Continuous synthesis of gold nanoparticles in micro- and millifluidic systems
-
He Huang
He Huang received her BS degree from the department of Chemical Engineering, Dalian University of Technology (Liaoning Province, China) in 2012. She obtained an MSc degree in 2014 and continued her study as a PhD candidate at the department of Chemical Engineering, University College London under the supervision of Prof. Gavriilidis. Her research area is the controlled synthesis of gold nanocrystals in microfluidic systems.Hendrik du Toit received an MEng in Chemical Engineering from Imperial College London in 2011. He subsequently received his PhD from the University of Bath in 2015 whilst conducting research into the production of biocompatible biosensors and biofuel cells for implantable medical devices. He then joined Prof. Gavriilidis’ research group at University College London where his focus has been on developing continuous flow gold nanoparticle synthesis systems. His research interests include fuel cells, biosensing, nanomaterials, fluidic technologies and reactor design.Luca Panariello received both a BS (2014) and MSc (2016) degree in Chemical Engineering with honours at the University of Naples “Federico II” (Italy). He joined the department of Chemical Engineering at University College London in 2016 in a joint research project with the University of Naples “Federico II”. He was then awarded a Marie Skłodowska-Curie PhD scholarship in 2017 to work on process intensification of nanomaterials production under the supervision of Prof. Gavriilidis.Luca Mazzei graduated in Chemical Engineering from the University of Naples “Federico II” (Italy) in 2001. He spent 3 years working for Technip KTI as a process and start-up engineer on sulphur recovery and refinery tail gas treatment. Subsequently he joined the Department of Chemical Engineering at University College London, first as a student, where he was awarded a PhD in 2008, and then promoted to Lecturer in 2009. His research activities deal with experimental and modelling of polydisperse multiphase systems, with focus on crystallization processes and nanoparticles synthesis, relying on advanced mathematical modelling and CFD.Asterios Gavriilidis obtained a Diploma from the University of Thessaloniki (Greece) in 1988, and an MSc in 1990 and PhD in 1993 from the University of Notre Dame, USA, all in Chemical Engineering. He joined the Department of Chemical Engineering at University College London (UK) in 1993, where he has been professor of Chemical Reaction Engineering since 2004. His research interests include chemical and catalytic reaction engineering, microreaction and microprocess technology, continuous nanomaterials synthesis.
Abstract
Gold nanomaterials have diverse applications ranging from healthcare and nanomedicine to analytical sciences and catalysis. Microfluidic and millifluidic reactors offer multiple advantages for their synthesis and manufacturing, including controlled or fast mixing, accurate reaction time control and excellent heat transfer. These advantages are demonstrated by reviewing gold nanoparticle synthesis strategies in flow devices. However, there are still challenges to be resolved, such as reactor fouling, particularly if robust manufacturing processes are to be developed to achieve the desired targets in terms of nanoparticle size, size distribution, surface properties, process throughput and robustness. Solutions to these challenges are more effective through a coordinated approach from chemists, engineers and physicists, which has at its core a qualitative and quantitative understanding of the synthesis processes and reactor operation. This is important as nanoparticle synthesis is complex, encompassing multiple phenomena interacting with each other, often taking place at short timescales. The proposed methodology for the development of reactors and processes is generic and contains various interconnected considerations. It aims to be a starting point towards rigorous design procedures for the robust and reproducible continuous flow synthesis of gold nanoparticles.
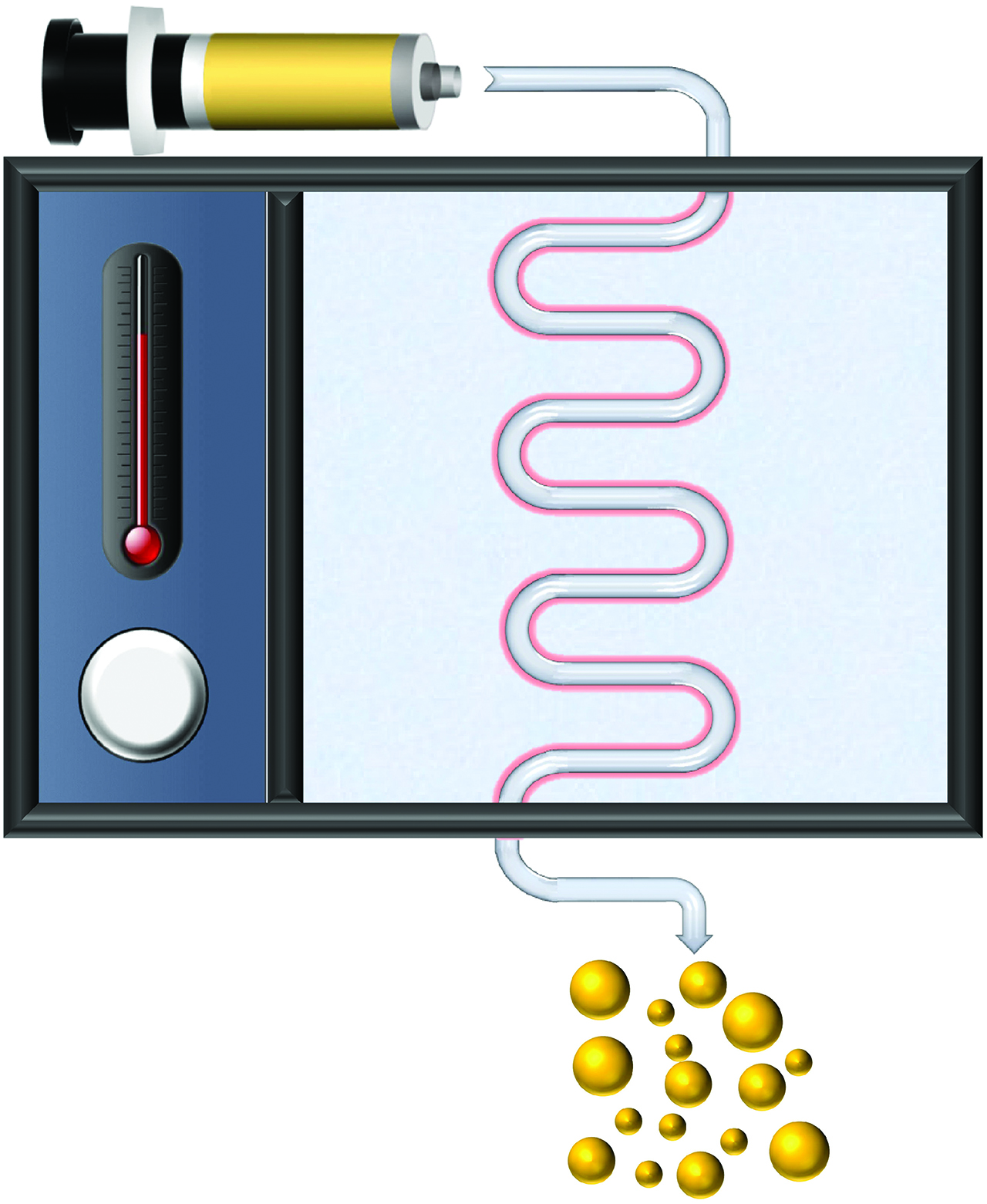
Graphical Abstract:
About the authors

He Huang received her BS degree from the department of Chemical Engineering, Dalian University of Technology (Liaoning Province, China) in 2012. She obtained an MSc degree in 2014 and continued her study as a PhD candidate at the department of Chemical Engineering, University College London under the supervision of Prof. Gavriilidis. Her research area is the controlled synthesis of gold nanocrystals in microfluidic systems.

Hendrik du Toit received an MEng in Chemical Engineering from Imperial College London in 2011. He subsequently received his PhD from the University of Bath in 2015 whilst conducting research into the production of biocompatible biosensors and biofuel cells for implantable medical devices. He then joined Prof. Gavriilidis’ research group at University College London where his focus has been on developing continuous flow gold nanoparticle synthesis systems. His research interests include fuel cells, biosensing, nanomaterials, fluidic technologies and reactor design.

Luca Panariello received both a BS (2014) and MSc (2016) degree in Chemical Engineering with honours at the University of Naples “Federico II” (Italy). He joined the department of Chemical Engineering at University College London in 2016 in a joint research project with the University of Naples “Federico II”. He was then awarded a Marie Skłodowska-Curie PhD scholarship in 2017 to work on process intensification of nanomaterials production under the supervision of Prof. Gavriilidis.

