A case-matched comparison of ER-α and ER-β expression between malignant and benign cystic pancreatic lesions
-
Panagis M. Lykoudis
, Ioannis Papaconstantinou
Abstract
Objectives
Older studies failed to establish a link between estrogen receptor (ER) expression and pancreatic adenocarcinoma, with findings either statistically insignificant or contradictory. The recent discovery of two distinct isoforms, namely ER-α and ER-β, with potentially differential expression in various pancreatic cells and with radically different effects, might explain preexisting contradictions. Therefore, the identification of two new ER types necessitates a reassessment of their role in the development and prognosis of pancreatic adenocarcinoma. This study aimed to investigate their differential expression in benign and malignant pancreatic lesions.
Methods
A case-matched study was conducted comparing benign and malignant pancreatic lesions in patients who underwent surgery at University Hospital ‘Aretaieio’, a tertiary referral centre for pancreatic disease. The expression of ER-α and ER-β receptors was assessed by immunohistochemistry and scored using two established scoring systems. Data regarding age, gender, histological diagnosis, grade, TNM stage, adjuvant treatment, follow-up, recurrence, and survival were recorded, and respective correlations were assessed.
Results
ER-α was detected in 31.25 % of benign tumours but not in any malignant tumours (p=0.003). ER-β was positive in 31.25 % of benign and 37.50 % of malignant tumours (p=0.757). Disease-free survival (p=0.910) and overall survival (p=0.623) were similar between ER-β negative and ER-β positive patients.
Conclusions
Pancreatic adenocarcinomas do not express ER-α, which might explain the failure of earlier trials targeting these receptors, but they do express ER-β at a considerable rate. Differences in the expression of ER-α and ER-β can be used to distinguish benign and malignant lesions. Moreover, further studies delineating the molecular function of ER-β and assessing it as a potential therapeutic target can lead to improved clinical outcomes.
Introduction
While research in the field of oncology has led to a significant improvement in survival from most types of malignancies, pancreatic cancer mortality is slowly rising [1]. Despite the advancements in surgical techniques and the technological progress in the equipment used, which has led to minimizing intra-operative tissue damage and postoperative complications, the overall mortality rate hasn’t been reduced accordingly. On the contrary, survival is steadily and slowly increasing in patients with cancer types originating from other tissues [2]. Pancreatic cancer remains the third cause of cancer-related mortality with a 5-year survival rate of 12 % [3],4]. The first reason for the dismal prognosis of pancreatic cancer is that only less than 20 % of patients are diagnosed at an operable stage, hence amenable to treatment with curative intent [5], rendering timely diagnosis extremely important.
Early diagnosis is correlated with a broad understanding of molecular patterns and pathways involved in the pathophysiologic mechanisms surrounding disease progression. The literature contains adequate data, contributing to the understanding of the genetic background [6]. Most patients are diagnosed with locally advanced or metastatic disease, with systematic chemotherapy being their only option. The second reason is that pancreatic cancer is a chemotherapy refractory disease, with minimal response even in modern agents [7]. This implies a complexity at a molecular level, which is interpreted as additional mechanisms contributing to malignant transformation, despite the oncological effectiveness of well-established drug compounds. These factors underline the continuous need for investigation of new prognostic markers and therapeutic targets for pancreatic cancer. Since diagnostic imaging modality interpretation has reached a peak level, attention should be drawn to deeper basic science comprehension, as well as combined drug therapies, which have already been proven efficient against similar pathogenetic mechanisms in different diseases [8].
Expression of estrogen receptors (ER) in pancreatic cells, including premalignant pancreatic lesions as well as pancreatic cancer, generated optimism that a novel target could have been discovered [9]. Unfortunately, subsequent preclinical and clinical studies did not confirm the efficacy of estrogen antagonists in the treatment of pancreatic cancer [10], [11], [12]. Therefore, research interest in the field declined as findings were considered conclusive. However, it recently became apparent that there are two types of estrogen receptors, demonstrating different expression in various types of normal and malignant pancreatic cells, different activity in terms of proliferation pathways and different responses to treatment with classic anti-estrogens [13], 14]. The limitations of these studies included the small and heterogeneous patient groups, as well as an inadvertent focus on ER-α. Small study sample neither allows extraction of results with adequate power, nor appropriate case-control matching of variables to further amplify the statistical significance of the evidence [15]. Recent studies focusing on the expression of the newest type of ER, namely ER-β, have demonstrated a potential prognostic as well as therapeutic role for the receptor [16], 17]. In fact, in a large-sample retrospective study in postmenopausal women, hormone replacement therapy was associated with a reduced risk of pancreatic cancer [18]. Despite limitations such as the inherent limitations of retrospective, cross-sectional study design, limiting the generalization of results to other populations and weakening causality, the results established a connection between hormone therapy against estrogen receptors and pancreatic cancer. Additionally, a study on murine syngeneic tumour models and human xenografts [19], suggested that G protein-coupled estrogen receptor agonists may be useful against a range of cancers, including those that are not classically considered sex hormone responsive, and those that arise from tissues outside of the reproductive system, such as pancreatic adenocarcinoma. In mice, 17β-estradiol protects β-cell survival through ER-α and ER-β via estrogen response element-independent, extra-nuclear mechanisms, as well as GPER-dependent mechanisms [20]. Additionally, tamoxifen inhibited the proliferation of β-cells in a dose-dependent manner, with dramatic reductions in β-cell turnover at the highest dose as demonstrated in mice models by Ahn et al. [21].
