Optimizing patient recovery: prospective study evaluating compliance and clinical outcomes of enhanced recovery protocols in ovarian cancer following cytoreductive surgery with HIPEC
-
S. P. Somashekhar
and K. R. Ashwin
Abstract
Objectives
To evaluate the implementation, compliance, and impact of the enhanced recovery after surgery (ERAS) protocol on perioperative outcomes in patients undergoing cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) for Stage IIIc ovarian cancer.
Methods
From September 2020 to March 2022, the ERAS protocol (62 perioperative and special consideration guidelines) was prospectively implemented in 75 patients. Based on compliance rates, patients were divided into three groups: Group A (<70 %, 13 patients), Group B (70 %–80 %, 52 patients), and Group C (>80 %, 10 patients). Compliance rates, length of stay, postoperative complications, and readmission rates were analyzed. Ethical committee approval was obtained.
Results
The cohort’s average compliance was 74.5 %, with group averages of 68.4 %, 74.4 %, and 82.5 % (p<0.001). Tolerance to normal diet (p=0.008), postoperative ileus (p=0.161), and mobilization rates (p<0.001) improved with higher compliance. Higher compliance also led to shorter hospital stays (p=0.008) and ICU stays (p<0.001). Complications like ileus and infections were lowest in Group C. No significant differences were found in re-surgery or mortality.
Conclusions
Implementation of the ERAS protocol in patients undergoing CRS and HIPEC for Stage IIIc ovarian cancer is feasible and associated with improved postoperative outcomes. Higher compliance with ERAS guidelines significantly reduced length of hospital and ICU stay, enhanced early mobilization, and improved tolerance to diet, while also decreasing postoperative complications. Compliance above 80 % is necessary for achieving optimal outcomes and protocol modifications may improve compliance.
Introduction
Cytoreductive surgery with hyperthermic intraperitoneal chemotherapy (CRS and HIPEC) is an important therapeutic strategy in the treatment of epithelial ovarian cancer (EOC). Randomized trials, retrospective studies and meta analysis has shown survival benefit [1], [2], [3], [4], [5], [6], following which HIPEC has been included as a treatment option in oncological guidelines [7]. Morbidity and mortality concerns are one of the deterrents for advocating CRS and HIPEC by clinicians [8]. The mortality and morbidity rates for CRS and HIPEC is 1–5 % and 10–30 %, respectively [9], [10], [11], [12].
Enhanced recovery after surgery (ERAS) is evidence-based perioperative measures designed to reduce the exaggerated postoperative metabolic and inflammatory response [13], 14]. ERAS challenges the conventional practices and traditional attitudes of clinicians. These multimodal pathways are widely used in various surgical fields for optimizing patient outcomes with reproducible clinical benefits [15], 16] ERAS pathway has shown to benefit patients by decreasing complications, length of stay and costs [17], [18], [19], [20]. It is personnel-intensive, requiring a multidisciplinary team to optimize patients from the initial assessment in outpatient department to the early days after surgery. The team is inclusive of ERAS coordinator, nutritionists, nurses, physiotherapists and doctors, spanning the entire continuum of care from preoperative counselling to return to normal function. Audit of results, assessing adherence and compliance rates is an essential component of the ERAS program [21].
Hubner et al. published the ERAS guidelines specific to CRS and HIPEC with several key elements including preoperative, intraoperative, post operative aspects and special considerations [22], 23]. The mere introduction of an ERAS protocol does not ensure success of program as there exists a wide heterogeneity in real world practice of perioperative patient care. Achieving high degree of compliance is imperative for patient benefit and satisfaction [24], 25]. Clinical studies have shown short and long-term prognoses of patients are closely related to the compliance to protocol [26], [27], [28], [29], [30], [31], [32], [33]. It is well known that minimally invasive surgery allows better outcomes with and without ERAS [34]. But can the same benefit of ERAS be reciprocated in a supra invasive surgery like CRS and HIPEC is not known.
This ERAS protocol represents a significant change in practice for CRS and HIPEC and ours is the first study to get evidence from the actual application of the guidelines in a clinical setting and analyze the course and effect of implementation of the new ERAS protocol for CRS and HIPEC in a PSM center of excellence. Considering the high morbidity and mortality, it is important that we study the effect of these perioperative protocols on outcomes, the barriers to achieve compliance and problems facing successful implementation.
Materials and methods
A retrospective analysis of prospectively maintained data at a tertiary referral hospital and centre of excellence for PSM. The centre has ERAS certification with a dedicated ERAS co-ordinator and the protocol is currently part of our routine perioperative care in gynaecological and colorectal surgeries. A total of 87 patients with Stage IIIc ovarian cancer between September 2020 and March 2022 participated in the study. Twelve patients dropped out during the study period, leaving data from 75 patients for outcome analyses. Figure 1 All 75 patients had ERAS protocol applied following informed consent and underwent CRS and HIPEC as per institution protocol [35]. The study was approved by the Institutional Review Board and ethics committee.
