Abstract
Background
The objectives were to perform an observation of the administration of injectable drugs in three ICUs, to identify injectable drugs administered by Y-site infusion or mixed in the same container, to compare with physical compatibility data available in the literature and to test the physical compatibility for missing data.
Methods
An observational study was realised over two weeks and patients receiving more than one injectable drug in the same line simultaneously were included. Physical compatibilities were assessed in pairs by comparing with three databases. For some missing data, three tests were realised for pairs including an anti-infective drug. Visual and subvisual evaluations were performed after the preparation, 1 and a 4-hour storage.
Results
A total of 389 combinations between two injectable drugs was observed for Y-site infusions and 31 mixtures in the same container. According to the literature, 21.1 % associations were physically compatible, 1.8 % as physically compatible potentially, 8.0 % as physically incompatible, 6.4 % have divergent data according to the databases and 62.7 % have no data. Two mixtures were documented. 37 pairs were tested and 70.3 % were physically compatible, 8.1 % were physically incompatible after visual evaluation and 21.6 % after subvisual evaluation.
Conclusions
In the majority of cases, no compatibility data are available in the literature. Laboratory tests give additional information.
Introduction
Patients hospitalized in Intensive Care Units (ICUs) often require the use of multiple drugs and the intravenous route is the most commonly way of administration. High concentrations of drug solutions with a minimum volume are used to avoid fluid overload and to rapidly obtain an optimal concentration. However, intravenous (I.V) accesses are limited and multiple I.V drugs have to be administered simultaneously to patients, leading to concomitant administration of different drugs in the same infusion line.
For Y-site infusions, drugs must be physically compatible, which means no precipitation, no change of colour or no gas formation [1]. Physical incompatibilities can lead to the formation of precipitate, catheter obstruction, venous irritation, and pulmonary or renal emboli [2]. Incompatibilities of drugs can also result in a decrease in drug activity, change of active drugs or formation of toxic compounds [3].
Bertsche et al. listed the five most important errors for parenteral drugs: hygiene defects, duration of administration, prescribing information (patient, brand, dosage, administration interval and formulation), labeling the preparation for a specific identification and incompatibilities [4]. Taxis et al. have reported that incompatibilities are the most common intravenous medication errors in hospital with a frequency of 25 % with 2 % having a severe clinical importance [5]. Tissot et al. have assessed medication-administration errors in an ICU: 18.6 % of physicochemical compatibility errors were recorded, 63.2 % of these errors had a potential clinical effect and 26.3 % were potentially life-threatening [6].
The objectives of this study were (1) to perform an observation of the administration of injectable drugs in three adults’ ICUs of our hospital, to identify injectable drugs administered by Y-site infusion or mixed in the same container, (2) to compare with physical compatibility data available in the literature and (3) in some cases, in the absence of physical compatibility data, to test the physical compatibility in our quality control laboratory.
Materials and methods
Observational analysis in ICUs
Between April and June 2018, an observational prospective study was conducted over two weeks in each of three different adults’ ICUs selected: one medical ICU and two surgical ICUs (cardiac and polyvalent).
Prescriptions of patients receiving more than one I.V drug in the same line simultaneously (Y-site infusion or drugs mixed in the same container) were included. Each prescription was counted only once per patient to avoid the over-representation of patients who had been hospitalised for a long time. Any modification of prescription for a patient already counted was included. All I.V. drugs were recorded for concentration, solvent and type of container.
The physical compatibility of the drugs used in the Y-site infusions was evaluated in pairs, even if more than two drugs were administered simultaneously in the same IV line. The mixtures in the same container were assessed as a whole, taking into account all the molecules mixed. For comparison with data available in the literature, three databases were used: the 19th edition of the Handbook on Injectable Drugs® [7], the 36th edition of “Stability of injectable drugs in infusion” [8] and Stabilis® [9].
The classification criteria according to the literature data on drug combinations and mixtures observed are presented in Table 1.
Criteria for the classification of drug combinations and mixtures observed in ICUs according to literature data.
| Pairs observed in Y-site infusion | Mixture observed in the same container | |||||
|---|---|---|---|---|---|---|
| Compatible | Potentially compatible | Incompatible | Controversial | No data | Stable or not stable | No data |
| Compatibility data available in the literature with concentrations equal to or higher than those observed | Compatibility data available in the literature with concentrations lower than those observed | Incompatibility data available in the literature regardless of the concentrations, the solvent and the container | Compatibility and incompatibility data available in the literature | Physico-chemical stability data available in the literature with identical concentrations or closer than observed | ||
Laboratory tests
Many pairs with no data were observed during this study and we focused laboratory tests on pairs including one or two anti-infective drugs (antibiotic, antiviral or antifungal drugs). To simulate the Y-site infusion, each drug in each pair tested was prepared separately at baseline. If the solvent used or the concentration of a drug was different between the three ICUs observed, we tested the different variables in the laboratory. Drugs were mixed and kept in glass tubes at room temperature, not protected from light, to simulate the conditions of storage observed in ICUs. Three tests were realised for each pair studied (drug A/drug B): a) 1 mL/9 mL; b) 9 mL/1 mL; c) 5 mL/5 mL. In the majority of physical compatibility studies, a single 1:1 ratio has been performed [10, 11]. For each pair of drugs tested, we performed these different ratios to simulate cases where the drug flow is different leading to higher or lower concentrations. Mixtures were manually stirred during 30 seconds.
Physical compatibility was defined as the absence of particulate formation, haze, colour change and gas evolution [1]. As recommended by the European Pharmacopeia [12], the samples were visually inspected against a white/black background with the unaided eye by two different technicians after the mixture and after a 1-hour and 4-hour storage. The subvisual aspect was assessed by using a UV spectrophotometer (Safas mc2, Monaco). The absorbance light was scanned at 350, 410 and 550 nm as recommended by the European Consensus Conference at each time of the analysis [1]. Drugs were considered as physically compatible if no visible change was detectable within 4 hours. A modification in absorbance value of the initial concentration was considered as turbidity. Table 2 gives the list of drugs and solvents used for laboratory tests.
