Abstract
Esophageal cancer is a highly aggressive disease with a poor prognosis, significantly impacting patients’ quality of life through symptoms such as difficulty in swallowing, malnutrition, and overall deterioration in health. Palliative care plays a crucial role, as median survival in advanced cases is typically limited to only a few months. This mini paper evaluates palliative treatment options for the management of swallowing difficulties, including self-expanding metal stents, internal radiation therapy, external beam radiation therapy, and combined chemotherapy and radiation therapy. It aims to clearly define the clinical significance and role of external beam radiation therapy as a palliative treatment option for swallowing difficulties in advanced esophageal cancer. Combining radiation therapy with chemotherapy or exploring novel radiation fractionation schedules may further improve treatment outcomes. The perspective summarizes recent clinical reports on the use of external beam radiation therapy in the palliative management of swallowing difficulties, compares its effectiveness with other treatment modalities, and discusses its potential to improve patient care through integrated therapeutic approaches and emerging strategies.
Introduction
Esophageal cancer is a highly malignant disease with a poor prognosis and is characterized by a rapidly increasing global incidence and mortality [1]. According to Liu et al., there were approximately 600,000 new cases and 540,000 deaths annually worldwide in 2020 [2]. They estimated that by 2030, there will be around 740,000 new cases and 720,000 deaths, and by 2040, the numbers are expected to rise to approximately 990,000 new cases and 910,000 deaths globally. Dysphagia is one of the most common and distressing symptoms in patients with advanced esophageal cancer, affecting 80–90 % of individuals with this terminal disease [3]. It significantly impairs the quality of life by restricting nutritional intake, leading to weight loss, malnutrition, and a decline in overall health [4]. For patients with a median survival of 3–6 months, effective palliative care is essential to alleviate symptoms and enhance comfort.
Several palliative interventions are available for managing dysphagia, including self-expanding metal stents (SEMS), external beam radiotherapy (EBRT), brachytherapy, and chemoradiotherapy [5], 6]. SEMS provide rapid relief from dysphagia but are associated with frequent complications and the need for repeated interventions [7]. Brachytherapy offers sustained symptom control but is less commonly used owing to its invasive nature and limited accessibility [8]. EBRT has become a widely used alternative, offering effective relief from gastrointestinal cancer symptoms with fewer complications [9], [10], [11]. The target field for palliative radiotherapy for dysphagia in esophageal cancer typically includes the radiological tumor volume with limited margins accounting for respiratory motion and