Abstract
The naturally occurring stilbenoid oxyresveratrol was shown to influence inflammatory and metabolic processes. During cellular immune activation, tryptophan breakdown and neopterin formation via the enzymes indoleamine 2,3-dioxygenase-1 (IDO-1) and GTP-cyclohydrolase, respectively, are induced. Neopterin and the kynurenine to tryptophan ratio are reliable and pertinent biomarkers of Th1-type immune response and are also used in vitro to monitor effects of active plant ingredients on peripheral blood mononuclear cells (PBMCs). We investigated the effects of oxyresveratrol on the activity of the above-mentioned pathways in mitogen-stimulated human PBMC and in the myelomonocytic cell line THP-1. Oxyresveratrol exerted suppressive effects on tryptophan breakdown in both stimulated cell models. Of note, in PBMC, tryptophan breakdown was induced at lower concentrations (5–20 µM) and suppressed at higher treatment concentrations only. Neopterin formation was decreased dose-dependently in stimulated PBMC. In unstimulated PBMC similar, albeit lesser effects were observed. Data indicate that oxyresveratrol exerts distinct and concentration-dependent effects on different immune cell types. IDO-1 is targeted by oxyresveratrol and its activity can be modulated in both directions. Detailed investigations of the interactions would be interesting to fully explore the activity of this phytocompound.
1 Introduction
Oxyresveratrol is a polyphenolic phytoalexin produced by many plants of which some prominent examples are Morus alba L. (Moraceae) – known as white mulberry, jackfruit, Veratrum nigrum L. (Melanthiaceae), and Smilax china L. (Smilacaceae) [1,2]. Structurally, oxyresveratrol is a stilbene related to resveratrol, which has been demonstrated to have multiple bioactivities and has therefore been extensively studied (Figure 1) [3,4]. Protocols for the purification of oxyresveratrol from plant material as well as for the qualitative and quantitative analyses are available [5]. Fewer studies, however, are available on the biological effects of oxyresveratrol. Reports point toward interesting biological effects bearing diverse therapeutic potentials including the interference with different inflammatory signaling cascades, among those the inhibition of inducible nitric oxide synthase (iNOS), the blocking of mitogen-activated protein kinase (MAPK), and NF-κB signaling pathways in murine macrophages are relevant to this study [6,7]. Furthermore, antihyperlipidemic [8], neuroprotective functions [9], and interferences with energy metabolism [2] have also been reported. In recent years, oxyresveratrol has attracted renewed attention as a drug candidate as there are formulations available outweighing its low bioactivity, e.g., microemulsion- and nanoparticle-based drug delivery systems [10,11]. However, its role in inflammatory processes and its potential to attenuate inflammation have not yet been elucidated to every detail.
![Figure 1
Structure of oxyresveratrol (C14H12O4 – IUPAC: 4-[(E)-2-(3,5-dihydroxyphenyl)ethenyl]benzene-1,3-diol; CAS: 29700-22-9).](/document/doi/10.1515/pteridines-2020-0029/asset/graphic/j_pteridines-2020-0029_fig_001.jpg)
Structure of oxyresveratrol (C14H12O4 – IUPAC: 4-[(E)-2-(3,5-dihydroxyphenyl)ethenyl]benzene-1,3-diol; CAS: 29700-22-9).
The aim of this study was to investigate immunomodulatory effects exerted by oxyresveratrol in more detail focusing on central metabolic pathways of the cellular immune response. In response to the major T helper (Th) cell type-1 cytokine interferon-γ (IFN-γ), the enzyme guanosine-triphosphate (GTP)-cyclohydrolase I (GTP-CH-I) is activated in human monocytes/macrophages and dendritic cells. These cells then form neopterin, a sensitive indicator of immune activation and oxidative stress [12]. In parallel, IFN-γ also stimulates the enzyme indoleamine 2,3-dioxygenase-1 (IDO-1) that converts the essential amino acid tryptophan (Trp) to kynurenine (Kyn). The ratio of Kyn to Trp (Kyn/Trp) can be used as an indicator of IDO-1 activity in inflammatory conditions, which are reflected by elevated neopterin concentrations [13]. Elevated neopterin concentrations and a high Kyn/Trp closely correlate with each other in patients with infections, autoimmune diseases, and malignant tumors [12,14].
Furthermore, these biomarkers turned out to be helpful for the assessment of immunomodulatory effects of a variety of plant extracts and phytochemicals in vitro engaging human peripheral blood mononuclear cells (PBMCs) [15]. Neopterin production and Trp breakdown were influenced by the lignan derivatives trachelogenin, arctigenin, and matairesinol [16], alkaloid preparations of Uncaria tomentosa [17], and lavender essential oil and constituents [18]. Changes in these biochemical pathways were efficiently applied as read-out in bio-guided fractionation procedures of extracts [19]. Moreover, though interferences are possible at different steps of the pathway, for some compounds interactions could be mapped with the binding site of IDO-1 [20].
In addition, for resveratrol, anti-inflammatory properties were previously shown via suppression of neopterin production and Trp breakdown [21]. Oxyresveratrol differs from resveratrol by having an additional hydroxyl group at position 2′ of the stilbene moiety, which seems to have striking functional consequences. For example, while resveratrol was able to downregulate iNOS protein expression, this was not the case for oxyresveratrol, which, however, was less cytotoxic and somewhat more efficient in protecting microglia cell cultures from radicals [22].
In this study, the in vitro effects of oxyresveratrol on Trp breakdown and neopterin formation were investigated. The established model of mitogen-stimulated PBMC was applied, which is composed of different types of blood cells including lymphocytes and monocytes, thus representing a coculture that could mimic interactions among different cell types. THP‐1 cells are at an advanced stage of myelomonocytic development and are a frequently used model due to their sensitivity toward lipopolysaccharide (LPS) treatment, thereby exhibiting macrophage-like properties [23].
2 Materials and method
2.1 Chemicals
Oxyresveratrol, dimethylsulfoxide (DMSO), phytohemagglutinin (PHA) isolated from Phaseolus vulgaris red kidney bean, and LPS were purchased from Sigma Aldrich (Vienna, Austria). LPS and PHA were dissolved in phosphate-buffered saline (PBS) and stored at −20°C until use.
2.2 Isolation of PBMC and cell culture maintenance
PBMCs were isolated from the whole blood of healthy donors from whom informed consent was obtained that their donated blood might be used for scientific purposes in the case when it was not selected for transfusion. PBMCs were separated using density centrifugation as reported previously [24].