List of drugs and solvents used for laboratory tests.
| Laboratory | Batch | |
|---|---|---|
| Anti-infective drugs | ||
| Amoxicillin 1 g | Panpharma | 305141, 305173 |
| Amoxicillin 2 g | Panpharma | 304826 |
| Amphothericin B liposomal (Ambisome®) | Gilead sciences | 0121476D1 |
| Cefazolin 2 g | Mylan | 171109, 171110 |
| Cefepim 2 g | Mylan | 4M2098FR |
| Cefotaxime 2 g | Mylan | R3051 |
| Cotrimoxazole (Bactrim®) 400/80 mg 5 mL | Roche | F3069F03 |
| Daptomycin 350 mg | Accord | 290/18 |
| Fluconazole 2 mg/mL | Fresenius Kabi | 15MF225F4 |
| Gentamicine 40 mg/mL | Panpharma | 70595 |
| Gentamicine 80 mg/mL | Panpharma | 70700 |
| Levofloxacine 250 mg/50 mL | Fresenius Kabi | 15ME507R22 |
| Linezolid 600 mg/300 mL | Fresenius kabi | 15MF170I1 |
| Ornidazole 1 g 6 mL | SERB/CSP | 2476 |
| Ornidazole 500 mg 3 mL | SERB/CSP | 2448 |
| Piperacillin/tazobactam 4 g | Mylan | 5M2914FR |
| Spiramycine (Rovamycine®) 1.5 MUI | Sanofi | 85088 |
| Vancomycin 1 g | Sandoz | EC0109 |
| Vancomycin 500 mg | Sandoz | CB0124 |
| Voriconazole (Vfend®) 200 mg | Pfizer | Z499509 |
| Other drugs | ||
| Heparin sodium 5000 UI/1 mL | Sanofi | 5A013 |
| Insulin (Umuline®) 100 UI/mL | Lilly | C875902 |
| Levetiracetam 100 mg/5 mL | Mylan | F2046 |
| Magnesium sulfate 15 % (1.5 g/10 mL) | Aguettant | 1802267 |
| Nefopam (Acupan®) 20 mg/mL | Biocodex | F0226 |
| Pantoprazole 40 mg | Arrow | PAG14 |
| Pyridoxine hydrochloride 250 mg/5 mL | Renaudin | 205548 |
| Thiamine (Bevitine®) 100 mg/2 mL | D.B Pharma | F1112 |
| Solvents | ||
| Water for injection 20 mL | Chaix et du marais | 8P345 |
| Water for injection 250 mL | Lavoisier | 8F031 |
| Sodium chloride 0.9 % 10 mL Proamp® | Aguettant | 1803445 |
| Sodium chloride 0.9 % 100 mL Ecoflac® | BBraun | 18281451 |
| Sodium chloride 0.9 % 250 mL | Lavoisier | 8F323 |
| Sodium chloride 0.9 % 500 mL | Lavoisier | 8F031, 8F368 |
| Sodium chloride 0.9 % 500 mL Ecoflac® | BBraun | 183358162 |
| Glucose 5 % 100 mL Ecoflac® | BBraun | 182018131 |
| Glucose 5 % 100 mL | Lavoisier | 4F1933 |
| Glucose 5 % 250 mL | Lavoisier | 4F1932 |
| Glucose 5 % 250 mL Easyflex® | MacoPharma | BMAF272 |
| Glucose 5 % 500 mL Ecoflac® | BBraun | 18314450 |
Results
Observational analysis in ICUs
Y-site evaluation
A total of 74 medical prescriptions were analysed. 389 different associations between two IV drugs have been reported and compared to physical compatibility data available in the three databases. According to the literature, the physical compatibility results obtained for the Y-site combinations are presented in Figure 1.
Table 3 illustrates the top 10 pharmacological classes used in Y-site infusion and Table 4 represents the main pharmacological classes with missing compatibility data in the literature, in descending order.
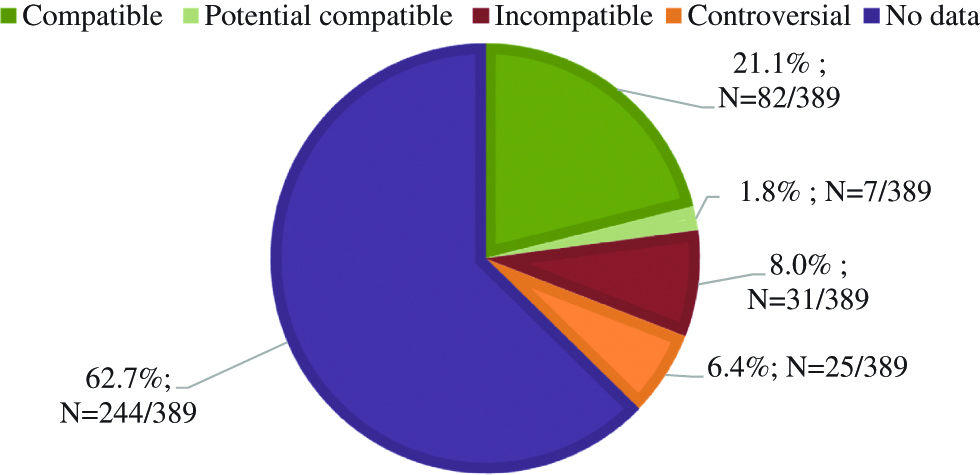
Drugs combinations for Y-site infusions: comparison with physical compatibility data in the literature.
The top 10 pharmacological classes and the main drugs used in Y-site infusion classified by ATC codes, in descending order.
| ATC Codes | Most used in Y-site infusion | Drugs |
|---|---|---|
| B05 | Blood substitutes and perfusion solutions | Cernevit®, Nutryelt®, magnesium sulfate, calcium chloride, potassium chloride, Phocytan®, Dipeptiven®, albumin |
| J01 | Antibiotics | Amikacin, amoxicilline, cefazolin, cefepim, cefotaxime, ceftazidime, ceftriaxone, cotrimoxazole, daptomycin, gentamicin, levofloxacin, linezolid, meropenem, metronidazole, ornidazole, piperacillin/tazobactam, spiramycine, vancomycin |
| N02 | Analgesics | Acetylsalicylic acid, morphine, nefopam, paracetamol, tramadol |
| A11 | Vitamins | Ascorbic acid, pyridoxine, thiamine |
| B01 | Anticoagulants | Heparin sodium |
| A10 | Antidiabetics | Insulin |
| C01 | Cardioactive drugs | Amiodarone, dobutamine, isosorbide dinitrate, isoprenaline, norepinephrine |
| A02 | Drugs for acid related disorders | Pantoprazole |
| A12 | Mineral supplements | Selenium, zinc |
| J02 | Antimycotics for systemic use | Caspofungin, fluconazole, voriconazole |
The 10 pharmacological classes with the least compatibility data in the literature classified by ATC codes, in descending order.
| ATC Codes | With missing compatibility data in the literature |
|---|---|
| B05 | Blood substitutes and perfusion solutions |
| J01 | Antibiotics |
| N02 | Analgesics |
| A11 | Vitamins |
| A12 | Mineral supplements |
| J02 | Antimycotics for systemic use |
| C01 | Cardioactive drugs |
| A10 | Antidiabetics |
| A02 | Drugs for acid related disorders |
| B01 | Anticoagulants |
Mixtures evaluation
A total of 31 drug mixtures in the same container were reported during this study. On average, 3.2 drugs were mixed in the same container and two mixtures have already been studied in the literature (6.5 %; N=2/31). The stability of the droperidol-morphine mixture was evaluated by Williams et al. [13] and the metoclopramide-ondansetron mixture by Stewart et al. [14]. For other mixtures, no data were reported. All the mixtures observed during this study with missing data in the literature are presented in Table 5.