Luca Mazzei graduated in Chemical Engineering from the University of Naples “Federico II” (Italy) in 2001. He spent 3 years working for Technip KTI as a process and start-up engineer on sulphur recovery and refinery tail gas treatment. Subsequently he joined the Department of Chemical Engineering at University College London, first as a student, where he was awarded a PhD in 2008, and then promoted to Lecturer in 2009. His research activities deal with experimental and modelling of polydisperse multiphase systems, with focus on crystallization processes and nanoparticles synthesis, relying on advanced mathematical modelling and CFD.

Asterios Gavriilidis obtained a Diploma from the University of Thessaloniki (Greece) in 1988, and an MSc in 1990 and PhD in 1993 from the University of Notre Dame, USA, all in Chemical Engineering. He joined the Department of Chemical Engineering at University College London (UK) in 1993, where he has been professor of Chemical Reaction Engineering since 2004. His research interests include chemical and catalytic reaction engineering, microreaction and microprocess technology, continuous nanomaterials synthesis.
Acknowledgements
We would like to acknowledge funding for our research from EPSRC (grant EP/M015157/1) and European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No. 721290. A special thanks to Prof. Peter Dobson and Prof. Ivan Parkin for their insightful feedback to this chapter and our work in general. He Huang acknowledges support from the program of China Scholarships. This publication reflects only the authors’ view, exempting the Community from any liability.
References
[1] Daniel MC, Astruc D. Gold nanoparticles: assembly, supramolecular chemistry, quantum-size related properties and applications toward biology, catalysis and nanotechnology. Chem Rev. 2004;104:293–346.10.1021/cr030698+Search in Google Scholar PubMed
[2] Barreto JA, O’Malley W, Kubeil M, Graham B, Stephan H, Spiccia L. Nanomaterials: applications in cancer imaging and therapy. Adv Mater. 2011;23:12.10.1002/adma.201100140Search in Google Scholar PubMed
[3] . In: Rai M., Shegokar R., editor(s). Metal nanoparticles in pharma. Cham: Springer, 2017Search in Google Scholar
[4] Thota S, Crans DC. Metal nanoparticles: synthesis and applications in pharmaceutical sciences. Weinheim: John Wiley & Sons, 201810.1002/9783527807093Search in Google Scholar
[5] Dykman L, Khlebtsov N. Gold nanoparticles in biomedical applications: recent advances and perspectives. Chem Soc Rev. 2012;41:2256–82.10.1039/C1CS15166ESearch in Google Scholar
[6] Yang X, Yang M, Pang B, Vara M, Xia Y. Gold nanomaterials at work in biomedicine. Chem Rev. 2015;115:10410–88.10.1201/9780429027819-5Search in Google Scholar
[7] Dreaden EC, Alkilany AM, Huang X, Murphy CJ, El-Sayed MA. The golden age: gold nanoparticles for biomedicine. Chem Soc Rev. 2012;41:2740–79.10.1039/C1CS15237HSearch in Google Scholar PubMed PubMed Central
[8] Sardar R, Funston AM, Mulvaney P, Murray RW. Gold nanoparticles: past, present, and future. Langmuir. 2009;25:13840–51.10.1021/la9019475Search in Google Scholar PubMed
[9] Willets KA, Van Duyne RP. Localized surface plasmon resonance spectroscopy and sensing. Annu Rev Phys Chem. 2007;58:267–97.10.1146/annurev.physchem.58.032806.104607Search in Google Scholar PubMed
[10] Wilson R. The use of gold nanoparticles in diagnostics and detection. Chem Soc Rev. 2008;37:2028–45.10.1039/b712179mSearch in Google Scholar PubMed
[11] Lane LA, Qian X, Nie S. SERS nanoparticles in medicine: from label-free detection to spectroscopic tagging. Chem Rev. 2015;115:10489–529.10.1021/acs.chemrev.5b00265Search in Google Scholar PubMed
[12] Saha K, Agasti SS, Kim C, Li X, Rotello VM. Gold nanoparticles in chemical and biological sensing. Chem Rev. 2012;112:2739–79.10.1021/cr2001178Search in Google Scholar PubMed PubMed Central
[13] Zhang Y, Chu W, Foroushani AD, Wang H, Li D, Liu J, et al. New gold nanostructures for sensor applications: a review. Materials. 2014;7:5169–201.10.3390/ma7075169Search in Google Scholar PubMed PubMed Central
[14] Matias AS, Carlos FF, Pedrosa P, Fernandes AR, Baptista PV. Gold nanoparticles in molecular diagnostics and molecular therapeutics. In: Rai M., Shegokar R, editor(s). Metal nanoparticles in pharma. Cham: Springer, 2017:365–87.10.1007/978-3-319-63790-7_16Search in Google Scholar
[15] Zhou W, Gao X, Liu D, Chen X. Gold nanoparticles for in vitro diagnostics. Chem Rev. 2015;115:10575–636.10.1021/acs.chemrev.5b00100Search in Google Scholar PubMed PubMed Central
[16] Vs AP, Joseph P, Scg KD, Lakshmanan S, Kinoshita T, Muthusamy S. Colorimetric sensors for rapid detection of various analytes. Mater Sci Eng: C. 2017;78:1231–45.10.1016/j.msec.2017.05.018Search in Google Scholar PubMed
[17] Singh J, Sharma S, Nara S. Evaluation of gold nanoparticle based lateral flow assays for diagnosis of enterobacteriaceae members in food and water. Food Chem. 2015;170:470–83.10.1016/j.foodchem.2014.08.092Search in Google Scholar PubMed
[18] Bahadır EB, Sezgintürk MK. Lateral flow assays: principles, designs and labels. TrAC Trends Anal Chem. 2016;82:286–306.10.1016/j.trac.2016.06.006Search in Google Scholar
[19] Quesada-González D, Merkoçi A. Nanoparticle-based lateral flow biosensors. Biosens Bioelectron. 2015;73:47–63.10.1016/j.bios.2015.05.050Search in Google Scholar PubMed
[20] Cordeiro M, Ferreira Carlos F, Pedrosa P, Lopez A, Baptista PV. Gold nanoparticles for diagnostics: advances towards points of care. Diagnostics. 2016;6:43.10.3390/diagnostics6040043Search in Google Scholar PubMed PubMed Central
[21] Huang X, Jain PK, El-Sayed IH, El-Sayed MA. Gold nanoparticles: interesting optical properties and recent applications in cancer diagnostics and therapy. Nanomedicine. 2007;2:681–93.10.2217/17435889.2.5.681Search in Google Scholar PubMed
[22] Kumar A, Zhang X, Liang X-J. Gold nanoparticles: emerging paradigm for targeted drug delivery system. Biotechnol Adv. 2013;31:593–606.10.1016/j.biotechadv.2012.10.002Search in Google Scholar PubMed
[23] Alkilany AM, Thompson LB, Boulos SP, Sisco PN, Murphy CJ. Gold nanorods: their potential for photothermal therapeutics and drug delivery, tempered by the complexity of their biological interactions. Adv Drug Deliv Rev. 2012;64:190–9.10.1016/j.addr.2011.03.005Search in Google Scholar PubMed
[24] Abadeer NS, Murphy CJ. Recent progress in cancer thermal therapy using gold nanoparticles. J Phys Chem C. 2016;120:4691–716.10.1201/9780429027819-3Search in Google Scholar
[25] Yao C, Zhang L, Wang J, He Y, Xin J, Wang S, et al. Gold nanoparticle mediated phototherapy for cancer. J Nanomater. 2016;2016:Article ID 549713610.1155/2016/5497136Search in Google Scholar
[26] Hwang S, Nam J, Jung S, Song J, Doh H, Kim S. Gold nanoparticle-mediated photothermal therapy: current status and future perspective. Nanomedicine. 2014;9:2003–22.10.2217/nnm.14.147Search in Google Scholar PubMed
[27] Haume K, Rosa S, Grellet S, Śmiałek MA, Butterworth KT, Solov’yov AV, et al. Gold nanoparticles for cancer radiotherapy: a review. Cancer Nanotechnol. 2016;7:8.10.1186/s12645-016-0021-xSearch in Google Scholar PubMed PubMed Central
