Abstract
Red algae can synthesize UV-absorbing mycosporine-like amino acid (MAA) compounds to minimize the damage caused by UV radiation. MAAs are molecules with low molecular weight and absorption maxima in the UV region (310–360 nm). Combined with their antioxidant activities, these features suggest a potential application in the prevention and therapeutic treatment of afflictions related to free-radical production and UV irradiation in humans. However, the use of MAAs in biotechnological products is limited by the low concentrations of these compounds in macroalgae harvested from the wild. Thus, species with high MAA concentrations are desirable. Information on red algae from Patagonia generally shows low concentrations of MAAs. However, increased MAA or at least changes in individual MAA concentration have been observed in certain species under stressful conditions. Additionally, methanolic extracts show an interesting absorption in the UVB region in certain red algae species, such as Lophurella hoockeriana.
Photoprotection in algae
Some marine algae that inhabit the intertidal zone are exposed to highly variable light environments. Changes in solar radiation could have important implications for the ecophysiology of algae because they influence key metabolic processes that not only affect the individual but also the population and community behaviors. UV radiation has been reported to have adverse effects on DNA [1, 2], growth [3], photosynthesis, pigments, enzyme activities [4–6], and increased reactive oxygen species (ROS) [7], among other indirect effects. A discussion of the UV radiation effects on algae is beyond the scope of this work, but the reader is referred to Franklin and Forster [3], Bischof et al. [8], and Pessoa [9] for more information. However, through evolution, the macrophyte metabolism has adapted to strongly changing conditions, especially in the upper intertidal zone.
Most macrophytes that are frequently exposed to solar radiation have various repair and protective mechanisms to minimize the damage caused by UVB. These mechanisms include the photorepair of DNA mediated by PAR and UVA [10, 11], the accumulation of antioxidant substances and antioxidant enzymes [12–15], dynamic photoinhibition [6], and increased thickness and density of the cell walls [16], which can help the organisms cope with and potentially adapt to high UVR conditions. Other UV protective mechanisms include the synthesis and accumulation of UV-absorbing compounds such as trihidroxicoumarins in green algae [17], polyphenols in brown algae [15, 18–21], and mycosporine-like amino acids (MAAs) in red algae species [22–28].
MAAs have been reported not only in red algae but also in organisms from other taxonomical groups, such as cyanobacteria, phytoplankton, lichens, gorgonians, corals and their associated biota, and many other marine organisms, such as cnidarians, sponges, brine shrimp, sea urchins, starfish, holothurids, clams, ascidians, and fish [29–31], as well as some species of green [32, 33] and brown algae [34]. More than 20 MAAs have been reported from diverse organisms (Table 1). However, continued surveying of organisms and methodological advances may yet reveal new MAAs, and hence, the diversity and distribution of these compounds may be greater than is currently recognized.
Mycosporine-like amino acid compounds identified in different organisms.
| MAAs | Absorption maxima (nm) |
|---|---|
| 1. Mycosporine-taurine | 309 |
| 2. Mycosporine-glycine | 310 |
| 3. Mycosporine-Glu | 311 |
| 4. Palythine | 320 |
| 5. Palythine-serine-sulfate | 321 |
| 6. Palythine-serine | 320 |
| 7. Palythine-threonine-sulfate | 321 |
| 8. Mycosporine-N-methylamine:serine | 325 |
| 9. Mycosporine-methylamine-serine | 325 |
| 10. Mycosporine-N-methylamine:threonine | 328 |
| 11. Mycosporine-methylamine-threonine | 330 |
| 12. Asterina-330 | 330 |
| 13. Mycosporine-glutamic acid-glycine | 330 |
| 14. Palythinol | 332 |
| 15. Mycosporine-glycine- serine | 332 |
| 16. Mycosporine-2-glycine | 334 |
| 17. Shinorine | 334 |
| 18. Porphyra-334 | 334 |
| 19.Mycosporine-glycine-valine | 335 |
| 20. Palythenic acid | 337 |
| 21. Usujirene | 357 |
| 22. Palythene | 360 |
| 23. Euhalothece-362 | 362 |
For more details and references, see Korbee et al. [29], and Pessoa [9, 31]. The most common MAAs in red macroalgae are shown in bold.
Functions of MAAs and their biotechnological applications
MAAs have been called “multipurpose” secondary metabolites [35] because they are thought to perform several roles in algal cells. First, MAAs are suggested to function as natural sunscreens that mitigate UV damage, as they mainly absorb in the UV region at wavelengths of 310–360 nm (Table 1) [24, 36, 37]. This absorbance prevents other cellular structures from being affected by the radiation [34, 38]. MAAs can function as passive shielding and dissipate the energy of the absorbed radiation as heat [39] without generating photochemical reactions or ROS [26, 40–42].