setup errors without elective nodal irradiation (Figure 1). This review aims to clearly define the clinical role of EBRT as a palliative treatment option for dysphagia in patients with advanced esophageal cancer. This evaluates the effectiveness and characteristics of EBRT in comparison with other palliative modalities, explores the potential of combination therapies and novel radiation schedules, and discusses future strategies to enhance the quality of patient care.
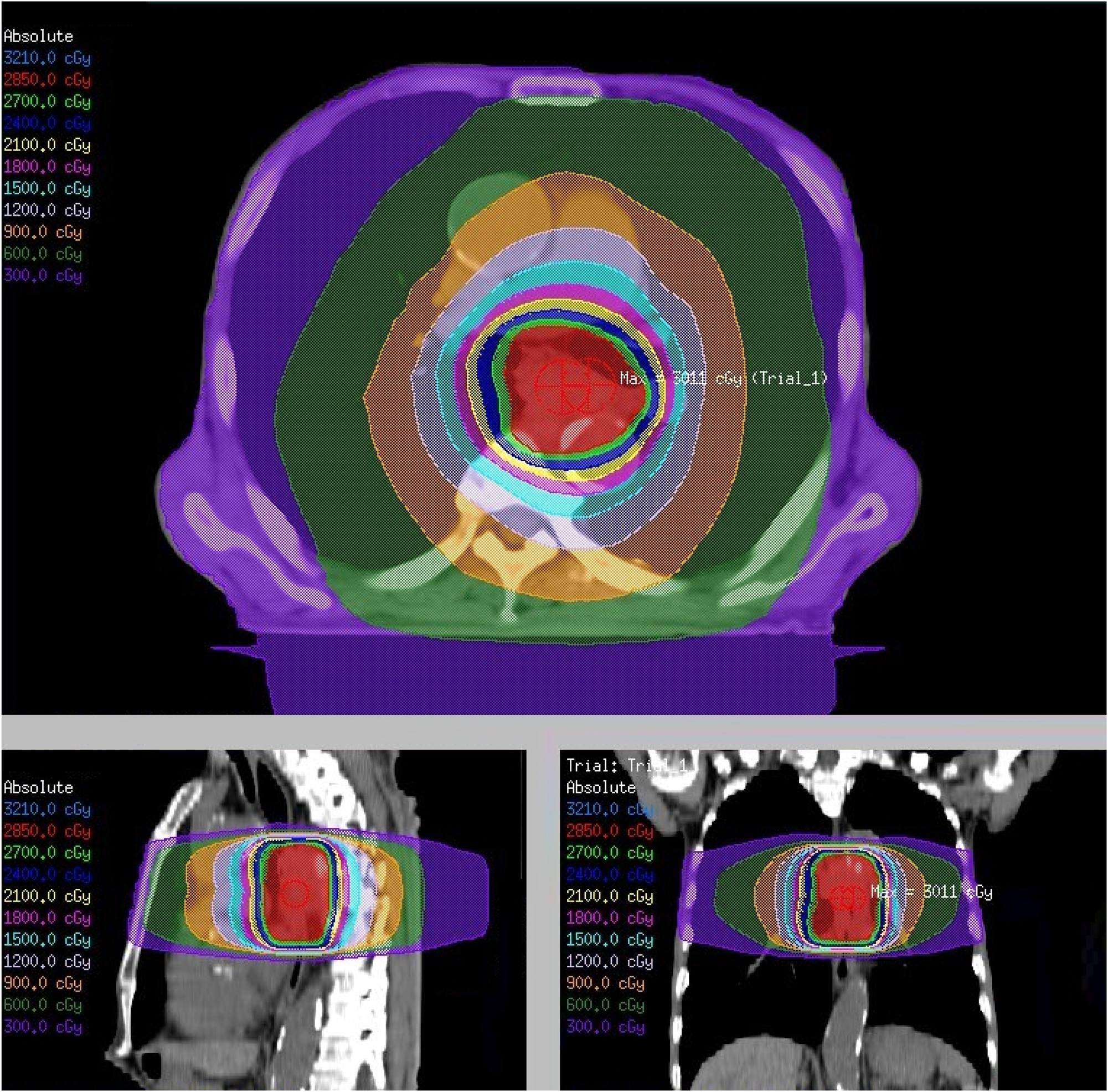
Palliative external beam radiotherapy for dysphagia in advanced esophageal cancer. A radiation dose distribution plan for a prescription dose of 30 Gy in 10 fractions. The top image represents an axial cross-section, the bottom left image represents a sagittal section, and the bottom right image represents a coronal section. The color-coded isodose lines indicate different radiation dose levels measured in centigray (cGy). The maximum dose is noted as “Max: 3011 cGy”. This figure is based on the institutional data and treatment planning system and has not been published elsewhere.
External beam radiotherapy
Palliative EBRT is an effective approach for symptom relief in patients with terminal esophageal cancer. However, there is no consensus on the optimal dose or fractionation schedule [12]. Ólafsdóttir et al. conducted a retrospective cohort study to evaluate the outcomes of short-course palliative radiotherapy, delivering 20 Gy in 5 fractions over 5 days, and long-course radiotherapy, delivering 30–39 Gy in 10–13 fractions over 10–13 days for esophageal cancer at the Karolinska University Hospital between 2009 and 2013 [13]. The dysphagia relief rates were similar between the groups (30.8 % vs. 22.0 %, p=0.341), and there was no significant difference in overall survival rates. Acute toxicity, including esophagitis and nausea (each p<0.05), was significantly lower with short-course radiotherapy, whereas re-interventions were more frequent after short-course treatment; however, the difference was not statistically significant (32.0 % vs. 18.9 %, p=0.098). The study concluded that short-course radiotherapy was equally effective, better tolerated, and associated with fewer acute toxicities than long-course RT. Vermeulen et al. conducted a multicenter retrospective study comparing low-dose radiation (LR; 20 Gy in 5 fractions EBRT) and high-dose radiation (HR; 30 Gy in 10 fractions EBRT or 12 Gy single-dose brachytherapy) for the palliation of dysphagia in inoperable or metastatic esophageal cancer [14]. Both regimens provided effective short-term relief, with dysphagia improvement at 6 weeks observed in 50 % of the LR patients and 66 % of HR patients (p=0.071). Persistent or recurrent dysphagia was significantly less frequent in the HR group (42 % vs. 64 %, p=0.012), and median survival was notably longer in the HR group (177 vs. 88 days, p<0.001). Both treatments were well-tolerated, with no significant differences in the severity of adverse events.
