Abstract
Our department of pharmacy takes over all the medical skin tests prescribed by the allergy department. The production takes place in specific premises, with qualified and calibrated equipment, by a qualified and regularly assessed staff—in compliance with the French preparation guidelines. The whole activity is under the responsibility of a pharmacist—handlings are performed by hospital pharmacy technicians. Each new intradermal skin test demand leads to a feasibility analysis—irritating nature, dilution solvent, concentration—this information is gathered in a thesaurus. The manufacturing steps are the following: prescription validation, production sheet and label printing, preparation of the needed equipment, batch numbers and expiration date checking, handling under a vertical laminar flow hood and control after production. The preparation activity increases continuously and the thesaurus currently contents 302 rows with following information: drug, dilution and reconstitution solvent, pure solution concentration and maximum concentration to test with intradermal tests. Work would prospect in costs reduction and resources optimization. Thanks to the allergists’ confidence, the partnership between the two departments can go on. This guarantees the quality of the preparations tested on patients but also the skin tests reproducibility.
Introduction
Drug hypersensitivity can account for up to 15 % of adverse drug reactions [1]. As part of the management of these patients, allergists use skin tests to confirm or refute the diagnosis. The available tests are skin prick tests, intradermal test (IDT) and patch tests. These tests are used in addition to interrogations, clinical examinations and in vitro tests. Skin test is the most commonly used procedure to confirm a sensitization in drug hypersensitivity; skin prick test and IDT with immediate readings are used for investigation of immediate hypersensitivity reactions.
The realization of IDT is standardized (injected volume, needle type, papule size) [2], so it is also necessary to standardize the preparation of solutions. These dilutions could be prepared by nurses or by the pharmacy department [3].
In our hospital, all the drug tested for the department of allergy are prepared by the pharmacy department. These preparations are made in accordance with the French preparation guidelines [4], according to validated procedures. A pharmacist is responsible for the activity, staff receives specific training, areas are reserved and equipment is qualified, controlled and regularly maintained.
This article relates the experience of our hospital and presents the quality assurance system set up in our institution’s pharmacy department to provide doctors with IDT dilutions of reproducible quality.
Context
The collaboration between the allergy and pharmacy departments has been ongoing since the 1990s. The preparation of skin medicated tests has been constantly rising since the partnership started. Each IDT prescription that is transmitted to a pharmacist includes the prescriber name, the patient name, the allergen to be tested – active ingredient or excipient – and the desired concentrations. For the preparation, the pharmacy uses the drugs available in hospital. Only sterile materials are used [5].
The quality assurance system ensures the quality, reliability and reproducibility of the production, and thus minimizes the risk of error inherent in this production. Given the intradermal nature of the test, they must be performed with injectable preparations [6]. The dilutions are carried out under a laminar airflow hood [7].
Description of the quality assurance system
Preparation area and equipment
The preparation of sterile drugs has to be done in accordance with the French guidelines [4]. It requires special conditions to reduce any risk of contamination – microbiological or particulate.
At the pharmacy, the preparation of skin tests for the allergy service is carried out in a dedicated room, reserved for this activity. It is a clean room, equipped with precision balances and two vertical laminar airflow hoods type IIa, class ISO 5 [5]. The preparation technique is aseptic preparation.
The number of people present in the preparation room is limited to avoid any contamination. Specific clothing is mandatory before entering the room, according to recommendations. It consists in a clean gown, dedicated shoes or overshoes and a headdress. All jewellery (watches, rings, bracelets) must be removed. Nails have to be short and without polish.
Maintenance, such as cleaning and disinfection of the room, is performed daily. Microbiological samples of air and surface are taken under the equipment after each production, allowing microbiological monitoring – according to the preparation guidelines.
The staff
The hospital pharmacist is responsible for the preparation, he “has the power of decision on the execution of the preparation” [4]. He also engages his responsibility in the realization and delivery of any preparation. He must train and supervise the personnel authorized to manipulate.
