving. The pinch technology is an approach for maximizing energy recovery and utilization in various systems. The pinch point of the system can be found through the temperature-enthalpy diagram (Liu et al. 2022), and the heat exchanger network with the largest energy recovery can be established to optimize process design and system utility. A typical example of this technology is the application of both feed and discharge heat exchangers in the heat exchange process. These heat exchangers facilitate heat transfer between the cold stream and hot stream, allowing the heat of the stream to be recycled (Seider et al. 2004). However, in such a process, if the heat exchange load is not enough, some of the required heat must be obtained from outside, mainly through the combustion of fossil fuels, leading to a great exergy loss (Caton 2014). Also, many developed energy-saving technologies have cascade heat utilization features or aim to save energy based on the first law of thermodynamics.
The first law of thermodynamics only focuses on the “quantity” of energy. However, the “quality” of energy can only be understood through the second law of thermodynamics (Zhao et al. 2021). To date, researchers have only calculated the efficiency of the availability of system (exergy efficiency) through exergy analysis methods based on the second law of thermodynamics, which cannot propose specific methods for energy saving (Liszka et al. 2012; Shayan et al. 2018; Xiong et al. 2011). To reduce exergy loss within the system, researchers proposed the utilization of a heat pump energy device (Ally and Fricke 2021). As well known, heat pumps can convert low-temperature heat energy that cannot be directly used into useful heat energy, thereby improving energy utilization and saving fuel (Zhang et al. 2020b). Although the heat pump can reduce the overall energy consumption of a system, the heat load and heat capacity of the stream are often different from the heat supply of the working medium. Therefore, a large exergy loss may still be caused during the heat exchange process of the heat pump. Commonly used heat pumps are named as compression (Wu et al. 2022), absorption (Li et al. 2011), steam injection (Zhang et al. 2020a) pumps and are defined as the second type of absorption heat pump (Wei et al. 2014) based on how they function. Among them, the mechanical vapor compression heat pump is mainly used in distillation (Vilardi 2021), evaporation (Han et al. 2021), and drying (Tu and Hwang 2019) processes. In the heat pump, the steam generated in the process is compressed to a higher pressure for upgrading and then condensed and exchanged to release heat. During vapor recompression, the latent heat in the vapor is recycled, but some of the sensible heat remains unused after the steam heat exchange. Consequently, the entire heat system cannot be effectively utilized, indicating potential energy savings even after the heat exchange process in the heat pump system.
Kuchonthara and Tsutsumi (2006) designed an advanced integrated coal gasification combined cycle power generation system (IGCC) based on exergy recovery. Thereafter, Kansha et al. (2009) proposed a self-heat recuperation (SHR) theory, focusing on maximizing exergy recovery within systems. It is considered that through adiabatic compression by the compressor, only a fraction of the high-quality electrical energy is consumed to convert low-quality thermal energy to a high-quality thermal value at a higher temperature. That is, by enhancing the quality of energy through processes like adiabatic compression, exergy losses can be minimized in the system, allowing maximum utilization of low-quality energy. As a new theory of technological energy saving (Feng 2004) in the energy saving pathways, SHR provides a bright direction for solving energy problems and the greenhouse effect.
SHR can guide the recycling and conversion of energy within a system to achieve maximum overall energy savings through the utilization of various technologies. For example, the quality of thermal energy can be improved initially by employing technologies, such as adiabatic compression with a compressor, magnetocaloric effect, thermoelectric device, absorption heat pump, or chemical heat pump. Subsequently, various heat integration technologies, such as pinch technology, heat exchanger network design, and others, can be used to improve thermal energy. The system could rematch the heat exchange of each logistics to achieve energy savings. Tsutsumi et al. (2010) proposed an advanced IGCC system based on SHR theory, featuring a triple-bed circulating fluidized bed (TBCFB). This system includes pyrolysis, gasification, and combustion units, which aim to enable the cascade clean and efficient utilization of low-rank coal. Wang et al. (2017, 2018) and Lian et al. (2019, 2020) conducted an in-depth research on the TBCFB system at different scales. They indicated that the theory of SHR could be widely used in various chemical unit operations and chemical processes for the optimization of energy conservation. The theory cannot only save energy, enhance production, reduce costs, and maintain sustainable social development, but it can also reduce carbon dioxide emissions and protect the environment. In this review, the principle of SHR theory will be revealed from three aspects: energy analysis, exergy analysis, and entransy analysis, and the application progress of SHR theory in distillation, drying, absorption, and other chemical unit operations will be introduced subsequently. Some unsolved issues will be put forward, and the development prospects of SHR theory will be discussed.
2 Principle of self-heat recuperation (SHR) theory
2.1 Energy analysis
The traditional thermal process entails providing the required heat through fuel combustion and then transferring the heat to the stream through heat exchange. However, during combustion, chemical energy of higher quality is converted into thermal energy of lower quality, leading to significant exergy losses. To reduce the energy consumption of the process through heat recovery, heat is generally exchanged between the incoming cold stream and the outgoing hot stream. However, the outgoing hot stream still needs to burn additional fuel to provide some of the heat needed to meet the heat transfer load, leading to exergy loss and energy consumption. To resolve this issue, in the SHR heat utilization process, it is proposed to convert high-quality electrical energy into heat energy using a compressor to improve the quality of the stream. This can make the output stream to match the heat transfer load required by the feed stream. In the process of SHR, the heat recovered through all process streams are recycled (Kotani et al. 2012). Hence, the energy consumption of the heat process based on SHR technology could be much lower than that of the traditional heat process using the input-discharge heat transfer method (Kansha et al. 2010a). Therefore, this process achieves great energy savings without needing additional heat from burning fuel, as indicated in Figure 1.

Thermal process of self-heat recuperation. Reproduced with permission from Kansha et al. (2009), copyright 2009 American Chemical Society.
Figure 1(a) shows the thermal process in which the gas stream does not undergo a phase change, based on the theory of SHR. In this process, the feed stream passes through the heat exchanger (1–2) and its temperature rises from T1 to T2. After the heat-exchanged stream passes through the compressor with an adiabatic compression for upgrading (3–4), the temperature further rises from T2 to T3. Thereafter, the stream passes through a heat exchanger to exchange heat with the feed stream (4–5), passes through an expander (5–6) to decompress the heat-exchanged stream, and further passes through a cooler (6–7) to reduce the temperature to T1. As such, a self-heating cycle process is realized. It can be seen in Figure 1(b) that using electric energy with the highest energy quality coefficient (or exergy rate) to upgrade the thermal energy of the stream can meet the heat load for heating the feed stream without requiring additional heat input. It should be noted that the energy required for this process is equal to the cooling load of the cooler, and the exergy loss occurs only in the heat exchanger for the hot and cold streams [as indicated in the shaded area of Figure 1(b)].
Figure 1(c) shows the thermal process of a gas/liquid stream thermally cycled based on the theory of SHR. In this process, the feed stream passes through the heat exchanger (1–2) and its temperature rises from T1 to T2. After the heat-exchanged stream passes through the compressor with the adiabatic compression and is upgraded (3–4), the temperature further rises from T2 to T3. Herein, the boiling point of the stream rises from T a to T b , which enables the latent heat to be exchanged between the feed and the discharge. Subsequently, the stream passes through a heat exchanger to exchange heat with the feed stream (4–5), then passes through a valve (5–6) to decompress the heat-exchanged stream, and further passes through a cooler (6–7) to reduce the temperature of the stream to T1. Similar to the above process, a self-heating cycle process is also achieved. It can be seen in Figure 1(d) that using electric energy with the highest energy quality coefficient (exergy rate) to upgrade the thermal energy of the stream can exchange the sensible heat of the gas and liquid in the feed stream with the outgoing stream. Simultaneously, the heat required for the evaporation of the feed stream is exchanged with the heat released during the condensation of the effluent stream without requiring additional heat input. Herein, the SHR theory integrates both the sensible and latent heats of the flow, to achieve optimum energy saving in the process (Kansha et al. 2009). It should be noted that the energy required for the process is also equal to the cooling load of the cooler, and the exergy loss of the process occurs only in the heat exchanger for the hot and cold streams, as indicated in the shaded area of Figure 1(d).
2.2 Exergy analysis
Kansha et al. (2013) conducted exergy analysis using temperature entropy diagram while Tsutsumi and Kansha (2017) and Chen et al. (2021) performed exergy analysis using energy conversion diagram and temperature entropy diagram. They carried out the thermodynamic analysis of thermal cycle process based on the theory of SHR, which further clarified the energy conversion process in the thermal cycle process. Figure 2(a)–(d) illustrates the process module, the temperature T-entropy S diagram, the energy conversion diagram, and the simplified energy conversion diagram, respectively, for the heat circulation system based on self-heat recuperation theory. Here, the heat Q1(Ex, An) represents the area of the region 1-2-7-5 in Figure 2(b), in which the feed stream temperature increases from T0 to T. The stream with heat Q1 is upgraded by the compressor input electric Win (Ex1 + Ex6, 0) with a further temperature increase from T to T + ΔT. It should be noted that Win(Ex1 + Ex6, 0) represents the electric input to the compressor, which includes the electric input from the outside and output from the system. As such, the net electric of the system is defined as Wmin(Ex1, 0), as shown in Figure 2(c).

Self-heat recuperation thermal circulation system. Reproduced with permission from Tsutsumi and Kansha (2017), copyright © 2017 AIDIC Servizi S.r.l.
Meanwhile, the heat flow formed after compression (the energy is represented by Q3, as shown in Figure 2(a)–(c)) is heat exchanged with the cold flow, and the heat flow is cooled to T0 + ΔT. According to the conservation of energy, since the heat absorbed in the heat transfer process is equal to the heat released, the area of 3-4-6-7 is equal to the area of 1-2-7-5 while the area of 2-3-4-11 is equal to the area of 1-5-6-11. One can see that the exergy of the heat flow formed after compression is actually equal to the area of 3-4-9-10, whereas the anergy is equal to the area of 6-7-10-9. Thus, the exergy of Q3 can be expressed as the total area of 2-11-9-10 and 1-5-6-11, that is, Ex + An2, whereas the anergy of Q3 is An − An2. Similarly, the exergy loss in the heat transfer process is the difference between the area of 3-4-9-10 and the area of 1-2-10-8, which is equal to the difference between the area of 2-3-4-11 and the area of 1-11-9-8, that is, the area of 5-6-9-8. Thus, Exloss = T0Sgen = An2.