Several studies have explored non-conventional dose and fractionation regimens for palliative EBRT. Hypofractionated volumetric modulated arc therapy (VMAT) was evaluated in 22 patients with esophageal cancer who were unsuitable for curative treatment. Patients received 40 Gy in 16 fractions [15]. The treatment was well tolerated, with no grade 3 acute toxicities or interruptions. Dysphagia improved by the end of treatment in 63.6 % of the patients, and 79 % achieved complete recovery (grade 0–1 dysphagia) during follow-up. Dosimetric results ensured effective target coverage and sparing of normal tissue. One-year progression-free and overall survival rates were 20 and 27.3 %, respectively. A study conducted by Powell et al. evaluated the efficacy and toxicity of continuous hyperfractionated accelerated radiotherapy (CHART) in patients with localized esophageal cancer compared with historical controls treated with conventional RT [16]. Among the 54 patients who underwent CHART, 65 % experienced improved dysphagia, with 52 % eating a normal diet by week 12. Dysphagia relief lasted for a median of 7.8 months compared to 5.5 months in controls. Strictures occurred in 61 % of CHART patients, with 18 patients confirmed to have recurrent disease. The median survival time was 12 months for CHART patients and 15 months for controls. CHART was well-tolerated and provided effective local control.
Comparison to brachytherapy
Intracavitary brachytherapy (BT) has also been used in palliative radiotherapy. In 2004, a phase 3 trial (Stent or Intraluminal Radiotherapy for inoperable Esophageal Cancer (SIREC) trial) compared single-dose brachytherapy at 12 Gy with stent placement for dysphagia in esophageal cancer [17]. The results demonstrated that brachytherapy provided superior long-term relief with fewer complications (21 % vs. 33 %) and comparable costs, establishing it as the preferred primary palliative treatment. In a propensity score-matched cohort analysis, Jeene et al. reported that dysphagia improvement at 3 months without reintervention was significantly higher with EBRT (83 %) than with BT (64 %; p=0.048). Moreover, dysphagia improvement occurred earlier with EBRT, observed in 67 % of patients at 2 weeks, compared to 35 % with brachytherapy [18]. The incidence of severe toxicity was lower with EBRT (3 % vs. 13 %). These findings suggest that EBRT is at least as effective as brachytherapy for dysphagia palliation and may be better tolerated.
van Rossum et al. compared patients with incurable esophageal cancer and demonstrated that short-course EBRT delivered at 20 Gy in 5 fractions achieved similar or better patient-reported outcomes at 3 months than single-dose brachytherapy for dysphagia palliation [19]. Although both treatments improved dysphagia, brachytherapy was associated with a significant decline in functioning and increased pain, appetite loss, and taste disturbances, further emphasizing the advantages of EBRT. A randomized trial conducted by Rosenblatt et al. investigated the efficacy of combining EBRT and brachytherapy to improve the palliation of esophageal cancer compared to brachytherapy alone [20]. Among the 219 patients, combination therapy significantly improved dysphagia relief (+18 % benefit at 200 days, p=0.019) and symptom scores, odynophagia, regurgitation, chest pain, and performance status. There were no differences in weight, toxicity, or overall survival rates between groups. They concluded that the combination of EBRT and brachytherapy provided enhanced symptom relief and was well-tolerated.
