Abstract
Formation of neopterin, a biomarker of the activated human immune system, is linked with tryptophan (TRP) and phenylalanine (PHE) metabolism. To obtain normal values, in this study, serum concentrations of neopterin as well as of TRP, PHE and their respective metabolites kynurenine (KYN) and tyrosine (TYR) were investigated in 100 successive blood donor serum specimens from the University Clinics of Innsbruck, Austria. In addition, nitrite concentrations were determined. Donors had passed anamnestic examination at entry and were therefore considered as healthy. The mean age of participants was 49±11.4 (mean±SD) years; 18% were older than 60 years. Both genders were included in the analysis. Neopterin concentrations measured by enzyme-linked immunosorbent assay were 5.9±1.6 nmol/L (mean±SD). Levels of amino acids and metabolites were determined by HPLC. Mean KYN and TRP concentrations were 1.78±0.42 μmol/L and 67.4±10.2 μmol/L, respectively. KYN to TRP ratio (KYN/TRP), an estimate for the activity of tryptophan-degrading enzyme indoleamine 2,3-dioxygenase, was 26.7±6.2 μmol/mmol. Mean PHE and TYR concentrations were 65.2±11.1 μmol/L and 90.6±22.9 μmol/L. PHE to TYR ratio (PHE/TYR), an estimate for the activity of PHE-converting enzyme phenylalanine hydroxylase, was 0.75±0.14 μmol/μmol. Nitrite concentrations, estimated by Griess-Ilosvay reagent, were 44.9±32.0 μmol/L. Males were taller and heavier than females (both p<0.01), but body mass index did not differ. Males presented with significantly higher TRP and TYR concentrations than females (both p<0.05). There existed significant correlations between neopterin and KYN (rs=0.368), KYN/TRP (rs=0.453), TYR (rs=–0.267; all p<0.01) and PHE/TYR (rs=0.236; p<0.05) concentrations. Data indicate that also in a population of healthy individuals an association exists between “low-grade” immune activation as is indicated by slightly higher neopterin concentrations and biochemical alterations in the amino acid metabolism. Although minor, such changes may interfere with psychoneuroimmunological regulatory networks and thus be of clinical relevance.
Introduction
Immune activation and inflammatory responses play a relevant role in the pathogenesis of a variety of diseases like cancer, infections and autoimmune disorders. Concentrations of 6-(D-erythro-1′,2′,3′-trihydroxypropyl)-pterin (neopterin), a well-established immune activation marker, were found to be elevated in these patients and to be of diagnostic value [1, 2]. Neopterin is primarily produced and released from activated human monocyte-derived macrophages, dendritic cells and astrocytes [3–5]. Thereby, formation of increased amounts of neopterin in macrophages is mainly initiated by Th1-type cytokine interferon-gamma (IFN-γ) [3], whereas type I interferons are also potent inducers of neopterin production in other cells like dendritic cells or astrocytes [4, 5]. The central enzyme involved in the biosynthesis of neopterin is GTP-cyclohydrolase-1 (GCH-1), which converts guanosine triphosphate (GTP) to 7,8-dihydroneopterintriphosphate [6].
IFN-γ is also the most important inducer of the antiproliferative and immunoregulatory enzyme indoleamine 2,3-dioxygenase (IDO) [7, 8]. IDO converts the essential amino acid L-tryptophan (TRP) to kynurenine (KYN), which is the first and rate-limiting step of tryptophan catabolism. IDO enzyme activity can be calculated by using the KYN to TRP quotient. Accelerated TRP breakdown and elevated KYN/TRP concentrations have been shown in many diseases and closely correlate with concentrations of immune activation markers like neopterin [1, 9–12]. In these situations, inflammation-activated IDO is driving the TRP catabolism and not tryptophan dioxygenase, a hepatic enzyme that is regulated via tryptophan levels or glucocorticoids. In many cases, the TRP breakdown rate not only correlates with neopterin concentrations, but also with the extent and the activity of the disease, e.g., in viral infections or malignant tumors [8–13].