All operations are described in detailed operating procedures, thus contributing to the quality assurance of the activity.
French preparation guidelines state that “pharmacy staff who manipulates has to be qualified and regularly trained”. Each new member of the staff is given both a theoretical and practical training session before taking part in any activity. A Pharmacy technician realizes preparations, a pharmacy resident analyses and validates each prescription. All staff works under the effective responsibility of the pharmacist.
Problems related to the activity of allergology, introduction of a new procedure, results of some studies or any other subject to consultation are discussed in staff meetings, held every four to six weeks, with all the staff assigned to the pharmacotechnical area.
Drugs and medical devices
All drugs are injectable solutions, emulsions or suspensions, powder for solution for injection [8]. The drugs and medical devices needed for the preparation activity are purchased by the pharmacy department – according to the purchase procedures. All storage conditions are controlled. It is possible to use the patients’ medicines, if prescribers agree and if the quality of the drugs is respected – respect of storage conditions and complete identification of the products, i. e. batch number and expiration date.
Feasibility analysis
For each new skin test prescription, the pharmacist must check the feasibility of the test. According to the French preparation guidelines, this feasibility study makes possible the assessment of the “conformity of a preparation with the state of scientific, medical and technical knowledge” [4]. An appropriate literature search gathers toxicological and clinical data on raw materials (active substances and excipients) from the reference works and publications available.
For each new IDT’ prescription, the pharmacist checks three points [5]:
The full-strength concentration: it corresponds to the concentration of use, either the concentration of the solution or the concentration of the solution after reconstitution of the powder. It is determined according to the maximum concentration administered according to available bibliographic data on the use of this molecule in IDT [2, 9, 10, 11].
The dilution’s solvent: it is chosen based on bibliographic data and reference works in order to guarantee the compatibility of the drug. This solvent is 0.9 % saline except for known incompatibilities.
The non-toxicity of the test: the risk of irritation is tolerated but must be mentioned. The data from literature and those provided by the laboratory make it possible to determine the toxicity of a molecule – local adverse effects such as reactions at the injection site, redness or necrosis.
Moreover, IDTs can be done only if an injectable form of the drug is commercialized [12]. All IDT’ data are stored in a thesaurus, the information collected are: INN (International Non-proprietary Name), pharmaceutical products, reconstitution and dilution solvents if applicable, concentration of the pure solution and the maximum concentration allowed in IDT.
If the drug does not exist in injectable form, it is necessary to find alternatives: use of the raw material (trometamol for example), dissolution and filtration on a 0.22 μm filter. Another alternative is the use of the eye drops form, which is presented in sterile form and can therefore be used for intradermal tests – carboxymethylcellulose for example [13].
The IDT’ manufacturing circuit at the pharmacy
Receipt and validation of the prescription
Prescriptions are sent by fax to the pharmacy. The pharmacy resident validates the prescription: he checks that the tests are not toxic (prescription of an irritating dilution for example), but also the homogeneity of the tests prescribed. He also takes into account, for insulins for example, the doses injected to the patient.
Edition of the manufacturing sheets and inscription to the prescription ledger
According to Article R 5125–45 and Article R 5132–10 of the French Public Health Code, any production or delivery by a pharmacist of a compounding preparation is immediately the subject of a transcription on a book register or record by any appropriate system. A serial number is chronologically given to any preparation. The mandatory information transcribed or recorded in the prescription is comprise of the date of preparation, the name of the prescriber, the name of the patient, the requesting service, the composition of the preparation, the quantity produced and the identification of the person who performed the preparation. The prescription must be kept for 10 years.
The preparation follows a written procedure validated by the pharmacist. All steps are detailed on a manufacturing sheet; the pharmacy technician must follow the instructions.
Manufacturing sheets are computerized on the Microsoft Office Excel® software (Figure 1). They include the mandatory information according to the guidelines [7]: the names, dosage and pharmaceutical form of the preparations, the order number, the date of manufacturing, the name of the manipulators, the drugs and devices used (batch numbers and expiration date). Each molecule has its file, only few Excel file cells can be modified, the others are protected by a password and can only be modified by the pharmacist. The calculations are predefined, as well as all volumes to be taken for manufacturing.