[28] Her S, Jaffray DA, Allen C. Gold nanoparticles for applications in cancer radiotherapy: mechanisms and recent advancements. Adv Drug Deliv Rev. 2017;109:84–101.10.1016/j.addr.2015.12.012Search in Google Scholar PubMed
[29] Corma A, Garcia H. Supported gold nanoparticles as catalysts for organic reactions. Chem Soc Rev. 2008;37:2096–126.10.1039/b707314nSearch in Google Scholar PubMed
[30] Stratakis M, Garcia H. Catalysis by supported gold nanoparticles: beyond aerobic oxidative processes. Chem Rev. 2012;112:4469–506.10.1021/cr3000785Search in Google Scholar PubMed
[31] Ciriminna R, Falletta E, Della Pina C, Teles JH, Pagliaro M. Industrial applications of gold catalysis. Angew Chem Int Ed. 2016;55:14210–7.10.1002/anie.201604656Search in Google Scholar PubMed
[32] Cebrián V, Martín-Saavedra F, Yagüe C, Arruebo M, Santamaría J, Vilaboa N. Size-dependent transfection efficiency of PEI-coated gold nanoparticles. Acta Biomater. 2011;7:3645–55.10.1016/j.actbio.2011.06.018Search in Google Scholar PubMed
[33] Chithrani BD, Ghazani AA, Chan WC. Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Lett. 2006;6:662–8.10.1021/nl052396oSearch in Google Scholar
[34] Leifert A, Pan-Bartnek Y, Simon U, Jahnen-Dechent W. Molecularly stabilised ultrasmall gold nanoparticles: synthesis, characterization and bioactivity. Nanoscale. 2013;5:6224–42.10.1039/c3nr00916eSearch in Google Scholar
[35] Dykman L, Khlebtsov N. Gold nanoparticles in biology and medicine: recent advances and prospects. Acta Nat. 2011;3:34–5510.32607/20758251-2011-3-2-34-55Search in Google Scholar
[36] Pan Y, Neuss S, Leifert A, Fischler M, Wen F, Simon U, et al. Size-dependent cytotoxicity of gold nanoparticles. Small. 2007;3:1941–9.10.1002/smll.200700378Search in Google Scholar
[37] Hvolbæk B, Janssens TV, Clausen BS, Falsig H, Christensen CH, Nørskov JK. Catalytic activity of Au nanoparticles. Nano Today. 2007;2:14–8.10.1016/S1748-0132(07)70113-5Search in Google Scholar
[38] Hashmi ASK, Rudolph M. Gold catalysis in total synthesis. Chem Soc Rev. 2008;37:1766–75.10.1039/b615629kSearch in Google Scholar PubMed
[39] Sebastian V, Arruebo M, Santamaria J. Reaction engineering strategies for the production of inorganic nanomaterials. Small. 2014;10:835–53.10.1002/smll.201301641Search in Google Scholar PubMed
[40] Valencia PM, Farokhzad OC, Karnik R, Langer R. Microfluidic technologies for accelerating the clinical translation of nanoparticles. Nat Nanotechnol. 2012;7:623.10.1201/9780429399039-3Search in Google Scholar
[41] Krishna KS, Li Y, Li S, Kumar CS. Lab-on-a-chip synthesis of inorganic nanomaterials and quantum dots for biomedical applications. Adv Drug Deliv Rev. 2013;65:1470–95.10.1016/j.addr.2013.05.006Search in Google Scholar PubMed
[42] Puigmartí-Luis J. Microfluidic platforms: a mainstream technology for the preparation of crystals. Chem Soc Rev. 2014;43:2253–71.10.1039/C3CS60372ESearch in Google Scholar
[43] Marre S, Jensen KF. Synthesis of micro and nanostructures in microfluidic systems. Chem Soc Rev. 2010;39:1183–202.10.1039/b821324kSearch in Google Scholar PubMed
[44] Tsuzuki T. Commercial scale production of inorganic nanoparticles. Int J Nanotechnol. 2009;6:567–78.10.1504/IJNT.2009.024647Search in Google Scholar
[45] Taifur-Rahman M, Rebrov E. Microreactors for gold nanoparticles synthesis: from Faraday to flow. Processes. 2014;2:466.10.3390/pr2020466Search in Google Scholar
[46] deMello AJ. Control and detection of chemical reactions in microfluidic systems. Nature. 2006;442:394–402.10.1038/nature05062Search in Google Scholar PubMed
[47] Nightingale AM, deMello JC. Segmented flow reactors for nanocrystal synthesis. Adv Mater. 2013;25:1813–21.10.1002/adma.201203252Search in Google Scholar PubMed
[48] Saldanha PL, Lesnyak V, Manna L. Large scale syntheses of colloidal nanomaterials. Nano Today. 2017;12:46–63.10.1016/j.nantod.2016.12.001Search in Google Scholar
[49] Maceiczyk RM, Lignos IG. Online detection and automation methods in microfluidic nanomaterial synthesis. Curr Opin Chem Eng. 2015;8:29–35.10.1016/j.coche.2015.01.007Search in Google Scholar
[50] Park JI, Saffari A, Kumar S, Günther A, Kumacheva E. Microfluidic synthesis of polymer and inorganic particulate materials. Annu Rev Mater Res. 2010;40:415–43.10.1146/annurev-matsci-070909-104514Search in Google Scholar
[51] Shestopalov I, Tice JD, Ismagilov RF. Multi-step synthesis of nanoparticles performed on millisecond time scale in a microfluidic droplet-based system. Lab Chip. 2004;4:316–21.10.1039/b403378gSearch in Google Scholar PubMed
[52] Zhao C-X, He L, Qiao SZ, Middelberg APJ. Nanoparticle synthesis in microreactors. Chem Eng Sci. 2011;66:1463–79.10.1016/j.ces.2010.08.039Search in Google Scholar
[53] Niu G, Ruditskiy A, Vara M, Xia Y. Toward continuous and scalable production of colloidal nanocrystals by switching from batch to droplet reactors. Chem Soc Rev. 2015;44:5806–20.10.1039/C5CS00049ASearch in Google Scholar PubMed
[54] Shang L, Cheng Y, Zhao Y. Emerging droplet microfluidics. Chem Rev. 2017;117:7964–8040.10.1021/acs.chemrev.6b00848Search in Google Scholar PubMed
[55] Pan LJ, Tu JW, Ma HT, Yang YJ, Tian ZQ, Pang DW, et al. Controllable synthesis of nanocrystals in droplet reactors. Lab Chip. 2018;18:41–56.10.1039/C7LC00800GSearch in Google Scholar PubMed
[56] Kim JH, Jeon TY, Choi TM, Shim TS, Kim S-H, Yang S-M. Droplet microfluidics for producing functional microparticles. Langmuir. 2013;30:1473–88.10.1021/la403220pSearch in Google Scholar PubMed
[57] Alexandridis P. Gold nanoparticle synthesis, morphology control, and stabilization facilitated by functional polymers. Chem Eng Technol. 2011;34:15–28.10.1002/ceat.201000335Search in Google Scholar
[58] Grzelczak M, Pérez-Juste J, Mulvaney P, Liz-Marzán LM. Shape control in gold nanoparticle synthesis. Chem Soc Rev. 2008;37:1783–91.10.1201/9780429295188-6Search in Google Scholar
[59] Guo S, Wang E. Synthesis and electrochemical applications of gold nanoparticles. Anal Chim Acta. 2007;598:181–92.10.1016/j.aca.2007.07.054Search in Google Scholar PubMed
[60] Zhao P, Li N, Astruc D. State of the art in gold nanoparticle synthesis. Coord Chem Rev. 2013;257:638–65.10.1016/j.ccr.2012.09.002Search in Google Scholar
[61] Günther A, Jensen KF. Multiphase microfluidics: from flow characteristics to chemical and materials synthesis. Lab Chip. 2006;6:1487–503.10.1039/B609851GSearch in Google Scholar PubMed
[62] Song Y, Hormes J, Kumar CS. Microfluidic synthesis of nanomaterials. Small. 2008;4:698–711.10.1002/smll.200701029Search in Google Scholar PubMed
[63] Shahbazali E, Hessel V, Noël T, Wang Q. Metallic nanoparticles made in flow and their catalytic applications in organic synthesis. Nanotechnol Rev. 2014;3:65–86.10.1515/ntrev-2013-0017Search in Google Scholar
[64] Sebastian V, Khan SA, Kulkarni AA. Flow synthesis of functional materials. J Flow Chem. 2017;7:96–105.10.1556/1846.2017.00028Search in Google Scholar
[65] Navin CV, Krishna KS, Theegala CS, Kumar CS. Lab-on-a-chip devices for gold nanoparticle synthesis and their role as a catalyst support for continuous flow catalysis. Nanotechnol Rev. 2014;3:39–63.10.1515/ntrev-2013-0028Search in Google Scholar
[66] Levenspiel O. Chemical reaction engineering, 3rd ed. Weinheim: John Wiley & Sons, 1999Search in Google Scholar
