Abstract
Background
The production of hospital-compounded medicines with a longer shelf life raises questions about drug-packaging interactions, especially desorption events involving extractables and leachables (E/L). A meta-synthesis of the literature was performed to describe which mass spectrometer is suitable for identifying and quantifying E/L.
Methods
A meta-synthesis of studies focused on the identification or quantification of E/L published between January 1997 and December 2017 was performed. Inclusion criteria were E/L studies dealing with pharmaceutical products, in which mass spectrometry (MS) coupled to liquid chromatography (LC) or gas chromatography (GC) was used. The full-text articles had to be available and written in English. Articles about food packaging, environmental contamination, counterfeit compounds, pharmacokinetics, or process-related impurity studies were excluded. Two researchers independently assessed the papers according to a score based on a seven-item questionnaire.
Results
In total, 32 papers matched our criteria and were included in the meta-synthesis. For qualitative analysis with LC, quadrupole time-of-flight (QTOF; n=4) and ion trap (n=4) mass detectors were used the most; and with GC, single quadrupole (n=8). For quantification studies with LC, QTOF (n=3) and triple quadrupole (n=2) were used the most; and with GC, single quadrupole (n=7).
Conclusions
For simultaneous qualitative and quantitative analysis of E/L with LC, QTOF or Orbitrap is a suitable detector. For quantitative analysis with LC only, triple quadrupole is suitable. For qualitative and quantitative analysis with GC, single quadrupole can be used.
Introduction
Pharmaceutical compounding has changed remarkably in hospital pharmacies in recent years. In fact, compounded products with a longer expiration date, such as ready-to-use syringes or chemotherapy dose banding, have been introduced in hospitals worldwide [1, 2, 3, 4]. Because the shelf-life of compounded products is increasing, it raises questions about drug-packaging interactions such as sorption and desorption [5].
Indeed, in hospital pharmacy, the choice of the container is mainly based on clinical needs and not, as in pharmaceutical industry, by taking account of the risk of primary packaging interactions with the product.
Sorption is generally assessed during stability studies of drug products. Conversely, desorption is rarely studied. However, there is a significant risk that substances migrate from the container into the drug product [6]. These substances are called extractables and leachables (E/L). Extractables correspond to substances that migrate from the packaging into the drug solution under extreme chemical and physical conditions (such as high temperature, organic solvents, etc.), whereas leachables are defined as substances that migrate from the packaging into the drug product under normal conditions [6].
E/L can be toxic or cause adverse effects in patients [6]. This problem is well exemplified by the Eprex® (epoetin alpha) case. The leaching of a substance from the rubber stopper led to a change in this product’s composition, which caused the aggregation of epoetin alpha, resulting in red cell aplasia in several patients [7]. Another paper described cases of nausea that might have been due to several leachables originating from sodium chloride-prefilled syringes used to flush IV tubing [8].
E/L may also cause compatibility concerns. Oxidation, precipitation, or aggregation phenomena have been observed and reported. One author observed a cloudy appearance in ceftriaxone sodium for injection that was due to a leachable compound named butylated hydroxytoluene [9]. Thus, based on these examples, it seems that E/L might affect the quality and safety of compounded products. Therefore, E/L should be a major concern in our hospital-compounded drug products. However, unlike to the marketed products for which regulatory law about drug-packaging interaction is strict, there are no mandatory specifications in the regulation for hospital preparations [10].
Several E/L can be detected in the same sample and may have very different physicochemical properties. They may be volatile or not, of an organic or inorganic nature, etc [11]. They are also very different in terms of structure; for example, they can be phenols, aromatic compounds, fatty acids, etc [12]. Because the E/L profile cannot be predicted, qualitative and structural analyses are required in order to be able to fully characterize every E/L that might be present in the sample [12]. Moreover, E/L are found at trace levels [11]. Therefore, a sensitive detector must be used for the quantitative analysis [12].
For the analysis of semivolatile and nonvolatile substances, liquid chromatography coupled to mass spectrometry (LC/MS) is frequently used for E/L studies; while for volatile compounds, gas chromatography coupled to MS (GC/MS) is the most common [11]. MS is frequently used for E/L analysis because it allows structural, qualitative, and quantitative analyses [12]. In fact, MS offers the possibility of identifying unknown compounds thanks to the existence of MS/MS spectral libraries [13].
A mass spectrometer is also very sensitive, which allows the quantification of E/L at trace levels [11]. As there are several types of mass spectrometers available (single quadrupole, quadrupole time-of-flight (QTOF), etc.), it is not straightforward to determine which mass spectrometer would be suitable for E/L analysis. To the best of our knowledge, there are no published studies describing which type of mass spectrometer should be used for E/L identification and quantification studies.
Therefore, by using a meta-synthesis approach, the aims of this study were to examine critically all identification and quantification studies involving E/L that have been published over the past twenty years and to discuss which type of mass spectrometer coupled to GC or LC would be suited to carry out E/L studies with a broad range of identification and quantification capacities. In most cases, the studied substances are not well known.
Methods
A meta-synthesis aims at selecting, comparing, and analyzing qualitative data or studies in order to better understand a specific topic [14, 15]. A meta-synthesis can be divided into five major steps [14, 15] : 1) the framing step, in which the research question is clearly defined; 2) the searching step, in which a search of articles with specific keywords and in several databases is carried out; 3) the screening step, in which the eligibility of the searched articles in the meta-synthesis is examined; 4) the quality appraisal step, in which the quality of the articles retained in the meta-synthesis is evaluated; and 5) the synthesis step, in which the qualitative data are sorted and analyzed. Our meta-synthesis obeyed the above procedure, as detailed below [14, 15].
Framing
In this study, we focused on E/L identification and quantification studies of pharmaceutical products or materials in which GC/MS or LC/MS was used for the analysis. The qualities of the structural, qualitative, and quantitative analyses of E/L by different types of mass spectrometers coupled to LC or GC were compared. A discussion about which type of mass spectrometer is suitable for E/L identification and quantification was then realized.
Searching
The following inclusion criteria were defined for the literature search: 1) the studies under selection had to focus exclusively on the E/L of a pharmaceutical product or material; 2) GC/MS or LC/MS had to be used in the analysis; 3) the packaging material tested as well as the type and model of the mass spectrometer used had to be described in the article; and 4) the article had to be published between January 1997 and December 2017. We decided to exclude papers dealing with food packaging, environmental contamination, counterfeit products, adulterants, process-related impurities, or pharmacokinetics.
The literature search was done in the PubMed database (with Mesh terms or keywords) and in Scifinder (with keywords) by using the following queries: 1) leachable, or leachables and extractable, or extractables; 2) drug contamination, and drug packaging; and 3) plasticizers/analysis and pharmaceutical solutions.