As stated by Prossnitz et al. G protein-coupled estrogen receptors hold the potential to become a diagnostic, prognostic and therapeutic target in clinical medicine. One of the suggested implementations concerns the repurposing of licensed drugs. Such an effort is reflected in the ongoing first-in-human clinical trial of the GPER-selective agonist G-1 [22], 23].
The recently published article by Liao et al. underlined that progesterone receptors potentiate macropinocytosis through CDC42 in pancreatic ductal adenocarcinoma [24]. To meet their increased energy demands, cancer cells can internalize extracellular proteins via macropinocytosis. These data deepen the understanding of how the endocrine system influences tumour progression via a non-classical pathway. Additionally, they provide a novel therapeutic option for these patients. Therefore, this study paves the way for future research regarding estrogen receptors and highlights their close interaction with progesterone receptors.
The present study primarily aimed to explore the differential expression profile of ER-α and ER-β, in benign and malignant neoplasms of the pancreas, and secondarily to correlate it with oncological outcomes. In addition, it attempted to offer a resolution for the existing disagreement in the relevant available literature. Preliminary results of the study were presented as a poster at the 42nd Congress of the European Society of Surgical Oncology (ESSO) in Florence, Italy, in 2023, and the corresponding conference abstract was published in the European Journal of Surgical Oncology (Lykoudis et al., EJSO, 50 (2), 107750) [25].
Materials and methods
Data collection
The archives of the 2nd Department of Surgery, University Hospital “Aretaieio”, serving as a tertiary centre for hepato-pancreato-biliary diseases, were searched. The records of patients who underwent pancreatic resections from 2002 to 2011, with curative intent, were retrieved. Data regarding age, sex, histological diagnosis, grade, and TNM stage were recorded. The prospectively maintained database of the affiliated clinical oncology clinic was accessed to record respective adjuvant treatment, follow-up, recurrence, and survival.
This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Approval was granted on 09-04-2012 by the Bioethics Committee of the University Hospital “Aretaieio” and the document ID is 6553. Informed consent was provided by all patients in written, with regard to data and tissue collection, maintenance and processing for research purposes.
Matching
Two groups were defined based on histological diagnosis: benign cystic lesions and pancreatic ductal adenocarcinomas. Manual case-matching was performed on a 1:2 ratio. Criteria for matching were sex, age (±5 years) and neoplasm size (±2 cm), as the most common parameters, currently used to assess the likelihood of malignancy in pancreatic lesions, prior to biopsy [26].
Immunohistochemistry
Corresponding formalin-fixed, paraffin-embedded archived tissue and histopathology slides were retrieved. Sections from the archived paraffin-embedded tumour tissues were obtained and processed by the Ventana BenchMark GX automatic immunostaining unit (Roche, Basel, Switzerland), according to manufacturers’ instructions with appropriate positive and negative controls. ER-α was assessed using the SP1 rabbit monoclonal antibody (Biocare Medical, Pacheco, CA, USA), at a dilution of 1:50. ER-β was assessed using the EMR02 rabbit monoclonal antibody (Novocastra, Newcastle, UK), at a dilution of 1:100. Evaluation was performed according to the established semi-quantitative Immunoreactive Score (IRS) [12] as well as according to the semi-quantitative Allred scoring system (AS) [13]. The monoclonal antibodies for ER-α and ER-β were tested previously and during the immunohistochemical staining using positive and negative controls. For ER-α positive control was normal mammary tissue. For ER-β positive control was prostatic tissue. As for negative controls skin (epidermis) tissue was used. For the histopathological evaluation, at least three tissue blocks were tested. From these blocks, the one with the more representative features was used for immunohistochemical staining. The typical nuclear staining pattern was observed. The microscope used was the Olympus BX-43F (Olympus Corporation, Tokyo, Japan).
Statistical analysis
Bivariate correlations were assessed using Fisher’s exact test for dichotomous categorical variables, chi-square for categorical variables with more than two categories, Spearman’s correlation for scale variables and log-rank for timelines (disease-free and overall survival). Medians were compared across groups using the Mann-Whitney U test for dichotomous categorical variables and the independent samples median test for categorical variables with more than two categories. Progression-free and overall survival were assessed using Cox regression analysis. A p-value of less than 0.05 was considered statistically significant. Two-tailed comparisons were consistently used where applicable. Statistical analysis was conducted using IBM SPSS Statistics version 25 (IBM Corp., Armonk, NY, USA).
Results
A total of 209 patients were identified, that underwent pancreatic resection with curative intent over the examined period. A benign lesion was identified in 22 of these, pancreatic adenocarcinoma was identified in 129 samples, while the remaining 58 cases consisted of neuroendocrine neoplasms (n=19), adenocarcinomas of enteric and biliary origin (n=25), pancreatitis (n=11), pseudocyst (n=1) and two metastatic tumours. Upon implementation of matching criteria, six cases of benign pancreatic lesions were excluded because of the size of a tumour being too large to be matched (n=2, tumour size of 11 and 17 cm) or because of age too young to be matched (n=4, age of 25, 27, 35 and 45 years). Relaxation of matching criteria was tested, with age ±10 years and size ±5 cm, but again no suitable matches were identified, even when looking for matching of two out of three parameters. Thus, these cases were excluded, and a total of 16 benign and 32 malignant tumour cases were included for immunohistochemical assessment. Table 1 presents the demographic characteristics of the cohort and relevant subgroups, as well as the matching quality of the finally examined groups of cases. Performed surgical procedure was pancreatoduodenectomy in 30 cases (62.50 %), distal pancreatectomy in 15 cases (31.25 %) and total pancreatectomy in 3 cases (6.25 %). The examined 16 cases of benign lesions consisted of nine serous cystic neoplasms (56.25 %), five mucinous cystic neoplasms (31.25 %) and two cystic pseudopapillary neoplasms (12.50 %).