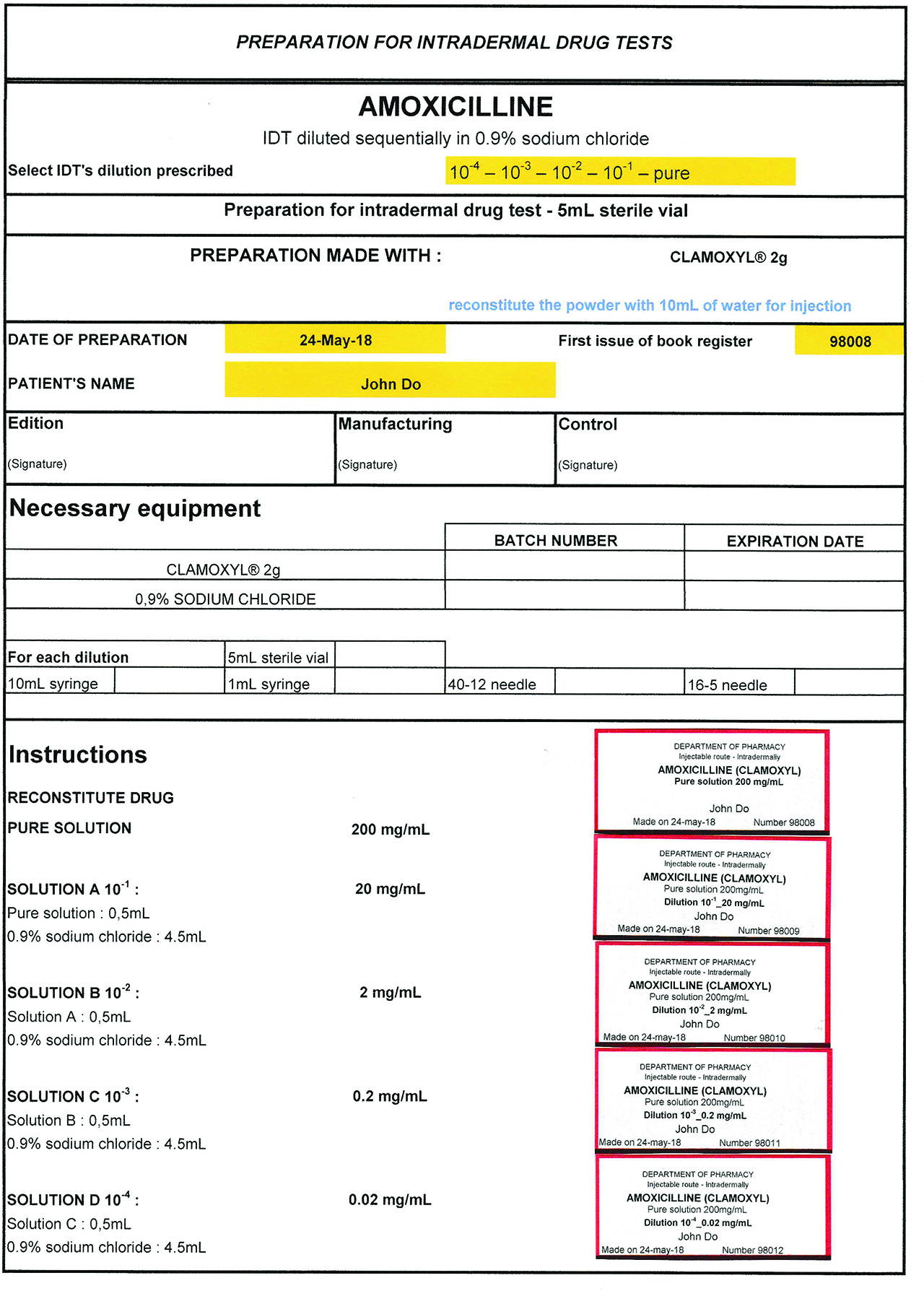
Manufacturing sheet and labels.
