Abstract
Chitin (β-(1-4)-poly-N-acetyl-D-glucosamine) is widely distributed in nature. A method for the preparation of chitin nanofibers (CNFs) is reported. CNFs are considered to have several potential applications because they have useful properties such as high specific surface area and porosity. More recently, beneficial effects of CNF as functional foods were reported. First, the anti-inflammatory effect of oral administration of chitin CNFs was demonstrated in a mouse model of inflammatory bowel disease (IBD). It was found that CNFs improved clinical symptoms and suppressed IBD. CNFs decreased the areas with nuclear factor-κB (NF-κB) staining in colon tissue. Second, the anti-obesity effects of surface-deacetylated chitin nanofibers (SDACNF) in a mouse model of high-fat diet-induced obesity was evaluated. SDACNFs suppressed the increase in body weight produced by the high-fat diet; however, CNFs did not suppress such weight gain. SDACNFs decreased serum levels of leptin. These results suggest that CNF and SDACNF are promising functional foods for patients with IBD or obesity.
Introduction
Chitin (β-(1-4)-poly-N-acetyl-D-glucosamine) is the second most abundant polysaccharide after cellulose [1]. As chitin is not readily dissolved in common solvents, it is often converted to its deacetylated derivative, chitosan [CS, (1,4)-linked 2-amino-deoxy-β-glucan; Fig. 1] [2], [3], [4].
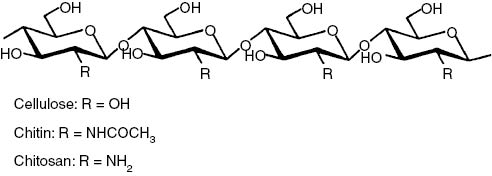
Chemical structure of linear polysaccharides: cellulose, chitin, and chitosan.
Recently, several methods for the preparation of chitin or CS nanofiber have been reported, including acid hydrolysis [5], [6], ultrasonication under acidic conditions [7], electrospinning [8], [9] and grinding [10], [11], [12]. Other approaches are also reported such as precipitation, regeneration, and gas bubbling. Precipitation, regeneration, and gas bubbling [13]. These nanofibers are considered to have great potential for various applications because they have several useful properties such as high specific surface area and high porosity [13]. Nanofibers containing chitin or CS are expected to be applied in areas such as tissue engineering, drug delivery, dental materials, wound healing, cosmetics, and medical implants [14], [15], [16], [17].
In this review, first, the applications of chitin and its derivatives for food industry are summarized. Next, the preparations of chitin nanofibers are described. Last, the applications of chitin nanofibers for functional foods are summarized.
Chitin and its derivatives for food industry
Chitin, chitosan, its monomers [N-acetyl-D-glucosamine (GlcNAc) and glucosamine (GlcN)] and oligomers [chitin oligosaccharide (NACOS) and chitosan oligosaccharide (COS)] are used as functional foods [18], [19], [20], [21].
GlcN is used as a supplement for osteoarthritis patients [18], [19]. Oral administration of GlcN repressed synovial cell hyperplasia, cartilage destruction, and inflammatory cell infiltration in rat adjuvant arthritis [20]. In addition, oral administration of GlcN improved the clinical symptoms, colonic inflammation, and tissue injury in dextran sulfate sodium (DSS)-induced colitis in rats [21]. We reported anti-inflammatory effects by oral administration of GlcNAc in an experimental model of rheumatoid arthritis [22]. We have also described that the anti-inflammatory mechanisms of the two monomers are different. In the GlcN group, serum tumor necrosis factor (TNF)-α and interleukin (IL)-6 concentrations were significantly decreased compared to the control group. In the GlcNAc group, serum IL-10, transforming growth factor β-1, and IL-2 concentrations were significantly increased compared to the control group.
It is also indicated that oral administrations of NACOS and COS also have many bioactivities [23]. We evaluated the anti-tumor activities of NACOS and COS after their oral administration in a tumor (colon 26)-bearing mouse model. Compared to the control group, NACOS and COS groups showed significantly suppressed tumor growth, and apparent, marked apoptosis in tumor tissues. Furthermore, serum levels of cytokines (IL-12p70 and interferon-γ) were significantly increased in those groups compared to the corresponding levels in the control group. Collectively, the results indicate the oral administration of NACOS and COS could enhance innate immunity. Moreover, it is indicated that the apparent effects were related to both Myd-88-dependent and Myd-88-independent pathways [24]. Kan investigated the therapeutic effect of NACOS, administered through the oral route, in patients with cancer [25]. A substantial regression of the cancer was observed in most patients, especially in those with early stage cancer. We also reported the anti-inflammatory effects of COS in DSS-induced colitis in mice. Oral administration of COS improved shortening of colon length and tissue injury (as assessed by histology) in mice. Oral administration of COS inhibited inflammation in the colonic mucosa by suppression of myeloperoxidase activation in inflammatory cells, as well as activation of nuclear factor-κB, cyclooxygenase-2, and inducible nitric oxide synthase. Oral administration of COS also reduced serum levels of pro-inflammatory cytokines (TNF-α and IL-6). Moreover, it prolonged survival time in mice [26].
CS is bioactive cationic polysaccharide. CS has many bioactivite properties including antibacterial, antifungal, antioxidant, antidiabetic, anti-inflammatory, anticancer, and hypocholesterolemic properties [27]. Anraku et al. reported the effect of water-soluble chitosan on indices of oxidative stress was investigated in human normal volunteers. Treatment with chitosan for 4 weeks produced a significant decrease in levels of plasma glucose, atherogenic index and led to increase in high density lipoprotein cholesterol (HDL) [28]. Chitosan treatment also lowered the ratio of oxidized to reduced albumin and increased total plasma antioxidant activity (TPA). They also reported the effect of high and low molecular weight chitosans (HMC; 1000 kDa, LMC; 30 kDa) on oxidative stress and hypercholesterolemia using male 6-week-old Wistar Kyoto rats as a normal model (Normal-rats) and spontaneously hypertensive rat/ND mcr-cp (SHP/ND) as a metabolic syndrome model (MS-rats), respectively. In Normal-rats, the ingestion of both chitosans over a 4-week period resulted in a significant decrease in total body weight (BW), glucose (Gl), triglyceride (TG), low density lipoprotein (LDL) and serum creatinine (Cre) levels. The ingestion of both chitosans also resulted in a lowered ratio of oxidized to reduced albumin and an increase in total plasma antioxidant activity. In addition to similar results in Normal-rats, the ingestion of only HMC over a 4-week period resulted in a significant decrease in total cholesterol levels in MS-rats. Further, the ingestion of LMC resulted in a significantly higher antioxidant activity than was observed for HMC in both rat models. It is also described that oral intakes of chitosan attenuates isoprenaline-induced oxidative stress in rat myocardium [29] and the anti-aging effect of chitosan intakes on glutathione-dependent antioxidant system in young and aged rats [30]. Anti-diabetic effects of chitin, chitosan and their derivatives have been reviewed recently [31]. Miura et al. reported that chitosan had anti-diabetic and anti-hyperlipidemia effects in neonatal streptozotocin-induced diabetic mice [32]. It was reported that low molecular-weight chitosan lactate also had an anti-diabetic effect in obese diabetic KK-Ay mice [33]. Chitosan decrease liver gluconeogenesis and increase glucose uptake and use in skeletal muscle [34]. Hypocholesterolemic effects of chitosan have been reported in many publications [35]. Recently, it has been demonstrated that the effect of media-milled chitosan on the decrease of serum triacylglycerol, total cholesterol and LDL cholesterol is higher compared to chitosan [35]. It has been also indicated that total cholesterol content in mice fed γ-irradiated chitosan (30–100 kGy: 12 weeks) was significantly lower than that of the control [36].
Preparations of chitin nanofiber
Nanofibers are known as fibers of <100 nm thickness and an aspect ratio of more than 100 [37], [38]. Because of their characteristic morphology, extremely high surface-to-volume ratio, unique optical properties, and high mechanical strength, the development of methods for the preparation of nanofibers became an important subject [39], [40]. Electrospinning is the most widely used technique for the preparation of artificial nanofibers. This technique produces nanofibers from a polymer solution using interactions between fluid dynamics, electrically charged surfaces, and liquids [41]. However, disadvantages of this procedure are its high environmental load and low processing ability. Moreover, the thus obtained non-woven nanofiber sheet has low mechanical strength. There is an increasing interest in nanofibers from biopolymers because of their biodegradability, biocompatibility, renewability, and sustainability. Various nanofibers can be found in nature, e.g. collagen triple helix fibers, fibroin fibrils, keratin fibers, and so on. These nanofibers have mainly a complex hierarchically organized structure. This indicates that bionanofibers are obtained by downsizing of the organized structure [13].