Second, MAAs can act as antioxidants to prevent damage from ROS resulting from UV radiation [43], desiccation [44], salinity [45–47], and heavy metal [48] stresses. The antioxidative capacities vary among different MAAs, with some of them having moderate to high antioxidant activity and others having weak activity or lacking it entirely, depending on the pH [49] and temperature [50]. Whereas shinorine and porphyra-334 have weak antioxidative activity, mycosporine-glycine acts as an effective antioxidant [43]. Recently, de la Coba et al. [49] reported that mycosporine-glycine presented the highest activity, with an IC50 that is 8 times the value of L-ascorbic acid at pH 8.5, followed by asterina-330 plus palythine. Porphyra-334 plus shinorine showed scarce activity for scavenging hydrosoluble free radicals. In contrast, dehydrated porphyra-334 that was produced after heat treatment (100 °C) showed a huge boost in antioxidative activity over the antioxidative activity of the original porphyra-334 [50].
Third, it has been suggested that MAAs may play a role in cell osmotic regulation as neutral, low-molecular-weight organic compounds that accumulate in the cytoplasm [35, 51]. MAAs may also be important in algal cells due to their potential additional roles as accessory light-harvesting pigments during photosynthesis, as intracellular nitrogen reservoirs, and in reproduction [35]. The MAAs can dissipate energy-excited thymine residues and prevent the formation of photoproducts [52]. However, there is no evidence of the importance of this mechanism in vivo [53]. The MAAs have also been identified as accessory pigments that transfer energy to the reaction centers of photosynthesis [54], although this issue remains controversial [35].
MAAs might be suitable candidates for the cosmetic industry to develop natural UV sunscreens due to their physico-chemical features of low molecular weight, absorption in the UV region [12, 42], high melting point, solubility in water and organic solvents [40], and extreme stability in a wide range of pH and temperature conditions [49]. In fact, examples of such sunscreens are available on the market. One of the most commercialized is Helioguard 365, which combines porphyra-334 and shinorine isolated from the red alga Porphyra umbilicalis. Although both porphyra-334 and shinorine present absorption maxima mainly in the UVA region, sunscreens manufactured with these types of MAAs can prevent not only the effects of UVA radiation such as aging and possibly the development of melanoma [55] but also sunburn [56], which is heavily weighted in the UVB range [55].
In addition to the protection of human skin, MAAs have been commercially explored as sunscreens for other materials. For example, they have been used as photostabilizing additives in plastics, paint, and varnish [40, 57]. Based on their antioxidant activities, MAAs from algae could have potential applications in the prevention and therapeutic treatment of infections related to free radical production and UV irradiation in humans [49] through the formulation and ingestion of multi-functional foods.
MAAs in species around the world
In red algae, MAAs are present in species from different water depths [25, 32] and geographical areas, ranging from polar to tropical regions [22] and including the Subantarctic [25, 28, 58, 59] and Antarctic regions [32]. Macroalgae can be classified according to their MAA content into three physiologically different groups: (1) species with no MAA biosynthesis capability, (2) species with a constant and relatively high MAA composition and concentration, and (3) species with a basic MAA concentration that adjusts in relation to changes in the quality and quantity of environmental radiation (e.g., UVA, UVB, and by PAR) [27, 28, 60–62], as well as in relation to other environmental variables such as salinity, temperature, desiccation [44, 63], and N availability [24, 26–28, 59, 64–66]. However, it has been suggested that there are no consistent MAA induction patterns, indicating that the induction, formation, and accumulation of MAAs are very flexible and species-specific mechanisms [28, 62]. Overall, the most common MAAs in red macroalgae are shinorine, palythine, palythinol, asterina-330, and porphyra-334, all of which have absorption maxima in the UVA region, along with mycosporine-glycine, which has its absorption maximum in the UVB region (310 nm).
MAAs in species from Patagonia
The Patagonian region is the only continental landmass along the mid-latitudes in the Southern Hemisphere. It includes Pacific and Atlantic lowlands and coasts, southern archipelagos, channels, fiords, valleys, tablelands, and high plains extending between the Andes and the Atlantic Ocean [67]. The marine flora of this area comprises unique communities characterized by the presence of endemic groups inter-mixed with sub-tropical and sub-Antarctic components [68, 69]. Studies on the presence and synthesis of MAAs in red macroalgae from Patagonia are limited compared with other areas, including the Antarctic. Some studies have screened MAAs in species from north Patagonia [25, 58, 70]. Korbee-Peinado collected 22 species from Chiloé Island in Chile (41° S, 73° O) and Camarones Bay in Argentina (44° 33′S, 65° 22′W) [70]. The species that showed the highest MAA concentrations were from the genera Bostrychia (4.7 mg.g–1 dw; 14% mycosporine-glycine and 75% shinorine) and Porphyra (4.5 mg.g–1 dw; 1.5% mycosporine-glycine and 86% porphyra-334). Huovinen et al. [25] studied the MAA content of 13 red macroalgae species collected from different sites and depths in southern Chile (Valdivia and Chiloé Island) and concluded that the MAA concentration is generally higher in species inhabiting locations with high intensities of solar irradiation. They also observed the highest MAA concentration in Porphyra columbina (7.2–10.6 mg.g–1 dw), while the lowest concentrations were observed in Polysiphonia sp. (less than 2 mg.g–1 dw). They identified six different MAAs, with shinorine being the most common and present in all algae species studied.