Mixtures of drugs in the same container, observed in ICUs, with missing data in the literature.
| Drugs | Dose | Concentration | Container | Solvent | Final volume |
|---|---|---|---|---|---|
| Mixtures of 2 drugs | |||||
| Cernevit® | one vial | Syringe | NaCl 0.9 % | 50 mL | |
| Nutryelt® | one vial | ||||
| Calcium chloride | 2 g | 4 mg/mL | Bag | G5 % | 500 mL |
| Thiamine | 100 mg | 0.2 mg/mL | |||
| Potassium chloride | 2 g–4 g | 8–16 mg/mL | Bag | G5 % | 250 mL |
| Thiamine | 100 mg | 0.4 mg/mL | |||
| Dobutamine | 500 mg | 10 mg/mL | Syringe | G5 % | 50 mL |
| Norepinephrine | 8 mg | 0.16 mg/mL | |||
| Droperidol | 1.25–2.5 mg | 0.025–0.052 mg/mL | Syringe | NaCl 0,9 % | 50 mL |
| Nefopam | 120 mg | 2,4 mg/mL | |||
| Glucose phosphate | 13.2 mmol | 0.053 mmol/mL | Bag | G5 % | 250 mL |
| Thiamine | 100 mg | 0.4 mg/mL | |||
| Magnesium sulfate | 1.5 g | 1,5 g/L | Bag | NaCl 0.9 % | 1000 mL |
| Thiamine | 100 mg | 0,1 mg/mL | |||
| Mixtures of 3 drugs | |||||
| Cernevit® | one vial | Bag | Parenteral nutrition | 2000 mL | |
| Clorazepate dipotassium | 10 mg | 5 µg/mL | |||
| Nutryelt® | one vial | ||||
| Cernevit | one vial | Bag | Parenteral nutrition | 1477 mL | |
| Nutryelt® | one vial | ||||
| Magnesium sulfate | 4,5 g | ||||
| Cernevit® | one vial | Syringe | NaCl 0.9 % | 100 mL | |
| Nutryelt® | one vial | ||||
| Thiamine | 100 mg | 1 mg/mL | |||
| Calcium chloride | 2 g | 16–2 g/L | Bag | NaCl 0.9 %(1) – | 125 mL(1) – |
| Nefopam | 80 mg | 0,64–0.08 mg/mL | Glucose 10 %(2) – | 1000 mL(2)(3) | |
| Magnesium sulfate | 3 g | 24–3 g/L | Isofundine® (3) | ||
| Calcium chloride | 2 g | 2 g/L | Bag | Isofundine® | 1000 mL |
| Nefopam | 80–100 mg | 0.08–0.1 mg/mL | |||
| Magnesium sulfate | 3–4.5 g | 3–4.5 g/L | |||
| Droperidol | 1.25–2.5 mg | 0.025–0.05 mg/mL | Syringe | NaCl 0.9 % | 50 mL |
| Ketamine | 30–45–50 mg | 0.6–0.9–1 mg/mL | |||
| Morphine sulfate | 20–50 mg | 0.4–1 mg/mL | |||
| Nefopam | 80 mg | 0.16 mg/mL | Bag | Isofundine® | 500 mL |
| Pyridoxine | 250 mg | 0.5 mg/mL | |||
| Thiamine | 250 mg | 0.5 mg/mL | |||
| Metoclopramide | 10 mg | 0.5 mg/mL | Syringe | – | 20 mL |
| Pantoprazole | 40 mg | 2 mg/mL | |||
| Phloroglucinol | 80 mg | 4 mg/mL | |||
| Pyridoxine | 250 mg | 0.5 mg/mL | Bag | NaCl 0,9 % | 500 mL |
| Magnesium sulfate | 3 g | 6 g/L | |||
| Thiamine | 100 mg | 0.2 mg/mL | |||
| Mixtures of 4 drugs | |||||
| Cernevit® | one vial | Syringe | NaCl 0.9 % | 100 mL | |
| Nutryelt® | one vial | ||||
| Magnesium sulfate | 4.5 g | 45 g/mL | |||
| Thiamine | 100 mg | 1 mg/mL | |||
| Cernevit® | one vial | Syringe | NaCl 0.9 % | 100 mL | |
| Nutryelt® | one vial | ||||
| Pyridoxine | 250 mg | 2.5 mg/mL | |||
| Thiamine | 100 mg | 1 mg/mL | |||
| Cernevit® | one vial | Bag | NaCl 0.9 % | 100 mL | |
| Nutryelt® | one vial | ||||
| Zinc | 10 mg | 0.1 mg/mL | |||
| Selenium | 100 µg | 1 µg/mL | |||
| Mixture of more than 4 drugs | |||||
| Ascorbic acid | 1g | 10 mg/mL | Bag | NaCl 0.9 % | 100 mL |
| Cernevit® | one vial | ||||
| Calcium folinate | 5 mg | 0.05 mg/mL | |||
| Nutryelt® | one vial | ||||
| Thiamine | 100 mg | 1 mg/mL | |||
| Pyridoxine | 250 mg | 2.5 mg/mL | |||
| Selenium | 100 µg | 0.1 mg/mL | |||
| Zinc | 10 mg | 0.1 mg/mL | |||
NaCl 0.9 %: sodium chloride 0.9 %; G5 %: glucose 5 %
Laboratory tests
37 pairs were tested in the laboratory. 70.3 % of pairs (N=26/37) were evaluated as physically compatible after visual and subvisual evaluation. 8.1 % of pairs (N=3/37) were found to be incompatible after the visual evaluation and 21.6 % of pairs (N=8/37) after only the subvisual evaluation. The results obtained are presented in Table 6.
Physical compatibility tests performed in laboratory.
