Photodynamic therapy in glioma cell culture
-
David Aebisher
, Kacper Rogóż
and Dorota Bartusik-Aebisher
Abstract
Photodynamic therapy (PDT) shows promise in the treatment of gliomas, the most prevalent primary malignant tumors in the central nervous system. Despite challenges such as tumor hypoxia and resistance to therapy, PDT can be used alone or in combination with other anticancer treatments. Research indicates that PDT can improve the survival of patients with malignant gliomas, although further efforts are required to standardize and optimize this therapy. Cell cultures are an indispensable tool in glioma research and PDT development. In vitro studies of PDT are crucial for assessing the effectiveness of various photosensitizing agents and light dosages on glioma cells. In vitro tests provide an initial assessment of the efficacy of a substance under controlled conditions, predicting potential effects before moving on to in vivo studies. Interest in glioma research is increasing, and a deep understanding of the molecular basis of PDT is essential to advance this therapeutic approach. This review aims to summarize current knowledge in vitro PDT in glioma cell cultures. The review highlights the importance of in vitro testing for PDT in gliomas, the underlying molecular mechanisms, and the factors that influence the efficacy of PDT. Recent advances and the necessity for in vitro studies are underscored.
Introduction
Photodynamic therapy (PDT) is a therapeutic system consisting of a photosensitizer (PS) with a high affinity for cancer tissue, visible light (usually with a wavelength of 600–800 nm) and molecular oxygen. Activation of PS with an appropriate wavelength of light results in the production of the reactive oxygen species (ROS) (1O2), superoxide anion radical (O2 −), hydroxyl radical (OH·) and hydrogen peroxide (H2O2) which leads to cell necrosis and apoptosis [1, 2]. The formation of active oxygen species can occur in two ways. Type I reactions involve electron transfer between the excited dye and oxygen. Type II reactions result in the formation of 1O2. by energy transfer from the excited dye to ground-state oxygen [3]. PDT induces a strong response from the immune system, recruits cells such as neutrophils, macrophages, and lymphocytes into tumor tissue and stimulates the production of homogenate, a specific antigen of cancer cells [1]. However, due to the mechanism of hypoxia of human cells and various types of unknown resistance, the use of PDT in clinics is limited [2]. Figure 1 shows the general principle of PDT.

The general principle of photodynamic therapy (PDT).
PDT was developed in the 1970s and is still being studied and refined for treatment. PDT is used in dermatology, dental treatment, and oncology [4, 5]. According to data from the literature, the most sensitive cells are neural fibers, endothelial cells, and fibroblasts. In turn, keratinocytes and chondrocytes are not as sensitive to the photodynamic effect[6]. The key role of PDT in inhibiting the negative and destructive impact of the therapeutic effect on healthy tissues is the appropriate selection of the exposure time and dose of PS [7]. Another way to limit the effect of PDT on noncancerous tissues is the endogenous application of a light source to cancer cells, which is extremely important in the context of treating the brain area, and one of such method is bioluminescence [8].
PDT of brain tumors is difficult due, among other reasons, insufficient light penetration and potential damage to peripheral tissues; therefore, the combination of PDT with chemotherapy, radiation therapy, immunotherapy, or other anticancer therapies has been and continues to be explored [1, 9]. Gliomas constitute approximately 77 % of brain tumors [10]. The World Health Organization (WHO) updated its classification of gliomas in the 2021 edition of the WHO Classification of Tumors of the Central Nervous System. This classification integrates molecular and histopathological data to provide a more accurate diagnosis and prognosis of gliomas (Table 1). The 2021 classification emphasizes the use of molecular diagnostics, incorporating genetic and molecular markers to refine tumor classification and prognosis [14]. Key markers include mutation status of isocitrate dehydrogenase (IDH), co-deletion 1p/19q, and telomerase (TERT) promoter mutations. The WHO classification of central nervous system (CNS) tumors has evolved from a system that aimed to standardized grading across all types to one that grades tumors within each specific type [15]. Traditionally, the lowest grade 1 was assigned to the least aggressive tumors, while grade IV indicated a highly aggressive tumor. In the newer system, tumors are classified according to their type, although this shift is gradual to maintain consistency with older classifications. For example, IDH-mutant astrocytomas start at grade 2, and glioblastoma, IDH wild type, is always grade 4 [16]. In addition, there is a growing recognition that factors such as tumor location and available treatments are often more critical than tumor grade alone in determining prognosis. For example, WNT-activated medulloblastoma, despite being a grade 4 tumor, has a good prognosis with appropriate treatment.
The WHO Classification of tumors of the central nervous system.
