The dialogue between died and viable cells: in vitro and in vivo bystander effects and 1H-NMR-based metabolic profiling of soluble factors
-
Elisa Panzarini
, Cristian Vergallo
Abstract
The bystander effect (BE) is an important biological phenomenon that induces damages in distant and not directly affected by a chemical/physical stress cells. This effect, well known in ionizing radiation treatment, relies on reactive signals released by exposed cells and transmitted via cell–cell interaction or culture medium. In this study, cycloheximide (CHX)-induced apoptotic U937 cells and untreated THP-1 cells were chosen to investigate the chemical-induced BE. The effects of apoptotic U937 cells culture medium, Conditioned Medium (CM), on THP-1 cells were evaluated by morphological and immunohistochemical analysis performed by light microscopy; 1D 1H and 2D J-resolved (JRES) NMR metabolomic analysis has been used to characterize the molecules involved in the BE. In summary, this study indicates that: CM of CHX-treated U937 cells induces a time-dependent induction of toxicity, probably apoptotic cell death, and macrophagic differentiation in THP-1 cells; CM contains different metabolites respect fresh culture medium; CM recruits in vivo activated fibroblasts, endothelial cells, macrophages and mononuclear inflammatory cells in rat calf muscles. These data suggest that CHX exposed cells could cause BE through the release, during the apoptotic process, of soluble factors into the medium that could be exploited in anticancer protocols.
Introduction
The bystander effect (BE) is the ability of chemically or physically stressed cells to induce biological responses in other cells not directly targeted by stress agent via transmitting signals through cell–cell interaction or culture medium. In 1992, Nagasawa and Little [1], first proposed the radiation-induced BE to indicate the phenomenon in which cells adjacent to irradiated cells but not themselves exposed display a broad spectrum of biological responses as much as radiation exposed cells. The BE includes reduced clonogenic survival [2], [3], apoptosis [4], [5], [6], [7], increased mitochondrial mass [8], increased number of micronuclei [5], [9], sister chromatid exchanges [1], [10], chromosomal aberrations [11] and DNA double-strand breaks [12], [13], [14]. A large number of papers suggest that radiation-induced BE can be divided in two categories, i.e. gap junction-mediated requiring cell-to-cell contact [13], [15], and culture medium-mediated which requires soluble factors extracellularly secreted [2], [16], [17], [18], [19]. Along with the well known radiation-induced BE [20], [21], [22], also chemotherapeutic drugs, such as chloroethylnitrosurea [24], mitomycin C [23], [25], [26], [27], phleomycin [25], [26], [27], paclitaxel, and actinomycin D [28], [29], and photodynamic stress agents [30], [31], [32] have also been shown to be involved in BE. Unfortunately, the molecules mediating the BE, both radiation and chemically induced, are still poor characterized. These factors include Reactive Oxygen Species (ROS) [33], [34], Nitric Oxide (NO) [35], [36] and cytokines [2], [16], [17], [18], [19], [20], [37]. Since the distinctive aspect of BE is its influence on cell viability of non-target cells [38], the phenomenon is extensively investigated also in cancer field mainly for the propagation of injury in cancer therapy to overcome the inaccessibility or the resistance of tumoural cells to therapeutic stress [39], [40]. Also, in cancer management very important is the role played by immune cells present in tumoural site that are able to ignite immune responses acting both in progression and in regression of cancer. The monocytes are among the first immune cells to infiltrate the tumoural lesions; once activated and differentiated into macrophages, they cooperate in the tumour microenvironment homeostasis leading to tumour inhibition or progression [41].
Here, we evaluated the BE elicited by conditioned medium of cycloheximide (CHX)-treated U937 cells on viable THP-1 cells. Moreover, we adopted a NMR-based cell metabolomics approach to analyze the metabolome of the conditioned medium of treated U937 cells to provide new insights into the potential applications of CHX.
Materials and methods
U937 and THP-1 cell lines
Human U937 myeloid leukemia cells and human THP-1 acute monocytic leukemia cells were cultured in RPMI-1640 medium (Cambrex, Verviers, Belgium) supplemented with 10% fetal calf serum (FCS), 2 mmol/L L-glutamine (Cambrex), 100 IU/mL penicillin and streptomycin solution (Sigma, St Louis, MO, USA) and 10000 U/mL antimicotic solution (Cambrex), in a 5% CO2 and 99% humidified atmosphere at 37°C. The cells were maintained in 75 cm2 flasks at a concentration of 5×105 cells/mL by passage every 3–4 days.
Rats
Male Wistar rats (150–200 g) were housed in a temperature-controlled (22±1°C) animal holding room with free access to commercial rat diet and tap water. Experiments were approved by the National and Local Ethics Committee and carried out in accordance with local and national guidelines for animal experimentation.
Chemical treatment and medium transfer
U937 cells were induced to apoptosis by treatment with cycloheximide (CHX) 10−2 M for 18 h in 10% FCS RPMI. The cells were washed three times with fresh medium to remove residual chemicals and cultured in 10% FCS RPMI fresh medium for various time points, i.e. 1, 2, 3 and 4 h, in a 5% CO2 humidified atmosphere at 37°C. The culture medium, called Conditioned Medium (CM), were collected and indicated as 1, 2, 3 and 4 h CM, respectively. To ensure no cells in the collected media, they were 1000 rpm 10 min centrifuged and filtered through a 0.22-μm filter (Corning, NY, USA). The 1 h CM was used to grow THP-1 cells (bystander cells) for 15, 30, 60, 120, 180, and 240 min to observe the BE (detection of cell death and macrophagic differentiation induction by morphological observation).
The 1 h CM and 4 h CM were inoculated in rat calf muscles to evaluate the BE (detection of immune cells, e.g. macrophages, fibroblasts, by immunohistochemical detection of CD68 antigen, and type II TGFβ and type I IL-1 receptors.
For each experiment, three independent replicates were performed. The collected media were analysed by 1D 1H and 2D J-resolved (JRES) NMR spectroscopy to evaluate bystander factors released from CHX-treated U937 cells in culture medium.
Analysis of cell death induced by CHX: Annexin-V/PI staining
THP-1 dead cells were detected by the Annexin-V–fluorescein isothiocyanate (FITC) Apoptosis Detection kit (Sigma, St. Louis, MO, USA). Cells were rinsed twice with 0.2 M PBS (pH 7.4) and incubated for 10 min in complete culture medium containing 0.5 mg/mL FITC-conjugated Annexin-V and 2 mg/mL PI. Dead cells were recognised with a fluorescence microscope Eclipse 80i (Nikon, Tokyo, Japan) for their positivity to Annexin-V that binds the phosphatidylserine residues translocated to the outer leaflet of the plasma membrane. Early apoptotic cells were stained only by Annexin-V–FITC, necrotic cells were simultaneously stained by PI and Annexin-V–FITC and living cells were not stained. Counts were performed on at least 20 randomly chosen microscopic fields (40X) and at least 500 cells were analysed.
Morphological observation
THP-1 cells (1×106 cells/mL of medium) were cultured for different period, i.e. 15, 30, 60, 120, 180, and 240 min, in the 1 h CM in 75 cm2 flasks in a 5% CO2 and 99% humidified atmosphere at 37°C.
At the end of the treatment, the culture medium was removed by flasks and centrifuged (1000 rpm, 5 min) to obtain the cells that were fixed in formalin (4% in phosphate-buffered saline (PBS) 0.2 M, pH 7.4), placed onto gelatine-coated slides, stained with Ematoxylin/eosin and processed for light microscope observation. The number of morphologically modified THP-1 cells were evaluated by scoring at least at least 20 randomly chosen microscopic fields (40X) and at least 500 cells for each time point under the light microscope Eclipse 80i (Nikon, Tokyo, Japan). The results were reported as percentage of modified cells by considering number of untreated THP-1 cells (i.e. culture in RPMI-1640 medium (Cambrex, Verviers, Belgium) supplemented with 10% fetal calf serum (FCS), 2 mmol/L L-glutamine (Cambrex), 100 IU/mL penicillin and streptomycin solution (Sigma, St Louis, MO, USA) and 10000 U/mL antimicotic solution (Cambrex), in a 5% CO2 and 99% humidified atmosphere at 37°C) as 100%.