While in human monocytic cells enzyme GCH-1 is responsible for the production of neopterin, it also induces the synthesis of 5,6,7,8-tetrahydrobiopterin (BH4), an essential cofactor of aromatic amino acid monooxygenases such as phenylalanine 4-hydroxylase (PAH), tyrosine 3-monooxygenase, tryptophan 5-hydroxylase, and also of the nitric oxide synthases (NOS) and of alkylglycerol monooxygenase [14–16]. PAH converts the essential amino acid phenylalanine (PHE) to tyrosine (TYR), which is further hydroxylated by tyrosine 3-monooxygenase to L-3,4-dihydroxyphenylalanine, a precursor of the neurotransmitter dopamine. Tryptophan 5-hydroxylase converts TRP to 5-hydroxytryptophan, the precursor molecule of the neurotransmitter serotonin. NOS produces nitric oxide (NO) by converting L-arginine to L-citrulline.
Decreased phytohaemagglutinin activity leads to an increase of PHE concentrations and of the phenylalanine to tyrosine ratio (PHE/TYR). PHE/TYR is well known to be highly elevated in patients suffering from inborn errors of PHE metabolism, e.g., classical phenylketonuria. In addition, a moderate form of hyperphenylalaninaemia is reported for patients who suffer from chronic inflammatory diseases. Notably, in such patients the increased PHE and PHE/TYR concentrations were also found to correlate with neopterin levels [17–19].
The aim of this investigation was to establish reference values for the above mentioned interrelated biomarkers in a reasonably sized healthy population of 100 blood donors of both genders, who had passed anamnestic examination and therefore can be considered as widely healthy and free from overt infections. In addition, potential associations between the concentrations of the different biomarkers were examined.
Materials and methods
Participants
One hundred participants were recruited from the Central Institute of Blood Transfusion and Immunology of the University Clinics Innsbruck. The participants had passed the regular anamnestic check before they were allowed to donate one blood unit, and no signs of infections or disease were noticed. All laboratory measurements were performed in a blinded manner. Donors gave informed consent that blood donations not selected for transfusion can be used in a blinded way for further scientific testing. The samples were stored at –20°C until the measurement.
Methods
Neopterin concentrations were determined by enzyme-linked immunosorbent assay (ELISA) (BRAHMS GmbH, Hennigsdorf, Germany) following the manufacturer’s instructions. Sensitivity of the test was 2 nmol/L neopterin.
Measurement of KYN and TRP concentrations was performed by reverse-phase HPLC method as described [20], using a Varian ProStar HPLC system equipped with a solvent delivery module (model 210), an autosampler (model 400, both Varian ProStar), an UV-spectrometric detector (SPD-6A, Shimadzu) and a fluorescence detector (model 360, Varian ProStar). Varian Star Chromatography Workstation (version 6.30) software was used. 3-nitro-L-tyrosine, KYN and TRP were purchased from Sigma (Steinheim, Germany); trichloroacetic acid and PHE were purchased from Merck KGaA (Darmstadt, Germany), TYR was purchased from Dr. Ehrensdorfer GmbH (Augsburg, Germany) and albumin was from Serva (Heidelberg, Germany). In brief, 100 μL of serum, 100 μL of internal standard (25 μmol/L 3-nitro-L-tyrosine) and 25 μL of 2 mol/L trichloroacetic acid were vortexed and centrifuged to precipitate proteins and generate the supernatants that were measured. An albumin-based mixture with 100 μmol/L TRP and 10 μmol/L KYN was treated in the same way as serum specimens and served as an external calibrator. Analytes were separated by reverse-phase HPLC using a LiChrosorb C18 column (5 μm particle size, Merck, Darmstadt, Germany) and 15 mmol/L acetic acid-sodium acetate solution (pH=4.0) as mobile phase. KYN and internal standard 3-nitro-L-tyrosine concentrations were determined by a UV detector at a wavelength of 360 nm. TRP was detected by its natural fluorescence at an excitation wavelength of 286 nm and an emission wavelength of 366 nm. The KYN/TRP ratio was calculated and expressed as μmol/mmol [9, 20].
PHE and TYR concentrations were determined simultaneously by HPLC by monitoring their natural fluorescence at an excitation wavelength of 210 nm and an emission wavelength of 302 nm [21]. In brief, 30 μL of plasma was diluted with 30 μL of 0.015 mol/L potassium dihydrogen phosphate and 300 μL of 500 μmol/L 3-nitro-L-tyrosine (internal standard), and 75 μL of 2 mol/L trichloroacetic acid was used to precipitate proteins. After centrifugation, 370 μL of supernatants was diluted with 400 μL potassium dihydrogen phosphate (0.015 mol/L), which was also used as elution buffer on HPLC. An albumin-based calibration mixture was prepared, which contained 100 μmol/L each of PHE and TYR, and underwent the same pre-analytical procedures as the plasma specimens. The PHE/TYR ratio was calculated and expressed as μmol/μmol [21].