Each preparation must be labelled, label contains: name and address of the pharmacy department, name of the preparation (route of administration and dosage in active ingredient), order number, expiration date. The mention “prepared on” indicates the date of preparation.
Label editing is automatic with the manufacturing sheet.
Manufacturing
Before starting the activity, technicians gather all necessary equipment to produce each IDT’ series, devices and materials required for manufacturing and packaging bottles.
The preparations are administered intradermal, they must be sterile and are carried out in vertical laminar airflow hood. All the manufacturing steps are described in the procedures known and followed by all the staff: simple washing of the hands, decontamination of the benches and the hood, surgical friction of the hands, put on sterile gloves and installation of the workstation. During manufacturing, two manipulators are present. The first decontaminates the material, controls the handling. The second manipulates under the laminar airflow hood according to the instructions of the manufacturing sheet. Preparation are made into 5 mL sterile vials. For each sampling, in-process control is performed: the volume taken, the vial in which the sample was taken, the vial into which the sample is introduced, are checked. These checks are recorded progressively, by affixing the initials on the sheet of manufacture. This guarantees the quality of the production.
Intradermal tests are performed using a sterile solution of the suspected drug, diluted sequentially (pure, 10–1, 10–2, 10–3, 10–4) in 0.9 % sodium chloride or dextrose 5 % if incompatible. Because of the risk posed by their handling, the preparation of tests with anticancer drugs is done in reserved preparation areas, under specific equipment and by specifically trained personnel.
After manufacturing, all labelled preparations are quarantined with the completed manufacturing form.
Control and release of IDT
At the end of the manufacturing process, the pharmacist checks the manufacturing sheet: initials of the manipulators, products used (in particular when generic drugs are used), batch numbers and expiration date, recording of the control of the volumes taken. It also controls the general appearance of the preparations and the correspondence between the newly-made preparations (molecule, dilutions) and the medical prescription. He then validates the preparations by signing the manufacturing sheet and the prescription, IDTs are dispensed to the allergy department.
Activity report
Table 1 presents the number of preparations for IDT performed annually since 2007. All full-strength solutions and dilutions manufactured are called “IDT preparations”. The tests are usually prescribed by battery. Thus, the battery betalactam antibiotics tests several antibiotics (benzylpenicillin, amoxicillin, piperacillin …). Each new preparation is recorded in the IDT thesaurus. It currently contains 302 rows; an extract is available in Table 2.
Activity report. Number of IDT dilutions prepared by our department of pharmacy.
| Year | 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| IDT (number of dilutions) | 2,723 | 2,988 | 4,019 | 4,227 | 5,010 | 5,091 | 4,496 | 7,536 | 7,968 | 8,561 | 8,832 |
Thesaurus tests for IDT. Extract.