Demographics and case-matching details.
| Full cohort | Matched cases | |||||
|---|---|---|---|---|---|---|
| Total (n=209) | Pancreatic adenocarcinomas (n=129) | Benign lesions (n=22) | Pancreatic adenocarcinomas (n=32) | Benign lesions (n=16) | p-Valuee | |
| Agea | 66 (24–87) | 67 (45–87) | 63 (24–82) | 64 (45–87) | 63 (49–82) | 0.734 |
| Sexb | 91 (43.54 %) | 96 (74.42 %) | 17 (77.27 %) | 24 (75.00 %) | 12 (75.00 %) | >0.99 |
| Sizec | 4.00 (1.20–17.00) | 3.50 (1.20–8.00) | 5.00 (1.80–17.00) | 3.50 (1.20–9.00) | 4.50 (1.80–10.00) | 0.187 |
| Graded | ||||||
| I | 29 (22.48 %) | 4 (12.50 %) | ||||
| II | 60 (46.51 %) | 22 (68.75 %) | ||||
| III | 40 (31.01 %) | 6 (18.75 %) | ||||
-
aIn years, median (range); bfemale sex cases, n (percentage); cin cm, median (range); dn (percentage); ecomparison between case-match groups.
With regards to the 32 patients with pancreatic adenocarcinomas that were included in the immunohistochemical assessment, two of them had a T2 disease (6.25 %), 28 had a T3 disease (87.50 %) and two had a T4 disease (6.25 %). Seven patients (21.88 %) had no positive lymph nodes and the remaining 25 patients (78.13 %) were node-positive. Three patients (11.54 %) did not receive adjuvant chemotherapy because of comorbidity-related contraindications, while the remaining 23 (88.46 %) received gemcitabine-based chemotherapy. Median follow-up was 11 months (range: 4–60 months). Two patients were lost to follow up and four remained disease-free until the last follow-up, 5 years after the operation. All patients with recurrence eventually died of the disease. Thus, excluding the two patients who were lost to follow-up, the recurrence rate was 86.67 % and the survival rate was 13.33 %. Median disease-free survival was 5 months (range: 1–60 months) and median overall survival was 11 months (range: 4–60 months). Table 2 presents the main characteristics of the included patients.
Main characteristics of included patients.
| Variable | n (%)/median (range) |
|---|---|
| Sexa | 12 (25 %) |
| Age, yearsb | 63 (45–87) |
| Histology | |
| Benign | 16 (33.3 %) |
| Malignant | 32 (66.7 %) |
| Tumour size (maximal diameter in cm2) | 4.00 (1.20–10.00) |
| Tumour gradec | |
| I | 4 (12.5 %) |
| II | 22 (68.8 %) |
| III | 6 (18.8 %) |
| T stagec | |
| 2 | 2 (6.3 %) |
| 3 | 28 (87.5 %) |
| 4 | 2 (6.3 %) |
| n stagec | |
| 0 | 7 (21.9 %) |
| 1 | 25 (78.1 %) |
| Type of operation | |
| Pancreatoduodenectomy | 30 (62.5 %) |
| Distal pancreatectomy | 15 (31.3 %) |
| Total pancreatectomy | 3 (6.3 %) |
| Histological type | |
| Serous cystic neoplasm | 9 (18.8 %) |
| Mucinous cystic neoplasm | 6 (12.5 %) |
| Cystic intraductal papillary mucinous neoplasm | 1 (2.1 %) |
| Adenocarcinoma | 32 (66.7 %) |
| Adjuvant chemotherapyc | 23 (88.5 %) |
| Follow-up (in months)b,c | 11 (8–28) |
| Disease-free survival in monthsb,c | 6 (4, 18) |
| Recurrencec | 26 (86.7 %) |
| Overall survivalb,c | 11 (8–28) |
-
aFemale gender individuals, n (%); bmedian (range); creferring only to malignant cases.