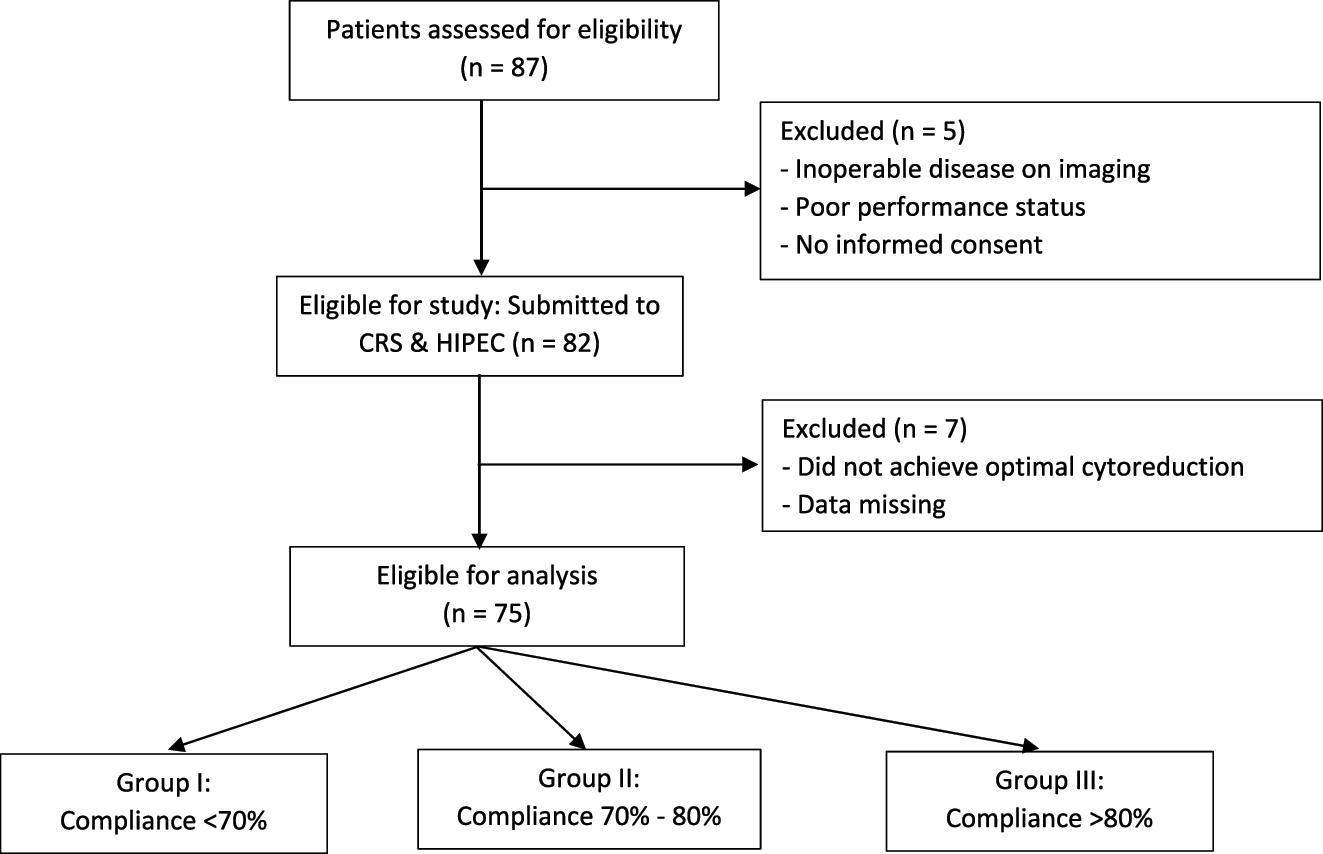
Flowchart of the study participants.
Perioperative care was provided in all patients based on the enhanced recovery after surgery society guidelines for perioperative care in CRS and HIPEC, consisting of 52 essential and 10 non-essential components (preoperative, intraoperative and postoperative and special considerations). Ten elements that were defined as non-essential were based on the weak negative or no consensus recommendation in the guidelines [22], 23] Table 1 The team responsible for implementation of the protocol included, 3 surgeons, 2 anaesthetists, 2 nurses, a physiotherapist, a dietician and ERAS coordinator. The ERAS coordinator was responsible for data collection, continuous auditing and monitoring the course of implementation, ensure adherence to the protocol, assess the compliance rates, record variations in daily practice and identify problems encountered during the process. Demographic factors like age, medical comorbidity, upfront surgery or interval debulking surgery pre-, intra-, and postoperative data was collected. Patients were discharged if they met the discharge criteria: no intravenous fluids, no infection, tolerating solid food, adequate pain control with oral analgesics and independent ambulation. Patients were contacted by telephone at 30 and 90 days following surgery.
ERAS protocol for CRS + HIPEC.
| Parametrs | |
|---|---|
| PREOPERATIVE | |
|
|
|
ESSENTIAL
|
|
|
|
|
| INTRAOPERATIVE | |
|
|
|
ESSENTIAL
|
|
|
|
|
| POST OPERATIVE | |
|
|
|
ESSENTIAL
|
|
We analysed the degree of implementation of the protocol and compliance to protocol. The compliance rate for each patient was calculated as the number of interventions fulfilled for the 52 essential elements of protocol. Primary outcome of the study was to analyse the effect of the ERAS protocol compliance rate on length of hospital stay (LOS), recovery parameters (diet tolerance, mobilization), complications, mortality and readmission rate. Complication was defined using the Clavien–Dindo classification. Readmission was identified as any patient rehospitalisation within 30 days of surgery after discharge.
Statistical analysis: The results are presented as mean ± standard deviation (SD), median and interquartile range (IQR), when appropriate. Tests were selected depending on the type of the variables. For the qualitative variables the chi-square test was used. In cases of quantitative variables, where no normal distribution was observed, we used the Kruskal-Wallis test. To compare the two groups, when non-normally distributed quantitative variables were present, the U Mann-Whitney test was used. The relationship between the compliance with the protocol and LOS was examined using Pearson’s correlation. Statistical significance is accepted at p<0.05.
Results
We analysed the compliance with the ERAS protocol and the entire cohort was divided into 3 sub groups according to the compliance score achieved: <70 % (Group A – 13 patients), 70–80 % (Group B – 52 patients) and >80 % (Group C – 10 patients). The average compliance achieved in the study was 74.5 %. The average compliance with the protocol differed significantly between subgroups, 68.4 % in group A, 74.4 % in group B and 82.5 % in group C (p<0.001, Figure 2). Demographic characteristics and operative details in the subgroups were comparable to each other in terms of age, gender, body mass index (BMI), performance score, co-morbidity, peritoneal carcinomatosis index (PCI) and operative times. Table 2.
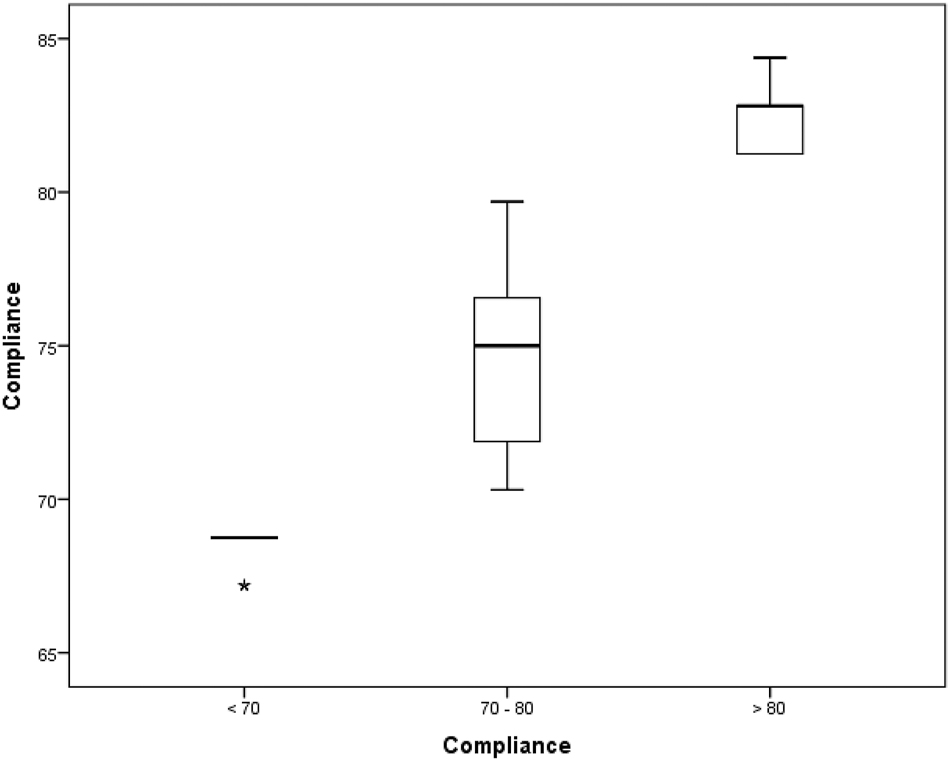
The average compliance in the patient subgroups.