| Drugs | Concentration | Solvent | Results | ||
|---|---|---|---|---|---|
| 1 | Amphothericin B liposomal (Ambisome®) | 1.16 mg/mL | G5 % | Incompatible | Precipitation |
| Vancomycin | 10, 62.5 and 83.3 mg/mL | G5 % | |||
| 2 | Amoxicillin | 20.83 and 83.3 mg/mL | NaCl 0.9 % | Physically compatible | |
| Heparin sodium | 208 UI/mL | NaCl 0.9 % | |||
| 3 | Amoxicillin | 20.83 and 83.3 mg/mL | NaCl 0.9 % | Physically compatible | |
| Insulin (Umuline®) | 1 UI/mL | NaCl 0.9 % | |||
| 4 | Amoxicillin | 20.83 mg/mL | G5 % | Incompatible | Increase of UV absorbance |
| Magnesium sulfate | 6 mg/mL | NaCl 0.9 % | |||
| 5 | Amoxicillin | 20.83 mg/mL | G5 % | Incompatible | Increase of UV absorbance |
| Nefopam | 0.16 mg/mL | NaCl 0.9 % | |||
| 6 | Amoxicillin | 20.83 mg/mL | G5 % | Incompatible | Increase of UV absorbance |
| Pyridoxine | 0.5 mg/mL | NaCl 0.9 % | |||
| 7 | Amoxicillin | 20.83 mg/mL | G5 % | Incompatible | Increase of UV absorbance |
| Thiamine (Bevitine®) | 0.2 mg/mL | G5 % | |||
| 8 | Amoxicillin | 20.83 mg/mL | G5 % | Incompatible | Precipitation (Figure 2) |
| Vancomycin | 31.25 mg/mL | G5 % | |||
| 9 | Cefazolin | 41.6 mg/mL | G5 % | Incompatible | Increase of UV absorbance |
| Cotrimoxazole (Bactrim®) | 3.2/0.64 and 80/16 mg/mL | G5 % or none | |||
| 10 | Cefazolin | 41.6 mg/mL | G5 % | Physically compatible | |
| Levetiracetam | 5.21 and 20.83 mg/mL | NaCl 0.9 % | |||
| 11 | Cefazolin | 41.6 mg/mL | G5 % | Physically compatible | |
| Levofloxacine | 5 mg/mL | – | |||
| 12 | Cefazolin | 41.6 mg/mL | G5 % | Incompatible | Increase of UV absorbance |
| Nefopam | 0.16 mg/mL | NaCl 0.9 % | |||
| 13 | Cefazolin | 41.6 mg/mL | G5 % | Physically compatible | |
| Pantoprazole | 4 mg/mL | NaCl 0.9 % | |||
| 14 | Cefepim | 83.3 mg/mL | G5 % | Physically compatible | |
| Daptomycin | 21 mg/mL | NaCl 0.9 % | |||
| 15 | Cefepim | 83.3 mg/mL | G5 % | Physically compatible | |
| Thiamine (Bevitine®) | 0.2 mg/mL | NaCl 0.9 % | |||
| 16 | Cefepim | 83.3 mg/mL | G5 % | Physically compatible | |
| Voriconazole | 8 mg/mL | G5 % | |||
| 17 | Cefotaxime | 41.6 and 125 mg/mL | G5 % or NaCl 0.9 % | Physically compatible | |
| Heparin sodium | 208 UI/mL | NaCl 0.9 % | |||
| 18 | Cefotaxime | 83.3 mg/mL | G5 % | Incompatible | Increase of UV absorbance |
| Hydrocortisone | 2 mg/mL | NaCl 0.9 % | |||
| 19 | Cefotaxime | 83.3 mg/mL | G5 % | Physically compatible | |
| Insulin (Umuline®) | 1 UI/mL | NaCl 0.9 % | |||
| 20 | Cefotaxime | 83,3 mg/mL | G5 % | Physically compatible | |
| Levetiracetam | 20.83 mg/mL | NaCl 0.9 % | |||
| 21 | Cefotaxime | 41.6 mg/mL | G5 % | Physically compatible | |
| Ornidazole | 31.25 mg/mL | NaCl 0.9 % | |||
| 22 | Cefotaxime | 83.3 mg/mL | G5 % | Physically compatible | |
| Thiamine (Bevitine®) | 0.4 mg/mL | G5 % | |||
| 23 | Cotrimoxazole (Bactrim®) | 3.2/0.64 and 80/16 mg/mL | G5 % or none | Incompatible | Precipitation for undiluted Bactrim® (Figure 3) |
| Heparin sodium | 208 UI/mL | NaCl 0.9 % | |||
| 24 | Cotrimoxazole (Bactrim®) | 3.2/0.64 and 80/16 mg/mL | G5 % | Incompatible | Increase of UV absorbance |
| Insulin (Umuline®) | 1 UI/mL | NaCl 0.9 % | |||
| 25 | Daptomycin | 21 mg/mL | NaCl 0.9 % | Physically compatible | |
| Thiamine (Bevitine®) | 0.2 mg/mL | NaCl 0.9 % | |||
| 26 | Fluconazole | 2 mg/mL | – | Physically compatible | |
| Thiamine (Bevitine®) | 0.2 mg/mL | NaCl 0.9 % | |||
| 27 | Gentamicin | 4.17 mg/mL | G5 % | Physically compatible | |
| Nefopam | 0.16 mg/mL | NaCl 0.9 % | |||
| 28 | Linezolid | 2 mg/mL | – | Physically compatible | |
| Insulin (Umuline®) | 1 UI/mL | NaCl 0.9 % | |||
| 29 | Ornidazole | 31.25 mg/mL | NaCl 0.9 % | Physically compatible | |
| Heparin sodium | 208 UI/mL | NaCl 0.9 % | |||
| 30 | Ornidazole | 31.25 mg/mL | NaCl 0.9 % | Physically compatible | |
| Insulin (Umuline®) | 1 UI/mL | NaCl 0.9 % | |||
| 31 | Piperacillin/tazobactam | 166/20.83 mg/mL | G5 % | Physically compatible | |
| Insulin (Umuline®) | 1 UI/mL | NaCl 0.9 % | |||
| 32 | Piperacillin/tazobactam | 166/20.83 mg/mL | G5 % | Physically compatible | |
| Levetiracetam | 20.83 mg/mL | NaCl 0.9 % | |||
| 33 | Piperacillin/tazobactam | 166/20.83 mg/mL | G5 % | Physically compatible | |
| Thiamine (Bevitine®) | 0.4 mg/ml | G5 % | |||
| 34 | Spiramycine (Rovamycine®) | 0.0625 MUI/mL | NaCl 0.9 % | Physically compatible | |
| Heparin sodium | 208 UI/mL | NaCl 0.9 % | |||
| 35 | Vancomycin | 31.25 mg/mL | G5 % | Physically compatible | |
| Nefopam | 0.16 mg/mL | NaCl 0.9 % | |||
| 36 | Voriconazole (Vfend®) | 8 mg/mL | G5 % | Physically compatible | |
| Heparin sodium | 208 UI/mL | NaCl 0.9 % | |||
| 37 | Voriconazole (Vfend®) | 8 mg/mL | G5 % | Physically compatible | |
| Thiamine (Bevitine®) | 0.2 mg/mL | NaCl 0.9 % | |||
Figure 2 presents the large white precipitate occurring immediately after mixing amoxicillin with vancomycin. Figure 3 presents the precipitation formed after the mixture of cotrimoxazole with heparin sodium. This precipitation occurs after a 4-hour contact.