With regards to ER-α assessment, only five samples were found positive (10.42 %), and they were all benign tumours (31.25 % of benign tumours). All malignant tumours were negative for ER-α, demonstrating a statistically significantly different expression between benign and malignant lesions (p=0.003). With regards to ER-β assessment, there was good concordance between the two assessment methods (Table 3). Thirty-one cases were identified as negative by both methods, and there was no case defined as negative by only one method. Fifteen cases were identified as weakly positive by IRS and positive with a small chance of benefit by AS. Two cases were identified as weakly positive by IRS and positive with a moderate chance of benefit by AS. ER-β expression was further analyzed as a binary variable (positive or negative) since there was good concordance between the two methods regarding negatives. A total of 17 tumours were found positive for ER-β (35.42 %), of which five were benign (31.25 % of benign tumours) and 12 were malignant (37.50 % of malignant tumours, Figure 1), which was not statistically significantly different between the two groups (p=0.757). Focusing on benign lesions, there was a statistically significant correlation between the expression of the two receptors (p=0.013). One lesion was positive only for ER-α, one lesion was positive only for ER-β, 10 lesions were negative for both receptors and four lesions were positive for both receptors. A similar statistically significant correlation was found in the total of 48 examined samples (p=0.047), where one lesion was positive only for ER-α, 13 were positive only for ER-β, 30 lesions were negative for both receptors and four were positive for both receptors. The respective correlation for malignant lesions could not be assessed since there were no ER-α positive malignant lesions. None of the other histological parameters demonstrated a statistically significant correlation with ER-β expression. Disease-free survival and overall survival were similar between ER-β negative and ER-β positive patients (p=0.910 and p=0.623 respectively).
Crosschecking of ER-β expression assessment along the two different scoring systems.
| Immunoreactive score (IRS) | ||||
|---|---|---|---|---|
| 0 | 2 | 3 | ||
| Allred score (AS) | ||||
| 0 | n=32 | – | – | |
| 2 | – | n=2 | – | |
| 3 | – | n=11 | n=2 | |
| 4 | – | – | n=2 | |
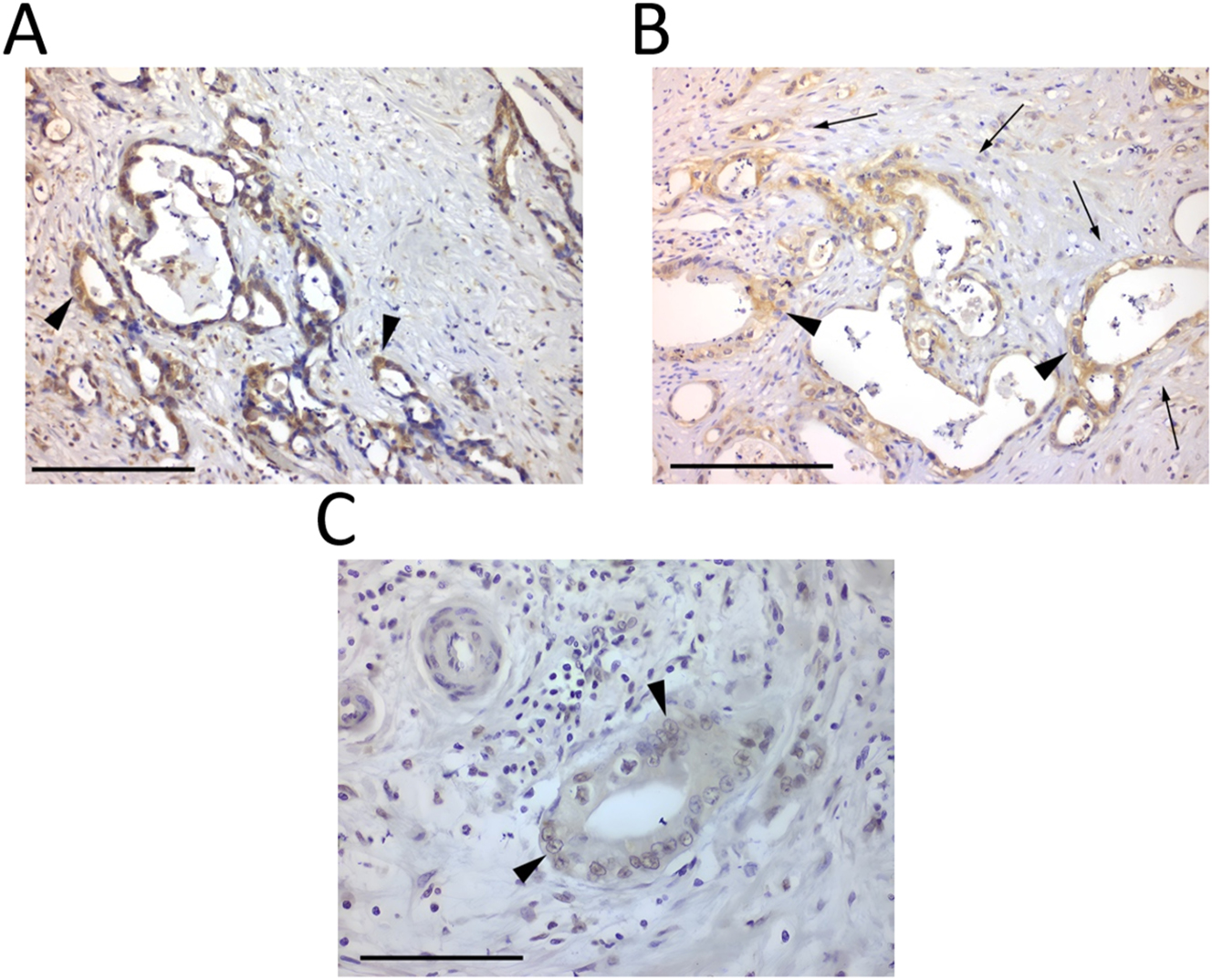
Immunohistochemistry images of pancreatic neoplasm tissues. (A) Pancreatic ductal adenocarcinoma demonstrating positive immunostaining for ER-β (black arrowhead at representative stained tumour cells) (×200, scale bar=100 μm). (B) Dense fibroblastic tissue (black arrows) surrounding neoplastic glands (black arrowhead at representative stained tumour cells), positive for ER-β (×200, scale bar=100 μm). (C) ER-β nuclear staining (black arrowhead at representative stained tumour cells) (×400, scale bar=50 μm).