After isolation, PBMCs were washed in PBS supplemented with 1 mM EDTA and transferred into culture medium RPMI 1640 (Sigma Aldrich, Vienna, Austria) supplemented with 10% heat inactivated fetal calf serum (Biochrom, Berlin, Germany), 2 mM l-glutamine (Serva, Heidelberg, Germany) and 50 μg/mL gentamicin (Bio-Whittaker, Walkersville, MD, USA) and immediately used for the experiments.
The myelomonocytic THP-1 cell line (DMSZ, Braunschweig, Germany) was maintained in RPMI 1640 (Sigma-Aldrich, Vienna, Austria) supplemented with 10% fetal calf serum, 2 mM l-glutamine and 50 μg/mL gentamicin.
-
Informed consent: Informed consent has been obtained from all individuals included in this study.
-
Ethical approval: The research related to human use has been complied with all the relevant national regulations, institutional policies and in accordance with the tenets of the Helsinki Declaration, and has been approved by the authors’ institutional review board.
2.3 Cell treatments with oxyresveratrol
For the experiments, PBMC and THP-1 cells were seeded at a final density of 1 × 106 cells/mL/well and of 5 × 105 cells/mL/well, respectively, in 48-well plates. Cells were pre-incubated for 30 min with or without different concentrations of oxyresveratrol (5–80 µM).
Maximum vehicle concentrations were 0.01% (v/v) DMSO. Then, cells were stimulated with 10 µg/mL PHA (for PBMC) or 1 µg/mL LPS (for THP-1) and incubated for 48 h under standard culture conditions. Each of the experiments was run in duplicates, and for each PBMC experiment cells were prepared freshly from different donors.
2.4 Estimation of cell viability
Cell viability was determined using the CellTiter-Blue assay (Promega, Mannheim, Germany), which is based on the ability of living cells to convert the redox dye resazurin into the fluorescent end product resorufin. Fluorescence [560 nmex/590 nmem] was measured using a Tecan infinite F200 PRO plate reader (Tecan Group Ltd., Männedorf, Switzerland).
2.5 Determination of tryptophan and kynurenine concentrations
After 48 h, culture supernatants were harvested by centrifugation at 1,000 rpm for 20 min. Concentrations of Trp and Kyn were analyzed using high-performance liquid chromatography (HPLC) as described previously using Purospher STAR RP-18 endcapped (3 µm) HPLC columns (Merck, Darmstadt, Germany) and 3-nitro-l-tyrosine as an internal standard [25]. Kyn/Trp was calculated to estimate the IDO activity [13,15,16,17,18,19,20,21]
2.6 Determination of neopterin levels
Neopterin concentrations in PBMC supernatants were measured using a commercially available quantitative enzyme-linked immunosorbent assay system (BRAHMS Diagnostics, Hennigsdorf, Germany) according to the manufacturer’s instructions.
2.7 Statistical analysis
Data were analyzed by using the Statistical Package for the Social Sciences (version 21, SPSS, Chicago, IL, USA). To take into account that not all collected data followed a normal distribution, group comparisons were performed using non-parametric Friedman’s and Wilcoxon signed-rank tests for paired samples. p-values <0.05 were considered statistically significant. The half maximal (50% inhibitory) concentration (IC50) was calculated by using the CalcuSyn software (Biosoft, UK) [26], in cases of only two available data points a linear extrapolation approach was used.
3 Results
3.1 Immunobiochemical pathways can be activated by oxyresveratrol in PBMC and THP-1 cells
After 48 h of culture, mean Trp concentrations were 28.1 ± 1.2 µM (mean ± SEM) in unstimulated compared to 10.8 ± 1.0 µM in PHA-stimulated PBMC, representing a reduction of the initial medium content of approximately 24 and 71%, respectively (Figure 2a). In unstimulated cells, the Kyn concentration was 0.9 ± 0.2 µM and Kyn/Trp was 34.1 ± 8.6 µM/mM and increased to 9.5 ± 0.7 µM kynurenine and 984 ± 211 µM/mM Kyn/Trp in stimulated PBMC (Figure 2b and c). Stimulation with the mitogen PHA led to a 20% increase in cell viability compared to non-stimulated cells.
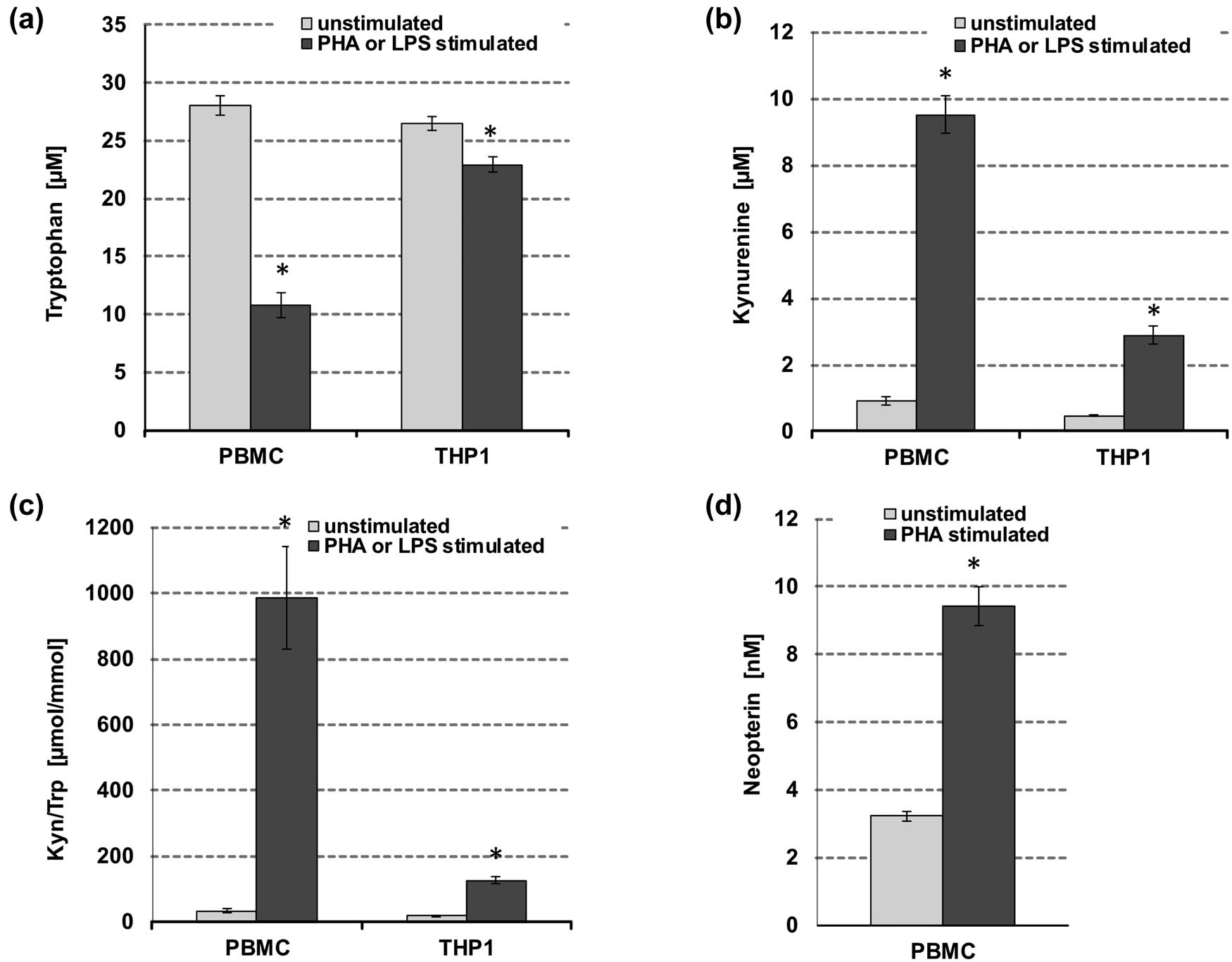
Effect of PHA stimulation on tryptophan (a), kynurenine (b), kynurenine to tryptophan ratio (Kyn/Trp) (c), and neopterin (d) concentrations in unstimulated (grey bars) and stimulated freshly isolated human PBMCs and THP1 cells (black bars), respectively; PHA was used to stimulate PBMC, while THP-1 cells were activated with LPS. Results shown are mean values ± SEM of four independent experiments run in duplicates (*p < 0.05, compared to baseline).