However, the flow after heat transfer still contains some heat values. So the temperature of the flow (T0 + ΔT) is reduced to T1 through the compressor turbine, and then cooled to T0, so that the flow returns to the initial state (that is the state of energy Q1). In this process, the compressor turbine emits electrical energy Wout, which is the difference between T0 + ΔT and T1 multiplied by the difference between S1 and S0, and can be expressed by the area of Ex6 in Figure 2(b). In addition, the exergy of the heat released during the cooling from T1 to T0 is Ex2 = Ex1 − An2, and the anergy is An2, which also explains the reason why An2 was used in the derivation of Q3 expression in the previous literature (Tsutsumi and Kansha 2017).
As shown in Figure 2(c), the cold flow (heat Q1) is compressed by the compressor (power consumption, Wmin) to form the hot flow, and the heat in the hot flow Q3 is used to achieve the heat transfer of the cold flow. Due to the limitation of the minimum heat exchange temperature difference, the other part of the heat Qout is not enough to further heat exchange of the cold flow; thus, the hot flow is expanded and cooled into the cold flow with heat of Q1. Here, the exergy loss during heat transfer is An2. It can be seen from Figure 2(d) that in the SHR thermal cycle, the net input work is used to make up for the exergy loss that occurs in the heat exchange process. At the same time, it is cooled and converted into heat Qout.
2.3 Entransy analysis
Generally, the exergy rate is only applicable to the heat-to-power conversion process, not to the pure heating/cooling heat exchange process (Chen et al. 2013). As such, another energy analysis named as entransy analysis is always used. Here, the entransy represents the total ability of an object for the heat transfer. In the reversible process, the object has no entransy dissipation. On the contrary, in the irreversible process, the object will have entransy dissipation (Chen et al. 2011), which will reduce the ability of the object to transfer heat. The pure heating/cooling heat exchange process is irreversible; thus, the irreversibility of the heat exchange process can be measured by the entransy dissipation rate (Guo et al. 2007). Wu and Guo (2014) carried out entransy analysis on the thermal process of gas and steam/liquid based on the theory of SHR and revealed the reason for the energy saving based on the theory of SHR from the perspective of heat transfer.
Figure 3(a) shows the comparison of the entransy dissipation rates of processes I–III. Here, process I represents the simple thermal process of the gas, process II stands for the heat exchange process of the gas inlet and outlet, and process III is the gas SHR process. Figure 3(b) shows the comparison of the transmissive dissipation rates for processes IV–VI. Here, process IV represents the steam/liquid simple thermal process, process V represents the steam/liquid inlet and outlet heat exchange process, and process VI represents the steam/liquid SHR process. The shaded part in the figure represents the entransy dissipation rate of each thermal power process. The entransy analysis of the SHR heat process shows that its entransy dissipation rate is lower than those of the simple heat process and the heat exchange process of the incoming and outgoing materials. Since the compressor can compress and upgrade the stream adiabatically, the enzymatic dissipation rate of the heat exchange process is reduced. Therefore, the fundamental reason for energy saving based on the theory of SHR is the reduction of the irreversibility of the heat exchange process by inputting part of the electrical work.

Comparison of the entransy-dissipation rates. Reproduced with permission from Wu et al. (2014), copyright 2014 American Chemical Society.
3 Application of self-heat recuperation (SHR) theory
3.1 Distillation
Although distillation is widely used in chemical industry separation processes, the reboiler always consumes a lot of heat, which is generally supplied by fuel combustion to generate steam. This process does not only result in significant exergy loss but also leads to the emission of a large amount of carbon dioxide. Various energy-saving processes, such as vapor recompression distillation, thermally coupled distillation column, and dividing wall distillation column, have been proposed. However, in these processes, only the heat loads of the reboiler and the condenser can be matched, whereas the utilization of the sensible heat of the compressed steam is not considered. Based on the theory of SHR, the distillation process can be divided into two modules: preheating and rectification modules. In the preheating module, the effluent stream from the rectification module is heat-matched with the feed stream. In the rectification module, the heats of the reboiler and condenser are matched. Therefore, the gas–liquid sensible heat of the feed stream can be exchanged with the sensible heat of the effluent stream, and the heat of evaporation can be exchanged with the heat of condensation of each module.
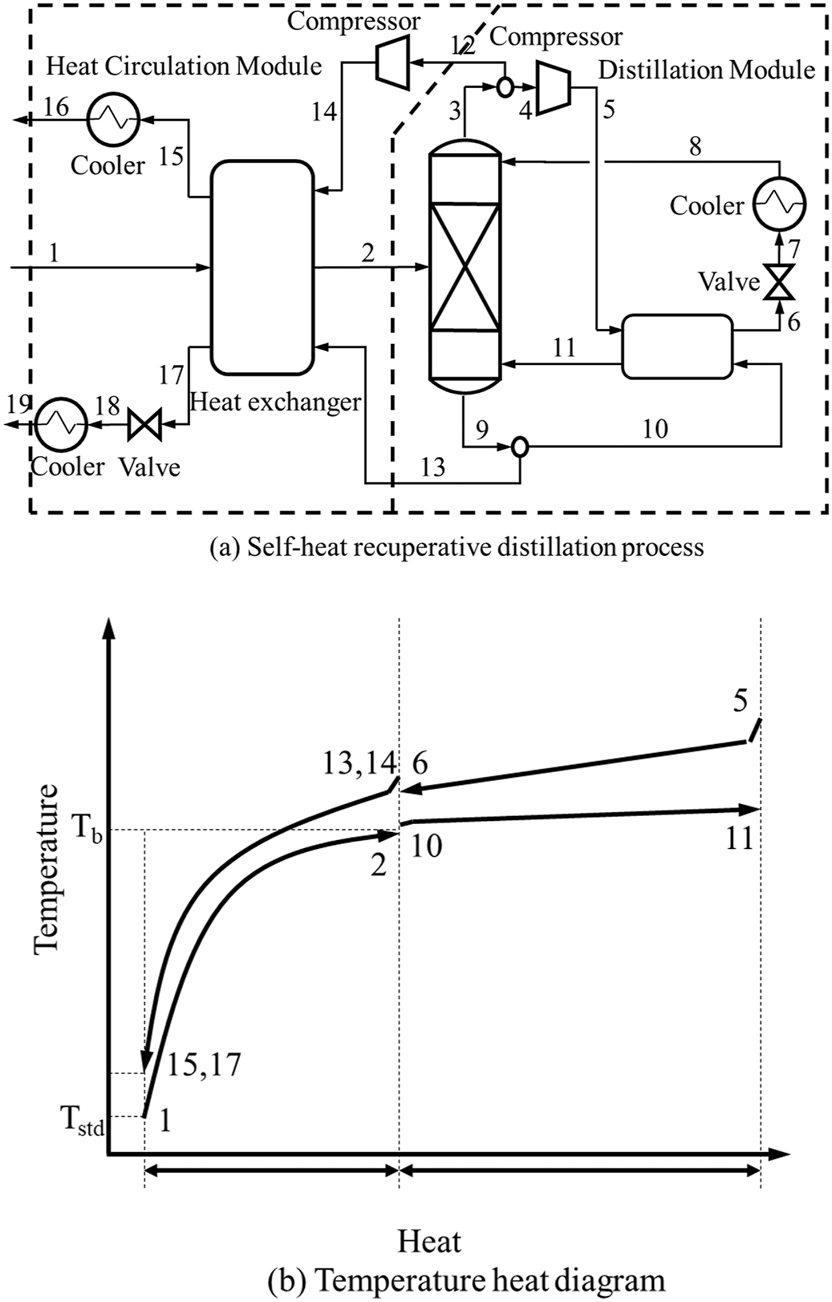
Based on the theory of SHR, Kansha et al. (2010b, 2012a) proposed a new integrated energy-saving process module for the distillation, as shown in Figure 4(a). In the preheating module, the distillation overhead vapor compression stream and the bottom product stream are heat-matched to the feed stream. In the distillation module, the compressor is used to compress and upgrade the vapor at the top of the column, which is matched with the reboiler at the bottom of the column for heat exchange. Therefore, the gas–liquid sensible heat of the feed stream can be exchanged with the sensible heat of the effluent stream, and the heat of evaporation can be exchanged with the heat of condensation of each module. Figure 4(b) shows the temperature calorific diagram of the SHR distillation process, in which each number corresponds to the flow number in Figure 4(a), and Tstd and Tb are the standard temperature and boiling temperature of the feed stream, respectively. The latent and sensible heats of the feed stream are exchanged with the effluent stream, and the heat of condensation overhead of the rectification column is heat exchanged with the heat of evaporation of the reboiler. As such, the heats of streams 4 and 12 can meet the heat load of the process after being upgraded by the compressor with the full utilization of the heat, thereby reducing energy loss. Matsuda et al. (2011) conducted a preliminary feasibility study on the application of SHR in the heavy chemical industry. They used the actual operation data of the benzene distillation process to examine the benefits of SHR from an industrial perspective. They developed an advanced SHR industrial distillation method. The study considered economic factors, applying a compressor. The comparison revealed that the energy consumption of the advanced process was 51 % of conventional industrial distillation, at the same time, the exergy input and energy input of the system were 24.4 and 60.1 % of those for the conventional industrial distillation, respectively. By utilization of the electrical energy, the energy introduced to the process as well as the energy consumption were significantly reduced.
Self-heat recuperation distillation process. Reproduced with permission from Kansha et al. (2012a), copyright 2012 Elsevier.
Azeotropic distillation can be used to separate azeotropic mixtures, but it requires two distillation columns and consumes a large amount of energy. In recent years, the use of membrane separation or pressure swing adsorption to replace azeotropic distillation, vapor recompression distillation column, and thermally coupled distillation column has been proposed. However, none of these alternatives have fully utilized the thermal energy of the process stream, and they still require additional heat input. Here, the emergence of SHR theory provides the possibility to solve this problem. Researchers have verified the feasibility of its application in azeotropic rectification through theoretical simulation (Kansha et al. 2010c). It can maximize energy utilization rate within systems by only considering energy consumption. Li et al. (2022) used a multiobjective genetic algorithm method to simulate and optimize SHR azeotropic distillation using ethanol-water azeotropic distillation as an example. By identifying the appropriate design point on the Pareto front of the process, calculations show that the SHR distillation (SHRAD) system obtains great results compared to the traditional azeotropic distillation method. Specifically, the total annual cost (TAC) and CO2 emissions were reduced significantly and the thermodynamic efficiency increased by 74.31 %. Also, two dynamic control structures are simultaneously proposed that differ from the conventional azeotropic distillation. They can maintain the product purity near the set point with a small overshoot. However, since the azeotropic distillation based on the theory of SHR requires multiple compressors, system energy saving and economic analysis need to be considered. Fan et al. (2019a) and Chen et al. (2018) carried out heat exchanger network optimization for multiple processes. Their work included detailed economic analysis and environmental studies (Yang et al. 2020). Wu et al. (2024) designed two novel heterogeneous azeotropic distillation processes by adding pure ethyl acetate as a self-solvent (HAD-PE) and refluxing part of the organic phase (HAD-OPR). They introduced SHR into the process (HAD-PE-SHR and HAD-OPR-SHR). The introduction of SHR sufficiently improves the heat utilization of the conventional process system, significantly reduces the total energy consumption, improves the exergy efficiency, and significantly reduces the TAC and CO2 emissions of the process.