| Drug classes and drug | Reconstitution solvent (if necessary) | Concentration (after reconstitution) | Full-strength concentration* | Maximum concentration tested in IDT | Dilution solvent |
|---|---|---|---|---|---|
| Nonsteroidal anti-inflammatory drug | |||||
| Aspirin | NS | 200 mg/mL | 200 mg/mL | 200 mg/mL | NS |
| Diclofenac | – | 25 mg/mL | 25 mg/mL | 25 mg/mL | NS |
| Ibuprofen | – | 5 mg/mL | 5 mg/mL | 5 mg/mL | NS |
| Ketoprofene | – | 25 mg/mL | 20 mg/mL | 2 mg/mL | NS |
| Piroxicam | – | 20 mg/mL | 20 mg/mL | 2 mg/mL | NS |
| Antibiotics | |||||
| Amoxicillin | Water | 200 mg/mL | 200 mg/mL | 20 mg/mL | NS |
| Amoxicillin/clavulaniq acid | Water | 200 mg/mL(1) | 200 mg/mL(1) | 20 mg/mL(1) | NS |
| Cefazolin | Water | 200 mg/mL | 200 mg/mL | 20 mg/mL | NS |
| Cefotaxime | NS | 200 mg/mL | 200 mg/mL | 20 mg/mL | NS |
| Ceftriaxone | Water | 200 mg/mL | 200 mg/mL | 20 mg/mL | NS |
| Cefuroxime | Water | 250 mg/mL | 250 mg/mL | 25 mg/mL | NS |
| Ciprofloxacin | – | 2 mg/mL | 2 mg/mL | 0,02 mg/mL | NS |
| Erythromycin | Water | 50 mg/mL | 50 mg/mL | 0,5 mg/mL | NS |
| Imipenem cilastatin | NS | 25 mg/mL(2) | 5 mg/mL(2) | 0,5 mg/mL(2) | NS |
| Vancomycin | Water | 50 mg/mL | 1 mg/mL | 1 mg/mL | NS |
| Levofloxacin | – | 5 mg/mL | 5 mg/mL | 0,05 mg/mL | NS |
| Meropenem | NS | 50 mg/mL | 50 mg/mL | 50 mg/mL | NS |
| Ofloxacin | – | 5 mg/mL | 5 mg/mL | 0,05 mg/mL | NS |
| Benzylpenicillin | NS | 1 000 000 UI/mL | 1 000 000 UI/mL | 10 000 UI/mL | NS |
| Piperacillin tazobactam | Water | 200 mg/mL(3) | 200 mg/mL(3) | 20 mg/mL(3) | NS |
| Rifampicin | Specific solvent | 60 mg/mL | 2,4 mg/mL | 2,4 mg/mL | NS |
| Spiramycin | Water | 375 000 UI/mL | 375 000 UI/mL | 375 UI/mL | NS |
| Teicoplanin | Water | 50 mg/mL | 50 mg/mL | 50 mg/mL | NS |
| Chemotherapeutic drugs | |||||
| Carboplatin | – | 10 mg/mL | 10 mg/mL | 10 mg/mL | D5W |
| Cisplatin | – | 1 mg/mL | 1 mg/mL | 1 mg/mL | NS |
| Docetaxel | – | 20 mg/mL | 1 mg/mL | 1 mg/mL | NS |
| Oxaliplatin | – | 5 mg/mL | 1 mg/mL | 1 mg/mL | D5W |
| Paclitaxel | – | 6 mg/mL | 1 mg/mL | 1 mg/mL | NS |
| Glucocorticoids | |||||
| Betamethasone sodium phosphate | – | 4 mg/mL | 4 mg/mL | 4 mg/mL | NS |
| Betamethasone dipropionate | – | 7 mg/mL | 7 mg/mL | 7 mg/mL | NS |
| Dexamethasone phosphate sodique | – | 4 mg/mL | 4 mg/mL | 4 mg/mL | NS |
| Hydrocortisone sodium hemisuccinate | Water | 100 mg/mL | 100 mg/mL | 10 mg/mL | NS |
| Methylprednisolone | Water | 100 mg/mL | 100 mg/mL | 10 mg/mL | NS |
| Prednisolone | – | 25 mg/mL | 25 mg/mL | 25 mg/mL | NS |
| Triamcinolone | – | 40 mg/mL | 40 mg/mL | 40 mg/mL | NS |
| Heparins | |||||
| Danaparoid | – | 1 250 UI antiXa/mL | 1 250 UI antiXa/mL | 1 250 UI antiXa/mL | NS |
| Enoxaparin | – | 10 000 UI antiXa/mL | 10 000 UI antiXa/mL | 10 000 UI antiXa/mL | NS |
| Fondaparinux | – | 5 mg/mL | 5 mg/mL | 5 mg/mL | NS |
| Heparin calcium | – | 25 000 UI/mL | 25 000 UI/mL | 25 000 UI/mL | NS |
| Heparin sodium | – | 5 000 UI/mL | 5 000 UI/mL | 5 000 UI/mL | NS |
| Nadroparin | – | 9 500 UI antiXa/mL | 9 500 UI antiXa/mL | 9 500 UI antiXa/mL | NS |
| Tinzaparin | – | 20 000 UI antiXa/mL | 20 000 UI antiXa/mL | 20 000 UI antiXa/mL | NS |
| Iodinated contrast media | |||||
| Iobitridol | – | 350 mgIode/mL | 350 mgIode/mL | 350 mgIode/mL | NS |
| Iodixanol | – | 320 mgIode/mL | 320 mgIode/mL | 320 mgIode/mL | NS |
| Iohexol | – | 300 mgIode/mL | 300 mgIode/mL | 300 mgIode/mL | NS |
| Iomeprol | – | 350 mgIode/mL | 350 mgIode/mL | 350 mgIode/mL | NS |