A total of 5333 papers were found to comply with our selection criteria. In order to refine the search results, the following border conditions were added to the queries: 1) the criteria “gas chromatography mass spectrometry or chromatography, high pressure liquid chromatography, liquid” were implemented to the three queries; 2) the articles had to be written in English; 3) the full text of the articles had to be available; and 4) duplicates were removed. The inclusion of these additional criteria restricted our search results to 73 papers.
Screening
We then reviewed the titles and abstracts of the 73 papers to determine which articles were eligible for the meta-synthesis. When the abstract summary was not sufficient to decide the suitability of an article, its full text was studied. In this step, 49 articles were identified as unsuitable for the following reasons: 1) The articles were about food packaging, environmental contamination, counterfeit products, adulterants, process-related impurities, stability, or pharmacokinetics; 2) it was a general review about E/L with no case study; 3) no mass detector was used for identifying or quantifying E/L; 4) the type and the model of the mass spectrometer used were not available; and 5) the material tested was not specified. This led us to a selection of 24 articles retained for the literature review. The references of these 24 articles were examined, and the E/L papers listed were also analyzed according to our protocol, yielding 9 additional papers meeting our selection criteria. In total, the meta-synthesis features 33 articles.
Quality appraisal
The relevance and the credibility of the 33 retained papers were assessed independently by two hospital pharmacist researchers by using a score based on seven binary questions inspired from the Critical Appraisals Skills Programme (CASP) Qualitative Checklist [14, 15, 16]. For some questions, subquestions were added to clarify the main question. The questions and subquestions were as follows:
Was the purpose of the study clear?
Is the aim of the research relevant? (Major)
Was the methodology appropriate?
Is it clear that the paper describes an identification or quantification study about E/L in a pharmaceutical product or material and that the analysis was done by LC/MS or GC/MS? (Major)
Was the research design appropriate to address the aims of the research?
Is the pharmaceutical material or product clearly defined?
Are the types and the model of the detector used specified? (Major)
Are the analytical conditions clearly described?
Are the screened E/L specified and are there E/L from the pharmaceutical container or material? (Major)
Did the results address the objectives of the research?
Could E/L have been identified or quantified by LC/MS or GC/MS? (Major)
If there was a change in the method, did the authors explain why this was done? (In case of a change, major)
Were the results strong enough?
Is the method validated? (For quantification studies, major)
b. Are the results of identification confirmed? (For identification studies, major)
Did the discussion and conclusion address the objectives of the research?
Is the research valuable?
Do the results and discussion provide a research perspective? (Major)
Do they propose new research fields?
A positive answer to a question gave one point, and a negative answer gave zero points [14, 15]. A positive answer to the subquestions considered as major was mandatory to get the point of the question. To be retained, an article needed to score at least four points [14, 15]. After independently assessing the quality of the papers, the score given for each article was compared and discussed by the two researchers. A consensus overall score was then assigned to each article. One article did not reach the minimum score of four points. In total, 32 papers were used for the meta-synthesis.
Synthesis
A summary of the different steps followed to determine whether papers were to be included in the meta-synthesis is shown in Figure 1. To help focus our discussion on the qualitative and structural analysis of E/L by GC/MS or LC/MS, the identification studies were grouped into Table 1 and the quantification studies were grouped into Table 2. For each of the selected articles, the raw material or packaging tested, the composition of the packaging tested, the equipment, the type of the mass spectrometer used, and the score obtained for the quality appraisal are provided.
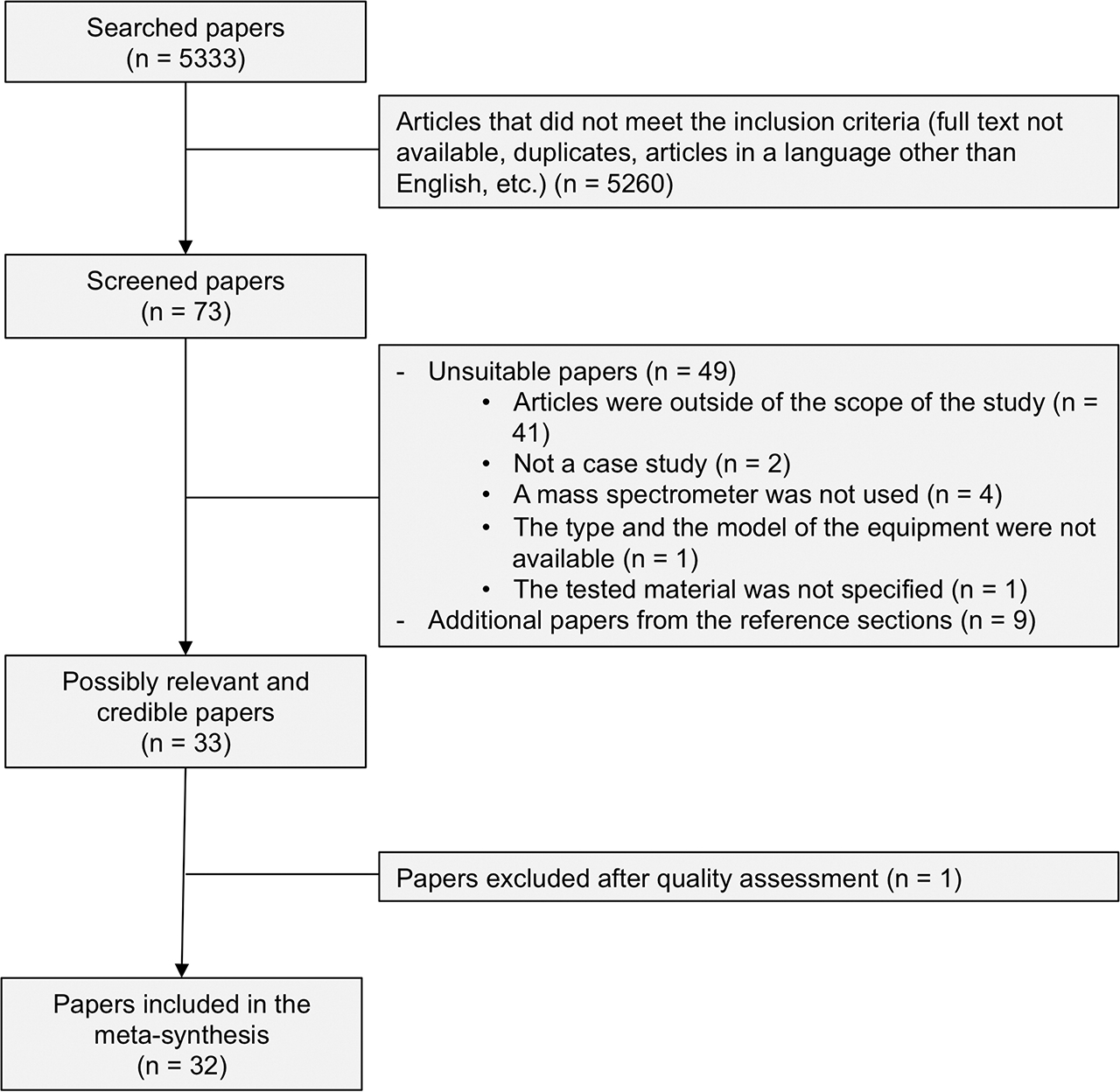
Summary diagram.