The chitin crystal consists of CNF. These nanofibers are embedded in a protein matrix. Similarly, cellulose in crab shells has also a hierarchical structure consisting of nanofibers (Fig. 2) [42], [43], [44], [45]. Therefore, chitin nanofibers are also obtained through the downsizing approach. However, compared to cellulose, there have been few reports about the preparation of nano-chitin using the downsizing approach. Chitin nano-crystals have been obtained by acid hydrolysis treatment using hydrochloric acid [46]. The hydrolyzed nano-crystals with low aspect ratio are different from the natural chitin fibers that exist in crab shells.
![Fig. 2: Schematic presentation of the exoskeleton structure of crab shells. Reprinted with permission from ref. [11].](/document/doi/10.1515/pac-2016-0504/asset/graphic/j_pac-2016-0504_fig_002.jpg)
Schematic presentation of the exoskeleton structure of crab shells. Reprinted with permission from ref. [11].
Preparations of CNF from crab shells
A simple method for the preparation of chitin nanofibers has been reported [10]. Figure 2 shows the structure of crab shells at various levels. Chitin polymers form a nanofiber with an extended crystalline structure. The nanofiber is covered with proteins. These nanofibers form a bundle of chitin/protein composite fibers with thicker diameters. A planar woven and branched network of bundles of nanofibers formed from the thicker fibers is embedded in minerals, mainly calcium carbonate. This woven network forms a twisted plywood pattern with helicoidal stacking structure.
Crab shell powder was used as the starting material for the preparation of nanofibers. To extract chitin nanofibers from the shells, proteins and minerals were removed using aqueous solutions of KOH and HCl, respectively [6], [46]. Most of these impurities were removed by these alkali and acid treatments. The strong hydrogen bonds that result from the drying process make it difficult to obtain thin and uniform nanofibers. The substance should be kept wet after the removal of proteins and minerals [47]. Figure 3 shows field emission scanning electron microscopic (FE-SEM) images of the crab shell surface after removal of proteins and calcium carbonate. Chitin nanofibers were observed (approximately 10-nm thickness). Thicker chitin-protein fibers with a diameter of approximately 100 nm were also observed and confirmed to be bundles of nanofibers of 10-nm width. The obtained chitin slurry (in neutral water) was passed through a grinder. The width of the fibers derived from crab shells after grinder treatment was distributed over a wide range, i.e. from 10 to 100 nm (Fig. 3a). The twisted plywood structure (Fig. 1) seems disintegrated after application of a one-time grinder treatment. As a result, 100-nm-thick fibers were isolated from chitin-protein fibers. However, thicker fibers were not successfully disintegrated by grinder treatment, even though the protein layers were removed under a never-dried condition.
![Fig. 3: FE-SEM micrographs of the crab shell surface after removal of the matrix.The length of the scale bars are (a) 1000 nm and (b) 100 nm, respectively. Reprinted with permission from ref. [10].](/document/doi/10.1515/pac-2016-0504/asset/graphic/j_pac-2016-0504_fig_003.jpg)
FE-SEM micrographs of the crab shell surface after removal of the matrix.
The length of the scale bars are (a) 1000 nm and (b) 100 nm, respectively. Reprinted with permission from ref. [10].
Fan et al. reported the preparation of chitin nanofibers made from squid pen β-chitin in acidic water [48]. Cationization of the amino groups under acidic conditions is necessary to maintain a stable dispersion state by electrostatic repulsion in water. Therefore, the purified β-chitin from crab shells would also be homogeneously dispersed under acidic conditions by the cationization of the amino groups on the fiber surface, which would facilitate nano-fibrillation. Thus, purified chitin was dispersed in aqueous acetic acid and subjected to grinder treatment. The chitin slurry thus obtained formed a gel after a single grinder treatment, suggesting that nano-fibrillation was accomplished because of the high dispersion property in acidic water and the high surface-to-volume ratio of the nanofiber. The disintegrated chitin was observed as highly uniform nanofibers, suggesting that the fibrillation process was facilitated in acidic water (Fig. 4b and c). Broken fibers were not observed over a wide observation area. The aspect ratios of the nanofibers were very high, indicating that chitin nanofibers were successfully isolated from crab shells while maintaining their original structure. CNF is prepared from prawn shells and mushrooms [49], [50].
![Fig. 4: FE-SEM micrographs of chitin nanofibers from crab shells after one pass through the grinder.(a) Without acetic acid (pH 7), (b) and (c): with acetic acid (pH 3). The length of the scale bar is (a) 200 nm, (b) 200 nm, and (c) 100 nm, respectively. Reprinted with permission from ref. [10].](/document/doi/10.1515/pac-2016-0504/asset/graphic/j_pac-2016-0504_fig_004.jpg)
FE-SEM micrographs of chitin nanofibers from crab shells after one pass through the grinder.
(a) Without acetic acid (pH 7), (b) and (c): with acetic acid (pH 3). The length of the scale bar is (a) 200 nm, (b) 200 nm, and (c) 100 nm, respectively. Reprinted with permission from ref. [10].
Anti-inflammatory effects of chitin nanofiber
Inflammatory bowel disease (IBD) is a common disorder of mucosa inflammation in the intestinal tract. Crohn’s disease and ulcerative colitis (UC) account for the majority of cases of these conditions [51]. Currently, some experimental animal models are used in IBD research. A model of dextran sulfate sodium (DSS)-induced colitis is one of the commonly used models of IBD, in which animals develop acute and chronic colitis resembling UC [52]. Currently, many drugs are used for IBD patients: including 5-aminosalicylic acid drugs such as sulfasalazine or balsalazide, immunomodulators such as thiopurines (azathioprine, 6-mercaptopurine), methotrexate, and biologic therapies that target TNF-α or IL-6 [51], [53]. Biologic therapies are used for induction treatment (to get the disease under control) and long-term maintenance of moderately to severely active forms of the disease that do not respond to conventional treatment. However, immunomodulators and biologic therapies increase the risk of serious infections. An optimal therapy for IBD has not been established yet. Some nutritional supplements have been reported to be beneficial in IBD, including amino acids [54], omega-3 fatty acids [55], dietary fibers [56], and probiotics [57].
We evaluated the anti-inflammatory effects of chitin nanofibrils in a mouse model of experimental IBD by oral administration of chitin nanofibrils [58]. We compared the anti-inflammatory effects of chitin nanofibrils with those of chitin suspensions (chitin-PS) using the clinical score (disease activity index: DAI), colon length, colon weight/length ratio, and histological observations. The results for the effect of chitin nanofibrils on the DAI in mice with DSS-induced acute UC are shown in Table 1. Weight loss, loose stools, and bleeding were observed on day 3 in the control and chitin-PS groups and on day 4 in the chitin nanofibrils group. The chitin nanofibrils group exhibited a significantly reduced DAI on days 4–6 compared with that of the control group and on days 5 and 6 compared with that of the chitin-PS group. In the chitin nanofibrils group, the colon length was significantly increased compared to those in the control and chitin-PS groups on days 3, 5, and 6. The colon weight/length ratios of the chitin nanofibrils and chitin-PS groups were significantly decreased compared to that of the control group. On day 6, the colon weight/length ratio of the chitin nanofibrils group was significantly decreased compared to those of the control and chitin-PS groups. The histological findings on day 6 are shown in Fig. 5. In the control and chitin-PS groups, severe erosions, crypt destruction, and edema were observed; moreover, some ulcers were observed. In the chitin nanofibrils group, erosions, crypt destruction, and edema were markedly suppressed compared to the control and chitin-PS groups. In addition, the severity of tissue damage was evaluated by histologically scoring (Fig. 6). The histological scores of the chitin nanofibrils group were significantly lower on day 5 compared to those of the control group and on day 6 compared to those of the control and chitin-PS groups.
Effect of chitin nanofibrils administration on the disease activity index in mice with DSS-induced acute UC.
| Day 0 | Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | |
|---|---|---|---|---|---|---|---|
| Control | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.3 ± 0.2 | 1.1 ± 0.4 | 3.6 ± 0.3 | 6.9 ± 0.5 |
| Chitin-NF | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.2 ± 0.1a | 2.3 ± 0.3a,b | 5.1 ± 0.4a,c |
| Chitin-PS | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.0 ± 0.0 | 0.3 ± 0.2 | 1.0 ± 0.4 | 4.0 ± 0.5 | 7.0 ± 0.8 |
ap < 0.05 compared with chitin-NF (+) and control (+) groups, bp < 0.01 compared with chitin-NF (+) and chitin-PS (+) groups, and cp < 0.05 compared with chitin-NF (+) and chitin-PS (+) groups. Reprinted with permission from ref. [26].