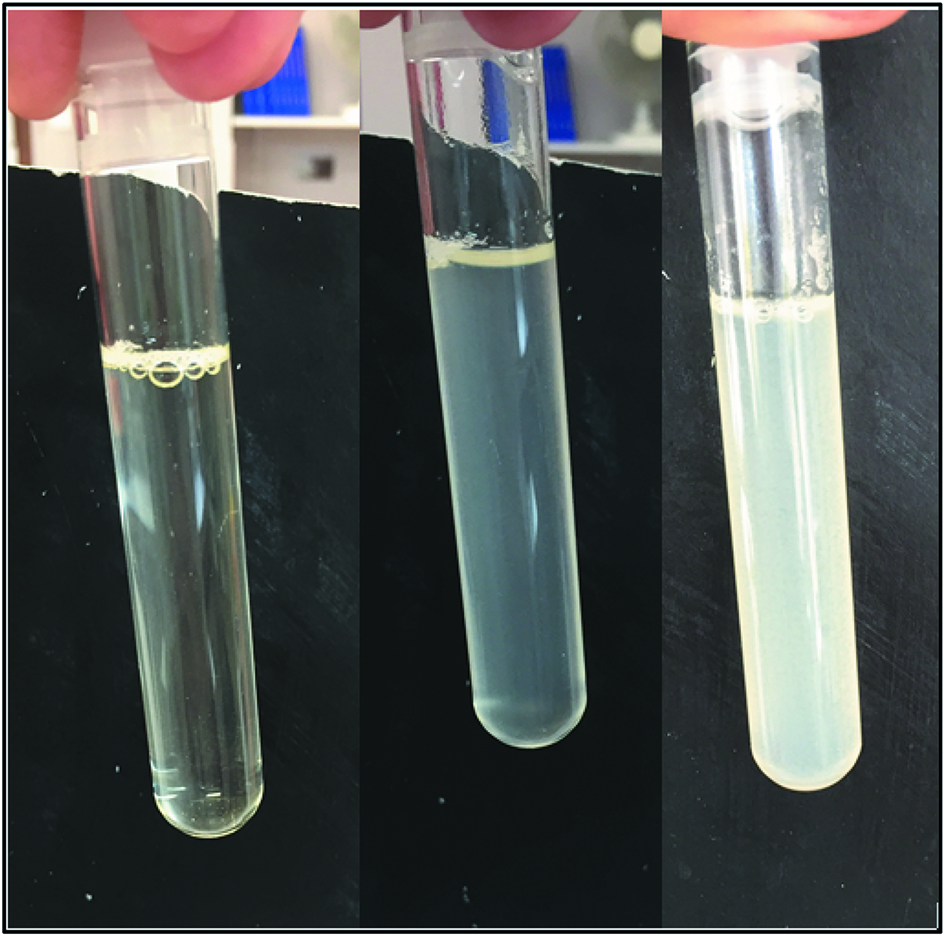
Mixture of amoxicillin (A) 20.83 mg/mL diluted in 5 % glucose with vancomycin (V) 31.25 mg/mL diluted in 5 % glucose after the mixture (left), after 5 minutes (middle) et after 30 minutes (right) (A:V (v:v): 5:5).
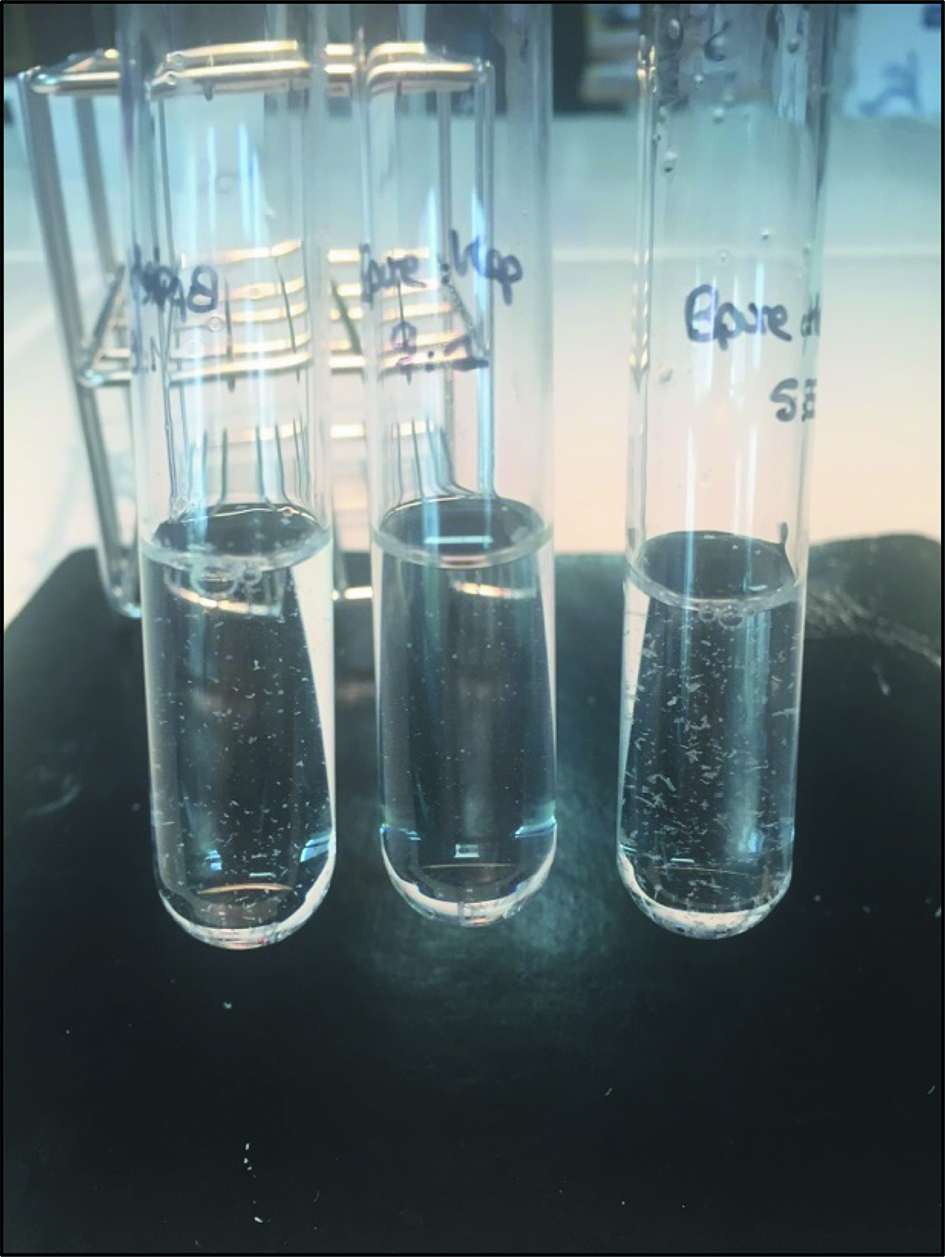
Mixture of cotrimoxazole (C) undiluted with heparin sodium (H) at 208 UI/mL diluted in 0.9 % sodium chloride after 4 hours (C:H (v:v): glass tube left to right, n°1: 1:9; n°2: 9:1; n°3: 5:5).
Discussion
Patients in ICUs are subjected to a high rate of incompatibilities.
Despite many physical compatibility studies published in the literature, physical compatibility data between injectable drugs are not always available.
Observational study
Patients admitted to ICUs constitute a high-risk population, with higher drug concentrations in the majority of cases than for other patients. The observational study has demonstrated that in the majority of cases, no physical compatibility data were available in the literature with only 22.9 % of the combinations documented as physically compatible and 8.0 % as incompatible. After laboratory tests, the percentage of combinations with missing data was 56.0 %. We obtained a final percentage of incompatible and physical compatible combinations of 9.8 % and 27.8 % respectively.