Although the red macroalgae species from Patagonia studied to date have not shown high MAA concentrations, some of these species could vary their MAA concentrations during the day or under stressful conditions. In this context, Helbling et al. [58] worked with four species collected from Playa Barrancas Blancas-Rawson, Chubut, Argentina (43° 19′S, 65° 3′W), and observed that the concentration of UV-absorbing MAA compounds in Ceramium sp. varied as a function of solar irradiance, with maximum values occurring around local noontime. They also observed that the differential responses of UV-absorbing compound concentrations were more associated with the previous light history of the algae (i.e., resulting from their position in the intertidal zone) than with the radiation treatment imposed on the samples. They determined the presence of MAAs in red algae using a simple UV-visible spectra analysis of methanolic extracts, and the total concentration was estimated as the area under the curve in the UV region. Afterward, each MAA was identified using high-performance liquid chromatography (HPLC).
Because many laboratories do not have the ability to detect these compounds via HPLC, a simple UV-visible spectrum analysis of methanolic extracts can be used as a prospecting technique to discriminate species with high concentrations of total MAAs and to determine the absorption maxima of the extracts. Studies on phytoplankton species [71] and macroalgae [44, 58] have shown that this technique can be a good estimator of the concentrations of MAA.
Methanolic extracts of the most conspicuous red algae species from the Strait of Magellan (South Patagonia: 53° 37′ S, 70° 59′ W) were analyzed in a spectrophotometer (290–400 nm, Spectroquant® Pharo 300) (Fig. 1). The concentration of UV-absorbing compounds (as area under the curve) was standardized by dry weight (mg.g–1 dw). While most species showed absorption maxima at 332–336 nm (UVA), other species such as Lophurella hoockeriana showed a peak at 304 nm (UVB). Because no MAAs with absorption maxima below 309 nm have been reported (Table 1), the peak at 304 nm in L. hoockeriana could be attributed to Gadusol, a compound usually found together with MAAs in marine organisms that shows a maximum at 296 nm at physiological pH, shifting to lower wavelengths with decreasing pH [72]. However, the peak at 304 nm could be due to phenolic compounds, substances that have also been found in red macroalgae [73, 74]. The concentration of UV-absorbing compounds was highest in Pyropia columbina, as expected. However, other species showed similar concentrations to P. columbina: Bostrychia sp., Lophurella hoockeriana, Polysiphonia sp., and Ceramium rubrum (Fig. 2).
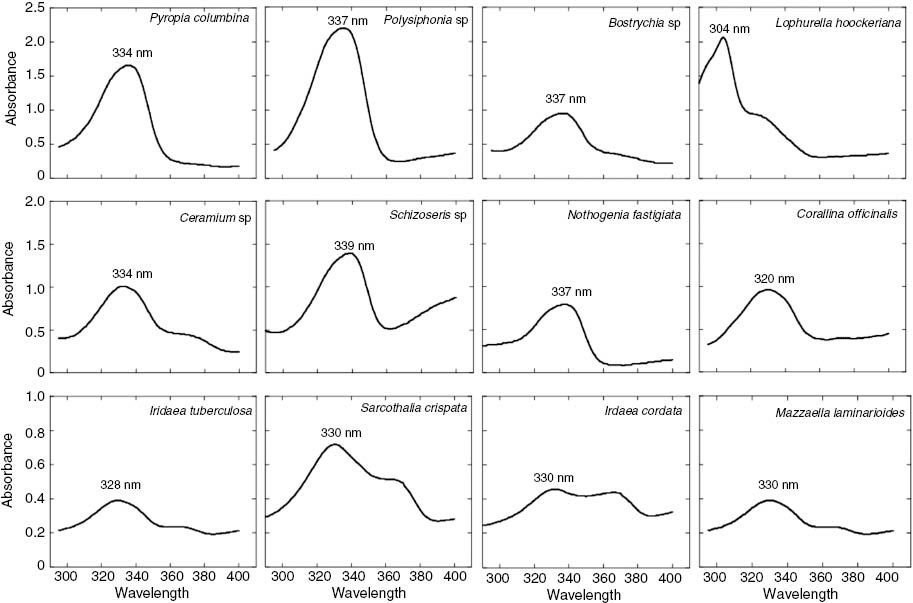
Absorption spectra of 100% methanol extracts of different red macroalgal species from the coasts of the Strait of Magellan. Each curve is an average of five measurements.