| Type | Description |
|---|---|
| Adult-type diffuse gliomas [11, 12] | Glioblastoma (GBM), wild-type IDH. This category includes glioblastomas that do not have IDH mutations and are characterized by specific genetic alterations such as the TERT promoter mutation, EGFR amplification, and +7/−10 chromosome copy number changes. |
| Astrocytoma, IDH-mutant. Classified into grades according to histological and molecular characteristics, ranging from grade 2 (low grade) to grade 4 (high-grade, corresponding to glioblastoma-like biology). | |
| Oligodendroglioma, IDH-mutant, and 1p/19q-co-deleted. These tumors have both IDH mutations and 1p/19q co-deletion and are classified as grade 2 or 3 based on histological characteristics. | |
| Pediatric-type diffuse low- and high-grade gliomas [13] | Pediatric-type diffuse low-grade gliomas. These include several distinct entities, such as diffuse astrocytoma, altered MYB or MYBL1, and polymorphous low-grade neuroepithelial tumors of the young. |
| Pediatric-type diffuse high-grade gliomas. Includes entities such as diffuse midline glioma, altered H3 K27, and diffuse hemispheric glioma, H3 G34. | |
| Circumscribed astrocytic gliomas [11, 12] | Entities such as pilocytic astrocytoma, pleomorphic xanthoastrocytoma, and subependymal giant cell astrocytoma are included in this category and are typically less aggressive. |
| Other glioma subtypes [11, 12] | New entities and subtypes have been recognized, and some previous categories have been redefined or merged on emerging molecular insights. |
Some gliomas are caused by congenital disorders such as tuberous sclerosis, neurofibromatosis, and Li-Fraumani syndrome [17]. All gliomas are growing rapidly and rapidly undergo angiogenesis and invade adjacent tissues. These factors significantly complicate glioma resection [10, 18]. Furthermore, the presence of blood-brain and blood-tumor barriers limits the possibility of metastases, but at the same time significantly complicates treatment. At the beginning of treatment, the glioma is resected followed by adjuvant radiation therapy and chemotherapy with temozolomide. However, the median survival ranges from 14.6 to 16.7 months [10]. Malignant gliomas are characterized by large central necrosis surrounded by a “crust” of invasive cells that migrate beyond the therapeutic margins and are difficult to remove, leading to tumor recurrence [19]. Glioma recurs in 90 % of patients [10]. The median survival after recurrence is 6.2 months [20]. Due to the above characteristics, the search for new therapeutic approaches is crucial in the fight against gliomas. The introduction of innovative therapies can significantly improve the prognosis of patients with this type of cancer. It is also important to strive to improve the quality of life of patients with gliomas by reducing symptoms and side effects and extending survival and disease-free periods. PDT seems to be an appropriate therapy for the treatment of this type of tumor [21, 22]. The pioneers in photodynamic brain therapy were Perria et al., who in 1980, as an adjunct to surgery, used PDT with hematoporphyrin as a PS and a He–Ne laser (632.8 nm) [23]. The effect of PDT on reducing the invasiveness of glioma cancer cells has also been demonstrated, but more work on the therapy is still needed to explain and exclude inconsistencies and variability of results in different patients [21].
This review compiles current knowledge on the treatment of gliomas using PDT, emphasizing studies on cell cultures and in vitro research. A thorough search in PubMed, Web of Science, and Embase revealed that PDT, which uses a light-sensitive agent and specific wavelengths of light to generate reactive oxygen species, shows promise in selectively targeting and destroying glioma cells. The in vitro results indicate that PDT can induce cell death through apoptosis or necrosis while sparing healthy tissue, depending on the type of PS used. Future directions focus on improving PS targeting, light penetration, and combining PDT with other treatments for better outcomes.
In vitro cell cultures of gliomas
Moving away from animal-based research, cell-based preclinical research is becoming more and more popular [2]. The traditional cell culture is based on a single layer of cells grown in a culture flask or in a Petri dish [23]. It has played an important role in research since the beginning of the 20th century [24]. This is a simple and cheap method that allows you to observe simple mechanisms and reactions of cell lines to the tested factors. The time required to create a cell culture is quick and the culture itself is characterized by high efficiency, repeatability, and ease of interpretation. However, such cultures are not very accurate since they do not imitate cell-cell and cell-extracellular environment interactions, as is the case in a cancer tumor. The morphology of cells of such a monolayer is changed compared to tissue of a living organism, affecting their functioning [23]. There are fewer cell connections between cells than in tissue [25]. Cells in 2D culture lose their differentiation and polarity. Such cells have unlimited access to nutrients, oxygen, metabolites, and signaling molecules, which do not reflect in vivo conditions [23]. For this reason, most cells are in the same stage of the cell cycle at the same time [25]. A simplified diagram of a two-dimensional culture based on the example of a monolayer of cells cultured on an agar substrate in a Petri dish 23], [24], [25 (Figure 2).
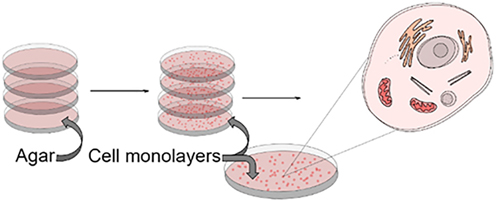
A simplified diagram of a two-dimensional culture based on the example of a monolayer of cells cultured on an agar substrate in a Petri dish.
The classic 2D culture model
The classic 2D culture model, although cheaper and simpler, is increasingly being replaced by 3-D (3D) cultures due to the reconstruction of the three-dimensional organization and functions of cells in tissues, such as glial tissue 26], [27], [28. This culture appears to be more similar to physiological tissue and this model allows the study of communication between different cells (cell-cell interactions) and with the extracellular matrix (cell-extracellular matrix interactions), and also allows the creation of a gradient of solutes across the matrix, providing a mechanism similar to normal tissue [28]. Organoid culture also seems interesting as it can reflect the structural and functional heterogeneity of the primary tumor [26]. Organoids can resemble proliferative and differentiated tissue fragments. Furthermore, due to the presence of stem cells, they can be stored in biobanks, enabling their use in experimental and clinical research. This enables the observation of proper tissue development, disease progression, and monitoring of the effects of drugs. Moreover, thanks to modern methods of genome interference, it is possible to cultivate cancer organoids, also from the same donor. This enables the testing of therapies and their effects on cancer cells and healthy cells [29].
A 3D cell culture
A 3D cell culture is defined as one in which growth is achieved without the use of a substrate for cell attachment or a substrate that promotes cell adhesion. Multicellular tumor spheroids (MCTS) are then formed with neoplastic features (heterogeneous proliferation rate and different concentrations of nutrients and oxygen, centrally located focus of necrosis, intercellular communication, and between cells and the extracellular matrix [30]. They allow observation of the regulation of proliferation, differentiation, cell death, metabolism, invasion, immune response, and angiogenesis [31, 32].
Inch et al. were the first to study tumor response to treatment [33]. MCTS allows you to obtain conditions like those in the body, imitating, among others, oxygen gradients and intracellular adhesion occurring in spheroids. Meanwhile, the presence of the extracellular matrix is also important [34]. The three-dimensionality of spheroids provides different conditions for the cell cycle, nutrient supply, oxygen concentration, pH, and other environmental factors [3]. MCTSs are an ideal system for testing the basic dosimetric parameters of PDT, including the PS dosage. Therefore, the data from in vitro studies can be used to optimize treatment. It is important that such tests can be performed without the involvement of complex factors, such as the presence of tumor blood vessels [35]. To develop primitive vascular structures within the tumor, umbilical vein endothelial cells should be added to spheroid culture [36].