Conversely, the cells attached to substrate were fixed in formalin (4% in phosphate-buffered saline (PBS) 0.2 M, pH 7.4) directly in the flask and observed under the inverted phase contrast microscope. An area of 10 cm×10 cm was observed and the cells attached were counted for each time point.
The parameters of morphological modifications considered have been (i) emission of filopodia and substrate adhesion (macrophagic differentiation induction) and (ii) cell surface blebbing and chromatin condensation e/o fragmentation (cell death induction).
1D 1H and 2D J-resolved (JRES) NMR spectroscopy
The collected media were dried under vacuum at room temperature. All lyophilized extracts were dissolved in 0.4 mL of D2O containing 1.8 mM 4,4-dimethyl-4-silapentane-1-sulfonic acid (DSS), which served as an internal chemical shift standard and placed in a 5 mm NMR tube. All NMR spectra were measured at 400.13 MHz using Avance DRX-400 (T=298 K) spectrometer (Bruker, Fremont, CA, USA). The acquisition and processing of NMR spectra were done by Bruker Xwin-NMR (Version 2.6; Bruker) and TopSpin-NMR (Version 2.0; Bruker) software. Quantitative evaluation were performed by using Windows 7 Office Excel software. In all 1D and 2D spectra the water signal was suppressed by a watergate or presaturation sequences, zgpr and jresgpprqf respectively. 1D 1H spectra were acquired with 32K data points, spectral width of 4795.396 Hz, 1024 scans with a 2s repetition delay. 2D 1H JRES spectra were acquired with 4K data points, a spectral width of 4795396 Hz, 2s repetition delay, 16 dummy scans, and 64 scans for 128 series.
Detection of immune cells recruited by bystander effect: immunohistochemistry of muscle tissue sections
Twelve male Wistar rats were sedated with ether anesthesia and divided in 2 groups according to the inoculation types, reported below performed in bilateral anterior and posterior calf muscles of each rat. Inoculation volume was 500 μL and cell number 5×105. Rats were sacrificed at 3, 7 and 15 Post-Inoculation (PI) days.
Inoculation types: (1) sterile pyrogen free water; (2) viable U937 cells; (3) RPMI (10% FCS); (4) apoptotic CHX (10−2 M 18 h)-induced U937 cells; (5) 1 h CM; (6) 4 h CM.
Muscles explanted and fixed by immersion in 4% paraformaldehyde for 2 h, were, then, dehydrated and embedded in 54°C paraffin. Three micrometer-thick sections were cut by using a microtome Reichert-Jung 2050 Supercut (Leica Microsystems GmbH, Wetzlar, Germany). Immunohistochemical analysis of CD68, type II TGFβ receptor (TGFβ RII) and type I IL-1 receptor (IL-1 RI) was performed on muscle sections placed onto gelatine-coated slides. Antibody anti-CD68 recognizes tissue macrophages, fibroblasts and activated endothelial cells, antibody anti-type II TGFβ receptor recognizes fibroblasts and activated endothelial cells, while anti-type I IL-1 receptor recognizes endothelial cells and mononuclear inflammatory cells. Endogenous peroxidase activity was quenched by treating the sections with 3% H2O2 in methanol for 20 min at room temperature. Slides were incubated with 0.5% trypsin in Tris Buffered Saline (TBS) for 5 min at 37°C for antigen retrieval and then with blocking solution (1% BSA in TBS) for 20 min in a humidified chamber to block excess proteins and prevent nonspecific antibody binding. Sections were incubated with the primary antibodies (Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-TGFβ RII (2 μg/mL), anti-CD68 (90 μg/mL), and anti-IL-1 RI (2 μg/mL) in blocking solution, for 60 min at room temperature in a humidified chamber. Slides were washed two times with TBS and incubated with biotinylated secondary antibody (5 μg/mL in blocking solution) for 60 min at room temperature in a humidified chamber. Slides were further washed two times with TBS before incubation with avidin-biotin complex (ABC; Vector Laboratories Inc., Burlingame, CA, USA) for 15 min at room temperature in a humidified chamber. Immunoreactivity, indicated by the appearance of brown colour, was developed with 0.05% of 3,3′-diaminobenzidine tetrahydrochloride (Sigma Aldrich, St. Louis, MO, USA) in 0.1% H2O2 in TBS for 3 min. Sections were rinsed in distilled water and counterstained with haematoxylin and processed for light microscope observation using the light microscope Eclipse 80i (Nikon, Tokyo, Japan). The number of cells positive to immunostaining was analysed on both cross and longitudinal muscles sections; at least 20 randomly chosen microscopic fields (40X) were counted and the number of cells was reported per mm2.
The specificity of immunostaining was controlled by omitting incubation with the primary or secondary antibodies or with both of them.
Statistical analysis
The two-tailed Student’s t-test was used to analyze differences between controls and treated samples. Data are presented as mean value±SD and all tests were performed at the 0.05 significance level.
Results
Establishment of apoptosis model of U937 cells induced by cycloheximide
Effect of cycloheximide (CHX) on cell death was shown in Table 1. Apoptosis and necrosis rates were measured after Annexin V-FITC/PI staining. Our results demonstrated that 10−2 M CHX causes the highest apoptosis rate (of about 88%) upon 1 h of recovery post CHX treatment; conversely, the highest percentage number of necrotic cells (of about 85%) was measured at 4 h of recovery post CHX treatment. We establish that the optimum condition to obtain the conditioned medium (CM) of apoptotic cells or necrotic cells is 1 h and 4 h incubation of U937 cells post treatment with CHX 10−2 M for 18 h respectively.
Effect of cycloheximide (CHX) on apoptosis and necrosis rates (%) of U937 cells.
| Untreated U937 cells |
CHX-treated U937 cells |
||||
|---|---|---|---|---|---|
| Apoptosis | Necrosis | Apoptosis | Necrosis | ||
| 18 h | 1.85±0.02 | 1.23±0.004 | CHX 18 h | 21.52±2.65a | 5.91±1.05a |
| 18 h+1 h recovery | 1.86±0.009 | 1.22±0.024 | CHX 18 h+1 h recovery | 88.09±5.64a | 4.89±1.11a |
| 18 h+2 h recovery | 2.01±0.05 | 1.24±0.047 | CHX 18 h+2 h recovery | 52.76±8.47a | 40.16±5.54a |
| 18 h+3 h recovery | 2.65±0.028 | 1.31±0.008 | CHX 18 h+3 h recovery | 28.41±4.69a | 64.54±5.89a |
| 18 h+4 h recovery | 2.64±0.008 | 1.29±0.041 | CHX 18 h+4 h recovery | 7.61±1.96a | 85.41±7.41a |
-
The fractions of apoptotic and necrotic dead cells were determined by counting Annexin-V-positive/Propidium Iodide negative and Annexin-V-positive/Propidium Iodide positive U937 cells respectively. At least 500 cells were scored for each time point using a fluorescence microscope. The values are the mean±S.D. of three independent experiments. aIndicates significant values (p<0.05) versus those in untreated ones.
Cycloheximide-induced bystander effect
To determine the effect of conditioned medium on morphology of bystander cells, we cultured the THP-1 cells with conditioned medium collected from cycloheximide-induced apoptotic U937 cells (called 1 h CM).
In particular, we considered two types of morphological features: cell surface blebbing (Fig. 1b) and chromatin condensation e/o fragmentation (Fig. 1c) to evaluate bystander toxic effects, and emission of filopodia (Fig. 2a) and substrate adhesion (Fig. 2b) for evaluating macrophagic differentiation induction.