For THP-1 cells, after 48 h of culture, mean Trp concentrations were 26.5 ± 1.0 µM (mean ± SEM) in unstimulated and 22.9 ± 1.6 µM in LPS-stimulated cells (approximately 28 and 38% reduction of initial medium content, respectively) (Figure 2a). The Kyn concentration was 0.5 ± 0.0 µM and Kyn/Trp was 17.9 ± 1.1 µmol/mmol in unstimulated cells and increased to 2.9 ± 0.3 µM and 126 ± 17.5 µmol/mmol in LPS-treated THP-1 (Figure 2b and c). No difference was observed regarding the cell viability of untreated versus LPS-treated cells.
3.2 Oxyresveratrol affects tryptophan breakdown and neopterin formation in PBMC
As shown in Figure 3, PHA-stimulated cells reacted in a more sensitive way to oxyresveratrol treatment than unstimulated PBMC. A dose-dependent increase of Trp concentrations was observed, which was significant in the concentration range from 40 to 80 µM oxyresveratrol (Figure 3a). Tryptophan concentrations in supernatants of PHA-stimulated PBMC coincubated with 80 µM oxyresveratrol were even higher compared to supernatants of PHA-only treated control cells. Interestingly, the addition of 5 or 10 µM oxyresveratrol led to a superinduction of kynurenine formation/Trp breakdown compared to PHA-stimulated cells (Figure 3b and c). On the other hand, higher oxyresveratrol concentrations reduced kynurenine production, thus lowering Kyn/Trp. Treatment with 80 µM oxyresveratrol decreased Kyn/Trp strongly.
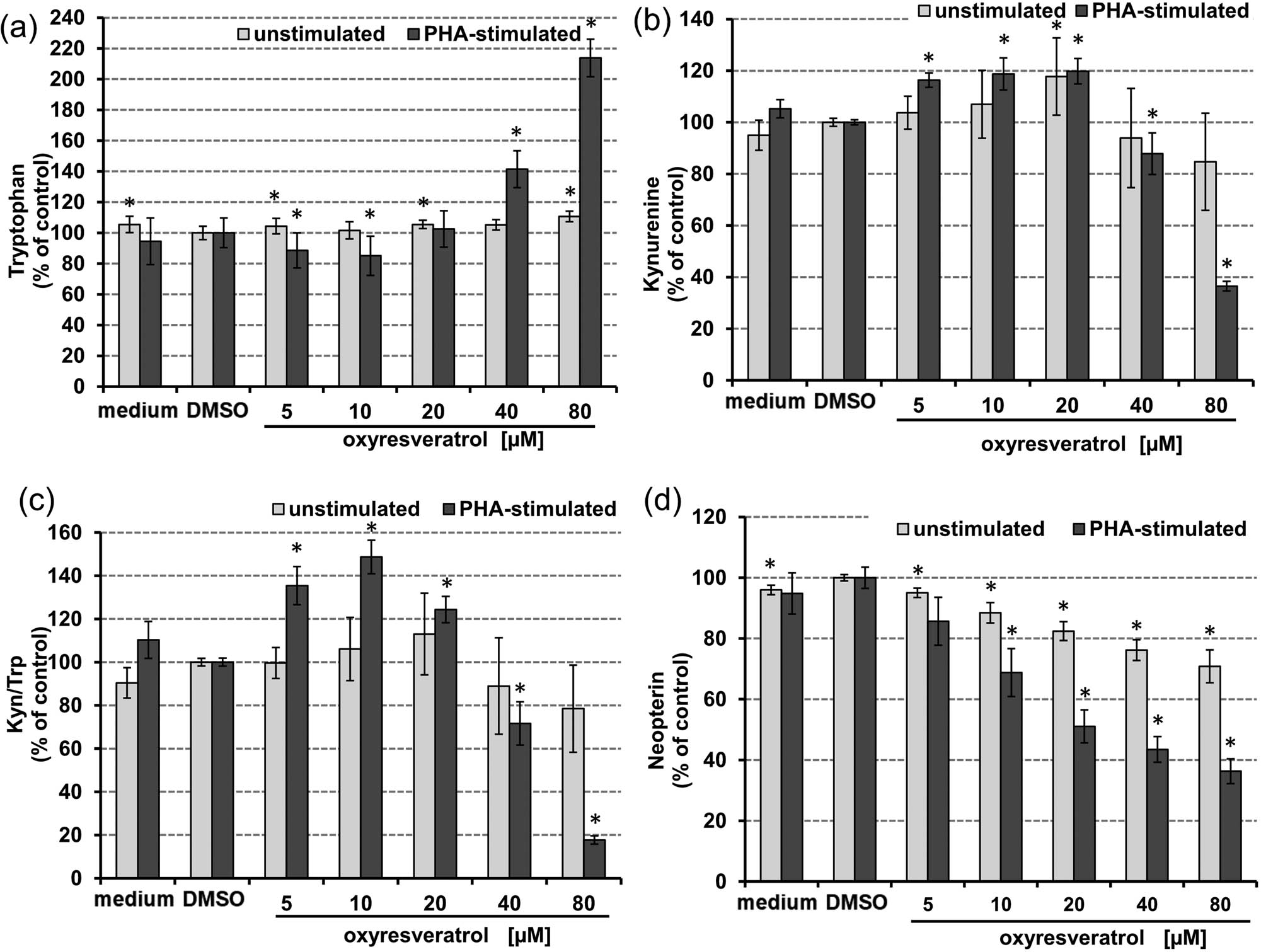
Effect of oxyresveratrol after 48 h treatment on tryptophan (a), and kynurenine (b) concentrations, kynurenine to tryptophan ratio (Kyn/Trp) (c), and neopterin (d) concentrations in unstimulated (grey bars) and PHA-stimulated freshly isolated human PBMCs (black bars); expressed as % of baseline (control cells treated with or without PHA, respectively, and DMSO). Tryptophan concentrations are expressed as % of medium control. Results shown are mean values ± SEM of four independent experiments run in duplicates (*p < 0.05, compared to baseline).