A previous similar study was performed in 2006 in the same hospital center in two ICUs. After consulting literature, Serrurier et al. had obtained 23 % of Y-site admixtures physically compatible, 5 % were physically incompatible, 2 % with conflict between two literature references and 70 % without any data about stability or compatibility. We can notice that the percentage of combinations without data has decreased by 14 %. On the other hand, a more important percentage of associations documented as incompatible are administered in this study compared to 2006 [15].
Review of observational studies performed in ICUs
In the literature, other studies have identified the percentage of drug incompatibilities in ICUs. We obtained a similar percentage of incompatibilities to that found in a retrospective ICU study performed by Bertsche et al. with 7.2 % of physically incompatible pairs administered [16]. Tardy et al. observed 12 % of incompatible combinations and 50 % of these incompatible combinations included an anti-infective drug [17]. Neininger et al. analysed prescriptions in pediatric ICUs and identified 10 % of incompatible drug administration [18]. The most common drugs involved were cefotaxime, pantoprazole and vancomycin. Kanji et al. conducted an observational study in thirteen ICUs and observed a prevalence of inappropriate associations in 18.7 % of patients with more than one drug infusion [19]. They also quantified the published physical and chemical stability data for drugs commonly used in continuous infusion in ICUs. Physical and/or chemical compatibility data existed for 54 % of the combinations with 15 % of incompatible combinations and 9 % of conflicting data [20]. Bertsche et al. have cited a publication of Kähny-Simonius et al. who observed that compatibility is unknown or ambiguous for up to 45 % of co-infusions in an ICU [16, 21].
A study obtained a more important percentage of incompatible combinations. Marisilio et al. found a percentage of incompatibilities in 68 % of prescriptions analysed in an ICU [22]. One study found a lower percentage of incompatibilities. After comparing clinical practices in ICUs with the literature, Humbert-Delaloye et al. obtained 4.8 % of incompatible combinations [23].
Data available in the literature
In this study, antibiotics are one of the most commonly used pharmacologic classes for Y-site infusion. This class is both the one that contains the most compatibility data available in the literature for responding to ICUs’ combinations, but also one of the classes in which the data was the most missing. This can be explained by the large number of molecules present in this class and used in ICUs. In our study, out of 77 molecules, 18 were antibiotics (23.4 %; N=18/77). The same observation can be made for the class of blood substitutes and perfusion solutions.
For the class of anticoagulants represented only by heparin sodium in this study, in the majority of cases, physical compatibility data are available in the literature to respond to the combinations performed in ICUs. For mineral supplements or anti-mycotics for systemic use, in the majority of cases, no compatibility data are available in the literature.
One of the limits of this study is that physical compatibility data in the literature often relate to drug pairs and the information available on triplets or quadruplets is limited. Overall, in clinical practice, more than two drugs are infused simultaneously by Y-site infusion or mixed in the same container. In this study, a mean of 3 drugs were mixed in the same container.
Incompatible combinations in our ICUs
Of the 31 combinations performed in ICUs and reported as incompatible in the literature, 9 combinations included heparin sodium (29.0 %; N=9/31). Stabilis® database reported 132 physical incompatibilities including heparin sodium [9]. Regarding the incompatible associations observed in ICUs, the incompatible association of heparin sodium with caspofungin has been demonstrated by Chan et al. with the formation of a large white precipitate immediately after contact between the two drugs [24]. For the combination of heparin sodium with vancomycin, the Summary of Product Characteristics of vancomycin reports this association as incompatible [25]. The association of heparin sodium with amiodarone has been demonstrated as incompatible by Perez Juan et al. [11] and Charlmers et al. [26].
We observed 8 incompatible combinations including pantoprazole (25.8 %; N=8/31). Pere et al. tested the compatibility of pantoprazole with other injectable drugs during Y-site infusion [27]. Among the mixtures observed in ICUs during our study, they had already observed a significant precipitation of the pantoprazole-amiodarone combination, a precipitation formed after 15 minutes for the association of pantoprazole with metoclopramide and a precipitation after one hour during the combination of pantoprazole with heparin or piperacillin/tazobactam. Neininger et al. had also highlighted the significant risk of precipitation with this molecule [18].
Of the 25 molecules observed in an incompatible combination, 9 anti-infective drugs were involved (caspofungin, cefazolin, cefotaxime, cefepim, cotrimoxazole, gentamicine, meropenem, metronidazole, vancomycin). The combination of cotrimoxazole with caspofungin has already been demonstrated to be incompatible, with the formation of a significant white precipitate formed immediately after the contact [24].
Mixtures of drugs in the same container in our ICUs
Physical compatibility data are not sufficient to evaluate a mixture and physico-chemical stability data are necessary. In the majority of cases, no information is available in the literature on the physicochemical stability of a mixture. Only two mixtures observed in ICUs have already been studied in the literature. Humbert-Delaloye et al. studied the mixture of 5 mg/mL dobutamine and 0.06 mg/mL norepinephrine [28]. This mixture was observed during our study but with higher concentrations of dobutamine (10 mg/mL) and norepinephrine (0.16 mg/mL). A new stability study should be performed to confirm the physicochemical stability with higher concentrations. Another mixture was also observed in ICUs, combining droperidol, ketamine and morphine sulfate. In the literature, a study evaluated the stability of a comparable mixture of droperidol, ketamine and fentanyl with a 30-day stability at 25 °C [29].
More than a third of the mixtures observed include thiamine (vitamin B1) (33.3 %; N=10/31), a molecule with limited stability data in literature. The mixture of nefopam with droperidol was observed in 12 different patients during this study, without any stability data published to date.
Laboratory tests
Physical compatibility tests on anti-infective drugs
In this work, we focused on laboratory tests including an anti-infective drug. Antibiotics and antifungal drugs are in the top 10 of the most-used pharmacological classes in Y-site infusion in ICUs, as well as in the top 10 of pharmacological class for which physical compatibility data are the most missing. Schneider et al. observed that parenteral drugs and particularly anti-infective agents were more frequently involved in administration errors than other compounds or forms of administration [30]. Bertsche et al. also observed that antibiotics were the most common incompatible pairs of drugs [4]. As demonstrated by Roberts et al. [31], continued administration of antibiotics such as β-lactams versus intermittent administration is associated with decreased hospital mortality in critically ill patients with severe sepsis. In contrast, continued administration of antibiotics may increase the risk of incompatibility.
Visually incompatible pairs
The duration of a physical compatibility study is different between the authors in the literature. Trissel et al. observed the tests at different intervals up to 4 hours [32]. As explained by Nemec et al., the in-line contact time between drugs in the Y-infusion is at most four hours [33].