Articles included in the meta-synthesis of the identification studies.
| Article | Raw material/ packaging | Composition | Equipment | Type of MS | Score |
|---|---|---|---|---|---|
| Wei, 2017 [17] | Rubber stopper | Chlorinated butyl rubber | GC/MS and LC/MS/MS | Single quadrupole and QTOF | 7 |
| Petrusevski, 2016 [18] | Bottle and caps | LDPE and PP | LC/MS/MS | Ion trap | 6 |
| Valente, 2016 [19] | Syringe and rubber stopper | Type I borosilicate glass and rubber | LC/MS/MS | Orbitrap + ion trap | 7 |
| Zdravkovic, 2015 [20] | Bag | PVC | GC/MS | Single quadrupole | 6 |
| Jenke, 2013 [21] | Raw material | PVC, brominated isobutylene-isoprene, LDPE, PC, COC | GC/MS, LC/MS, LC/MS/MS, and Headspace GC/MS | GC/MS: 2 single quadrupoles and TOF, LC/MS: single quadrupole, TOF, ion trap + FT-ICR, QTOF and ion trap | 7 |
| Norwood, 2013 [11] | Raw material | COC | LC/MS | TOF | 7 |
| Chang, 2010 [22] | Bag + stopper | PVC + rubber and PO + rubber | LC/MS/MS | Orbitrap | 6 |
| Nashed-Samuel, 2010 [23] | Syringe | Glass (quality of glass not available) | LC/MS/MS | Ion trap | 5 |
| Corredor, 2009 [24] | Rubber closure | Rubber | LC/MS | Single quadrupole | 5 |
| Pan, 2008 [25] | Bottle | LDPE | LC/MS/MS and GC/MS/MS | Ion trap and dual stage quadrupole | 6 |
| Maus, 2007 [26] | Foam | PE | GC/MS/MS | Quadrupole ion trap | 5 |
| Pang, 2007 [7] | Stopper and prefilled syringe | Rubber | GC/MS and LC/MS/MS | Single quadrupole and QTOF | 6 |
| Zhao, 2007 [9] | Vial + septum | Rubber | GC/MS | Ion trap | 6 |
| Jenke, 2005 [27] | Film | Polyolefin | GC/MS and LC/MS/MS | Triple quadrupole, single quadrupole, double focusing magnetic sector mass spectrometer, and QTOF | 6 |
| Nassar, 2005 [28] | Glass vial, aluminium cap, and septum | Type I flint glass vial + aluminium cap + rubber | LC/MS/MS and LC/MS | Ion trap and TOF | 4 |
| Zhang, 2004 [13] | Rubber closures | Rubber | GC/MS | Ion trap and single quadrupole | 7 |
| Milano, 1999 [29] | Rubber stopper | Rubber | GC/MS/MS | Ion trap | 4 |
| Wahl, 1999 [30] | Plastic tubing | PVC | GC/MS | Single quadrupole | 6 |
Articles included in the meta-synthesis of the quantification studies.
| Article | Raw material/ packaging | Composition | Equipment | Type of MS | Score |
|---|---|---|---|---|---|
| Dorival-Garcia, 2017 [6] | Single-use bag | EVA + PE + LLDPE + LDPE + ULDPE | LC/MS/MS | QTOF | 5 |
| Bourdeaux, 2016 [31] | Medical device | PVC | GC/MS | Single quadrupole | 7 |
| Moreta, 2015 [32] | Film, multilayer packaging made of PE, aluminium foil, polyester, or paper | PE, aluminum foil, polyester and paper | LC/MS/MS | QTOF | 6 |
| Gimeno, 2014 [33] | 1) Medicaldevice2) Raw material | PVC | GC/MS/MS | Triple quadrupole | 5 |
| Pahl, 2014 [34] | Bag | Various films | LC/MS + GC/MS and Headspace GC/MS | Single quadrupole | 5 |
| Zdravkovic, 2014 [35] | IV bag, bottle, metered dose inhaler | PVC, PP, acetal, polyester, ethylene propylene diene, monomer rubber, stainless steel | LC/MS | Single quadrupole | 5 |
| Pouech, 2013 [36] | Raw material | PP, Poly-cyclo-olefin, and copolyester | LC/MS/MS | Triple quadrupole/ion trap | 7 |
| Reisinger, 2013 [37] | Raw material | PP and PE | LC/MS/MS | Triple quadrupole and QTOF | 4 |
| Strac, 2013 [38] | Bag | PVC | GC/MS | Single quadrupole | 6 |
| Yamaji, 2012 [39] | Ampoule | PE | GC/MS | Single quadrupole | 6 |
| Soeborg, 2006 [40] | Tube | Epoxy phenol lacquered aluminium | LC/MS/MS | Triple quadrupole | 7 |
| Garrido-Lopez, 2005 [41] | Films | PE | LC/MS | Single quadrupole | 5 |
| Story, 2005 [42] | Bag | PVC | GC/MS | Single quadrupole | 4 |
| Loff, 2004 [43] | Perfusion lines | PVC with DEHP, PVC DEHP-free, PE, PVC with DEHP/PU, and PVC with DEHP/PE | GC/MS | Single quadrupole | 5 |
Results
Among the 32 articles included in this meta-synthesis of E/L contamination, 18 identification studies and 14 quantification studies were flagged (Tables 1–2) [6, 7, 9, 11, 13, 17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43].
Equipment and detectors used for E/L identification
Among the E/L identification studies, LC/MS was used for the analyses in seven studies, GC/MS was used in six studies, and both GC/MS and LC/MS were employed together in five studies (Table 1). In particular, LC/MS was utilized in 6 studies and LC/MS/MS was utilized in 12 studies (Table 3). Meanwhile, GC/MS/MS was only used in 3 studies, while GC/MS was employed in 12 studies (Table 3).