In unstimulated PBMC, the oxyresveratrol treatment influenced IDO-1-activity only slightly, however, at the highest treatment concentration of 80 µM the Kyn/Trp ratio tended to be lower.
Importantly, oxyresveratrol suppressed the neopterin formation in a dose-dependent manner in both unstimulated and PHA-stimulated cells. In stimulated cells, this effect was more pronounced and the highest concentration of 80 µM oxyresveratrol decreased neopterin levels to about 38% of control values (Figure 3d).
IC50 concentrations could be determined for inhibition of tryptophan breakdown in stimulated cells only. The IC50 for Kyn/Trp was reached at a treatment concentration of 47.1 µM oxyresveratrol (upper and lower 95% confidence interval [CI] of 21.1–105 µM, correlation coefficient (r) = 0.849) in PHA-stimulated PBMC. The IC50 for neopterin formation was reached at a much higher treatment concentration in unstimulated PBMC (305 µM, CI: 164–565 µM, r = 0.985) compared to stimulated cells (45.6 µM, CI: 31.2–66.6 µM, r = 0.958).
3.3 Interferences of oxyresveratrol with cell viability in PBMC
After 48 h incubation of PBMC with 5–80 µM oxyresveratrol, cytotoxicity was evaluated measuring resazurin to resorufin conversion (Figure 4). At 80 µM treatment concentration, oxyresveratrol decreased cell viability by approximately 20% in both stimulated and unstimulated cells, compared to the vehicle control-treated cells. The vehicle DMSO slightly increased cell viability compared to control cells only, still, the effect was less than 5%.
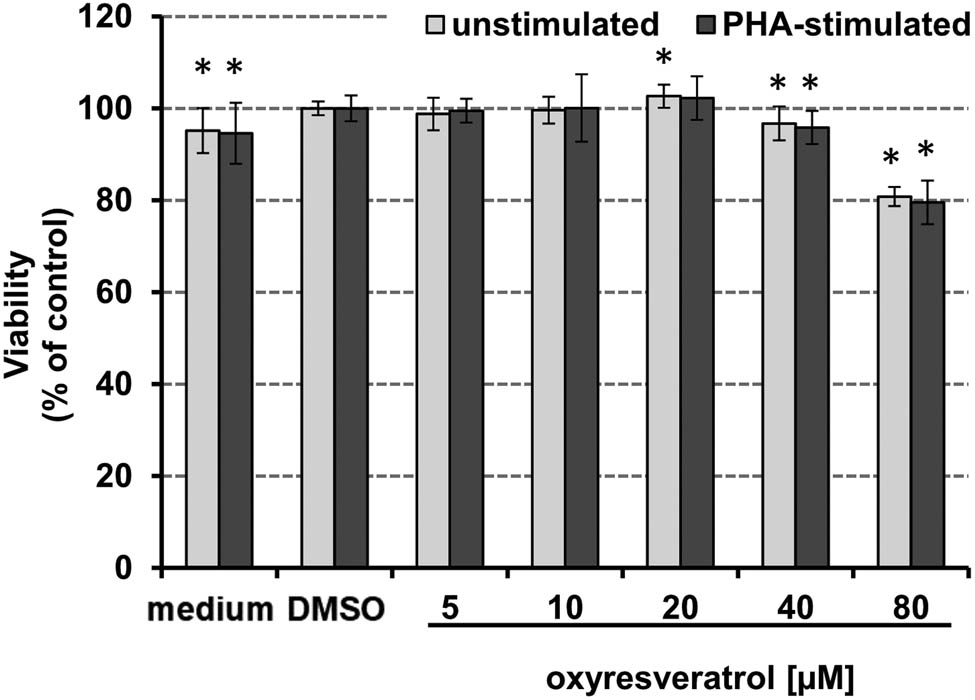
Effect of increasing concentrations of oxyresveratrol on the viability of freshly isolated human PBMCs. Viability of unstimulated (grey bars) and PHA-stimulated (black bars) PBMCs after incubation with increasing concentrations of oxyresveratrol for 48 h, expressed as % of baseline of PHA-stimulated and unstimulated PBMC with DMSO (*p < 0.05). The results shown are mean values ± SEM of four independent experiments run in duplicates.
3.4 Oxyresveratrol affects tryptophan breakdown in THP-1 cells
In LPS-stimulated cells, oxyresveratrol treatment affected tryptophan breakdown stronger than in unstimulated THP-1 cells. Trp concentrations slightly increased upon treatment, in both stimulated and unstimulated THP-1 cells compared to controls (Figure 5a). This effect was significant only at a treatment concentration of 80 µM oxyresveratrol. Kyn concentrations did not change in unstimulated THP-1 supernatants upon treatment (Figure 5b), and also Kyn/Trp decreased only somewhat upon treatment with 80 µM oxyresveratrol (Figure 5c). In LPS-treated THP-1 cells, Kyn concentrations decreased significantly and dose-dependently (Figure 5b). Likewise, Kyn/Trp was reduced significantly compared to control cells (Figure 5c). The IC50 for Trp breakdown was reached at a concentration of 50.7 µM oxyresveratrol (CI: 20.1–128 µM, r = 0.824) in LPS-stimulated THP-1 cells.
