Abstract
Objectives
Pressurized intraperitoneal aerosol chemotherapy (PIPAC) gives encouraging results in the treatment of peritoneal metastasis (PM). The current recommendations require at least 3 sessions of PIPAC. However, some patients do not complete the full treatment course and stop after only 1 or 2 procedures, hence the limited benefit. A literature review was performed, with search terms including “PIPAC” and “pressurised intraperitoneal aerosol chemotherapy.”
Content
Only articles describing the causes for premature termination of the PIPAC treatment were analysed. The systematic search identified 26 published clinical articles related to PIPAC and reporting causes for stopping PIPAC.
Summary
The series range from 11 to 144 patients, with a total of 1352 patients treated with PIPAC for various tumours. A total of 3088 PIPAC treatments were performed. The median number of PIPAC treatments per patient was 2.1, the median PCI score at the time of the first PIPAC was 19 and the number of patients who did not complete the recommended 3 sessions of PIPAC was 714 (52.8%). Disease progression was the main reason for early termination of the PIPAC treatment (49.1%). The other causes were death, patients’ wishes, adverse events, conversion to curative cytoreductive surgery and other medical reasons (embolism, pulmonary infection, etc…).
Outlook
Further investigations are necessary to better understand the causes for interrupting PIPAC treatment and also improving the selection of patients who are most likely to benefit from PIPAC.
Introduction
Peritoneal metastasis (PM) is associated with a poor prognosis in the absence of effective multimodal therapeutic approaches. Pressurized intraperitoneal aerosol chemotherapy (PIPAC) has emerged with encouraging results in the treatment of PM [1], [2], [3] in the last ten years. The current recommendations require at least 3 PIPAC procedures planned every 4–6 weeks [4, 5]. Unfortunately, many patients do not complete the 3 planned procedures and stop PIPAC prematurely. There are many reasons for discontinuing after only 1 or 2 procedures, with the main reason being the progression of the oncological disease [6, 7]. We therefore aimed to investigate reasons for stopping PIPAC, to better select patients.
The aim of the present study is to propose a systematic review of the literature in order to evaluate the number and reasons for discontinuing PIPAC procedures.
Materials and methods
A literature review was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [8]. A literature search was performed by using the Medline database (via PubMed). This first search step was performed without any language restriction. Search terms included “PIPAC” and “pressurized intraperitoneal aerosol chemotherapy.” After removing duplicates, a total of 244 articles were identified up to August 1, 2022. Only articles published in English were analysed in the second steep. The following publications were excluded: case reports of 5 patients or fewer, preclinical experimental studies, including ex vivo and animal models, pharmacological or histological study, ongoing trials, case reports, technique development and safety studies, review articles, editorials and unrelated/erratum (flowchart, Figure 1). We only analysed articles that described the reasons for discontinuing PIPAC treatment.
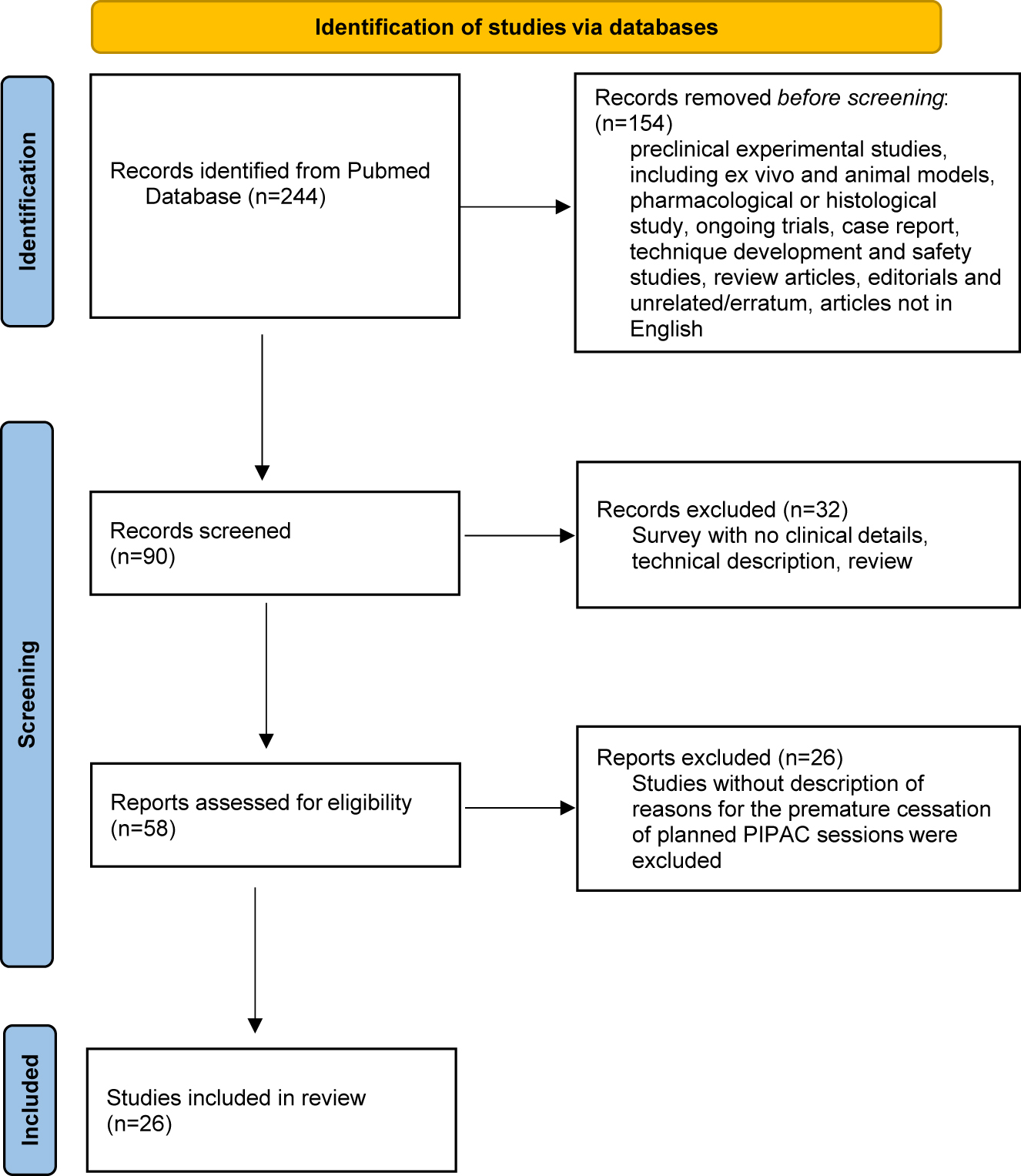
Flow-chart.
For each article, when available, the following items were recorded for each study: authors, title, year of publication, primary tumour origin, number of patients, details of patients (median age of patients), previous chemotherapy and number of previous lines of systemic chemotherapy, total number of PIPAC procedures, details of number of PIPAC and protocol (bimodal treatment or not), median number of PIPAC sessions per patient, details on the surgical intervention (rate of non-access, peritoneal carcinomatosis index [PCI], ascites at PIPAC#1 and intraoperative complications). Postoperative outcome measures included postoperative surgical complications (morbidity), mortality, and reasons for stopping PIPAC before three sessions had been completed, which were separated into 6 groups: non-access, conversion to curative cytoreductive surgery, tumour progression intra- or extraperitoneal, patient wishes, postoperative complications/adverse events (compromising other PIPAC), death or other medical reasons (pulmonary embolism, infection, myocardial infarction, protocol violation or other cancer).