The industry obtains pure oxygen and nitrogen provided by an air separation unit. However, the commonly used cryogenic distillation needs to consume a lot of energy. To reduce the energy consumption, Kansha et al. (2011a) verified the feasibility of SHR theory in low-temperature air separation process. In recent years, the double-tower structure has been optimized, whereas the single-tower type low-temperature air separation process has been proposed. In fact, many factors could affect the process energy consumption, such as oxygen and nitrogen purity, compressor efficiency, a minimum temperature difference of the main condensers, and others. To explore the role of the SHR theory in the single-column low-temperature air separation process, air separation in oxy-combustion units (Fu et al. 2014, 2016a) and IGCC (Fu et al. 2016b) systems have been analyzed in detail. Although systems that fully utilize the latent heat and sensible heat of the process steam can greatly reduce energy consumption, research on the economic and environmental aspects of such processes is still insufficient.
To address the high energy consumption issue in distillation columns, various improved distillation column structures, such as heat-integrated distillation column (HIDiC) and dividing wall distillation column (DWC), have been proposed. However, energy matching, energy upgrading, and reuse in new energy-saving distillation towers are seldom considered. By using the theory of SHR, HIDiC (Kansha et al. 2011b) and DWC (Xia et al. 2018a) could be optimized. Kansha et al. (2010a) matched the condensing heat recovery and evaporative heat in the column to achieve the optimal heat transfer. This was done by combining the SHR technology to change the process pressure based on the conventional thermally coupled distillation column. It is found that with the use of SHR, the energy consumption of HIDiC can be greatly reduced compared to the conventional HIDiC. In addition, the theory of SHR can consider not only the upgrading of energy using electrical energy with a high exergy rate but also the utilization of chemical energy with a higher exergy rate. For example, the coupling of reactive distillation with a dividing wall distillation column essentially utilizes the chemical energy generated by the reaction (Novita et al. 2015). Similarly, various explorations have been made on this process, where not only the total energy consumption, total cost, and environmental impact are analyzed, but also various schemes using heat exchanger networks for comparison are designed (Fan et al. 2019b; Li et al. 2019a,b). Wen et al. (2023) coupled the conventional double-tower distillation air separation process with dividing wall column and proposed the cryogenic air separation process with dividing wall column process based on the SHR theory. As shown in Figure 5, the process couples single distillation column with a crude argon column (CC) and the dividing wall column (DC). After the rectification process of DC, a large amount of heat is released from the top of the column, which is compressed and improved by SHR through the compressor to match the heat transfer with the material at the bottom of the column. By establishing a systematic calculation sequence, the process parameters satisfying the purity of the product are optimized with the aim of the lowest TAC. As a result, the process energy consumption of the cryogenic air separation process with dividing wall column process based on SHR is reduced by 26.19 %, with a reduction of the TAC by 31.93 % and the CO2 emission reduction by 25.18 %.

Process flowsheet of the cryogenic air separation process with dividing wall column process. Reproduced with permission from Wen et al. (2023), copyright 2023 CIESC Journal.
Pressure swing distillation (PSD) is an effective method for separating pressure-sensitive azeotropes and has been widely used in industries. However, it consumes high energy. Various optimization processes, such as double-effect rectification, mechanical heat pump technology, and HIDiC, have been proposed. However, the full use of system energy reserves remains a problem. To further realize energy savings in the PSD process, the theory of SHR has been also applied, revealing that more energy savings can be realized compared to the traditional PSD and thermally integrated PSD processes (Xia et al. 2017). In the PSD process applying the theory of SHR, the energy of various processes has been optimized using the heat exchanger network. A detailed analysis of the total annual cost of the process (although it is concluded that the addition of a compressor will increase the process cost) (Xia et al. 2018b) shows that it not only improves energy efficiency but also reduces carbon dioxide emissions (Cui et al. 2020). Also, the economic advantages of the process can be reflected with increased operating time (Li et al. 2019a,b). Xia et al. (2017) coupled SHR with PSD (SHR-PSD) process and optimized the thermal integration of the process by heat exchanger network. They concluded that this SHR-PSD process can reduce the energy consumption by 72.39 % and TAC by 36.65 % compared to the conventional PSD process, while simultaneously reducing CO2 emissions. Later, Luyben (2018) proposed a simpler heat exchanger system based on the study of Xia et al. (2017). They studied the dynamics and plant-wide controllability, which showed lower energy cost. Thereafter, Cui et al. (2020) proposed a novel electrical-driven self-heat recuperative pressure-swing azeotropic distillation technology, comparing it with the conventional thermal-driven PSD and PSDs integrated with heat integration and heat pump technologies. In the subsequent work (Cui et al. 2022; Yang et al. 2023), it is found that the total investment cost of PSD based on SHR can be reduced by 20 % and carbon emissions by 25 %. In addition, the study of the dynamic control structure and the process of highly integrated and interacted process shows that SHR theory has no conflict between the steady-state advantage and dynamic controllability, and thus it should be a broader and deeper study way for the SHR-PSD.
The theory of SHR has also been applied to other distillation processes, such as methanol-water distillation (Zhen et al. 2015a, 2015b), crude oil distillation (Chen 2015; Kansha et al. 2012b), natural gas recovery (Kim et al. 2012; Long and Lee 2013, 2014), propane-propylene separation (Christopher et al. 2017) processes, etc. And, all of them show good energy saving effect. As a unit operation with high energy consumption in chemical industry, the distillation has attracted the attention of many researchers. As a new energy-saving theory, SHR can be widely used in various distillation processes. Combined with other energy-saving technologies, the distillation unit has been optimized by SHR theory in terms of energy consumption, economy, and environment. Thus, the advantages of applying the SHR theory for distillation is reflected not only in the recompression and upgrading of the top steam but also in the reuse of reaction heat in the reactive distillation column. As stated above, in terms of energy conversion, the theory of SHR uses energy with a higher exergy rate to increase the energy performance of the thermal energy of the stream. It does not use additional heat to increase the stream’s exergy rate, which could effectively reduce the exergy loss in the process.
3.2 Drying
In chemical unit operations, drying reduces transportation costs by reducing product weight and size. This enables stable long-term storage of products and improves thermal efficiency. However, the drying process is very energy-intensive due to the high latent heat of the evaporation of water. There are two ways to save energy in the drying process: one is to strengthen the heat and mass transfer processes, and the other is heat recovery to achieve efficient energy utilization. For the latter, energy-saving methods, such as exhaust gas recirculation heat recovery, pinch technology, and mechanical recompression heat pump, have been proposed. However, these methods still cannot fully recover the heat of the drying medium, drying product, and evaporating water.
To improve the energy utilization rate in the drying system, Fushimi et al. (2010) proposed a drying system based on the theory of SHR, which was verified by simulation to show an energy-saving effect. Aziz et al. (2011a) also proposed a lignite drying system based on the theory of SHR, in which the hot stream is adiabatically compressed and upgraded by the compressor. Consequently, the exergy rate of the thermal energy of the hot stream is improved by using electric energy with a higher exergy rate. Since this system matches the heat load with the cold stream in the system, the sensible heat recovered from the drying medium during the drying process, the latent and sensible heat of evaporating water, and the sensible heat of the dried product can be all recycled. Figure 6 shows a structural diagram of a fluidized bed drying system based on the theory of SHR. Here, the wet lignite is heated to the specified temperature by the preheater (1a), and then the main drying process is carried out in the fluidized bed dryer (2). The moisture in the wet lignite is heated and evaporated, and then the fluidized medium is heated through the heat exchanger (1b). The heat exchanger in the dryer provides the heat required for drying by mixing steam and air, and the heat-exchanged steam and air mixture is circulated into the fluidized bed through the preheater (1a). The steam and air mixture coming out of the fluidized bed is superheated by the heat exchanger (3) and sent to the compressor for adiabatic compression and upgrading to form a closed cycle of drying medium, leading to the realization of the optimal energy savings. Subsequently, Aziz et al. (2012) proposed an improved SHR drying process for low-rank coal drying. Compared to the previously proposed SHR drying process, although the energy consumption is slightly increased, the heat exchange performance in the fluidized bed dryer is improved due to the use of pure steam condensation heat exchange. It reduces the size of the heat exchanger, and the heat load matching of the heat is optimized, reducing the exergy loss of the heat exchange process.

Self-heat recuperation brown coal drying. Reproduced with permission from Aziz et al. (2012), copyright 2012 Elsevier.
Biomass has great potential to mitigate global warming. However, its high moisture content leads to higher transportation costs and lower thermal efficiency. Drying is one solution to the problem, but the biomass drying process requires a lot of heat. To solve this problem, Aziz et al. (2011b) applied the theory of SHR for biomass drying and verified its feasibility. However, since many factors affect the energy consumption of biomass drying systems, detailed simulation and experimental analysis (Liu et al. 2012a) by considering factors such as fluidization velocity, fluidizer type (Liu et al. 2014), and fluidization medium (Liu et al. 2015) have been carried out. It is found that the fluidized bed dryer can reduce drying time and drying surface area, and simultaneously carbon dioxide can replace air as the drying medium. To make full use of the energy in the drying system and improve the energy utilization rate of the system, researchers improved the process and used exergy analysis to optimize the system (Liu et al. 2012b, 2013). Fushimi and Fukui (2014), Fushimi and Dewi (2015) simplified the SHR drying system with a complicated heat exchange process and found that simplifying the feed preheating can reduce energy consumption. This also shows that the simple drying heat exchange system design can also achieve a certain energy saving effect. Sun et al. (2022) introduced SHR into the biomass drying system, achieving an energy-saving ratio of up to 93.4 %. The theory of SHR has also been applied to other drying processes, such as paddy fluidized bed drying system (Yao et al. 2017), wood fixed bed drying system (Bai et al. 2017), freeze-drying (Bando et al. 2017), etc. Simultaneously, thermodynamic and economic analyses have also been carried out (Chen et al. 2022).
The drying process applying the theory of SHR increases the thermal energy performance of the hot stream by adiabatic compression of the hot stream of steam and air. It makes the thermal energy of the hot stream to be more fully utilized and greatly reduces the energy loss of the system. Also, through the circulation of the drying medium, the heat in the system can be fully recovered and utilized. Most research on the drying process focuses on the reduction of energy consumption, which could be achieved through continuous improvement of the drying system based on the theory of SHR. In addition, the influencing factors such as fluidizing medium, dryer type, compressor adiabatic efficiency, compression ratio, drying medium flow rate, and fluidization speed can be analyzed by it. But there are few studies on system economy and environment.