Nitrite concentrations were measured with the modified Griess-Ilosvay diazotization reaction assay (Merck KGaA, Darmstadt, Germany) [22]. Thereby, sulfanilamide was quantitatively converted to a diazonium salt by reaction with NO2– present in samples under acidic (phosphoric acid) conditions. The diazonium salt was then coupled with N-(1-naphthyl)ethylenediamine dihydrochloride, forming an azo dye that was analyzed at 562 nm in a spectrophotometer (KC4 reader, Bio-Tek Instruments Inc.).
Data analyses
Because not all data sets showed normal distribution, data were analyzed by applying non-parametric tests such as Mann-Whitney U-test for group comparisons and Spearman’s rank correlation method to test for correlations between concentrations of measured analytes. All statistical analyses were performed with the Statistical Package for the Social Sciences (SPSS) software version 20 (SPSS, Chicago, Illinois). Significance level below 0.05 was considered as statistically significant.
Results
One hundred subjects were included in the study. The mean age of participants was 49±11.4 (mean±SD) years; 52 persons were older than 50 years (18 older than 60 years). Further demographic information is given in Table 1, as are the determined concentrations of neopterin, nitrite and amino acids. Seven blood donors presented with neopterin concentrations above the upper limit of the normal for age of 19–65 years (95th percentile), which is 8.7 nmol/L [23]. Two donation samples contained neopterin concentrations higher than 10 nmol/L; the threshold has been defined earlier as the 98th percentile in blood donors [2]. More information on the distribution of concentrations of the other parameters tested is given in Table 1.
Serum concentrations of neopterin, amino acids and nitrite in the whole study population of 100 individuals.
| Mean (SD) | Median | 25th–75th percentile | 95th percentile | |
|---|---|---|---|---|
| Neopterin, nmol/L | 5.94 (1.58) | 4.96 | 4.71–7.21 | 9.10 |
| Tryptophan, μmol/L | 67.4 (10.2) | 66.8 | 60.9–72.7 | 53.1a |
| Kynurenine, μmol/L | 1.78 (0.42) | 1.72 | 1.52–2.06 | 2.48 |
| KYN/TRP, μmol/mmol | 26.7 (6.2) | 26.0 | 22.8–29.9 | 35.1 |
| Tyrosine, μmol/L | 90.6 (22.9) | 87.8 | 73.8–108 | 59.5a |
| Phenylalanine, μmol/L | 65.2 (11.1) | 64.1 | 56.8–74.3 | 83.4 |
| PHE/TYR, μmol/μmol | 0.75 (0.14) | 0.74 | 0.65–0.85 | 0.95 |
| Nitrite, μmol/L | 44.9 (32.0) | 33.2 | 19.2–63.5 | 105 |
SD, standard deviation; KYN/TRP, kynurenine to tryptophan ratio; PHE/TYR, phenylalanine to tyrosine ratio. aLower limit of the normal=5th percentile.
Forty-two participants were women with a mean body mass index (BMI) of 25.4±4.91 kg/m2; the 58 males had a mean BMI of 25.9±3.30 kg/m2. In Table 2, more group comparisons regarding gender are shown. Men were older, taller and heavier than females (all p<0.001). TRP and TYR concentrations were higher in males than in females (p<0.02). Also, PHE concentrations were slightly elevated in males compared to females, but the difference failed to reach the level of significance (U=1.777. p=0.075).