The review conducted by Lancellotta et al. evaluated the efficacy and safety of palliative brachytherapy for esophageal cancer, demonstrating a median dysphagia-free survival of 99 days and a median overall survival of 175.5 days, with acceptable toxicity profiles [21]. Despite these favorable outcomes supporting brachytherapy as an effective treatment option, its clinical use remains limited. A survey of 1,510 members of the Italian Association of Radiation Oncologists revealed that among 40 surveyed brachytherapy centers, only 17.5 % (7 centers) performed esophageal brachytherapy, and 7.5 % (3 centers) considered it a first-line treatment [22].
Combination with systemic therapy
Palliative chemotherapy can effectively manage dysphagia in patients with esophageal cancer, particularly those with mild symptoms [23]. However, the concurrent use of palliative radiotherapy remains controversial. A multicenter randomized trial (TROG 03.01) compared palliative chemoradiotherapy (CRT) with radiotherapy (RT) alone for malignant dysphagia in patients with advanced esophageal cancer [24]. Dysphagia relief was achieved in 45 % of patients undergoing CRT compared to 35 % of those undergoing RT alone (p=0.13), with no significant differences in progression-free survival (4.1 vs. 3.4 months, p=0.58) or overall survival (6.9 vs. 6.7 months, p=0.88) rates. CRT was associated with significantly higher rates of grade 3–4 toxicity (36 % vs. 16 %, p=0.0017), including increased anemia, neutropenia, esophagitis, and mucositis. The study concluded that short-course RT alone is a safe and well-tolerated option for the palliative treatment of malignant dysphagia, with limited benefits from combining chemotherapy.
Borg et al. conducted a phase II trial investigating the combination of short-course RT (20 Gy in 5 fractions) followed by adjuvant chemotherapy (FOLFOX regimen) as a treatment strategy for patients with terminal esophageal adenocarcinoma experiencing dysphagia [25]. Among the 29 patients, dysphagia improvement was achieved in 79 %, lasting a median of 6.7 months (12.2 months for responders), with a median overall survival of 9.9 months. In a per-protocol analysis, the dysphagia improvement rate was 91 %, with a median duration of 12.2 months and an overall survival of 16.0 months. Common grade 3-4 adverse events included neutropenia (29 %), infection (25 %), and esophagitis (11 %). Harney et al. conducted a prospective study to evaluate the feasibility, efficacy, and toxicity of concurrent chemotherapy combined with CHARTWEL (continuous, hyperfractionated, accelerated radiotherapy weekend less) in 19 patients with locally advanced, inoperable esophageal cancer [26]. RT doses ranged from 40.5 to 49.5 Gy and were combined with the administration of cisplatin and 5-fluorouracil. All patients completed RT with acceptable acute toxicity and no treatment-related deaths. The median dysphagia-free time was 9.6 months, with 38 % of the patients being dysphagia-free at 42 months.
A retrospective study evaluated CRT in patients with inoperable squamous esophageal carcinoma and compared its outcomes with those of endoscopic stenting [27]. CRT involved the administration of 5-fluorouracil, cisplatin, and concurrent RT (50–60 Gy over 6 weeks). Among the 36 patients who underwent CRT, treatment was completed by 89 %, with no mortality. CRT reduced tumor volume in 53 % of cases, and 11 % of patients underwent salvage esophagectomy. Compared with the stenting group, CRT significantly improved the 5-year survival (15 % vs. 0 %, p=0.01) and median survival (10.8 vs. 4.0 months, p<0.005) and reduced the need for stenting (22 % vs. 100 %, p=0.005). CRT is effective for palliative care and offers superior survival outcomes.
Over the past decade, immune checkpoint inhibitor therapy has emerged as a major advancement in the treatment of esophageal cancer, demonstrating substantial improvements in patient survival [28], 29]. Zhao et al. reported that combining immune checkpoint inhibitors with radiotherapy is both effective and safe for patients with advanced esophageal squamous cell carcinoma, with radiotherapy significantly enhancing survival outcomes and treatment response [30].
Combination with self-expanding metal stents
SEMS are widely used for dysphagia palliation owing to their prompt effects. Stent placement significantly improved dysphagia in over 80 % of patients, with technical success in 100 % of cases [31]. However, complications such as stent migration, reflux, tumor overgrowth, and tissue hyperplasia can occur. Esophageal stent complications include migration, chest pain, bleeding, and perforation, affecting approximately 30 % of the patients [32].