3.3 Absorption
With global warming, the disposal of the greenhouse gas carbon dioxide has attracted widespread attention. Carbon capture and storage (CCS) technology has been developed as one of the technologies for carbon dioxide processing, which entails the collection of carbon dioxide produced in industry and storing it in a specific location. It includes three stages: capture, transportation, and storage (Bassano et al. 2014). The capture stage has three ways: precombustion capture, combustion capture, and postcombustion capture. The application of SHR theory in the capture stage has been mainly studied.
The most commonly used carbon dioxide capture way is the chemical absorption via ethanolamine (MEA). Figure 7(a) shows the schematic diagram of conventional carbon dioxide capture, which includes an absorption column, a heat exchanger, a desorption column with a condenser, and a reboiler. After combustion, flue gas and ethanolamine solution containing a small amount of carbon dioxide (lean liquid) enter the absorption tower. The lean liquid absorbs carbon dioxide in the flue gas and becomes a carbon dioxide-rich ethanolamine solution (rich liquid) and enters the desorption tower after heat exchange with the lean liquid from the desorption tower. The heat required for the desorption column is provided by the bottom reboiler. The absorption process in the absorption tower will release heat, and part of the heat will enter the rich liquid for heat exchange with the poor liquid. The desorption tower requires additional heat input from the bottom reboiler due to the mismatch between the overhead condenser and the bottom reboiler. This results in high energy consumption in the carbon dioxide absorption process.

CO2 absorption process. Reproduced with permission from Kishimoto et al. (2011), copyright 2011 American Chemical Society.
To achieve higher energy saving, Kishimoto et al. (2011) proposed a carbon dioxide absorption process based on the theory of SHR. By upgrading and recycling chemical energy and thermal energy, a small amount of electrical energy is used to form a self-heating cycle, which can reduce energy input. Figure 7(b) shows the schematic diagram of the carbon dioxide absorption process of SHR, in which the mixture of carbon dioxide and steam discharged from the top of the desorption tower is subjected to adiabatic compression and upgraded by the compressor. Then, the heat exchange occurs with the reboiler at the bottom of the tower. This process can effectively reduce the energy consumption of the desorption tower. Some of the heat-exchanged mixtures is refluxed, while the remaining part is sent to the subsequent section. In addition, the reaction heat released by the absorption tower is upgraded by the heat pump cycle and then used in the desorption tower, which reduces the heat loss in the process. Also, Kishimoto et al. (2012) applied the theory of SHR to the carbon monoxide shift unit and precombustion carbon dioxide capture unit in IGCC to realize energy recycling and reuse of the whole process. Compared with the traditional process, the energy consumption of this new process is reduced by 2/3, indicating a good application prospect. Subsequently, Kansha et al. (2017) investigated the CO2 absorption process based on SHR through simulation and experimental methods, in which the reaction heat that accompanies absorption is successfully supplied through a heat pump to achieve thermal decomposition in a regenerator, thereby achieving recirculation of all process heat, indicating great energy saving potential.
SHR can also be applied in the process of removing acid gas. For example, Qian et al. (2023) established a model of acid gas removal (AGR) coupled with a power generation unit based on SHR theory. Here, steam compression and mechanical steam recompression are introduced respectively into AGR unit. The calculated energy and exergy balance of each unit shows that AGR based on SHR theory can enhance the resolution of acidic gas in the analyzer, and the introduction of steam compression SHR greatly improves the economy of AGR with a reduction of the operating cost by 3.75 %. In addition, the improved process uses low-pressure steam phase change cooling to replace part of the water cooling process, resulting in the reduced exergy loss in the distillation cooling process by 17.67 %. Thus, in the AGR process, energy saving and consumption reduction can be realized through SHR. Long and Lee (2017) proposed several distillation columns based on SHR with improved waste heat utilization modules to increase the AGR energy efficiency. They can be applied for both close-boiling and wide-boiling mixtures. Such proposed cases address the problem of high compressor cost resulting from the large temperature difference between the top and bottom of the tower through SHR technology with side reboiler or side SHR. In addition, several other proposals can be considered by using the generated steam from the top steam heat of the tower to provide steam for other consumers. After the SHR is further coupled into an integrated generator, the simulated results indicated that the load and operating cost of the reboiler of the SHR-based AGR can be reduced by 62.5 % and 45.9 % compared to the conventional AGR, respectively, with lower TAC and CO2 emissions.
The research on the carbon dioxide absorption process is not only limited in terms of energy consumption; the economic and environmental aspects have been also discussed in detail (Andika et al. 2017). Chen et al. (2019) took carbon dioxide from combustion flue gas in a high-density triple bed circulating fluidized bed (TBCFB) system as the capture object and used the theory of SHR to couple the waste heat recovery of flue gas and carbon dioxide capture (as the detail description in Section 3.4). It recycles the reaction heat and condensation heat in the system after compression and upgrading, thereby reducing the exergy loss in the process and improving the energy utilization rate. Also, from the energy consumption and economic analysis, it is found that the system is superior to the traditional carbon dioxide capture process. Song et al. (2014, 2015) also used the SHR theory for the PSA carbon dioxide capture process. They successfully recycled the heat generated from the adsorption reaction to reduce energy consumption.
3.4 TBCFB polygeneration system
Based on the theory of SHR, Hou et al. (2022) proposed a coal-based polygeneration carbon cycle system with a TBCFB unit for efficient, clean, and low-carbon utilization of coal. As shown in Figure 8, this system mainly includes three parts: gasification island, chemical island, and power island. In this system, the pyrolysis gas generated by TBCFB is circulated as the pyrolysis atmosphere, and the generated flue gas is used as the power generation working medium of the gas turbine to achieve the purpose of carbon cycle.

Process diagram of coal-based polygeneration carbon cycle (CBPCC) system. Reproduced with permission from Hou (2022), copyright 2022 Taiyuan University of Technology.
Before the proposal of Hou et al. (2022), Wang et al. (2018) started from the gasification island and used Aspen plus to simulate the TBCFB auto thermal cycle system as shown in Figure 9. Taking Huolinhe lignite in Inner Mongolia as an example, the operation parameters, such as suitable gasification reaction temperature, gasification agent dosage, and solid heat carrier circulation amount, were explored, and the material balance and energy balance in the system were analyzed. Using semi-coke as heat-carrying particles, on one hand, on the premise of satisfying heat transfer, the circulation amount of heat-carrying particles can be greatly reduced, which can reduce the difficulty and cost of system operation. On the other hand, the catalytic activity of semi-coke can promote pyrolysis and gasification reactions, improving its efficiency. With this, TBCFB can realize self-heating cycle by using circulating heat-carrying particles. This state represents the minimization of energy within the system, harnessing the advantages of SHR theory fully.

Diagram simulation of TBCFB system by Aspen Plus. Reproduced with permission from Wang et al. (2018), copyright 2018 CIESC Journal.
Thereafter, Chen et al. (2019) used the theory of SHR to optimize the chemical absorption CO2 capture process. As shown in Figure 10, this process utilizes the heat pump 1 to recover the reaction heat released by the absorption tower for the desorption tower to realize the recycling of the reaction heat between the exothermic reaction and the endothermic reaction in the system. At the same time, the compressor 2 is used to upgrade the vapor at the top of the absorption tower, which is used for the reboiler at the bottom of the absorption tower. The heat pump 3 is used to upgrade and recycle the waste heat of the system. Compared with the traditional process, the optimized process reduces the energy consumption of the capture process by 41.36 %, improves the exergy efficiency by 25.36 %, saves the standard coal consumption by 37 % per year, and reduces the total capture cost by 12 %, despite the increase of the investment cost by 27.76 %. Thus, by using the theory of SHR, the utilization of low-level thermal energy in the system can be greatly enhanced, indicating its great application potential for energy saving.

Process simulation of CO2 capture by chemical absorption method based on self-heat recuperation. Reproduced with permission from Chen et al. (2019), copyright 2019 CIESC Journal.
Furthermore, as shown in Figure 11, Liu et al. (2021) modeled the process of the low-temperature methanol washing unit and methanol rectification unit in the methanol synthesis process of the chemical island. The energy-saving optimization of the two units is also carried out using the theory of SHR, in which the heat exchanger network design is carried out for the optimized process. As a result, compared to the traditional methanol synthesis process, the total energy consumption of the Rectisol process is saved by 25.8 %, whereas the total energy consumption of the methanol distillation unit is saved by 32.3 %. Hence, this process can improve the exergy rate of the material through adiabatic compression of the compressor and redesign the heat exchange process of the system through the design of the heat exchanger network, achieving energy savings within the system.

Flowchart of synthesis gas to methanol in TBCFB system. Reproduced with permission from Liu et al. (2021), copyright 2021 CIESC Journal.
Based on the above research, Hou et al. (2022) used Aspen plus to simulate the carbon cycle system of coal-based polygeneration by carrying out material, energy, and exergy balance calculations for the system, in which the optimal operating conditions of the system with energy utilization efficiency as the optimization goal are determined. The results show that the energy saving of the coal-based polygeneration carbon cycle (CBPCC) system based on SHR is 13 %, which is equivalent to 149,000 tonnes/year of CO2 emission reduction compared with the conventional coal-based polygeneration (CBP). CBPCC has higher energy utilization efficiency and exergy efficiency, especially, the weak link of the TBCFB gasification unit system can be known through exergy analysis. Here, the coal-based polygeneration carbon cycle system fully integrates the various units based on SHR theory. Although the energy utilization efficiency of the system has been improved by optimizing the SHR energy of each unit, the close connection between the various parts of the system is not reflected. Thus, it is necessary to upgrade the local SHR theory optimization to the overall system SHR theory optimization, which could fully reflect the advantages of the SHR theory in the system energy optimization level. In response to this, Hou (2022) compared the coal-based polygeneration carbon cogeneration (CBPCC), the coal-based polygeneration carbon cogeneration with partial SHR (PSHR-CBPCC), and the coal-based polygeneration carbon cogeneration with integral SHR (ISHR-CBPCC) in subsequent study. Compared with the conventional CBPCC, the energy utilization efficiencies of the PSHR-CBPCC and ISHR-CBPCC systems are improved by 2.7 and 4.2 %, respectively, and the exergy efficiencies are improved by 2 and 4 %. The energy amounts saved by both are converted into reductions of 28 and 90.1 % of carbon dioxide emissions, respectively, which can effectively reduce carbon dioxide emissions in CBPCC. The comparison of the three process systems is shown in Tables 1 and 2.