Equipment and detector inventory list for the identification studies.
| IDENTIFICATION | |||
|---|---|---|---|
| Equipment | LC/MS (n = 18) | GC/MS (n = 15) | |
| Type of detector | Number of studies using this type of detector | Number of studies using this type of detector | |
| MS | Single quadrupole | 2 | 8 |
| Ion trap | 1 | 2 | |
| TOF | 3 | 1 | |
| Double focusing mass spectrometer | 0 | 1 | |
| MS/MS | QTOF | 4 | 0 |
| Orbitrap | 1 | 0 | |
| Triple quadrupole | 1 | 0 | |
| Quadrupole/ion trap | 0 | 1 | |
| Ion trap | 4 | 1 | |
| Ion trap-Orbitrap | 1 | 0 | |
| Ion trap-FT-ICR | 1 | 0 | |
| Dual stage quadrupole | 0 | 1 | |
| Total | 18 | 15 | |
Equipment and detectors used for E/L quantification
Among the E/L quantification studies, LC/MS was used for the analyses in seven studies, GC/MS was used in six studies, and both GC/MS and LC/MS were employed together in one study (Table 2). In particular, LC/MS was utilized in three studies and LC/MS/MS was utilized in six studies (Table 4). Meanwhile, GC/MS/MS was used in only one study, and GC/MS was employed in seven studies (Table 4).
Equipment and detector inventory list for the quantification studies.
| QUANTIFICATION | |||
|---|---|---|---|
| Equipment | LC/MS (n = 9) | GC/MS (n = 8) | |
| Type of detector | Number of studies using this type of detector | Number of studies using this type of detector | |
| MS | Single quadrupole | 3 | 7 |
| MS/MS | QTOF | 3 | 0 |
| Triple quadrupole | 2 | 1 | |
| Triple quadrupole/ion trap | 1 | 0 | |
| Total | 9 | 8 | |
Discussion
Equipment and detectors used for E/L identification
LC/MS for E/L identification
Identifying E/L and their degradation products is a critical issue, given that ignoring the identity of the compounds of interest prevents a proper safety assessment. Identification studies are facilitated by the development of new technologies such as MS/MS, especially for untargeted substances [44, 45]. Due to its ability to generate precursor ions and product ions, this technology enables one to obtain much more structural information about E/L than with MS alone [45]. The specificity is higher with MS/MS because the generation of a product ion enables the separation of two compounds with the same molecular weight, which is not possible with MS alone [46]. This may explain why MS/MS has been used in most identification studies. Indeed, among the identification studies in our meta-synthesis, analysis was performed by MS/MS in 12 studies and by MS in 6 studies.
It became more common to use LC/MS/MS, compared with LC/MS, in identification studies after 2007. This corresponds to the launching of MS/MS databases specifically for E/L, such as the E/L Safety Information Exchange database (ELSIE) founded in 2007 [47, 48]. The ELSIE database contains safety data on E/L and E/L profiles [47, 48]. The marketing of MS/MS spectral libraries by instrument suppliers in recent years also has provided a helpful tool for compound identification. In fact, the databases suggest a potential molecular structure for each compound of interest based on the accurate masses of precursor and product ions. It is noteworthy that the analyzed papers were published in the last twenty years and even the most of them in the last ten years. The development of the databases was quite spectacular but only for some providers.
Among the identification studies using LC/MS, an ion trap (n=4) or a QTOF (n=4) mass detector was used the most frequently. In addition, an Orbitrap detector (n=2) was used for the identification of E/L. Based on these results, we wondered which mass spectrometer would have the capacity to identify E/L and what the difference would be between these three detectors.
Comparison of QTOF with ion trap mass detection
To date, no identification studies of E/L have compared ion trap and QTOF mass detection. In the articles included in this meta-synthesis, the identification of E/L with only ion trap detection coupled to LC was successful in one study out of four [18, 23, 25, 28]. Petrusevski et al. successfully identified two E/L (Irganox 1010 and Irgafos 168) with an ion trap mass detector [18]. In the three other articles using ion trap detection, the identification of E/L was possible by using other techniques such as nuclear magnetic resonance (NMR), Fourier-transform infrared spectroscopy, and GC/MS [23, 25, 28].
The identification of E/L with only QTOF mass detection was not possible in any of the articles included in this meta-synthesis [7, 17, 21, 27]. However, much structural information could be obtained with QTOF mass detection. For example, Pang et al. described a tentative structural identification of 2,2’-thiobis[6-(1,1-dimethylethyl)-4-methyl] phenol with QTOF mass detection [7]. Because no reference standard was available, the identity of this substance could not be confirmed [7]. Nevertheless, this discovery helped to identify the source of the leachable component: it came from additives used in rubber processing [7].
Based on these results, it appears that the identification of E/L compounds with QTOF mass detection coupled to LC is difficult. This is due to the fact that the bond connectivity cannot be completely solved with QTOF mass detection [17]. Therefore, the identification of an unknown compound cannot be achieved without a reference standard to confirm its identity [17]. Moreover, the identification of compounds with QTOF is complex for constitutional isomers and stereoisomers due to the absence of some structural information [49].
Thanks to the ability of ion trap mass detection to do multiple fragmentations of precursor ions, a more complex structure can be solved with ion trap MS than with QTOF MS [49]. The identification of isomers or unknown compounds may be feasible [49]. However, ion trap mass detection has a lower resolution than QTOF mass detection, which may compromise the identification processes due to a low mass accuracy measurement [49].
Based on the information found in the literature, it can be concluded that both QTOF and ion trap mass detection are techniques that contribute to the identification and confirmation of E/L [49]. Using only QTOF or ion trap mass detection may not be sufficient for the full identification of an unknown E/L. However, the limits and abilities of these two techniques are complementary [49]. Combining an accurate mass measurement with multiple fragmentations might help to solve the molecular structure of an unknown compound [49].
Comparison of QTOF with orbitrap mass detection
The identification of E/L with Orbitrap mass detection was possible [19, 22]. In fact, for example, Chang et al. identified 2-mercapto-benzothiazole thanks to the accurate mass and fragmentation pattern given by the Orbitrap mass detector [22].
QTOF and Orbitrap are both high-performance detectors suitable for identification studies of E/L [50]. Orbitrap is well known for its high mass resolving power (70,000 at m/z 200 for Qexactive focus, Thermofisher), but it has a slower scan acquisition [50]. Moreover, its scanning speed is inversely proportional to the resolution, which means that the faster the scan acquisition, the lower the resolution [50]. QTOF mass detection has a lower resolving power (up to 40,000) than Orbitrap mass detection, but its resolving power is independent of the acquisition rate [51].
Choosing between a QTOF and an Orbitrap mass detector is a difficult dilemma because both detectors are able to identify compounds accurately [50]. Several studies comparing the performance of these two detectors have been presented in the literature, but none was specific to E/L identification [50, 51, 52]. A study on forensic hair analysis concluded that the resolution of Orbitrap and QTOF mass detectors was sufficient to differentiate tetrahydrocannabinol from isobaric ions [52].