![Fig. 5: Effect of administration of chitin nanofibrils on histopathological changes in mice with DSS-induced acute UC.The colon was fixed, and tissue sections were stained with hematoxylin and eosin. Data are presented for one mouse each from the control (a), chitin nanofibrils (b), and chitin-PS (c) groups on day 6. Erosion indicated by arrow. Bar = 100 μm. Reprinted with permission from ref. [26].](/document/doi/10.1515/pac-2016-0504/asset/graphic/j_pac-2016-0504_fig_005.jpg)
Effect of administration of chitin nanofibrils on histopathological changes in mice with DSS-induced acute UC.
The colon was fixed, and tissue sections were stained with hematoxylin and eosin. Data are presented for one mouse each from the control (a), chitin nanofibrils (b), and chitin-PS (c) groups on day 6. Erosion indicated by arrow. Bar = 100 μm. Reprinted with permission from ref. [26].
![Fig. 6: Effect of administration of chitin nanofibrils on the histological damage score of the intestinal mucosa in mice with DSS-induced acute UC.Data represent the means ± S.E. of 30 fields/100× field in each group. Values are compared among control (+), chitin nanofibrils (+) and chitin-PS (+) groups. **p < 0.01. Reprinted with permission from ref. [26].](/document/doi/10.1515/pac-2016-0504/asset/graphic/j_pac-2016-0504_fig_006.jpg)
Effect of administration of chitin nanofibrils on the histological damage score of the intestinal mucosa in mice with DSS-induced acute UC.
Data represent the means ± S.E. of 30 fields/100× field in each group. Values are compared among control (+), chitin nanofibrils (+) and chitin-PS (+) groups. **p < 0.01. Reprinted with permission from ref. [26].
The number of MPO-positive cells was significantly lower in the chitin nanofibrils group than in the control group on days 3, 5, and 6. Moreover, significantly fewer MPO-positive cells were counted in the chitin nanofibrils group than in the chitin-PS group on days 3, 5, and 6. Furthermore, in the chitin nanofibrils group, the serum IL-6 level was significantly lower than that of the control group. Because MPO is a marker of oxidative stress, high MPO activities were observed in a mouse model of DSS-induced UC [59], [60]. IL-6 is a central cytokine in IBD that contributes to enhanced T-cell survival and apoptosis resistance in the lamina propria at sites of inflammation [61]. Chitin nanofibrils suppressed inflammation caused by acute UC by suppressing the MPO-mediated activation of inflammatory cells such as leukocytes and decreasing serum IL-6 concentrations.
Furthermore, we examined the protective mechanism of chitin nanofibrils focusing on the activation of nuclear factor-κB (NF-κB) and anti-fibrosis effects in a mouse model of DSS-induced acute UC [62]. The results of the immunohistochemical detection of NF-κB are shown in Fig. 7. In the control and chitin-PS groups, large areas of NF-κB-positive epithelium were observed. In the chitin nanofibrils group, the areas of NF-κB-positive epithelium were markedly reduced. In the chitin nanofibrils group, the NF-κB-positive areas were significantly smaller compared to the control group. The area of collagen deposition is indicated by arrows in Fig. 8. In the control and chitin-PS groups, marked collagen deposition was observed. In the chitin nanofibrils group, the areas of collagen deposition were slightly smaller. The percentages of areas of collagen deposition in mucosal and submucosal layers are shown in Fig. 8. In the chitin nanofibrils group, the score was significantly lower than that in the control group. Furthermore, in the chitin nanofibrils group, the serum level of monocyte chemotactic protein 1 (MCP-1) was significantly decreased compared with that of the control group. It was reported that MCP-1 induced a fibrogenic response of the gut in an IBD model [63]. Chitin nanofibrils suppressed fibrosis and decreased the serum MCP-1 concentration in a mouse model of DSS-induced acute UC. These results indicated that α-chitin nanofibrils exert suppressive effects on fibrosis in a mouse model of DSS-induced acute UC. Suppression of the action of MCP-1 was proposed as mechanism of the suppressive effect of α-chitin nanofibrils on fibrosis.
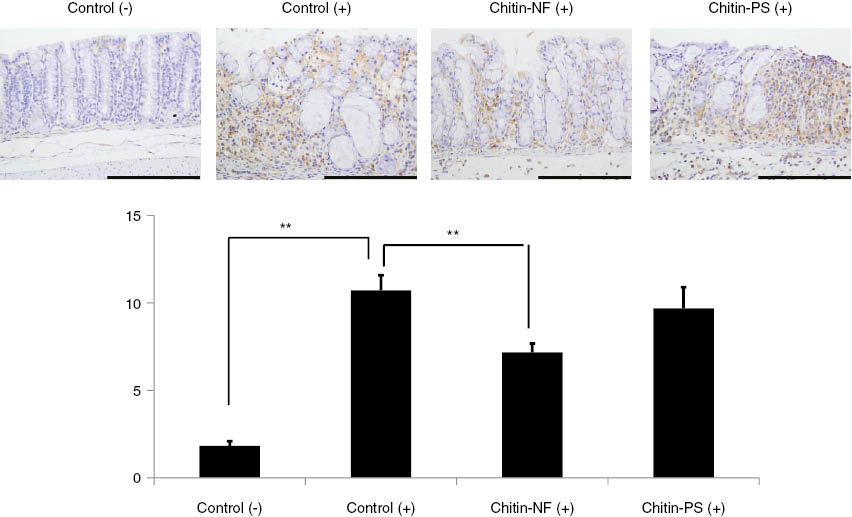
To evaluate the effects of α-chitin nanofibrils on NF-κB of the colon epithelium, immunohistochemical detections of NF-κB were performed. The results of immunohistochemical detections of NF-κB were shown. In the control (+) and chitin-PS (+) group, much positive areas of NF-κB in epithelium cells were observed. In the α-chitin nanofibrils (+) group, positive areas of NF-κB in epithelium cells were markedly decreased. To evaluate the effects of α-chitin nanofibrils on NF-κB activations in the epithelium cells, we performed digital image analysis. The percentages of positive areas of NF-κB in epithelium cells are shown in this slide. In the α-chitin nanofibrils (+) group, the score was significantly lower than that in the control (+) group (p < 0.05).
![Fig. 8: Effects of α-chitin nanofibrils on colon fibrosis in a mouse model of DSS-induced acute UC.(a) Masson’s trichrome staining results are shown. Data are presented for one mouse each from the control (+) (A), -chitin nanofibrils (+) (B), chitin-PS (C), and control (–) groups. Areas of collagen deposition are indicated by arrows. Bar = 200 μm. (b) Data represent the means ± S.E. of 30 fields/×100 field in each group. The statistical analyses were performed with a Steel–Dwass test. *p < 0.05, **p < 0.01. Reprinted with permission from ref. [62].](/document/doi/10.1515/pac-2016-0504/asset/graphic/j_pac-2016-0504_fig_008.jpg)
Effects of α-chitin nanofibrils on colon fibrosis in a mouse model of DSS-induced acute UC.
(a) Masson’s trichrome staining results are shown. Data are presented for one mouse each from the control (+) (A), -chitin nanofibrils (+) (B), chitin-PS (C), and control (–) groups. Areas of collagen deposition are indicated by arrows. Bar = 200 μm. (b) Data represent the means ± S.E. of 30 fields/×100 field in each group. The statistical analyses were performed with a Steel–Dwass test. *p < 0.05, **p < 0.01. Reprinted with permission from ref. [62].
NF-κB plays an important role in several innate immune signaling pathways. So far, it has been shown that NF-κB is a critical transcription factor required for the expression of genes associated with a pro-inflammatory response [64]. NF-κB activity is increased in the colon during active episodes of IBD [65]. Chitin nanofibrils suppressed the activation of NF-κB in the colonic epithelium in a model of DSS-induced acute colitis. MCP-1 has been shown to play an important role in the pathogenesis of experimental colitis, i.e. it is involved in the recruitment of immune and enterochromaffin cells. The absence of MCP-1 was associated with a significant reduction in inflammation in a model of experimental colitis [66]. Ju et al. demonstrated that pro-inflammatory cytokines induced the expression of MCP-1 via p38 mitogen-activated protein kinase and NF-κB signaling [67]. Compared to the control group, chitin nanofibrils decreased the serum MCP-1 concentration. These results indicated that chitin nanofibrils suppressed the increase in the level of serum MCP-1 via suppression of NF-κB activation.