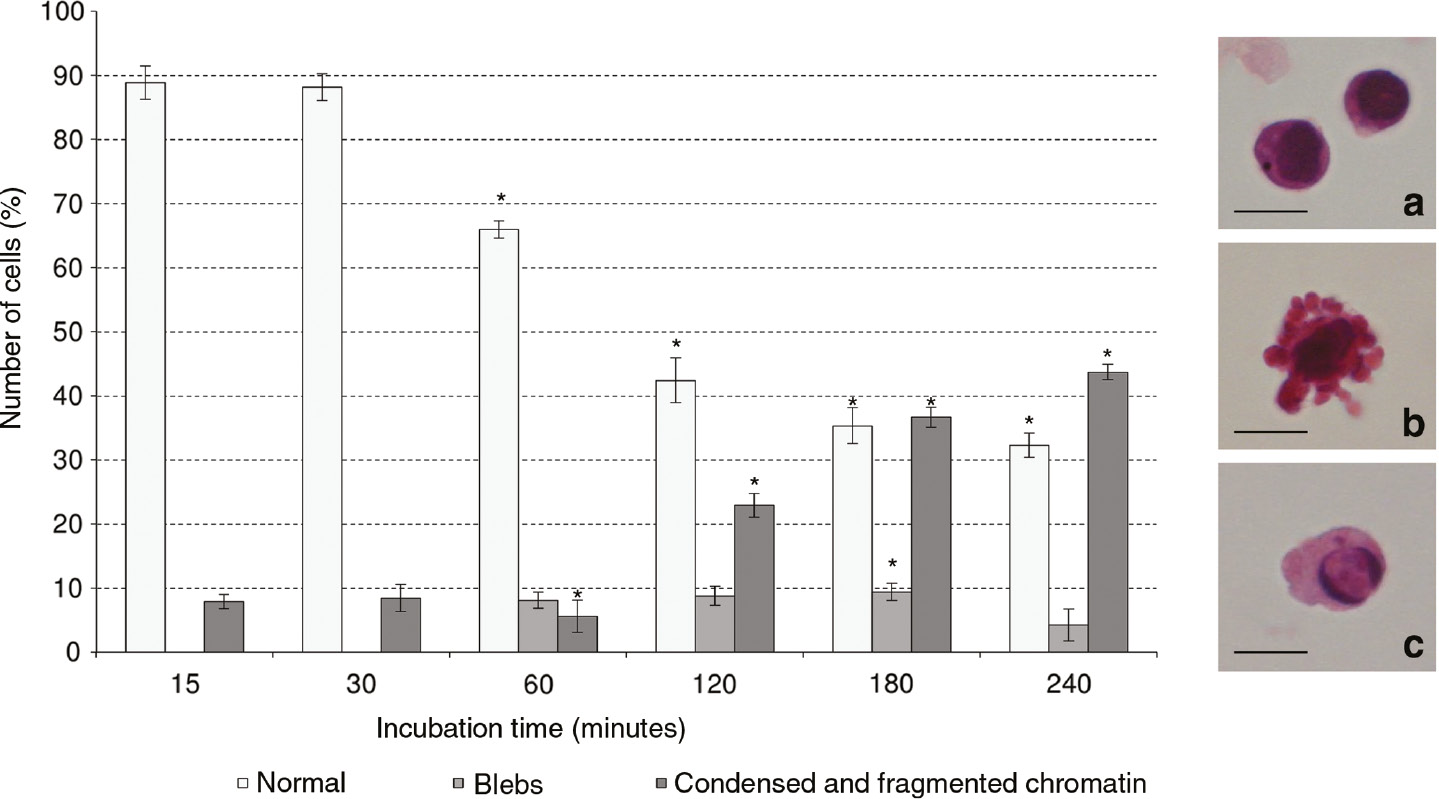
Effect of 1 h CM derived by apoptotic U937 cells on morphology of THP-1 cells (bystander cells) cultured for different times (15, 30, 60, 120, 180 and 240 min). The morphological changes of bystander cells were observed by using the light microscope after haematoxylin/eosin staining. At least 500 cells were scored for each time point. The values are the mean±S.D. of three independent experiments. The asterisks indicate significant values (p<0.05) vs. culture in RPMI medium ones at the same time point. (a) Normal THP-1 cells; (b) THP-1 cells with blebs; (c) THP-1 cells with condensed and/or fragmented chromatin. Bars: 10 μm.
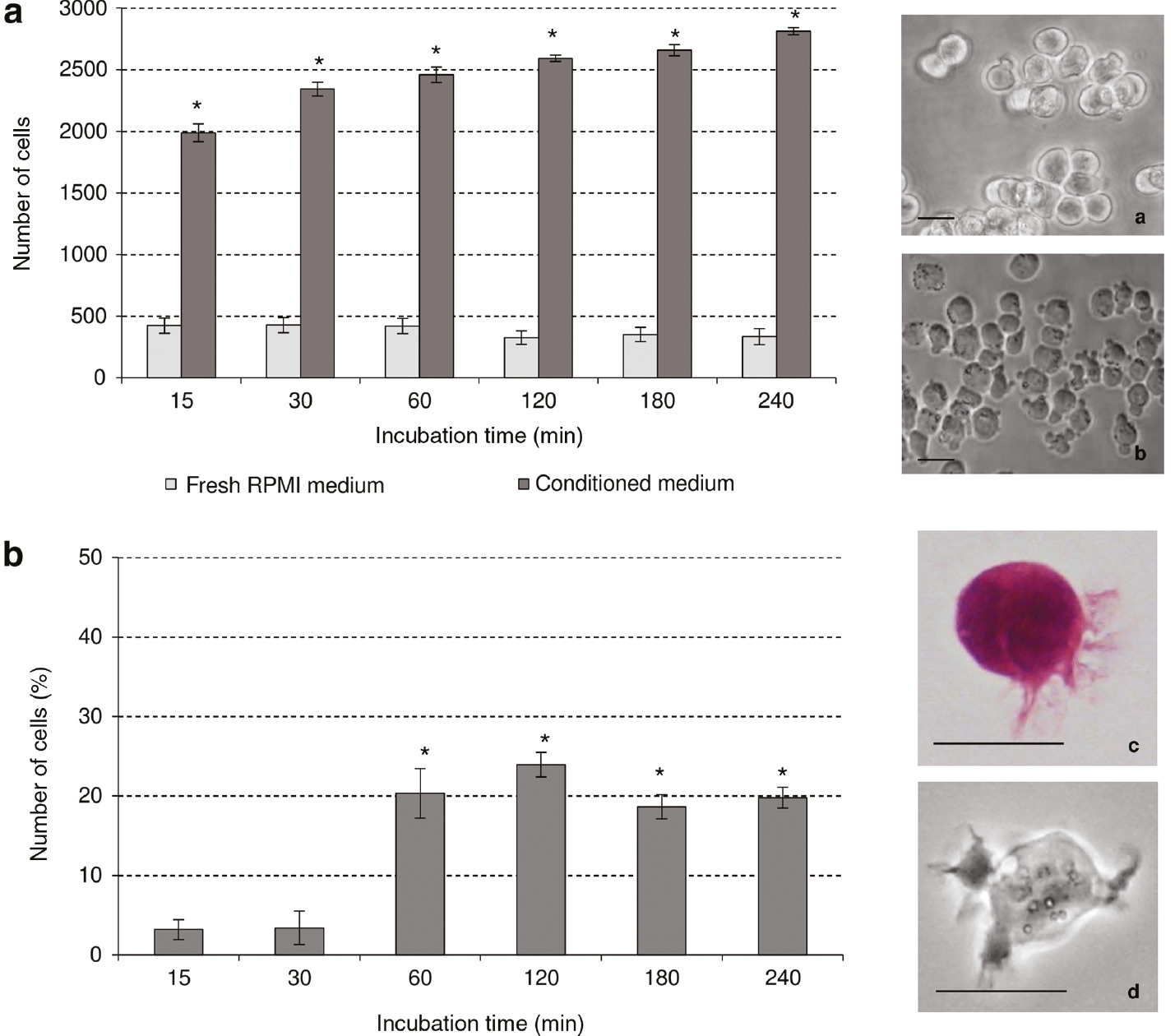
Effect of 1 h CM derived by apoptotic U937 cells on morphological differentiation signs of THP-1 cells (bystander cells) cultured for different times (15, 30, 60, 120, 180 and 240 min). The adhesion to substrate was observed by phase contrast microscope (b) and the attached cells in an area 10 cm×10 cm were counted for each time point. The emission of filopodia in bystander cells was observed by phase contrast microscope (d) and light microscope after haematoxylin/eosin staining (c); at least 500 cells were scored for each time point. The values are the mean±S.D. of three independent experiments. The asterisks indicate significant values (p<0.05) vs. culture in RPMI 1640 medium ones at the same time point. (a) Floating THP-1 cells; (b) attached to substrate THP-1 cells; (c), (d) THP-1 cells with filopodia. Bars: 10 μm.