Thus, it appears that there is not much of a difference in the performance between an Orbitrap and a QTOF mass detector. The choice between these two detectors for the identification of E/L may depend on others factors. Nevertheless, the main criteria are the existence and the quality of the E/L libraries provided by the supplier.
GC/MS for E/L identification
Many GC/MS libraries exist, such as the National Institute of Standards and Technology (NIST) library, and are used for the identification of unknown compounds [53]. In the present meta-analysis, the NIST database was used in seven articles to identify or confirm the identity of E/L analyzed by GC/MS
The results of our literature review demonstrated that triple quadrupole mass detection is rarely used to identify E/L and that single quadrupole mass detection is the most frequently used detector combined with GC for identification studies (n=8). This finding coincides with the results of comparable literature reviews [54, 55]. In fact, several literature reviews of pesticide residue analysis in food confirm that GC coupled to a single quadrupole mass detector is the most frequently used equipment for identification studies [55, 56, 57]. Indeed, more than half of the cited articles used GC coupled with a single quadrupole mass detector to identify pesticide residues [55, 56, 57]. This might be due to the fact that single quadrupole detectors are robust, affordable, and sensitive, especially in the selected ion monitoring mode [53]. Thus, using GC coupled to a single quadrupole mass detector for the identification of E/L seems to be the most reasonable choice.
Equipment and detectors used for E/L quantification
LC/MS for E/L quantification
Similar to the studies on the identification of E/L, LC/MS/MS was a more frequently used technique than LC/MS. The most frequently used MS/MS detectors in quantification studies of E/L are QTOF (n=3) and triple quadrupole (n=2).
Comparison of QTOF with triple quadrupole mass detection
There are no studies available in the literature that specifically compare the quantification of E/L with triple quadrupole and QTOF mass detection. Reisinger et al. simultaneously used QTOF and triple quadrupole mass detection to analyze E/L substances (Tin622, Tin770…) [37]. In this paper, the limit of quantification (LOQ) was much lower with triple quadrupole mass detection than with QTOF mass detection [37]. In fact, the LOQ with triple quadrupole mass detection was ten times lower than with QTOF mass detection for the analysis of Tin770 [37]. This corresponds to the literature findings for studies with similar applications (mostly regarding food or water contamination).
Several studies mention that a better dynamic range and lower detection limits are observed with triple quadrupole mass detection [58, 59]. One study shows that the results were more repeatable when using triple quadrupole mass detection [59]. However, all comparative studies found in the literature conclude that triple quadrupole mass detection yields better results only for the quantitative analysis of targeted compounds and that it cannot provide some qualitative information [58, 59, 60, 61, 62]. In fact, in contrast with QTOF, with a triple quadrupole mass detector, there is no possibility of obtaining structural information about unknown compounds [58, 59, 60, 61, 62]. As discussed above, QTOF mass detection has the advantage of being able to obtain a full MS and MS/MS spectra of any compound in the same run at a high acquisition rate and a high resolution, which are useful for compound identification and quantification [58, 59, 60, 61, 62].
GC/MS for E/L quantification
As observed with the identification studies, single quadrupole is the most frequently used type of mass detector with GC (n=7). This result may be explained by the same reasons described for the identification studies: a single quadrupole mass detector coupled to GC is sensitive enough for trace analysis and is robust and cost-effective [53].
Conclusions
The aim of the present meta-synthesis was to determine which detection method was suitable to identify and quantify E/L. The results of the search suggest that there is no specific type of LC or GC/MS instrument that is the best for E/L analysis. As a limitation of this study, no detailed analysis of the ionization source could have been done, because of the large range of equipment and type of spectrometer included in the study.
The choice of a mass detector will depend on the aims of the study. If the objective is to use a mass detector that might be able to do qualitative, structural, and quantitative studies of E/L with LC, a QTOF or Orbitrap mass detector would be a good choice because these detectors can identify and quantify compounds simultaneously at high resolution and with good sensitivity. If the aim of the study is to quantify targeted E/L with LC, a triple quadrupole mass detector would be a good choice because it has a better dynamic range and can reach a lower LOQ. For GC/MS, a single quadrupole mass detector is the most frequently used to analyze volatile E/L because it is sensitive and robust. Compound identification is also possible with GC/MS due to the high reproducibility of electrospray ionization and the availability of MS libraries.
Future perspectives
It is obvious that doing a complete assessment of E/L might not be possible by using only GC/MS or LC/MS In fact, some structural information, especially regarding bond connectivity, may be missing when using only a mass spectrometer particularly for complex structures [11]. In several identification studies included in this literature review, NMR was used for identifying unknown E/L [11, 17, 19]. This technology can yield much more structural information about compounds and therefore offers a chance to elucidate the structures of unknown substances such as isomers [11, 17, 19]. Finally, the analysis of inorganic E/L such as aluminum, magnesium, and calcium cannot be performed with LC/MS/MS or GC/MS However, elemental analysis should be done because these substances may also cause toxicity and compatibility concerns. This is why Inductively Coupled Plasma coupled to MS is also used for the analysis of inorganic E/L [47]. Thus, to do a complete E/L study, several types of detection techniques are needed.
These warnings raise questions about the feasibility of such studies in hospital pharmacies, due to the resources needed. Moreover, analysis of E/L is only part of the work. Doing a complete E/L study implies a toxicological assessment of each substance extracted and identified [17]. Fundamentally, it seems clear that to ensure safety and quality in our pharmaceutical products, drug-packaging interactions should be assessed. But given the huge variety of containers and pharmaceutical products, working alone might be difficult. Rarely, it is possible for a hospital pharmacy to have a full access to the leachable and extractable profile of a container, but mostly the information file is not provided leading to an extensive screening of E/L substances. It is also difficult to find the right challenge between time and cost invested compared to the need of identification/quantification of peaks revealed. Therefore, the choice of the equipment for hospital pharmacists has also to take into account this balance.
Based on this idea, a European group named the L/E Group of Hospital Pharmacies in Europe has been created in order to share work on E/L and to discuss the main issues in such E/L studies [63]. Thus, it seems that it is only by collaborating with major European hospitals that we may be able to cope with the problem of E/L.
Acknowledgments
The author thanks Prof. Bernard Testa and Prof. Serge Rudaz for their comments and advice during the writing of this article.
Conflict of interest statement: none declared.