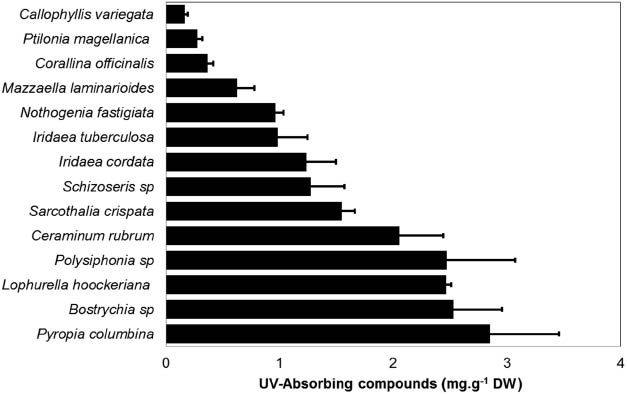
Content of UV-absorbing compounds in different red macroalgal species from the coast of the Strait of Magellan. UV-absorbing compounds were estimated from the area under the curve in the UV region and standardized by dry weight biomass (expressed as mg.g–1 dw) (n=5).
Increased concentrations of MAAs in species from the Strait of Magellan were observed by Navarro et al. [28, 59]. The MAA content was increased in Mazzaella laminarioides (from 2.1 to 3.24 mg.g–1 dw) under solar radiation and nitrogen supply [59]. However, one of the most important and promising results of this study was the accumulation of mycosporine-glycine. This increase occurred when M. laminarioides was exposed to high solar radiation, depending on the nitrate concentration, with the accumulation becoming saturated at approximately 0.18 mM of nitrate [59]. The importance of this result lies in the fact that mycosporine-glycine is the only MAA in red macroalgae that can absorb in the UVB region. In the case of Pyropia columbina, the total MAA concentration increased (from 3 to 10 mg.g–1 dw) after 4 h of exposure to solar radiation [28]. This result is promising in an economic context because this species accumulated 4 times more total MAAs after only 4 h of exposure to the full solar spectrum. Thus, both species could be used as a source of photoprotectors: P. columbina for its high total MAA concentration, and M. laminarioides for its mycosporine-glycine content. The results observed in P. columbina and M. laminarioides from the Strait of Magellan are compared with other species cultivated under nitrogen supply in Table 2.
Concentrations of MAAs (mg.g–1 dw) observed in P. columbina and M. laminarioides from the Strait of Magellan compared with other species cultivated under different quantities of nitrogen supply.
| Species and culture condition | Seawater without additives | Enriched seawater, using different quantities of nitrogen | References |
|---|---|---|---|
| Asparagopsis armata (Solar outdoor) | 2.5±0.2 | [65] | |
| Gracilaria tenuistipitata | 0.3±0.03 | 2.5±0.06 | [66] |
| Artificial PAR+UV (7 days) | |||
| Grateloupia lanceola | 1.9±0.12 | 3.4±0.25 | [64] |
| Artificial PAR+UV (14 days) | |||
| Porphyra leucosticta | 4.3±0.63 | 9.7±0.45 | [26] |
| Artificial PAR+UV (6 days) | |||
| Porphyra columbina | 3.7±0.76 | 8.9±0.90 | [24] |
| Artificial PAR+UV (6 days) | |||
| Pyropia (= Porphyra ) columbina | 2.2±0.6 | 10.4±1.1 | [28] |
|
Solar outdoor (4 h)
Mazzaella laminarioides |
|||
| Solar outdoor (18 days) | 2.27±0.3 | 3.03±0.31 | [59] |
| Mycosporine-glycine | 0.04±0.01 | 0.11±0.06 |
Bold values indicate studies performed with algae from South Patagonia.
Perspective
The use of MAAs and other products derived from algae in biotechnological products is limited by the low concentrations of MAAs reported in algae and by the inherent difficulty of ensuring sufficient algal biomass for product extraction. Thus, species with high MAA concentrations and a high and sustainable year-round production of biomass are desirable. In general, biomass limitations and the possible over-harvest of species have been overcome through the development of intensive macroalgae-growing techniques under controlled conditions [75]. Another alternative is to obtain two or more compounds (including MAAs) from the same biomass using the biorefinery concept to take advantage of the biomass in a sustainable way.
Another plausible solution is the diversification of MAA sources among various Patagonian red macroalgae species. This diversification would ensure sufficient algal biomass for MAA extraction, even though the concentrations observed in these species are not significantly higher than in other species worldwide. L. hoockeriana from South Patagonia showed absorption maxima at 304 nm and a high MAA concentration, and this species could become a model to determine the types of compounds present and how to increase their concentration. It is important to note that few macroalgae have MAAs that absorb in the UVB region, and their concentrations are generally low (e.g., mycosporine-glycine) [25, 59].