Demographic data and concentrations of neopterin, amino acids and nitrite from the study population split into two groups according to gender.
| Males (n=58) | Females (n=42) | U, p-value | |
|---|---|---|---|
| Mean (SD) | Mean (SD) | ||
| Age, years | 51.1 (11.0) | 46.2 (11.4) | 2.198, <0.001 |
| Height, cm | 179 (5.18) | 165 (6.07) | 7.755, <0.001 |
| Weight, kg | 82.9 (12.0) | 69.1 (13.6) | 5.456, <0.001 |
| BMI, kg/m2 | 25.9 (3.30) | 25.4 (4.91) | n.s. |
| Neopterin, nmol/L | 5.93 (1.58) | 5.95 (1.59) | n.s. |
| Tryptophan, μmol/L | 69.3 (9.88) | 64.8 (10.2) | 2.500, 0.012 |
| Kynurenine, μmol/L | 1.79 (0.44) | 1.76 (0.39) | n.s. |
| KYN/TRP, μmol/mmol | 26.2 (6.87) | 27.4 (5.12) | n.s. |
| Tyrosine, μmol/L | 95.0 (21.5) | 84.5 (23.7) | 2.427, 0.015 |
| Phenylalanine, μmol/L | 67.1 (11.8) | 62.8 (9.49) | (1.777, 0.075) |
| PHE/TYR, μmol/μmol | 0.73 (0.13) | 0.78 (0.15) | (1.692, 0.091) |
| Nitrite, μmol/L | 47.8 (32.8) | 40.8 (30.8) | n.s. |
Means and standard deviations (SD) are shown; U and p values indicate results of statistical comparison between the two groups (Mann-Whitney U-test; n.s., not significant, p>0.1).
For the whole set of participants, a significant correlation existed between the BMI and TRP concentrations (rs=0.237; p<0.02). However, when analyzing male and female data separately, only in females but not in males, a significant correlation between body mass and the concentrations of amino acids TRP (rs=0.332), TYR (rs=0.318) and PHE (rs=0.306; all p values <0.05) was apparent (Figure 1).
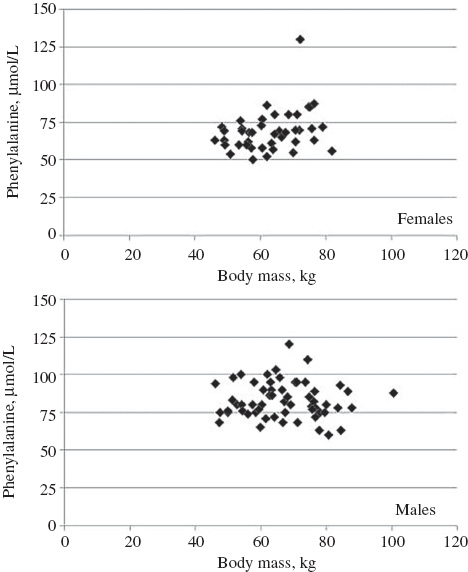
Scattergrams of body mass vs. phenylalanine concentrations in 42 female (upper) and 58 male (lower) blood donors (females rs=0.306, p<0.05; males rs=–0.061. p=not significant).
No significant correlation was found for BMI and neopterin concentrations and also not for age and neopterin (rs=0.012), KYN/TRP (rs=–0.028) or PHE/TYR (rs=–0.153; all not significant) concentrations in the whole data set including male and female data.
Neopterin concentrations and tryptophan metabolism were significantly associated in the whole data set (Table 3); neopterin correlated with kynurenine (rs=0.368) and KYN/TRP (rs=0.453; both p<0.001). Also, PHE/TYR (rs=0.236; p=0.018) correlated with neopterin concentrations, and there was an inverse correlation between tyrosine and neopterin concentrations (rs=–0.267; p<0.001). In both females and males, a significant correlation existed between neopterin and KYN/TRP, and interestingly in females the correlation (rs=0.621, p<0.001) was substantially stronger than in males (rs=0.366, p=0.005).
Spearman’s rank correlations (rs and p values) between neopterin concentrations and the concentrations of amino acids and nitrite in the whole data set and in the subgroups split by gender.
| Tryptophan, μmol/L | Kynurenine, μmol/L | KYN/TRP, μmol/mmol | Tyrosine, μmol/L | Phenylalanine, μmol/L | PHE/TYR, μmol/μmol | Nitrite, μmol/L |
|---|---|---|---|---|---|---|
| All (n=100): | ||||||
| –0.105 | 0.368 | 0.453 | –0.267 | –0.138 | 0.236 | –0.118 |
| n.s. | <0.001 | <0.001 | <0.001 | n.s. | 0.018 | n.s. |
| Females (n=42): | ||||||
| –0.150 | 0.422 | 0.621 | –0.366 | –0.202 | 0.340 | –0.114 |
| n.s. | 0.005 | <0.001 | 0.017 | n.s. | 0.028 | n.s. |
| Males (n=58): | ||||||
| –0.055 | 0.346 | 0.366 | –0.200 | –0.085 | 0.186 | –0.139 |
| n.s. | 0.008 | 0.005 | n.s. | n.s. | n.s. | n.s. |
n.s., not significant.