References
[1] Griffiths W, Ing H, Fleury-Souverain S, Kern C, Matthey B, Sadeghipour F, et al. The stability of ready-to-use (RTU) ephedrine hydrochloride in polypropylene syringes for use in maternal hypotension. EJHP Sci 2005;11:107–10.Search in Google Scholar
[2] Stucki C, Fleury-Souverain S, Sautter AM, Sadeghipour F, Bonnabry P. Development of ready-to-use ketamine hydrochloride syringes for safe use in post-operative pain. EJHP Sci 2008;14:14–8.Search in Google Scholar
[3] Karande IS, Goff Z, Kewley J, Mehta S, Snelling T. Dose-banding of intravenous Piperacillin-Tazobactam in pediatric surgical inpatients. J Pediatr Pharmacol Ther 2017;22:364–8.10.5863/1551-6776-22.5.364Search in Google Scholar PubMed PubMed Central
[4] O’Leary CE, Collins A, Henman MC, King F. Introduction of a dose-banding system for parenteral chemotherapy on a haematology-oncology day ward. J Oncol Pharm Pract 2017;0:1–11.10.1177/1078155217736376Search in Google Scholar PubMed
[5] Speaker TJ, Turco SJ, Nardone DA, Miripol JE. A study of the interaction of selected drugs and plastic syringes. J Parenter Sci Technol 1991;45:212–7.Search in Google Scholar
[6] Dorival-García N, Bones J. Evaluation of solvent systems for optimized extractables studies of single use bioprocessing solutions. J Chromatogr A 2017;1513:69–77.10.1016/j.chroma.2017.06.066Search in Google Scholar PubMed
[7] Pang J, Blanc T, Brown J, Labrenz S, Villalobos A, Depaolis A, et al. Recognition and identification of UV-absorbing leachables in EPREX pre-filled syringes: an unexpected occurrence at a formulation-component interface. PDA J Pharm Sci Technol 2007;61:423–32.Search in Google Scholar
[8] Kongsgaard UE, Andersen A, Øien M, Oswald IA, Bruun LI. Experience of unpleasant sensations in the mouth after injection of saline from prefilled syringes. BMC Nurs 2010;9:1.10.1186/1472-6955-9-1Search in Google Scholar PubMed PubMed Central
[9] Zhao X, Jin SH, Hu CQ. The effect of rubber closures on the haze state of ceftriaxone sodium for injection. Drug Dev Ind Pharm 2007;33:35–44.10.1080/03639040600815228Search in Google Scholar PubMed
[10] Alarcon A, Barcelo B, Caire-Maurisier M, Delaire M, Feuilloley M, Genot S, et al. Interaction contenant-contenu. II. Methodologie. S.T.P. Pharma Pratiques 2007;17:143–60.Search in Google Scholar
[11] Norwood DL, Mullis JO, Davis M, Pennino S, Egert T, Gonnella NC. Automated solid phase extraction (SPE) LC/NMR applied to the structural analysis of extractable compounds from a pharmaceutical packaging material of construction. PDA J Pharm Sci Technol 2013;67:267–87.10.5731/pdajpst.2013.00920Search in Google Scholar PubMed
[12] Norwood DL, Jenke D, Manolescu C, Pennino S, Grinberg N. HPLC and LC/MS analysis of pharmaceutical container closure system leachables and extractables. J Liq Chromatogr Relat Technol 2009;32:1768–827.10.1080/10826070902959497Search in Google Scholar
[13] Zhang F, Chang A, Karaisz K, Feng R, Cai J. Structural identification of extractables from rubber closures used for pre-filled semisolid drug applicator by chromatography, mass spectrometry, and organic synthesis. J Pharm Biomed Anal 2004;34:841–9.10.1016/j.jpba.2003.08.003Search in Google Scholar PubMed
[14] Salter K, Hellings C, Foley N, Teasell R. The experience of living with stroke: a qualitative meta-synthesis. J Rehabil Med 2008;40:595–602.10.2340/16501977-0238Search in Google Scholar PubMed
[15] Abbott LS, Williams CL. Influences of social determinants of health on African Americans living with HIV in the rural Southeast: A qualitative meta-synthesis. J Assoc Nurses AIDS Care 2015;26:340–56.10.1016/j.jana.2015.03.004Search in Google Scholar PubMed
[16] Critical Appraisals Skills Programme (CASP). https://casp-uk.net/casp-tools-checklists/ (accessed 2018 Jan 11).Search in Google Scholar
[17] Wei Y, Wu Y, Zhu T, Li Z, Zhang Y. Identification of UV-absorbing extractables from rubber closures used in containers of injectable powder and safety assessment of leachables in the drug. J Pharm Biomed Anal 2017;138:256–66.10.1016/j.jpba.2017.02.023Search in Google Scholar PubMed
[18] Petrusevski V, Jolevska ST, Ribarska JT, Chachorovska M, Petkovska A, Ugarkovic S. Development of complementary HPLC-DAD/APCI MS methods for chemical characterization of pharmaceutical packaging materials. J Pharm Biomed Anal 2016;124:228–35.10.1016/j.jpba.2016.03.005Search in Google Scholar PubMed
[19] Valente JJ, Peddicord MB, Rinaldi FA, Kelly KA, Bolgar MS. Identification of leachables observed in the size exclusion chromatograms of a low concentration product stored in prefilled syringes. J Pharm Biomed Anal 2017;139:133–42.10.1016/j.jpba.2017.02.039Search in Google Scholar PubMed
[20] Zdravkovic SA. Solid phase extraction in tandem with GC/MS for the determination of semi-volatile organic substances extracted from pharmaceutical packaging/delivery systems via aqueous solvent systems. J Pharm Biomed Anal 2015;112:126–38.10.1016/j.jpba.2015.04.031Search in Google Scholar PubMed
[21] Jenke D, Castner J, Egert T, Feinberg T, Hendricker A, Houston C, et al. Extractables characterization for five materials of construction representative of packaging systems used for parenteral and ophthalmic drug products. PDA J Pharm Sci Technol 2013;67:448–511.10.5731/pdajpst.2013.00933Search in Google Scholar PubMed
[22] Chang JY, Xiao NJ, Zhu M, Zhang J, Hoff E, Russell SJ, et al. Leachables from saline- containing IV bags can alter therapeutic protein properties. Pharm Res 2010;27:2402–13.10.1007/s11095-010-0193-8Search in Google Scholar PubMed
[23] Nashed-Samuel Y, Torraca G, Liu D, Fujimori K, Zhang Z, Wen ZQ, et al. Identification of an extraneous black particle in a glass syringe: extractables/leachables case study. PDA J Pharm Sci Technol 2010;64:242–8.Search in Google Scholar
[24] Corredor C, Tomasella FP, Young J. Drug interactions with potential rubber closure extractables. Identification of thiol-disulfide exchange reaction products of captopril and thiurams. J Chromatogr A 2009;1216:43–8.10.1016/j.chroma.2008.11.021Search in Google Scholar PubMed