To eliminate the possibility that residual chemicals might be responsible for the effect observed in the bystander cells, we washed the chemical-exposed cells three times in fresh media. Media from the final wash were used to culture unexposed cells. No appearance of morphological signs of toxicity was observed (data not shown). Thus, the morphological alterations observed in the THP-1 cells in conditioned medium were solely due to the factors released by exposed cells.
The culture in 1 h CM caused a high percentage of cells with morphological modification, of about 50% of cells show condensed and/or fragmented chromatin and blebs on cell surface at the end of treatment considered, i.e. 240 min.
In particular, cells showed a time-responsive increase in condensed and/or fragmented chromatin population, yielding approximately a 9 fold increase compared to THP-1 cells cultured in RPMI 1640 fresh medium at 240 min of culture. The appearance of blebs on plasma membrane (10% of cell population for all times of treatment) starts at 60 min of incubation in 1 h CM (Fig. 1).
Another endpoint of the bystander effect considered was morphological signs of differentiation, i.e. emission of filopodia and adhesion to substrate. The major differentiation sign in THP-1 cells cultured in 1 h CM was the adhesion to substrate. In fact, the number of THP-1 cells attached to the substrate increased after 240 min of about 9 fold respect the THP-1 cells cultured in RPMI 1640 fresh medium (Fig. 2a). Emission of filopodia on cell surface in 1 h CM-treated cells showed a 5 fold increase (of about 25% of cell population at 120 min) compared with RPMI 1640 fresh medium (Fig. 2b).
Experiments in vivo
Inflammation due to the mechanical stress is resolved within 3 days post-inoculation (PI) in the rat calf muscles inoculated with pyrogen-free water, considered as the negative control. The number of CD68 positive macrophages infiltrating the site of inoculum with viable U937 cells or RPMI 1640 culture medium is low during 15 days PI and it is not significantly different from the negative control.
A significant increase (3-fold respect to viable U937 cells and RPMI culture medium) of CD68 positive cells was observed in muscles inoculated with CHX-treated U937 cells. The number of CD68 positive cells was high at 3 days PI and progressively decreased during 15 days of observation. The highest number (5-fold respect to viable U937 cells and RPMI 1640 culture medium) of infiltrating cells is found after 3 days PI in the muscles inoculated with conditioned medium. The amount of CD68 positive cells is always high (3.5 fold respect control) until 15 days PI in the muscles inoculated with 4 h CM (Table 2).
Number of cells recruited by dying U937 cells and their Conditioned Medium (CM) per mm2 of calf muscles.
| Type of inoculum into calf rat muscles | CD 68 |
TGFβ RII |
IL-1 RI |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Post-inoculation days |
Post-inoculation days |
Post-inoculation days |
|||||||
| 3 | 7 | 15 | 3 | 7 | 15 | 3 | 7 | 15 | |
| Pyrogen free water | 82.5±9.1 | 79.6±7.9 | 81.1±8.2 | 113.5±19 | 109.4±21.2 | 115.4±17 | 95.6±7.8 | 89.8±9.2 | 91.3±8.7 |
| RPMI 1640 | 93.7±10.6 | 89.6±15.9 | 79.4±13.2 | 121.1±19.12 | 126.4±16.1 | 116.4±23 | 112.4±6.9 | 109.3±7.2 | 102.9±8.4 |
| Viable cells | 85.2±8.2 | 80.3±8.5 | 81.5±3.2 | 122.5±12.3 | 125.1±21.1 | 122.3±11.5 | 119.7±5.9 | 115.6±8.2 | 113.7±5.4 |
| CHX-treated cells | 283.8±5.3a | 181.6±17.2a | 93.2±18.5a | 205.3±20.7a | 215.6±11.4a | 213.5±9.5a | 340.6±15.1a | 231.2±11.2a | 135.7±9.2a |
| 1 h CM | 305.1±12.6a,b | 190.6±23.5a | 99.9±13.2a | 351.1±21.1a,b | 229.8±12.3a | 149.9±10.9a,b | 229.6±10.2a,b | 120.5±9.6a,b | 103.3±8.3b |
| 4 h CM | 317.7±25.6a | 214.5±17.8a,b | 233.34±21.2a,b | 361.3±23.1a,b | 359.2±21.5a,b | 221.7±21.7a | 352.3±23.8a,b | 118.4±19.2a,b | 119.2±21.2a |
-
The average number of cells recruited in Wistar rat calf muscles inoculated with dead U937 cells was measured after immunohistochemical analysis of cells expressing the CD68 antigen, type II of TGFβ and type I IL-1 receptor. Values are the average±SD of two independent experiments.
-
aValues significantly different (p<0.05) with respect to RPMI 1640 and viable U937 cells.
-
bValues significantly different (p<0.05) with respect to CHX-treated cells.
-
Viable cells: muscles inoculated with untreated U937 cells; RPMI 1640: muscles inoculated with RPMI 1640 (10% FCS); CHX-treated cells: muscles inoculated with U937 cells treated 18 h with CHX 10−2 M; 1 h CM: muscles inoculated with conditioned medium of apoptotic U937 cells, 1 h of recovery post CHX treatment; 4 h CM: muscles inoculated with conditioned medium of necrotic U937 cells, 4 h of recovery post CHX treatment.
1 h CM and 4 h CM stimulate the recruitment of fibroblasts and endothelial cells expressing TGFβ RII, as detected by the increased number of TGFβ RII positive cells. This number progressively decreased from 3 to 15 days PI. The number of TGFβ RII positive cells was significantly higher, of about 1.5-fold at 7 and 15 days PI, in muscles inoculated with 4 h CM compared with 1 h CM one (Table 2).
A significant increase (3-fold respect to viable U937 cells and RPMI 1640 culture medium) of IL-1 RI positive cells was observed in muscles inoculated with CHX-treated U937 cells and 4 h CM. The number of positive cells was high at 3 days PI and progressively decreased during 15 days PI (Table 2).
Figure 3 shows CD68, TGFβ RII and IL-1 RI positive cells infiltrated in rats calf muscles.