Demographic characteristics of compliance sub groups.
| Parameter | Compliance group <70 % | Compliance group <70–80 % | Compliance group >80 % | p-Value |
|---|---|---|---|---|
| No of patients | 13 | 52 | 10 | |
| Age, mean | 56.3 ± 7.36 | 54.1 ± 9.31 | 55.7 ± 7.17 | 0.657 |
| Comorbidity | 10 (76.9) | 31 (59.6) | 4 (40.0) | 0.200 |
| Mean ECOG performance score ECOG 0 ECOG 1 |
8 5 |
25 27 |
4 6 |
0.511 |
| Haemoglobin (mean ± SD) | 11.3 ± 1.52 | 11.1 ± 1.57 | 11.2 ± 2.08 | 0.868 |
| Albumin (mean ± SD) | 3.9 ± 0.44 | 3.9 ± 0.41 | 4.0 ± 0.37 | 0.861 |
| Mean BMI (kg/m2) (mean ± SD) | 23.3 ± 3.88 | 23.1 ± 3.16 | 21.7 ± 3.62 | 0.456 |
| CRS - Upfront - Interval |
3 10 |
17 35 |
2 8 |
0.622 |
| PCI (median) | 14.0 (2–31) | 11.0 (3–39) | 9.0 (3–18) | 0.669 |
| Mean duration of surgery, hours (Median) |
330 (190–380) | 370 (230–450) | 290 (170–350) | 0.470 |
Clinical parameters
An inverse correlation between compliance LOS and ICU stay was noticed in the study. The LOS was shorter in the higher compliance subgroups; 11.0 (6–22), vs. 10.0 (5–21), vs. 8.0 (7–10) p=0.008) days respectively. Figure 3 and 4 The ICU stay was shorter in better compliant patients; (4.0 (2–8) vs. 2.0 (1–5) vs. 1.0 (0–2) p<0.001), respectively. The on table extubation rate although not statistically significant (p<0.083) did show earlier extubation and avoidance of ICU in higher compliance subgroups. There were no statistically significant differences in postoperative 30-day readmission rate (p<0.438), reoperation rate (p<0.799) and 30-day hospital mortality rate (p<0.438) between the three subgroups. The analysis of recovery parameters showed differences between the subgroups: tolerance of normal diet (10.7 ± 3.42 vs. 8.3 ± 3.03 vs. 5.7 ± 1.42 days; p 0.008), post operative ileus 46.2 % vs. 38.5 % vs. 20 %; p=0.161), mobilization of a patient on the day of surgery (15.4 % vs. 21.2 % vs. 90 % p<0.001). Table 3.
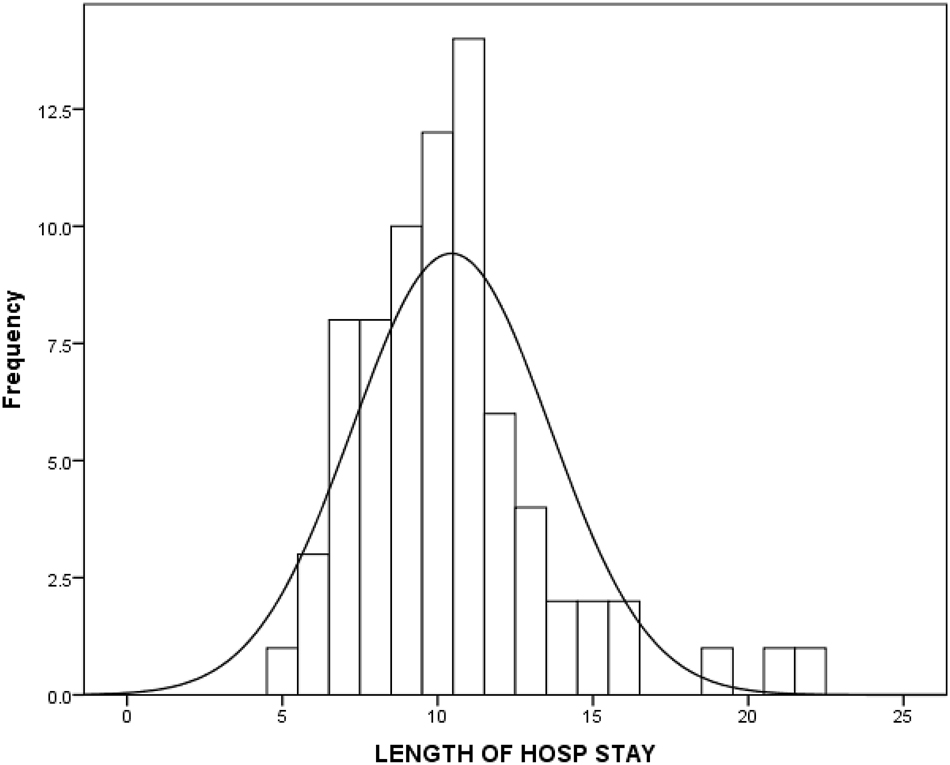
Length of hospital stay in the cohort.
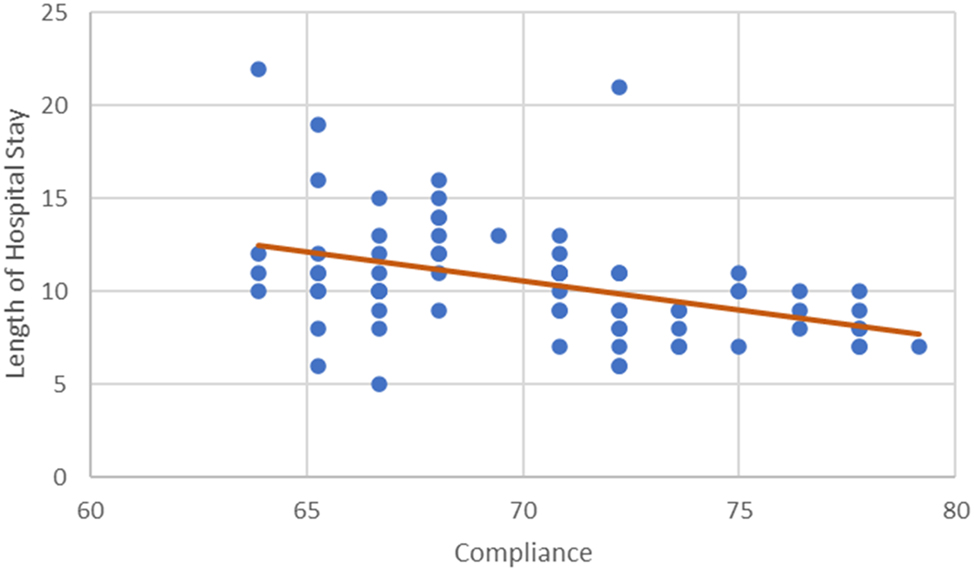
Correlation between compliance and length of hospital stay.