In our study, for the majority of laboratory tests, pairs of drugs were physically compatible. Three pairs were visually incompatible: amphotericin B liposomal with vancomycin (pair 1), amoxicillin with vancomycin (pair 2) and undiluted cotrimoxazole with heparin sodium (pair 3). The precipitation occured immediately after mixing for pair 1, after 5 minutes for pair 2 and after 4 hours for pair 3. For each test performed with cotrimoxazole, we studied two concentrations used in ICUs: undiluted concentration for patients with fluid restriction and diluted concentration as recommended by the Summary of Product Characteristics. There was no visual precipitation for the diluted cotrimoxazole solution with heparin sodium but an increase of absorbance during the subvisual evaluation was observed. For cotrimoxazole, the risk of precipitation is higher with undiluted solution.
Subvisually incompatible pairs
No visual precipitation occurred but an increase of absorbance was observed after a 1-hour or a 4-hour contact. These combinations cannot be recommended safely.
Critical drugs
Of the three tests performed for cotrimoxazole, all were considered as incompatible. Two out of three tests performed on vancomycin were visually incompatible. Of the 7 tests performed on amoxicillin with another drug, five combinations were considered as incompatible.
Solutions and limits
Stabilis® has also created a tool for creating compatibility charts for clinicians [9]. This tool has already shown its usefulness [11, 34]. Conducting compatibility studies is one of the scientific missions of pharmacists [2, 35]. In the ICUs of our Hospital, the prescriptions are not fully computerised. The handwritten prescriptions are realised and accessible only in the care units, which limits the knowledge of all the drugs administered to a patient by the pharmacist. In addition, the administration scheme is not specified on the handwritten prescriptions.
One of the limitations of this study is that only physical compatibility has been evaluated. The absence of physical modification does not allow to conclude to the chemical stability. The concentrations of the drug and the degradation products have not been evaluated in our study. Another limitation is that the evaluation has been investigated only by pairs and the results cannot be extrapolated for mixtures of 3 drugs or more.
Additional Y-site compatibility studies have to be performed after this evaluation. A program of stability studies will be set up to evaluate the stability of frequently-used mixtures observed during this evaluation.
Conclusion
The present study provides new information on the physical compatibility of anti-infective drugs used in Y-infusion and on mixtures performed in ICUs. Physical compatibility data can contribute to safe medication practices.
This observational study highlighted that in most cases, physical compatibility data were missing from the literature and did not meet the clinical needs of ICUs.
The laboratory tests carried out focused on anti-infective drugs, one of the most-used pharmacological classes in ICUs care and represented by a large number of molecules. Many other compatibility tests need to be performed, especially on blood substitutes and perfusion solutions, mineral supplements and antifungal drugs. Mixture studies must also be performed, especially on vitamins, such as thiamine or pyridoxine. Critical molecules with high precipitation potential have been identified, including amoxicillin, cotrimoxazole, heparin sodium, pantoprazole and vancomycin.
In the absence of compatibility data, these drugs must be infused alone.
Acknowledgements
Thank you to Jacques Kuhnlé for reading through it all and making corrections. Thank you to Franck Blaise, Nathalie Sobalak and Hubert Zenier for their technical assistance and their help during this study.
Research funding: none.
Conflict of interest statement: The authors state no conflict of interest. The authors have read the journal’s Publication ethics and publication malpractice statement available at the journal’s website and hereby confirm that they comply with all its parts applicable to the present scientific work.
References
[1] Bardin C, Astier A, Vulto A, Sewell G, Vigneron J, Trittler R, et al. Guidelines for the practical stability studies of anticancer drugs: a European consensus conference. Ann Pharm Fr 2011;69:221–31.10.1016/j.pharma.2011.07.002Search in Google Scholar PubMed
[2] Guignard B, Gschwind L, Fonzo-Christe C. Les incompatibilités médicamenteuses en 2015: encore une mission du pharmacien d’établissement de santé ? Pharmactuel 2015;48:132–4.Search in Google Scholar
[3] Tatro DS. Drug interaction facts: the authority on drug interactions. St. Louis: Facts and Comparisons, 2006.Search in Google Scholar
[4] Bertsche T, Niemann D, Mayer Y, Ingram K, Hoppe-Tichy T, Haefeli WE. Prioritising the prevention of medication handling errors. Pharm World Sci 2008;30:907–15.10.1007/s11096-008-9250-3Search in Google Scholar PubMed
[5] Taxis K, Barber N. Incidence and severity of intravenous drug errors in a German hospital. Eur J Clin Pharmacol 2004;59:815–17.10.1007/s00228-003-0689-9Search in Google Scholar PubMed
[6] Tissot E, Cornette C, Demoly P, Jacquet M, Barale F, Capellier G. Medication errors at the administration stage in an intensive care unit. Intensive Care Med 1999;25:353–9.10.1007/s001340050857Search in Google Scholar PubMed
[7] Trissel LA. Handbook on injectable drugs, 19th ed. Bethesda, MD: American Society of Health-System Pharmacist, 2017.Search in Google Scholar
[8] Hecq JD. Stability of injectable drugs infusion, 36th ed. Belgium: Association belge des Pharmaciens Hospitaliers, 2018.Search in Google Scholar
[9] Vigneron J. Stabilis®. Available at: www.stabilis.org. Accessed: 2 Dec 2018.Search in Google Scholar
[10] Allen LV, Levinson RS, Phisutsinthop D. Compatibility of various admixtures with secondary additives at Y-injection sites of intravenous administration sets. Am J Hosp Pharm 1977;34:939–43.10.1093/ajhp/34.9.939Search in Google Scholar
[11] Perez Juan E, Maqueda Palau M, Amoros Cerda S, Arevalo Rubert M, Ribas Nicolau B. Compatibilité physique de médicaments administrés dans l’unité de soins intensifs. Pharmactuel 2015;48:146–2.Search in Google Scholar
[12] European Pharmacopeia 7.0. Chapter 2.2. Physical and physicochemical methods. 2.2.2: Degree of coloration of liquids. 22 p.Search in Google Scholar
[13] Williams OA, Middleton M, Henderson P, Reilly CS. Stability of morphine and droperidol separately and combined, for use as an infusion. Hosp Pharm Pract 1992;2:597–600.Search in Google Scholar
[14] Stewart JT, Warren FW, King DT, Venkateshwaran TG, Fox JL. Stability of ondansetron hydrochloride and 12 medications in plastic syringes. Am J Health-Syst Pharm 1998;55:2630–4.10.1093/ajhp/55.24.2630Search in Google Scholar PubMed