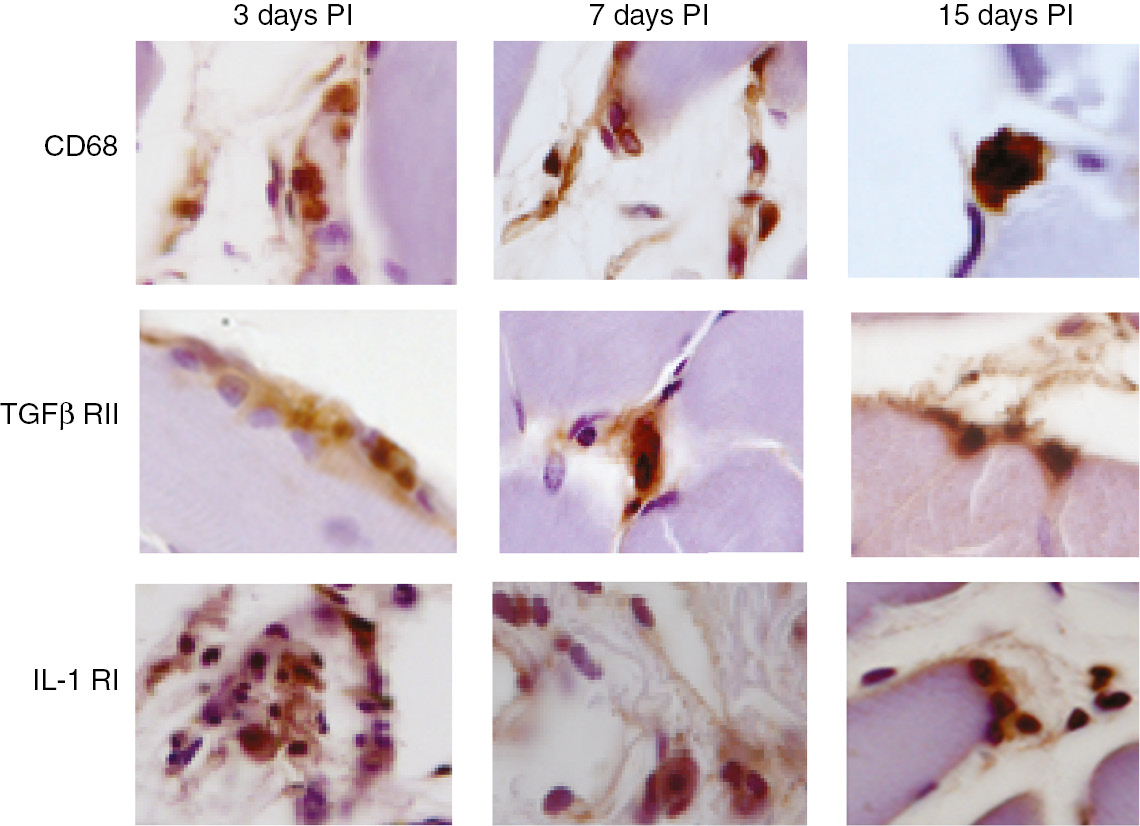
Recruitment of macrophages and activated fibroblasts and endothelial cells in vivo.
Light microscope micrographs of anti-CD68, anti-TGFβ RII and anti-IL-1 RI positive cells infiltrated in rat calf muscles inoculated with U937 cells 4 h conditioned medium.
Metabolomic profiling of conditioned media of U937 cells treated with CHX
We performed NMR-based metabolomic analysis of culture medium corresponding to 1 h of recovery and 4 h of recovery post treatment with CHX 10−2 M for 18 h corresponding to media of apoptotic and necrotic cells, respectively.
Identified metabolites are summarized in Table 3. The metabolic biomarkers obtained from culture media include amino acids (alanine, glutamine, asparagine, lysine, methionine, cysteine, proline, OH-proline, tyrosine, phenylalanine, istidine, choline), organic acids (lactate, citrate, glutamate, pyruvate, succinate, isocitrate, acetate) and energy storage compounds (α-glucose, β-glucose). There are 31 metabolites in cell culture conditioned media. Totally, 14 discrepant metabolites were assigned in conditioned medium corresponding to 1 h of recovery post CHX treatment, including propionate, lactate, alanine, acetate, citrate, glutamate, glutamine, pyruvate, succinate, aspartate, choline, β-glucose, α-glucose, and asparagine. Conversely, in conditioned medium corresponding to 4 h of recovery post CHX treatment there are 20 metabolites different respect to those present in RPMI 1640 medium, including propionate, alanine, acetate, glutamine, pyruvate, aspartate, α-glucose, OH-pyruvate, lysine, methionine, cysteine, proline, OH-proline, tyrosine, phenylalanine, istidine, formiate. Increased levels of lactate, acetate, citrate, glutamate, pyruvate, succinate, choline and asparagine were found in the 1 h CM compared to fresh RPMI 1640. In addition, the levels of propionate, alanine, glutamine, aspartate and glucose, both α and β, were decreased. In 4 h CM increased levels of acetate, pyruvate, OH-pyruvate, cysteine, proline, OH-proline, tyrosine, phenylalanine, istidine and formiate have been measured; conversely, levels of propionate, alanine, glutamine, aspartate, α-glucose, lysine, methionine decreased.
Quantitative analysis of metabolites derived from 1H NMR from cell conditioned medium.
| Metabolites | RPMI 1640 (g/L) | 1 h CM (g/L) | FC | 4 h CM (g/L) | FC |
|---|---|---|---|---|---|
| Val Leu Ile | 2.3171 | 2.3224 | 2.4431 | ||
| Propionate | 0.3629 | 0.1566 | 0.431 | 0.2497 | 0.688 |
| OH-butyrate | 0.1141 | 0.1311 | 0.1295 | ||
| Lactate | 26.3088 | 31.9022 | 1.213 | 29.0737 | 1.105 |
| Alanine | 1.1258 | 0.9643 | 0.856 | 0.8627 | 0.766 |
| OH-pyruvate | 0.0195 | 0.0393 | 0.2063 | 10.579 | |
| Acetate | 0.3849 | 0.7879 | 2.047 | 0.9323 | 2.422 |
| Lysine | 0.5159 | 0.5263 | 0.4026 | 0.781 | |
| Methyonine | 0.3159 | 0.3290 | 0.1919 | 0.607 | |
| Citrate | 0.6197 | 0.8613 | 1.389 | 0.6209 | |
| Glutamate | 1.3295 | 1.5028 | 1.130 | 1.4174 | 1.066 |
| Glutamine | 5.8725 | 5.3669 | 0.914 | 3.9742 | 0.677 |
| Pyruvate | 1.7074 | 2.2906 | 1.341 | 2.2129 | 1.296 |
| Succinate | 0.1884 | 0.4651 | 2.468 | 0.2132 | 1.132 |
| Monoamine | 0.0493 | 0.0223 | 0 | ||
| Dimethylamine | 0.0476 | 0.0511 | 0.0624 | ||
| Asparagine | 1.2244 | 2.4769 | 2.023 | 0.9839 | 0.803 |
| Aspartate | 1.2263 | 1.0708 | 0.873 | 0.5463 | 0.445 |
| Creatine | 0.1589 | 0.1697 | 0.1562 | ||
| Creatinine | 0.0108 | 0.0183 | 0.0176 | ||
| Cysteine | 0.3477 | 0.3722 | 0.4222 | 1.214 | |
| Choline | 0.1520 | 0.2137 | 1.406 | 0.1994 | 1.312 |
| Proline | 0.4437 | 0.4217 | 1.5030 | 3.387 | |
| OH-proline | 0 | 0.0964 | 0.4212 | ||
| β-Galactose | 0.2209 | 0.2040 | 0.2299 | ||
| β-Glucose | 42.1076 | 36.2086 | 0.859 | 41.7751 | |
| α-Glucose | 12.4388 | 10.3428 | 0.831 | 9.9521 | 0.800 |
| Tyrosine | 0.1076 | 0.0900 | 0.1442 | 1.340 | |
| Phenylalanine | 0 | 0.0438 | 0.1598 | ||
| Istidine | 0.0539 | 0.0989 | 0.1652 | 3.065 | |
| Formiate | 0.1685 | 0.1629 | 0.2933 | 1.741 |
-
1 h CM: conditioned medium corresponding to 1 h recovery of THP-1 cells after 18 h CHX 10−2 M treatment.
-
4 h CM: conditioned medium corresponding to 4 h recovery of THP-1 cells after 18 h CHX 10−2 M treatment.
-
FC: fold change, FC>1 indicate that the amount of metabolites is higher in CM group than RPMI group, and vice versa.
Discussion and conclusions
The major findings of this study are: (1) CHX-treated culture media (CM) of human pro-monocytes U937 cells induce BE in human monocytes THP-1 cells; (2) morphological evaluation of THP-1 cells cultured in CM shows signs of toxicity, that suggests an induction of cell death, probably apoptosis as THP-1 cells undergo blebbing of cell surface and chromatin condensation and fragmentation, and macrophagic differentiation, as THP-1 cells emit filopodia on cell surface and adhere to substrate; (3) CM recruit in in vivo model macrophages, fibroblasts and immune cells; (4) the CM display a changed metabolomic profile.