[67] Wang ZL. Transmission electron microscopy of shape-controlled nanocrystals and their assemblies. J Phys Chem B. 2000;104:1153–75.10.1021/jp993593cSearch in Google Scholar
[68] Young NP, Van Huis MA, Zandbergen HW, Xu H, Kirkland AI. Transformations of gold nanoparticles investigated using variable temperature high-resolution transmission electron microscopy. Ultramicroscopy. 2010;110:506–16.10.1016/j.ultramic.2009.12.010Search in Google Scholar PubMed
[69] Georgiev P, Bojinova A, Kostova B, Momekova D, Bjornholm T, Balashev K. Implementing atomic force microscopy (AFM) for studying kinetics of gold nanoparticle’s growth. Colloids Surf. A. 2013;434:154–63.10.1016/j.colsurfa.2013.05.064Search in Google Scholar
[70] Khlebtsov BN, Khlebtsov NG. On the measurement of gold nanoparticle sizes by the dynamic light scattering method. Colloid J. 2011;73:118–27.10.1134/S1061933X11010078Search in Google Scholar
[71] Du Toit H, Macdonald T, Huang H, Parkin I, Gavriilidis A. Continuous flow synthesis of citrate capped gold nanoparticles using UV induced nucleation. RSC Adv. 2017;7:9632–38.10.1039/C6RA27173ASearch in Google Scholar
[72] Chaki NK, Negishi Y, Tsunoyama H, Shichibu Y. Ubiquitous 8 and 29 kDa gold: alkanethiolate cluster compounds: mass-spectrometric determination of molecular formulas and structural implications. J Am Chem Soc. 2008;130:8608–10.10.1021/ja8005379Search in Google Scholar PubMed
[73] Kettemann F, Birnbaum A, Witte S, Wuithschick M, Pinna N, Kraehnert R, et al. Missing piece of the mechanism of the Turkevich method: the critical role of citrate protonation. Chem Mater. 2016;28:4072–81.10.1021/acs.chemmater.6b01796Search in Google Scholar
[74] Abécassis B, Testard F, Spalla O, Barboux P. Probing in situ the nucleation and growth of gold nanoparticles by small-angle X-ray scattering. Nano Lett. 2007;7:1723–27.10.1021/nl0707149Search in Google Scholar
[75] Polte J, Erler R, Thünemann AF, Emmerling F, Kraehnert R. SAXS in combination with a free liquid jet for improved time-resolved in situ studies of the nucleation and growth of nanoparticles. Chem Commun. 2010;46:9209–11.10.1039/c0cc03238gSearch in Google Scholar
[76] Polte J, Erler R, ThüNemann AF, Sokolov S, Ahner TT, Rademann K, et al. Nucleation and growth of gold nanoparticles studied via in situ small angle X-ray scattering at millisecond time resolution. ACS Nano. 2010;4:1076–82.10.1021/nn901499cSearch in Google Scholar
[77] Abécassis B, Testard F, Kong Q, Francois B, Spalla O. Influence of monomer feeding on a fast gold nanoparticles synthesis: time-resolved XANES and SAXS experiments. Langmuir. 2010;26:13847–54.10.1021/la1020274Search in Google Scholar
[78] Polte J, Ahner TT, Delissen F, Sokolov S, Emmerling F, Thunemann AF, et al. Mechanism of gold nanoparticle formation in the classical citrate synthesis method derived from coupled in situ XANES and SAXS evaluation. J Am Chem Soc. 2010;132:1296–301.10.1021/ja906506jSearch in Google Scholar
[79] Kwon K, Lee KY, Lee YW, Kim M, Heo J, Ahn SJ, et al. Controlled synthesis of icosahedral gold nanoparticles and their surface-enhanced Raman scattering property. J Phys Chem C. 2007;111:1161–65.10.1021/jp064317iSearch in Google Scholar
[80] Allabashi R, Stach W, De La Escosura-Muñiz A, Liste-Calleja L, Merkoçi A. ICP-MS: a powerful technique for quantitative determination of gold nanoparticles without previous dissolving. J Nanopart Res. 2008;11:2003.10.1007/s11051-008-9561-2Search in Google Scholar
[81] Laborda F, Bolea E, Jiménez-Lamana J. Single particle inductively coupled plasma mass spectrometry: a powerful tool for nanoanalysis. Anal Chem. 2014;86:2270–8.10.1021/ac402980qSearch in Google Scholar
[82] Dequaire M, Degrand C, Limoges B. An electrochemical metalloimmunoassay based on a colloidal gold label. Anal Chem. 2000;72:5521–8.10.1021/ac000781mSearch in Google Scholar
[83] González García MB, Costa García A. Adsorptive stripping voltammetric behaviour of colloidal gold and immunogold on carbon paste electrode. Bioelectrochem Bioenerg. 1995;38:389–95.10.1016/0302-4598(95)01813-TSearch in Google Scholar
[84] Pumera M, Aldavert M, Mills C, Merkoçi A, Alegret S. Direct voltammetric determination of gold nanoparticles using graphite-epoxy composite electrode. Electrochim Acta. 2005;50:3702–7.10.1016/j.electacta.2005.01.035Search in Google Scholar
[85] Welz B, Becker-Ross H, Florek S, Heitmann U. High-resolution continuum source AAS: the better way to do atomic absorption spectrometry. Weinheim: John Wiley & Sons, 2006Search in Google Scholar
[86] Elzey S, Tsai DH, Rabb SA, Yu LL, Winchester MR, Hackley VA. Quantification of ligand packing density on gold nanoparticles using ICP-OES. Anal Bioanal Chem. 2012;403:145–9.10.1007/s00216-012-5830-0Search in Google Scholar PubMed
[87] Hendel T, Lesnyak V, Kühn L, Herrmann AK, Bigall NC, Borchardt L, et al. Mixed aerogels from Au and CdTe nanoparticles. Adv Funct Mater. 2013;23:1903–11.10.1002/adfm.201201674Search in Google Scholar
[88] Liu X, Atwater M, Wang J, Huo Q. Extinction coefficient of gold nanoparticles with different sizes and different capping ligands. Colloids Surf. B. 2007;58:3–7.10.1016/j.colsurfb.2006.08.005Search in Google Scholar PubMed
[89] Kattumuri V, Katti K, Bhaskaran S, Boote EJ, Casteel SW, Fent GM, et al. Gum arabic as a phytochemical construct for the stabilization of gold nanoparticles: in vivo pharmacokinetics and X-ray-contrast-imaging studies. Small. 2007;3:333–41.10.1002/smll.200600427Search in Google Scholar PubMed
[90] Park J, Shumaker-Parry JS. Structural study of citrate layers on gold nanoparticles: role of intermolecular interactions in stabilizing nanoparticles. J Am Chem Soc. 2014;136:1907–21.10.1021/ja4097384Search in Google Scholar PubMed
[91] Koziej D. Revealing complexity of nanoparticle synthesis in solution by in situ hard X-ray spectroscopy – today and beyond. Chem Mater. 2016;28:2478–90.10.1021/acs.chemmater.6b00486Search in Google Scholar
[92] Ohyama J, Teramura K, Higuchi Y, Shishido T, Hitomi Y, Aoki K, et al. An in situ quick XAFS spectroscopy study on the formation mechanism of small gold nanoparticles supported by porphyrin-cored tetradentate passivants. Phys Chem Chem Phys. 2011;13:11128–35.10.1039/c1cp20231fSearch in Google Scholar PubMed
[93] Zhang P, Sham T. X-ray studies of the structure and electronic behavior of alkanethiolate-capped gold nanoparticles: the interplay of size and surface effects. Phys Rev Lett. 2003;90:245502.10.1103/PhysRevLett.90.245502Search in Google Scholar PubMed
[94] Park J-W, Shumaker-Parry JS. Strong resistance of citrate anions on metal nanoparticles to desorption under thiol functionalization. ACS Nano. 2015;9:1665–82.10.1021/nn506379mSearch in Google Scholar PubMed
[95] Badia A, Cuccia L, Demers L, Morin F, Lennox RB. Structure and dynamics in alkanethiolate monolayers self-assembled on gold nanoparticles: a DSC, FT-IR, and deuterium NMR study. J Am Chem Soc. 1997;119:2682–92.10.1021/ja963571tSearch in Google Scholar
[96] Ji X, Song X, Li J, Bai Y, Yang W, Peng X. Size control of gold nanocrystals in citrate reduction: the third role of citrate. J Am Chem Soc. 2007;129:13939–48.10.1021/ja074447kSearch in Google Scholar