Comparison of system economic performances.
| Process | CBPCC | PSHR-CBPCC | ISHR-CBPCC |
|---|---|---|---|
| Operating expense (106 $ year−1) | 1.0 | 1.4 | 0.8 |
| Additional equipment cost (106 $) | 255.9 | 258.9 | 180.8 |
|
|
|||
| Payback period | Total annual cost (10 6 $·year − 1) | ||
|
|
|||
| 5 | 52.2 | 53.2 | 37 |
| 8 | 33 | 33.8 | 23.4 |
Comparative analysis of system environment performances.
| Process | CBPCC | PSHR-CBPCC | ISHR-CBPCC |
|---|---|---|---|
| Total amount of fuel burned (kW) | 23,615 | 15,978 | 2901 |
| Steam CO2 emissions (kg h−1) | 9,644 | 6,525 | 857 |
| Electricity consumption (kW) | 7,099 | 6,606 | 10,503 |
| Power generation CO2 emissions (kg h−1) | 2001 | 1862 | 296 |
| Total CO2 emissions (kg h−1) | 11,645 | 8,387 | 1,153 |
3.5 Other processes
The theory of SHR has also been applied in other processes, such as evaporation systems, magnetocaloric cyclers, thermoelectric devices, and chemical production systems. As well known, solution evaporation crystallization is widely used in industry. However, due to the large amount of heat required for the phase transition of the solvent evaporation, the evaporation process always consumes a lot of energy. To solve this problem, the theory of SHR has been applied to those evaporation systems, such as ammonium sulfate and vitamin production (Wu et al. 2014). It is found that SHR of single-stage and two-stage evaporation systems differ in terms of energy savings. The two-stage evaporation system further recovers the energy in the outlet stream of the single-stage evaporation system, improving the heat recycling within the system and the energy utilization efficiency of the system. The effects of different concentration solutions and different boiling point solutions on the evaporation system have been also investigated. Han et al. (2014a) found that the energy-saving effect of the double-stage mechanical vapor recompression (MVR) evaporation system based on SHR for ammonium sulfate solution processing rises with increased evaporation concentration of the ammonium sulfate solution. They found an increase in energy savings from 30 to 55 % at the saturated concentration compared to the single-stage MVR evaporation system. Subsequently, Han et al. (2014b) also found that the overall energy efficiency of the MVR evaporation concentration system could be improved through SHR with the boiling point elevation in the effective range. The double-stage MVR evaporation concentration system based on SHRT is limited in its energy-saving characteristics when dealing with highly concentrated solutions with a high boiling point elevation.
In the theory of SHR, it is recommended not only to use adiabatic compression for upgrading but also to consider stream upgrading by applying magnetocaloric effect and thermoelectric devices, which can be useful. For example, the magnetocaloric cycler based on the theory of SHR has no heat input, in which only the magnetization and demagnetization of ferromagnetic materials are utilized for thermal cycling (Kotani et al. 2013a). The experimental and simulation results on the magnetocaloric cycler have verified its feasibility, but the fluid flow, bed geometry, and so on need to be further optimized (Kotani et al. 2013b, 2014). Here, the use of adiabatic magnetization and demagnetization instead of compression and expansion is an extension of the theory of SHR. Although the temperature of ferromagnetic materials is affected by the Curie temperature, they have application prospects in energy cascade utilization within systems. Thermoelectric devices use the Peltier effect to improve the quality of logistics. They can be applied for either gaseous streams or nongaseous streams. Here, the influencing factors such as surface area and heat transfer coefficient can be simulated and analyzed (Rasfuldi et al. 2015) to provide a theoretical basis for the application of thermoelectric devices in nongaseous streams.
The theory of SHR can also be applied in various chemical production processes, by which the energy-saving optimization can be carried out in various stages using the exergy analysis and energy analysis. For example, for the naphtha hydrodesulfurization reaction system, Matsuda et al. (2010) carried out exergy analysis and energy analysis by using the theory of SHR, comparing it with the traditional methods. The process of applying SHR can lead to a great energy-saving effect. It is pointed out that the reason for energy saving is to replace the direct input heat by input work. Also, the load of the cooler as well as the exergy loss can be greatly reduced. Other processes, such as the indirect production process of dimethyl ether (Kansha et al. 2015), methanol synthesis process (Kansha et al. 2014, 2019; Liu et al. 2021), carbon dioxide conversion methanol process (Chaniago et al. 2019), biodiesel production process (Fu et al. 2015), seawater desalination process (Mizuno et al. 2013) process, etc., have also been studied in detail. All these studies indicated that the energy-saving effect can be achieved by using the theory of SHR in each stage of the process.
4 Conclusion and outlook
The theory of SHR shows a good energy-saving merit in any unit operation. At present, the pilot test has been completed in the rectification unit, but it is still in the theoretical simulation stage in other chemical production processes. Although many optimization designs have been carried out in the theoretical simulation stage, the specific design process for the application of the SHR theory has not yet been determined. By the analyses, SHR has embodied better energy saving effect; however, additional considerations of environment, economy, and safety should be necessary during the application of SHR theory. For example, the life cycle assessment (LCA) of it could determine the environmental sustainability performance of such a process technology. That is, although SHR has a high energy and carbon saving effect, the environmental impacts of the process technology under boundary conditions should be also considered and especially further optimized using LCA analysis. In the economic aspect, comparing with the traditional process technology, there is a certain increase, but for some process routes that use high energy consuming utilities, SHR can achieve significant energy savings and carbon emission reductions with only a small amount of power consumption. In general, although SHR inevitably increases equipment costs, it should have an important role in energy saving and carbon reduction in the future. In addition, for the SHR theory, it also needs to consider the safety factor in the application process. For the high temperature and high pressure situation in the application process, it is necessary to take abundant safety measures and develop machinery and equipment with suitable safety performance.
The theory of SHR has a very promising future, and its applications and research should be not only limited to the aspects described in this study, and the future development of SHR in industry will be diverse with more meaningful, which includes the following:
The optimal design steps should be clarified in combination with the theory of SHR, and it should be integrated into engineering thermochemistry (Han et al. 2023; Xu et al. 2023) so that this theory can be applied to a wider range of fields.
To obtain the optimal solution, the application of SHR theory in unit operation should consider not only energy consumption, but also comprehensive analyses of both economic and environmental aspects with LCA to compare multiple energy-saving schemes.
The theory of SHR should not be limited in the vapor compression upgrading, other exergy rate improvement ways should be also explored. That is, besides self-heating recuperation, those exergy rate improvement ways such as using the thermoelectric device to upgrade nongas phase energy quality could be also studied.
The SHR theory should be widely applied to optimize various unit operations. It is expected to further improve any energy utilization process and chemical production system. Meanwhile, economic and environmental issues in the design of SHR-based system must be considered and those exergy rate enhancement devices should be designed and made for the practical application in the meantime.
Funding source: the National Nature Science Fund of China
Award Identifier / Grant number: U1710101
Acknowledgments
This review article is written by ourselves. It is an original review articles.
-
Research ethics: Not applicable.
-
Informed consent: Informed consent was obtained from all individuals included in this study, or their legal guardians or wards.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Use of Large Language Models, AI and Machine Learning Tools: None declared.
-
Conflict of interests: The authors state no conflict of interest.
-
Research funding: This work was supported by the National Nature Science Fund of China (U1710101), and the Shanxi Science and Technology Service Co. Ltd., China.
-
Data availability: Not applicable.
References
Ally, M.R. and Fricke, B. (2021). Heat transfer, refrigeration and heat pumps. Energies 14: 1–3, https://doi.org/10.3390/en14237988.Search in Google Scholar
Andika, R., Nhien, L.C., and Lee, M. (2017). Techno-economic study of enhanced absorber–regenerator configurations for improving an industrial sulfinol-m-based acid gas removal processes. J. Ind. Eng. Chem. 54: 454–463, https://doi.org/10.1016/j.jiec.2017.06.028.Search in Google Scholar
Aziz, M., Kansha, Y., and Tsutsumi, A. (2011a). Self-heat recuperative fluidized bed drying of brown coal. Chem. Eng. Process. 50: 944–951, https://doi.org/10.1016/j.cep.2011.07.005.Search in Google Scholar
Aziz, M., Fushimi, C., Kansha, Y., Mochidzuki, K., Kaneko, S., Tsutsumi, A., Matsumoto, K., Hashimoto, T., Kawamoto, N., Oura, K., et al.. (2011b). Innovative energy-efficient biomass drying based on self-heat recuperation technology. Chem. Eng. Technol. 34: 1095–1103, https://doi.org/10.1002/ceat.201100065.Search in Google Scholar