We used α-chitin nanofibrils in a mouse model of experimental IBD [58], [62]. Chitin exists in different allomorphic forms in nature, which vary in terms of their polymer chain structure and crystallinity [68], [69]. Most chitins, e.g. insect and crustacean chitins, are composed of α-chitin in the native state, whereas the rarer β-chitin allomorph can be found in squid pen and some diatoms. α-Chitin exhibits a two-chain, antiparallel structure, whereas β-chitin has a one-chain unit cell with a parallel-chain structure and intramolecular hydrogen-bonding [70], [71], [72]. The weaker hydrogen bonds in the parallel-chain structure of β-chitin may account for its higher chemical reactivity [73], [74]. In addition, β-chitin has the unique feature of incorporating small molecules, including water, into its crystal lattice to form crystalline complexes [75]. The α-chitin chains give rise to strong hydrogen bonds and, consequently, higher stability [69]. We evaluated the differences of the biological effects between α-chitin nanofibrils and β-chitin nanofibrils using a mouse model of IBD. α-Chitin nanofibrils significantly suppressed the increases in the histological scores and number of MPO-positive cells. Furthermore, α-chitin nanofibrils significantly decreased the NF-κB-positive areas and areas of fibrosis. On the other hand, no anti-inflammatory effects were observed in the β-chitin nanofibrils group in the mouse model of experimental IBD. These results suggested that the α-crystal structure is crucial for chitin nanofibrils to exert an anti-inflammatory effect [76]. These findings indicate that chitin nanofibrils prepared from α-chitin have a potential as new functional food for IBD patients.
Anti-obesity effects of surface-deacetylated chitin nanofibers
Obesity is a growing health problem worldwide and has been associated with the metabolic syndrome [77]. It has been reported that in rodents, a high-fat diet is a major contributor to obesity [78]. The metabolic syndrome is a constellation of risk factors, including atherogenic dyslipidemia, impaired fasting glucose, hypertension, and central adiposity, which predispose affected individuals to a higher risk of type 2 diabetes, cardiovascular diseases, and cancer [77], [79], [80], [81], [82]. The increasing incidence of obesity suggests that this epidemic will worsen in the future [83]. Recently, the increased incidence of obesity has been recognized as a major cause for the promotion of metabolic diseases, including non-alcoholic fatty liver diseases (NAFLD). NAFLD is not only linked to other metabolic diseases such as diabetes but can also progress toward more severe liver diseases, including non-alcoholic steatohepatitis, hepatic cirrhosis, and hepatocellular carcinoma [84]. NAFLD can be characterized by increased lipid accumulation in the liver, which can be caused by multiple factors. Increased lipolysis from adipocytes or the increased intake of dietary fat, followed by an increase in free fatty acids can explain this phenomenon [85]. Mitochondrial dysfunction, which is associated with insulin resistance and normally precedes NAFLD, could also cause lipid accumulation due to impaired fatty acid beta oxidation [86]. In addition, de novo lipogenesis in the liver contributes greatly to hepatic steatosis [87]. Finally, reduced lipid clearance, which is often associated with insulin resistance, can also exacerbate the condition [88]. Some dietary modifications have been indicated to be beneficial for preventing or suppressing the metabolic syndrome, including catechins, flavonoids, and chitosan [89], [90], [91]. Furthermore, the cholesterol-lowing effects of CS have been studied extensively [78], [92], [93]. It is, in general, accepted that the origin of this effect lies in the unique ability of CS to bind lipids and bile acids [93], [94], [95], [96], [97], [98], [99]. Such binding results in an increase in the fecal excretion of fat, decrease in bile acid recycling, and induction of the hepatic synthesis of new bile acid constituents from cholesterol [100].
We evaluated the anti-obesity effects of chitin nanofibrils (CN) and surface-deacetylated chitin nanofibrils (sda-CN) in a mouse model of high-fat diet-induced obesity. The changes in body weight over the experimental period are shown in Fig. 9. Sda-CN and CS suppressed the increase in body weight; on the other hand, CN did not suppress weight gain. Sda-CN and CS suppressed the increase in epididymal tissue weight. Moreover, sda-CN decreased serum levels of leptin and TNF-α (Fig. 10). These results could be explained by the high surface areas of nanofibrils. Surface-deacetylation of CS resulted in equally or more potent anti-obesity effects in experimental models. It has previously been indicated that the adipocytes in adipose tissue secrete various proteins, known as adipocytokines, including TNF-α, IL-6, resistin, leptin, and adiponectin [101]. Plasma leptin concentrations are positively correlated with adiposity (excessive body fat) and body weight changes in humans and rodents [102]. Adiponectin contributes to insulin sensitivity and fatty acid oxidation, and circulating concentrations of adiponectin are inversely correlated with body mass [103]. The results of our study indicated that sda-CN suppressed the secretion of leptin from adipocytes. Liver steatohepatitis was markedly suppressed by oral administration of sda-CN. In NAFLD patients, insulin resistance leads to hepatic steatosis by multiple mechanisms. Greater uptake rates of plasma non-esterified fatty acids are attributable to increased release from the expanded adipose tissue mass because the insulin sensitivity is reduced. Hyperinsulinemia promotes the transcriptional up-regulation of genes that promote de novo lipogenesis in the liver. The increase in hepatic lipid accumulation does not result in enhanced fatty acid oxidation or increased secretion rates of triglyceride-rich lipoproteins. The molecular mechanisms by which hepatic triglyceride homeostasis is achieved under normal conditions, as well as the metabolic alterations that occur in the setting of insulin resistance, contribute to the pathogenesis of NAFLD [104], [105], [106].
![Fig. 9: Effects of surface-deacetylated chitin nanofibrils (sda-CN) on body weight changes in a mouse model of high-fat diet-induced obesity.(a) The body weight changes of mice are shown. In the high-fat diet (HFD) + sda-CN group, the body weights were significantly decreased compared with those of the HFD and HFD + sda-CN groups on days 6, 9, 15, 18, 21, and 45 (p < 0.05). (b) The gross appearances of mice were shown. In the ND group, epididymal fat tissue was slightly observed (A). In the HFD group, much epididymal fat tissue was observed compared with that of the ND group (B). In the HFD + sda-CN group, less epididymal fat tissue was observed compared with that of the HFD group (C). Reprinted with permission from ref. [13].](/document/doi/10.1515/pac-2016-0504/asset/graphic/j_pac-2016-0504_fig_009.jpg)
Effects of surface-deacetylated chitin nanofibrils (sda-CN) on body weight changes in a mouse model of high-fat diet-induced obesity.
(a) The body weight changes of mice are shown. In the high-fat diet (HFD) + sda-CN group, the body weights were significantly decreased compared with those of the HFD and HFD + sda-CN groups on days 6, 9, 15, 18, 21, and 45 (p < 0.05). (b) The gross appearances of mice were shown. In the ND group, epididymal fat tissue was slightly observed (A). In the HFD group, much epididymal fat tissue was observed compared with that of the ND group (B). In the HFD + sda-CN group, less epididymal fat tissue was observed compared with that of the HFD group (C). Reprinted with permission from ref. [13].
![Fig. 10: Effects of sda-CN on serum cytokines in a mouse model of HFD-induced obesity.(a) The result of serum leptin concentrations are shown. In the sda-CN group, the level of serum leptin was significantly decreased compared with those of the HFD and HFD + CN groups (p < 0.05). (b) The result of serum TNF-α concentration was shown. In the sda-CN group, the level of the serum TNF-α was significantly decreased compared with those of the HFD and HFD + chitosan groups (p < 0.05). Reprinted with permission from ref. [13]. *p < 0.05, **p < 0.01.](/document/doi/10.1515/pac-2016-0504/asset/graphic/j_pac-2016-0504_fig_010.jpg)
Effects of sda-CN on serum cytokines in a mouse model of HFD-induced obesity.
(a) The result of serum leptin concentrations are shown. In the sda-CN group, the level of serum leptin was significantly decreased compared with those of the HFD and HFD + CN groups (p < 0.05). (b) The result of serum TNF-α concentration was shown. In the sda-CN group, the level of the serum TNF-α was significantly decreased compared with those of the HFD and HFD + chitosan groups (p < 0.05). Reprinted with permission from ref. [13]. *p < 0.05, **p < 0.01.