Statistical analysis
Small sample size and heterogeneity in the original data did not allow for proper statistical meta-analysis. Microsoft Excel version 16.60 and R version 4.1.3 was used respectively for data collection and statistical analysis. Continuous data are presented using descriptive statistics mean ± standard deviation or median (25th; 75th percentiles). Categorical data are presented using frequencies and percentages. Quantitative parameters were compared between groups using the Student’s t-test or Mann–Whitney test when normality was rejected. Qualitative parameters were compared between groups using the Chi-square test or Fisher exact test, as appropriate. A threshold of 5 % was used to define the significance of the statistical tests.
Results
In the literature, a limited number of studies include the reasons for the early discontinuation of PIPAC procedures especially after 1 or 2 sessions. The systematic search identified 26 published clinical articles related to PIPAC with inclusion criteria (Figure 1, Flow chart).
The studies included 11 to 183 patients, with the number of reported PIPAC sessions of 17–517. The origin of the peritoneal metastasis of the overall group was colorectal (5; 19.2 %), biliary tract and pancreatic (3; 11.6 %), gastric (5; 19.2 %), ovarian (1; 3.8 %), mesothelioma (1; 3.8 %), gynaecological (1; 3.8 %) and various (10; 38.6 %). Studies and patient characteristics are summarised in Table 1. A total of 3,088 PIPAC procedures were performed in 1,278 patients.
Characteristics of PIPAC studies.
| Reference | Primary tumour | Numbers of patients, n | Median age, years | Primary no abdominal access, n | Number PIPAC procedure, n | Median number PIPAC/patient | Median PCI | Acites, n | Bimodal treatment, n | Previous chemotherapy, n |
|---|---|---|---|---|---|---|---|---|---|---|
| Alyami et al. [9] | Various | 73 | 57.1 | 0 | 164 | 2.6 | 19 | 35 | 64 | 64 |
| Balmer et al. [6] | Various | 183 | 64 | 0 | 517 | 19 | 42 | 48 | ||
| De Simone et al. [10] | Various | 67 | 59 | 4 | 171 | 1.8 | ||||
| Di Giorgio et al. [11] | Gastric | 28 | 50 | 2 | 46 | 1.7 | 20 | 8 | 26 | |
| Di Giorgio et al. [12] | Bilary tract and pancreatic | 20 | 64 | 1 | 45 | 1.7 | 20 | 10 | 11 | 20 |
| Ellebæk et al. [13] | Colorectal | 24 | 64 | 0 | 74 | 3 | 10 | 7 | 3 | 22 |
| Ellebæk et al. [14] | Gastric | 20 | 58 | 0 | 52 | 3.2 | 13 | 8 | 9 | 19 |
| Falkenstein et al. [15] | Bilary tract and pancreatic | 11 | 58 | 2 | 17 | 1.3 | 20 | 13 | 3 | 10 |
| Graversen et al. [16] | Various | 33 | 59 | 3 | 65 | 1.9 | 12 | 32 | ||
| Graversen et al. [17] | Various | 35 | 65 | 0 | 129 | 3 | 14 | 0 | 5 | 32 |
| Hübner et al. [18] | Various | 42 | 66 | 2 | 91 | 2.1 | 10 | 40 | ||
| Giger-Pabst et al. [19] | Mesothelioma | 29 | 62 | 4 | 74 | 2.6 | 19 | 7 | 21 | |
| Khomyakov et al. [20] | Various | 31 | 52 | 0 | 56 | 1.8 | 16 | 7 | ||
| Khosrawipour et al. [21] | Bilary tract and pancreatic | 20 | 64 | 0 | 41 | 2.1 | 26 | 20 | ||
| Kim et al. [22] | Various | 17 | 62 | 1 | 24 | 1.4 | 17 | 11 | 16 | |
| Kurtz et al. [23] | Various | 71 | 58 | 8 | 142 | 2 | 19 | 24 | 42 | 60 |
| Lurvink et al. [24] | Colorectal | 20 | 64 | 0 | 59 | 3 | 29 | 13 | 12 | |
| Nadiradze et al. [25] | Gastric | 24 | 56 | 1 | 60 | 2.4 | 16 | 8 | 19 | |
| Rovers et al. [26] | Colorectal | 22 | 64 | 1 | 59 | 3 | 29 | 16 | 12 | |
| Sgarbura et al. [27] | Various | 101 | 59 | 5 | 251 | 2.5 | 19 | 47 | 93 | |
| Struller et al. [28] | Gastric | 25 | 55.1 | 0 | 43 | 1.7 | 15 | 18 | 0 | 25 |
| Tempfer et al. [29] | Ovarian | 64 | 62 | 11 | 130 | 2 | 16 | 22 | ||
| Taibi et al. [30] | Colorectal | 131 | 59 | 2 | 343 | 2.2 | 20 | 21 | 30 | 127 |
| Tabchouri et al. [31] | Colorectal | 102 | 64 | 22 | 185 | 2.3 | 14 | 0 | 58 | 99 |
| Sindayigaya et al. [32] | Gastric | 144 | 57 | 11 | 216 | 2 | 15 | 131 | ||
| Tempfer et al. [33] | Gynaecologic | 15 | 60 | 1 | 34 | 2.3 | ||||
| Total, n (%) | 1,352 | 81 | 3,088 | 248 | 373 | 881 | ||||
| Median, Q1–Q3 per study | 30 (20–70) | 60 (58–64) | 1 (0–3.7) | 69 (47–158) | 2.1 (1.8–2.5) | 19 (15.1–20) | 13 (8–21.2) | 11.5 (6.5–43.2) | 22 (19–60) | |
| Mean, SD per study | 52 ± 45.5 | 60.2 ± 4.1 | 3.1 ± 5 | 118.8 ± 113.4 | 2.2 ± 0.5 | 18.2 ± 5 | 15.5 ± 11.4 | 23.3 ± 21.8 | 42 ± 38.6 |
The median number of PIPAC procedures per patient was 2.1 (1.8–2.6). Only 373 patients (27.6 %) received concomitant chemotherapy (bimodal treatment). The median PCI at the time of the first PIPAC was 19 [15], [16], [17], [18], [19], [20].
In patients who underwent PIPAC, adverse events were graded according to the Common Terminology Criteria for Adverse Events (v4.0 CTCAE) in all studies. The median rate of major postoperative complications (CTCAE grade 3 and 4) was 3.1 % (0.8–6.2 %). Mortality rate was 0.5 % (0–3.9 %) (Table 2).