MCTSs can also be used to study the diffusion kinetics of drugs and PSs, including the 5-ALA pro-sensitizer, which is done by adding lipophilic ester groups 37], [38], [39. MCTS research helped discover and better understand the formation of free radicals during PDT treatment [35], and the assessment of the impact of PDT on the invasiveness of grade IV gliomas cancer cells [40, 41]. It also allows for long-term observations of repeated PDT [19].
Unlike animal models, in in vitro cell cultures, tumor cells are not difficult to visualize and continuously track, and they are not so expensive and repeatable cell models can be obtained [42]. Spheroid cultures are characterized by high phenotypic diversity of stem cells, which may be both an advantage and a disadvantage in studies that determine the behavior of cancer cells [43]. Cell differentiation is the main factor responsible for the acquisition of resistance to chemotherapy; therefore, it is important to thoroughly understand tumor heterogeneity, including the use of a single stem cell microfluidic culture or adherent culture [43, 44]. This makes 3D bioprinting of scaffolds is becoming increasingly important in cell cultures [45]. 3D breeding models can be broadly categorized into two types. 3D models are independent of cell anchorage. These models do not require cells to adhere to a surface and include techniques such as the use of low-adhesion plates (e.g., plates coated with polyhydroxyethyl methacrylate (poly-HEMA) or agarose). Examples include the liquid overlay technique (LOT), the hanging drop method, the soft agar method, and methods that use porous scaffolds to promote growth. Additionally, they include the use of microwell arrays, microplates, bioreactors (e.g., centrifuge flasks, rotary shakers, or microgravity bioreactors), and the magnetic manipulation method. The second type includes 3D models dependent on cell anchorage. These models require cell attachment to a substrate, such as a membrane, or the use of microfluidic channels in the microfluidic method [29, 36, 46]. Figure 3 shows the advantages and disadvantages of 3D cell culture.

The advantages and disadvantages of 3D cell culture.
Clinical therapies traditionally rely on 2D cell culture systems, which often do not allow an accurate representation of tissue complexity. As a result, 3D cell cultures have emerged as more promising platforms for integrating various signals from the extracellular matrix and cells, thus offering closer in vivo conditions. An example of the development of a three-dimensional cell model adapted to in vitro assessment of oxygen- and drug-dependent therapies, in the example of PDT, is the use of synthetic self-assembling peptides (RAD16-I) as the so-called cellular scaffold. The creation process is related to the diffusion of oxygen and drugs, along with their biological effects, such as the emergence of cellular resistance to therapy. The findings, presented by Alemany-Ribes et al., showed that the basic mechanism of action remains consistent in both 2D and 3D systems [47]. However, the dynamic effects of mass transfer significantly influenced efficacy, highlighting the importance of considering these factors in therapeutic evaluations [47]. The appropriate selection of the culture method can provide controlled experimental conditions that are difficult or impossible to study in vivo. Despite this, to date, no in vitro system can fully simulate the structure of the brain and the microenvironment in which glioma invasion occurs [42]. Three-dimensional cultures are also relatively expensive compared to 2D cultures, and the cell proliferation rate is relatively slow and depends on the type of culture and cells used 45], [46], [47], [48], [49], [50.
The importance of 2D and 3D cell cultures
2D and 3D cell cultures have significantly advanced PDT research by providing complementary insights into the efficacy and mechanisms of PDT. 2D cell cultures are valuable for the initial screening of PSs, offering a straightforward platform to evaluate their phototoxicity, cellular uptake, and activation mechanisms. These models allow for detailed studies of cellular responses to PDT, including apoptosis and cell cycle changes.
Advances in overcoming tumor immunotolerance, addressing immunosuppressive tumor microenvironments, and combining PDT with other therapies are crucial for its clinical success. Innovations in nanotechnology to improve PS delivery and integrating immunostimulants are promising. However, most PDT research has relied on 2D cell cultures, which do not accurately reflect the complexity of human tumors. This has led to the development of 3D tumor models, which better simulate tumor environments and immune interactions. 3D cell cultures, including spheroids and organoids, better mimic the physiological conditions of tumors, such as cell-cell interactions and extracellular matrix components. They provide insight into PS diffusion and accumulation within a tumor, revealing how PDT penetrates and affects heterogeneous tumor cells [51]. 3D models are crucial for studying tumor resistance mechanisms, such as hypoxia, and the interactions between PDT and the tumor microenvironment. This helps to identify how tumors adapt and resist PDT over time. Kucinska et al. highlighted how cell culture models and conditions, including 3D nonscaffold and hydrogel-based models under normoxic and hypoxic conditions, impact the efficacy of PDT [52]. The research demonstrated that zinc phthalocyanine M2TG3 induces apoptotic cancer cell death, with effectiveness varying based on the cell culture model and oxygen levels. In addition, the study showcased the benefits of bioluminescence assays for repetitive and simultaneous analysis of various parameters in 3D cultures. In general, M2TG3’s effectiveness is influenced by cell culture conditions, underscoring the importance of these factors in PDT research [52]. Together, these models have improved our understanding of PDT’s effects, leading to better development and optimization of PSs and PDT protocols. Integrating 2D and 3D approaches with advanced imaging and omics technologies continues to drive progress towards more effective, personalized PDT strategies 53], [54], [55], [56], [57.