Discussion
This case-matched study demonstrated a statistically significantly different expression of ER-α between benign and malignant lesions of the pancreas, with approximately 31 % of benign tumours being positive while all malignant tumours were negative. No previous study has attempted such a comparison and studies on benign pancreatic lesions are mainly case reports and very small case series. The overall lack of ER-α expression in malignant lesions of the pancreas is in line with previous medical literature [27]. Therefore, this study provides further support to the theory that early studies for ER expression in pancreatic cancer and early clinical trials with tamoxifen for pancreatic adenocarcinomas failed because they were designed to target mainly ER-α before ER-β was described [28]. Moreover, in this study, 37.5 % of malignant tumours were positive for ER-β. This finding is in accordance with the recent study by Seeliger et al. [16], which reports a respective rate of 31 % while a larger study from the same research group reported a rate of over 60 % [29]. While the first study did not identify any statistically significant correlation between ER-β expression and other histological or clinical parameters, in accordance with present findings, the second study demonstrated that ER-β expression and particularly phosphorylated ER-β was an independent prognostic factor.
A recent review summarizes the current problems in the incorporation of estrogen/progesterone receptors in the management of pancreatic ductal adenocarcinoma and postulates reasons for current discrepancies [30]. The topic has re-emerged very recently, therefore very few original studies have addressed it including up-to-date molecular knowledge [31].
Widely used Selective Estrogen Receptor Modulators (SERMs) which have been proven effective in hormone-sensitive tissues such as breast and endometrium, may play a significant role in pancreatic adenocarcinoma as well. In vitro study by Pozios et al. showed in vitro growth inhibition of pancreatic adenocarcinoma cells by raloxifene, an SERM widely used for postmenopausal osteoporosis as well as breast cancer. The interaction of raloxifene with interleukin-6 and glycoprotein-130 further complicates molecular signalling understanding [32]. Decreased efficacy issues may be overcome by novel nanoparticle carriers which can increase drug penetration, specifically to malignant cells. An interesting paper by Fahmy et al. demonstrates augmented raloxifene efficacy when combined with a carrier system consisting of phospholipid-based vesicles with melittin [33]. Okuni et al. have even gone further and combined raloxifene with a classic anticancer drug used in T-cell lymphoma, romidepsin, which proved to suppress FOXM1 [34]. FOXM1 is a tumorigenesis factor contributing to pancreatic cancer progression [35]. On the other hand, potent immunotherapy has shown promising results aiming to overcome other drug limitations. KRAS, one of the primary modulators of malignant turnover, seems to be another possible target. The mutated, permanently activated KRAS protein acts as a molecular switch to activate a variety of signalling pathways and transcription factors inducing cell proliferation, migration, transformation, and survival. Immunotherapy drugs previously perceived as ineffective [36] now seem promising and may lead to a possible breakthrough in the field of pharmacologic intervention toward pancreatic cancer management [37].
The findings of this study provide a potential explanation for the conflicting results of previous trials. The predominant detection and targeting of ER-α over ER-β may explain why traditional ER blockers failed to show significant clinical benefits in the past. SERMs specifically designed to target ER-β could have a more pronounced effect on ER-β positive tumours, potentially offering tangible clinical benefits.
The focus of this study is to compare benign and malignant pancreatic lesions, and to the best of the authors’ knowledge, this is the first study that has attempted such a comparison. It was a case-matched study which means that parameters such as gender, age and tumour size have been excluded as confounding factors for this comparison. However, the retrospective design and the small sample size should be reported as limitations. Especially the lack of correlation between ER-β expression and oncological outcomes could possibly be attributed to the small sample size and might thus represent a type II statistical error. Besides, such an objective would require a multi-factorial analysis, including all known predictive factors, and consequently a significantly larger sample size. Given the rarity of pancreatic cancer, even the largest hospitals are limited to small sample sizes for such studies. Therefore, accelerating the conduction of multi-centre studies is of great importance if the scientific community seeks to make advancements in pancreatic malignancy comprehension.
Conclusions
Overall, this study provides further evidence that different types of pancreatic lesions exhibit differential expression of the two ER subtypes. Early studies that failed to demonstrate ER expression in pancreatic cancer or clinical benefit in treatment with tamoxifen, were probably targeting ER-α, which is indeed expressed only in benign lesions. Approximately 30 % of pancreatic adenocarcinomas express ER-β and this should be further explored as a possible therapeutic target. The development of therapeutic regimens targeting specifically the ER-β subtype could perhaps reshape the management of pancreatic cancer. Previous attempts had moderate efficacy due to the non-specific targeting of estrogen receptors. Additionally, this study offers valuable data that can be used to calculate sample sizes for future multifactorial studies, essential for accurately evaluating the role of ER-β in pancreatic cancer.
-
Research ethics: This study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). Approval was granted by the Bioethics Committee of the University Hospital “Aretaieio” on 09-04-2012 and the document ID is 6553.