PIPAC clinical data summary.
| Reference | Number patients at least 1 PIPAC | PIPAC <3, n | PIPAC >3, n | Morbidity (CTCAE 3 ou 4)/PIPAC | Mortality/study |
|---|---|---|---|---|---|
| Alyami et al. [9] | 73 | 42 | 31 | 9.7 % | 6.8 % |
| Balmer et al. [6] | 183 | 88 | 95 | 2.3 % | 1.6 % |
| De Simone et al. [10] | 63 | 43 | 24 | 2.4 % | |
| Di Giorgio et al. [11] | 26 | 17 | 11 | 4 % | 3.8 % |
| Di Giorgio et al. [12] | 19 | 13 | 7 | 0 % | 0 % |
| Ellebæk et al. [13] | 24 | 9 | 15 | 2.7 % | 0 % |
| Ellebæk et al. [14] | 20 | 10 | 10 | 3.8 % | 0 % |
| Falkenstein et al. [15] | 11 | 10 | 1 | 0 % | |
| Graversen et al. [16] | 30 | 21 | 12 | 0 % | 6 % |
| Graversen et al. [17] | 35 | 8 | 27 | 3.9 % | |
| Hübner et al. [18] | 40 | 24 | 18 | 1 % | 1 % |
| Giger-Pabst et al. [19] | 25 | 13 | 16 | 4 % | 4 % |
| Khomyakov et al. [20] | 31 | 23 | 8 | 3.2 % | 0 % |
| Khosrawipour et al. [21] | 20 | 10 | 10 | 0 % | 2.4 % |
| Kim et al. [22] | 16 | 16 | 1 | 0 % | 0 % |
| Kurtz et al. [23] | 63 | 43 | 28 | 0.7 % | 0 % |
| Lurvink et al. [24] | 20 | 7 | 13 | 0 % | 0 % |
| Nadiradze et al. [25] | 23 | 14 | 10 | 11.7 % | 8.3 % |
| Rovers et al. [26] | 20 | 8 | 14 | 11.9 % | 5 % |
| Sgarbura et al. [27] | 101 | 47 | 54 | 15.9 % | 0 % |
| Struller et al. [28] | 25 | 19 | 6 | 12 % | 0 % |
| Tempfer et al. [29] | 53 | 19 | 45 | 6.9 % | 0 % |
| Taibi et al. [30] | 131 | 72 | 59 | 9.3 % | 0 % |
| Tabchouri et al. [31] | 80 | 46 | 56 | 3.9 % | 0.5 % |
| Sindayigaya et al. [32] | 131 | 86 | 58 | 1.4 % | 1.5 % |
| Tempfer et al. [33] | 15 | 6 | 9 | 2.9 % | 6.6 % |
| Total, n | 1,278 | 714 | 638 | ||
| Median (Q1–Q3) per study | 28 (20–63) | 18 (10–42.7) | 14.5 (10–30.2) | 3.1 % (0.8–6.2 %) | 0.5 % (0–3.9 %) |
| Mean SD±, per study | 49.2 ± 43.8 | 27.5 ± 24 | 24.5 ± 23.1 | 4.4 % ± 4 0.6 % | 2.1 % ± 2.7 % |
The number of patients who did not complete the 3 planned PIPAC sessions was 714 (52.8 %) and the median failure of 3 planned PIPAC sessions was about 56.1 % (44.9–62.9 %). After analysing the literature, the main reason for discontinuing PIPAC treatment was disease progression (49.1 %). All reasons for PIPAC interruption are described in Figure 2 and Table 3.
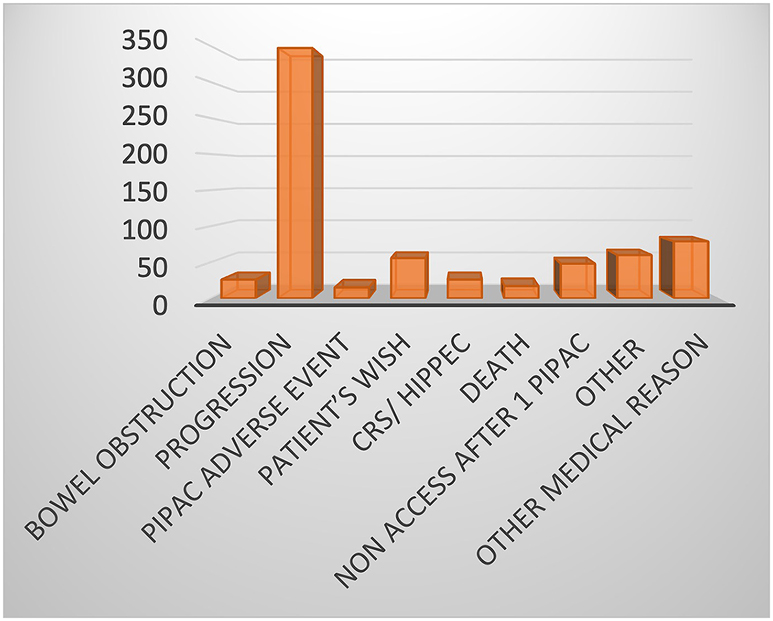
Reason for discontinuing PIPAC treatment.
Reasons for PIPAC interruption before 3 planned PIPAC.
| Reference | PIPAC <3, n | Bowel obstruction or perforation, n; % | Progression, n; % | PIPAC adverse event, n; % | Patient’s wish, n; % | CRS/HIPEC, n; % | Death, n; % | Non access after 1 PIPAC, n; % | Other (missing patient), n; % | Other medical reason, n; % |
|---|---|---|---|---|---|---|---|---|---|---|
| Alyami et al. [9] | 42 | 7; 16.7 % | 2; 4.8 % | 0 % | 0 % | 2; 4.8 % | 5; 11.9 % | 4; 9.5 % | 0 % | 22; 52.4 % |
| Balmer et al. [6] | 88 | 13; 14.8 % | 44; 50 % | 1; 1.1 % | 9; 10.2 % | 6; 6.8 % | 3; 3.4 % | 2; 2.3 % | 2; 2.3 % | 8; 9.1 % |
| De Simone et al. [10] | 43 | 0 % | 43; 100 % | 0 % | 0 % | 0 % | 0 % | 0 % | 0 % | 0 % |
| Di Giorgio et al. [11] | 17 | 0 % | 9; 52.9 % | 2; 11.8 % | 5; 29.4 % | 1; 5.9 % | 0 % | 0 % | 0 % | 0 % |
| Di Giorgio et al. [12] | 13 | 0 % | 7; 53.8 % | 0 % | 0 % | 0 % | 0 % | 0 % | 1; 7.7 % | 5; 38.5 % |
| Ellebæk et al. [13] | 9 | 1; 11.1 % | 2; 22 % | 0 % | 5; 55.6 % | 0 % | 1; 11.1 % | 0 % | 0 % | 0 % |
| Ellebæk et al. [14] | 10 | 0 % | 6; 60 % | 0 % | 0 % | 0 % | 0 % | 0 % | 0 % | 4; 40 % |
| Falkenstein et al. [15] | 10 | 2; 20 % | 4; 40 % | 0 % | 0 % | 0 % | 0 % | 0 % | 0 % | 4; 40 % |
| Graversen et al. [16] | 21 | 0 % | 9; 42.9 % | 0 % | 3; 14.3 % | 0 % | 2; 9.5 % | 0 % | 4; 19 % | 3; 14.3 % |
| Graversen et al. [17] | 8 | 3; 37.5 % | 1; 12.5 % | 0 % | 3; 37.5 % | 0 % | 1; 12.5 % | 0 % | 0 % | 0 % |
| Hübner et al. [18] | 24 | 0 % | 5; 20.8 % | 0 % | 3; 12.5 % | 0 % | 0 % | 0 % | 1; 4.2 % | Incompleted for 2 PIPAC |
| Giger-Pabst et al. [19] | 13 | 0 % | 6; 46.2 % | 0 % | 2; 15.4 % | 1; 7.7 % | 1; 7.7 % | 3; 23.1 % | 0 % | 0 % |
| Khomyakov et al. [20] | 16 | 0 % | 8; 50 % | 0 % | 0 % | 0 % | 0 % | 0 % | 3; 18.8 % | 5; 31.3 % |
| Khosrawipour et al. [21] | 10 | 0 % | 7; 70 % | 0 % | 0 % | 0 % | 0 % | 3; 30 % | 0 % | 0 % |
| Kim et al. [22] | 16 | 0 % | 5; 31.3 % | 0 % | 3; 18.8 % | 0 % | 0 % | 0 % | 0 % | Incomplead for 2 PIPAC |