In vitro glioma cells
Cell cultures used in PDT
In general, we can divide the cell lines used in in vitro studies into three types. The first group includes primary cells, derived directly from human tissue (e.g., fibroblasts from the skin and hepatocytes from the liver). In the second group, we can include transformed cells, immortalized cells, created naturally or by genetic manipulation. In the third group, we can include self-renewing cells, which can differentiate into a variety of other cell types (e.g., embryonic stem cells, induced pluripotent stem cells, neural stem cells, and intestinal stem cells) [48]. In vitro glioma cell cultures use various types of cell lines, which when combined with growth stimulants, form colonies used in laboratory studies. We can find in the literature the use of various glioma cell lines, such as: U-105MG [58], U-251MG [59], U-87MG 59], [60], [61, U118MG [60], LN18 [62], SNB19 [63], HEK293T [62], UP007 [64], UP029 [64], G112 [63], GL261 [65], C6 [62, 66], 9L [67], GIC7 [68], PG88 [68], HGG13 [69], HGG30 [69], HGG1123 [69], HGG146 [69], HGG157 [69], GSC C6 [70], ULM-GBM-PC128 [71], ULM-GBM-PC38 [71], ULM-GBM-PC40 [71]. However, by far the most frequently repeated lineage is U-87MG 50], [51], [52, 62], [63], [64. Cells in this line have nuclei whose genetic material is burdened with many significant chromosomal aberrations. Despite this, it is characterized by exceptional stability and has not undergone rapid changes over the years [39]. Using the U-87MG line in vitro, Reburn et al. showed that the iron chelating protoporphyrin IX (PpIX) prodrug AP2-18 causes an increase in the accumulation of PpIX fluorescence in these cells [61]. In another study, Teng et al. using this line demonstrated that downregulation of ferrochelatase (FECH) mRNA expression, whose protein product catalyzes the conversion reaction of PpIX to heme, is responsible for the accumulation of PpIX in glioma cells [63].
The use of different cell lines in photodynamic treatment research is important because it allows a more comprehensive understanding of how PDT affects different types of cancer cells. Different cell lines have different properties, so studying their response to PDT helps to better understand the mechanisms of action and identify possible resistance or toxicity problems. When different cell lines, it is also possible to better assess the effectiveness of PDT and its potential applications for different types of cancer.
Factors affecting the effect of PDT in in vitro cell cultures in glioma
Modification of PDT components, i.e. PS synthesis, type of light or type of reactive oxygen species, affects the phenotypic variability of tumor cells and variability of the tumor environment. Therefore, the use of different PSs produces different results at the cellular level. In general, the ideal PS for glioma therapy is one that can absorb light at red or near-infrared wavelengths and, under in vivo conditions can penetrate the blood-brain barrier. It should be highly selective to tumor tissue, low in toxicity, and rapidly eliminated from the body. The above factors should already be considered when selecting the appropriate PS for in vitro studies [72, 73]. The effectiveness of this therapy for glioma, as for other cancers, is directly proportional to the amount of singlet oxygen produced [74].
Successful PDT depends on the conditions of the tumor environment. The oxygen concentration in healthy brain tissue is usually 5–15 %, while in gliomas it can reach 0.1 % [74]. The effectiveness of PDT also depends on the use of analgesics such as phenytoin [75, 76], the ambient temperature [77], and the wavelength of light [77]. Cell resistance to PDT depends on oxidative stress (superoxide dismutase, catalase, NO synthase), as well as glutathione and derivatives such as glutathione peroxidase, glutathione S-transferase, glutathione omega-1 transferase, glutathione synthase, hemoxygenase-1, and apurin/apyrimidin endonuclease 1/redox factor-1 [78]. Müller et al. showed that the susceptibility of glioblastoma cells (U251MG line) to PDT depends on the expression of the PpIX transporter – ABCG2 [79]. Other researchers also observed that high expression of this receptor reduces PS accumulation, and then a higher dose of light is required for the appropriate effect of phototherapy 80], [81], [82. In addition, silencing of the ferrochelatase FECH gene causes an induction of apoptosis in glioma [26]. Substances such as calcitriol, arsenic trioxide, methadone, motexafine gadolinium, and Shikonin may increase glioma cell death 83], [84], [85], [86], [87. In vitro tests should be conducted with these and other factors in mind.
Latest findings in PDT on glioma cells in vitro
In Table 2 some of the latest in vitro studies of PDT in glioma cells are listed.
In vitro studies – summary.
| Year | Authors | Study focus | Methodology | Key findings |
|---|---|---|---|---|
| 2018 | Abdel Gaber et al. [88] | Chlorin e6 in U87 & U251 glioblastoma cells | Tumor spheres, PDT, KO143 inhibitor | ABCG2 reduces chlorin e6 accumulation, KO143 restores efficacy |
| 2017 | Fontana et al. [89] | PDT with photodithazine (PDZ) in gliosarcoma cells | 9L/lacZ cells, LED irradiation, MTT assay | PDZ led to 100 % cell death, located in nuclear and cytoplasmic regions. |
| 2019 | De Groof et al. [90] | Targeted PDT with nanobody-photosensitizer conjugates | IRDye700DX linked to nanobodies, near-IR light | Selective killing of US28-expressing glioblastoma cells |
| 2018 | Mishchenko et al. [91] | Novel porphyrazine derivatives in glioma | Accumulation studies in murine cells | pz II is effective against glioma and non-toxic to neuronal cells |
| 2018 | Fujishiro et al. [92] | PpIX biosynthesis in GSCs and A172 cells, | GSLCs, qRT-PCR, flow cytometry | GSLCs showed higher PpIX levels and greater sensitivity to ALA-PDT |
| 2023 | Pedrosa et al. [68] | 5-ALA PDT in GICs and cerebral organoids | Uptake and dose-response, apoptosis | Increased PpIX fluorescence, reduced proliferation, induced apoptosis |
| 2018 | Müller et al. [79] | ABCG2 effect on 5-ALA PDT in U251MG cells | Doxycycline-inducible ABCG2, KO143 | The induction of ABCG2 reduced PpIX, KO143 increased PDT efficiency |
| 2023 | Christie et al. [93] | Macrophage-mediated delivery of AlPcS2a | Hybrid spheroids, F98 glioma cells, LED light | Comparable efficacy of PDT with macrophages as the carrier |
| 2018 | Turubanova et al. [65] | PS-PDT and PD-PDT in GL261 glioma | Lysosomal and ER localization, immune response | Induced apoptosis/ferroptosis, immune activation |
| 2023 | Redkin et al. [94] | pz I & pz III in glioma ICD | Golgi and ER targeting, immune response | Effective tumor vaccination in a mouse mode |
| 2013 | Masubuchi et al. [95] | PpIX fluorescence in brain tissue | 5-ALA imaging, BBB studies | Tumor and normal brain contribute to PpIX fluorescence |
| 2023 | Omura et al. [69] | ALA-PDT in MES-GSCs | PpIX accumulation, hypoxia conditions | Effective in MES-GSCs, lower sensitivity under hypoxia |
Action of 5-aminolevulinic acid on gliomas cell cultures
Ideally, PS should accumulate preferentially in tumors, have a high singlet oxygen quantum yield, have low activity in the absence of light, be rapidly eliminated from the patient, have amphiphilicity and a light absorption peak between approximately 600 and 800 nm [96, 97]. Ideally, PS should be a single pure compound to enable production under good manufacturing practice conditions with quality control and low production costs and leading to better storage stability. The most effective PSs are generally relatively hydrophobic compounds that rapidly diffuse into cancer cells and localize to structures of the intracellular membrane such as mitochondria and the endoplasmic reticulum [97].