UVB-absorbing compounds could be used in commercial preparations to prevent sunburn, which is heavily weighted in the UVB range [55]. At present, most of the sunscreens available on the market protect mainly against UVA radiation and its effects of aging and possibly the development of melanoma [76]. A valuable combination of highly efficient and photostable filters that afford optimally balanced protection against both UVA and UVB could be obtained by combining mycosporine-glycine with other MAAs, such as shinorine and pophyra-334. Information about the content and composition of MAAs in different species around the world is accumulating, and a database of information on the presence or absence of specific MAAs in different groups of organisms has become available (e.g., http://www.fyboa.uma.es/?page_id=276). However, the search for species with high MAA content or the ability to synthesize these compounds in culture conditions is an unfinished task.
Article note
A collection of invited papers based on presentations at the 16th International Congress on Photobiology (ICP-16), Córdoba, Argentina, 7–12 September 2014.
Acknowledgments
This work was financed by program number 027110, Universidad de Magallanes, Chile.
References
[1] W. van de Poll, A. Eggert, A. Buma, A. Breeman. J. Phycol.37, 30 (2001).10.1046/j.1529-8817.2001.037001030.xSearch in Google Scholar
[2] M. Y. Roleda, W. H. van de Poll, D. Hanelt, C. Wiencke. Mar. Ecol.Prog. Ser. 281, 37 (2004).10.3354/meps281037Search in Google Scholar
[3] L. Franklin, R. Forster. Eur. J. Phycol.32, 207 (1997).10.1017/S0967026297001327Search in Google Scholar
[4] F. L. Figueroa, S. Salles, J. Aguilera, C. Jiménez, J. Mercado, B. Viñegla, A. Flores-Moya, M. Altamirano. Mar. Ecol. Prog. Ser.151, 81 (1997).10.3354/meps151081Search in Google Scholar
[5] I. Gómez, E. Pérez-Rodríguez, B. Viñegla, F. L. Figueroa, U. Karsten. J. Photoch. Photobiol. B: Biol.47, 46 (1998).10.1016/S1011-1344(98)00199-7Search in Google Scholar
[6] F. L. Figueroa, I. Gómez. J. Appl. Phycol.13, 235 (2001).10.1023/A:1011126007656Search in Google Scholar
[7] C. T. Shiu, T. M. Lee. J. Exp. Bot.56, 2851 (2005).10.1093/jxb/eri277Search in Google Scholar
[8] K. Bischof, I. G.mez, M. Molis, D. Hanelt, U. Karsten, U. Lüder, M. Y. Roleda, K. Zacher, C. Wiencke. Rev. Environ. Sci. Biotechnol.5, 141 (2006).10.1007/s11157-006-0002-3Search in Google Scholar
[9] M. F. Pessoa. Emir. J. Food Agric.24, 510 (2012).10.9755/ejfa.v24i6.510526Search in Google Scholar
[10] D. L. Mitchell, D. Karentz. “The induction and repair of DNA photodamage in the environment”, in Environment UV Photobiology, A. R. Young, L. O. Björn, J. Moan, W. Nultsch, (Eds.), pp. 345–377, Plenum Press, New York (1993).10.1007/978-1-4899-2406-3_12Search in Google Scholar
[11] H. Pakker, C. A. C. Beekman, A. Breeman. Eur. J. Phycol.35, 109 (2000).10.1080/09670260010001735691Search in Google Scholar
[12] C. S. Cockell, J. Knowland. Biol. Rev.74, 311 (1999).10.1017/S0006323199005356Search in Google Scholar
[13] J. Aguilera, A. Dummermuth, U. Karsten, R. Schrick, C. Wiencke. Polar Biol.25, 432 (2002).10.1007/s00300-002-0362-2Search in Google Scholar
[14] A. L. Dummermuth, U. Karsten, K. M. Fisch, G. M. König, C. Wiencke. J.Exp. Mar. Biol. Ecol.289, 103 (2003).10.1016/S0022-0981(03)00042-XSearch in Google Scholar
[15] S. Connan, F. Goulard, V. Stiger, E. Deslandes, E. Ar Gall. Bot. Mar.47, 410 (2004).10.1515/BOT.2004.057Search in Google Scholar
[16] N. P Navarro, A. Mansilla, E. M. Plastino. Micron41, 899 (2010).10.1016/j.micron.2010.06.004Search in Google Scholar PubMed
[17] E. Pérez-Rodríguez, J. Aguilera, I. Gómez, F. L. Figueroa. Mar. Biol.139, 633 (2001).Search in Google Scholar