Effects of surface-deacetylated chitin nanofibers in hypercholesterolemia
Cardiovascular disease is the leading cause of death and disability. The population at risk for atherosclerotic cardiovascular disease is ever increasing as the obesity epidemic and its complications, including diabetes, hypertension, and dyslipidemia, continue to grow among young adults [107]. To date, several authors have reported that certain foods and nutraceuticals are beneficial in the management of serum cholesterol levels [108], [109]. For example, dietary fiber, phytosterols, soy protein, nuts, and red yeast rice are beneficial supplements that decrease the level of low-density lipoprotein (LDL) cholesterol [108]. In addition, the use of policosanol and berberine have been reported for the treatment of hypercholesterolemia [109]. We evaluated the effects of oral administration of SDACNFs on hypercholesterolemia using an experimental hypercholesterolemia model. Moreover, the effects of SDACNFs were compared with those of CS and cellulose nanofibers (CLNFs) [110].
In the SDACNF and CS groups, serum total-cholesterol (T-Cho) levels were significantly lower than that of the control group on day 14 (p < 0.01). In the CLNF group, serum T-Cho level was significantly lower than that of the control group on day 14 (p < 0.05). In the SDACNF, CS, and CLNF groups, serum TG levels were slightly less than the control group, but the difference was not significant. In the SDACNF group, serum phospholipid (PL) level was significantly decreased compared to the CLNF and control groups (p < 0.01).
The results of serum chemistry on day 29 are shown in Table 2. In the SDACNF group, serum T-Cho and T-TG levels were significantly decreased compared to that of the CLNF groups. In the SDACNF group, moreover, serum ALT level was significantly less than the CLNF group (p < 0.05).
Effects of oral administration of SDACNFs on serum chemistry.
| Control | SDACNF | CS | CLNF | |
|---|---|---|---|---|
| (a) Day 14 | ||||
| T-Cho (mg/dL) | 105.2 ± 21.0 | 66.5 ± 6.2a | 68.2 ± 7.0a | 83.7 ± 8.2b |
| T-TG (mg/dL) | 78.7 ± 22.8 | 68.8 ± 14.9 | 60.0 ± 5.8 | 59.7 ± 10.8 |
| PL (mg/dL) | 152.0 ± 23.8 | 110.8 ± 6.5c | 133.7 ± 10.1 | 153.2 ± 11.5 |
| (b) Day 29 | ||||
| T-Cho (mg/dL) | 60.2 ± 18.5 | 47.6 ± 3.8c | 58.2 ± 10.6 | 63.2 ± 11.0 |
| T-TG (mg/dL) | 47.7 ± 19.4 | 44.0 ± 7.0c | 44.5 ± 10.3 | 57.7 ± 10.6 |
| PL (mg/dL) | 89.8 ± 19.6 | 75.5 ± 9.6 | 83.2 ± 12.7 | 83.0 ± 5.5 |
| ALT (U/L) | 40.8 ± 7.2 | 33.8 ± 2.6d | 40.0 ± 7.2 | 43.2 ± 5.5 |
Each table indicates the results of serum chemistry on day 14 (a), and on day 29 (b). Data are presented as mean ± standard error. ap < 0.01 compared with the control group. bp < 0.05 compared with the control group. cp < 0.05 compared with the control and CLNF groups. dp < 0.05 compared with the CLNF group. Reprinted with permission from ref. [110].
Serum lipid levels on day 29 were lower than those on day 14. The difference might come from the consist of the fasting. Normally, cholesterol homeostasis is maintained by the absorption of alimentary cholesterol and the endogenous synthesis of cholesterol. In fact, the differences of serum lipids levels were reported by the status of the fasting or non-fasting. Previously, it was demonstrated that CS decreased serum TG and T-Cho levels in the MS-rats, and this was likely due to the unique ability of CS to bind lipids and bile acids. Such binding can promote elimination of fat in the stool, reduce the level of bile acid recycling, and induce hepatic synthesis of new bile acid constituents from cholesterol. In this study, SDACNF and CS similarly decreased serum T-Cho levels. Moreover, oral administration of SDACNF suppressed the increase of serum PL level. The transfer of PL is promoted by phospholipid transfer protein (PLTP), and PLTP is widely expressed in organs and cells. High levels of PLTP mRNA have been found in the brain, lungs, and gonads, suggesting specific functions of PLTP in these organs. In addition to promoting transfer of phospholipids from very-low-density lipoprotein (VLDL) and chylomicrons (CM) into high-density lipoprotein (HDL), PLTP contributes to the remodeling of HDL particles. Our results indicated that oral administration of SDACNF may affect the activation of the PLTP, and further studies are needed to address this possibility.
As shown in Table 3, serum CM levels in the SDACNF and CS groups were significantly less than the control group on day 14 (p < 0.05). In the SDACNF group, serum VLDL level was significantly less than the CLNF group (p < 0.05). No change was observed for LDL and HDL serum levels on day 14 among the experimental groups, and there was no difference in serum CM, VLDL, LDL, and HDL levels among experimental groups on day 29. In this study, oral administration of SDACNF and CS equally suppressed the increases of serum CM and VLDL on day 14. Triacylglycerols, PLs, and cholesterols were the predominant dietary lipids. An important step in the intestinal digestion of these lipids is their emulsification with bile salts. A CM acts to transport ingested fat and fat-soluble vitamins, and VLDL acts to transport synthesized glyceride. Our results indicated that one possible mechanism of orally administered SDACNFs may be is their binding to lipids like CS. However, no studies have yet examined the binding ability of SDACNFs, and further experiments are necessary.
Effects of oral administration of SDACNFs on serum T-Cho contents.
| mg/dL | Control | SDACNF | CS | CLNF |
|---|---|---|---|---|
| (a) Day 14 | ||||
| CM | 33.9 ± 23.0 | 18.9 ± 13.4a | 18.5 ± 15.6a | 23.6 ± 15.4 |
| VLDL | 76.2 ± 44.9 | 54.4 ± 32.5b | 62.9 ± 40.6 | 77.5 ± 44.9 |
| LDL | 25.0 ± 13.2 | 24.1 ± 12.7 | 24.8 ± 13.4 | 29.7 ± 15.1 |
| HDL | 34.0 ± 18.2 | 33.7 ± 17.5 | 39.9 ± 23.8 | 32.8 ± 18.0 |
| (b) Day 29 | ||||
| CM | 14.6 ± 17.3 | 7.6 ± 6.2 | 7.4 ± 8.2 | 15.2 ± 11.5 |
| VLDL | 38.6 ± 27.8 | 28.8 ± 15.8 | 23.9 ± 22.1 | 29.8 ± 17.8 |
| LDL | 12.5 ± 6.0 | 11.6 ± 7.0 | 10.1 ± 6.0 | 11.3 ± 6.7 |
| HDL | 25.6 ± 14.2 | 27.5 ± 15.1 | 30.5 ± 17.8 | 25.6 ± 15.1 |
Each table summarizes serum T-Cho contents on day 14 (a) and day 29 (b). Data are presented as mean ± standard error. ap < 0.05 compared with the control group. bp < 0.05 compared with the control and CLNF groups. Reprinted with permission from ref. [110].
The present study indicated that oral administration of SDACNF suppressed the increase in serum total cholesterol, chylomicron, VLDL, and PL levels on day 14. Moreover, oral administration of SDACNFs suppressed the increase of alanine transaminase levels on day 29 and suppressed vacuolar degeneration and accumulation of lipid droplets in liver tissue. These data indicate that SDACNF may be a potential functional food for patients with hypercholesterolemia.
Conclusion
The anti-inflammatory effect of oral administration of chitin CNFs was revealed in a experimental model. It was found that CNFs improved clinical symptoms and suppressed IBD. CNFs decreased the sizes of areas with NF-κB staining in colon tissue. Moreover, it was indicated the anti-obesity effects of SDACNF in a mouse model of high-fat diet-induced obesity. SDACNFs suppressed the increase in body weight produced by the high-fat diet; however, CNFs did not suppress such weight gain. SDACNFs decreased serum levels of leptin. These results suggest that CNF and SDACNF are promising functional foods for patients with IBD or obesity.
Article note:
A collection of invited papers based on presentations at the 12th Conference of the European Chitin Society (12th EUCHIS)/13th International Conference on Chitin and Chitosan (13th ICCC), Münster, Germany, 30 August–2 September 2015.