The clinical efficacy of PDT depends on complex dosimetry, including total light dose, light exposure time, and light delivery method [98, 99]. PpIX, the precursor of which is 5-ALA, is widely known and is used as a PS in glioma PDT therapy. 5-ALA-PDT was approved for dermatological treatment in the late 1990s and in 2006, Stummer et al. used it to facilitate surgical procedures in brain tumors [100]. 5-ALA is selectively converted by cancer cells to PpIX that subsequently accumulates in glioma cells [101, 102]. Activated by a 635 nm light beam, it generates reactive oxygen species, which was introduced into oncological practice by Schipmann et al. in 2020 [103]. Schwake et al. demonstrated the positive effect of PDT on brain tumor cell lines in children [104]. The cell lines used were medulloblastoma (DAOY, UW228), pNET primary neuroectodermal tumor (PFSK-1), and rhabdomyoblastoma (BT16), which were incubated for 4 h with 5-ALA and irradiated at 635 nm. It was noted that neither 5-ALA incubation nor irradiation alone caused cell death, and the DAOY and PFSK lines were more sensitive than UW228 and BT16. Furthermore, significant cell death occurred only after the use of 5-ALA at a concentration of at least 50 μg/mL [104]. This study may confirm the study by Cornelius et al., who a year earlier showed the effect of therapy with 5-ALA on U-CH2 human chordoma cells [105]. Similarly, Schimanski’s research demonstrated the effect of the same combination in PDT on human grade IV glioma stem cells. Spheroids from the GD3, GD5, BT273, BT275, and BT379 cell lines were used, as well as U373 line cells cultured as monolayers [106]. All cells tested were destroyed using PDT [106]. PDT using PpIX as PS also gives good results when acting on C6 spheroids from the ACBT glioma cell line [107].
The response of spheroids depends on the total fluence and the rate of fluence delivery. Lower fluence values (50 J cm−2) were more effective [108]. In in vitro studies in glioma cell lines, ALA-PDT continues to be the most studied PS [59, 63, 66, 68, 70]. The successful use of 5-ALA in the treatment of glioma was approved by the US Food and Drug Administration (FDA) [109]. 5-ALA is the most studied PS in gliomas. Its advantages include rapid elimination from the body, which reduces the risk of skin hypersensitivity. 5-ALA is also a physiologically synthesized endogenous metabolite by mitochondria; therefore the use of this drug is not associated with side effects. The proton-dependent peptide transporter 2 (PEPT2) and the organic anion transporter Na+/HCO3 − in the choroid plexus are believed to be responsible for 5-ALA uptake in the CNS. The expression of PEPT2 encoded mRNA is much higher in HGG than in LGG [110]. The uptake of radioactive glycine, beta-alanine, GABA, delta-aminovaleric acid, and epsilon-aminocaproic acid was examined in rabbit retina, both in vivo and in vitro. The results showed that these amino acids were predominantly taken up and retained by specific cells of amacrine type. Within neurons, omega-amino acids appeared to be stored in a protected manner. Uptake of delta-aminovaleric acid and epsilon-aminocaproic acid exhibited temperature dependence, saturation kinetics, and high-affinity characteristics (with Km values of 3.02 × 10−5 M for delta-aminovaleric acid and 9.38 × 10−5 M for epsilon-aminocaproic acid). Additionally, GABA competitively inhibited the uptake of both delta-aminovaleric acid and epsilon-aminocaproic acid, but not by glycine or several other amino acids considered as potential neurotransmitters. In comparison, the uptake of delta-aminolevulinic acid, taurine, and alpha-amino-isobutyric acid did not show selective neuronal uptake. This suggests that certain amacrine cells selectively accumulate delta amino acids like beta-alanine, GABA, delta-aminovaleric acid, and epsilon-aminocaproic acid. Moreover, it has been speculated that the mechanism responsible for accumulating GABA might also function with other omega-amino acids. This raises the possibility that omega-amino acids could act as false neurotransmitters [111]. The fluorescent properties of PpIX make it suitable for intraoperative imaging. Light activation induces red fluorescence, which can be used in real time to assess tumor location and margins. Combining the properties of PpIX used in diagnostics with its properties as a PS is called theranostics [112]. The limit of PDT is the depth of light penetration, so the use of PDT alone in the context of tumors of significant size could also be insufficient. There are already clinical studies that support this and indicate the possible use of PpIX as a theranostic agent in the treatment of highly malignant gliomas 113], [114], [115], [116], [117], [118], [119], [120.