| Iopamidol | – | 370 mgIode/mL | 370 mgIode/mL | 370 mgIode/mL | NS |
| Iopromide | – | 300 mgIode/mL | 300 mgIode/mL | 300 mgIode/mL | NS |
| Ioversol | – | 350 mgIode/mL | 350 mgIode/mL | 350 mgIode/mL | NS |
*Full-strength concentration: maximum concentration that can be administered by intravenous route.
(1)Expressed in amoxicillin
(2)Expressed in piperacillin.
(3)Expressed in imipenem.
NS: normal saline (0.9 % sodium chloride) – D5W: Dextrose 5 % in water.
To optimize resources and in agreement with prescribers, the pharmacy department manufactures once a week all drugs antibiotic batteries, iodinated contrast media and corticosteroids. None stability studies are available, this preparation in advance is based on clinical data (there is no more false positives or false negatives).
Discussion
Our activity is important compared to that of other centres, in terms of number of dilutions and labour time [14]. Regarding the drug classes inducing reactions, anti-infective are most often involved (40–50 % of cases), in particular beta-lactams, then analgesics, antipyretics and non-steroidal anti-inflammatory drugs (between 15 and 20 %). Then come the contrast products (6 %) and other drugs [15].
The true incidence of hypersensitivity to drugs including allergies, is not really known. Since drug allergies are not predictable, they cannot be studied or detected in clinical trials during the drug’s development phase. Estimated data reveal that drug allergies account for 5 to 15 % of drug side effects [1]. They affect more than 7 % of the general population; drug allergies are reported to account for 3 % of hospital admissions on average [16, 17].
The approach to drug hypersensitivity always comprises a clinical and a diagnostic workup [18]. Immediate-reading skin tests (pricks and intradermal tests) are indicated in patients reporting symptoms suggesting immediate-type hypersensitivity reactions. The diagnostic and/or predictive value of these tests is good with antibiotics [11]. For immediate iodinated contrast media reactors, the intradermal tests were the most sensitive, whereas delayed intradermal tests in combination with patch tests were needed for optimal sensitivity in non-immediate reactors [19]. In some cases, controlled provocation testing is required to clarify drug reactions [2]. Drug skin tests induce only rarely adverse reactions. False-positive results can occur and should be considered by testing new products [12]. The goal of a diagnostic workup is not only to identify the culprit drug, but also to defer the patient from similar drugs in order to prevent a second episode [18]. All results must be documented in an “allergy passport” in order to ensure targeted avoidance in the future and allow the use of alternative drugs where possible [2]. This global approach allows a diagnosis of allergy or non-allergy and thus avoids therapeutic escalations and the use of more expensive drugs.
Currently allergists agree on the need to standardize skin tests and guidelines are available [2, 8, 20], but few studies speak of the test’s preparation by pharmacists [5]. This will require further studies in terms of products used, concentrations and stability data. The collaboration between allergy department and pharmacy department must go on, it allows the optimization of organization: dedicated days for IDT’s realization, dilution made for several patients and then a decrease of the global cost.