[15] Serrurier C, Chenot E-D, Vigneron J, May I, Demoré B. Assessment of injectable drugs’ administration in two intensive care units and determination of potential physico-chemical incompatibilities. EJHP 2006;12:96–9.Search in Google Scholar
[16] Bertsche T, Mayer Y, Stahl R, Hoppe-Tichy T, Encke J, Haefeli WE. Prevention of intravenous drug incompatibilities in an intensive care unit. Am J Health-Syst Pharm 2008;65:1834–40.10.2146/ajhp070633Search in Google Scholar PubMed
[17] Tardy C, Maison O, Faudel A, Sarfati L, Iroir G, Rioufol C, et al. Incompatibilités médicamenteuses physico-chimiques en unités de soins intensifs: états des lieux et mise en place de mesures préventives. Le Pharmacien Hospitalier et Clinicien 2017;52:18.10.1016/j.phclin.2017.01.047Search in Google Scholar
[18] Neininger MP, Buchholz P, Frontini R, Kiess W, Siekmeyer W, Bertsche A, et al. Incompatible intravenous drug combinations and respective physician and nurses knowledge: a study in routine paediatric intensive care. Eur J Hosp Pharm 2017. Published Online First: 24 July 2017. doi: 10.1136/ejhpharm-2017-001248Search in Google Scholar PubMed PubMed Central
[19] Kanji S, Lam J, Goddard RD, Johanson C, Singh A, Petrin L, et al. Turgeon AF: inappropriate medication administration practices in Canadian adult ICUs: a multicenter, cross-sectional observational study. Ann Pharmacother 2013;47:637–43.10.1345/aph.1R414Search in Google Scholar PubMed
[20] Kanji S, Lam J, Johanson C, Singh A, Goddard R, Fairbairn J, et al. Sytematic review of physical and chemical compatibility of commonly used medications administered by continuous infusion in intensive care units. Crit Care Med 2010;38:1890–8.10.1097/CCM.0b013e3181e8adccSearch in Google Scholar PubMed
[21] Kähny-Simonius J. Drug incompatibilities. Problems in the simultaneous administration of drugs in infusions. Schweiz Rundsch Med Prax 1993;82:1320–7.Search in Google Scholar
[22] Marsilio NR, Da Silva D, Bueno D. Drug incompatibilities in the adult intensive care unit of a university hospital. Res Bras Ter Intensiva 2016;28:147–53.10.5935/0103-507X.20160029Search in Google Scholar PubMed PubMed Central
[23] Humbert-Delaloye V. Administration des médicaments par voie intraveineuse aux soins intensifs adultes: évaluation et validation des pratiques par la littérature et des essais en laboratoire. Thèse de doctorat: Univ. Genève, 2012, no. Sc. 4440.Search in Google Scholar
[24] Chan P, Heatherly K, Kupiec TC, Trissel LA. Compatibility of caspofungin acetate injection with other drugs during simulated Y-site co-administration. Int J Pharma Compound 2008;12:276–8.Search in Google Scholar
[25] Vancomycin 1000 mg. Powder for Concentrate for Solution for Infusion. Summary of Product Characteristics. Updated November-2018. Sandoz.Search in Google Scholar
[26] Chalmers JR, Bobek MB, Militello MA. Visual compatibility of amiodarone hydrochloride injection with intravenous drugs. Am J Health Syst Pharm 2001;58:504–6.10.1093/ajhp/58.6.504Search in Google Scholar PubMed
[27] Péré H, Chassé V, Forest JM, Hildgen P. Compatibilité du pantoprazole injectable lors d’administration en Y. Pharmactuel 2004;37:193–6.Search in Google Scholar
[28] Humbert-Delaloye V, Berger-Gryllaki M, Voirol P, Gattlen L, Pannatier A. In vitro compatibility of various cardioactive drugs during simulated Y-site administration. EJHP 2013;20:110–16.10.1136/ejhpharm-2012-000239Search in Google Scholar
[29] Lee DKT, Wang DP, Harsono R, Wong CY. Compatibility of fentanyl citrate, ketamine hydrochloride, and droperidol in 0.9 % sodium chloride injection stored in polyvinyl chloride bags. Am J Hosp Pharm 2005;62:1190–2.10.1093/ajhp/62.11.1190Search in Google Scholar PubMed
[30] Schneider MP, Cotting J, Pannatier A. Evaluation of nurses’errors associated in the preparation and administration of medication in a pediatric intensive care unit. Pharm World Sci 1998;20:172–82.10.1023/A:1012087727393Search in Google Scholar
[31] Roberts JA, Abdul-Aziz MH, Davis JS, Dulhunty JM, Cotta MO, Myburgh J, et al. Continuous versus intermittent β-Lactam infusion in severe sepsis. A Meta-analysis of individual patient data from randomized trials. Am J Respir Crit Care Med 2016;194:681–1.10.1164/rccm.201601-0024OCSearch in Google Scholar PubMed
[32] Trissel LA, Martinez JF. Physical compatibility of melphalan with selected drugs during simulated Y-site administration. Am J Hosp Pharm 1993;50:2959–63.10.1093/ajhp/50.11.2359Search in Google Scholar
[33] Nemec K, Kopelent-Frank GR. Standardization of infusion solutions to reduce the risk of incompatibility. Am J Health-Syst Pharm 2008;65:1648–54.10.2146/ajhp070471Search in Google Scholar PubMed
[34] Compatibilité des médicaments injectables administrés en Y. Update August 18th, 2018. Hôpitaux universitaires de Genève. Available at: https://pharmacie.hug-ge.ch/infomedic/utilismedic/HUG_CompatAdm_DCI.pdf. Accessed: 2 Dec 2018.Search in Google Scholar
[35] Vigneron J. Stability studies: a scientific mission of the hospital pharmacist. Pharm Technol Hosp Pharm 2017;2:143–4.10.1515/pthp-2017-0032Search in Google Scholar
Article note
Preliminary data accepted as a poster during the 24th EAHP Congress in Barcelona, Spain, in March 2019.
© 2019 Walter de Gruyter GmbH, Berlin/Boston
Articles in the same Issue
- Frontmatter
- Editorial
- The Importance of a Scientific Journal in the Field of Pharmaceutical Technology in Hospitals
- Review
- Investigation of Drug-Packaging Interactions with Mass Spectroscopy Detectors: A Meta-Synthesis of the Literature
- Research Articles
- Automation of Aseptic Sterile Preparation: Risk Analysis and Productivity Comparison with Manual Process
- Physical Compatibility of Intravenous Drugs Commonly Used in Intensive Care Units: An Observational Study and Physical Compatibility Laboratory Tests on Anti-Infective Drugs
- Short Communication
- Assessment of an Online Training Tool for the Automated Unit-Dose Dispensing System (ADS) Process
- Corrigendum
- Corrigendum to: Formulation of a 3-months Stability Oral Viscous Budesonide Gel and Development of an Indicating Stability HPLC Method
Articles in the same Issue
- Frontmatter
- Editorial
- The Importance of a Scientific Journal in the Field of Pharmaceutical Technology in Hospitals
- Review
- Investigation of Drug-Packaging Interactions with Mass Spectroscopy Detectors: A Meta-Synthesis of the Literature
- Research Articles
- Automation of Aseptic Sterile Preparation: Risk Analysis and Productivity Comparison with Manual Process
- Physical Compatibility of Intravenous Drugs Commonly Used in Intensive Care Units: An Observational Study and Physical Compatibility Laboratory Tests on Anti-Infective Drugs
- Short Communication
- Assessment of an Online Training Tool for the Automated Unit-Dose Dispensing System (ADS) Process
- Corrigendum
- Corrigendum to: Formulation of a 3-months Stability Oral Viscous Budesonide Gel and Development of an Indicating Stability HPLC Method