Resistance to anti-glioma PDT
Resistance to antiglioma PDT involves several mechanisms that help tumor cells evade the cytotoxic effects of PDT. However, resistance mechanisms can significantly reduce their effectiveness. One of the main mechanisms is the active efflux of PS from glioma cells, primarily mediated by ATP-binding cassette transporters such as P-glycoprotein (P-gp). This reduces the intracellular concentration of PSs, reducing the cytotoxic effects. Up-regulation of P-gp in glioma cells can lead to resistance to various therapeutic agents, including PDT PSs [121].
The tumor microenvironment is a critical factor in resistance to PDT. Hypoxia within the glioma environment decreases the efficacy of PDT, as oxygen is essential for ROS generation. Hypoxia-inducible factors, which are upregulated in hypoxic regions of gliomas, contribute to resistance to treatment by promoting angiogenesis and survival of tumor cells under stressful conditions [122]. Glioma cells can increase their antioxidant defenses to neutralize ROS generated by PDT. Glutathione and superoxide dismutase are two key antioxidants that are upregulated in response to oxidative stress, effectively reducing PDT-induced cell damage [123]. PDT-induced damage, particularly DNA damage, can be mitigated by glioma cells by up-regulation of DNA repair pathways, including base excision repair and nucleotide excision repair. These repair mechanisms help glioma cells survive PDT by reversing the damage caused by ROS [124].
The resistance of glioblastoma cells to PDT-induced photokilling can be linked to the production of low levels of nitric oxide (NO) by up-regulation of inducible nitric oxide synthase (iNOS/NOS2). Studies have shown that following an ALA and light challenge, glioblastoma cells can increase iNOS expression, leading to the production of NO. This NO acts as a signaling molecule that promotes cell survival and reduces the efficacy of PDT. Specifically, low levels of NO can activate survival pathways and contribute to resistance to apoptosis. A study demonstrated that iNOS is upregulated in various cancer cells, including glioblastomas, in response to PDT. The production of NO through this pathway can modulate the sensitivity of glioma cells to PDT by activating survival signaling pathways such as the PI3K/Akt pathway. This is particularly evident when glioblastoma cells are subjected to PDT with ALA as PS [125]. It is also well-established that NO can mitigate oxidative stress by modulating the cellular redox environment, which is crucial during PDT-induced ROS production. By reducing oxidative stress, NO produced by iNOS helps tumor cells avoid photo-killing [126]. Studies have also shown that NO can interfere with apoptotic pathways, particularly in gliomas. This interference is mediated by S-nitrosylation of key apoptotic proteins, which reduces cell death and contributes to resistance to PDT. This phenomenon is especially pronounced in cells with increased expression of iNOS after ALA/PDT.
Summary and outcome
Recent breakthroughs in PDT for gliomas have significantly advanced our understanding and capabilities in this field. Key innovations include the development of more specific PSs, novel delivery systems, and combination therapies. Advances in BBB-crossing nanomedicine and the synthesis of new PSs with enhanced stability and efficacy have improved treatment outcomes. Additionally, integration of PDT with immunotherapy and other modalities has shown synergistic effects, offering new hope for treating gliomas resistant to conventional therapies. One of the most important future directions of research in PDT for gliomas is to further improve the synthesis of PSs, especially the development of types that will bind more strongly to cancer cells than to normal cells. Additionally, it is important to synthesize components capable of absorbing light in the red or near-red wavelength range and PSs that will be able to penetrate the blood-brain barrier. Further research on the third generation of photosensitizing compounds, especially those that bind to target molecules on the surface of cancer cells, which increases their selectivity and effectiveness, is crucial.
Another interesting direction is to better understand the effects of modifying PDT components on the phenotypic variability of tumor cells and the tumor environment. Research on optimization of therapeutic conditions, such as light dose, ambient temperature, and the use of adjunctive drugs, may also be beneficial in improving the effectiveness of therapy. Furthermore, investigating the mechanisms of cellular resistance to PDT and the search for new substances or strategies that can enhance the effectiveness of treatment are important areas for further research. Another potential direction for the development of PDT for gliomas is to further investigate the efficacy of other PSs in addition to 5-ALA. Although 5-ALA remains the most studied PS in gliomas, many other substances may be effective in PDT therapy. Further research on these compounds may lead to the discovery of new and more effective treatments for gliomas. Additionally, the further refinement of theranostics techniques, which combine diagnostics and therapeutics using the fluorescent and photosensitizing properties of PpIX, could be an exciting development in medicine.
Combining PDT with other therapies, such as immunotherapy or targeted therapies, is another important research direction. Exploring the synergistic effects of PDT with other treatments may lead to the development of more effective therapeutic strategies, especially in the treatment of cancers resistant to traditional therapies. Understanding the effects of PDT on body immune mechanisms, such as the CD8+ T cell response or activation of the complement system, may provide information on the interactions between PDT and the immune system and potential strategies to improve the efficacy of therapy by stimulating the immune response.
Future research directions may include the further development of in vitro cellular models to better reflect the complexity of tumor tissue and the microenvironment. The refinement of three-dimensional (3D) cell cultures, including organoids, cell spheroids, and bioprinted structures, is needed to better mimic the physiological properties of tissue. This will enable a more precise study of the mechanisms of intercellular communication and communication between cells and the extracellular matrix. The development of advanced 3D models may allow for a more precise study of the mechanisms of response to PDT and the identification of factors that affect the efficacy and toxicity of this type of therapy. Another interesting research direction may be the further use of different cell lines in PDT studies to understand how different types of cancer cells respond to this therapy. The study of different cell lines allows for a better understanding of the mechanism of action and the identification of possible resistance or toxicity issues. Furthermore, studying different cell lines can help to better evaluate the efficacy of PDT and potential applications for the treatment of different types of cancer.
-
Research ethics: Not applicable.
-
Informed consent: Not applicable.