References
[1] R. A. A. Muzzarelli. In Chitosan-Based Systems for Biopharmaceuticals, B. Sarmento, J. DasNeves (Eds.), pp. 3–22, Wiley, New York (2012).Search in Google Scholar
[2] K. Kurita. Prog. Polym. Sci.269, 1921 (2001).10.1016/S0079-6700(01)00007-7Search in Google Scholar
[3] M. Rinaudo. Prog. Polym. Sci.31, 603 (2006).10.1016/j.progpolymsci.2006.06.001Search in Google Scholar
[4] K. S. Pillai, W. Paul, C. P. Sharma. Prog. Polym. Sci.34, 641 (2009).10.1016/j.progpolymsci.2009.04.001Search in Google Scholar
[5] J. F. Revol, R. H. Marchessault. Int. J. Biol. Macromol.15, 329 (1993).10.1016/0141-8130(93)90049-RSearch in Google Scholar
[6] N. K. Gopalan, A. Dufresne. Biomacromolecules4, 657 (2003).10.1021/bm020127bSearch in Google Scholar PubMed
[7] Y. Fan, T. Saito, A. Isogai. Biomacromolecules9, 1919 (2008).10.1021/bm800178bSearch in Google Scholar PubMed
[8] R. Jayakumar, M. Prabaharan, S. V. Nair, H. Tamura. Biotechnol. Adv. 28, 142 (2010).10.1016/j.biotechadv.2009.11.001Search in Google Scholar PubMed
[9] R. Jayakumar, M. Prabaharan, P. T. Sudheesh Kumar, S. V. Nair, H. Tamura. Biotechnol. Adv.29, 322 (2011).10.1016/j.biotechadv.2011.01.005Search in Google Scholar PubMed
[10] S. Ifuku, M. Nogi, K. Abe, M. Yoshioka, M. Morimoto, H. Saimoto, H. Yano. Biomacromolecules10, 1584 (2009).10.1021/bm900163dSearch in Google Scholar PubMed
[11] S. Ifuku, H. Saimoto. Nanoscale4, 3308 (2012).10.1039/C2NR30383CSearch in Google Scholar
[12] S. Ifuku. Molecules19, 18367 (2014).10.3390/molecules191118367Search in Google Scholar PubMed PubMed Central
[13] K. Azuma, S. Ifuku, T. Osaki, Y. Okamoto, S. Minami. J. Biomed. Nanotechnol.10, 2891 (2014).10.1166/jbn.2014.1882Search in Google Scholar PubMed
[14] C. Zhang, X. Yuan, L. Wu, Y. Han, J. Sheng. Eur. Polym. J.41, 423 (2005).10.1016/j.eurpolymj.2004.10.027Search in Google Scholar
[15] Y. Zhang, C. T. Lim, S. Ramakrishna, Z. M. Huang. J. Mater. Sci. Mater. Med.16, 933 (2005).10.1007/s10856-005-4428-xSearch in Google Scholar PubMed
[16] J. Fang, H. Niu, T. Lin, X. Wang. Chin. Sci. Bull.53, 2265 (2008).10.1360/csb2008-53-19-2265Search in Google Scholar
[17] R. A. A. Muzzarelli, P. Morganti, G. Morganti, P. Palombo, M. Palombo, G. Biagini, M. M. Belmonte, F. Giantomassi, F. Orlandi, C. Muzzarelli. Carbohydr. Polym. 70, 274 (2007).10.1016/j.carbpol.2007.04.008Search in Google Scholar
[18] V. De Silva, A. El-Metwally, E. Ernst, G. Lewith, G. J. Macfarlane. Rheumatology (Oxford)50, 911 (2011).10.1093/rheumatology/keq379Search in Google Scholar PubMed
[19] J. Y. Reginster, A. Neuprez, M. P. Lecart, N. Sarlet, O. Bruyere. Rheumatol. Int.32, 2959 (2012).10.1007/s00296-012-2416-2Search in Google Scholar PubMed PubMed Central
[20] J. Hua, S. Suguro, S. Hirano, K. Sakamoto, I. Nagaoka. Inflamm. Res.54, 127 (2005).10.1007/s00011-004-1333-6Search in Google Scholar PubMed
[21] S. Yomogida, Y. Kojima, Y. Tsutsumi-Ishii, J. Hua, K. Sakamoto, I. Nagaoka. Int. J. Mol. Med.22, 3217 (2008).Search in Google Scholar
[22] K. Azuma, T. Osaki, T. Wakuda, T. Tsuka, T. Imagawa, Y. Okamoto, S. Minami. Inflammation35, 1462 (2012).10.1007/s10753-012-9459-0Search in Google Scholar PubMed
[23] K. Azuma, T. Osaki, S. Minami, Y. Okamoto. J. Func. Biomater.6, 33 (2015).10.3390/jfb6010033Search in Google Scholar PubMed PubMed Central
[24] S. Masuda, K. Azuma, S. Kurozumi, M. Kiyose, T. Osaki, T. Tsuka, N. Itoh, T. Imagawa, S. Minami, K. Sato, Y. Okamoto. Carbohydr. Polym.111, 783 (2014).10.1016/j.carbpol.2014.04.102Search in Google Scholar PubMed
[25] K. Kan. J. Chitin Chitosan Sci.2, 205 (2014).10.1166/jcc.2014.1077Search in Google Scholar
[26] K. Azuma, T. Osaki, S. Kurozumi, M. Kiyose, T. Tsuka, Y. Murahata, T. Imagawa, N. Itoh, S. Minami, K. Sato, Y. Okamoto. Carbohydr. Polym.115, 448 (2015).10.1016/j.carbpol.2014.09.012Search in Google Scholar PubMed
[27] G. Kerch. Mar. Drugs.13, 2158 (2015).10.3390/md13042158Search in Google Scholar PubMed PubMed Central
[28] M. Anraku, T. Fujii, N. Furutani, D. Kadowaki, T. Maruyama, M. Otagiri, J. M. Gebicki, H. Tomida. Food Chem. Toxicol.47, 104 (2009).10.1016/j.fct.2008.10.015Search in Google Scholar PubMed
[29] R. Anandan, B. Ganesan, T. Obulesu, S. Mathew, R. S. Kumar, P. T. Lakshmanan, A. A. Zynudheen. Int. J. Biol. Macromol.51, 783 (2012).10.1016/j.ijbiomac.2012.07.016Search in Google Scholar PubMed
[30] R. Anandan, B. Ganesan, T. Obulesu, S. Mathew, K. K. Asha, P. T. Lakshmanan, A. A. Zynudheen. Cell Stress Chaperon18, 121 (2013).10.1007/s12192-012-0354-2Search in Google Scholar PubMed PubMed Central
[31] F. Karadeniz, S. K. Kim. In Marine Carbohydrates: Fundamentals and Applications, Part B, S. K. Kim (Ed.), pp. 15–31, Elsevier Inc., Oxford, UK (2014).Search in Google Scholar
[32] T. Miura, M. Usami, Y. Tsuura, H. Ishida, Y. Seino. Biol. Pharm. Bull.18, 1623 (1995).10.1248/bpb.18.1623Search in Google Scholar PubMed
[33] K. Hayashi, M. Ito. Biol. Pharm. Bull.25, 188 (2002).10.1248/bpb.25.188Search in Google Scholar PubMed
[34] S. H. Liu, Y. H. Chang, M. T. Chiang. J. Agric. Food Chem.58, 5795 (2010).10.1021/jf100662rSearch in Google Scholar
[35] W. Zhang, W. Xia. Int. J. Biol. Macromol.72, 1402 (2015).10.1016/j.ijbiomac.2014.10.049Search in Google Scholar
[36] T. U. Rashid, S. M. Shamsuddin, M. A. Khan, M. M. Rahman. Soft Mater.12, 262 (2014).10.1080/1539445X.2014.880720Search in Google Scholar
[37] Y. Xia, P. Yang, Y. Sun, Y. Wu, B. Mayers, B. Gates, Y. Yin, F. Kim, H. Yan. Adv. Mater.15, 353 (2013).10.1002/adma.200390087Search in Google Scholar
[38] D. Li, Y. Xia. Adv. Mater.16, 1151 (2004).10.1002/adma.200400719Search in Google Scholar
[39] Z. M. Huang, Y. Z. Zhang, M. Kotaki, S. Ramakrishna. Compos. Sci. Technol.63, 2223 (2003).10.1016/S0266-3538(03)00178-7Search in Google Scholar
[40] S. Ramakrishna, K. Fujihara, W. E. Teo, T. Yong, Z. Ma, R. Ramaseshan. Mater. Today9, 40 (2006).10.1016/S1369-7021(06)71389-XSearch in Google Scholar