[18] H. Pavia, G. Cervin, A. Lindgren, P. Aberg. Mar. Ecol. Prog. Ser.157, 139 (1997).10.3354/meps157139Search in Google Scholar
[19] R. T. Abdala-Díaz, A. Cabello-Pasini, E. Pérez-Rodríguez, R. M. Conde -Ávarez, F. L. Figueroa. Mar. Biol.148, 459 (2006).10.1007/s00227-005-0102-6Search in Google Scholar
[20] I. Gómez, P. Huovinen. Photochem. Photobiol.86, 1056 (2010).10.1111/j.1751-1097.2010.00786.xSearch in Google Scholar
[21] P. S. M. Celis-Plá, N. Korbee, A. Gómez-Garreta, F. L. Figueroa. Scientia Marina78, 377 (2014).10.3989/scimar.04053.05ASearch in Google Scholar
[22] U. Karsten, T. Sawall, D. Hanelt, K. Bischof, F. L. Figueroa, A. Flores-Moya, C. Wiencke. Bot. Mar.41, 443 (1998).10.1515/botm.1998.41.1-6.443Search in Google Scholar
[23] U. Karsten, L. A. Franklin, K. Lüning, C. Wiencke. Planta.205, 257 (1998).10.1007/s004250050319Search in Google Scholar
[24] N. Korbee-Peinado, R. Abdala-Díaz, F. L. Figueroa. J. Phycol.40, 248 (2004).10.1046/j.1529-8817.2004.03013.xSearch in Google Scholar
[25] P. Huovinen, I. Gómez, F. L. Figueroa, N. Ulloa, V. Morales, C. Lovengreen. Bot. Mar.47, 21 (2004).10.1515/BOT.2004.003Search in Google Scholar
[26] N. Korbee, P. Huovinen, F. L. Figueroa, J. Aguilera, U. Karsten. Mar. Biol.146, 645 (2005).10.1007/s00227-004-1484-6Search in Google Scholar
[27] N. Korbee, F. L. Figueroa, J. Aguilera. J. Photochem. Photobiol. B: Biol.80, 71 (2005).10.1016/j.jphotobiol.2005.03.002Search in Google Scholar
[28] N. P. Navarro, A. Mansilla, F. L. Figueroa, N. Korbee, J. Jofre, E. M. Plastino. Bot. Mar.57, 9 (2014).10.1515/bot-2013-0090Search in Google Scholar
[29] N. Korbee, F. L. Figueroa, J. Aguilera. Rev. Chil. Hist. Nat.79, 119 (2006).10.4067/S0716-078X2006000100010Search in Google Scholar
[30] J. I. Carreto, M. O. Carignan. Mar. Drugs9, 387 (2011).10.3390/md9030387Search in Google Scholar PubMed PubMed Central
[31] M. F. Pessoa. Emir. J. Food Agric.24, 527 (2012).10.9755/ejfa.v24i6.527545Search in Google Scholar
[32] K. Hoyer, U. Karsten, T. Sawall, C. Wiencke. Mar. Ecol. Prog. Ser.211, 117 (2001).10.3354/meps211117Search in Google Scholar
[33] U. Karsten, T. Friedl, R. Schumann, K. Hoyer, S. Lembcke. J. Phycol.41, 557 (2005).10.1111/j.1529-8817.2005.00081.xSearch in Google Scholar
[34] R. P. Sinha, M. Klisch, A. Gröniger, D. P. Häder. J. Photoch. Photobio. B: Biol.47, 83 (1998).10.1016/S1011-1344(98)00198-5Search in Google Scholar
[35] A. Oren, N. Gunde-Cimerman. FEMS Microbiol. Lett.269, 1 (2007).10.1111/j.1574-6968.2007.00650.xSearch in Google Scholar
[36] W. C. Dunlap, B. E. Chalker, J. K. Oliver. J. Exp. Mar. Biol. Ecol.104, 239 (1986).10.1016/0022-0981(86)90108-5Search in Google Scholar
[37] W. C. Dunlap, J. M. Shick. J. Phycol.34, 418 (1998).10.1046/j.1529-8817.1998.340418.xSearch in Google Scholar
[38] D. Karentz, J. E. Cleaver, D. L. Mitchell. J. Phycol.27, 326 (1991).10.1111/j.0022-3646.1991.00326.xSearch in Google Scholar
[39] F. R. Conde, M. S. Churio, C. M. Previtali. Photochem. Photobiol. Sci.3, 960 (2004).10.1039/b405782aSearch in Google Scholar
[40] W. M. Bandaranayake. Nat. Prod. Rep.15, 159 (1998).10.1039/a815159ySearch in Google Scholar PubMed
[41] F. R. Conde, M. S. Churio, C. M. Previtali. J. Photoch. Photobiol. B: Biol.56, 139 (2000).10.1016/S1011-1344(00)00066-XSearch in Google Scholar
[42] J. M. Shick, W. C. Dunlap. Annu. Rev. Physiol.64, 223 (2002).10.1146/annurev.physiol.64.081501.155802Search in Google Scholar
[43] W. C. Dunlap, Y. Yamamoto. Comp. Biochem. Physiol.112B, 105 (1995).Search in Google Scholar
[44] H. Jiang, K. Gao, E. W. Helbling. J. Appl. Phycol.20, 387 (2008).10.1007/s10811-007-9268-2Search in Google Scholar