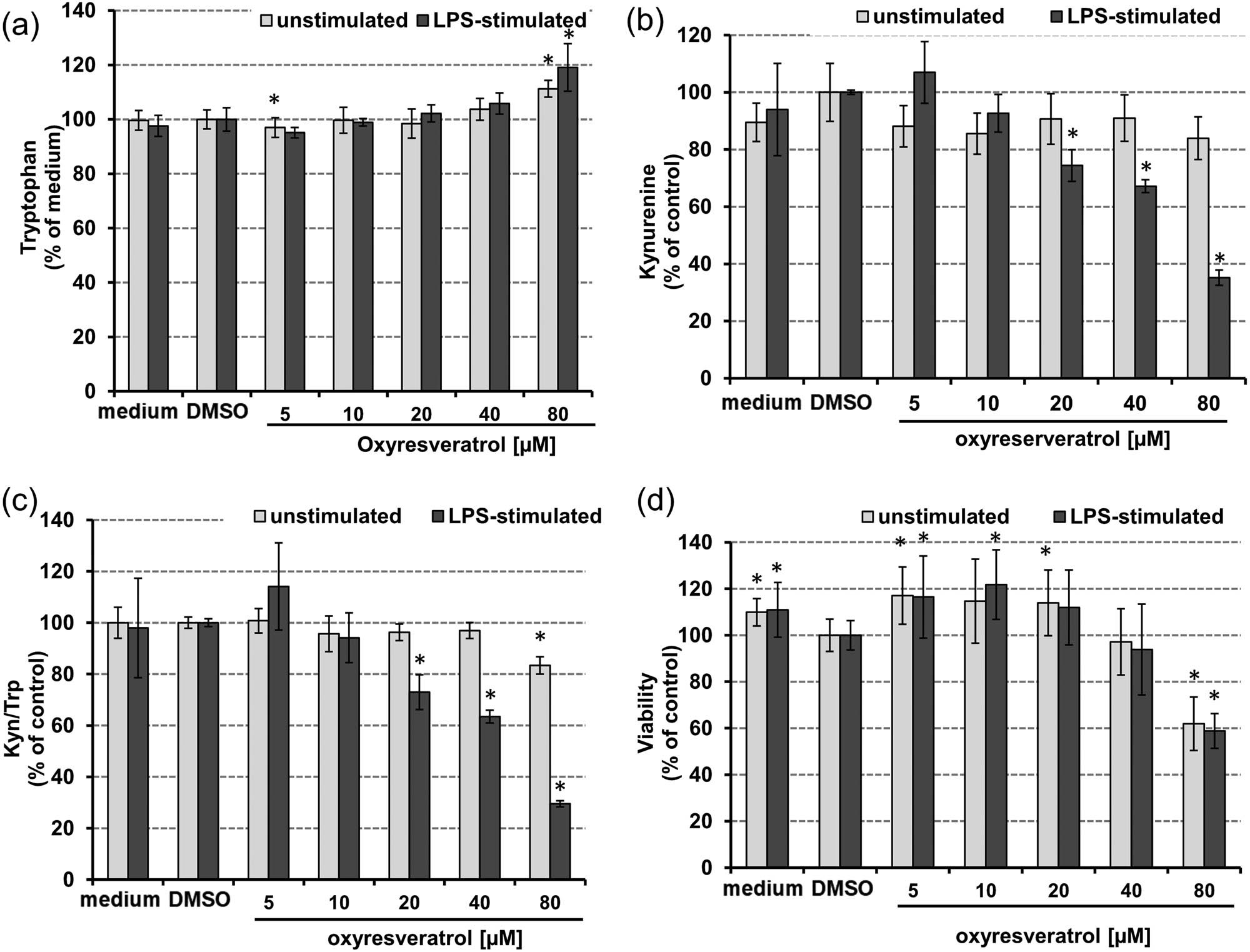
Effect of oxyresveratrol 48 h after treatment on the tryptophan (a), kynurenine (b) concentrations kynurenine to tryptophan ratio (Kyn/Trp) (c), and on cell viability (d) in unstimulated (grey bars) and LPS-stimulated THP-1 cells (black bars), expressed as % of baseline (control cells treated with or without LPS and DMSO, respectively). Tryptophan concentrations are expressed as % of medium control. Results shown are mean values ± SEM of three independent experiments run in duplicates (*p < 0.05, compared to baseline).
3.5 Interferences of oxyresveratrol with cell viability in THP-1
THP-1 cell viability was approximately 40% reduced at the highest treatment concentration of 80 µM oxyresveratrol in both stimulated and unstimulated cells compared to vehicle-treated controls (Figure 5d). Lower concentrations increased the viability up to 20% compared to the vehicle concentrations. This elevation remained significant only for few treatment concentrations when being compared to untreated control cells. The vehicle, DMSO, led to a decreased cell viability by about 10% compared to control cells.
4 Discussion
During inflammation, pro-inflammatory cytokines such as IFN-γ induce and regulate a variety of immune response pathways. Mitogen-stimulated PBMCs were efficiently applied in a variety of studies to investigate the immunomodulatory properties of phytochemicals and extracts [15,19,24]. PBMC release IFN-γ upon stimulation with the mitogen PHA, simultaneously also interleukin (IL)-2 concentrations rise [27]. As Trp breakdown and neopterin formation are activated in parallel upon IFN-γ signaling in human immune cells (in particular in monocytes/macrophages and dendritic cells) [28,29], determination of the concentrations of Trp, Kyn, and neopterin in cell culture supernatants of PBMC can be employed as a read-out to evaluate the effects of phytochemicals on the immune response in vitro [15].
Resveratrol was shown previously to suppress these immunobiochemical pathways in PHA- and concanavalin-stimulated PBMC dose-dependently in a concentration range from 10 to 100 µM. Moreover, phorbol 12-myristate 13-acetate (PMA)/ionomycin-stimulated production of IFN-γ, IL-2, and tumor necrosis factor alpha (TNF-α) were suppressed by resveratrol [21]. This study and the poor solubility of oxyresveratrol, also in the presence of a vehicle, have been considered for dose selection.
Our data show that also oxyresveratrol is able to suppress Trp breakdown and neopterin formation in PBMC and in addition, to decrease IDO-activity in the cell line THP-1. Thus, both the stilbenes, resveratrol and oxyresveratrol, are very effective to suppress immune activation pathways, but there were some differences, which shall now be described in more detail: In stimulated PBMCs all concentrations of resveratrol suppressed neopterin production and IDO-activation [21], while oxyresveratrol stimulated kynurenine formation and IDO-activation at 5–20 µM and decreased it dose-dependently at higher concentrations.
Interestingly, we could confirm that stilbenes induce nonmonotonic dose-dependent responses on kynurenine production in unstimulated PBMC: the IDO activity was stimulated by low concentrations of oxyresveratrol and resveratrol [21], while it was suppressed by high concentrations (80 and 100 µM, respectively) in unstimulated PBMC. Neopterin formation of PBMC was influenced similarly by oxyresveratrol and resveratrol treatment: It was suppressed dose-dependently in stimulated as well as unstimulated PBMC.