[97] Wuithschick M, Birnbaum A, Witte S, Sztucki M, Vainio U, Pinna N, et al. Turkevich in new robes: key questions answered for the most common gold nanoparticle synthesis. ACS Nano. 2015;9:7052–71.10.1021/acsnano.5b01579Search in Google Scholar
[98] Boleininger J, Kurz A, Reuss V, Sönnichsen C. Microfluidic continuous flow synthesis of rod-shaped gold and silver nanocrystals. Phys Chem Chem Phys. 2006;8:3824–7.10.1039/B604666ESearch in Google Scholar
[99] Pellegrino T, Sperling RA, Alivisatos AP, Parak WJ. Gel electrophoresis of gold-DNA nanoconjugates. Journal of Biomedicine and Biotechnology. 2007;2007:Article ID 26796.10.1155/2007/26796Search in Google Scholar
[101] Haiss W, Thanh NTK, Aveyard J, Fernig DG. Determination of size and concentration of gold nanoparticles from UV–Vis spectra. Anal Chem. 2007;79:4215–21.10.1021/ac0702084Search in Google Scholar
[102] Hendel T, Wuithschick M, Kettemann F, Birnbaum A, Rademann K, Polte J. In situ determination of colloidal gold concentrations with UV–Vis spectroscopy: limitations and perspectives. Anal Chem. 2014;86:11115–24.10.1021/ac502053sSearch in Google Scholar
[103] Liz-Marzán LM. Tailoring surface plasmons through the morphology and assembly of metal nanoparticles. Langmuir. 2006;22:32–41.10.1201/9780429295188-4Search in Google Scholar
[104] Schneider G, Decher G. From functional core/shell nanoparticles prepared via layer-by-layer deposition to empty nanospheres. Nano Lett. 2004;1833–9.10.1021/nl0490826Search in Google Scholar
[105] Peck JA, Tait CD, Swanson BI, Brown GE. Speciation of aqueous gold (III) chlorides from ultraviolet/visible absorption and Raman/resonance Raman spectroscopies. Geochim Cosmochim Acta. 1991;55:671–6.10.1016/0016-7037(91)90332-YSearch in Google Scholar
[106] Becker R, Doring W. Kinetic treatment of the nucleation in supersaturated vapors. Washington: National Advisory Committee for Aeronautics, 1954Search in Google Scholar
[107] LaMer VK, Dinegar RH. Theory, production and mechanism of formation of monodispersed hydrosols. J Am Chem Soc. 1950;72:4847–54.10.1021/ja01167a001Search in Google Scholar
[108] Bogush GH, Zukoski CF. IV, Uniform silica particle precipitation: an aggregative growth model. J Colloid Interface Sci. 1991;142:19–34.10.1016/0021-9797(91)90030-CSearch in Google Scholar
[109] Watzky MA, Finke RG. Transition metal nanocluster formation kinetic and mechanistic studies. a new mechanism when hydrogen is the reductant: slow, continuous nucleation and fast autocatalytic surface growth. J Am Chem Soc. 1997;119:10382–400.10.1021/ja9705102Search in Google Scholar
[110] Zeng XC, Oxtoby DW. Gas–Liquid nucleation in Lennard–Jones fluids. J Chem Phys. 1991;94:4472–8.10.1063/1.460603Search in Google Scholar
[111] Xia Y, Xiong Y, Lim B, Skrabalak SE. Shape-controlled synthesis of metal nanocrystals: simple chemistry meets complex physics? Angew Chem Int Ed. 2009;48:60–103.10.1002/anie.200802248Search in Google Scholar PubMed PubMed Central
[112] Polte J. Fundamental growth principles of colloidal metal nanoparticles – A new perspective. Cryst Eng Comm. 2015;17:6809–30.10.1039/C5CE01014DSearch in Google Scholar
[113] Luty-Blocho M, Pacławski K, Jaworski W, Streszewski B, Fitzner K. Kinetic studies of gold nanoparticles formation in the batch and in the flow microreactor system. Trends in colloid and interface science XXIV, Progr Colloid Polym Sci Vol. 138. Berlin: Springer, 2011:39–44.10.1007/978-3-642-19038-4_7Search in Google Scholar
[114] Pacławski K, Streszewski B, Jaworski W, Luty-Błocho M, Fitzner K. Gold nanoparticles formation via gold (III) chloride complex ions reduction with glucose in the batch and in the flow microreactor systems. Colloids Surf. A. 2012;413:208–15.10.1016/j.colsurfa.2012.02.050Search in Google Scholar
[115] De Jonge N, Ross FM. Electron microscopy of specimens in liquid. Nat Nanotechnol. 2011;6:695–704.10.1038/nnano.2011.161Search in Google Scholar PubMed
[116] Kim BH, Yang J, Lee D, Choi BK, Hyeon T, Park J. Liquid-phase transmission electron microscopy for studying colloidal inorganic nanoparticles. Adv Mater. 2018;30:1–20.10.1002/adma.201703316Search in Google Scholar PubMed
[117] Baumgartner J, Dey A, Bomans PHH, Le Coadou C, Fratzl P, Sommerdijk NAJM, et al. Nucleation and growth of magnetite from solution. Nat Mater. 2013;12:310–14.10.1038/nmat3558Search in Google Scholar PubMed
[118] McKenzie LC, Haben PM, Kevan SD, Hutchison JE. Determining nanoparticle size in real time by small-angle X-ray scattering in a microscale flow system. J Phys Chem C. 2010;114:22055–63.10.1021/jp1077533Search in Google Scholar
[119] Watt J, Hance BG, Anderson RS, Huber DL. Effect of seed age on gold nanorod formation: a microfluidic, real-time investigation. Chem Mater. 2015;27:6442–9.10.1021/acs.chemmater.5b02675Search in Google Scholar
[120] Buining PA, Humbel BM, Philipse AP, Verkleij AJ. Preparation of functional silane-stabilized gold colloids in the (sub) nanometer size range. Langmuir. 1997;13:3921–6.10.1021/la962071aSearch in Google Scholar
[121] Tofighi G, Lichtenberg H, Pesek J, Sheppard TL, Wang W, Schöttner L, et al. Continuous microfluidic synthesis of colloidal ultrasmall gold nanoparticles: in situ study of the early reaction stages and application for catalysis. React Chem Eng. 2017;2:876–84.10.1039/C7RE00114BSearch in Google Scholar
[122] Yue J, Falke FH, Schouten JC, Nijhuis TA. Microreactors with integrated UV/Vis spectroscopic detection for online process analysis under segmented flow. Lab Chip. 2013;13:4855–63.10.1039/c3lc50876eSearch in Google Scholar PubMed
[123] Sai Krishna K, Navin CV, Biswas S, Singh V, Ham K, Bovenkamp GL, et al. Millifluidics for time-resolved mapping of the growth of gold nanostructures. J Am Chem Soc. 2013;135:5450–6.10.1021/ja400434cSearch in Google Scholar PubMed
[124] Barnard AS. Modelling of nanoparticles: approaches to morphology and evolution. Rep Prog Phys. 2010;73:086502.10.1088/0034-4885/73/8/086502Search in Google Scholar
[125] Ojea-Jiménez I, Campanera JM. Molecular modeling of the reduction mechanism in the citrate-mediated synthesis of gold nanoparticles. J Phys Chem C. 2012;116:23682–91.10.1021/jp305830pSearch in Google Scholar
[126] Grochola G, Snook IK, Russo SP. Computational modeling of nanorod growth. J Chem Phys. 2007;127:194707.10.1063/1.2789420Search in Google Scholar PubMed
[127] Häkkinen H. The gold–Sulfur interface at the nanoscale. Nat Chem. 2012;4:443.10.1038/nchem.1352Search in Google Scholar
[128] Taylor MG, Mpourmpakis G. Thermodynamic stability of ligand-protected metal nanoclusters. Nat Commun. 2017;8:15988.10.1038/ncomms15988Search in Google Scholar
[129] Ramkrishna D. Population balances – theory and applications to particulate systems in engineering, 1. Academic Press, 2000:355.Search in Google Scholar
[130] Crowley T. Control of particle size distribution described by a population balance model of semibatch emulsion polymerization. J Process Control. 2000;10:419–32.10.1016/S0959-1524(00)00017-2Search in Google Scholar
[131] Immanuel CD, Doyle FJ, III. Computationally efficient solution of population balance models incorporating nucleation, growth and coagulation: application to emulsion polymerization. Chem Eng Sci. 2003;58:3681–98.10.1016/S0009-2509(03)00216-1Search in Google Scholar