Aziz, M., Kansha, Y., Kishimoto, A., Kotani, Y., Liu, Y., and Tsutsumi, A. (2012). Advanced energy saving in low rank coal drying based on self-heat recuperation technology. Fuel Process. Technol. 104: 16–22, https://doi.org/10.1016/j.fuproc.2012.06.020.Search in Google Scholar
Bai, G., Han, D., Yao, Y., and Faizan, A. (2017). Thermodynamic analysis of wood drying process based on self-heat recuperation technology. Energ. Chem. Ind. 38: 1–7.Search in Google Scholar
Bando, K., Kansha, Y., Ishizuka, M., and Tsutsumi, A. (2017). Innovative freeze-drying process based on self-heat recuperation technology. J. Clean. Prod. 168: 1244–1250, https://doi.org/10.1016/j.jclepro.2017.09.088.Search in Google Scholar
Bassano, C., Deiana, P., and Girardi, G. (2014). Modeling and economic evaluation of the integration of carbon capture and storage technologies into coal to liquids plants. Fuel 116: 850–860, https://doi.org/10.1016/j.fuel.2013.05.008.Search in Google Scholar
Caton, J.A. (2014). On the destruction of availability (exergy) due to combustion processes-with specific application to internal-combustion engines. Energy 25: 1097–1117, https://doi.org/10.1016/s0360-5442(00)00034-7.Search in Google Scholar
Chaniago, Y.D., Qyyum, M.A., Andika, R., Ali, W., Qadeer, K., and Lee, M. (2019). Self-recuperative high temperature co-electrolysis-based methanol production with vortex search-based exergy efficiency enhancement. J. Clean. Prod. 239: 118029, https://doi.org/10.1016/j.jclepro.2019.118029.Search in Google Scholar
Chen, Y. (2015). The application of self-heat recuperation technology in crude oil distillation units. M.E. thesis. Heilongjiang Province, Harbin Institute of Technology.Search in Google Scholar
Chen, Q., Zhu, H., Pan, N., and Guo, Z. (2011). An alternative criterion in heat transfer optimization. Proc. R. Soc. A: Math. Phys. Eng. Sci. 467: 1012–1028, https://doi.org/10.1098/rspa.2010.0293.Search in Google Scholar
Chen, Q., Liang, X., and Guo, Z. (2013). Entransy theory for the optimization of heat transfer-A review and update. Int. J. Heat Mass Tran.: 65–81, https://doi.org/10.1016/j.ijheatmasstransfer.2013.03.019.Search in Google Scholar
Chen, J., Ye, Q., Liu, T., Xia, H., and Feng, S. (2018). Improving the performance of heterogeneous azeotropic distillation via self-heat recuperation technology. Chem. Eng. Res. Des. 141: 516–528, https://doi.org/10.1016/j.cherd.2018.11.022.Search in Google Scholar
Chen, D., Zhang, Z., Yang, J., Ma, X., Li, P., Hao, X., and Guan, G. (2019). Process simulation and energy saving analysis of CO2 capture by chemical absorption method based on self-heat recuperation. CIESC J. 70: 2938–2945.Search in Google Scholar
Chen, L., Shikazono, N., and Tsutsumi, A. (2021). Thermodynamic mechanism of self-heat recuperative heat circulation system with non-isentropic compression and expansion for a continuous heating and cooling gas cycle process. J. Chem. Eng. Jpn. 54: 313–323, https://doi.org/10.1252/jcej.20we065.Search in Google Scholar
Chen, J., Han, D., Bai, G., Zheng, M., Si, Z., Song, Y., and Gu, J. (2022). Thermodynamic analysis of a novel wood drying system based on self-heat recuperation technology. Energy Sources, Part A Recovery, Util. Envir. Eff. 44: 2385–2401, https://doi.org/10.1080/15567036.2019.1649752.Search in Google Scholar
Christopher, C.E., Dutta, A., Farooq, S., and Karimi, A.I. (2017). Process synthesis and optimization of propylene-propane separation using vapor recompression and self-heat recuperation. Ind. Eng. Chem. Res. 56: 14557–14564, https://doi.org/10.1021/acs.iecr.7b03432.Search in Google Scholar
Cui, C., Long, N.V.D., Sun, J., and Lee, M. (2020). Electrical-driven self-heat recuperative pressure-swing azeotropic distillation to minimize process cost and CO2 emission: process electrification and simultaneous optimization. Energy 195: 116998, https://doi.org/10.1016/j.energy.2020.116998.Search in Google Scholar
Cui, C., Zhang, Q., Zhang, X., Sun, J., and Chien, I. (2022). Dynamics and control of thermal-versus electrical-driven pressure-swing distillation to separate a minimum-boiling azeotrope. Sep. Purif. Technol. 280: 119839, https://doi.org/10.1016/j.seppur.2021.119839.Search in Google Scholar
Fan, Y., Ye, Q., Chen, J., Chen, X., Liu, T., and Cen, H. (2019a). A systematic method for optimum heterogeneous azeotropic distillation systems with vapor recompression. Chem. Eng. Process 143: 107597, https://doi.org/10.1016/j.cep.2019.107597.Search in Google Scholar
Fan, Y., Ye, Q., Cen, H., Chen, J., and Liu, T. (2019b). Design and optimization of reactive distillation processes for synthesis of isopropanol based on self-heat recuperation technology. Chem. Eng. Res. Des. 147: 171–186, https://doi.org/10.1016/j.cherd.2019.05.004.Search in Google Scholar
Feng, X. (2004). Chemical energy conservation principle and technology, 2nd ed. Chemical Industry Press, Beijing.Search in Google Scholar
Fu, Q., Kansha, Y., Song, C., Liu, Y., Ishizuka, M., and Tsutsumi, A. (2014). An Advanced cryogenic air separation process based on self-heat recuperation for CO2 separation. Energy Procedia 61: 1673–1676, https://doi.org/10.1016/j.egypro.2014.12.189.Search in Google Scholar
Fu, Q., Song, C., Kansha, Y., Liu, Y., Ishizuka, M., and Tsutsumi, A. (2015). Energy saving in a biodiesel production process based on self-heat recuperation technology. Chem. Eng. J. 278: 556–562, https://doi.org/10.1016/j.cej.2014.11.027.Search in Google Scholar
Fu, Q., Kansha, Y., Song, C., Liu, Y., Ishizuka, M., and Tsutsumi, A. (2016a). A cryogenic air separation process based on self-heat recuperation for oxy-combustion plants. Appl. Energy 162: 1114–1121, https://doi.org/10.1016/j.apenergy.2015.03.039.Search in Google Scholar
Fu, Q., Kansha, Y., Song, C., Liu, Y., Ishizuka, M., and Tsutsumi, A. (2016b). An Elevated-pressure cryogenic air separation unit based on self-heat recuperation technology for integrated gasification combined cycle systems. Energy 103: 440–446, https://doi.org/10.1016/j.energy.2015.09.095.Search in Google Scholar
Fushimi, C. and Dewi, N.W. (2015). Energy efficiency and capital cost estimation of superheated steam drying processes combined with integrated coal gasification combined cycle. J. Chem. Eng. Jpn. 48: 872–880, https://doi.org/10.1252/jcej.14we401.Search in Google Scholar
Fushimi, C. and Fukui, K. (2014). Simplification and energy saving of drying process based on self-heat recuperation technology. Dry. Technol. 32: 667–678, https://doi.org/10.1080/07373937.2013.851085.Search in Google Scholar
Fushimi, C., Kansha, Y., Aziz, M., Mochidzuki, K., Kaneko, S., Tsutsumi, A., Matsumoto, K., Yokohama, K., Kosaka, K., Kawamoto, N., et al.. (2010). Novel drying process based on self-heat recuperation technology. Dry. Technol. Int J. 29: 105–110, https://doi.org/10.1080/07373937.2010.482719.Search in Google Scholar
Guo, Z., Zhu, H., and Liang, X. (2007). Entransy – a physical quantity describing heat transfer ability. Int. J. Heat Mass Transf. 50: 2545–2556, https://doi.org/10.1016/j.ijheatmasstransfer.2006.11.034.Search in Google Scholar
Han, D., Peng, T., He, W., Yue, C., Pu, W., and Liang, L. (2014a). Advanced energy saving in the evaporation system of ammonium sulfate solution with self-heat recuperation technology. Energy Procedia 61: 131–136, https://doi.org/10.1016/j.egypro.2014.11.923.Search in Google Scholar
Han, D., He, W., Yue, C., Pu, W., and Liang, L. (2014b). Analysis of energy saving for ammonium sulfate solution processing with self-heat recuperation principle. Appl. Therm. Eng. 73: 641–649, https://doi.org/10.1016/j.applthermaleng.2014.08.026.Search in Google Scholar
Han, D., Si, Z., and Chen, J. (2021). Analysis of an intermittent mechanical vapor recompression evaporation system. Appl. Therm. Eng. 193: 116996, https://doi.org/10.1016/j.applthermaleng.2021.116996.Search in Google Scholar
Han, Z., Jia, X., Song, X., An, P., Fu, L., Yue, J., Yu, J., Liu, X., Zhang, Z., Jin, Y., et al.. (2023). Engineering thermochemistry to cope with challenges in carbon neutrality. J. Clean Prod. 416: 137943, https://doi.org/10.1016/j.jclepro.2023.137943.Search in Google Scholar
Hou, Q. (2022). Design simulation of coal-based polygeneration carbon cycle system and optimization of self-heating regeneration energy. Taiyuan university of technology, Shanxi.Search in Google Scholar
Hou, Q., Wen, Z., Zhang, Z., Liu, Y., Yang, J., Chen, D., Hao, X., and Guan, G. (2022). Design and evaluation of a coal-based polygeneration system with carbon cycle. CIESC J. 73: 2073–2082.Search in Google Scholar
Kansha, Y., Tsuru, N., Sato, K., Fushimi, C., and Tsutsumi, A. (2009). Self-heat recuperation technology for energy saving in chemical processes. Ind. Eng. Chem. Res. 48: 7682–7686, https://doi.org/10.1021/ie9007419.Search in Google Scholar
Kansha, Y., Kishimoto, A., and Tsutsum, A. (2010a). A new design methodology for heat integrated distillation column based on self-heat recuperation. Chem. Eng. Trans. 21: 43–48.Search in Google Scholar
Kansha, Y., Tsuru, N., Fushimi, C., and Tsutsumi, A. (2010b). Integrated process module for distillation processes based on self-heat recuperation technology. J. Chem. Eng. Jpn. 43: 502–507, https://doi.org/10.1252/jcej.43.502.Search in Google Scholar
Kansha, Y., Tsuru, N., Fushimi, C., and Tsutsumi, A. (2010c). New design methodology based on self-heat recuperation for production by azeotropic distillation. Energy Fuels 24: 6099–6102, https://doi.org/10.1021/ef100671w.Search in Google Scholar
Kansha, Y., Kishimoto, A., Nakagawa, T., and Tsutsumi, A. (2011a). A Novel cryogenic air separation process based on self-heat recuperation. Sep. Purif. Technol. 77: 389–396, https://doi.org/10.1016/j.seppur.2011.01.012.Search in Google Scholar
Kansha, Y., Kishimoto, A., and Tsutsumi, A. (2011b). Process design methodology for high-energy saving HIDiC based on self-heat recuperation. Asia-Pacific J. Chem. Eng. 6: 320–326, https://doi.org/10.1002/apj.582.Search in Google Scholar