Comparison of compliance sub groups with clinical parameters.
| Parameter | Compliance group<70 % | Compliance group<70–80 % | Compliance group>80 % | p-Value |
|---|---|---|---|---|
| No of patients | 13 | 52 | 10 | |
| Length of ICU | 4.0 (2–8) | 2.0 (1–5) | 1.0 (0–2) | 0.001 |
| Length of hospital stay, days | 11.0 (6–22) | 10.0 (5–21) | 8.0 (7–10) | 0.008 |
| Extubation – on table | 16 (61.5 %) | 82 (78.8 %) | 20 (100.0 %) | 0.083 |
| Readmission | 1 | 1 | 0 | 0.438 |
| Re surgery | 0 | 1 | 0 | 0.799 |
| In hosp mortality | 1 | 1 | 0 | 0.438 |
| Tolerance to normal diet, days | 10.7 ± 3.42 | 8.3 ± 3.03 | 5.7 ± 1.42 | 0.008 |
| Post operative ileus | 6 (46.2 %) | 20 (38.5 %) | 1 (10 %) | 0.161 |
| Mobilization on day of surgery | 4 (15.4 %) | 22 (21.2 %) | 19 (90 %) | < 0.001 |
Postoperative complications
Complications were compared in subgroups and was not statistically significant (p=0.849). Grade 1–2 complications were seen in majority of the patients. Eighteen patients (12 %) had grade 3–5 complications; in group A and B (23.1 % and 11.5 % respectively) and none in group C. The most common complications were post operative ileus (36.6 %), surgical site infections (20.6 %), pulmonary complications (17.3 %) and intraabdominal collections (13.3 %). Two patients were readmitted, and both treated conservatively. 0ne patient required reoperation in post operative period because of fecal peritonitis secondary to anastomotic leak. Table 4.
The incidence of postoperative complications in compliance subgroups.
| Calvien Dindo | Compliance | Total | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| <70 | 70–80 | > 80 | |||||||
| Count | % | Count | % | Count | % | Count | % | ||
| 1 | 2 | 15.4 | 9 | 17.3 | 2 | 20.0 | 13 | 17.3 | |
| 2 | 8 | 61.5 | 37 | 71.2 | 8 | 80.0 | 53 | 70.7 | |
| 3 | 2 | 15.4 | 4 | 7.7 | 0 | 0.0 | 6 | 8.0 | |
| 4 | 0 | 0.0 | 1 | 1.9 | 0 | 0.0 | 1 | 1.3 | |
| 5 | 1 | 7.7 | 1 | 1.9 | 0 | 0.0 | 2 | 2.7 | |
| Total | 13 | 100.0 | 54 | 100.0 | 10 | 100.0 | 75 | 100.0 | p=0.849 |
Compliance to individual elements of protocol
Compliance to the various modalities varied considerably and was poorest during the postoperative period. Lowest compliance rates between the groups were found for following elements: nasogastric drainage, removal of urinary catheter by post operative day (POD) 3, early oral intake (solid food from POD1), mobilisation on day of surgery>2 h, physical exercises on POD 2 for >6 h, no usage of post operative antibiotic prophylaxis, abdominal and thoracic drains, post-operative fluid overload monitoring and avoidance, which was statistically significant (p<0.001); Routine mechanical bowel preparation and pre-emptive parenteral nutrition showed mild significant difference. Table 5.
Comparison of ERAS factors with low compliance rate in the compliance sub groups.
| Parameter | Recommendation | Compliance group<70 % (n=13) | Compliance group<70–80 % (n=52) | Compliance group>80 % (n=10) | p-Value |
|---|---|---|---|---|---|
| Nasogastric drainage | Weak negative | 3 (23.1) | 35 (67.3) | 10 (100.0) | <0.001 |
| Removal of urinary catheter by POD 3 | Strong positive | 3 (23.1) | 39 (75.0) | 10 (100.0) | <0.001 |
| Early oral intake (solid food from POD1) | Strong positive | 3 (23.1) | 35 (67.3) | 10 (100.0) | <0.001 |
| Mobilisation: Day of surgery>2 h | Strong positive | 2 (15.4) | 11 (21.2) | 9 (90.0) | <0.001 |
| Physical exercises for POD 2 and >6 h | Strong positive | 2 (15.4) | 16 (30.8) | 9 (90.0) | <0.001 |
| No post operative antibiotic prophylaxis | Weak positive | 0 (0.0) | 4 (7.7) | 9 (90.0) | <0.001 |
| Abdominal and thoracic drains | Weak positive | 2 (15.4) | 19 (36.5) | 10 (100.0) | <0.001 |
| Limiting postoperative weight gain | Strong positive | 4 (30.8) | 38 (73.1) | 10 (100.0) | <0.001 |
| Avoid routine mechanical bowel preparation | Weak positive | 12 (92.3) | 52 (100.0) | 10 (100.0) | 0.089 |
| Pre-emptive parenteral nutrition | Strong positive | 7 (53.8) | 30 (75.0) | 10 (100.0) | 0.041 |
Discussion
Managing advanced stage EOC patients with elderly age, post chemotherapy, previous surgery multiple comorbidities with complex multimodality treatment and significant morbidity represents a clinical challenge. ERAS protocols are designed to offset stress caused by conventional and counter intuitive practices and achieve pretherapy baseline function. There are only few retrospective studies on the use of ERAS protocol in CRS and HIPEC patients till now [36], [37], [38], [39], [40]. It represents a significant change in practice for CRS and HIPEC and poses a challenge for adherence and compliance.
Impact on clinical outcomes
Compliance issues
The dilemma is not only the implementation of ERAS protocols but also ensuring clinical adherence and patient compliance. The impact of different levels of compliance and specific elements is poorly understood. Studies have also shown that the key element for the improvement of recovery and convalescence parameters is to increase compliance [27], 41], 42]. The mean compliance rate varies between 60 % and 80 %, even in centres that use it on a routine basis [43], 44]. In the present study improved compliance resulted in shortened LOS and ICU stay in the higher compliance groups but patients with compliance of less than 70 % failed to achieve benefit. Interestingly a study has also shown that an increase in compliance to the ERAS protocol from>80 % to>90 % was not associated with further improvement in short-term outcomes [32].