| Kurtz et al. [23] | 43 | 0 % | 18; 41.9 % | 11; 25.6 % | 0 % | 0 % | 0 % | 0 % | 0 % | 4; 9.3 % |
| Lurvink et al. [24] | 7 | 0 % | 5; 71.4 % | 0 % | 0 % | 0 % | 1; 14.3 % | 1; 14.3 % | 0 % | 0 % |
| Nadiradze et al. [25] | 14 | 0 % | 10; 71.4 % | 0 % | 0 % | 0 % | 0 % | 1; 7.1 % | 0 % | 3; 21.4 % |
| Rovers et al. [26] | 8 | 0 % | 6; 75 % | 0 % | 0 % | 0 % | 0 % | 0 % | 0 % | 2; 25 % |
| Sgarbura et al. [27] | 47 | 0 % | 18; 38.3 % | 0 % | 2; 4.3 % | 3; 6.4 % | 0 % | 6; 12.8 % | 10; 21.3 % | 8; 17 % |
| Struller et al. [28] | 19 | 0 % | 12; 63.2 % | 0 % | 2; 10.5 % | 2; 10.5 % | 0 % | 2; 10.5 % | 1; 5.3 % | 0 % |
| Tempfer et al. [29] | 19 | 0 % | 19; 100 % | 0 % | 0 % | 0 % | 0 % | 0 % | 0 % | 0 % |
| Taibi et al. [30] | 72 | 0 % | 38; 52.8 % | 0 % | 1; 1.4 % | 5; 6.9 % | 0 % | 2; 2.8 % | 17; 23.6 % | 9; 12.5 % |
| Tabchouri et al. [31] | 46 | 0 % | 7; 15.2 % | 1; 2.2 % | 14; 30.4 % | 5; 10.9 % | 3; 6.5 % | 14; 30.4 % | 0 % | 2; 4.3 % |
| Sindayigaya et al. [32] | 86 | 0 % | 54; 62.8 % | 0 % | 2; 2.3 % | 1; 1.2 % | 0 % | 8; 9.3 % | 21; 24.4 % | 0 % |
| Tempfer et al. [33] | 6 | 0 % | 2; 33.3 % | 0 % | 2; 33.3 % | 0 % | 0 % | 2; 33.3 % | 0 % | 0 % |
| Total, n (%) | 707 | 26; 3.7 % | 347; 49.1 % | 15; 2.1 % | 56; 7.9 % | 26; 3.7 % | 17; 2.4 % | 48; 6.8 % | 60; 8.5 % | 79; 11.2 % |
| Median (Q1–Q3) per study | 16.5 (10–42.7) | 0 % (0–0%) | 50 % (34.6–63 %) | 0 % (0–0%) | 19 % (0–15.1 %) | 0 % (0–5.6 %) | 0 % (0–5.7 %) | 0 % (0–10.3 %) | 0 % (0–5%) | 6.7 % (0–22.3 %) |
| Mean SD±, per study | 27.2 ± 24.1 | 3.8 ± 9 % | 49.3 ± 24.2 % | 1.6 ± 5.4 % | 10.6 ± 15 % | 2.3 ± 3.7 % | 3 ± 4.9 % | 7.1 ± 10.7 % | 4.9 ± 8.5 % | 13.1 ± 16.4 % |
Other reasons for the premature cessation of planned PIPAC sessions were bowel obstruction, adverse events (problem with healing, toxicity, etc.), patient wishes, curative intent cytoreductive surgery (CRS) and Hyperthermic IntraPEritoneal Chemotherapy (HIPEC), no secondary peritoneal access, medical reasons (embolism, sepsis, etc.) and other reasons (protocol violation, loss of view, no reason found).
We performed a subgroup analysis between studies with a failure rate of 3 planned PIPAC sessions ≥50 % (FAILURE Group) and studies with a failure rate <50 % (SUCCESS Group) (Table 4). The number of studies is small, but notably, in the >50 % FAILURE group, all the treated series include PM from gastric and hepato-biliary tumours. In the SUCCESS group, we observed more patients treated for colorectal cancer or mesothelioma. Statistical analysis showed that patients in the “SUCCESS group” had a higher average mean age (58 vs. 63; p=0.007), but no statistical difference was found in terms of the presence of ascites (p=0.285), bimodal treatment (p=0.263) or previous chemotherapy (p=0.224).
Comparison between SUCCESS and FAILURE group.
| FAILURE group | SUCCESS group | p-Value | |
|---|---|---|---|
| Number of studies | 16 | 10 | |
| Mean age of patients, years | 58 ± 4 | 63 ± 2 | 0.007 |
| Origin | |||
| Various | 7 (43.7 %) | 3 (30 %) | – |
| Colorectal | 1 (6.25 %) | 4 (40 %) | 0.217 |
| Biliary tract and pancreatic | 3 (18.75 %) | 0 | <0.0001 |
| Mesothelioma | 0 | 1 (10 %) | <0.0001 |
| Gastric | 5 (31.25 %) | 0 | <0.0001 |
| Ovarian | 0 | 1 (10 %) | <0.0001 |
| Gynaecologic | 0 | 1 (10 %) | <0.0001 |
| PCI | 18 ± 4 | 19 ± 6 | 0.951 |
| Ascites presence | 52 ± 30 % | 32 ± 29 % | 0.285 |
| Bimodal treatment | 46 ± 29 % | 30 ± 18 % | 0.263 |
| Previous chemotherapy | 88 ± 20 % | 80 ± 17 % | 0.224 |
We performed a subgroup analysis by creating a group of studies with >50 % PIPAC discontinuation due to progression and a group with <30 % PIPAC discontinuation due to progression (Table 5). By focusing on cancer types, it appears that colorectal origin causes less progression than cancer of gastric or biliopancreatic origin. Statistical analysis showed that patients in studies stopping PIPAC due to progression in more than 50 % of cases had a higher average PCI (14 ± 4 vs. 20 ± 5 p=0.014), but no statistical difference was found in terms of the presence of ascites (p=0.134), bimodal treatment (p=0.927) or previous chemotherapy (p=0.828). We observed more discontinuation due to progression in cases of gastric cancer.