These data may indicate that oxyresveratrol and resveratrol may modulate Trp metabolism differentially and also independent from IFN-γ, possibly by interacting with intracellular signaling pathways dependent on reactive oxygen species. Decreased IFN-γ formation by T-cells and decreased ROS formation by monocytes/macrophages (due to the potent antioxidants resveratrol and oxyresveratrol) might explain this effect. The direct interaction with the relevant enzymes IDO-1 and GTP-CH-I may also contribute to this effect.
In fact, the interplay of T cells and macrophages could be responsible for the non-monotonic response, as demonstrated in unstimulated as well as in stimulated PBMC treated with oxyresveratrol; in THP-1 myelomonocytes, oxyresveratrol led to a significant, dose-dependent suppression of Trp breakdown when cells were stimulated with LPS. On the other hand, the fact that THP-1 cells are tumor cells may account for differences: Oxyresveratrol treatment decreased cell viability much stronger in THP-1 cells than in PBMC (nearly twice at 80 µM). Tumor cells proliferate stronger and are probably more susceptible to the effects of antioxidants; a study in breast cancer cells even described pro-apoptotic effects of oxyresveratrol [30]. Earlier experiments with resveratrol were only performed with PBMC, but not with THP-1 cells [21]. It is worthy to mention that the vehicle DMSO, despite being used at a very low concentration, slightly increased cell viability with PBMC, while in THP-1 cells the opposite effect was observed. The differences in metabolism and redox homeostasis of the applied cell models may also be responsible for this diverse response.
A preliminary study, assessing the effects of oral resveratrol on these biomarkers in human healthy volunteers, further points toward a potential stimulatory activity of resveratrol, as indicated by a slightly elevated serum Kyn/Trp ratio compared to placebo controls 2.5 and 5 h after intake, while neopterin concentrations did not change significantly [31]. Thus, low concentrations of stilbenes might stimulate Trp breakdown slightly also in vivo, while higher concentrations might rather decrease its activity. Apart from that, oxyresveratrol might act via different mechanisms or may elicit other effects on intracellular signaling pathways dependent on the concentration used, although it is structurally similar to resveratrol.
Limitations of this study are that neither a potential bioconversion nor the stability of oxyresveratrol during the incubation time was investigated. In plants, oxyresveratrol occurs in both free and glycosidic forms. In humans, oxyresveratrol is absorbed by the liver after deglycosylation by intestinal bacteria. Hepatic conjugation reveals oxyresveratrol 2-O-β-d-glucuronide and sulfate [5,32]. The impact of inter-individual variations of uridine diphospho-glucuronosyltransferase (UGT) enzymes and their role in the metabolism of oxyresveratrol need to be studied in more detail when aiming for a therapeutic application [33]. However, this aspect as well as the discussion of a potential conversion by extrahepatic enzymes is beyond the scope of the recent study. Moreover, the period immediately after incubation of cells with oxyresveratrol and upon stimulation with PHA is most likely critical when focusing on the antioxidative potential and its impact on immunometabolic pathways investigated in this study. Nevertheless, in addition to counteracting ROS formation and attenuation of the PHA-driven stimulation of IFN-γ release, direct inhibition of relevant enzymes may play a role.
There is accumulating evidence demonstrating the capacity of oxyresveratrol to interfere with pharmaceutically relevant circuits. For example, oxyresveratrol is suggested to strengthen antioxidative strategies by interfering with nuclear factor (erythroid-derived 2)-like 2 (Nrf-2) and extracellular signal-regulated kinase 1/2 (ERK1/2), which was shown in the human hepatocarcinoma cell line HepG2 [34]. The same study described liver-protective effects of oxyresveratrol administration in carbon tetrachloride-treated mice. Estrogen receptor agonistic effects are discussed due to induction of increased proliferation of MCF-7 human breast cancer cells, activation of ER-target gene transcription, and interferences with NF-κB signaling [35]. Moreover, Chung et al. reported an inhibitory effect on iNOS expression and nitrite accumulation in supernatants, on translocation of nuclear factor kappa B (NF-κB) and on activity of cyclooxygenase (COX)-2 in LPS-stimulated murine macrophage RAW 264.7 cell line [6,7].
5 Conclusion
In conclusion, the results reported here show the capacity of oxyresveratrol to interfere with immunobiochemical pathways in human cell models. Moreover, differential effects on Trp breakdown and the formation of neopterin in stimulated PBMC suggest variable effects on individual cell populations in particular at lower concentrations, probably by attenuating monocyte/macrophage activity, while activating T cells. Certainly, further studies are warranted to decipher the molecular background of these activities.
Acknowledgements
The authors kindly acknowledge Stefanie Hofer for assistance in preparing the figures. This research received no external funding. All authors have read and agreed to the published version of the manuscript.
-
Conflict of interest: Dietmar Fuchs is the Editor-in-Chief, and Katharina Kurz is the Editorial Board member of Pteridines. Other authors state no conflict of interest.