It will be difficult to compare our current research with other ERAS studies because of the difference in nature of surgery. It is well known that minimally invasive surgery allows better outcomes compared to open surgery. Research that has demonstrated benefit and safety with ERAS has been with colorectal or gynaecological surgery where minimally invasive surgeries are very common [26], 30], 45]. In a surpra invasive surgery like CRS and HIPEC there is extensive multiquadrant procedures(peritonectomy with multivisceral resections) leading to significant physiological changes and achieving full protocol compliance is impossible in majority of patients [25].
We analysed each individual element of the protocol based on the compliance rate. Elements like prehabilitation, optimization of nutrition, short duration of fasting with carbohydrate loading, multimodal analgesia, postoperative nausea and vomiting (PONV) prophylaxis, anaesthesia induction and ventilation protocols, goal directed fluid therapy, DVT prophylaxis and maintenance of intraoperative normothermia were well adopted with highest compliance rates. There was no statistical significance between the compliance groups for these elements.
We observed deviations from the pathway in certain clinical situations resulting in lower compliance rates for some aspects of protocol, like routine bowel preparation in case of extensive disease in anticipation of multiple resections and anastomosis, prolonged urinary catheter after bladder peritonectomy or resection. In the lowest compliance group, items with lower implementation rates included: nasogastric drainage, removal of urinary catheter by post operative day (POD) 3, early oral intake (solid food from POD1), mobilisation on day of surgery>2 h, physical exercises on POD 2 for>6 h, no usage of post operative antibiotic prophylaxis, abdominal and thoracic drains, post-operative fluid overload monitoring and avoidance. Unfortunately, some clinical situations do not allow full protocol realization due to medical considerations. Although routine NG decompression is not recommended by the protocol, in presence of extensive upper abdominal disease; total supra colonic omentectomy, lesser omentectomy, gastric resections or splenectomy when performed can lead to delayed gastric emptying necessitating post op NG drainage [46]. Guidelines have also recommended against the routine use of peritoneal drains since placement of drains can stimulate serous fluid production, and may lead to an increased risk of surgical-site infection and adhesions without any benefit of early detection of anastomotic leak. After CRS and HIPEC the possibility of intraperitoneal collections is higher and therefore most surgeons prefer placement of intraperitoneal drains after extensive CRS [47], 48]. Aggressive mobilisation and early normal diet initiation is again difficult to comply as most patients who undergo CRS and HIPEC deal with major hemodynamic, respiratory and metabolic derangements needing ICU care with or without ventilatory support [49], 50].
The improvement in recovery outcomes is not an effect of one particular element, but rather an aggregation of gains from all the elements of guidelines. Even though it is not always possible to fully adhere to the protocol, as a whole they are proven to work, which is clearly confirmed in our analysis.
Difficulties in ERAS implementation
The full implementation of the comprehensive ERAS guidelines into daily practice for CRS and HIPEC meets certain difficulties. Despite the existence of strong evidence of benefit in surgical practice, introducing a multielement protocol represents a shift from the ingrained conventional practices and its adoption with systematic implementation by HIPEC surgeons will be gradual [51]. Effective implementation of ERAS requires counselling of patients, education and close collaboration of a multidisciplinary team consisting of surgeons, anaesthetists, intensive care specialists, nurses, physiotherapists and dieticians [52], [53], [54].
The support by the hospital administrators and recommendation of guidelines by scientific societies are also significant for success of the program [55].
Recommendations
Seventy-two items in the protocol should be stratified as considered as ERAS core items, those with highest level of evidence and with maximum impact on patient outcomes. Peri-operative care items without a specific focus on enhanced recovery could be removed from the guidelines to make them shorter, more specific, and easier to implement. Modifying few elements of protocol depending on clinical requirements and centre capabilities will make ERAS more practical and easier to implement.
There were few limitations in the study. We did not analyze cost effect of ERAS in our study. This was a single-center observational study, multicenter and large-scale trials are needed to verify the current results.
Conclusions
Compliance at the level of 80 % or more is needed to achieve patient benefit, faster and safer patient recovery with early resumption of adjuvant therapies and ultimately improved quality of life and patient satisfaction. Training and formation of a dedicated team is imperative for success. Although there is evidence of benefit in other specialties where minimally invasive surgery is a major component, the reciprocation of same benefit in highly invasive surgery like CRS and HIPEC requires conclusive evidence to legitimise the use. There is room to improve the protocol and standardize it to achieve higher adherence and compliance.
-
Research ethics: The Institutional Review Board approval has been obtained. Ref no ASTER/IEC/Thesis/020/2024-25. Date: 30/07/2024. The authors declare that the study was conducted in accordance with the Declaration of Helsinki (as revised in 2013).
-
Informed consent: Informed consent was obtained from all individuals included in this study, or their legal guardians or wards.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Use of Large Language Models, AI and Machine Learning Tools: None declared.
-
Conflict of interest: The authors state no conflict of interest.
-
Research funding: None declared.
-
Data availability: Not applicable.