Comparison between group with less than 30 % of failure and group with more than 50 % of failure.
| Rate of stop for progression <30 % | Rate of stop for progression ≧50 % | p-Value | |
|---|---|---|---|
| Number of studies | 5 | 14 | |
| Mean age of patients, years | 63 ± 3 | 59 ± 4 | 0.062 |
| Origin | |||
| Various | 3 (60 %) | 3 (21 %) | |
| Colorectal | 2 (40 %) | 3 (21 %) | |
| Biliary tract and pancreatic | 0 | 2 (14 %) | |
| Mesothelioma | 0 | 0 | |
| Gastric | 0 | 5 (36 %) | |
| Ovarian | 0 | 1 (7 %) | |
| Gynaecologic | 0 | ||
| PCI median | 14 ± 3 | 19 ± 6.4 | 0.014 |
| Ascites presence | 15 ± 31 % | 32 ± 29 % | 0.134 |
| Bimodal treatment | 36 ± 3 % | 30 ± 18 % | 0.927 |
| Previous chemotherapy | 92 ± 3 % | 80 ± 17 % | 0.828 |
Discussion
Reasons for discontinuing PIPAC before 3 recommended PIPAC sessions are oncological progression, conversion to CRS/HIPEC, patient wishes, adverse events after PIPAC, medical reasons or death [6]. Fewer studies reported a reason for stopping PIPAC, as our review demonstrated; among all the articles >200, only 26 described the reason for discontinuing PIPAC before 3 cycles.
The results of this review of the available literature suggest that early discontinuation of the 3 planned sessions is a problem that is repeatedly reported, with a median number of 2.3 PIPAC sessions. In 2018, Nowacki et al. reported similar results in a multicenter study [7]. Furthermore, the standard treatment protocol for PIPAC consists of 3 procedures and completion of this treatment model has been shown to lead to improved survival and good tumoural response [4, 34, 35]. Our analysis of success rate when performing the 3 planned PIPAC sessions showed that failure and the premature cessation of PIPAC is more frequent in young patients with gastric PM.
The main reason for the interruption of treatment before the 3 planned PIPAC sessions in our review was oncological progression. The mean rate was 49.3 ± 24.2 %. In some series, this rate increases to over 70 % [10, 21, 24], [25], [26, 29] which can be partially explained by the fact that most patients treated with PIPAC are in palliative situations with advanced, aggressive and refractory disease. Discontinuing before 3 planned PIPAC sessions for progression or clinical deterioration raises questions around the selection of patients. The potential indications for PIPAC are PM from colorectal cancer, gastric cancer, ovarian cancer, pancreatic and biliary tract cancer, peritoneal mesothelioma and appendiceal cancer1. In our analysis of discontinuation due to progression, in more than 50 % of cases, we found that young patients with a higher PCI stop the PIPAC protocol earlier.
PIPAC therapy also appears to be legitimate for patients who are not eligible for CRS/HIPEC and do not tolerate or have developed systemic chemotherapy intolerance [1]. For many patients, the absence of proof of efficiency for second- or third-line systemic treatments makes PIPAC a very promising option. It is probably this hope that makes us consider this treatment for our patients and overestimate the potential results.
Other reasons for stopping PIPAC before the 3 planned procedures are abdominal non-access; our review showed a rate of 6.8 %. Some studies have reported rates as high as 30 % [21, 31, 33]. After at least one PIPAC session, intraperitoneal chemotherapy and repeated PIPAC are well known causes for the induction of peritoneal sclerosis [36]. Moreover, patients who are eligible for PIPAC treatment often have prior abdominal surgery as CRS or HIPEC. This may explain the observed failure rate which is higher due to attempts to access the abdomen to perform PIPAC [37, 38].
For the treatment of resectable peritoneal metastasis, cytoreductive surgery and HIPEC could be performed for colorectal, ovarian and gastric peritoneal metastasis as well as for pseudomyxoma peritonei and peritoneal mesothelioma. For unresectable PM, systemic chemotherapy and targeted therapy remain the standard of care [1]. Since 2011, PIPAC has been introduced as a novel treatment for PM, with an alternative for patients who are not eligible for CRS and HIPEC [19, 29, 39]. The use of PIPAC as a neoadjuvant treatment has been proposed for different types of cancer [40, 41], with safe and feasible conclusions. Recently, PIPAC demonstrated encouraging results in patients with unresectable PM and Alyami et al. [41] demonstrated that CRS and HIPEC can be achieved in strictly selected patients with unresectable PM at diagnosis after repeated PIPAC. The reason to stop PIPAC had been identified, but with a limited frequency.
Another reason for the discontinuation of the 3 planned PIPAC sessions was patient wishes. The review showed a mean of 7.9 %, but no further explanation was found in the literature. According to Balmer et al. [6] fear of surgery or general anaesthesia and the refusal for repeated hospitalizations are some of the reasons for discontinuing PIPAC.
Bowel obstruction or perforation is some of the reasons for interrupting the 3 planned PIPAC sessions. Bowel obstruction is often a reflection of oncological disease progression with occlusion on carcinosis. Balmer et al. [6] demonstrated that the absence of a prior history of bowel obstruction before the first PIPAC session was associated with the completion of PIPAC treatment.
Post-operative morbidity is a reason for discontinuing PIPAC. Our review showed that adverse events/post-operative complications are reasons for discontinuing PIPAC and found a mean morbidity rate with CTCAE grade 3 and 4 of 8 %. This is in accordance with other reviews of the literature [2, 42], [43], [44]. Recently, Alyami et al. summarised 45 clinical studies with 1,810 PIPAC procedures in 838 patients [1]. The review found that repeated PIPAC sessions were feasible, with a 3 % incidence of post-operative surgical complications. Among adverse events, the most common intraoperative complication was an iatrogenic bowel injury (0–3 % of total PIPAC procedures) [45]. In a previous literature review, Winkler et al. [43] found that bowel injury related to a Veress needle or trocar insertion was the most common complication. For this reason, clinical teams continue to search for improved means of access to the peritoneal cavity to avoid complications and the deterioration of therapeutic management [37].
The main limitations of our study were the missing data for all retrospective studies and the lack of case series describing the motivations to stop PIPAC. Therefore, it would be useful to better describe the reasons of stopping PIPAC in the upcoming studies.
Conclusions
Many treatment parameters of PIPAC, such as the minimum number of PIPAC applications to induce maximum tumour regression, are still empirical with limited available evidence. It is assumed by most pioneers of PIPAC therapy that at least 3 PIPAC sessions are probably required to induce a substantial regression of PM. Many patients do not complete the 3 planned PIPAC sessions, especially due to rapid oncological progression. This review seemed to demonstrate that PIPAC could be more successful in the case of colorectal, ovarian or mesothelioma PM. It appears that PIPAC in gastric PM gives poor results, especially in young patients with high PCI. As reported by Balmer et al. investigations are necessary to better understand the causes, in order to improve the selection of patients who are most likely to benefit from PIPAC.