Kansha, Y., Kishimoto, A., and Tsutsumi, A. (2012a) Dynamic characteristics of self-heat recuperative distillation process. In: International Symposium on Process Systems Engineering. National University of Singapore, Singapore.10.1016/B978-0-444-59507-2.50132-3Search in Google Scholar
Kansha, Y., Kishimoto, A., and Tsutsumi, A. (2012b). Application of the self-heat recuperation technology to crude oil distillation. Appl. Therm. Eng. 43: 153–157, https://doi.org/10.1016/j.applthermaleng.2011.10.022.Search in Google Scholar
Kansha, Y., Kotani, Y., Aziz, M., Kishimoto, A., and Tsutsumi, A. (2013). Evaluation of a self-heat recuperative thermal process based on thermodynamic irreversibility and exergy. J. Chem. Eng. Jpn. 46: 87–91, https://doi.org/10.1252/jcej.12we084.Search in Google Scholar
Kansha, Y., Ishizuka, M., Song, C., and Tsutsumi, A. (2014). An Innovative methanol synthesis process based on self-heat recuperation. Appl. Therm. Eng. 70: 1189–1194, https://doi.org/10.1016/j.applthermaleng.2014.05.002.Search in Google Scholar
Kansha, Y., Ishizuka, M., Song, C., and Tsutsumi, A. (2015). Process intensification for dimethyl ether production by self-heat recuperation. Energy 90: 122–127, https://doi.org/10.1016/j.energy.2015.05.037.Search in Google Scholar
Kansha, Y., Ishizuka, M., Mizuno, H., and Tsutsumi, A. (2017). Design of energy-saving carbon dioxide separation process using fluidized bed. Appl. Therm. Eng. 126: 134–138, https://doi.org/10.1016/j.applthermaleng.2017.07.156.Search in Google Scholar
Kansha, Y., Ishizuka, M., Tsutsumi, A., Kambe, Y., and Yoshihara, J. (2019). Simulated application of self-heat recuperation and pressure swing system to industrial methanol synthesis process. J. Chem. Eng. Jpn. 52: 650–655, https://doi.org/10.1252/jcej.18we177.Search in Google Scholar
Kim, M.G., Long, N.V.D., and Lee, M. (2012). Application of the self-heat recuperation technology to deisobutanizer column. Int. J. Chem. Eng. Appl. 3: 424–426, https://doi.org/10.7763/ijcea.2012.v3.234.Search in Google Scholar
Kishimoto, A., Kansha, Y., Fushimi, C., and Tsutsumi, A. (2011). Exergy recuperative CO2 gas separation in post-combustion capture. Ind. Eng. Chem. Res. 50: 10128–135, https://doi.org/10.1021/ie200852b.Search in Google Scholar
Kishimoto, A., Kansha, Y., Fushimi, C., and Tsutsumi, A. (2012). Exergy recuperative CO2 gas separation in pre-combustion capture. Clean. Technol. Envir. 14: 465–474, https://doi.org/10.1007/s10098-011-0428-3.Search in Google Scholar
Kotani, Y., Kansha, Y., and Tsutsum, A. (2012). Self-heat recuperation using magnetocaloric effect. Chem. Eng. Trans. 29: 373–378.Search in Google Scholar
Kotani, Y., Aziz, M., Kansha, Y., Fushimi, C., and Tsutsumi, A. (2013a). Magnetocaloric heat circulator based on self-heat recuperation technology. Chem. Eng. Sci. 101: 5–12, https://doi.org/10.1016/j.ces.2013.05.064.Search in Google Scholar
Kotani, Y., Kansha, Y., and Tsutsumi, A. (2013b). Conceptual design of an active magnetic regenerative heat circulator based on self-heat recuperation technology. Energy 55: 127–133, https://doi.org/10.1016/j.energy.2013.03.014.Search in Google Scholar
Kotani, Y., Kansha, Y., Ishizuka, M., and Tsutsumi, A. (2014). Experimental investigation of an active magnetic regenerative heat circulator applied to self-heat recuperation technology. Appl. Therm. Eng. 70: 1202–1207, https://doi.org/10.1016/j.applthermaleng.2014.04.015.Search in Google Scholar
Kuchonthara, P. and Tsutsumi, A. (2006). Energy-recuperative coal-integrated gasification/gas turbine power generation system. J. Chem. Eng. Jpn. 39: 545–552, https://doi.org/10.1252/jcej.39.545.Search in Google Scholar
Li, Y., Fu, L., Zhang, S., and Zhao, X. (2011). A new type of district heating system based on distributed absorption heat pumps. Energy 36: 4570–4576, https://doi.org/10.1016/j.energy.2011.03.019.Search in Google Scholar
Li, J., Zhang, F., Pan, Q., Yang, Y., and Sun, L. (2019a). Performance enhancement of reactive dividing wall column based on self-heat recuperation technology. Ind. Eng. Chem. Res. 58: 12179–12191, https://doi.org/10.1021/acs.iecr.9b02363.Search in Google Scholar
Li, X., Cui, C., Li, H., and Gao, X. (2019b). Process synthesis and simulation-based optimization of ethylbenzene/styrene separation using double-effect heat integration and self-heat recuperation technology: a techno-economic analysis. Sep. Purif. Technol. 228: 115760, https://doi.org/10.1016/j.seppur.2019.115760.Search in Google Scholar
Li, L., Yu, N., and Zhu, Y. (2022). Multi-objective optimization and control of self-heat recuperative azeoropic distillation for separating an ethanol/water mixture. ACS Omega 7: 11382–11394, https://doi.org/10.1021/acsomega.2c00478.Search in Google Scholar PubMed PubMed Central
Lian, W., Pan, X., Zheng, S., Zhang, W., Zhang, H., Fushimi, C., Tsutsumi, A., Hao, X., Huang, W., and Guan, G. (2019). Mechanism analysis of the solids holdup variations in downer reactors based on volumetric flux. Chem. Eng. Sci. 205: 259–268, https://doi.org/10.1016/j.ces.2019.04.045.Search in Google Scholar
Lian, W., Pan, X., Li, Z., Yang, J., Hao, X., Zhang, H., Fushimi, C., Tsutsumi, A., Huang, W., and Guan, G. (2020). A drag model considering the particle size distribution via multi-subgrid for the simulation of downer. Sep. Purif. Technol. 214: 115363–115373, https://doi.org/10.1016/j.ces.2019.115363.Search in Google Scholar
Linnhoff, B. (1993). Pinch analysis-a state-of-the-art overview. Chem. Eng. Res. Des. 71: 503–522.Search in Google Scholar
Linnhoff, B. and Eastwood, A.R. (1997). Overall site optimisation by pinch technology. Chem. Eng. Res. Des. 75: 138–144.10.1016/S0263-8762(97)80011-1Search in Google Scholar
Linnhoff, B. and Hindmarsh, E. (1983). The pinch design method for heat exchanger networks. Sep. Purif. Technol. 38: 745–763, https://doi.org/10.1016/0009-2509(83)80185-7.Search in Google Scholar
Linnhoff, B., Mason, D.R., and Wardle, I. (1979). Understanding heat exchanger networks. Comput. Chem. Eng. 3: 295–302, https://doi.org/10.1016/0098-1354(79)80049-6.Search in Google Scholar
Liszka, M., Malik, T., and Manfrida, G. (2012). Energy and exergy analysis of hydrogen-oriented coal gasification with CO2 capture. Energy 45: 142–150, https://doi.org/10.1016/j.energy.2012.03.054.Search in Google Scholar
Liu, Y., Aziz, M., Fushimi, C., Kansha, Y., Mochidzuki, K., Kaneko, S., Tsutsumi, A., Yokohama, K., Kazuyuki, Myoyo., Koji, Oura., et al.. (2012a). Exergy analysis of biomass drying based on self-heat recuperation technology and its application to industry: a simulation and experimental study. Ind. Eng. Chem. Res. 51: 997–10007, https://doi.org/10.1021/ie2027298.Search in Google Scholar
Liu, Y., Aziz, M., Kansha, Y., and Tsutsumi, A. (2012b). Drying energy saving by applying self-heat recuperation technology to biomass drying system. Chem. Eng. Trans. 29: 577–582.Search in Google Scholar
Liu, Y., Aziz, M., Kansha, Y., and Tsutsumi, A. (2013). A Novel exergy recuperative drying module and its application for energy-saving drying with superheated steam. Sep. Purif. Technol. 100: 392–401, https://doi.org/10.1016/j.ces.2013.01.044.Search in Google Scholar
Liu, Y., Aziz, M., Kansha, Y., Bhattacharya, S., and Tsutsumi, A. (2014). Application of the self-heat recuperation technology for energy saving in biomass drying system. Fuel Process. Technol. 117: 66–74, https://doi.org/10.1016/j.fuproc.2013.02.007.Search in Google Scholar
Liu, Y., Kansha, Y., Ishizuka, M., Fu, Q., and Tsutsumi, A. (2015). Experimental and simulation investigations on self-heat recuperative fluidized bed dryer for biomass drying with superheated steam. Fuel Process. Technol. 136: 79–86, https://doi.org/10.1016/j.fuproc.2014.10.005.Search in Google Scholar
Liu, Y., Zhang, Z., Hou, Q., Yang, J., Chen, D., and Hao, X. (2021). Process design and simulation of synthesis gas to methanol in TBCFB system. CIESC J. 72: 4838–4846.Search in Google Scholar
Liu, J., Xu, Y., Zhang, Y., Shuai, Y., and Li, B. (2022). Multi-objective optimization of low temperature cooling water organic rankine cycle using dual pinch point temperature difference technologies. Energy 240: 122740, https://doi.org/10.1016/j.energy.2021.122740.Search in Google Scholar
Long, N.V.D. and Lee, M. (2013). A Novel NGL (natural gas liquid) recovery process based on self-heat recuperation. Energy 57: 663–670, https://doi.org/10.1016/j.energy.2013.04.078.Search in Google Scholar
Long, N.V.D. and Lee, M. (2014). Review of retrofitting distillation columns using thermally coupled distillation sequences and dividing wall columns to improve energy efficiency. J. Chem. Eng. Jpn. 47: 87–108, https://doi.org/10.1252/jcej.13we067.Search in Google Scholar
Long, N.V.D. and Lee, M. (2017). Novel acid gas removal process based on self-heat recuperation technology. Int. J. Greenh. Gas Con. 64: 34–42.10.1016/j.ijggc.2017.07.003Search in Google Scholar
Luyben, W.L. (2018). Design and control of a pressure-swing distillation process with vapor recompression. Chem. Eng. Process. 123: 174–184, https://doi.org/10.1016/j.cep.2017.09.020.Search in Google Scholar
Matsuda, K., Kawazuishi, K., Hirochi, Y., Sato, R., Kansha, Y., Fushimi, C., Shikatani, Y., Kunikiyo, H., and Tsutsumi, A. (2010). Advanced energy saving in the reaction section of the hydro-desulfurization process with self-heat recuperation technology. Appl. Therm. Eng. 30: 2300–2305, https://doi.org/10.1016/j.applthermaleng.2010.05.003.Search in Google Scholar
Matsuda, K., Kawazuishi, K., Kansha, Y., Fushimi, C., Nagao, M., Kunikiyo, H., Masuda, F., and Tsutsumi, A. (2011). Advanced energy saving in distillation process with self-heat recuperation technology. Energy 36: 4640–4645, https://doi.org/10.1016/j.energy.2011.03.042.Search in Google Scholar