References
1. van Driel, WJ, Koole, SN, Sikorska, K, Schagen van Leeuwen, JH, Schreuder, HW, Hermans, RH, et al.. Hyperthermic intraperitoneal chemotherapy in ovarian cancer. N Engl J Med 2018;378:230–40. https://doi.org/10.1056/NEJMoa1708618.Search in Google Scholar PubMed
2. Bakrin, N, Bereder, JM, Msika, S, Lorimier, G. FROGHI (FRench Oncologic and Gynecologic HIPEC) Group. Peritoneal carcinomatosis treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (HIPEC) for advanced ovarian carcinoma: a French multicentre retrospective cohort study of 566 patients. Eur J Surg Oncol 2013;39:1435–43. https://doi.org/10.1016/j.ejso.2013.09.030.Search in Google Scholar PubMed
3. Cascales-Campos, PA, Gil, J, Gil, E, Feliciangeli, E, González-Gil, A, Parrilla, JJ, et al.. Treatment of microscopic disease with hyperthermic intraoperative intraperitoneal chemotherapy after complete cytoreduction improves disease-free survival in patients with stage IIIC/IV ovarian cancer. Ann Surg Oncol 2014;21:2383–9. https://doi.org/10.1245/s10434-014-3599-4.Search in Google Scholar PubMed
4. Cascales-Campos, P, López-López, V, Gil, J, Arévalo-Pérez, J, Nieto, A, Barceló, F, et al.. Hyperthermic intraperitoneal chemotherapy with paclitaxel or cisplatin in patients with stage III-C/IV ovarian cancer: is there any difference? Surg Oncol 2016;25:164–70. https://doi.org/10.1016/j.suronc.2016.05.010.Search in Google Scholar PubMed
5. Di Giorgio, A, De Iaco, P, De Simone, M, Garofalo, A, Scambia, G, Pinna, AD, et al.. Cytoreduction (peritonectomy procedures) combined with hyperthermic intraperitoneal chemotherapy (HIPEC) in advanced ovarian cancer: retrospective Italian multicenter observational study of 511 cases. Ann Surg Oncol 2017;24:914–22. https://doi.org/10.1245/s10434-016-5686-1.Search in Google Scholar PubMed PubMed Central
6. Zhang, G, Zhu, Y, Liu, C, Chao, G, Cui, R, Zhang, Z. The prognosis impact of hyperthermic intraperitoneal chemotherapy (HIPEC) plus cytoreductive surgery (CRS) in advanced ovarian cancer: the meta-analysis. J Ovarian Res 2019;12:33. https://doi.org/10.1186/s13048-019-0509-1.Search in Google Scholar PubMed PubMed Central
7. Armstrong, DK, Alvarez, RD, Bakkum-Gamez, JN, Barroilhet, L, Behbakht, K, Berchuck, A, et al.. NCCN guidelines insights: ovarian cancer, version 1. 2019. J Natl Compr Cancer Netw 2019;17:896–909. https://doi.org/10.6004/jnccn.2019.0039.Search in Google Scholar PubMed
8. Somashekhar, SP, Rohit, KC, Deo, SVS, Ashwin, KR. Practice patterns, attitudes, and knowledge among clinicians regarding hyperthermic intraperitoneal chemotherapy and pressurized intraperitoneal aerosol chemotherapy: a national survey by Indian society of peritoneal surface malignancies (ISPSM). Pleura Peritoneum 2020;5:20200120. https://doi.org/10.1515/pp-2020-0120.Search in Google Scholar PubMed PubMed Central
9. Elias, D, Goere, D, Blot, F, Billard, V, Pocard, M, Kohneh-Shahri, N, et al.. Optimization of hyperthermic intraperitoneal chemotherapy with oxaliplatin plus irinotecan at 43 degrees C after compete cytoreductive surgery: mortality and morbidity in 106 consecutive patients. Ann Surg Oncol 2007;14:1818–24. https://doi.org/10.1245/s10434-007-9348-1.Search in Google Scholar PubMed
10. Kusamura, S, Younan, R, Baratti, D, Costanzo, P, Favaro, M, Gavazzi, C, et al.. Cytoreductive surgery followed by intraperitoneal hyperthermic perfusion: analysis of morbidity and mortality in 209 peritoneal surface malignancies treated with closed abdomen technique. Cancer 2006;106:1144–53. https://doi.org/10.1002/cncr.21708.Search in Google Scholar PubMed
11. Ceelen, WP, Peeters, M, Houtmeyers, P, Breusegem, C, De Somer, F, Pattyn, P. Safety and efficacy of hyperthermic intraperitoneal chemoperfusion with high-dose oxaliplatin in patients with peritoneal carcinomatosis. Ann Surg Oncol 2008;15:535–41. https://doi.org/10.1245/s10434-007-9648-5.Search in Google Scholar PubMed
12. Desantis, M, Bernard, J-L, Casanova, V, Cegarra-Escolano, M, Benizri, E, Rahili, AM, et al.. Morbidity, mortality, and oncological outcomes of 401 consecutive cytoreductive procedures with hyperthermic intraperitoneal chemotherapy (HIPEC). Langenbecks Arch Surg 2015;400:37–48. https://doi.org/10.1007/s00423-014-1253-z.Search in Google Scholar PubMed
13. Ljungqvist, O, Scott, M, Fearon, KC. Enhanced recovery after surgery: a review. JAMA Surg 2017;152:292–8. https://doi.org/10.1001/jamasurg.2016.4952.Search in Google Scholar PubMed
14. Kehlet, H. Fast-track colorectal surgery. Lancet 2008;371:791–3. https://doi.org/10.1016/s0140-6736(08)60357-8.Search in Google Scholar
15. ERAS Compliance Group. The impact of enhanced recovery protocol compliance on elective colorectal cancer resection: results from an international registry. Ann Surg 2015;261:1153–9. https://doi.org/10.1097/SLA.0000000000001029.Search in Google Scholar PubMed
16. Wijk, L, Udumyan, R, Pache, B, Altman, AD, Williams, LL, Elias, KM, et al.. International validation of Enhanced Recovery after Surgery Society guidelines on enhanced recovery for gynecologic surgery. Am J Obstet Gynecol 2019;221:237 e1ee11. https://doi.org/10.1016/j.ajog.2019.04.02.Search in Google Scholar
17. Khoo, CK, Vickery, CJ, Forsyth, N, Vinall, NS, Eyre-Brook, IA. A prospective randomized controlled trial of multimodal perioperative management protocol in patients undergoing elective colorectal resection for cancer. Ann Surg 2007;245:867–72. https://doi.org/10.1097/01.sla.0000259219.08209.36.Search in Google Scholar PubMed PubMed Central
18. Muller, S, Zalunardo, MP, Hübner, M, Clavien, PA, Demartines, N. Zurich fast track study group, A fast-track program reduces complications and length of hospital stay after open colonic surgery. Gastroenterology 2009;136:842–7. https://doi.org/10.1053/j.gastro.2008.10.030.Search in Google Scholar PubMed
19. Miralpeix, E, Nick, AM, Meyer, LA, Cata, J, Lasala, J, Mena, GE, et al.. A call for new standard of care in perioperative gynecologic oncology practice: impact of enhanced recovery after surgery (ERAS) programs. Gynecol Oncol 2016;141:371–8. https://doi.org/10.1016/j.ygyno.2016.02.019.Search in Google Scholar PubMed PubMed Central
20. Wijk, L, Udumyan, R, Pache, B, Altman, AD, Williams, LL, Elias, KM, et al.. International validation of enhanced recovery after surgery society guidelines on enhanced recovery for gynecologic surgery. Am J Obstet Gynecol 2019;221:237 e1ee11. https://doi.org/10.1016/j.ajog.2019.04.028.Search in Google Scholar PubMed