In a phase 3 trial, combining EBRT with SEMS for advanced esophageal cancer did not improve dysphagia outcomes, survival, or quality of life compared to usual care. EBRT reduces the risk of bleeding but increases costs and fatigue. Routine EBRT with SEMS is not recommended, although it may benefit patients at high risk of tumor bleeding [33]. Chen et al. investigated whether SEMSs affected radiation dosing during the management of malignant dysphagia [34]. Using a tissue-mimicking model, dose enhancement was observed on the anterior surface of the stents, depending on the design and material. This study concluded that dose adjustments are unnecessary if multiple radiation fields are used because dose enhancement is limited to specific stent designs and surfaces.
Conclusions
Palliative strategies for advanced esophageal cancer must balance efficacy, toxicity, and the patient’s quality of life. EBRT remains an important treatment option, offering a reasonable therapeutic effect with acceptable toxicity. Future research should focus on personalized treatment algorithms to optimize palliative care and resource utilization in this patient population.
-
Research ethics: Not applicable.
-
Informed consent: Not applicable.
-
Author contributions: Atsuto Katano was the sole contributor to this work. Conceptualization, methodology, literature review, data curation, formal analysis, writing – original draft, writing – review and editing, visualization, and project administration were all performed by the author. The author read and approved the final manuscript
-
Use of Large Language Models, AI and Machine Learning Tools: None declared.
-
Conflict of interest: The author states no conflict of interest.
-
Research funding: None declared.
-
Data availability: Not applicable.
References
1. Zhang, C, Chen, L, Xiu, Y, Zhang, H, Zhang, Y, Ying, W. Burden of esophageal cancer in global, regional and national regions from 1990 to 2021 and its projection until 2050: results from the GBD study 2021. Front Oncol 2024;14:1518567. https://doi.org/10.3389/fonc.2024.1518567.Search in Google Scholar PubMed PubMed Central
2. Liu, CQ, Ma, YL, Qin, Q, Wang, PH, Luo, Y, Xu, PF, et al.. Epidemiology of esophageal cancer in 2020 and projections to 2030 and 2040. Thorac Cancer 2023;14:3–11. https://doi.org/10.1111/1759-7714.14745.Search in Google Scholar PubMed PubMed Central
3. Coia, LR, Soffen, EM, Schultheiss, TE, Martin, EE, Hanks, GE. Swallowing function in patients with esophageal cancer treated with concurrent radiation and chemotherapy. Cancer 1993;71:281–6. https://doi.org/10.1002/1097-0142(19930115)71:2<281::aid-cncr2820710202>3.0.co;2-0.10.1002/1097-0142(19930115)71:2<281::AID-CNCR2820710202>3.0.CO;2-0Search in Google Scholar
4. Wang, Y, Xie, Z, Liu, Y, Wang, J, Liu, Z, Li, S. Symptom clusters and impact on quality of life in esophageal cancer patients. Health Qual Life Outcomes 2022;20:168. https://doi.org/10.1186/s12955-022-02084-9.Search in Google Scholar
5. Guyer, DL, Almhanna, K, McKee, KY. Palliative care for patients with esophageal cancer: a narrative review. Ann Transl Med 2020;8:1103. https://doi.org/10.21037/atm-20-3676.Search in Google Scholar
6. DeCarli, K, Guyer, D, Almhanna, K. Palliative care for patients with gastroesophageal cancer at all stages: a narrative review. Ann Palliat Med 2024;13:641–53. https://doi.org/10.21037/apm-22-1243.Search in Google Scholar
7. van der Bogt, RD, Vermeulen, BD, Reijm, AN, Siersema, PD, Spaander, MCW. Palliation of dysphagia. Best Pract Res Clin Gastroenterol 2018;36–37:97–103. https://doi.org/10.1016/j.bpg.2018.11.010.Search in Google Scholar