-
Research funding: None declared.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission. Conceptualization, data curation and original draft writing: AC Ezanno. Writing – Review: B Malgras. Supervision, Writing review: M Pocard.
-
Competing interests: A.C. Ezanno and B. Malgras have no conflict of interest. M. Pocard receives Research funding by Capnomed GmbH, IDImed, as INSERM laboratory unit U 1275 responsible for peritoneal metastasis research.
-
Informed consent: Not applicable.
-
Ethical approval: Not applicable.
References
1. Alyami, M, Hübner, M, Grass, F, Bakrin, N, Villeneuve, L, Laplace, N, et al.. Pressurised intraperitoneal aerosol chemotherapy: rationale, evidence, and potential indications. Lancet Oncol 2019;20:e368–77. https://doi.org/10.1016/s1470-2045(19)30318-3.Search in Google Scholar
2. Grass, F, Vuagniaux, A, Teixeira-Farinha, H, Lehmann, K, Demartines, N, Hübner, M. Systematic review of pressurized intraperitoneal aerosol chemotherapy for the treatment of advanced peritoneal carcinomatosis. Br J Surg 2017;104:669–78. https://doi.org/10.1002/bjs.10521.Search in Google Scholar PubMed
3. Girardot-Miglierina, A, Clerc, D, Alyami, M, Villeneuve, L, Sgarbura, O, Reymond, MA, et al.. Consensus statement on safety measures for pressurized intraperitoneal aerosol chemotherapy. Pleura Peritoneum 2021;6:139–49. https://doi.org/10.1515/pp-2021-0125.Search in Google Scholar PubMed PubMed Central
4. Sgarbura, O, Villeneuve, L, Alyami, M, Bakrin, N, Torrent, JJ, Eveno, C, et al.. Current practice of pressurized intraperitoneal aerosol chemotherapy (PIPAC): still standardized or on the verge of diversification? Eur J Surg Oncol 2021;47:149–56. https://doi.org/10.1016/j.ejso.2020.08.020.Search in Google Scholar PubMed
5. Horvath, P, Beckert, S, Struller, F, Königsrainer, A, Reymond, MA. Pressurized intraperitoneal aerosol chemotherapy (PIPAC) for peritoneal metastases of pancreas and biliary tract cancer. Clin Exp Metastasis 2018;35:635–40. https://doi.org/10.1007/s10585-018-9925-7.Search in Google Scholar PubMed
6. Balmer, A, Clerc, D, Toussaint, L, Sgarbura, O, Taïbi, A, Hübner, M, et al.. Selection criteria for pressurized intraperitoneal aerosol chemotherapy (PIPAC) treatment in patients with peritoneal metastases. Cancers 2022;14:2557. https://doi.org/10.3390/cancers14102557.Search in Google Scholar PubMed PubMed Central
7. Nowacki, M, Alyami, M, Villeneuve, L, Mercier, F, Hubner, M, Willaert, W, et al.. Multicenter Multicentre comprehensive methodological and technical analysis of 832 pressurized intraperitoneal aerosol chemotherapy (PIPAC) interventions performed in 349 patients for peritoneal carcinomatosis treatment: an international survey study. Eur J Surg Oncol J Eur Soc Surg Oncol Br Assoc Surg Oncol 2018;44:991–6. https://doi.org/10.1016/j.ejso.2018.02.014.Search in Google Scholar PubMed
8. Shamseer, L, Moher, D, Clarke, M, Ghersi, D, Liberati, A, Petticrew, M, et al.. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ 2015;350:g7647. https://doi.org/10.1136/bmj.g7647.Search in Google Scholar PubMed
9. Alyami, M, Gagniere, J, Sgarbura, O, Cabelguenne, D, Villeneuve, L, Pezet, D, et al.. Multicentric initial experience with the use of the pressurized intraperitoneal aerosol chemotherapy (PIPAC) in the management of unresectable peritoneal carcinomatosis. Eur J Surg Oncol J Eur Soc Surg Oncol Br Assoc Surg Oncol 2017;43:2178–83. https://doi.org/10.1016/j.ejso.2017.09.010.Search in Google Scholar PubMed
10. De Simone, M, Vaira, M, Argenziano, M, Berchialla, P, Pisacane, A, Cinquegrana, A, et al.. Pressurized intraperitoneal aerosol chemotherapy (PIPAC) with oxaliplatin, cisplatin, and doxorubicin in patients with peritoneal carcinomatosis: an open-label, single-arm, phase II clinical trial. Biomedicines 2020;8:E102. https://doi.org/10.3390/biomedicines8050102.Search in Google Scholar PubMed PubMed Central
11. Di Giorgio, A, Schena, CA, El Halabieh, MA, Abatini, C, Vita, E, Strippoli, A, et al.. Systemic chemotherapy and pressurized intraperitoneal aerosol chemotherapy (PIPAC): a bidirectional approach for gastric cancer peritoneal metastasis. Surg Oncol 2020;34:270–5. https://doi.org/10.1016/j.suronc.2020.05.006.Search in Google Scholar PubMed
12. Di Giorgio, A, Sgarbura, O, Rotolo, S, Schena, CA, Bagalà, C, Inzani, F, et al.. Pressurized intraperitoneal aerosol chemotherapy with cisplatin and doxorubicin or oxaliplatin for peritoneal metastasis from pancreatic adenocarcinoma and cholangiocarcinoma. Ther Adv Med Oncol 2020;12:1–10. https://doi.org/10.1177/1758835920940887.Search in Google Scholar PubMed PubMed Central
13. Ellebæk, SB, Graversen, M, Detlefsen, S, Lundell, L, Fristrup, CW, Pfeiffer, P, et al.. Pressurized intraperitoneal aerosol chemotherapy (PIPAC)-directed treatment of peritoneal metastasis in end-stage colorectal cancer patients. Pleura Peritoneum 2020;5:20200109. https://doi.org/10.1515/pp-2020-0109.Search in Google Scholar PubMed PubMed Central
14. Ellebæk, SB, Graversen, M, Detlefsen, S, Lundell, L, Fristrup, CW, Pfeiffer, P, et al.. Pressurized intraperitoneal aerosol chemotherapy (PIPAC) of peritoneal metastasis from gastric cancer: a descriptive cohort study. Clin Exp Metastasis 2020;37:325–32. https://doi.org/10.1007/s10585-020-10023-5.Search in Google Scholar PubMed
15. Falkenstein, TA, Götze, TO, Ouaissi, M, Tempfer, CB, Giger-Pabst, U, Demtröder, C. First clinical data of intraperitoneal aerosol chemotherapy (PIPAC) as salvage therapy for peritoneal metastatic biliary tract cancer. Anticancer Res 2018;38:373–8. https://doi.org/10.21873/anticanres.12232.Search in Google Scholar PubMed
16. Graversen, M, Detlefsen, S, Ellebaek, SB, Fristrup, C, Pfeiffer, P, Mortensen, MB. Pressurized intraperitoneal aerosol chemotherapy with one minute of electrostatic precipitation (ePIPAC) is feasible, but the histological tumour response in peritoneal metastasis is insufficient. Eur J Surg Oncol J Eur Soc Surg Oncol Br Assoc Surg Oncol 2020;46:155–9. https://doi.org/10.1016/j.ejso.2019.08.024.Search in Google Scholar PubMed