Discussion
The average neopterin concentrations observed in the 100 blood donations compare rather well with yet published data. Neopterin concentrations in males and females were not different, and also the 95th and 98th percentiles agree rather well with earlier reported values [23, 24], where similar immunoassays (radio-immunometric assays or ELISA systems) were used. However, concentrations estimated via HPLC or liquid chromatography-mass spectrometry analytics tend to be higher [25]. Most probably, this discrepancy depends on the precipitation method applied for serum protein separation. When acidic milieu is used, partial oxidation may occur, which contributes to higher neopterin levels due to oxidation of 7,8-dihydroneopterin to neopterin [26]. Only separation of unwanted serum protein with molecular sieves or precipitation with acetonitrile (CH3CN) could be employed as alternative techniques that are not associated with 7,8-dihydroneopterin oxidation. Also, minor differences may depend on the distinct analytical procedures, e.g., the manual workup as was done in this study in contrast to the automated pipetting systems used in larger studies [24].
Concentrations of amino acids are in the expected range, although the comparison with data from the literature can be more difficult due to the fact that methods used for measurements differ widely. In most recent analyses, HPLC or other liquid chromatography methods are applied. However, common protocols include detection of analytes after derivatization reaction with ninhydrin [21], whereas in this study the natural fluorescence of compounds (for PHE and TYR measurements: excitation wavelength 210 nm and emission wavelength 302 nm; TRP: excitation wavelength 286 nm and emission wavelength 366 nm) was used, and only the kynurenine was detected via UV absorption at 360 nm.
Differences between concentrations in male and female donors are apparent: Lower TRP, PHE and TYR concentrations were observed in females as compared with males. Thereby, the TRP results agree well with earlier observations [20]. In the whole data set there existed correlations between body mass and levels of several amino acid, but gender appears to be an additional factor influencing amino acid concentrations, because there exists a correlation between body mass and amino acid levels in females but not in males. Previous investigations showed a correlation between age and neopterin and KYN/TRP [27, 28], which was not observed in this study. In this regard, the rather narrow age range of donors in this current study might be of relevance; the maximum age of our study participants was 70 years. Further, slight differences in the correlation of the immune biomarkers neopterin and KYN/TRP were found in female and male data.
Because it is well documented that immune activation influences the metabolism of the amino acid TRP and also of PHE and TYR, results of this study support the hypothesis that low-grade inflammation is responsible for the significant correlations found between immune system biomarkers like neopterin, KYN/TRP or PHE/TYR. Increased KYN levels and an elevated KYN/TRP are closely linked to accelerated TRP breakdown due to activation of the enzyme IDO [10, 11]. Deprivation of TRP is an efficient strategy during the Th1-type immune response to counteract unwanted proliferation of pathogens, infected cells and tumor cells [29]. The disturbed PHE metabolism and thus higher PHE/TYR, also correlating with immune activation, probably results from disturbed activity of PHE-metabolizing enzyme PAH, due to insufficient supply with its cofactor BH4, which is sensitive to oxidation [17].
The alterations of amino acids concentrations found in the present study indicate that these metabolic disturbances could be of relevance not only in patients who suffer from diseases with overt immune activation and inflammation but also in healthy individuals. At least, in elderly people it was already observed that the disturbed TRP, PHE and TYR metabolism was associated with signs of depression and/or neurovegetative symptoms [30].
Nitrite concentrations showed large inter-individual variability and did not correlate with neopterin concentrations. Aside from many other factors, nitrite concentrations can be influenced by endogenous NOS activity, which oxidizes L-arginine to L-citrulline thereby releasing NO. However, in contrast to rodents, human macrophages are not a major source for inducible nitric oxide synthase derived NO [31]. An important exogenous factor influencing serum nitrite concentrations is food. Diet containing high-nitrate vegetables may heavily exceed recommended daily intake values [32]. Commensal bacteria in the oral cavity are able to reduce nitrate to nitrite [33], and also, mammalian xanthine oxidoreductase was shown to be able to reduce nitrate [34]. A number of mammalian enzymes and redox active compounds can convert nitrite into NO, and vice versa. While these new findings further put into question to assess serum nitrite in regard to immune activation, recent literature discusses the potential of a high-nitrate diet as an alternative nitrate/NO source to lower blood pressure [32, 35].