-
Informed consent: All patients had provided written informed consent, for maintaining clinical data and tissue samples for biomedical research.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission. Panagis M. Lykoudis – Concept, Design, Data Collection, Lab Work, Data Analysis, Drafting; Ioannis Papaconstantinou – Data Collection, Critical Revision; Nikolaos Dafnios – Data Analysis, Critical Revision; Charalampos Charalampous – Data Analysis, Drafting; Charis Konti – Data Analysis, Drafting; Dimitrios Vlachodimitropoulos – Design, Lab Work; John Contis – Concept, Drafting.
-
Use of Large Language Models, AI and Machine Learning Tools: None declared.
-
Conflict of interest: The authors state no conflict of interest.
-
Research funding: None declared.
-
Data availability: The raw data are available upon request from the corresponding author. Preliminary results of the study were presented as a poster at the 42nd Congress of the European Society of Surgical Oncology (ESSO) in Florence, Italy, in 2023, and the corresponding conference abstract was published in the European Journal of Surgical Oncology (Lykoudis et al., EJSO, 50 (2), 107750). Compared to the conference abstract, the current study presents an analysis of demographics of included papers, the details of manual case-matching, an analysis of comparisons, as well as the concordance between the two immunohistochemical scoring systems.
References
1. Didier, AJ, Nandwani, S, Fahoury, AM, Craig, DJ, Watkins, D, Campbell, A, et al.. Trends in pancreatic cancer mortality in the United States 1999-2020: a CDC database population-based study. Cancer Causes Control 2024;35:1509–16. https://doi.org/10.1007/s10552-024-01906-z.Search in Google Scholar PubMed PubMed Central
2. Carioli, G, Bertuccio, P, Boffetta, P, Levi, F, La Vecchia, C, Negri, E, et al.. European cancer mortality predictions for the year 2020 with a focus on prostate cancer. Ann Oncol 2020;31:650–8. https://doi.org/10.1016/j.annonc.2020.02.009.Search in Google Scholar PubMed
3. Siegel, RL, Giaquinto, AN, Jemal, A. Cancer statistics, 2024. CA Cancer J Clin 2024;74:12–49. https://doi.org/10.3322/caac.21820. [Erratum in CA Cancer J Clin 2024;74(2):203].Search in Google Scholar PubMed
4. Reshkin, SJ, Cardone, RA, Koltai, T. Genetic signature of human pancreatic cancer and personalized targeting. Cells 2024;13:602. https://doi.org/10.3390/cells13070602.Search in Google Scholar PubMed PubMed Central
5. Park, W, Chawla, A, O’Reilly, EM. Pancreatic cancer: a review. JAMA 2021;326:851–62. https://doi.org/10.1001/jama.2021.13027. [Erratum in: JAMA 2021;326(20):2081].Search in Google Scholar PubMed PubMed Central
6. Wang, S, Zheng, Y, Yang, F, Zhu, L, Zhu, XQ, Wang, ZF, et al.. The molecular biology of pancreatic adenocarcinoma: translational challenges and clinical perspectives. Signal Transduct Target Ther 2021;6:249. https://doi.org/10.1038/s41392-021-00659-4.Search in Google Scholar PubMed PubMed Central
7. Principe, DR, Narbutis, M, Kumar, S, Park, A, Viswakarma, N, Dorman, MJ, et al.. Long-term gemcitabine treatment reshapes the pancreatic tumor microenvironment and sensitizes murine carcinoma to combination immunotherapy. Cancer Res 2020;80:3101–15. https://doi.org/10.1158/0008-5472.can-19-2959.Search in Google Scholar PubMed PubMed Central
8. Conlon, K, Coppola, R. Updates on pancreatic cancer treatment - invited special issue. Int J Surg 2024;110:6049–51. https://doi.org/10.1097/js9.0000000000002086.Search in Google Scholar
9. Zhao, Z, Liu, W. Pancreatic cancer: a review of risk factors, diagnosis, and treatment. Technol Cancer Res Treat 2020;19:1533033820962117. https://doi.org/10.1177/1533033820962117.Search in Google Scholar PubMed PubMed Central
10. Bakkevold, KE, Pettersen, A, Arnesjø, B, Espehaug, B. Tamoxifen therapy in unresectable adenocarcinoma of the pancreas and the papilla of Vater. Br J Surg 1990;77:725–30. https://doi.org/10.1002/bjs.1800770704.Search in Google Scholar PubMed
11. Wong, A, Chan, A, Arthur, K. Tamoxifen therapy in unresectable adenocarcinoma of the pancreas. Cancer Treat Rep 1987;71:749–50.Search in Google Scholar
12. Wong, A, Chan, A. Survival benefit of tamoxifen therapy in adenocarcinoma of pancreas. A case-control study. Cancer 1993;71:2200–3. https://doi.org/10.1002/1097-0142(19930401)71:7<2200::aid-cncr2820710706>3.0.co;2-2.10.1002/1097-0142(19930401)71:7<2200::AID-CNCR2820710706>3.0.CO;2-2Search in Google Scholar
13. Barkhem, T, Carlsson, B, Nilsson, Y, Enmark, E, Gustafsson, J, Nilsson, S. Differential response of estrogen receptor alpha and estrogen receptor beta to partial estrogen agonists/antagonists. Mol Pharmacol 1998;54:105–12. https://doi.org/10.1124/mol.54.1.105.Search in Google Scholar