[132] Kotoulas C, Kiparissides C. A generalized population balance model for the prediction of particle size distribution in suspension polymerization reactors. Chem Eng Sci. 2006;61:332–46.10.1016/j.ces.2005.07.013Search in Google Scholar
[133] Marchal P, David R, Klein JP, Villermaux J. Crystallization and precipitation engineering-I. An efficient method for solving population balance in crystallization with agglomeration. Chem Eng Sci. 1988;43:59–67.10.1016/0009-2509(88)87126-4Search in Google Scholar
[134] Puel F, Févotte G, Klein JP. Simulation and analysis of industrial crystallization processes through multidimensional population balance equations. Part 1: a resolution algorithm based on the method of classes. Chem Eng Sci. 2003;58:3715–27.10.1016/S0009-2509(03)00254-9Search in Google Scholar
[135] Puel F, Févotte G, Klein JP. Simulation and analysis of industrial crystallization processes through multidimensional population balance equations. Part 2: a study of semi-batch crystallization. Chem Eng Sci. 2003;58:3729–40.10.1016/S0009-2509(03)00253-7Search in Google Scholar
[136] Bogush GH, Zukoski CF, IV. Studies of the kinetics of the precipitation of uniform silica particles through the hydrolysis and condensation of silicon alkoxides. J Colloid Interface Sci. 1991;142:1–18.10.1016/0021-9797(91)90029-8Search in Google Scholar
[137] Park J, Joo J, Soon GK, Jang Y, Hyeon T. Synthesis of monodisperse spherical nanocrystals. Angew Chem Int Ed. 2007;46:4630–60.10.1002/anie.200603148Search in Google Scholar PubMed
[138] Stolzenburg P, Garnweitner G. Experimental and numerical insights into the formation of zirconia nanoparticles: a population balance model for the nonaqueous synthesis. React Chem Eng. 2017;2:337–48.10.1039/C7RE00005GSearch in Google Scholar
[139] Rempel JY, Bawendi MG, Jensen KF. Insights into the kinetics of semiconductor nanocrystal nucleation and growth. J Am Chem Soc. 2009;131:4479–89.10.1021/ja809156tSearch in Google Scholar PubMed
[140] Maceiczyk RM, Bezinge L, deMello AJ. Kinetics of nanocrystal synthesis in a microfluidic reactor: theory and experiment. React Chem Eng. 2016;1:261–71.10.1039/C6RE00073HSearch in Google Scholar
[141] Lazzari S, Abolhasani M, Jensen KF. Modeling of the formation kinetics and size distribution evolution of II–VI quantum dots. React Chem Eng. 2017;2:567–76.10.1039/C7RE00068ESearch in Google Scholar
[142] Perala SRK, Kumar S. On the mechanism of metal nanoparticle synthesis in the Brust-Schiffrin method. Langmuir. 2013;29:9863–73.10.1021/la401604qSearch in Google Scholar PubMed
[143] Kumar S, Gandhi K, Kumar R. Modeling of formation of gold nanoparticles by citrate method. Ind Eng Chem Res. 2007;46:3128–36.10.1021/ie060672jSearch in Google Scholar
[144] Agunloye E, Gavriilidis A, Mazzei L. A mathematical investigation of the Turkevich organizer theory in the citrate method for the synthesis of gold nanoparticles. Chem Eng Sci. 2017;173:275–86.10.1016/j.ces.2017.07.032Search in Google Scholar
[145] Turkevich J, Stevenson PC, Hillier J. A study of the nucleation and growth processes in the synthesis of colloidal gold. Discuss Faraday Soc. 1951;11:55–75.10.1039/df9511100055Search in Google Scholar
[146] Biggs S, Chow M, Zukoski CF, Grieser F. The role of colloidal stability in the formation of gold sols. J Colloid Interface Sci. 1993;160:511–13.10.1006/jcis.1993.1430Search in Google Scholar
[147] Frens G. Controlled nucleation for the regulation of the particle size in monodisperse gold suspensions. Nature. 1973;241:20–2.10.1038/physci241020a0Search in Google Scholar
[148] Kimling J, Maier M, Okenve B, Kotaidis V, Ballot H, Plech A. Turkevich method for gold nanoparticle synthesis revisited. J Phys Chem B. 2006;110:15700–7.10.1021/jp061667wSearch in Google Scholar PubMed
[149] Sivaraman SK, Kumar S, Santhanam V. Monodisperse sub-10 nm gold nanoparticles by reversing the order of addition in Turkevich method–The role of chloroauric acid. J Colloid Interface Sci. 2011;361:543–47.10.1016/j.jcis.2011.06.015Search in Google Scholar PubMed
[150] Xia H, Bai S, Hartmann JR, Wang D. Synthesis of monodisperse quasi-spherical gold nanoparticles in water via silver (I)-assisted citrate reduction. Langmuir. 2009;26:3585–9.10.1021/la902987wSearch in Google Scholar PubMed
[151] Schulz F, Homolka T, Bastús NG, Puntes V, Weller H, Vossmeyer T. Little adjustments significantly improve the Turkevich synthesis of gold nanoparticles. Langmuir. 2014;30:10779–84.10.1021/la503209bSearch in Google Scholar PubMed
[152] Bastús NG, Comenge J, Puntes V. Kinetically controlled seeded growth synthesis of citrate-stabilized gold nanoparticles of up to 200 nm: size focusing versus Ostwald ripening. Langmuir. 2011;27:11098–105.10.1021/la201938uSearch in Google Scholar PubMed
[153] Ziegler C, EychmüLler A. Seeded growth synthesis of uniform gold nanoparticles with diameters of 15–300 nm. J Phys Chem C. 2011;115:4502–06.10.1021/jp1106982Search in Google Scholar
[154] Piella J, Bastús NG, Puntes V. Size-controlled synthesis of sub-10-nanometer citrate-stabilized gold nanoparticles and related optical properties. Chem Mater. 2016;28:1066–75.10.1021/acs.chemmater.5b04406Search in Google Scholar
[155] Slot JW, Geuze HJ. A new method of preparing gold probes for multiple-labeling cytochemistry. Eur J Cell Biol. 1985;38:87–93.Search in Google Scholar
[156] Brown KR, Fox AP, Natan MJ. Morphology-dependent electrochemistry of cytochrome c at Au colloid-modified SnO2 electrodes. J Am Chem Soc. 1996;118:1154–7.10.1021/ja952951wSearch in Google Scholar
[157] Singh A, Shirolkar M, Lalla NP, Malek CK, Kulkarni S. Room temperature, water-based, microreactor synthesis of gold and silver nanoparticles. Int J Nanotechnol. 2009;6:541–51.10.1504/IJNT.2009.024645Search in Google Scholar
[158] Brust M, Walker M, Bethell D, Schiffrin DJ, Whyman R. Synthesis of thiol-derivatised gold nanoparticles in a two-phase liquid–liquid system. J Chem Soc, Chem Commun. 1994;0:801–210.1039/C39940000801Search in Google Scholar
[159] Jun H, Fabienne T, Florent M, Coulon P-E, Nicolas M, Olivier S. Understanding of the size control of biocompatible gold nanoparticles in millifluidic channels. Langmuir. 2012;28:15966–74.10.1021/la303439fSearch in Google Scholar PubMed
[160] Shalom D, Wootton RC, Winkle RF, Cottam BF, Vilar R, Wilde CP. Synthesis of thiol functionalized gold nanoparticles using a continuous flow microfluidic reactor. Mater Lett. 2007;61:1146–50.10.1016/j.matlet.2006.06.072Search in Google Scholar
[161] Tsunoyama H, Ichikuni N, Tsukuda T. Microfluidic synthesis and catalytic application of PVP-stabilized, ~1 nm gold clusters. Langmuir. 2008;24:11327–30.10.1021/la801372jSearch in Google Scholar PubMed
[162] Luty-Błocho M, Fitzner K, Hessel V, Löb P, Maskos M, Metzke D, et al. Synthesis of gold nanoparticles in an interdigital micromixer using ascorbic acid and sodium borohydride as reducers. Chem Eng J. 2011;171:279–90.10.1016/j.cej.2011.03.104Search in Google Scholar