Conclusion
The pharmacy department of our university hospital performs skin tests for the exploration of allergic hypersensitivity to drugs. The IDTs are sterile and intended for intradermal use, they are made on medical prescription. The manufacturing is carried out in a room dedicated to the activity by trained, qualified and regularly evaluated personnel, according to procedures validated by the pharmacist, according to French preparation guidelines. Work prospects are part of a process of reducing manufacturing costs and optimizing resources. The confidence of the allergists allows the partnership between the service and the pharmacy to continue, guaranteeing the quality of the preparations tested on the patients, as well as the reproducibility of the cutaneous tests.
Acknowledgements
Assistance with the study: thank you to Jean Vigneron for reading through it all and making meticulous corrections
Financial support and sponsorship: none
Conflicts of interest: Authors state no conflict of interest. All authors have read the journal’s Publication ethics and publication malpractice statement available at the journal’s website and hereby confirm that they comply with all its parts applicable to the present scientific work.
References
1. Alfirevic A, Pirmohamed M. Drug-induced hypersensitivity reactions and pharmacogenomics: past, present and future. Pharmacogenomics 2010;11:497–9.10.2217/pgs.10.12Search in Google Scholar
2. Brockow K, Przybilla B, Aberer W, Bircher AJ, Brehler R, Dickel H, et al. Guideline for the diagnosis of drug hypersensitivity reactions. Allergo J Int 2015;24:94–105.10.1007/s40629-015-0052-6Search in Google Scholar
3. Thong BY, Mirakian R, Castells M, Pichler W, Romano A, Bonadonna P, et al. A World Allergy Organization International Survey on Diagnostic Procedures and Therapies in Drug Allergy/Hypersensitivity. World Allergy Organ J 2011;4:257–70.10.1097/WOX.0b013e31823dc02cSearch in Google Scholar
4. Agence Nationale de Sécurité du Médicament et des Produits de Santé. Bonnes Pratiques de Préparation. Available at: http://ansm.sante.fr/var/ansm_site/storage/original/application/a5d6ae4b3d5fdee013ca463462b7b296.pdf. Accessed 23 May 2018.Search in Google Scholar
5. Zemmouche S, Barbaud A, Commun N. Démarche pharmaceutique pour la réalisation de préparations pour tests allergologiques: expérience de la pharmacie des hôpitaux Maringer-Villemin-Fournier du CHU de Nancy. J Pharm Clin 2004;23:157–68.Search in Google Scholar
6. Brockow K, Garvey LH, Aberer W, Atanaskovic-Markovic M, Barbaud A, Bilo MB, et al. Skin test concentrations for systemically administered drugs – an ENDA/EAACI Drug Allergy Interest Group position paper. Allergy 2013;68:702–12.10.1111/all.12142Search in Google Scholar
7. Barbaud A, Gonçalo M, Bruynzeel D, Bircher A. Guidelines for performing skin tests with drugs in the investigation of cutaneous adverse drug reactions. Contact Dermatitis 2001;45:321–8.10.1034/j.1600-0536.2001.450601.xSearch in Google Scholar
8. Barbaud A. Drug skin tests and systemic cutaneous adverse drug reactions: an update. Expert Rev Dermatol 2014;2:481–95.10.1586/17469872.2.4.481Search in Google Scholar
9. Brockow K, Romano A, Blanca M, Ring J, Pichler W, Demoly P. General considerations for skin test procedures in the diagnosis of drug hypersensitivity. Allergy 2002;57:45–51.10.1046/j.0105-4538.2001.00001.x-i8Search in Google Scholar
10. Empedrad R, Liebl Darter A, Earl HS, Gruchalla RS. Nonirritating intradermal skin test concentrations for commonly prescribed antibiotics. J Allergy Clin Immunol 2003;112:629–30.10.1016/S0091-6749(03)01783-4Search in Google Scholar