[41] T. J. Sill, H. A. Recum. Biomaterials29, 1989 (2008).10.1016/j.biomaterials.2008.01.011Search in Google Scholar
[42] M. M. Giraud-guille. Tissue Cell16, 75 (1984).10.1016/0040-8166(84)90020-XSearch in Google Scholar
[43] D. Raabe, C. Sachs, P. Romano. Acta Mater.53, 4281 (2005).10.1016/j.actamat.2005.05.027Search in Google Scholar
[44] D. Raabe, P. Romano, C. Sachs, H. Fabritius, A. Al-Sawalmih, S. B. Yi, G. Servos, H. G. Hartwig. Mater. Sci. Eng. A421, 143 (2006).10.1016/j.msea.2005.09.115Search in Google Scholar
[45] K. Chen, J. B. Lindsey, A. Khera, J. A. De Lemos, C. R. Ayers, A. Goyal, G. L. Vega, S. A. Murphy, S. M. Grundy, D. K. McGuire. Diab. Vasc. Dis. Res.5, 96 (2008).10.3132/dvdr.2008.016Search in Google Scholar PubMed
[46] J. B. Zeng, Y. S. He, S. L. Li, Y. A. Wang. Biomacromolecules13, 1 (2012).10.1021/bm201564aSearch in Google Scholar PubMed
[47] K. Abe, S. Iwamoto, H. Yano. Biomacromolecules8, 3276 (2007).10.1021/bm700624pSearch in Google Scholar PubMed
[48] S. Ifuku, S. Morooka, M. Morimoto, H. Saimoto. Biomacromolecules11, 1326 (2010).10.1021/bm100109aSearch in Google Scholar PubMed
[49] S. Ifuku, M. Nogi, K. Abe, M. Yoshioka, M. Morimoto, H. Saimoto, H. Yano. Carbohyd. Polym.84, 762 (2011).10.1016/j.carbpol.2010.04.039Search in Google Scholar
[50] S. Ifuku, R. Nomura, M. Morimoto, H. Saimoto. Materials4, 1417 (2011).10.3390/ma4081417Search in Google Scholar PubMed PubMed Central
[51] G. Morrison, B. Headon, P. Gibson. Aust. Fam. Physician.38, 956 (2009).Search in Google Scholar
[52] S. Melgar, A. Karlsson, E. Michaëlsson. Am. J. Physiol. Gastrointest. Liver Physiol.288, G1328 (2005).10.1152/ajpgi.00467.2004Search in Google Scholar PubMed
[53] K. Nakamura, K. Honda, T. Mizutani, H. Akiho, N. Harada. World J. Gastroenterol.12, 4628 (2006).10.3748/wjg.v12.i29.4628Search in Google Scholar PubMed PubMed Central
[54] M. Coëffier, R. Marion-Letellier, P. Déchelotte. Inflamm. Bow. Dis.16, 518 (2010).10.1002/ibd.21017Search in Google Scholar PubMed
[55] N. Rajendran, D. Kumar. World J. Gastroenterol.16, 1442 (2010).10.3748/wjg.v16.i12.1442Search in Google Scholar PubMed PubMed Central
[56] M. E. Rodríguez-Cabezas, M. D. Gálvez, J. Lorente, A. Concha, D. Camuesco, S. Azzouz, A. Osuna, L. Redondo, A. Zarzuelo. J. Nutr.132, 3263 (2002).10.1093/jn/132.11.3263Search in Google Scholar PubMed
[57] C. Vanderpool, F. Yan, D. B. Polk. Inflamm. Bow. Dis.14, 1585 (2008).10.1002/ibd.20525Search in Google Scholar PubMed
[58] K. Azuma, T. Osaki, T. Wakuda, S. Ifuku, H. Saimoto, T. Tsuka, T. Imagawa, Y. Okamoto, S. Minami. Carbohydr. Polym.87, 1399 (2012).10.1016/j.carbpol.2011.09.036Search in Google Scholar
[59] Y. Naito, T. Takagi, T. Yoshikawa. J. Clin. Biochem. Nutr.41, 18 (2007).10.3164/jcbn.2007003Search in Google Scholar PubMed PubMed Central
[60] R. K. Schindhelm, L. P. van der Zwan, T. Teerlink, P. G. Scheffer. Clin. Chem.55, 1462 (2009).10.1373/clinchem.2009.126029Search in Google Scholar PubMed
[61] J. Mudeter, M. F. Neurath. Inflamm. Bowel Dis.13, 1016 (2007).10.1002/ibd.20148Search in Google Scholar PubMed
[62] K. Azuma, T. Osaki, S. Ifuku, H. Saimoto, T. Tsuka, T. Imagawa, Y. Okamoto, S. Minami. Carbohydr. Polym.90, 197 (2012).10.1016/j.carbpol.2012.05.023Search in Google Scholar PubMed
[63] Y. Motomura, W. I. Khan, R. T. El-Sharkawy, M. Verma-Gandhu, E. F. Verdu, J. Gauldie, S. M. Collins. Gut55, 662 (2006).10.1136/gut.2005.068429Search in Google Scholar PubMed PubMed Central
[64] C. O. Elson, Y. Cong, V. J. McCracken, R. A. Dimmitt, R. G. Lorenz, C. T. Weaver. Immunol. Rev.206, 260 (2005).10.1111/j.0105-2896.2005.00291.xSearch in Google Scholar PubMed
[65] T. Zarubin, J. Han. Cell Res.15, 11 (2005).10.1038/sj.cr.7290257Search in Google Scholar
[66] W. I. Khan, Y. Motomura, H. Wang, R. T. El-Sharkawy, E. F. Verdu, M. Verma-Gandhu, B. J. Rollins, S. M. Collins. Am. J. Physiol. Gastrointest. Liver Physiol.291, G803 (2006).10.1152/ajpgi.00069.2006Search in Google Scholar
[67] Y. Ju, J. Hua, K. Sakamoto, H. Ogawa, I. Nagaoka. Int. J. Mol. Med. 22, 809 (2008).Search in Google Scholar
[68] F. Khoushab, F. M. Yamabhai. Mar. Drugs.8, 1988 (2010).10.3390/md8071988Search in Google Scholar
[69] M. K. Jang, B. G. Kong, Y. I. Jeong, C. H. Lee, J. W. Nah. J. Polym. Sci. A Polym. Chem. 42, 3423 (2004).10.1002/pola.20176Search in Google Scholar
[70] J. Blackwell. Biopolymers7, 281 (1969).10.1002/bip.1969.360070302Search in Google Scholar
[71] R. Minke, J. Blackwell. J. Mol. Biol.120, 167 (1978).10.1016/0022-2836(78)90063-3Search in Google Scholar
[72] K. M. Rudall, W. Kenchington. Biol. Rev.48, 597 (1973).10.1111/j.1469-185X.1973.tb01570.xSearch in Google Scholar
[73] E. Atkins. J. Biosci.8, 375 (1985).10.1007/BF02703990Search in Google Scholar
[74] K. Kurita, K. Sugita, N. Kodaira, M. Hirakawa, J. Yang. Biomacromolecules6, 1414 (2005).10.1021/bm049295pSearch in Google Scholar PubMed
[75] P. Sikorski, R. Hori, M. Wada. Biomacromolecules10, 1100 (2009).10.1021/bm801251eSearch in Google Scholar PubMed
[76] K. Azuma, T. Osaki, S. Ifuku, H. Saimoto, T. Tsuka, T. Imagawa, Y. Okamoto, S. Minami. J. Chitin Chitosan Sci.1, 144 (2013).10.1166/jcc.2013.1020Search in Google Scholar
[77] I. Vucenik, J. P. Stains. Ann. N Y Acad. Sci.1271, 37 (2012).10.1111/j.1749-6632.2012.06750.xSearch in Google Scholar
[78] J. Y. Je, P. J. Park, S. K. Kim. Food Chem. Toxicol.42, 381 (2004).10.1016/j.fct.2003.10.001Search in Google Scholar
[79] S. H. Park, B. I. Kim, S. H. Kim, H. J., Kim, D. I. Park, Y. K. Cho, I. K. Sung, C. I. Sohn, H. Kim, D. K. Keum, H. D. Kim, J. H. Park, J. H. Kang, J.H. Jeon. J. Am. Coll. Nutr.26, 321 (2007).10.1080/07315724.2007.10719618Search in Google Scholar
[80] I. Grattagliano, V. O. Palmieri, P. Portincasa, A. Moschetta, G. Palasciano. J. Nutr. Biochem.19, 491 (2008).10.1016/j.jnutbio.2007.06.011Search in Google Scholar
[81] P. Y. Chen, A. Y. M. Lin, A. J. McKittrick, M. A. Meyers. Acta Biomater.4, 587 (2008).10.1016/j.actbio.2007.12.010Search in Google Scholar