17. Graversen, M, Detlefsen, S, Bjerregaard, JK, Fristrup, CW, Pfeiffer, P, Mortensen, MB. Prospective, single-implementation and response evaluation of pressurized intraperitoneal aerosol chemotherapy (PIPAC) for peritoneal metastasis. Ther Adv Med Oncol 2018;10:1–11. https://doi.org/10.1177/1758835918777036.Search in Google Scholar PubMed PubMed Central
18. Hübner, M, Teixeira Farinha, H, Grass, F, Wolfer, A, Mathevet, P, Hahnloser, D, et al.. Feasibility and safety of pressurized pressurised intraperitoneal aerosol chemotherapy for peritoneal carcinomatosis: a retrospective cohort study. Gastroenterol Res Pract 2017;2017:6852749. https://doi.org/10.1155/2017/6852749.Search in Google Scholar PubMed PubMed Central
19. Giger-Pabst, U, Demtröder, C, Falkenstein, TA, Ouaissi, M, Götze, TO, Rezniczek, GA, et al.. Pressurized intraperitoneal aerosol chemotherapy (PIPAC) for the treatment of malignant mesothelioma. BMC Cancer 2018;18:442. https://doi.org/10.1186/s12885-018-4363-0.Search in Google Scholar PubMed PubMed Central
20. Khomyakov, V, Ryabov, A, Ivanov, A, Bolotina, L, Utkina, A, Volchenko, N, et al.. Bidirectional chemotherapy in gastric cancer with peritoneal metastasis combining intravenous XELOX with intraperitoneal chemotherapy with low-dose cisplatin and doxorubicin administered as a pressurized aerosol: an open-label, Phase-2 study (PIPAC-GA2). Pleura Peritoneum 2016;1:159–66. https://doi.org/10.1515/pp-2016-0017.Search in Google Scholar PubMed PubMed Central
21. Khosrawipour, T, Khosrawipour, V, Giger-Pabst, U. Pressurized intraperitoneal aerosol chemotherapy in patients suffering from peritoneal carcinomatosis of pancreatic adenocarcinoma. PLoS One 2017;12:e0186709. https://doi.org/10.1371/journal.pone.0186709.Search in Google Scholar PubMed PubMed Central
22. Kim, G, Tan, HL, Sundar, R, Lieske, B, Chee, CE, Ho, J, et al.. PIPAC-OX: a phase I study of oxaliplatin-based pressurized intraperitoneal aerosol chemotherapy in patients with peritoneal metastases. Clin Cancer Res Off J Am Assoc Cancer Res 2021;27:1875–81. https://doi.org/10.1158/1078-0432.ccr-20-2152.Search in Google Scholar
23. Kurtz, F, Struller, F, Horvath, P, Solass, W, Bösmüller, H, Königsrainer, A, et al.. Feasibility, safety, and efficacy of intraperitoneal aerosol chemotherapy (PIPAC) for peritoneal metastasis: a Registry Study. Gastroenterol Res Pract 2018;2018:2743985. https://doi.org/10.1155/2018/2743985.Search in Google Scholar PubMed PubMed Central
24. Lurvink, RJ, Rovers, KP, Wassenaar, ECE, Bakkers, C, Burger, JWA, Creemers, GM, et al.. Patient-reported outcomes during repetitive oxaliplatin-based pressurized intraperitoneal aerosol chemotherapy for isolated unresectable colorectal peritoneal metastases in a multicenter, single-arm, phase 2 trial (CRC-PIPAC). Surg Endosc 2021;10:4486–98. https://doi.org/10.1007/s00464-021-08802-6.Search in Google Scholar PubMed PubMed Central
25. Nadiradze, G, Giger-Pabst, U, Zieren, J, Strumberg, D, Solass, W, Reymond, MA. Pressurized intraperitoneal aerosol chemotherapy (PIPAC) with low-dose cisplatin and doxorubicin in gastric peritoneal metastasis. J Gastrointest Surg Off J Soc Surg Aliment Tract 2016;20:367–73. https://doi.org/10.1007/s11605-015-2995-9.Search in Google Scholar PubMed PubMed Central
26. Lurvink, RJ, Rovers, KP, Wassenaar, ECE, Bakkers, C, Burger, JWA, Creemers, GM, et al.. Intraperitoneal aerosol chemotherapy (oxaliplatin) for unresectable colorectal peritoneal metastases: a multicenter, single-arm, phase II trial (CRC-PIPAC). Ann Surg Oncol 2021;28:5311–26. https://doi.org/10.1245/s10434-020-09558-4.Search in Google Scholar PubMed
27. Sgarbura, O, Hübner, M, Alyami, M, Eveno, C, Gagnière, J, Pache, B, et al.. Oxaliplatin use in pressurized intraperitoneal aerosol chemotherapy (PIPAC) is safe and effective: a multicenter study. Eur J Surg Oncol J Eur Soc Surg Oncol Br Assoc Surg Oncol 2019;45:2386–91. https://doi.org/10.1016/j.ejso.2019.05.007.Search in Google Scholar PubMed
28. Struller, F, Horvath, P, Solass, W, Weinreich, FJ, Strumberg, D, Kokkalis, MK, et al.. Pressurized intraperitoneal aerosol chemotherapy with low-dose cisplatin and doxorubicin (PIPAC C/D) in patients with gastric cancer and peritoneal metastasis: a phase II study. Ther Adv Med Oncol 2019;11:1–12. https://doi.org/10.1177/1758835919846402.Search in Google Scholar PubMed PubMed Central
29. Tempfer, CB, Winnekendonk, G, Solass, W, Horvat, R, Giger-Pabst, U, Zieren, J, et al.. Pressurized intraperitoneal aerosol chemotherapy in women with recurrent ovarian cancer: a phase 2 study. Gynecol Oncol 2015;137:223–8. https://doi.org/10.1016/j.ygyno.2015.02.009.Search in Google Scholar PubMed
30. Taibi, A, Sgarbura, O, Hübner, M, Bardet, SM, Alyami, M, Bakrin, N, et al.. Feasibility and safety of oxaliplatin-based pressurized intraperitoneal aerosol chemotherapy with or without intraoperative intravenous 5-fluorouracil and leucovorin for colorectal peritoneal metastases: a multicenter comparative cohort study. Ann Surg Oncol 2022;29:5243–51. https://doi.org/10.1245/s10434-022-11577-2.Search in Google Scholar PubMed
31. Tabchouri, N, Buggisch, J, Demtröder, CR, Thiery, J, Rezniczek, G, Tempfer, CB, et al.. Pressurized intraperitoneal aerosol chemotherapy for colorectal peritoneal metastases. Ann Surg Oncol 2021;28:5275–86. https://doi.org/10.1245/s10434-020-09508-0.Search in Google Scholar PubMed
32. Sindayigaya, R, Dogan, C, Demtröder, CR, Fischer, B, Karam, E, Buggisch, JR, et al.. Clinical outcome for patients managed with low-dose cisplatin and doxorubicin delivered as pressurized intraperitoneal aerosol chemotherapy for unresectable peritoneal metastases of gastric cancer. Ann Surg Oncol 2022;29:112–23. https://doi.org/10.1245/s10434-021-10860-y.Search in Google Scholar PubMed
33. Tempfer, CB, Giger-Pabst, U, Seebacher, V, Petersen, M, Dogan, A, Rezniczek, GA. A phase I, single-arm, open-label, dose escalation study of intraperitoneal cisplatin and doxorubicin in patients with recurrent ovarian cancer and peritoneal carcinomatosis. Gynecol Oncol 2018;150:23–30. https://doi.org/10.1016/j.ygyno.2018.05.001.Search in Google Scholar PubMed