11. Ponvert C. Valeurs diagnostique et prédictive des tests cutanés aux médicaments et substances biologiques. Rev Fr Allerg Immunol Clin 2006;46:14–28.10.1016/j.allerg.2005.11.002Search in Google Scholar
12. Barbaud A. Skin testing and patch testing in non-IgE-mediated drug allergy. Curr Allergy Asthma Rep 2014;14:442.10.1007/s11882-014-0442-8Search in Google Scholar PubMed
13. Robert S, Ménétré S, Poreaux C, Brault F, Freling E, Demoré B. Comment réaliser des intradermoréactions de carboxyméthylcellulose? Rev Fr Allerg 2018;58:260–1.10.1016/j.reval.2018.02.106Search in Google Scholar
14. Queuille E, Favier B, Savet M, Cousin F, Bureau J, Nicolas JF. Pharmaco-allergologie : place du pharmacien dans le diagnostic et le suivi des intolérances médicamenteuses. J Pharm Clin 2002;21:255–9.Search in Google Scholar
15. Ponvert C. Grands principes du diagnostic étiologique des réactions d’hypersensibilité immédiate aux médicaments et substances biologiques. Rev Fr Allergol Immunol Clin 2007;47:292–7.10.1016/j.allerg.2006.12.002Search in Google Scholar
16. Demoly P, Viola M, Gomes ER, Romano A. Epidemiology and causes of drug hypersensitivity. In: Pichler WL, editor. Drug hypersensitivity. Basel: Karger, 2007:2–17.10.1159/000104184Search in Google Scholar
17. Demoly P. Les allergies médicamenteuses. M T Pédiatrie 2007;10:34–43.Search in Google Scholar
18. Harr T. Diagnostic approach to drug allergy. Chem Immunol Allergy 2012;97:47–60.10.1159/000335615Search in Google Scholar PubMed
19. Brockow K, Romano A, Aberer W, Bircher AJ, Barbaud A, Bonadonna P, et al. Skin testing in patients with hypersensitivity reactions to iodinated contrast media – a European multicenter study. Allergy 2009;64:234–41.10.1111/j.1398-9995.2008.01832.xSearch in Google Scholar PubMed
20. Ruëff F1, Bergmann KC, Brockow K, Fuchs T, Grübl A, Jung K, et al. Skin tests for diagnostics of allergic immediate-type reactions. Guideline of the German Society for Allergology and Clinical Immunology). Pneumologie 2011;65:484–95.10.1055/s-0030-1256476Search in Google Scholar PubMed
© 2018 Walter de Gruyter GmbH, Berlin/Boston
Articles in the same Issue
- Frontmatter
- Editorial
- Pharmaceutical Technology to Improve Patient Care
- Research Articles
- A Proof of Principle Study of the Terminal Sterilization of Prefilled Syringes Using A Water Cascade Process
- Validation of an HPLC Assay Method for Routine QC Testing and Stability Study of Compounded Low-Dose Capsules of Acetylsalicylic Acid
- Development of a Stability Indicating Method for Simultaneous Analysis of Five Water-Soluble Vitamins by Liquid Chromatography
- Stability of a 50 mg/mL Ceftazidime Eye-Drops Formulation
- Opinion Paper
- Pharmaceutical Preparations for Intradermal Drug Tests
- Reviewer Acknowledgment
Articles in the same Issue
- Frontmatter
- Editorial
- Pharmaceutical Technology to Improve Patient Care
- Research Articles
- A Proof of Principle Study of the Terminal Sterilization of Prefilled Syringes Using A Water Cascade Process
- Validation of an HPLC Assay Method for Routine QC Testing and Stability Study of Compounded Low-Dose Capsules of Acetylsalicylic Acid
- Development of a Stability Indicating Method for Simultaneous Analysis of Five Water-Soluble Vitamins by Liquid Chromatography
- Stability of a 50 mg/mL Ceftazidime Eye-Drops Formulation
- Opinion Paper
- Pharmaceutical Preparations for Intradermal Drug Tests
- Reviewer Acknowledgment