[45] K. Bischof, R. Rautenberger. Seaweed responses to environmental stress: reactive oxygen and antioxidative strategies, in Seaweed biology. Novel insights into ecophysiology, ecology and utilization Ecological studies, Vol. 219, C. Wiencke, K. Bischof, (Eds.), pp. 109–132, Springer, Heidelberg (2012).10.1007/978-3-642-28451-9_6Search in Google Scholar
[46] J. Collen, I. R. Davison. J. Phycol.35, 54 (1999).10.1046/j.1529-8817.1999.3510054.xSearch in Google Scholar
[47] J. Collen, I. R. Davison. Plant Cell and Environment, 22, 1143 (1999).10.1046/j.1365-3040.1999.00477.xSearch in Google Scholar
[48] L. Contreras, A. Moenne, J. A. Correa. J. Phycol.41, 1184 (2005).10.1111/j.1529-8817.2005.00151.xSearch in Google Scholar
[49] J. de la Coba, J. Aguilera, F. L. Figueroa, M. V. Gálvez, E. Herrera. J. Appl. Phycol.21, 161 (2009).10.1007/s10811-008-9345-1Search in Google Scholar
[50] M. Yoshiki, K. Tsuge, Y. Tsuruta, T. Yoshimura, K. Koganemaru, T. Sumi, T. Matsui, K. Matsumoto. Food Chem.113, 1127 (2009).10.1016/j.foodchem.2008.08.087Search in Google Scholar
[51] N. N. Rosic, S. Dove. Applied and Environmental Microbiology. 77, 8478 (2011).10.1128/AEM.05870-11Search in Google Scholar
[52] T. Misonou, J. Saitoh, S. Oshiba, Y. Tokimoto, M. Maegawa, Y. Inoue, H. Hori, T. Sakurai. Mar. Biotechnol.5, 194 (2003).10.1007/s10126-002-0065-2Search in Google Scholar
[53] M. Klisch, D. P. Häder. Mar. Drugs6, 147 (2008).10.3390/md6020147Search in Google Scholar
[54] W. C. Dunlap. Redox Rep.4, 304 (1999).10.1179/135100099101535142Search in Google Scholar PubMed
[55] A. F. McKinlay, B. L. Diffey. CIE J.6, 17 (1987).10.1177/030631287017003003Search in Google Scholar
[56] F. de la Coba, J. Aguilera, M. V. de Gálvez, M. Alvarez, E. Gallego, F. L. Figueroa, E. Herrera. J. Dermatol. Sci.55, 161 (2009).10.1016/j.jdermsci.2009.06.004Search in Google Scholar
[57] K. Cardozo, T. Guaratini, M. P. Barros, V. R. Falcão, A. P. Tonon, N. P. Lopes, S. Campos, M. A. Torres, A. O. Souza, P. Colepicolo, E. Pinto. Comp. Biochem. Physiol.146C, 60 (2007).10.1016/j.cbpc.2006.05.007Search in Google Scholar
[58] E. W. Helbling, E. S. Barbieri, R. P. Sinha, V. E. Villafañe, D. P. Häder. J. Photochem. Photobiol. B: Biol.75, 63 (2004).10.1016/j.jphotobiol.2004.05.006Search in Google Scholar
[59] N. P. Navarro, F. L. Figueroa, N. Korbee, A. Mansilla, B. Matsuhiro, T. Barahona, E. M. Plastino. Photochem. Photobiol.90, 1299 (2014).10.1111/php.12344Search in Google Scholar
[60] J. M. Shick, S. D. Romaine-Lioud, C. Ferrier-Pages, J. P. Gattuso. Limnol. Oceanogr.44, 1667 (1999).10.4319/lo.1999.44.7.1667Search in Google Scholar
[61] L. A. Franklin, G. Kräbs, R. Kuhlenkamp. J. Phycol.37, 257 (2001).10.1046/j.1529-8817.2001.037002257.xSearch in Google Scholar
[62] K. Hoyer, U. Karsten, C. Wiencke. Mar. Biol.41, 619 (2002).Search in Google Scholar
[63] U. Karsten, A. Dummermuth, K. Hoyer, C. Wiencke Polar Biol.26, 249 (2003).10.1007/s00300-002-0462-zSearch in Google Scholar
[64] P. Huovinen, J. Matos, I. S. Pinto, F. L. Figueroa. Aquat. Bot.84, 308 (2006).10.1016/j.aquabot.2005.12.002Search in Google Scholar
[65] F. L. Figueroa, A. Bueno, N. Korbee, R. Santos, L. Mata, A. Schuenhoff. J. World Aquacul. Soc.39, 692 (2008).10.1111/j.1749-7345.2008.00199.xSearch in Google Scholar
[66] J. Bonomi-Barufi, N. Korbee, M. Oliveira, F. L. Figueroa. 2011. J. Appl. Phycol.23, 457 (2011).10.1007/s10811-010-9603-xSearch in Google Scholar
[67] A. M. J. Coronato, F. Coranato, E. Mazzoni, M. Vásquez. The Physical Geography of Patagonia and Tierra del Fuego, in The late cenozoic of Patagonia and Tierra del Fuego, J. Rabassa, (Ed.), Elsevier, Amsterdam (2009).10.1016/S1571-0866(07)10003-8Search in Google Scholar