21. Pędziwiatr, M, Kisialeuski, M, Wierdak, M, Stanek, M, Natkaniec, M, Matłok, M, et al.. Early implementation of enhanced recovery after surgery (ERAS®) protocol – compliance improves outcomes: a prospective cohort study. Int J Surg 2015;21:75–81. https://doi.org/10.1016/j.ijsu.2015.06.087.Search in Google Scholar PubMed
22. Hübner, M, Kusamura, S, Villeneuve, L, Al-Niaimi, A, Alyami, M, Balonov, K, et al.. Guidelines for perioperative care in cytoreductive surgery (CRS) with or without hyperthermic intraperitoneal chemotherapy (HIPEC): enhanced recovery after surgery (ERAS®) society recommendations – Part II: postoperative management and special considerations. Eur J Surg Oncol 2020;46:2311–23. https://doi.org/10.1016/j.ejso.2020.08.006.Search in Google Scholar PubMed
23. Hübner, M, Kusamura, S, Villeneuve, L, Al-Niaimi, A, Alyami, M, Balonov, K, et al.. Guidelines for perioperative care in cytoreductive surgery (CRS) with or without hyperthermic intraperitoneal chemotherapy (HIPEC): enhanced recovery after surgery (ERAS®) society recommendations – Part I: preoperative and intraoperative management. Eur J Surg Oncol 2020;46:2292–310. https://doi.org/10.1016/j.ejso.2020.07.041.Search in Google Scholar PubMed
24. Maessen, J, Dejong, CH, Hausel, J, Nygren, J, Lassen, K, Andersen, J, et al.. A protocol is not enough to implement an enhanced recovery programme for colorectal resection. Br J Surg 2007;94:224–31. https://doi.org/10.1002/bjs.5468.Search in Google Scholar PubMed
25. Kahokehr, A, Sammour, T, Zargar-Shoshtari, K, Thompson, L, Hill, AG. Implementation of ERAS and how to overcome the barriers. Int J Surg 2009;7:16–9. https://doi.org/10.1016/j.ijsu.2008.11.004.Search in Google Scholar PubMed
26. Pędziwiatr, M, Kisialeuski, M, Wierdak, M, Stanek, M, Natkaniec, M, Matłok, M, et al.. Early implementation of Enhanced Recovery after Surgery (ERAS®) protocol – compliance improves outcomes: a prospective cohort study. Int J Surg 2015;21:75–81. https://doi.org/10.1016/j.ijsu.2015.06.087.Search in Google Scholar
27. Gustafsson, UO, Hausel, J, Thorell, A, Ljungqvist, O, Soop, M, Nygren, J. Adherence to the enhanced recovery after surgery protocol and outcomes after colorectal cancer surgery. Arch Surg 2011;146:571–7. https://doi.org/10.1001/archsurg.2010.309.Search in Google Scholar PubMed
28. Geltzeiler, CB, Rotramel, A, Wilson, C, Deng, L, Whiteford, MH, Frankhouse, J. Prospective study of colorectal enhanced recovery after surgery in a community hospital. JAMA Surg 2014;149:955–61. https://doi.org/10.1001/jamasurg.2014.675.Search in Google Scholar PubMed
29. Bisch, SP, Wells, T, Gramlich, L, Faris, P, Wang, X, Tran, DT, et al.. Enhanced recovery after surgery (ERAS) in gynecologic oncology: system-wide implementation and audit leads to improved value and patient outcomes. Gynecol Oncol 2018;151:117–23. https://doi.org/10.1016/j.ygyno.2018.08.007.Search in Google Scholar PubMed
30. Pisarska, M, Pędziwiatr, M, Małczak, P, Major, P, Ochenduszko, S, Zub-Pokrowiecka, A, et al.. Do we really need the full compliance with ERAS protocol in laparoscopic colorectal surgery? A prospective cohort study. Int J Surg 2016;36:377–82. Part A https://doi.org/10.1016/j.ijsu.2016.11.088.Search in Google Scholar PubMed
31. Pache, B, Joliat, GR, Hübner, M, Grass, F, Demartines, N, Mathevet, P, et al.. Cost-analysis of enhanced recovery after surgery (ERAS) program in gynecologic surgery. Gynecol Oncol 2019;154:388–93. https://doi.org/10.1016/j.ygyno.2019.06.004.Search in Google Scholar PubMed
32. Gustafsson, UO, Oppelstrup, H, Thorell, A, Nygren, J, Ljungqvist, O. Adherence to the ERAS protocol is associated with 5-year survival after colorectal cancer surgery: a retrospective cohort study. World J Surg 2016;40:1741–7. https://doi.org/10.1007/s00268-016-3460-y.Search in Google Scholar PubMed
33. Vlug, MS, Wind, J, Hollmann, MW, Ubbink, DT, Cense, HA, Engel, AF, et al.. LAFA Study Group. Laparoscopy in combination with fast track multimodal management is the best perioperative strategy in patients undergoing colonic surgery: a randomized clinical trial (LAFA-study). Ann Surg 2011;254:868–75. https://doi.org/10.1097/SLA.0b013e31821fd1ce.Search in Google Scholar
34. Vlug, MS, Wind, J, Hollmann, MW, Ubbink, DT, Cense, HA, Engel, AF, et al.. Laparoscopy in combination with fast track multimodal management is the best perioperative strategy in patients undergoing colonic surgery: a randomized clinical trial (LAFA-study). Ann Surg 2011;254:868–75. https://doi.org/10.1097/sla.0b013e31821fd1ce.Search in Google Scholar
35. P, SS, R, AK, Kumar, R, Naidu, N, Zaveri, SS, et al.. Standardization of patient selection and hyperthermic intraperitoneal chemotherapy protocol for peritoneal surface malignancy in Indian patients. Indian J Gynecol Oncolog 2017;15:55–63. https://doi.org/10.1007/s40944-017-0154-9.Search in Google Scholar
36. Duzgun, O. Evaluation of enhanced recovery after following a surgical protocol for cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for peritoneal carcinomatosis. Med Arch 2019;73:331–7. https://doi.org/10.5455/medarh.2019.73.331-337.Search in Google Scholar PubMed PubMed Central
37. Solanki, SL, Mukherjee, S, Agarwal, V, Thota, RS, Balakrishnan, K, Shah, SB, et al.. Society of onco-anaesthesia and perioperative care consensus guidelines for perioperative management of patients for cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (CRS-HIPEC). Indian J Anaesth 2019;63:972–87. https://doi.org/10.4103/ija.ija_765_19.Search in Google Scholar
38. Aarts, MA, Rotstein, OD, Pearsall, EA, Victor, JC, Okrainec, A, McKenzie, M, et al.. Postoperative ERAS interventions have the greatest impact on optimal recovery: experience with implementation of ERAS across multiple hospitals. Ann Surg 2018;267:992–7. https://doi.org/10.1097/sla.0000000000002632.Search in Google Scholar PubMed
39. Veerapong, J, King, BH, Baumgartner, JM, Kelly, KJ, Lowy, AM, Gabriel, RA. The use of predictive modelling for prolonged hospital length of stay for the development of an enhanced recovery after surgery program for patients undergoing cytoreductive surgery with/without hyperthermic intra peritoneal chemotherapy. Clin Nutr 2018;25:166–209.10.1016/j.clnesp.2018.03.063Search in Google Scholar