-
Data availability statement: The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
[1] Xu L, Liu C, Xiang W, Chen H, Qin X, Huang X. Advances in the study of oxyresveratrol. Int J Pharmacol. 2014;10(1):44–54.10.3923/ijp.2014.44.54Search in Google Scholar
[2] Chan EW, Lye PY, Wong SK. Phytochemistry, pharmacology, and clinical trials of Morus alba. Chin J Nat Med. 2016 Jan;14(1):17–30.Search in Google Scholar
[3] de Sá Coutinho D, Pacheco MT, Frozza RL, Bernardi A. Anti-inflammatory effects of resveratrol: mechanistic insights. Int J Mol Sci. 2018 Jun;19(6):1812.10.3390/ijms19061812Search in Google Scholar PubMed PubMed Central
[4] Nawaz W, Zhou Z, Deng S, Ma X, Ma X, Li C, et al. Therapeutic versatility of resveratrol derivatives. Nutrients. 2017 Oct;9(11):E1188.10.3390/nu9111188Search in Google Scholar PubMed PubMed Central
[5] Likhitwitayawuid K. Oxyresveratrol: sources, productions, biological activities, pharmacokinetics, and delivery systems. Molecules. 2021 Jul;26(14):4212.10.3390/molecules26144212Search in Google Scholar PubMed PubMed Central
[6] Chung KO, Kim BY, Lee MH, Kim YR, Chung HY, Park JH, et al. In-vitro and in-vivo anti-inflammatory effect of oxyresveratrol from Morus alba L. J Pharm Pharmacol. 2003 Dec;55(12):1695–700.10.1211/0022357022313Search in Google Scholar PubMed
[7] Lee HS, Kim DH, Hong JE, Lee JY, Kim EJ. Oxyresveratrol suppresses lipopolysaccharide-induced inflammatory responses in murine macrophages. Hum Exp Toxicol. 2015 Aug;34(8):808–18.10.1177/0960327114559989Search in Google Scholar PubMed
[8] Jo SP, Kim JK, Lim YH. Antihyperlipidemic effects of stilbenoids isolated from Morus alba in rats fed a high-cholesterol diet. Food Chem Toxicol. 2014 Mar;65:213–8.10.1016/j.fct.2013.12.040Search in Google Scholar PubMed
[9] Chao J, Yu MS, Ho YS, Wang M, Chang RC. Dietary oxyresveratrol prevents parkinsonian mimetic 6-hydroxydopamine neurotoxicity. Free Radic Biol Med. 2008 Oct;45(7):1019–26.10.1016/j.freeradbiomed.2008.07.002Search in Google Scholar PubMed
[10] Sangsen Y, Sooksawate T, Likhitwitayawuid K, Sritularak B, Wiwattanapatapee R. A self-microemulsifying formulation of oxyresveratrol prevents amyloid beta protein-induced neurodegeneration in mice. Planta Med. 2018 Jul;84(11):820–8.10.1055/s-0043-125337Search in Google Scholar PubMed
[11] Donini M, Gaglio SC, Laudanna C, Perduca M, Dusi S. Oxyresveratrol-loaded PLGA nanoparticles inhibit oxygen free radical production by human monocytes: role in nanoparticle biocompatibility. Molecules. 2021 Jul;26(14):4351.10.3390/molecules26144351Search in Google Scholar PubMed PubMed Central
[12] Murr C, Widner B, Wirleitner B, Fuchs D. Neopterin as a marker for immune system activation. Curr Drug Metab. 2002 Apr;3(2):175–87.10.2174/1389200024605082Search in Google Scholar
[13] Fuchs D, Möller AA, Reibnegger G, Werner ER, Werner-Felmayer G, Dierich MP, et al. Increased endogenous interferon-gamma and neopterin correlate with increased degradation of tryptophan in human immunodeficiency virus type 1 infection. Immunol Lett. 1991 Jun;28(3):207–11.10.1016/0165-2478(91)90005-USearch in Google Scholar
[14] Schröcksnadel K, Wirleitner B, Winkler C, Fuchs D. Monitoring tryptophan metabolism in chronic immune activation. Clin Chim Acta. 2006 Feb;364(1–2):82–90.10.1016/j.cca.2005.06.013Search in Google Scholar PubMed
[15] Becker K, Schroecksnadel S, Gostner J, Zaknun C, Schennach H, Uberall F, et al. Comparison of in vitro tests for antioxidant and immunomodulatory capacities of compounds. Phytomedicine. 2014 Jan;21(2):164–71.10.1016/j.phymed.2013.08.008Search in Google Scholar PubMed
[16] Kuehnl S, Schroecksnadel S, Temml V, Gostner JM, Schennach H, Schuster D, et al. Lignans from Carthamus tinctorius suppress tryptophan breakdown via indoleamine 2,3-dioxygenase. Phytomedicine. 2013 Oct;20(13):1190–5.10.1016/j.phymed.2013.06.006Search in Google Scholar PubMed PubMed Central
[17] Winkler C, Wirleitner B, Schroecksnadel K, Schennach H, Mur E, Fuchs D. In vitro effects of two extracts and two pure alkaloid preparations of Uncaria tomentosa on peripheral blood mononuclear cells. Planta Med. 2004 Mar;70(3):205–10.10.1055/s-2004-815536Search in Google Scholar PubMed
[18] Gostner JM, Ganzera M, Becker K, Geisler S, Schroecksnadel S, Überall F, et al. Lavender oil suppresses indoleamine 2,3-dioxygenase activity in human PBMC. BMC Complement Altern Med. 2014 Dec;14(1):503.10.1186/1472-6882-14-503Search in Google Scholar PubMed PubMed Central
[19] Becker K, Schwaiger S, Waltenberger B, Fuchs D, Pezzei CK, Schennach H, et al. Immunomodulatory effects of diterpene quinone derivatives from the roots of Horminum pyrenaicum in human PBMC. Oxid Med Cell Longev. 2018 Jan;2018:2980295.10.1155/2018/2980295Search in Google Scholar PubMed PubMed Central
[20] Temml V, Kuehnl S, Schuster D, Schwaiger S, Stuppner H, Fuchs D. Interaction of Carthamus tinctorius lignan arctigenin with the binding site of tryptophan-degrading enzyme indoleamine 2,3-dioxygenase. FEBS Open Bio. 2013 Sep;3(1):450–2.10.1016/j.fob.2013.08.008Search in Google Scholar PubMed PubMed Central
[21] Wirleitner B, Schroecksnadel K, Winkler C, Schennach H, Fuchs D. Resveratrol suppresses interferon-gamma-induced biochemical pathways in human peripheral blood mononuclear cells in vitro. Immunol Lett. 2005 Sep;100(2):159–63.10.1016/j.imlet.2005.03.008Search in Google Scholar PubMed
[22] Lorenz P, Roychowdhury S, Engelmann M, Wolf G, Horn TF. Oxyresveratrol and resveratrol are potent antioxidants and free radical scavengers: effect on nitrosative and oxidative stress derived from microglial cells. Nitric Oxide. 2003 Sep;9(2):64–76.10.1016/j.niox.2003.09.005Search in Google Scholar
[23] Eperon S, Jungi TW. The use of human monocytoid lines as indicators of endotoxin. J Immunol Methods. 1996 Aug;194(2):121–9.10.1016/0022-1759(96)00073-7Search in Google Scholar
[24] Jenny M, Klieber M, Zaknun D, Schroecksnadel S, Kurz K, Ledochowski M, et al. In vitro testing for anti-inflammatory properties of compounds employing peripheral blood mononuclear cells freshly isolated from healthy donors. Inflamm Res. 2011 Feb;60(2):127–35.10.1007/s00011-010-0244-ySearch in Google Scholar
[25] Widner B, Werner ER, Schennach H, Wachter H, Fuchs D. Simultaneous measurement of serum tryptophan and kynurenine by HPLC. Clin Chem. 1997 Dec;43(12):2424–6.10.1093/clinchem/43.12.2424Search in Google Scholar