In conclusion, the establishment of the normal range measured in a reasonably high number of healthy controls provides the basis for further studies of the highly relevant topics around the metabolism of aromatic amino acids. Most importantly, the associations found in healthy donors may indicate that there exists a strong interference between immune status and amino acid metabolism that is of relevance not only in patients but also in the healthy population. This is of special interest in the chronic situation, as also prevailing minor changes in amino acids may lead to clinical relevant symptoms. In particular, this may be of relevance for the later development of especially neuropsychiatric symptoms associated with immune response [36, 37].
References
1. Murr C, Widner B, Wirleitner B, Fuchs D. Neopterin as a marker for immune system activation. Curr Drug Metab 2002;3:175–87.10.2174/1389200024605082Search in Google Scholar PubMed
2. Hönlinger M, Fuchs D, Hausen A, Reibnegger G, Schönitzer D, Werner ER, et al. Serum-Neopterinbestimmung zur zusätzlichen Sicherung der Bluttransfusion. Deutsch Med Wochenschr 1989;114:172–6.10.1055/s-2008-1066571Search in Google Scholar PubMed
3. Huber C, Batchelor JR, Fuchs D, Hausen A, Lang A, Niederwieser D, et al. Immune response-associated production of neopterin. Release from macrophages primarily under control of interferon-gamma. J Exp Med 1984;160:310–6.10.1084/jem.160.1.310Search in Google Scholar PubMed PubMed Central
4. Wirleitner B, Reider D, Ebner S, Böck G, Widner B, Jaeger M, et al. Monocyte-derived dendritic cells release neopterin. J Leukoc Biol 2002;72:1148–53.10.1189/jlb.72.6.1148Search in Google Scholar
5. Cano OD, Neurauter G, Fuchs D, Shearer GM, Boasso A. Differential effect of type I and type II interferons on neopterin production and amino acid metabolism in human astrocyte-derived cells. Neurosci Lett 2008;438:22–5.10.1016/j.neulet.2008.04.046Search in Google Scholar PubMed PubMed Central
6. Werner ER, Werner-Felmayer G, Fuchs D, Hausen A, Reibnegger G, Wachter H. Parallel induction of tetrahydrobiopterin biosynthesis and indoleamine 2,3-dioxygenase activity in human cells and cell lines by interferon-gamma. Biochem J 1989;262:861–6.10.1042/bj2620861Search in Google Scholar PubMed PubMed Central
7. Byrne GI, Lehmann LK, Landry GJ. Induction of tryptophan catabolism is the mechanism for gamma-interferon-mediated inhibition of intracellular Chlamydia psittaci replication in T24 cells. Infect Immun 1986;53:347–51.10.1128/iai.53.2.347-351.1986Search in Google Scholar PubMed PubMed Central
8. Pfefferkorn ER, Rebhun S, Eckel M. Characterization of an indoleamine 2,3-dioxygenase induced by gamma-interferon in cultured human fibroblasts. J Interferon Res 1986;6:267–79.10.1089/jir.1986.6.267Search in Google Scholar PubMed
9. Fuchs D, Moeller AA, Reibnegger G, Stoeckle E, Werner ER, Wachter H. Decreased serum tryptophan in patients with HIV-1 infection correlates with increased serum neopterin and with neurologic/psychiatric symptoms. J Acquir Immune Defic Syndr 1990;3:873–6.Search in Google Scholar
10. Schroecksnadel K, Wirleitner B, Winkler C, Fuchs D. Monitoring tryptophan metabolism in chronic immune activation. Clin Chim Acta 2006;364:82–90.10.1016/j.cca.2005.06.013Search in Google Scholar PubMed
11. Huengsberg M, Winer JB, Gompels M, Round R, Ross J, Shahmanesh M. Serum kynurenine-to-tryptophan ratio increases with progressive disease in HIV-infected patients. Clin Chem 1998;44:858–62.10.1093/clinchem/44.4.858Search in Google Scholar
12. Weinlich G, Murr C, Richardsen L, Winkler C, Fuchs D. Decreased serum tryptophan concentration predicts poor prognosis in malignant melanoma patients. Dermatology 2007;214:8–14.10.1159/000096906Search in Google Scholar PubMed