40. Siddharthan, R, Dewey, E, Billingsley, K, Gilbert, E, Tsikitis, VL. Feasibility and benefits of an enhanced recovery after surgery protocol for patients undergoing cytoreductive surgery and heated intraperitoneal chemotherapy: a single institution experience. Am J Surg 2020;219:1073–5. https://doi.org/10.1016/j.amjsurg.2019.06.019.Search in Google Scholar PubMed
41. Feroci, F, Lenzi, E, Baraghini, M, Garzi, A, Vannucchi, A, Cantafio, S, et al.. Fast- track colorectal surgery: protocol adherence influences postoperative out- comes. Int J Colorectal Dis 2012;28:103e109. https://doi.org/10.1007/s00384-012-1569-5.Search in Google Scholar PubMed
42. Alcantara-Moral, M, Serra-Aracil, X, Gil-Egea, MJ, Frasson, M, Flor-Lorente, B, Garcia-Granero, E, et al.. Observational cross-sectional study of compliance with the fast track protocol in elective surgery for colon cancer in Spain. Int J Colorectal Dis 2014;29:477–83. https://doi.org/10.1007/s00384-013-1825-3.Search in Google Scholar PubMed
43. Greco, M, Capretti, G, Beretta, L, Gemma, M, Pecorelli, N, Braga, M. Enhanced recovery program in colorectal surgery: a meta- analysis of randomized controlled trials. World J Surg 2014;38:1531–41. https://doi.org/10.1007/s00268-013-2416-8.Search in Google Scholar PubMed
44. Kummer, A, Slieker, J, Grass, F, Hahnloser, D, Demartines, N, Hübner, M. Enhanced recovery pathway for right and left colectomy: comparison of functional recovery. World J Surg 2016;40:2519–27. https://doi.org/10.1007/s00268-016-3563-5.Search in Google Scholar PubMed
45. Iniesta, MD, Lasala, J, Mena, G, Rodriguez-Restrepo, A, Salvo, G, Pitcher, B, et al.. Impact of compliance with an enhanced recovery after surgery pathway on patient outcomes in open gynecologic surgery. Int J Gynecol Cancer 2019;29:1417–24. https://doi.org/10.1136/ijgc-2019-000622.Search in Google Scholar PubMed
46. Carmichael, JC, Keller, DS, Baldini, G, Bordeianou, L, Weiss, E, Lee, L, et al.. Clinical practice guidelines for enhanced recovery after colon and rectal surgery from the American society of colon and rectal surgeons and society of American gastrointestinal and endoscopic surgeons. Dis Colon Rectum 2017;60:761–84. https://doi.org/10.1097/dcr.0000000000000883.Search in Google Scholar
47. Puleo, F, Mishra, N, Hall, J. Use of intra-abdominal drains. Clin Colon Rectal Surg 2013;26:174–7. https://doi.org/10.1055/s-0033-1351134.Search in Google Scholar PubMed PubMed Central
48. Mujagic, E, Zeindler, J, Coslovsky, M, Hoffmann, H, Soysal, SD, Mechera, R, et al.. The association of surgical drains with surgical site infections – a prospective observational study. Am J Surg 2019;217:17–23. https://doi.org/10.1016/j.amjsurg.2018.06.015.Search in Google Scholar PubMed
49. Alyami, M, Kim, BJ, Villeneuve, L, Vaudoyer, D, Képénékian, V, Bakrin, N, et al.. Ninety-day post-operative morbidity and mortality using the National Cancer Institute’s common terminology criteria for adverse events better describe post-operative outcome after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Int J Hyperther 2018;34:532–7. https://doi.org/10.1080/02656736.2017.1367846.Search in Google Scholar PubMed
50. Padmakumar, AV. Intensive care management of patient after cytoreductive surgery and HIPEC – a concise review. Indian J Surg Oncol 2016;7:244–8. https://doi.org/10.1007/s13193-016-0511-7.Search in Google Scholar PubMed PubMed Central
51. Lyon, A, Solomon, MJ, Harrison, JD. A qualitative study assessing the barriers to implementation of enhanced recovery after surgery. World J Surg 2014;38:1374–80. https://doi.org/10.1007/s00268-013-2441-7.Search in Google Scholar PubMed
52. Francis, N, Kennedy, RH, Ljungqvist, O, Mythen, MG. Manual of fast track recovery for colorectal surgery. Berlin, Germany: Springer Science & Business Media; 2012.10.1007/978-0-85729-953-6Search in Google Scholar
53. Pasero, C, Belden, J. Evidence-based perianesthesia care: accelerated post- operative recovery programs. J. Perianesth. Nurs. 2006;21:168–76. https://doi.org/10.1016/j.jopan.2006.03.010.Search in Google Scholar PubMed
54. Egbert, LD, Battit, GE, Welch, CE, Bartlett, MK. Reduction of postoperative pain by encouragement and instruction of patients. A study of doctor-patient rapport. N Engl J Med 1964;270:825–7. https://doi.org/10.1056/NEJM196404162701606.Search in Google Scholar PubMed
55. Kehlet, H, Wilmore, DW. Evidence-based surgical care and the evolution of fast-track surgery. Ann Surg 2008;248:189e198. https://doi.org/10.1097/SLA.0b013e31817f2c1a.Search in Google Scholar PubMed
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- Review Article
- The microbiome of pseudomyxoma peritonei: a scoping review
- Research Articles
- Implementation of an enhanced recovery after surgery (ERAS) program in patients undergoing cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: study protocol for a prospective multicenter interventional trial (EPICH study)
- The impact of surgical staging in patients with colorectal peritoneal metastases scheduled for CRS-HIPEC
- Immediate postoperative effects of cytoreductive surgery with hyperthermic intraperitoneal chemotherapy using carboplatin on peritoneal tissue inflammatory and ischemic responses: an explorative porcine study
- Visceral adipose tissue is associated with occult synchronous peritoneal metastasis in colorectal cancer
- Optimizing patient recovery: prospective study evaluating compliance and clinical outcomes of enhanced recovery protocols in ovarian cancer following cytoreductive surgery with HIPEC
Articles in the same Issue
- Frontmatter
- Review Article
- The microbiome of pseudomyxoma peritonei: a scoping review
- Research Articles
- Implementation of an enhanced recovery after surgery (ERAS) program in patients undergoing cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: study protocol for a prospective multicenter interventional trial (EPICH study)
- The impact of surgical staging in patients with colorectal peritoneal metastases scheduled for CRS-HIPEC
- Immediate postoperative effects of cytoreductive surgery with hyperthermic intraperitoneal chemotherapy using carboplatin on peritoneal tissue inflammatory and ischemic responses: an explorative porcine study
- Visceral adipose tissue is associated with occult synchronous peritoneal metastasis in colorectal cancer
- Optimizing patient recovery: prospective study evaluating compliance and clinical outcomes of enhanced recovery protocols in ovarian cancer following cytoreductive surgery with HIPEC