14. Mosselman, S, Polman, J, Dijkema, R. ER beta: identification and characterization of a novel human estrogen receptor. FEBS Lett 1996;392:49–53. https://doi.org/10.1016/0014-5793(96)00782-x.Search in Google Scholar
15. Chan, KS, Ho, BCS, Shelat, VG. A pilot study of estrogen receptor (ER) expression in pancreatic ductal adenocarcinoma (PDAC). Transl Gastroenterol Hepatol 2021;6:9. https://doi.org/10.21037/tgh.2020.02.16.Search in Google Scholar
16. Seeliger, H, Pozios, I, Assmann, G, Zhao, Y, Müller, MH, Knösel, T, et al.. Expression of estrogen receptor beta correlates with adverse prognosis in resected pancreatic adenocarcinoma. BMC Cancer 2018;18:1049. https://doi.org/10.1186/s12885-018-4973-6.Search in Google Scholar
17. Younes, M, Ly, CJ, Singh, K, Ertan, A, Younes, PS, Bailey, JM. Expression of estrogen receptor beta isoforms in pancreatic adenocarcinoma. Oncotarget 2018;9:37715–20. https://doi.org/10.18632/oncotarget.26503.Search in Google Scholar
18. Liu, L, Wang, X, Guo, D, Ma, R, Gong, H, Wang, C. Hormone replacement therapy and risk of pancreatic cancer in postmenopausal women: evidence from the US National Inpatient Sample 2008–2018. Heliyon 2024;10:e37588. https://doi.org/10.1016/j.heliyon.2024.e37588.Search in Google Scholar
19. Natale, CA, Li, J, Pitarresi, JR, Norgard, RJ, Dentchev, T, Capell, BC, et al.. Pharmacologic activation of the G protein-coupled estrogen receptor inhibits pancreatic ductal adenocarcinoma. Cell Mol Gastroenterol Hepatol 2020;10:868–80.e1. https://doi.org/10.1016/j.jcmgh.2020.04.016.Search in Google Scholar
20. Liu, S, Le May, C, Wong, WP, Wong, WPS, Ward, RD, Clegg, DJ, et al.. Importance of extranuclear estrogen receptor-alpha and membrane G protein-coupled estrogen receptor in pancreatic islet survival. Diabetes 2009;58:2292–302. https://doi.org/10.2337/db09-0257.Search in Google Scholar
21. Ahn, SH, Granger, A, Rankin, MM, Lam, CJ, Cox, AR, Kushner, JA. Tamoxifen suppresses pancreatic β-cell proliferation in mice. PLoS One 2019;14:e0214829. https://doi.org/10.1371/journal.pone.0214829.Search in Google Scholar
22. Prossnitz, ER, Barton, M. The G protein-coupled oestrogen receptor GPER in health and disease: an update. Nat Rev Endocrinol 2023;19:407–24. https://doi.org/10.1038/s41574-023-00822-7.Search in Google Scholar PubMed PubMed Central
23. Prossnitz, ER, Barton, M. The G-protein-coupled estrogen receptor GPER in health and disease. Nat Rev Endocrinol 2011;7:715–26. https://doi.org/10.1038/nrendo.2011.122.Search in Google Scholar PubMed PubMed Central
24. Liao, YN, Gai, YZ, Qian, LH, Pan, H, Zhang, YF, Li, P, et al.. Progesterone receptor potentiates macropinocytosis through CDC42 in pancreatic ductal adenocarcinoma. Oncogenesis 2024;13:10. https://doi.org/10.1038/s41389-024-00512-7.Search in Google Scholar PubMed PubMed Central
25. Lykoudis, P, Papaconstantinou, I, Dafnios, N, Vlachodimitropoulos, D, Contis, J. Differential expression of ER-α and ER-β in pancreatic neoplasms: a case-matched study. Eur J Surg Oncol 2024;50:107750. https://doi.org/10.1016/j.ejso.2023.107750.Search in Google Scholar
26. Dell’Aquila, E, Fulgenzi, CAM, Minelli, A, Citarella, F, Stellato, M, Pantano, F, et al.. Prognostic and predictive factors in pancreatic cancer. Oncotarget 2020;11:924–41. https://doi.org/10.18632/oncotarget.27518.Search in Google Scholar PubMed PubMed Central
27. Iwao, K, Miyoshi, Y, Ooka, M, Ishikawa, O, Kasugai, T, Egawa, C, et al.. Quantitative analysis of estrogen receptor-alpha and -beta messenger RNA expression in human pancreatic cancers by real-time polymerase chain reaction. Cancer Lett 2001;170:91–7. https://doi.org/10.1016/s0304-3835(01)00563-8.Search in Google Scholar PubMed
28. Konduri, S, Schwarz, RE. Estrogen receptor beta/alpha ratio predicts response of pancreatic cancer cells to estrogens and phytoestrogens. J Surg Res 2007;140:55–66. https://doi.org/10.1016/j.jss.2006.10.015.Search in Google Scholar PubMed
29. Pozios, I, Knösel, T, Zhao, Y, Assmann, G, Pozios, I, Müller, MH, et al.. Expression of phosphorylated estrogen receptor beta is an independent negative prognostic factor for pancreatic ductal adenocarcinoma. J Cancer Res Clin Oncol 2018;144:1887–97. https://doi.org/10.1007/s00432-018-2717-2.Search in Google Scholar PubMed
30. Lykoudis, PM, Contis, J. Estrogen receptor expression in pancreatic adenocarcinoma: time to reconsider evidence. Pancreas 2021;50:1250–3. https://doi.org/10.1097/mpa.0000000000001921.Search in Google Scholar
31. Wu, H, Fu, M, Wu, M, Cao, Z, Zhang, Q, Liu, Z. Emerging mechanisms and promising approaches in pancreatic cancer metabolism. Cell Death Dis 2024;15:553. https://doi.org/10.1038/s41419-024-06930-0.Search in Google Scholar PubMed PubMed Central