13. Sucher R, Schroecksnadel K, Weiss G, Margreiter R, Fuchs D, Brandacher G. Neopterin, a prognostic marker in human malignancies. Cancer Lett 2010;287:13–22.10.1016/j.canlet.2009.05.008Search in Google Scholar
14. Werner ER, Blau N, Thöny B. Tetrahydrobiopterin: biochemistry and pathophysiology. Biochem J 2011;438:397–414.10.1042/BJ20110293Search in Google Scholar
15. Watschinger K, Werner ER. Alkylglycerol monooxygenase. IUBMB Life 2013;65:366–72.10.1002/iub.1143Search in Google Scholar
16. Werner ER, Werner-Felmayer G, Fuchs D, Hausen A, Reibnegger G, Yim JJ, et al.Tetrahydrobiopterin biosynthetic activities in human macrophages, fibroblasts, THP-1, and T 24 cells. GTP-cyclohydrolase I is stimulated by interferon-gamma, and 6-pyruvoyl tetrahydropterin synthase and sepiapterin reductase are constitutively present. J Biol Chem 1990;265:3189–92.10.1016/S0021-9258(19)39752-2Search in Google Scholar
17. Neurauter G, Schrocksnadel K, Scholl-Bürgi S, Sperner-Unterweger B, Schubert C, Ledochowski M, et al. Chronic immune stimulation correlates with reduced phenylalanine turnover. Curr Drug Metab 2008;9:622–7.10.2174/138920008785821738Search in Google Scholar PubMed
18. Ploder M, Neurauter G, Spittler A, Schroecksnadel K, Roth E, Fuchs D. Serum phenylalanine in patients post trauma and with sepsis correlate to neopterin concentrations. Amino Acids 2008;35:303–7.10.1007/s00726-007-0625-xSearch in Google Scholar PubMed
19. Zangerle R, Kurz K, Neurauter G, Kitchen M, Sarcletti M, Fuchs D. Increased blood phenylalanine to tyrosine ratio in HIV-1 infection and correction following effective antiretroviral therapy. Brain Behav Immun 2010;24:403–8.10.1016/j.bbi.2009.11.004Search in Google Scholar PubMed
20. Widner B, Werner ER, Schennach H, Wachter H, Fuchs D. Simultaneous measurement of serum tryptophan and kynurenine by HPLC. Clin Chem 1997;43:2424–6.10.1093/clinchem/43.12.2424Search in Google Scholar
21. Neurauter G, Scholl-Bürgi S, Haara A, Geisler S, Mayersbach P, Schennach H, et al. Simultaneous measurement of phenylalanine and tyrosine by high performance liquid chromatography (HPLC) with fluorescence detection. Clin Biochem 2013;46:1848–51.10.1016/j.clinbiochem.2013.10.015Search in Google Scholar PubMed
22. Giustarini D, Dalle-Donne I, Colombo R, Milzani A, Rossi R. Adaptation of the Griess reaction for detection of nitrite in human plasma. Free Radic Res 2004;38:1235–40.10.1080/10715760400017327Search in Google Scholar PubMed
23. Werner ER, Bichler A, Daxenbichler G, Fuchs D, Fuith LC, Hausen A, et al. Determination of neopterin in serum and urine. Clin Chem 1987;33:62–6.10.1093/clinchem/33.1.62Search in Google Scholar
24. Mayersbach P, Fuchs D, Schennach H. Performance of a fully automated quantitative neopterin measurement assay in a routine voluntary blood donation setting. Clin Chem Lab Med 2010;48 373–7.10.1515/CCLM.2010.072Search in Google Scholar
25. Pedersen ER, Midttun Ø, Ueland PM, Schartum-Hansen H, Seifert R, Igland J, et al. Systemic markers of interferon-γ-mediated immune activation and long-term prognosis in patients with stable coronary artery disease. Arterioscler Thromb Vasc Biol 2011;31:698–704.10.1161/ATVBAHA.110.219329Search in Google Scholar
26. Flavall EA, Crone EM, Moore GA, Gieseg SP. Dissociation of neopterin and 7,8-dihydroneopterin from plasma components before HPLC analysis. J Chromatogr B Analyt Technol Biomed Life Sci 2008;863:167–71.10.1016/j.jchromb.2007.12.019Search in Google Scholar