[82] N. Ishizaka, Y. Ishizaka, M. Yamakado, E. Toda, K. Koike, R. Nagai. Atherosclerosis204, 619 (2009).10.1016/j.atherosclerosis.2008.10.022Search in Google Scholar
[83] D. W. Haslam, W. P. James. Lancet366, 1197 (2005).10.1016/S0140-6736(05)67483-1Search in Google Scholar
[84] J. D. Browning, L. S. Szczepaniak, R. Dobbins, P. Nuremberg, J. D. Horton, J. C. Cohen, S. M. Grundy, H. H. Hobbs. Hepatology40, 1387 (2004).10.1002/hep.20466Search in Google Scholar PubMed
[85] K. L. Donnelly, C. I. Smith, S. J. Schwarzenberg, J. Jessurun, M. D. Boldt, E. J. Parks. J. Clin. Invest.115, 1343 (2005).10.1172/JCI23621Search in Google Scholar PubMed PubMed Central
[86] B. Fromenty, M. A. Robin, A. Igoudjil, A. Mansouri, D. Pessayre. Diabetes. Metab.30, 121 (2004).10.1016/S1262-3636(07)70098-8Search in Google Scholar
[87] C. Postic, J. Girard. J. Clin. Invest.118, 829 (2008).10.1172/JCI34275Search in Google Scholar
[88] E. Fabbrini, B. S. Mohammed, F. Magkos, K. M. Korenblat, B. W. Patterson, S. Klein. Gastroenterology134, 424 (2008).10.1053/j.gastro.2007.11.038Search in Google Scholar
[89] F. Thielecke, M. Boschmann. Phytochemistry70, 11 (2009).10.1016/j.phytochem.2008.11.011Search in Google Scholar
[90] J. Ahn, H. Lee, S. Kim, J. Park, T. Ha. Biochem. Biophys. Res. Commun.373, 545 (2008).10.1016/j.bbrc.2008.06.077Search in Google Scholar
[91] F. R. Seiva, L. G. Chuffa, C. P. Braga, J. P. Amorim, A. A. Fernandes. Food Chem. Toxicol.50, 3726 (2012).10.1016/j.fct.2012.07.009Search in Google Scholar
[92] J. Y. Je, S. K. Kim. Adv. Food Nutr. Res.65, 121 (2012).10.1016/B978-0-12-416003-3.00007-XSearch in Google Scholar
[93] G. R. Kaats, J. E. Michalek, H. G. Preuss. J. Am. Coll. Nutr.25, 389 (2006).10.1080/07315724.2006.10719550Search in Google Scholar
[94] N. G. Schipper, K. M. Vârum, P. Stenberg, G. Ocklind, H. Lennernäs, P. Artursson. Eur. J. Pharm. Sci.8, 335 (1999).10.1016/S0928-0987(99)00032-9Search in Google Scholar
[95] M. Anraku, A. Michihara, T. Yasufuku, K. Akasaki, D. Tsuchiya, H. Nishio, T. Maruyama, M. Otagiri, Y. Maezaki, Y. Kondo, H. Tomida. Biol. Pharm. Bull.33, 1994 (2010).10.1248/bpb.33.1994Search in Google Scholar PubMed
[96] J. E. Pie, J. H. Park, Y. H. Park, Y. M. Ryu, K. N. Kim, S. W. Suh, K. G. Becker, Y. S. Cho-Chung, M.K. Kim. J. Nutr. Biochem.17, 157 (2006).10.1016/j.jnutbio.2005.06.002Search in Google Scholar PubMed
[97] D. J. Ormrod, C. C. Holmes, T. E. Miller. Atherosclerosis138, 329 (1998).10.1016/S0021-9150(98)00045-8Search in Google Scholar
[98] C. M. Gallaher, J. Munion, R. Hesslink Jr, J. Wise, D. D. Gallaher. J. Nutr.130, 2753 (2000).10.1093/jn/130.11.2753Search in Google Scholar
[99] M. Sugano, T. Fujikawa, Y. Hiratsuji, K. Nakashima, N. Fukuda, Y. Hasegawa. Am. J. Clin. Nutr.33, 787 (1980).10.1093/ajcn/33.4.787Search in Google Scholar
[100] M. Fasshauer, R. Paschke. Diabetologia46, 1594 (2003).10.1007/s00125-003-1228-zSearch in Google Scholar
[101] M. Gnacińska, S. Małgorzewicz, M. Stojek, W. Łysiak-Szydłowska, K. Sworczak. Adv. Med. Sci.54, 150 (2009).10.2478/v10039-009-0035-2Search in Google Scholar
[102] E. D. Rosen, B. M. Spiegelman. Nature444, 847 (2006).10.1038/nature05483Search in Google Scholar
[103] Y. Arita, S. Kihara, N. Ouchi, M. Takahashi, K. Maeda, J. Miyagawa, K. Hotta, I. Shimomura, T. Nakamura, K. Miyaoka, H. Kuriyama, M. Nishida, S. Yamashita, K. Okubo, K. Matsubara, M. Muraguchi, Y. Ohmoto, T. Funahashi, Y. Matsuzawa. Biochem. Biophys. Res. Commun.257, 79 (1999).10.1006/bbrc.1999.0255Search in Google Scholar
[104] M. W. Am. J. Physiol. Gastrointest. Liver Physiol.290, G194 (2006).10.1152/ajpgi.00413.2005Search in Google Scholar
[105] H. Doege, A. Stahl. Physiology (Bethesda)21, 259 (2006).10.1152/physiol.00014.2006Search in Google Scholar
[106] Y. Kawano, D. E. Cohen. J. Gastroenterol.48, 434 (2013).10.1007/s00535-013-0758-5Search in Google Scholar
[107] D. J. McNamara. Biochim Biophys Acta1529, 310 (2000).10.1016/S1388-1981(00)00156-6Search in Google Scholar
[108] P. S. Nijjar, F. M. Burke, A. Bloesch, D. J. Rader. J Clin Lipidol4, 248 (2010).10.1016/j.jacl.2010.07.001Search in Google Scholar PubMed
[109] M. R. Mannarino, S. Ministrini, M. Pirro. Eur J Intern Med25, 592 (2014).10.1016/j.ejim.2014.06.008Search in Google Scholar PubMed
[110] K. Azuma, T. Nagae, T. Nagai, H. Izawa, M. Morimoto, Y. Murahata, T. Osaki, T. Tsuka, T. Imagawa, N. Ito, Y. Okamoto, H. Saimoto, S. Ifuku. Int. J. Mol. Sci.16, 17445 (2015).10.3390/ijms160817445Search in Google Scholar PubMed PubMed Central
©2016 IUPAC & De Gruyter. This work is licensed under a Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License. For more information, please visit: http://creativecommons.org/licenses/by-nc-nd/4.0/
Articles in the same Issue
- Frontmatter
- In this issue
- Editorial
- VIII Trans-Mediterranean Colloquium on Heterocyclic Chemistry (TRAMECH VIII)
- Conference papers
- Heterocycle-based bifunctional organocatalysts in asymmetric synthesis
- Arylxanthones and arylacridones: a synthetic overview
- Esterification of chitosan with L-alanine and a study on their effect in removing the heavy metals and total organic carbon (TOC) from wastewater
- Nanofibers based on chitin: a new functional food
- Dissolution, gelation, functionalization, and material preparation of chitin using ionic liquids
Articles in the same Issue
- Frontmatter
- In this issue
- Editorial
- VIII Trans-Mediterranean Colloquium on Heterocyclic Chemistry (TRAMECH VIII)
- Conference papers
- Heterocycle-based bifunctional organocatalysts in asymmetric synthesis
- Arylxanthones and arylacridones: a synthetic overview
- Esterification of chitosan with L-alanine and a study on their effect in removing the heavy metals and total organic carbon (TOC) from wastewater
- Nanofibers based on chitin: a new functional food
- Dissolution, gelation, functionalization, and material preparation of chitin using ionic liquids