32. Pozios, I, Seel, NN, Hering, NA, Hartmann, L, Liu, V, Camaj, P, et al.. Raloxifene inhibits pancreatic adenocarcinoma growth by interfering with ERβ and IL-6/gp130/STAT3 signaling. Cel Oncol (Dordr) 2021;44:167–77. https://doi.org/10.1007/s13402-020-00559-9.Search in Google Scholar PubMed PubMed Central
33. Fahmy, UA, Badr-Eldin, SM, Aldawsari, HM, Alhakamy, NA, Ahmed, OAA, Radwan, MF, et al.. Potentiality of raloxifene loaded melittin functionalized lipidic nanovesicles against pancreatic cancer cells. Drug Deliv 2022;29:1863–77. https://doi.org/10.1080/10717544.2022.2072544.Search in Google Scholar PubMed PubMed Central
34. Okuni, N, Honma, Y, Urano, T, Tamura, K. Romidepsin and tamoxifen cooperatively induce senescence of pancreatic cancer cells through downregulation of FOXM1 expression and induction of reactive oxygen species/lipid peroxidation. Mol Biol Rep 2022;49:3519–29. https://doi.org/10.1007/s11033-022-07192-9.Search in Google Scholar PubMed
35. Xie, D, Yu, S, Li, L, Quan, M, Gao, Y. The FOXM1/ATX signaling contributes to pancreatic cancer development. Am J Transl Res 2020;12:4478–87.Search in Google Scholar
36. Bannoura, SF, Khan, HY, Azmi, AS. KRAS G12D targeted therapies for pancreatic cancer: has the fortress been conquered? Front Oncol 2022;12:1013902. https://doi.org/10.3389/fonc.2022.1013902.Search in Google Scholar PubMed PubMed Central
37. Cowzer, D, Zameer, M, Conroy, M, Kolch, W, Duffy, AG. Targeting KRAS in pancreatic cancer. J Personalized Med 2022;12:1870. https://doi.org/10.3390/jpm12111870.Search in Google Scholar PubMed PubMed Central
© 2025 the author(s), published by De Gruyter on behalf of Tech Science Press (TSP)
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- Review Articles
- Unveiling the hidden role of tumor-educated platelets in cancer: a promising marker for early diagnosis and treatment
- Multiple roles of mitochondria in tumorigenesis and treatment: from mechanistic insights to emerging therapeutic strategies
- The impact of JMJD5 on tumorigenesis: a literature review
- Research Articles
- A case-matched comparison of ER-α and ER-β expression between malignant and benign cystic pancreatic lesions
- Salivary gamma-glutamyltransferase activity as an indicator of redox homeostasis in breast cancer
- Cancer can be suppressed by alkalizing the tumor microenvironment: the effectiveness of “alkalization therapy” in cancer treatment
- Percutaneous-assisted laparoscopic bilateral salpingo-oophorectomy in BRCA-mutated patients: a retrospective comparative study
- ACAT2 contributes to cervical cancer tumorigenesis by regulating the expression of the downstream gene LATS1
- Rapid Communication
- Efficacy of mild hyperthermia in cancer therapy: balancing temperature and duration
- Case Report
- Orbital marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue with amyloidosis: a case series and review of the literature
- Commentary
- Palliative external beam radiotherapy for dysphagia management in advanced esophageal cancer: a narrative perspective
- Endometriosis and endometriosis-associated ovarian cancer, possible connection and early diagnosis by evaluation of plasma microRNAs
Articles in the same Issue
- Frontmatter
- Review Articles
- Unveiling the hidden role of tumor-educated platelets in cancer: a promising marker for early diagnosis and treatment
- Multiple roles of mitochondria in tumorigenesis and treatment: from mechanistic insights to emerging therapeutic strategies
- The impact of JMJD5 on tumorigenesis: a literature review
- Research Articles
- A case-matched comparison of ER-α and ER-β expression between malignant and benign cystic pancreatic lesions
- Salivary gamma-glutamyltransferase activity as an indicator of redox homeostasis in breast cancer
- Cancer can be suppressed by alkalizing the tumor microenvironment: the effectiveness of “alkalization therapy” in cancer treatment
- Percutaneous-assisted laparoscopic bilateral salpingo-oophorectomy in BRCA-mutated patients: a retrospective comparative study
- ACAT2 contributes to cervical cancer tumorigenesis by regulating the expression of the downstream gene LATS1
- Rapid Communication
- Efficacy of mild hyperthermia in cancer therapy: balancing temperature and duration
- Case Report
- Orbital marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue with amyloidosis: a case series and review of the literature
- Commentary
- Palliative external beam radiotherapy for dysphagia management in advanced esophageal cancer: a narrative perspective
- Endometriosis and endometriosis-associated ovarian cancer, possible connection and early diagnosis by evaluation of plasma microRNAs