Mizuno, H., Kansha, Y., Kishimoto, A., and Tsutsumi, A. (2013). Thermal seawater desalination based on self-heat recuperation. Clean Technol. Envir. 15: 765–769, https://doi.org/10.1007/s10098-012-0539-5.Search in Google Scholar
Novita, F., Lee, H., and Lee, M. (2015). Self-heat recuperative dividing wall column for enhancing the energy efficiency of the reactive distillation process in the formic acid production process. Chem. Eng. Process. 97: 144–152, https://doi.org/10.1016/j.cep.2015.09.007.Search in Google Scholar
Qian, Z., Zhai, R., Feng, L., and Mei, W. (2023). Performance analysis of the acid gas removal from syngas based on self-heat recuperation technology. Appl. Therm. Eng. 219: 119506, https://doi.org/10.1016/j.applthermaleng.2022.119506.Search in Google Scholar
Rasfuldi, R., Kotani, Y., Kansha, Y., Ishizuka, M., and Tsutsumi, A. (2015). Self-heat recuperative heat circulation processing with thermoelectric device. Appl. Energy 160: 836–842, https://doi.org/10.1016/j.apenergy.2015.03.055.Search in Google Scholar
Seider, W.D., Seader, J.D., and Lewin, D.R. (2004). Product & process design principles synthesis, analysis, and evaluation, 2nd ed. John Wiley & Sons, New York.Search in Google Scholar
Shayan, E., Zare, V., and Mirzaee, I. (2018). Hydrogen production from biomass gasification; a theoretical comparison of using different gasification agents. Energ. Convers. Manage. 159: 30–41, https://doi.org/10.1016/j.enconman.2017.12.096.Search in Google Scholar
Song, C., Kansha, Y., Ishizuka, M., Fu, Q., and Tsutsumi, A. (2014). Design of a low-cost CO2 capture process based on heat integration technology. Energy Procedia 61: 365–368, https://doi.org/10.1016/j.egypro.2014.11.1126.Search in Google Scholar
Song, C., Kansha, Y., Ishizuka, M., Fu, Q., and Tsutsumi, A. (2015). Conceptual design of a novel pressure swing CO2 adsorption process based on self-heat recuperation technology. Chem. Eng. Process. 94: 20–28, https://doi.org/10.1016/j.cep.2015.03.008.Search in Google Scholar
Sun, Z., Wang, S., and Aziz, M. (2022). Highly integrated system for ammonia and electricity production from biomass employing direct chemical looping: exergy and exergoeconomic analyses. Energ. Convers. Manage. 251: 115013, https://doi.org/10.1016/j.enconman.2021.115013.Search in Google Scholar
Tsutsumi, A. and Kansha, Y. (2017). Thermodynamic mechanism of self-heat recuperative and self-heat recovery heat circulation system for a continuous heating and cooling gas cycle process. Chem. Eng. Trans. 61: 1759–1763.Search in Google Scholar
Tsutsumi, A., Guan, G., Fushimi, C., Ikeda, M., Nakamura, Y., Suda, T., Ishizuka, M., Hatano, H., Matsuda, S., and Suzuki, Y. (2010). Flow behaviors in a high solid flux circulating fluidized bed composed of a riser, a downer and a bubbling fluidized bed. In: Fluidization ⅩⅢ: New Paradigm in Fluidization Engineering, Korea. Engineering Conferences International, pp. 407–414.Search in Google Scholar
Tu, R. and Hwang, Y. (2019). Performance analyses of a new system for water harvesting from moist air that combines multi-stage desiccant wheels and vapor compression cycles. Energ. Convers. Manage. 198: 111811, https://doi.org/10.1016/j.enconman.2019.111811.Search in Google Scholar
Vilardi, G. (2021). The electrification of conventional industrial processes: the use of mechanical vapor compression in an EtOH–water distillation tower. Energies 14: 1–18.10.3390/en14217267Search in Google Scholar
Wang, J., Lian, W., Li, P., Zhang, Z., Yang, J., Hao, X., Huang, W., and Guan, G. (2017). Simulation of pyrolysis in low rank coal particle by using DAEM kinetics model: reaction behavior and heat transfer. Fuel 207: 126–135, https://doi.org/10.1016/j.fuel.2017.06.078.Search in Google Scholar
Wang, Y., Yang, J., Zhang, Z., Ma, X., Li, P., Hao, X., and Guan, G. (2018). TBCFB system simulation and optimization for pyrolysis-gasification-combustion of low rank coal. CIESC J. 69: 3596–3604.Search in Google Scholar
Wei, F., Zhang, S., and Xiao, Y. (2014). Performance and exergy analysis of open absorption heat pump. Adv. Mater. Res. 953: 667–672, https://doi.org/10.4028/www.scientific.net/amr.953-954.667.Search in Google Scholar
Wen, Z., Li, P., Zhang, Z., Du, X., Hou, Q., Liu, Y., Hao, X., and Guan, G. (2023). Design and optimization of cryogenic air separation process with dividing wall column based on self-heat regeneration. CIESC J. 74: 2988–2998.Search in Google Scholar
Wu, J. and Guo, Z. (2014). Application of entransy analysis in self-heat recuperation technology. Ind. Eng. Chem. Res. 53: 1274–1285, https://doi.org/10.1021/ie4031506.Search in Google Scholar
Wu, Y., Han, D., He, W., Zhen, P., Pu, W., and Yue, C. (2014). Advanced energy saving in vitamin evaporation crystallization section at low temperature with self-heat recuperation technology. CIESC J.: 831–4838.Search in Google Scholar
Wu, W., Zhai, C., Huang, S., Sui, Y., Sui, Z., and Ding, Z. (2022). A hybrid H2O/IL absorption and CO2 compression air-source heat pump for ultra-low ambient temperatures. Energy 239: 122180, https://doi.org/10.1016/j.energy.2021.122180.Search in Google Scholar
Wu, H., Ye, Q., Li, J., Xu, Z., and Pan, J. (2024). Novel energy-efficient designs of heterogeneous azeotropic distillation for separating ternary organic wastewater based on self-heat recuperation technology. Process Saf. Environ. 183: 1038–1050, https://doi.org/10.1016/j.psep.2024.01.061.Search in Google Scholar
Xia, H., Ye, Q., Feng, S., Li, R., and Suo, X. (2017). A Novel energy-saving pressure swing distillation process based on self-heat recuperation technology. Energy 141: 770–781, https://doi.org/10.1016/j.energy.2017.09.108.Search in Google Scholar
Xia, H., Chen, R., Ye, Q., Feng, S., Chen, J., Liu, T., and Wu, W. (2018a). Application of a self-heat recuperation technology-based novel energy-saving pressure swing distillation process in separation of azeotropes. Mod. Chem. Ind. 38: 193–196.Search in Google Scholar
Xia, H., Ye, Q., Feng, S., Chen, J., and Liu, T. (2018b). Energy-efficient design of downstream separation to produce n-butanol by several heat integrated technologies. Ind. Eng. Chem. Res. 57: 13205–13216, https://doi.org/10.1021/acs.iecr.8b02077.Search in Google Scholar
Xiong, J., Zhao, H., Chen, M., and Zheng, C. (2011). Exergy analysis of a 600 MWe oxy-combustion pulverized-coal-fired power plant. Energy Fuels 25: 3854–3864, https://doi.org/10.1021/ef200702k.Search in Google Scholar
Xu, G., Bai, D., Xu, C., and He, M. (2023). Challenges and opportunities for engineering thermochemistry in carbon-neutralization technologies. Natl. Sci. Rev. 10: nwac217, https://doi.org/10.1093/nsr/nwac217.Search in Google Scholar PubMed PubMed Central
Yang, S., Zhang, Q., Zeng, A., Ma, Y., and Yuan, X. (2020). Eco-efficient self-heat recuperative vapor recompression-assisted side-stream pressure-swing distillation arrangement for separating a minimum-boiling azeotrope. Ind. Eng. Chem. Res. 59: 13190–13203, https://doi.org/10.1021/acs.iecr.0c01964.Search in Google Scholar
Yang, D., Zhang, Q., Zhang, Q., and Cui, C. (2023). Dynamics and control of electrified pressure-swing distillation for separating a maximum-boiling azeotrope featuring small pressure-induced shift. Sep. Purif. Technol. 312: 123360, https://doi.org/10.1016/j.seppur.2023.123360.Search in Google Scholar
Yao, Y., Han, D., and Bai, G. (2017). Design and study of paddy fluidized bed drying system based on self-heat recuperation theory. Energ. Conserv. Technol.: 199–203.Search in Google Scholar
Zhang, C., Li, Y., Zhang, J. (2020a). A Developed CRMC Design Modeling for the Ejector Component in the Steam Jet Heat Pumps, In Proceedings of the 11th International Symposium on Heating, Ventilation and Air Conditioning (ISHVAC 2019). Environmental Science and Engineering. July 12-15,2019: Springer, Singapore. 2020, pp 135–143.10.1007/978-981-13-9524-6_15Search in Google Scholar
Zhang, Q., Yang, S., Shi, P., Hou, W., Zeng, A., Ma, Y., and Yuan, X. (2020b). Economically and thermodynamically efficient heat pump-assisted side-stream pressure-swing distillation arrangement for separating a maximum-boiling azeotrope. Appl. Therm. Eng. 173: 115228, https://doi.org/10.1016/j.applthermaleng.2020.115228.Search in Google Scholar
Zhao, Z., Situmorang, Y.A., An, P., Yang, J., Hao, X., Rizkiana, J., Abudula, A., and Guan, G. (2021). A biomass-based small-scale power generation system with energy/exergy recuperation. Energ. Convers. Manage. 227: 113623.10.1016/j.enconman.2020.113623Search in Google Scholar
Zhen, P., Han, D., Wu, Y., He, W., Pu, W., and Yue, C. (2015a). Design and energy consumption analysis in methanol distillation system based on self-heat recuperation theory. Energy Chem. Ind. 36: 12–15.Search in Google Scholar
Zhen, P., Han, D., Wu, Y., He, W., Pu, W., and Yue, C. (2015b). Macro analysis of energy on methanol distillation system based on self-heat recuperation theory. Energ. Conserv. Technol. 33: 220–225.Search in Google Scholar
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- Reviews
- Trends and perspectives in deterministic MINLP optimization for integrated planning, scheduling, control, and design of chemical processes
- Self-heat recuperation (SHR) theory: principle and applications
- Heteropolyacid-alumina composites as heterogeneous catalysts for the synthesis of organic compounds: a comprehensive review
- Mini Review
- Hybrid MOF-COF structures for advanced gas separation membranes: a short review of synthesis, performance analysis and application potential
Articles in the same Issue
- Frontmatter
- Reviews
- Trends and perspectives in deterministic MINLP optimization for integrated planning, scheduling, control, and design of chemical processes
- Self-heat recuperation (SHR) theory: principle and applications
- Heteropolyacid-alumina composites as heterogeneous catalysts for the synthesis of organic compounds: a comprehensive review
- Mini Review
- Hybrid MOF-COF structures for advanced gas separation membranes: a short review of synthesis, performance analysis and application potential