Most of the research on the BE has focused on ionizing radiation and only very few studies have evaluated induction of chemical-induced effect (as reviewed in [42]). The present study indicates that medium conditioned for 1 h by cells induced to apoptosis with CHX, a eukaryote protein synthesis inhibitor, caused a high percentage (of about 80%) of cells with morphological modification at the end of treatment considered, i.e. 240 min.
In particular, cells showed morphological signs of cytotoxicity: condensed and/or fragmented chromatin population, yielding approximately a 9 fold increase compared to viable THP-1 cells, and appearance of blebs on plasma membrane (10% of cell population). This effect caused by chemical-induced BE has been demonstrated in cells treated with DNA-damaging agents: normal human B lymphoblastoid cells induced to apoptosis with mitomycin C and phleomycin [25]; Chinese hamster fibroblast V79-C3 treated with actinomycin D [28], [29].
Surprisingly, in addition to toxic effects the culture medium of apoptotic U937 cells is able to induce differentiation of monocytes THP-1 cells in macrophages. In fact, of about 25% of THP-1 cells emit filopodia on cell surface and consequently the cells change the culture modality (the number of THP-1 cells attached to the substrate increased about 9 fold respect the THP-1 cells cultured in RPMI 1640 fresh medium). A similar bystander-based communication has been demonstrated in mouse bone marrow derived macrophages cultured in the presence of N2O2 tumor spheroids [43] and in J774.2 macrophages cultured in the presence NIH3T3 fibroblasts [44].
To better understand the role of soluble factors present in the conditioned media in bystander communication, we performed an in vivo evaluation of BE by using a rat Wistar model. We have inoculated the calf muscles with conditioned media (both CM 1 h and CM 4 h) and then we have performed an immunohistochemical evaluation to detect CD68 positive cells (macrophages) and cells displaying TGF-β (macrophages and fibroblasts) and IL-1 (fibroblasts and inflammatory cells) receptors in the muscle tissue sections. Our analysis suggested that a massive recruitment of cells is induced in the sites of the inoculum, confirming the observations of the macrophagic differentiation induction in vitro.
Even if it has been demonstrated a BE caused by chemical-treated cells, the molecular mechanism of this effect has not been elucidated by metabolomics studies. Cell metabolomics can provide relevant information regarding the endogenous cellular metabolites for new drug discovery and cell culture metabolomics has demonstrated significant advantages in cancer research [44]. These considerations inspired us to research the alterations in the metabolic profile of culture media of cells treated with CHX by using a NMR-based cell metabolomic approach. There are 31 metabolites in cell culture conditioned media. In particular, 14 and 20 discrepant metabolites were assigned in conditioned medium corresponding to 1 h of recovery post CHX treatment and 4 h of recovery respectively. We consider that the increase of amount of molecules in CM corresponds to exit or secretion by cells; conversely, the molecules amount decrease corresponds to entry into the cells.
Based on the discovery of the relevant molecules found in culture media, we have elaborated the mechanisms in which the molecules are involved into the cells; so, they could represent key regulators of the BE observed (Fig. 4).
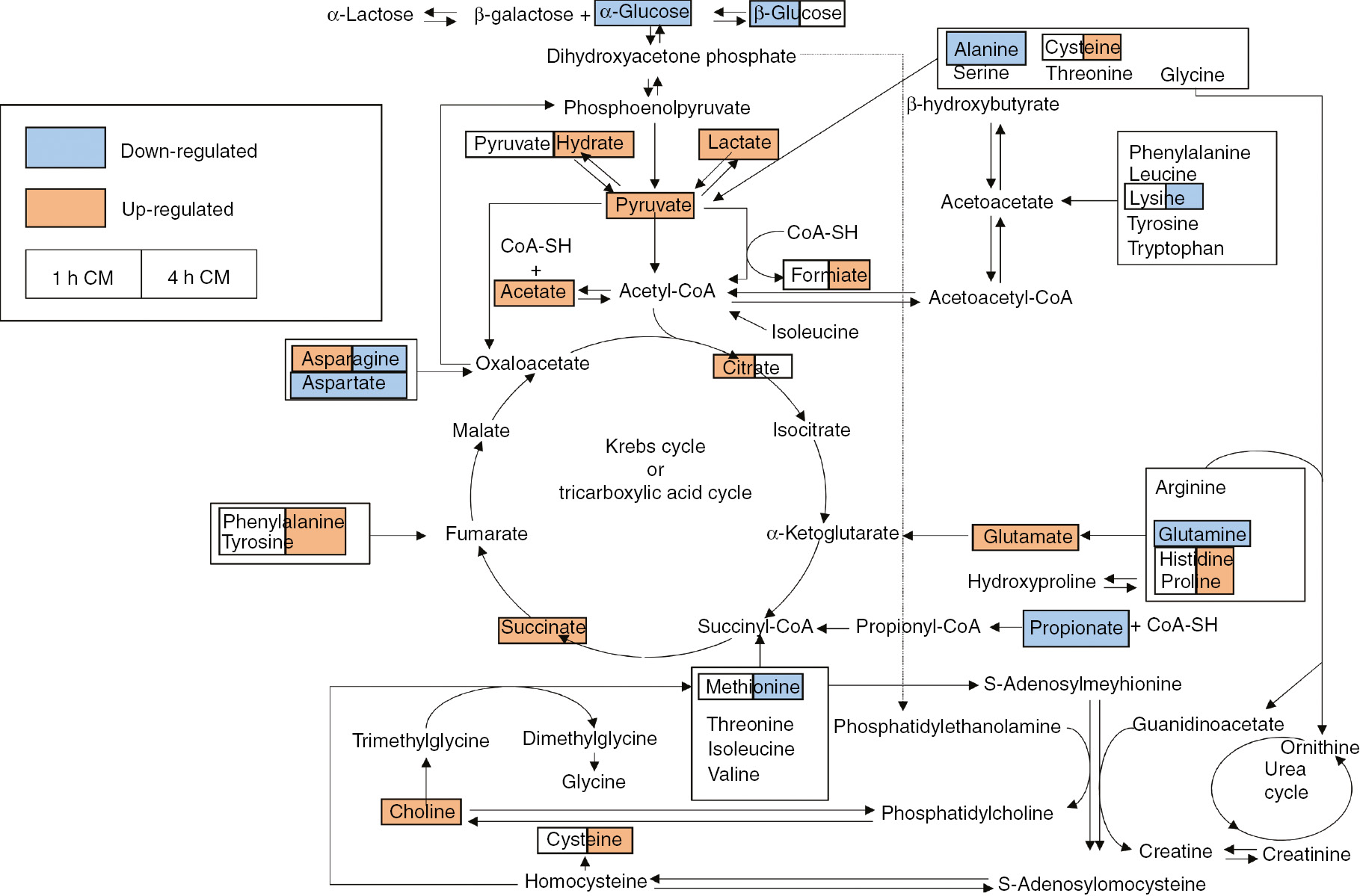
Schematic diagram of the perturbed metabolic pathways that were detected by the NMR analysis, showing the interrelationship of the identified metabolic pathways. The colour on the left side and right side of the box indicate that the metabolites were found in the 1 h CM and 4 h CM, respectively. The orange metabolites were upregulated in the CM groups compared to the RPMI 1640 fresh culture group, while the blue metabolites were downregulated in the CM groups compared to the RPMI 1640 fresh culture group.
In total, there are 11 metabolic pathways in this study, including alanine, aspartate and glutamate metabolism; arginine, histidine and proline metabolism; the tricarboxylic cycle (TCA cycle); pyruvate metabolism; phenylalanine and tyrosine metabolism; and choline and cysteine metabolism.