8. Kawamoto, T, Nakamura, N, Saito, T, Tonari, A, Wada, H, Harada, H, et al.. Palliative brachytherapy and external beam radiotherapy for dysphagia from esophageal cancer: a nationwide survey in Japan. Jpn J Clin Oncol 2021;51:950–5. https://doi.org/10.1093/jjco/hyab015.Search in Google Scholar
9. Katano, A, Yamashita, H. The impact of palliative radiation therapy on patients with advanced gastric cancer: results of a retrospective cohort study. Cureus 2022;14:e32971. https://doi.org/10.7759/cureus.32971.Search in Google Scholar
10. Katano, A, Yamashita, H. Usefulness of palliative radiotherapy in reducing the frequency of red blood cell transfusion in patients with malignant tumor bleeding. J Cancer Res Therapeut 2023;19:753–6. https://doi.org/10.4103/jcrt.jcrt_2090_21.Search in Google Scholar
11. Katano, A, Yamashita, H. The efficacy of hemostatic radiotherapy for advanced malignancies assessed by world health organization bleeding status. Cureus 2021;13:e19939. https://doi.org/10.7759/cureus.19939.Search in Google Scholar PubMed PubMed Central
12. Walterbos, NR, Fiocco, M, Neelis, KJ, van der Linden, YM, Langers, AMJ, Slingerland, M, et al.. Effectiveness of several external beam radiotherapy schedules for palliation of esophageal cancer. Clin Transl Radiat Oncol 2019;17:24–31. https://doi.org/10.1016/j.ctro.2019.04.017.Search in Google Scholar PubMed PubMed Central
13. Ólafsdóttir, HS, Klevebro, F, Ndegwa, N, von Döbeln, GA. Short-course compared to long-course palliative radiotherapy for oesophageal cancer: a single centre observational cohort study. Radiat Oncol 2021;16:153. https://doi.org/10.1186/s13014-021-01880-9.Search in Google Scholar PubMed PubMed Central
14. Vermeulen, BD, Jeene, PM, Sijben, J, Krol, R, Rütten, H, Bogers, JA, et al.. Low-dose versus high-dose radiation therapy for the palliation of dysphagia from esophageal cancer: a multicenter retrospective cohort study. Pract Radiat Oncol 2020;10:e255–e263. https://doi.org/10.1016/j.prro.2019.10.010.Search in Google Scholar PubMed
15. Deantonio, L, Cima, S, Leva, S, Richetti, A, Valli, M. Hypofractionated volumetric modulated arc therapy (VMAT) for fragile patients with oesophageal cancer. Clin Transl Oncol 2020;22:1532–8. https://doi.org/10.1007/s12094-020-02293-y.Search in Google Scholar PubMed
16. Powell, ME, Hoskin, PJ, Saunders, MI, Foy, CJ, Dische, S. Continuous hyperfractionated accelerated radiotherapy (CHART) in localized cancer of the esophagus. Int J Radiat Oncol Biol Phys 1997;38:133–6. https://doi.org/10.1016/s0360-3016(96)00582-2.Search in Google Scholar PubMed
17. Homs, MY, Steyerberg, EW, Eijkenboom, WM, Tilanus, HW, Stalpers, LJA, Bartelsman, JFWM, et al.. Single-dose brachytherapy versus metal stent placement for the palliation of dysphagia from oesophageal cancer: multicentre randomised trial. Lancet 2004;364:1497–504. https://doi.org/10.1016/s0140-6736(04)17272-3.Search in Google Scholar
18. Jeene, PM, Vermeulen, BD, Rozema, T, Braam, PM, Lips, I, Muller, K, et al.. Short-course external beam radiotherapy versus brachytherapy for palliation of dysphagia in esophageal cancer: a matched comparison of two prospective trials. J Thorac Oncol 2020;15:1361–8. https://doi.org/10.1016/j.jtho.2020.04.032.Search in Google Scholar PubMed
19. van Rossum, PSN, Jeene, PM, Rozema, T, Braam, PM, Lips, IM, Muller, K, et al.. Patient-reported outcomes after external beam radiotherapy versus brachytherapy for palliation of dysphagia in esophageal cancer: a matched comparison of two prospective trials. Radiother Oncol 2021;155:73–9. https://doi.org/10.1016/j.radonc.2020.10.009.Search in Google Scholar PubMed
20. Rosenblatt, E, Jones, G, Sur, RK, Donde, B, Salvajoli, JV, Ghosh-Laskar, S, et al.. Adding external beam to intra-luminal brachytherapy improves palliation in obstructive squamous cell oesophageal cancer: a prospective multi-centre randomized trial of the International Atomic Energy Agency. Radiother Oncol 2010;97:488–94. https://doi.org/10.1016/j.radonc.2010.09.001.Search in Google Scholar PubMed