[25] Pan C, Harmon F, Toscano K, Liu F, Vivilecchia R. Strategy for identification of leachables in packaged pharmaceutical liquid formulations. J Pharm Biomed Anal 2008;46:520–7.10.1016/j.jpba.2007.11.032Search in Google Scholar PubMed
[26] Maus RG, Li M, Clement CM, Kinzer JA. Gas-phase transfer of polymer cross-linking agents and by-products to solid oral pharmaceuticals. J Pharm Biomed Anal 2007;45:400–6.10.1016/j.jpba.2007.06.023Search in Google Scholar PubMed
[27] Jenke D, Swanson S, Edgcomb E, Couch T, Chacko M, Garber MJ, et al. Strategy for assessing the leachables impact of a material change made in a container/closure system. PDA J Pharm Sci Technol 2005;59:360–80.Search in Google Scholar
[28] Nassar MN, Nesarikar VV, Lozano R, Huang Y, Palaniswamy V. Degradation of a lyophilized formulation of BMS-204352: identification of degradants and role of elastomeric closures. Pharm Dev Technol 2005;10:227–32.10.1081/PDT-54429Search in Google Scholar
[29] Milano CJ, Bailey LC. Evaluation of current compendial physiochemical test procedures for pharmaceutical elastomeric closures and development of an improved HPLC procedure. PDA J Pharm Sci Technol 1999;53:202–10.Search in Google Scholar
[30] Wahl HG, Hoffmann A, Haring HU, Liebich HM. Identification of plasticizers in medical products by a combined direct thermodesorption-cooled injection system and gas chromatography-mass spectrometry. J Chromatogr A 1999;847:1–7.10.1016/S0021-9673(99)00138-7Search in Google Scholar
[31] Bourdeaux D, Yessaad M, Chennell P, Larbre V, Eljezi T, Bernard L, et al. Analysis of PVC plasticizers in medical devices and infused solutions by GC-MS. J Pharm Biomed Anal 2016;118:206–13.10.1016/j.jpba.2015.10.034Search in Google Scholar PubMed
[32] Moreta C, Tena MT. Determination of plastic additives in packaging by liquid chromatography coupled to high resolution mass spectrometry. J Chromatogr A 2015;1414:77–87.10.1016/j.chroma.2015.08.030Search in Google Scholar PubMed
[33] Gimeno P, Thomas S, Bousquet C, Maggio AF, Civade C, Brenier C, et al. Identification and quantification of 14 phthalates and 5 non-phthalate plasticizers in PVC medical devices by GC-MS. J Chromatogr B: Anal Technol Biomed Life Sci 2014;949–950:99–108.10.1016/j.jchromb.2013.12.037Search in Google Scholar PubMed
[34] Pahl I, Dorey S, Barbaroux M, Lagrange B, Frankl H. Analysis and evaluation of single- use bag extractables for validation in biopharmaceutical applications. PDA J Pharm Sci Technol 2014;68:456–71.10.5731/pdajpst.2014.00996Search in Google Scholar PubMed
[35] Zdravkovic SA, Bruss MD, Piccoli RF, Wood DM. A method utilizing ultra-high performance liquid chromatography with ultraviolet and mass spectrometric detection for the analysis of material extracts produced during a controlled extraction study. PDA J Pharm Sci Technol 2014;68:504–26.10.5731/pdajpst.2014.00992Search in Google Scholar PubMed
[36] Pouech C, Lafay F, Wiest L, Baudot R, Leonard D, Cren-Olivé C. Monitoring the extraction of additives and additive degradation products from polymer packaging into solutions by multi-residue method including solid phase extraction and ultra-high performance liquid chromatography-tandem mass spectrometry analysis. Anal Bioanal Chem 2014;406:1493–507.10.1007/s00216-013-7551-4Search in Google Scholar PubMed
[37] Reisinger M, Beissmann S, Buchberger W. Quantitation of hindered amine light stabilizers in plastic materials by high performance liquid chromatography and mass spectrometric detection using electrospray ionization and atmospheric pressure photoionization. Anal Chim Acta 2013;803:181–7.10.1016/j.aca.2013.07.061Search in Google Scholar PubMed
[38] Strac IV, Pusic M, Gajski G, Garaj-Vrhovac V. Presence of phthalate esters in intravenous solution evaluated using gas chromatography-mass spectrometry method. J Appl Toxicol 2013;33:214–9.10.1002/jat.1741Search in Google Scholar PubMed
[39] Yamaji K, Kawasaki Y, Yoshitome K, Matsunaga H, Sendo T. Quantitation and human monocyte cytotoxicity of the polymerization agent 1-hydroxycyclohexyl phenyl ketone (Irgacure 184) from 3 brands of aqueous injection solution. Biol Pharm Bull 2012;35:1821–5.10.1248/bpb.b12-00210Search in Google Scholar PubMed
[40] Soeborg T, Hansen SH, Halling-Sorensen B. Determination of bisphenol diglycidyl ethers in topical dosage forms. J Pharm Biomed Anal 2006;40:322–30.10.1016/j.jpba.2005.07.038Search in Google Scholar PubMed
[41] Garrido-Lopez A, Tena MT. Experimental design approach for the optimisation of pressurised fluid extraction of additives from polyethylene films. J Chromatogr A 2005;1099:75–83.10.1016/j.chroma.2005.09.005Search in Google Scholar PubMed
[42] Story DA, Leeder J, Cullis P, Bellomo R. Biologically active contaminants of intravenous saline in PVC packaging: Australasian, European, and North American samples. Anaesth Intensive Care 2005;33:78–81.10.1177/0310057X0503300113Search in Google Scholar PubMed
[43] Loff S, Subotic U, Reinicke F, Wischmann H, Brade J. Extraction of di-ethylhexyl-phthalate from perfusion lines of various material, length and brand by lipid emulsions. J Pediatr Gastroenterol Nutr 2004;39:341–5.10.1097/00005176-200410000-00008Search in Google Scholar PubMed
[44] Grebe SK, Singh RJ. LC-MS/MS in the clinical laboratory - where to from here? Clin Biochem Rev 2011;32:5–31.Search in Google Scholar
[45] Yuan C, Chen D, Wang S. Drug confirmation by mass spectrometry: identification criteria and complicating factors. Clin Chim Acta 2015;438:119–25.10.1016/j.cca.2014.08.021Search in Google Scholar PubMed
[46] Wong Y, Lewis R. Analysis of food toxins and toxicants, vol. 1. Oxford: John Wiley & sons, 2017:339.10.1002/9781118992685Search in Google Scholar
[47] Teasdale A, Jahn M, Bailey S, Feilden A, Taylor G, Corcoran ML, et al. Controlled extraction studies applied to polyvinyl chloride and polyethylene materials: conclusions from the ELSIE controlled extraction pilot study. AAPS PharmSciTech 2015;16:664–74.10.1208/s12249-014-0249-xSearch in Google Scholar PubMed PubMed Central