-
Author contributions: Conceptualization, DA., K.R., Z.A.Y., K.D., A.M., W.M., K.K.V., M.M., A.K.K. and D.B.A.; methodology, DA., K.R., Z.A.Y., K.D., A.M., W.M., K.K.V., M.M., A.K.K. and D.B.A.; validation, DA., K.R., K.D., A.M., W.M., K.K.V., M.M., A.K.K., and D.B.A.; formal analysis, DA., K.R., Z.A.Y., K.D., A.M., W.M., K.K.V., M.M., A.K.K. and D.B.A.; investigation, DA., K.R., Z.A.Y., K.D., A.M., W.M., K.K.V., M.M., A.K.K. and D.B.A.; resources, DA., K.R., Z.A.Y., K.D., A.M., W.M., K.K.V., M.M., A.K.K. and D.B.A.; data curation, DA., K.R., K.D., A.M., M.M., A.K.K., and D.B.A.; writing – original draft preparation, DA., K.R., Z.A.Y., K.D., A.M., W.M., K.K.V., M.M., A.K.K. and D.B.A.; writing – review and editing, DA., K.R., Z.A.Y., K.D., A.M., W.M., K.K.V., M.M., A.K.K. and D.B.A.; visualization, DA., K.R., Z.A.Y., K.D., A.M., W.M., K.K.V., M.M., A.K.K. and D.B.A.; supervision, D.A. All authors have read and agreed to the published version of the manuscript.
-
Use of Large Language Models, AI and Machine Learning Tools: Not applicable.
-
Conflict of interest: The authors declare that they have no conflicts of interest.
-
Research funding: This research received no external funding.
-
Data availability: All data have been included.
References
1. Zhang, Q, Li, L. Photodynamic combination therapy in cancer treatment. J BUON 2018;23:561–7.Search in Google Scholar
2. Kucinska, M, Murias, M, Nowak-Sliwinska, P. Beyond mouse cancer models: three-dimensional human-relevant in vitro and non-mammalian in vivo models for photodynamic therapy. Mutat Res Rev Mutat Res 2017;773:242–62. https://doi.org/10.1016/j.mrrev.2016.09.002.Search in Google Scholar PubMed
3. Terzis, AJ, Dietze, A, Bjerkvig, R, Arnold, H. Effects of photodynamic therapy on glioma spheroids. Br J Neurosurg 1997;11:196–205. https://doi.org/10.1080/02688699746249.Search in Google Scholar PubMed
4. Austin, E, Jagdeo, J. An in vitro approach to photodynamic therapy. J Vis Exp 2018;17:58190. https://doi.org/10.3791/58190.Search in Google Scholar PubMed PubMed Central
5. Plotino, G, Grande, NM, Mercade, M. Photodynamic therapy in endodontics. Int Endod J 2019;52:760–74. https://doi.org/10.1111/iej.13057.Search in Google Scholar PubMed
6. Zhou, C, Yang, W, Ding, Z, Wang, Y, Shen, H, Fan, X, et al.. The biological effects of photodynamic therapy on normal skin in mice-II. An electron microscopic study. Adv Exp Med Biol 1985;193:111–5. https://doi.org/10.1007/978-1-4613-2165-1_13.Search in Google Scholar PubMed
7. Correia, JH, Rodrigues, JA, Pimenta, S, Dong, T, Yang, Z. Photodynamic therapy review: principles, photosensitizers, applications, and future directions. Pharmaceutics 2021;13:1332. https://doi.org/10.3390/pharmaceutics13091332.Search in Google Scholar PubMed PubMed Central
8. Ng, J, Henriquez, N, Kitchen, N, Williams, N, Novelli, M, Oukrif, D, et al.. Suppression of tumour growth from transplanted astrocytoma cells transfected with luciferase in mice by bioluminescence mediated, systemic, photodynamic therapy. Photodiagnosis Photodyn Ther 2023;45:103923. https://doi.org/10.1016/j.pdpdt.2023.103923.Search in Google Scholar PubMed
9. Rothe, F, Patties, I, Kortmann, R-D, Glasow, A. Immunomodulatory effects by photodynamic treatment of glioblastoma cells in vitro. Molecules 2022;27:3384–4. https://doi.org/10.3390/molecules27113384.Search in Google Scholar PubMed PubMed Central
10. Jeising, S, Geerling, G, Guthoff, R, Hänggi, D, Sabel, M, Rapp, M, et al.. In-vitro use of verteporfin for photodynamic therapy in glioblastoma. Photodiagnosis Photodyn Ther 2022;40:103049. https://doi.org/10.1016/j.pdpdt.2022.103049.Search in Google Scholar PubMed
11. Choi, JY. Medulloblastoma: current perspectives and recent advances. Brain Tumor Res Treat. 2023;11:28–38.10.14791/btrt.2022.0046Search in Google Scholar PubMed PubMed Central
12. Yang, F, Wang, Y, Ma, C. Current WHO guidelines and the critical role of genetic parameters in the classification of glioma: opportunities for immunotherapy. Curr Treat Options Oncol 2022;23:188–98.10.1007/s11864-021-00930-4Search in Google Scholar PubMed
13. Sejda, A, Grajkowska, W, Trubicka, J, Szutowicz, E, Wojdacz, T, Kloc, W, et al.. WHO CNS5 2021 classification of gliomas: a practical review and road signs for diagnosing pathologists and proper patho-clinical and neuro-oncological cooperation. Folia Neuropathol 2022;60:137–52.10.5114/fn.2022.118183Search in Google Scholar PubMed
14. Louis, DN, Perry, A, Wesseling, P, Brat, DJ, Cree, IA, Figarella-Branger, D, et al.. The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol 2021;23:1231–51.10.1093/neuonc/noab106Search in Google Scholar PubMed PubMed Central
15. Labreche, K, Kinnersley, B, Berzero, G, Di Stefano, AL, Rahimian, A, Detrait, I, et al.. Diffuse gliomas classified by 1p/19q co-deletion, TERT promoter and IDH mutation status are associated with specific genetic risk loci. Acta Neuropathol 2018;135:743–55.10.1007/s00401-018-1825-zSearch in Google Scholar PubMed PubMed Central