27. Reibnegger G, Huber LA, Jürgens G, Schönitzer D, Werner ER, Wachter H, et al. Approach to define “normal aging” in man. Immune function, serum lipids, lipoproteins and neopterin levels. Mech Ageing Dev 1988;46:67–82.10.1016/0047-6374(88)90115-7Search in Google Scholar
28. Frick B, Schroecksnadel K, Neurauter G, Leblhuber F, Fuchs D. Increasing production of homocysteine and neopterin and degradation of tryptophan with older age. Clin Biochem 2004;37:684–7.10.1016/j.clinbiochem.2004.02.007Search in Google Scholar PubMed
29. Pfefferkorn ER. Interferon gamma blocks the growth of Toxoplasma gondii in human fibroblasts by inducing the host cells to degrade tryptophan. Proc Natl Acad Sci USA 1984;81:908–12.10.1073/pnas.81.3.908Search in Google Scholar PubMed PubMed Central
30. Capuron L, Schroecksnadel S, Féart C, Aubert A, Higueret D, Barberger-Gateau P, et al. Chronic low-grade inflammation in elderly persons is associated with altered tryptophan and tyrosine metabolism: role in neuropsychiatric symptoms. Biol Psychiatry 2011;70:175–82.10.1016/j.biopsych.2010.12.006Search in Google Scholar PubMed
31. Schneemann M, Schoedon G, Hofer S, Blau N, Guerrero L, Schaffner A. Nitric oxide synthase is not a constituent of the antimicrobial armature of human mononuclear phagocytes. J Infect Dis 1993;167:1358–63.10.1093/infdis/167.6.1358Search in Google Scholar PubMed
32. Hord NG, Tang Y, Bryan NS. Food sources of nitrates and nitrites: the physiologic context for potential health benefits. Am J Clin Nutr 2009;90:1–10.10.3945/ajcn.2008.27131Search in Google Scholar PubMed
33. Duncan C, Dougall H, Johnston P, Green S, Brogan R, Leifert C, et al. Chemical generation of nitric oxide in the mouth from the enterosalivary circulation of dietary nitrate. Nat Med 1995;1:546–51.10.1038/nm0695-546Search in Google Scholar
34. Millar TM, Stevens CR, Benjamin N, Eisenthal R, Harrison R, Blake DR. Xanthine oxidoreductase catalyses the reduction of nitrates and nitrite to nitric oxide under hypoxic conditions. FEBS Lett 1998;427:225–8.10.1016/S0014-5793(98)00430-XSearch in Google Scholar
35. Weitzberg E, Lundberg JO. Novel aspects of dietary nitrate and human health. Annu Rev Nutr 2013;33:129–59.10.1146/annurev-nutr-071812-161159Search in Google Scholar
36. Widner B, Laich A, Sperner-Unterweger B, Ledochowski M, Fuchs D. Neopterin production, tryptophan degradation, and mental depression – what is the link? Brain Behav Immun 2002;16:590–5.10.1016/S0889-1591(02)00006-5Search in Google Scholar
37. Haroon E, Raison CL, Miller AH. Psychoneuroimmunology meets neuropsychopharmacology: translational implications of the impact of inflammation on behavior. Neuropsychopharmacology 2012;37:137–62.10.1038/npp.2011.205Search in Google Scholar PubMed PubMed Central
©2015 by De Gruyter
Articles in the same Issue
- Frontmatter
- Review
- Folate receptor: a potential target in ovarian cancer
- Original articles
- Theoretical study on the relative energies of cationic pterin tautomers
- Binding affinities of folic acid and related pterins with biological macromolecules under physiological conditions
- Serum tryptophan, kynurenine, phenylalanine, tyrosine and neopterin concentrations in 100 healthy blood donors
- Correlation of trisomy 13 with atelencephalic aprosencephaly
Articles in the same Issue
- Frontmatter
- Review
- Folate receptor: a potential target in ovarian cancer
- Original articles
- Theoretical study on the relative energies of cationic pterin tautomers
- Binding affinities of folic acid and related pterins with biological macromolecules under physiological conditions
- Serum tryptophan, kynurenine, phenylalanine, tyrosine and neopterin concentrations in 100 healthy blood donors
- Correlation of trisomy 13 with atelencephalic aprosencephaly