[48] ELSIE. http://www.elsiedata.org/elsie-database/. (accessed 2018 Jan 11).Search in Google Scholar
[49] Chen XF, Wu HT, Tan GG, Zhu ZY, Chai YF. Liquid chromatography coupled with time-of-flight and ion trap mass spectrometry for qualitative analysis of herbal medicines. J Pharm Anal 2011;1:235–45.10.1016/j.jpha.2011.09.008Search in Google Scholar PubMed PubMed Central
[50] Bade R, Rousis NI, Bijlsma L, Gracia-Lor E, Castiglioni S, Sancho JV, et al. Screening of pharmaceuticals and illicit drugs in wastewater and surface waters of Spain and Italy by high resolution mass spectrometry using UHPLC-QTOF MS and LC-LTQ-Orbitrap MS Anal Bioanal Chem 2015;407:8979–88.10.1007/s00216-015-9063-xSearch in Google Scholar PubMed
[51] Glauser G, Veyrat N, Rochat B, Wolfender JL, Turlings TC. Ultra-high pressure liquid chromatography-mass spectrometry for plant metabolomics: a systematic comparison of high-resolution quadrupole-time-of-flight and single stage Orbitrap mass spectrometers. J Chromatogr A 2013;1292:151–9.10.1016/j.chroma.2012.12.009Search in Google Scholar PubMed
[52] Duvivier WF, van Beek TA, Nielen MW. Critical comparison of mass analyzers for forensic hair analysis by ambient ionization mass spectrometry. Rapid Commun Mass Spectrom 2016;30:2331–40.10.1002/rcm.7722Search in Google Scholar PubMed
[53] Lei Z, Huhman DV, Sumner LW. Mass spectrometry strategies in metabolomics. J Biol Chem 2011;286:25435–42.10.1074/jbc.R111.238691Search in Google Scholar PubMed PubMed Central
[54] Alder L, Greulich K, Kempe G, Vieth B. Residue analysis of 500 high priority pesticides: better by GC–MS or LC–MS/MS? Mass Spectrom Rev 2006;25:838–65.10.1002/mas.20091Search in Google Scholar PubMed
[55] Hernandez F, Cervera MI, Portoles T, Beltran J, Pitarch E. The role of GC-MS/MS with triple quadrupole in pesticide residue analysis in food and the environment. Anal Methods 2013;5:5875–94.10.1039/c3ay41104dSearch in Google Scholar
[56] Andreu V, Pico Y. Determination of currently used pesticides in biota. Anal Bioanal Chem 2012;404:2659–81.10.1007/s00216-012-6331-xSearch in Google Scholar PubMed
[57] Botitsi HV, Garbis SD, Economou A, Tsipi DF. Current mass spectrometry strategies for the analysis of pesticides and their metabolites in food and water matrices. Mass Spectrom Rev 2011;30:907–39.10.1002/mas.20307Search in Google Scholar PubMed
[58] Farre M, Gros M, Hernandez B, Petrovic M, Hancock P, Barcelo D. Analysis of biologically active compounds in water by ultra-performance liquid chromatography quadrupole time-of-flight mass spectrometry. Rapid Commun Mass Spectrom 2008;22:41–51.10.1002/rcm.3324Search in Google Scholar PubMed
[59] Wang J, Leung D. Analyses of macrolide antibiotic residues in eggs, raw milk, and honey using both ultra-performance liquid chromatography/quadrupole time-of-flight mass spectrometry and high-performance liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom 2007;21:3213–22.10.1002/rcm.3207Search in Google Scholar PubMed
[60] Marchese S, Gentili A, Perret D, Ascenzo GD, Pastori F. Quadrupole time-of-flight versus triple-quadrupole mass spectrometry for the determination of non-steroidal antiinflammatory drugs in surface water by liquid chromatography/tandem mass spectrometry. Rapid Commun Mass Spectrom 2003;17:879–86.10.1002/rcm.998Search in Google Scholar PubMed
[61] Stolker AA, Niesing W, Fuchs R, Vreeken RJ, Niessen WM, Brinkman UA. Liquid chromatography with triple-quadrupole and quadrupole-time-of-flight mass spectrometry for the determination of micro-constituents-a comparison. Anal Bioanal Chem 2004;378:1754–61.10.1007/s00216-003-2485-xSearch in Google Scholar PubMed
[62] Stolker AA, Niesing W, Hogendoorn EA, Versteeg JF, Fuchs R. Brinkman UAT Liquid chromatography with triple-quadrupole or quadrupole-time of flight mass spectrometry for screening and confirmation of residues of pharmaceuticals in water. Anal Bioanal Chem 2004;378:955–63.10.1007/s00216-003-2253-ySearch in Google Scholar PubMed
[63] Vigneron J The L/E-meeting in Spain Granada Infostab Newsletter n°32. http://www.stabilis.org/sourcesPDF/Fichier.10914.3.pdf (accessed 2018 Mar 16).Search in Google Scholar
© 2019 Walter de Gruyter GmbH, Berlin/Boston
Articles in the same Issue
- Frontmatter
- Editorial
- The Importance of a Scientific Journal in the Field of Pharmaceutical Technology in Hospitals
- Review
- Investigation of Drug-Packaging Interactions with Mass Spectroscopy Detectors: A Meta-Synthesis of the Literature
- Research Articles
- Automation of Aseptic Sterile Preparation: Risk Analysis and Productivity Comparison with Manual Process
- Physical Compatibility of Intravenous Drugs Commonly Used in Intensive Care Units: An Observational Study and Physical Compatibility Laboratory Tests on Anti-Infective Drugs
- Short Communication
- Assessment of an Online Training Tool for the Automated Unit-Dose Dispensing System (ADS) Process
- Corrigendum
- Corrigendum to: Formulation of a 3-months Stability Oral Viscous Budesonide Gel and Development of an Indicating Stability HPLC Method
Articles in the same Issue
- Frontmatter
- Editorial
- The Importance of a Scientific Journal in the Field of Pharmaceutical Technology in Hospitals
- Review
- Investigation of Drug-Packaging Interactions with Mass Spectroscopy Detectors: A Meta-Synthesis of the Literature
- Research Articles
- Automation of Aseptic Sterile Preparation: Risk Analysis and Productivity Comparison with Manual Process
- Physical Compatibility of Intravenous Drugs Commonly Used in Intensive Care Units: An Observational Study and Physical Compatibility Laboratory Tests on Anti-Infective Drugs
- Short Communication
- Assessment of an Online Training Tool for the Automated Unit-Dose Dispensing System (ADS) Process
- Corrigendum
- Corrigendum to: Formulation of a 3-months Stability Oral Viscous Budesonide Gel and Development of an Indicating Stability HPLC Method