[26] Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55.10.1016/0065-2571(84)90007-4Search in Google Scholar
[27] Katial RK, Sachanandani D, Pinney C, Lieberman MM. Cytokine production in cell culture by peripheral blood mononuclear cells from immunocompetent hosts. Clin Diagn Lab Immunol. 1998 Jan;5(1):78–81.10.1128/CDLI.5.1.78-81.1998Search in Google Scholar
[28] Werner ER, Werner-Felmayer G, Fuchs D, Hausen A, Reibnegger G, Wachter H. Parallel induction of tetrahydrobiopterin biosynthesis and indoleamine 2,3-dioxygenase activity in human cells and cell lines by interferon-gamma. Biochem J. 1989 Sep;262(3):861–6.10.1042/bj2620861Search in Google Scholar
[29] Werner ER, Werner-Felmayer G, Fuchs D, Hausen A, Reibnegger G, Yim JJ, et al. Tetrahydrobiopterin biosynthetic activities in human macrophages, fibroblasts, THP-1, and T 24 cells. GTP-cyclohydrolase I is stimulated by interferon-gamma, and 6-pyruvoyl tetrahydropterin synthase and sepiapterin reductase are constitutively present. J Biol Chem. 1990 Feb;265(6):3189–92.10.1016/S0021-9258(19)39752-2Search in Google Scholar
[30] Sunilkumar D, Drishya G, Chandrasekharan A, Shaji SK, Bose C, Jossart J, et al. Oxyresveratrol drives caspase-independent apoptosis-like cell death in MDA-MB-231 breast cancer cells through the induction of ROS. Biochem Pharmacol. 2020 Mar;173:113724.10.1016/j.bcp.2019.113724Search in Google Scholar PubMed
[31] Gualdoni GA, Fuchs D, Zlabinger GJ, Gostner JM. Resveratrol intake enhances indoleamine-2,3-dioxygenase activity in humans. Pharmacol Rep. 2016 Oct;68(5):1065–8.10.1016/j.pharep.2016.06.008Search in Google Scholar PubMed
[32] Junsaeng D, Anukunwithaya T, Songvut P, Sritularak B, Likhitwitayawuid K, Khemawoot P. Comparative pharmacokinetics of oxyresveratrol alone and in combination with piperine as a bioenhancer in rats. BMC Complement Altern Med. 2019 Sep;19(1):235.10.1186/s12906-019-2653-ySearch in Google Scholar PubMed PubMed Central
[33] Guillemette C, Lévesque É, Rouleau M. Pharmacogenomics of human uridine diphospho-glucuronosyltransferases and clinical implications. Clin Pharmacol Ther. 2014 Sep;96(3):324–39.10.1038/clpt.2014.126Search in Google Scholar PubMed
[34] Choi HY, Lee JH, Jegal KH, Cho IJ, Kim YW, Kim SC. Oxyresveratrol abrogates oxidative stress by activating ERK-Nrf2 pathway in the liver. Chem Biol Interact. 2016 Feb;245:110–21.10.1016/j.cbi.2015.06.024Search in Google Scholar PubMed
[35] Wei J, Chen JR, Pais EM, Wang TY, Miao L, Li L, et al. Oxyresveratrol is a phytoestrogen exerting anti-inflammatory effects through NF-κB and estrogen receptor signaling. Inflammation. 2017 Aug;40(4):1285–96.10.1007/s10753-017-0572-ySearch in Google Scholar PubMed
© 2021 Saziye Sezin Palabiyik-Yucelik et al., published by De Gruyter
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Research Articles
- Norepinephrine was superior in death risk reducing and hemodynamics compared to dopamine in treatment of patients with septic shock
- Clinical significance of serum homocysteine as a biomarker for early diagnosis of diabetic nephropathy in type 2 diabetes mellitus patients
- The urinary biopterin in autism spectrum disorder
- Correlation between MTHFR 677C > T polymorphism and response of pemetrexed-based chemotherapy in advanced NSCLC: A meta-analysis
- Homocysteine is potential serological marker for predicting the risk of deep venous thrombosis of the lower extremities in patients received operation of lower limb fracture
- Association of expression and genotypes of thymidylate synthase in non-small cell lung cancer patients with different clinicopathological characteristics
- Lipophilic vs. hydrophilic statins and psychiatric hospitalizations and emergency room visits in US Veterans with schizophrenia and bipolar disorder
- Oxyresveratrol modulates the immune response in vitro
- Screening and bioinformatics analysis of key biomarkers in acute myocardial infarction
- Circulating concentrations of citrulline, neopterin, kynurenine, and tryptophan during chemoradiation in patients with cervical carcinoma
- Methylene tetrahydrofolate dehydrogenase 2 (MTHFD2) is overexpressed in head and neck squamous cell carcinoma (HNSCC) and correlated with patient’s poor prognosis
- Ferroptosis-associated gene SLC7A11 is upregulated in NSCLC and correlated with patient’s poor prognosis: An integrated bioinformatics analysis
- Correlation between methylene tetrahydrofolate reductase (MTHFR) gene rs1801133 C>T polymorphisms and risk of osteoporosis
- Short Communication
- Laboratory diagnostic value of neopterin measurements in patients with COVID-19 infection
Articles in the same Issue
- Research Articles
- Norepinephrine was superior in death risk reducing and hemodynamics compared to dopamine in treatment of patients with septic shock
- Clinical significance of serum homocysteine as a biomarker for early diagnosis of diabetic nephropathy in type 2 diabetes mellitus patients
- The urinary biopterin in autism spectrum disorder
- Correlation between MTHFR 677C > T polymorphism and response of pemetrexed-based chemotherapy in advanced NSCLC: A meta-analysis
- Homocysteine is potential serological marker for predicting the risk of deep venous thrombosis of the lower extremities in patients received operation of lower limb fracture
- Association of expression and genotypes of thymidylate synthase in non-small cell lung cancer patients with different clinicopathological characteristics
- Lipophilic vs. hydrophilic statins and psychiatric hospitalizations and emergency room visits in US Veterans with schizophrenia and bipolar disorder
- Oxyresveratrol modulates the immune response in vitro
- Screening and bioinformatics analysis of key biomarkers in acute myocardial infarction
- Circulating concentrations of citrulline, neopterin, kynurenine, and tryptophan during chemoradiation in patients with cervical carcinoma
- Methylene tetrahydrofolate dehydrogenase 2 (MTHFD2) is overexpressed in head and neck squamous cell carcinoma (HNSCC) and correlated with patient’s poor prognosis
- Ferroptosis-associated gene SLC7A11 is upregulated in NSCLC and correlated with patient’s poor prognosis: An integrated bioinformatics analysis
- Correlation between methylene tetrahydrofolate reductase (MTHFR) gene rs1801133 C>T polymorphisms and risk of osteoporosis
- Short Communication
- Laboratory diagnostic value of neopterin measurements in patients with COVID-19 infection