Glutamine plays a critical role in cell growth and proliferation. In recent years, glutamine has come to the attention of researchers due to its increase and fast consumption in most cancers when compared to normal tissues. Glutamine restriction has been proved to be effective in suppressing cancer cell growth while glutamine supplementation can induce or inhibit cell death according to the cell type [45]. In any case, extracellular glutamine level affects the susceptibility of cancer cells to apoptosis. For example, glutamine deprivation has been reported to sensitize Hela cells to apoptosis [46] and in human lung fibroblasts IMR-90 [47]. Glutamate, an inessential amino acid with a significant role in mammalian cells and especially in cancer cells, has diverse biological functions, such as cell growth, protein synthesis and translation. It is involved in asparagine-proline metabolism and alanine, aspartate and glutamate metabolism. Aspartate and glutamine, acting as precursors, were converted to asparagine and glutamate under the catalysis of asparagine or glutamate synthetases, respectively. In addition, glutamine participates to the TCA cycle through the synthesis of 2-oxoglutarate. The increase of TCA cycle precursors, such as succinate and citrate, and pyruvate could provide assertive evidence for abnormal TCA cycle activity induction in THP-1 cells that undergo an altered energetic metabolism that, in turn, induces apoptotic death.
In the process of glycolysis, pyruvate is a crucial intermediate product and could be metabolized to acetyl-CoA, alanine, acetate or lactate [48]. Pyruvate could be transformed into acetyl-CoA and enters the TCA cycle to provide energy to the cells. Additionally, the decreased levels of acetate and alanine in culture media indicates that the decomposition of pyruvate is disturbed in the mitochondria, suggesting an apoptotic process that can be induced into THP-1 cells. Lactate could be also involved in THP-1 macrophage differentiation as reported in the study of Wu and coworkers [49] reporting that umbilical cord-derived MSC (UC-MSC) alter the phenotype and function of monocyte-derived dendritic cells (DC) through lactate-mediated metabolic reprogramming. UC-MSC can secrete large quantities of lactate and, when present during monocyte-to-DC differentiation, induce the acquisition of the M2-macrophage phenotype in terms of morphology, surface markers, migratory properties and antigen presentation capacity. Finally, the increase in culture medium of tyrosine, phenylalanine, proline and histidine could be involved in differentiation of monocytes to macrophages as reported also in Yoon et al. [50].
In conclusion, the metabolomic analysis showed that CHX causes variations in the metabolites content in cell culture medium of U937 cells. These metabolites induce toxicity and cell differentiation of THP-1 cells, suggesting that CHX is able to trigger BE that seem to be very potential in a cancer therapy. Moreover, the understanding how different metabolites are linked to cell metabolism provides new approach for the treatment of cancer.
Article note
A collection of invited papers based on presentations at the 15th Eurasia Conference on Chemical Sciences (EuAsC2S-15) held at Sapienza University of Rome, Italy, 5–8 September 2018.
References
[1] H. Nagasawa, J. B. Little. Cancer Res.52, 6394 (1992).Search in Google Scholar
[2] C. Mothersill, C. Seymour. Int. J. Radiat. Biol.71, 421 (1997).10.1093/mutage/13.5.421Search in Google Scholar PubMed
[3] S. G. Sawant, W. Zheng, K. M. Hopkins, G. Randers-Pehrson, H. B. Lieberman, E. J. Hall. Radiat. Res.157, 361 (2002).10.1667/0033-7587(2002)157[0361:TRIBEF]2.0.CO;2Search in Google Scholar
[4] F. M. Lyng, C. B. Seymour, C. Mothersill. Br. J. Cancer83, 1223 (2000).10.1054/bjoc.2000.1433Search in Google Scholar
[5] O. V. Belyakov, A. M. Malcolmson, M. Folkard, K. M. Prise, B. D. Michael. Br. J. Cancer84, 674 (2001).10.1054/bjoc.2000.1665Search in Google Scholar
[6] O. V. Belyakov, M. Folkard, C. Mothersill, K. M. Prise, B. D. Michael. Radiat. Prot. Dosim.99, 249 (2002).10.1093/oxfordjournals.rpd.a006775Search in Google Scholar
[7] C. Mothersill, R. J. Seymour, C. B. Seymour. Radiat. Res.165, 26 (2006).10.1667/RR3488.1Search in Google Scholar
[8] S. M. Nugent, C. E. Mothersill, C. Seymour, B. McClean, F. M. Lyng, J. E. Murphy. Radiat. Res.168, 134 (2007).10.1667/RR0769.1Search in Google Scholar
[9] K. M. Prise, O. V. Belyakov, M. Folkard, B. D. Michael. Int. J. Radiat. Biol.74, 793 (1998).Search in Google Scholar
[10] A. Deshpande, E. H. Goodwin, S. M. Bailey, B. L. Marrone, B. E. Lehnert. Radiat. Res.145, 260 (1996).10.2307/3578980Search in Google Scholar
[11] S. A. Lorimore, M. A. Kadhim, D. A. Pocock, D. Papworth, D. L. Stevens, D. T. Goodhead, E. G. Wright. Proc. Natl. Acad. Sci. U.S.A.95, 5730 (1998).10.1073/pnas.95.10.5730Search in Google Scholar
[12] M. V. Sokolov, L. B. Smilenov, E. J. Hall, I. G. Panyutin, W. M. Bonner, O. A. Sedelnikova. Oncogene24, 7257 (2005).10.1038/sj.onc.1208886Search in Google Scholar
[13] B. Hu, L. Wu, W. Han, L. Zhang, S. Chen, A. Xu, T. K. Hei, Z. Yu. Carcinogenesis27, 245 (2006).10.1093/carcin/bgi224Search in Google Scholar PubMed
[14] O. A. Sedelnikova, A. Nakamura, O. Kovalchuk, I. Koturbash, S. A. Mitchell, S. A. Marino, D. J. Brenner, W. M. Bonner. Cancer Res.67, 4295 (2007).10.1158/0008-5472.CAN-06-4442Search in Google Scholar PubMed
[15] E. I. Azzam, S. M. de Toledo, J. B. Little. Proc. Natl. Acad. Sci. U.S.A.98, 473 (2001).10.1073/pnas.98.2.473Search in Google Scholar PubMed PubMed Central
[16] B. E. Lehnert, E. H. Goodwin, A. Deshpande. Cancer Res.57, 2164 (1997).Search in Google Scholar
[17] M. Grifalconi, L. Celotti, M. Mognato. Mutat. Res.625, 102 (2007).10.1016/j.mrfmmm.2007.06.004Search in Google Scholar PubMed
[18] H. Yang, N. Asaad, K. D. Held. Oncogene24, 2096 (2005).10.1038/sj.onc.1208439Search in Google Scholar PubMed
[19] A. K. Mitra, M. Krishna. J. Cell. Biochem.100, 991 (2007).10.1002/jcb.21084Search in Google Scholar PubMed
[20] C. Mothersill, C. Seymour. Radiat. Res.155, 759 (2001).10.1667/0033-7587(2001)155[0759:RIBEPH]2.0.CO;2Search in Google Scholar
[21] C. Mothersill, C. B. Seymour. Nat. Rev. Cancer4, 158 (2004).10.1038/nrc1277Search in Google Scholar
[22] E. I. Azzam, J. B. Little. Hum. Exp. Toxicol.23, 61 (2004).10.1191/0960327104ht418oaSearch in Google Scholar
[23] R. E. Rugo, K. H. Almeida, C. A. Hendricks, V. S. Jonnalagadda, B. P. Engelward. Oncogene24, 5016 (2005).10.1038/sj.onc.1208690Search in Google Scholar
[24] A. Demidem, D. Morvan, J. C. Madelmont. Int. J. Cancer119, 992 (2006).10.1002/ijc.21761Search in Google Scholar PubMed