21. Lancellotta, V, Cellini, F, Fionda, B, De Sanctis, V, Vidali, C, Fusco, V, et al.. The role of palliative interventional radiotherapy (brachytherapy) in esophageal cancer: an AIRO (Italian Association of Radiotherapy and Clinical Oncology) systematic review focused on dysphagia-free survival. Brachytherapy 2020;19:104–10. https://doi.org/10.1016/j.brachy.2019.09.005.Search in Google Scholar PubMed
22. Fuccio, L, Guido, A, Hassan, C, Frazzoni, L, Arcelli, A, Farioli, A, et al.. Underuse of brachytherapy for the treatment of dysphagia owing to esophageal cancer. An Italian survey. Dig Liver Dis 2016;48:1233–6. https://doi.org/10.1016/j.dld.2016.07.003.Search in Google Scholar PubMed
23. Levy, A, Wagner, AD, Chargari, C, Moehler, M, Verheij, M, Durand-Labrunie, J, et al.. Palliation of dysphagia in metastatic oesogastric cancers: an international multidisciplinary position. Eur J Cancer 2020;135:103–12. https://doi.org/10.1016/j.ejca.2020.04.032.Search in Google Scholar PubMed
24. Penniment, MG, De Ieso, PB, Harvey, JA, Stephens, S, Au, HJ, O’Callaghan, CJ, et al.. Palliative chemoradiotherapy versus radiotherapy alone for dysphagia in advanced oesophageal cancer: a multicentre randomised controlled trial (TROG 03.01). Lancet Gastroenterol Hepatol 2018;3:114–24. https://doi.org/10.1016/s2468-1253(17)30363-1.Search in Google Scholar
25. Borg, D, Sundberg, J, Brun, E, Kjellén, E, Petersson, K, Hermansson, M, et al.. Palliative short-course hypofractionated radiotherapy followed by chemotherapy in esophageal adenocarcinoma: the phase II PALAESTRA trial. Acta Oncol 2020;59:212–8. https://doi.org/10.1080/0284186x.2019.1670861.Search in Google Scholar
26. Harney, J, Goodchild, K, Phillips, H, Glynne-Jones, R, Hoskin, PJ, Saunders, MI. A phase I/II study of CHARTWEL with concurrent chemotherapy in locally advanced, inoperable carcinoma of the oesophagus. Clin Oncol (R Coll Radiol) 2003;15:109–14. https://doi.org/10.1053/clon.2003.0200.Search in Google Scholar PubMed
27. Wong, SK, Chiu, PW, Leung, SF, Cheung, KY, Chan, ACW, Au-Yeung, ACM, et al.. Concurrent chemoradiotherapy or endoscopic stenting for advanced squamous cell carcinoma of esophagus: a case-control study. Ann Surg Oncol 2008;15:576–82. https://doi.org/10.1245/s10434-007-9679-y.Search in Google Scholar PubMed
28. Shoji, Y, Koyanagi, K, Kanamori, K, Tajima, K, Ogimi, M, Ninomiya, Y, et al.. Immunotherapy for esophageal cancer: where are we now and where can we go. World J Gastroenterol 2024;30:2496–501. https://doi.org/10.3748/wjg.v30.i19.2496.Search in Google Scholar PubMed PubMed Central
29. Moughnyeh, MM, Green, M, Katuwal, B, Hammoud, ZT. Current landscape of immunotherapy in esophageal cancer: a literature review. J Thorac Dis 2024;16:8807–14. https://doi.org/10.21037/jtd-24-1145.Search in Google Scholar PubMed PubMed Central
30. Zhao, XH, Gao, HM, Wen, JY, Wang, HS, Wu, LY, Song, CY, et al.. Immune checkpoint inhibitors combined with or without radio(chemo)therapy for locally advanced or recurrent/metastatic esophageal squamous cell carcinoma. Discov Oncol 2023;14:165. https://doi.org/10.1007/s12672-023-00783-3.Search in Google Scholar PubMed PubMed Central
31. Kim, KY, Tsauo, J, Song, HY, Kim, PH, Park, JH. Self-Expandable metallic stent placement for the palliation of esophageal cancer. J Kor Med Sci 2017;32:1062–71. https://doi.org/10.3346/jkms.2017.32.7.1062.Search in Google Scholar PubMed PubMed Central
32. Hindy, P, Hong, J, Lam-Tsai, Y, Gress, F. A comprehensive review of esophageal stents. Gastroenterol Hepatol (NY) 2012;8:526–34.Search in Google Scholar