[68] B. Santelices. Phycologia19, 1 (1980).10.2216/i0031-8884-19-1-1.1Search in Google Scholar
[69] K. Lüning. Seaweeds: their environment, biogeography and ecophysiology, Wiley Interscience, New York (1990).Search in Google Scholar
[70] N. Korbee-Peinado. Fotorregulación y efecto del nitrógeno inorgánico en la acumulación de aminoácidos tipo micosporina en algas rojas, Tesis Doctoral, Servicio de Publicaciones, Universidad de Málaga, España (2004).Search in Google Scholar
[71] W. C. Dunlap, G. A. Rae, E. W. Helbling, V. E. Villafañe, O. Holm-Hansen. Antarct. J. US30, 323 (1995).Search in Google Scholar
[72] P. A. Plack, N. W. Fraser, P. T. Grant, C. Middleton, A. I. Mitchel, R. H. Thomson. Biochem. J.199, 741 (1981).10.1042/bj1990741Search in Google Scholar
[73] Y. Athukorala, K. W. Lee, C. Song, C. B. Ahn, T. S. Shin, Y. J. Cha, F. Shahidi, Y. J. Jeon. J. Food Lipids10, 251 (2003).10.1111/j.1745-4522.2003.tb00019.xSearch in Google Scholar
[74] Q. Zhang, J. Zhang, Shen, A. Silva, D. A. Dennis, C. J. Barrow. J. Appl. Phycol. 18, 445 (2006).10.1007/s10811-006-9048-4Search in Google Scholar
[75] M. Freidlander, I. Levy. J. Appl. Phycol.7, 315 (1995).10.1007/BF00004005Search in Google Scholar
[76] C. S. Sander, H. Chang, F. Hamm, P. Elsner, J. J. Thiele. Int. J. Dermatol.43, 326 (2004).10.1111/j.1365-4632.2004.02222.xSearch in Google Scholar
©2015 IUPAC & De Gruyter
Articles in the same Issue
- Frontmatter
- Conference papers
- A brief illustrated history on sunscreens and sun protection
- Simulation of sunscreen performance
- Sunscreens of red algae from Patagonia: a biotechnological perspective
- In vitro photoprotection and antioxidant capacity of Sphagnum meridense extracts, a novel source of natural sunscreen from the mountains of Colombia
- Defect-rich ZnO quantum dots as a potential multifunctional sunscreen and cosmetic active ingredient
- Photochemistry and photophysics of mycosporine-like amino acids and gadusols, nature’s ultraviolet screens
- Vitamin B2-sensitized degradation of the multifunctional drug Evernyl, in the presence of visible light – microbiological implications
- Combined heterogeneous metal/organic catalysts for eco-friendly synthesis
- Amino acid sequence controls the self-assembled superstructure morphology of N-acetylated tri-β3-peptides
- Bioinspired infrared detection using thermoresponsive hydrogel nanoparticles
- IUPAC Technical Reports
- Brief guide to the nomenclature of inorganic chemistry
- Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report)
Articles in the same Issue
- Frontmatter
- Conference papers
- A brief illustrated history on sunscreens and sun protection
- Simulation of sunscreen performance
- Sunscreens of red algae from Patagonia: a biotechnological perspective
- In vitro photoprotection and antioxidant capacity of Sphagnum meridense extracts, a novel source of natural sunscreen from the mountains of Colombia
- Defect-rich ZnO quantum dots as a potential multifunctional sunscreen and cosmetic active ingredient
- Photochemistry and photophysics of mycosporine-like amino acids and gadusols, nature’s ultraviolet screens
- Vitamin B2-sensitized degradation of the multifunctional drug Evernyl, in the presence of visible light – microbiological implications
- Combined heterogeneous metal/organic catalysts for eco-friendly synthesis
- Amino acid sequence controls the self-assembled superstructure morphology of N-acetylated tri-β3-peptides
- Bioinspired infrared detection using thermoresponsive hydrogel nanoparticles
- IUPAC Technical Reports
- Brief guide to the nomenclature of inorganic chemistry
- Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report)