[25] R. S. Asur, R. A. Thomas, J. D. Tucker. Mutat. Res.676, 11 (2009).10.1016/j.mrgentox.2009.02.012Search in Google Scholar PubMed
[26] R. Asur, M. Balasubramaniam, B. Marples, R. A. Thomas, J. D. Tucker. Mutagenesis25, 271 (2010).10.1093/mutage/geq003Search in Google Scholar PubMed
[27] R. Asur, M. Balasubramaniam, B. Marples, R. A. Thomas, J. D. Tucker. Mutat. Res.686, 15 (2010).10.1016/j.mrfmmm.2009.12.007Search in Google Scholar PubMed
[28] C. Jin, S. Wu, X. Lu, Q. Liu, M. Qi, S. Lu, Q. Xi, Y. Cai. Toxicol. Lett.202, 178 (2011).10.1016/j.toxlet.2011.02.002Search in Google Scholar PubMed
[29] C. Jin, S. Wu, X. Lu, Q. Liu, L. Zhang, J. Yang, Q. Xi, Y. Cai. Toxicol. Lett.211, 45 (2012).10.1016/j.toxlet.2012.02.020Search in Google Scholar PubMed
[30] J. Dahle, E. Angell-Petersen, H. B. Steen, J. Moan. Photochem. Photobiol.73, 378 (2001).10.1562/0031-8655(2001)073<0378:BEICDI>2.0.CO;2Search in Google Scholar
[31] A. Dabrowska, M. Goś, P. Janik. Med. Sci. Monit.11, 316 (2005).Search in Google Scholar
[32] B. Calì, S. Ceolin, F. Ceriani, M. Bortolozzi, A. H. Agnellini, V. Zorzi, A. Predonzani, V. Bronte, B. Molon, F. Mammano. Oncotarget6, 10161 (2015).10.18632/oncotarget.3553Search in Google Scholar
[33] E. I. Azzam, S. M. De Toledo, D. R. Spitz, J. B. Little. Cancer Res.62, 5436 (2002).Search in Google Scholar
[34] C. Shao, Y. Furusawa, M. Aoki, K. Ando. Radiat. Res.160, 318 (2003).10.1667/RR3044Search in Google Scholar
[35] H. Matsumoto, S. Hayashi, M. Hatashita, K. Ohnishi, H. Shioura, T. Ohtsubo, R. Kitai, T. Ohnishi, E. Kano. Radiat. Res.155, 387 (2001).10.1667/0033-7587(2001)155[0387:IORBAN]2.0.CO;2Search in Google Scholar
[36] C. Shao, V. Stewart, M. Folkard, B. D. Michael, K. M. Prise. Cancer Res.63, 8437 (2003).Search in Google Scholar
[37] S. Burdak-Rothkamm, S. C. Short, M. Folkard, K. Rothkamm, K. M. Prise. Oncogene26, 993 (2007).10.1038/sj.onc.1209863Search in Google Scholar
[38] B. Djordjevic. Bioessays22, 286 (2000).10.1002/(SICI)1521-1878(200003)22:3<286::AID-BIES10>3.0.CO;2-SSearch in Google Scholar
[39] R. R. Chhipa, M. K. Bhat. J. Cell. Biochem.101, 68 (2007).10.1002/jcb.21153Search in Google Scholar
[40] I. Rios-Mondragon, X. Wang, H. H. Gerdes. Biomicrofluidics6, 24128 (2012).10.1063/1.4726349Search in Google Scholar
[41] S. I. Grivennikov, F. R. Greten, M. Karin. Cell140, 883 (2010).10.1016/j.cell.2010.01.025Search in Google Scholar
[42] N. Verma, A. B. Tiku. Mutat. Res.773, 104 (2017).10.1016/j.mrrev.2017.05.003Search in Google Scholar
[43] Y. Huang, C. Lee, P. Borgström, R. A. Gjerset. Mol. Ther.15, 524 (2007).10.1038/sj.mt.6300080Search in Google Scholar
[44] A. Zhang, H. Sun, H. Xu, S. Qiu, X. Wang. OMICS17, 495 (2013).10.1089/omi.2012.0090Search in Google Scholar
[45] B. C. Fuchs, B. P. Bode. J. Surg. Res.131, 26 (2006).10.1016/j.jss.2005.07.013Search in Google Scholar
[46] Y. G. Ko, E. Y. Kim, T. Kim, H. Park, H. S. Park, E. J. Choi, S. Kim. J. Biol. Chem.276, 6030 (2001).10.1074/jbc.M006189200Search in Google Scholar
[47] M. Yuneva, N. Zamboni, P. Oefner, R. Sachidanandam, Y. Lazebnik. J. Cell Biol.178, 93 (2007).10.1083/jcb.200703099Search in Google Scholar PubMed PubMed Central
[48] Y. Li, S. Man, J. Li, H. Chai, W. Fan, Z. Liu, W. Gao. Chem. Biol. Interact.220, 193 (2014).10.1016/j.cbi.2014.06.023Search in Google Scholar PubMed
[49] W. Wu, X. Zhang, X. Hu, X. Wang, L. Sun, X. Zheng, L. Jiang, X. Ni, C. Xu, N. Tian, S. Zhu, H. Xu. J. Orthop. Res.32, 253 (2014).10.1002/jor.22503Search in Google Scholar PubMed
[50] B. R. Yoon, Y. J. Oh, S. W. Kang, E. B. Lee, W. W. Lee. Front. Immunol.9, 53 (2018).10.3389/fimmu.2018.00053Search in Google Scholar PubMed PubMed Central
© 2020 IUPAC & De Gruyter, Berlin/Boston
Articles in the same Issue
- Frontmatter
- In this issue
- Conference papers
- Proficiency testing as a tool to assess quality of data: the experience of the EU Reference Laboratory for chemical elements in food of animal origin
- Chloroform desorption from poly(lactic acid) nanocomposites: a thermal desorption spectroscopy study
- The dialogue between died and viable cells: in vitro and in vivo bystander effects and 1H-NMR-based metabolic profiling of soluble factors
- Magnetic Pt single and double core-shell structures for the catalytic selective hydrogenation of cinnmaladehyde
- Application of calcium carbonate nanocarriers for controlled release of phytodrugs against Xylella fastidiosa pathogen
- Stimuli responsive microgel containing silver nanoparticles with tunable optical and catalytic properties
- Curcumin-loaded zeolite as anticancer drug carrier: effect of curcumin adsorption on zeolite structure
- Kinetics and thermodynamics of the hydroxylation products in the photodegradation of the herbicide Metolachlor
- Electronic cigarettes – an important progress or just another risk for health?
- A surprising mechanism lacking the Ni(0) state during the Ni(II)-catalyzed P–C cross-coupling reaction performed in the absence of a reducing agent – An experimental and a theoretical study
- Ion exchange of H+/Na+ in polyantimonic acid, doped with vanadium ions
- Spin-orbital exclusion principle and the periodic system
- IUPAC Technical Report
- Brief guide to the nomenclature of organic chemistry (IUPAC Technical Report)
Articles in the same Issue
- Frontmatter
- In this issue
- Conference papers
- Proficiency testing as a tool to assess quality of data: the experience of the EU Reference Laboratory for chemical elements in food of animal origin
- Chloroform desorption from poly(lactic acid) nanocomposites: a thermal desorption spectroscopy study
- The dialogue between died and viable cells: in vitro and in vivo bystander effects and 1H-NMR-based metabolic profiling of soluble factors
- Magnetic Pt single and double core-shell structures for the catalytic selective hydrogenation of cinnmaladehyde
- Application of calcium carbonate nanocarriers for controlled release of phytodrugs against Xylella fastidiosa pathogen
- Stimuli responsive microgel containing silver nanoparticles with tunable optical and catalytic properties
- Curcumin-loaded zeolite as anticancer drug carrier: effect of curcumin adsorption on zeolite structure
- Kinetics and thermodynamics of the hydroxylation products in the photodegradation of the herbicide Metolachlor
- Electronic cigarettes – an important progress or just another risk for health?
- A surprising mechanism lacking the Ni(0) state during the Ni(II)-catalyzed P–C cross-coupling reaction performed in the absence of a reducing agent – An experimental and a theoretical study
- Ion exchange of H+/Na+ in polyantimonic acid, doped with vanadium ions
- Spin-orbital exclusion principle and the periodic system
- IUPAC Technical Report
- Brief guide to the nomenclature of organic chemistry (IUPAC Technical Report)