33. Adamson, D, Byrne, A, Porter, C, Blazeby, J, Griffiths, G, Nelson, A, et al.. Palliative radiotherapy after oesophageal cancer stenting (ROCS): a multicentre, open-label, phase 3 randomised controlled trial. Lancet Gastroenterol Hepatol 2021;6:292–303. https://doi.org/10.1016/s2468-1253(21)00004-2.Search in Google Scholar
34. Chen, YK, Schefter, TE, Newman, F. Esophageal cancer patients undergoing external beam radiation after placement of self-expandable metal stents: is there a risk of radiation dose enhancement? Gastrointest Endosc 2011;73:1109–14. https://doi.org/10.1016/j.gie.2011.02.001.Search in Google Scholar PubMed
© 2025 the author(s), published by De Gruyter on behalf of Tech Science Press (TSP)
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- Review Articles
- Unveiling the hidden role of tumor-educated platelets in cancer: a promising marker for early diagnosis and treatment
- Multiple roles of mitochondria in tumorigenesis and treatment: from mechanistic insights to emerging therapeutic strategies
- The impact of JMJD5 on tumorigenesis: a literature review
- Research Articles
- A case-matched comparison of ER-α and ER-β expression between malignant and benign cystic pancreatic lesions
- Salivary gamma-glutamyltransferase activity as an indicator of redox homeostasis in breast cancer
- Cancer can be suppressed by alkalizing the tumor microenvironment: the effectiveness of “alkalization therapy” in cancer treatment
- Percutaneous-assisted laparoscopic bilateral salpingo-oophorectomy in BRCA-mutated patients: a retrospective comparative study
- ACAT2 contributes to cervical cancer tumorigenesis by regulating the expression of the downstream gene LATS1
- Rapid Communication
- Efficacy of mild hyperthermia in cancer therapy: balancing temperature and duration
- Case Report
- Orbital marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue with amyloidosis: a case series and review of the literature
- Commentary
- Palliative external beam radiotherapy for dysphagia management in advanced esophageal cancer: a narrative perspective
- Endometriosis and endometriosis-associated ovarian cancer, possible connection and early diagnosis by evaluation of plasma microRNAs
Articles in the same Issue
- Frontmatter
- Review Articles
- Unveiling the hidden role of tumor-educated platelets in cancer: a promising marker for early diagnosis and treatment
- Multiple roles of mitochondria in tumorigenesis and treatment: from mechanistic insights to emerging therapeutic strategies
- The impact of JMJD5 on tumorigenesis: a literature review
- Research Articles
- A case-matched comparison of ER-α and ER-β expression between malignant and benign cystic pancreatic lesions
- Salivary gamma-glutamyltransferase activity as an indicator of redox homeostasis in breast cancer
- Cancer can be suppressed by alkalizing the tumor microenvironment: the effectiveness of “alkalization therapy” in cancer treatment
- Percutaneous-assisted laparoscopic bilateral salpingo-oophorectomy in BRCA-mutated patients: a retrospective comparative study
- ACAT2 contributes to cervical cancer tumorigenesis by regulating the expression of the downstream gene LATS1
- Rapid Communication
- Efficacy of mild hyperthermia in cancer therapy: balancing temperature and duration
- Case Report
- Orbital marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue with amyloidosis: a case series and review of the literature
- Commentary
- Palliative external beam radiotherapy for dysphagia management in advanced esophageal cancer: a narrative perspective
- Endometriosis and endometriosis-associated ovarian cancer, possible connection and early diagnosis by evaluation of plasma microRNAs