[163] Köhler J, Wagner J, Albert J. Formation of isolated and clustered Au nanoparticles in the presence of polyelectrolyte molecules using a flow-through Si chip reactor. J Mater Chem. 2005;15:1924–30.10.1039/b417868hSearch in Google Scholar
[164] Wagner J, Köhler JM. Continuous synthesis of gold nanoparticles in a microreactor. Nano Lett. 2005;5:685–91.10.1021/nl050097tSearch in Google Scholar PubMed
[165] Wagner J, Tshikhudo TR, Köhler JM. Microfluidic generation of metal nanoparticles by borohydride reduction. Chem Eng J. 2007;135:104–9.10.1016/j.cej.2007.07.046Search in Google Scholar
[166] Sugano K, Uchida Y, Ichihashi O, Yamada H, Tsuchiya T, Tabata O. Mixing speed-controlled gold nanoparticle synthesis with pulsed mixing microfluidic system. Microfluid Nanofluidics. 2010;9:1165–74.10.1007/s10404-010-0637-9Search in Google Scholar
[167] Verma M, Kumaran V. Effect of ultra-fast mixing in a microchannel due to a soft wall on the room temperature synthesis of gold nanoparticles. Sadhana. 2015;40:973–83.10.1007/s12046-015-0351-0Search in Google Scholar
[168] Bandulasena MV, Vladisavljević GT, Odunmbaku OG, Benyahia B. Continuous synthesis of PVP stabilized biocompatible gold nanoparticles with a controlled size using a 3D glass capillary microfluidic device. Chem Eng Sci. 2017;171:233–43.10.1016/j.ces.2017.05.035Search in Google Scholar
[169] Baber R, Mazzei L, Thanh NTK, Gavriilidis A. An engineering approach to synthesis of gold and silver nanoparticles by controlling hydrodynamics and mixing based on a coaxial flow reactor. Nanoscale. 2017;9:14149–61.10.1039/C7NR04962ESearch in Google Scholar
[170] Yang SY, Cheng FY, Yeh CS, Lee GB. Size-controlled synthesis of gold nanoparticles using a micro-mixing system. Microfluid Nanofluidics. 2009;8:303–11.10.1007/s10404-009-0461-2Search in Google Scholar
[171] Kitson PJ, Rosnes MH, Sans V, Dragone V, Cronin L. Configurable 3D-printed millifluidic and microfluidic ‘lab on a chip’ reactionware devices. Lab Chip. 2012;12:3267–71.10.1039/c2lc40761bSearch in Google Scholar PubMed
[172] Ftouni J, Penhoat M, Addad A, Payen E, Rolando C, Girardon J-S. Highly controlled synthesis of nanometric gold particles by citrate reduction using the short mixing, heating and quenching times achievable in a microfluidic device. Nanoscale. 2012;4:4450–4.10.1039/c2nr11666aSearch in Google Scholar PubMed
[173] Jamal F, Jean-Sébastien G, Maël P, Edmond P, Christian R. Gold nanoparticle synthesis in microfluidic systems and immobilisation in microreactors designed for the catalysis of fine organic reactions. MicrosystTechnol. 2012;18:151–58.10.1007/s00542-011-1369-9Search in Google Scholar
[174] Gómez-De Pedro S, Puyol M, Alonso-Chamarro J. Continuous flow synthesis of nanoparticles using ceramic microfluidic devices. Nanotechnology. 2010;21:415603.10.1088/0957-4484/21/41/415603Search in Google Scholar PubMed
[175] Sugie A, Song H, Horie T, Ohmura N, Kanie K, Muramatsu A, et al. Synthesis of thiol-capped gold nanoparticle with a flow system using organosilane as a reducing agent. Tetrahedron Lett. 2012;53:4457–59.10.1016/j.tetlet.2012.06.056Search in Google Scholar
[176] Kumar DR, Kulkarni A, Prasad B. Microfluidic platform for continuous flow synthesis of triangular gold nanoplates. Colloids Surf., A. 2014;443:149–55.10.1016/j.colsurfa.2013.10.047Search in Google Scholar
[177] Fu Q, Ran G, Xu W. A microfluidic-based controllable synthesis of rolled or rigid ultrathin gold nanoplates. RSC Adv. 2015;5:37512–6.10.1039/C5RA02461GSearch in Google Scholar
[178] Sebastián V, Lee S, Zhou C, Kraus MF, Fujimoto JG, Jensen KF. One-step continuous synthesis of biocompatible gold nanorods for optical coherence tomography. Chem Commun. 2012;48:6654–6.10.1039/c2cc32969gSearch in Google Scholar PubMed PubMed Central
[179] Ishizaka T, Ishigaki A, Kawanami H, Suzuki A, Suzuki TM. Dynamic control of gold nanoparticle morphology in a microchannel flow reactor by glucose reduction in aqueous sodium hydroxide solution. J Colloid Interface Sci. 2012;367:135–8.10.1016/j.jcis.2011.10.027Search in Google Scholar PubMed
[180] Wagner J, Kirner T, Mayer G, Albert J, Köhler J. Generation of metal nanoparticles in a microchannel reactor. Chem Eng J. 2004;101:251–60.10.1016/j.cej.2003.11.021Search in Google Scholar
[181] Sebastian V, Khan SA, Kulkarni AA. Perspective article: flow synthesis of functional materials. J Flow Chem. 2017;7:96–105.10.1556/1846.2017.00028Search in Google Scholar
[182] Lohse SE, Eller JR, Sivapalan ST, Plews MR, Murphy CJ. A simple millifluidic benchtop reactor system for the high-throughput synthesis and functionalization of gold nanoparticles with different sizes and shapes. ACS Nano. 2013;7:4135–50.10.1021/nn4005022Search in Google Scholar PubMed
[183] Gomez L, Sebastian V, Irusta S, Ibarra A, Arruebo M, Santamaria J. Scaled-up production of plasmonic nanoparticles using microfluidics: from metal precursors to functionalized and sterilized nanoparticles. Lab Chip. 2014;14:325–32.10.1039/C3LC50999KSearch in Google Scholar PubMed
[184] Uson L, Sebastian V, Arruebo M, Santamaria J. Continuous microfluidic synthesis and functionalization of gold nanorods. Chem Eng J. 2016;285:286–92.10.1016/j.cej.2015.09.103Search in Google Scholar
[185] Bullen C, Latter MJ, D’Alonzo NJ, Willis GJ, Raston CL. A seedless approach to continuous flow synthesis of gold nanorods. Chem Commun. 2011;47:4123–5.10.1039/c0cc05175fSearch in Google Scholar PubMed
[186] Sans V, Glatzel S, Douglas FJ, Maclaren DA, Lapkin A, Cronin L. Non-equilibrium dynamic control of gold nanoparticle and hyper-branched nanogold assemblies. Chem Sci. 2014;5:1153–7.10.1039/c3sc53223bSearch in Google Scholar
[187] Bayazit MK, Yue J, Cao E, Gavriilidis A, Tang J. Controllable synthesis of gold nanoparticles in aqueous solution by microwave assisted flow chemistry. ACS Sustain Chem Eng. 2016;4:6435–42.10.1021/acssuschemeng.6b01149Search in Google Scholar
[188] Köhler JM, Li S, Knauer A. Why is micro segmented flow particularly promising for the synthesis of nanomaterials? Chem Eng Technol. 2013;36:887–99.10.1002/ceat.201200695Search in Google Scholar
[189] Liu H, Vandu CO, Krishna R. Hydrodynamics of Taylor flow in vertical capillaries: flow regimes, bubble rise velocity, liquid slug length, and pressure drop. Ind Eng Chem Res. 2005;44:4884–97.10.1021/ie049307nSearch in Google Scholar
[190] Sebastian Cabeza V, Kuhn S, Kulkarni AA, Jensen KF. Size-controlled flow synthesis of gold nanoparticles using a segmented flow microfluidic platform. Langmuir. 2012;28:7007–13.10.1021/la205131eSearch in Google Scholar PubMed
[191] Kulkarni AA, Sebastian Cabeza V. Insights in the diffusion controlled interfacial flow synthesis of Au nanostructures in a microfluidic system. Langmuir. 2017;33:14315–24.10.1021/acs.langmuir.7b03277Search in Google Scholar PubMed
[192] . In: Ngai T, Bon SAF, editor(s). Particle-stabilized emulsions and colloids. Cambridge: Royal Society of Chemistry, 201410.1039/9781782620143Search in Google Scholar
[193] Khan SA, Duraiswamy S. Controlling bubbles using bubbles – microfluidic synthesis of ultra-small gold nanocrystals with gas-evolving reducing agents. Lab Chip. 2012;12:1807–12.10.1039/c2lc21198jSearch in Google Scholar PubMed