34. Hübner, M, Grass, F, Teixeira-Farinha, H, Pache, B, Mathevet, P, Demartines, N. Pressurized intraperitoneal aerosol chemotherapy - practical aspects. Eur J Surg Oncol J Eur Soc Surg Oncol Br Assoc Surg Oncol 2017;43:1102–9. https://doi.org/10.1016/j.ejso.2017.03.019.Search in Google Scholar PubMed
35. Farinha, H, Hubner, M, Abba, J, Rao, R, Willaert, W. Treatment response after pressurized intraperitoneal aerosol chemotherapy (PIPAC) for peritoneal metastases of colorectal origin. Eur J Surg Oncol 2022;48:E114. https://doi.org/10.1016/j.ejso.2021.12.190.Search in Google Scholar
36. Graversen, M, Detlefsen, S, Pfeiffer, P, Lundell, L, Mortensen, MB. Severe peritoneal sclerosis after repeated pressurized intraperitoneal aerosol chemotherapy with oxaliplatin (PIPAC OX): report of two cases and literature survey. Clin Exp Metastasis 2018;35:103–8. https://doi.org/10.1007/s10585-018-9895-9.Search in Google Scholar PubMed
37. Glatz, T, Horvath, P, Lang, SA, Archid, R, Nadiradze, G. Staging laparoscopy and Pressurized intraperitoneal aerosol chemotherapy (PIPAC) for peritoneal metastasis: safe access to the abdomen. Pleura Peritoneum 2019;4:20190004. https://doi.org/10.1515/pp-2019-0004.Search in Google Scholar PubMed PubMed Central
38. Chi, DS, Abu-Rustum, NR, Sonoda, Y, Awtrey, C, Hummer, A, Venkatraman, ES, et al.. Ten-year experience with laparoscopy on a gynaecologic oncology service: analysis of risk factors for complications and conversion to laparotomy. Am J Obstet Gynecol 2004;191:1138–45. https://doi.org/10.1016/j.ajog.2004.05.004.Search in Google Scholar PubMed
39. Alyami, M, Bonnot, PE, Mercier, F, Laplace, N, Villeneuve, L, Passot, G, et al.. Pressurized intraperitoneal aerosol chemotherapy (PIPAC) for unresectable peritoneal metastasis from gastric cancer. Eur J Surg Oncol J Eur Soc Surg Oncol Br Assoc Surg Oncol 2021;47:123–7. https://doi.org/10.1016/j.ejso.2020.05.021.Search in Google Scholar PubMed
40. Girshally, R, Demtröder, C, Albayrak, N, Zieren, J, Tempfer, C, Reymond, MA. Pressurized Pressurised intraperitoneal aerosol chemotherapy (PIPAC) as a neoadjuvant therapy before cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. World J Surg Oncol 2016;14:253. https://doi.org/10.1186/s12957-016-1008-0.Search in Google Scholar PubMed PubMed Central
41. Alyami, M, Mercier, F, Siebert, M, Bonnot, PE, Laplace, N, Villeneuve, L, et al.. Unresectable peritoneal metastasis treated by pressurized intraperitoneal aerosol chemotherapy (PIPAC) leading to cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Eur J Surg Oncol J Eur Soc Surg Oncol Br Assoc Surg Oncol 2021;47:128–33. https://doi.org/10.1016/j.ejso.2019.06.028.Search in Google Scholar PubMed
42. Ploug, M, Graversen, M, Pfeiffer, P, Mortensen, MB. Bidirectional treatment of peritoneal metastasis with Pressurized intraperitoneal aerosol chemotherapy (PIPAC) and systemic chemotherapy: a systematic review. BMC Cancer 2020;20:105. https://doi.org/10.1186/s12885-020-6572-6.Search in Google Scholar PubMed PubMed Central
43. Winkler, CS, Sandhu, J, Pettke, E, Merchea, A, Fong, Y, Kumara, HMCS, et al.. Pressurized Pressurised intraperitoneal aerosol chemotherapy, a palliative treatment approach for patients with peritoneal carcinomatosis: description of method and systematic review of literature. Dis Colon Rectum 2020;63:242–55. https://doi.org/10.1097/dcr.0000000000001565.Search in Google Scholar
44. Garg, PK, Jara, M, Alberto, M, Rau, B. The role of pressurized intraperitoneal aerosol chemotherapy in the management of gastric cancer: a systematic review. Pleura Peritoneum 2019;4:20180127. https://doi.org/10.1515/pp-2018-0127.Search in Google Scholar PubMed PubMed Central
45. Lurvink, RJ, Van der Speeten, K, Rovers, KP, de Hingh, IHJT. The emergence of pressurized intraperitoneal aerosol chemotherapy as a palliative treatment option for patients with diffuse peritoneal metastases: a narrative review. J Gastrointest Oncol 2021;12:S259–70. https://doi.org/10.21037/jgo-20-497.Search in Google Scholar PubMed PubMed Central
© 2023 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- Review
- Pressurized intraperitoneal aerosol chemotherapy, reasons for interrupting treatment: a systematic review of the literature
- Research Articles
- Peritoneal regression grading score (PRGS): first evidence for independent predictive and prognostic significance
- Correlation between PSOGI pathological classification and survival outcomes of patients with pseudomyxoma peritonei treated using cytoreductive surgery and HIPEC: national referral centre experience and literature review
- The role of cytology in patients undergoing pressurized intraperitoneal aerosol chemotherapy (PIPAC) treatment for peritoneal carcinomatosis
- Overall survival and morbidity are not associated with advanced age for cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: a single centre experience
- Enhanced recovery after surgery in cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: national survey of peri-operative practice by Indian society of peritoneal surface malignancies
Articles in the same Issue
- Frontmatter
- Review
- Pressurized intraperitoneal aerosol chemotherapy, reasons for interrupting treatment: a systematic review of the literature
- Research Articles
- Peritoneal regression grading score (PRGS): first evidence for independent predictive and prognostic significance
- Correlation between PSOGI pathological classification and survival outcomes of patients with pseudomyxoma peritonei treated using cytoreductive surgery and HIPEC: national referral centre experience and literature review
- The role of cytology in patients undergoing pressurized intraperitoneal aerosol chemotherapy (PIPAC) treatment for peritoneal carcinomatosis
- Overall survival and morbidity are not associated with advanced age for cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: a single centre experience
- Enhanced recovery after surgery in cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: national survey of peri-operative practice by Indian society of peritoneal surface malignancies