[194] Song H, Tice JD, Ismagilov RF. A microfluidic system for controlling reaction networks in time. Angew Chem. 2003;115:792–6.10.1002/anie.200390203Search in Google Scholar PubMed
[195] Günther A, Jhunjhunwala M, Thalmann M, Schmidt MA, Jensen KF. Micromixing of miscible liquids in segmented gas–liquid flow. Langmuir. 2005;21:1547–55.10.1021/la0482406Search in Google Scholar PubMed
[196] Zhang L, Xia Y. Scaling up the production of colloidal nanocrystals: should we increase or decrease the reaction volume? Adv Mater. 2014;26:2600–6.10.1002/adma.201304897Search in Google Scholar PubMed
[197] Christopher GF, Anna SL. Microfluidic methods for generating continuous droplet streams. J Phys D Appl Phys. 2007;40:R319.10.1088/0022-3727/40/19/R01Search in Google Scholar
[198] Garstecki P, Fuerstman MJ, Stone HA, Whitesides GM. Formation of droplets and bubbles in a microfluidic T-junction – scaling and mechanism of break-up. Lab Chip. 2006;6:437–46.10.1039/b510841aSearch in Google Scholar PubMed
[199] Duraiswamy S, Khan SA. Droplet-based microfluidic synthesis of anisotropic metal nanocrystals. Small. 2009;5:2828–34.10.1002/smll.200901453Search in Google Scholar PubMed
[200] Duraiswamy S, Khan SA. Dual-stage continuous-flow seedless microfluidic synthesis of anisotropic gold nanocrystals. Part Part Syst Charact. 2014;31:429–32.10.1002/ppsc.201300266Search in Google Scholar
[201] Duraiswamy S, Khan SA. Plasmonic nanoshell synthesis in microfluidic composite foams. Nano Lett. 2010;10:3757–63.10.1021/nl102478qSearch in Google Scholar PubMed
[202] Lazarus LL, Yang AS, Chu S, Brutchey RL, Malmstadt N. Flow-focused synthesis of monodisperse gold nanoparticles using ionic liquids on a microfluidic platform. Lab Chip. 2010;10:3377–9.10.1039/c0lc00297fSearch in Google Scholar PubMed
[203] Lazarus LL, Riche CT, Marin BC, Gupta M, Malmstadt N, Brutchey RL. Two-phase microfluidic droplet flows of ionic liquids for the synthesis of gold and silver nanoparticles. ACS Appl Mater Interfaces. 2012;4:3077–83.10.1021/am3004413Search in Google Scholar PubMed
[204] Taifur-Rahman M, Krishnamurthy PG, Parthiban P, Jain A, Park CP, Kim D-P, et al. Dynamically tunable nanoparticle engineering enabled by short contact-time microfluidic synthesis with a reactive gas. RSC Adv. 2013;3:2897–900.10.1039/c2ra23216bSearch in Google Scholar
[205] Abalde-Cela S, Taladriz-Blanco P, De Oliveira MG, Abell C. Droplet microfluidics for the highly controlled synthesis of branched gold nanoparticles. Sci Rep. 2018;8:2440.10.1038/s41598-018-20754-xSearch in Google Scholar PubMed PubMed Central
[206] Hafermann L, Köhler JM. Small gold nanoparticles formed by rapid photochemical flow-through synthesis using microfluid segment technique. J Nanopart Res. 2015;17:1–8.10.1007/s11051-015-2914-8Search in Google Scholar
[207] Lee KG, Hong J, Wang KW, Heo NS, Kim DH, Lee SY, et al. In vitro biosynthesis of metal nanoparticles in microdroplets. ACS Nano. 2012;6:6998–7008.10.1021/nn302043qSearch in Google Scholar PubMed
[208] Gu T, Zheng C, He F, Zhang Y, Khan SA, Hatton TA. Electrically controlled mass transport into microfluidic droplets from nanodroplet carriers with application in controlled nanoparticle flow synthesis. Lab Chip. 2018;18:1330–40.10.1039/C8LC00114FSearch in Google Scholar
[209] Zhang L, Niu G, Lu N, Wang J, Tong L, Wang L, et al. Continuous and scalable production of well-controlled noble-metal nanocrystals in milliliter-sized droplet reactors. Nano Lett. 2014;14:6626–31.10.1021/nl503284xSearch in Google Scholar PubMed
[210] Yang Y, Serrano González LA, Guldin S. A versatile AuNP synthetic platform for decoupled control of size and surface composition. Langmuir. 2018;34:6820–610.1021/acs.langmuir.8b00353Search in Google Scholar PubMed
[211] Rossi D, Gargiulo L, Valitov G, Gavriilidis A, Mazzei L. Experimental characterization of axial dispersion in coiled flow inverters. Chem Eng Res Des. 2017;120:159–70.10.1016/j.cherd.2017.02.011Search in Google Scholar
[212] Trachsel F, Günther A, Khan S, Jensen KF. Measurement of residence time distribution in microfluidic systems. Chem Eng Sci. 2005;60:5729–37.10.1016/j.ces.2005.04.039Search in Google Scholar
[213] Günther A, Khan SA, Thalmann M, Trachsel F, Jensen KF. Transport and reaction in microscale segmented gas-liquid flow. Lab Chip. 2004;4:278–86.10.1039/B403982CSearch in Google Scholar PubMed
[214] Khan SA, Günther A, Schmidt MA, Jensen KF. Microfluidic synthesis of colloidal silica. Langmuir. 2004;20:8604–11.10.1021/la0499012Search in Google Scholar PubMed
[215] Krishnadasan S, Tovilla J, Vilar R, deMello AJ, deMello JC. On-line analysis of CdSe nanoparticle formation in a continuous flow chip-based microreactor. J Mater Chem. 2004;14:2655.10.1039/b401559bSearch in Google Scholar
[216] Panariello L, Mazzei L, Gavriilidis A. Modelling the synthesis of nanoparticles in continuous microreactors: the role of diffusion and residence time distribution on nanoparticle characteristics. Chem Eng J. 2018;350:1144–5410.1016/j.cej.2018.03.167Search in Google Scholar
[217] Marchisio DL, Rivautella L, Barresi AA. Design and scale-up of chemical reactors for nanoparticle precipitation. AIChE J. 2006; 1877–87.10.1002/aic.10786Search in Google Scholar
[218] Tae G, Lammertink RG, Kornfield JA, Hubbell JA. Facile hydrophilic surface modification of poly(tetrafluoroethylene) using fluoroalkyl-terminated poly(ethylene glycol)s. Adv Mater. 2003;15:66–9.10.1002/adma.200390013Search in Google Scholar
[219] Zhang L, Wang Y, Tong L, Xia Y. Synthesis of colloidal metal nanocrystals in droplet reactors: the pros and cons of interfacial adsorption. Nano Lett. 2014;14:4189–94.10.1021/nl501994qSearch in Google Scholar PubMed
[220] Weeranoppanant N, Adamo A, Saparbaiuly G, Rose E, Fleury C, Schenkel B, et al. Design of multistage counter-current liquid–Liquid extraction for small-scale applications. Ind Eng Chem Res. 2017;56:4095–103.10.1021/acs.iecr.7b00434Search in Google Scholar
[221] Sweeney SF, Woehrle GH, Hutchison JE. Rapid purification and size separation of gold nanoparticles via diafiltration. J Am Chem Soc. 2006;128:3190–97.10.1021/ja0558241Search in Google Scholar PubMed
© 2018 Walter de Gruyter GmbH, Berlin/Boston
Articles in the same Issue
- Frontmatter
- Green chemistry outreach
- Continuous synthesis of gold nanoparticles in micro- and millifluidic systems
- Ionic liquid-assisted biphasic systems for downstream processing of fermentative enzymes and organic acids
- Description of excited states in photochemistry with theoretical methods
- In situ neutron powder diffraction studies
- Cheminformatics techniques in antimalarial drug discovery and development from natural products 2: Molecular scaffold and machine learning approaches
Articles in the same Issue
- Frontmatter
- Green chemistry outreach
- Continuous synthesis of gold nanoparticles in micro- and millifluidic systems
- Ionic liquid-assisted biphasic systems for downstream processing of fermentative enzymes and organic acids
- Description of excited states in photochemistry with theoretical methods
- In situ neutron powder diffraction studies
- Cheminformatics techniques in antimalarial drug discovery and development from natural products 2: Molecular scaffold and machine learning approaches