16. van den Bent, MJ, French, PJ, Brat, D, Tonn, JC, Touat, M, Ellingson, BM, et al.. The biological significance of tumor grade, age, enhancement, and extent of resection in IDH-mutant gliomas: how should they inform treatment decisions in the era of IDH inhibitors? Neuro Oncol 2024;26:1805–22. [Erratum in 2024 Sep 14:noae185].10.1093/neuonc/noae107Search in Google Scholar PubMed PubMed Central
17. Gianno, F, Giovannoni, I, Cafferata, B, Diomedi-Camassei, F, Minasi, S, Barresi, S, et al.. Paediatric-type diffuse high-grade gliomas in the 5th CNS WHO Classification. Pathologica 2022;114:422–35. https://doi.org/10.32074/1591-951x-830.Search in Google Scholar
18. Ostrom, QT, Bauchet, L, Davis, FG, Deltour, I, Fisher, JL, Langer, CE, et al.. The epidemiology of glioma in adults: a “state of the science” review. Neuro Oncol 2014;16:896–913. https://doi.org/10.1093/neuonc/nou087.Search in Google Scholar PubMed PubMed Central
19. Claes, A, Idema, AJ, Wesseling, P. Diffuse glioma growth: a guerilla war. Acta Neuropathol 2007;114:443–58. https://doi.org/10.1007/s00401-007-0293-7.Search in Google Scholar PubMed PubMed Central
20. Madsen, SJ, Sun, C-H, Tromberg, BJ, Cristini, V, De Magalhães, N, Hirschberg, H. Multicell tumor spheroids in photodynamic therapy. Laser Surg Med 2006;38:555–64. https://doi.org/10.1002/lsm.20350.Search in Google Scholar PubMed
21. Stupp, R, Hegi, ME, Mason, WP, van den Bent, MJ, Taphoorn, MJ, Janzer, RC, et al.. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol 2009;10:459–66. https://doi.org/10.1016/s1470-2045(09)70025-7.Search in Google Scholar PubMed
22. Zavadskaya, ТS. Photodynamic therapy in the treatment of glioma. Exp Oncol 2015;37:234–41. https://doi.org/10.31768/2312-8852.2015.37(4):234-241.10.31768/2312-8852.2015.37(4):234-241Search in Google Scholar
23. Perria, C, Capuzzo, T, Cavagnaro, G, Datti, R, Francaviglia, N, Rivano, C, et al.. Fast attempts at the photodynamic treatment of human gliomas. J Neurosurg Sci 1980;24:119–29.Search in Google Scholar
24. Kapałczyńska, M, Kolenda, T, Przybyła, W, Zajączkowska, M, Teresiak, A, Filas, V, et al.. 2D and 3D cell cultures – a comparison of different types of cancer cell cultures. Arch Med Sci 2018;14:910–9. https://doi.org/10.5114/aoms.2016.63743.Search in Google Scholar PubMed PubMed Central
25. Ferreira, LP, Gaspar, VM, Mano, JF. Design of spherically structured 3D in vitro tumor models -Advances and prospects. Acta Biomater 2018;75:11–34. https://doi.org/10.1016/j.actbio.2018.05.034.Search in Google Scholar PubMed PubMed Central
26. Jensen, C, Teng, Y. Is it time to start transitioning from 2D to 3D cell culture? Front Mol Biosci 2020;7:33. https://doi.org/10.3389/fmolb.2020.00033.Search in Google Scholar PubMed PubMed Central
27. Kawai, S, Yamazaki, M, Shibuya, K, Yamazaki, M, Fujii, E, Nakano, K, et al.. Three-dimensional culture models mimic colon cancer heterogeneity induced by different microenvironments. Sci Rep 2020;10:3156. https://doi.org/10.1038/s41598-020-60145-9.Search in Google Scholar PubMed PubMed Central
28. Shaheen, S, Ahmed, M, Lorenzi, F, Nateri, AS. Spheroid-formation (colonosphere) assay for in vitro assessment and expansion of stem cells in colon cancer. Stem Cell Rev 2016;12:492–9. https://doi.org/10.1007/s12015-016-9664-6.Search in Google Scholar PubMed PubMed Central
29. Silva-Almeida, C, Ewart, M-A, Wilde, C. 3D gastrointestinal models and organoids to study metabolism in human colon cancer. Semin Cell Dev Biol 2020;98:98–104. https://doi.org/10.1016/j.semcdb.2019.05.019.Search in Google Scholar PubMed
30. Lovitt, CJ, Shelper, TB, Avery, VM. Advanced cell culture techniques for cancer drug discovery. Biology 2014;3:345–67. https://doi.org/10.3390/biology3020345.Search in Google Scholar PubMed PubMed Central
31. Sutherland, RM. Cell and environment interactions in tumor microregions: the multicell spheroid model. Science 1988;240:177–84. https://doi.org/10.1126/science.2451290.Search in Google Scholar PubMed
32. Kamoshima, Y, Terasaka, S, Kuroda, S, Iwasaki, Y. Morphological and histological changes of glioma cells immediately after 5-aminolevulinic acid mediated photodynamic therapy. Neurol Res 2011;33:739–46. https://doi.org/10.1179/1743132810y.0000000001.Search in Google Scholar PubMed
33. Inch, WR, McCredie, JA, Sutherland, RM. Growth of nodular carcinomas in rodents compared with multi-cell spheroids in tissue culture. Growth 1970;34:271–82.Search in Google Scholar
34. Santini, M, Rainaldi, G, Indovina, PL. Apoptosis, cell adhesion and the extracellular matrix in the three-dimensional growth of multicellular tumor spheroids. Crit Rev Oncol Hematol 2000;36:75–87. https://doi.org/10.1016/s1040-8428(00)00078-0.Search in Google Scholar PubMed
35. Wilson, BC, Patterson, MS, Lilge, L. Implicit and explicit dosimetry in photodynamic therapy: a New paradigm. Laser Med Sci 1997;12:182–99. https://doi.org/10.1007/bf02765099.Search in Google Scholar PubMed
36. Fennema, E, Rivron, N, Rouwkema, J, van Blitterswijk, C, de Boer, J. Spheroid culture as a tool for creating 3D complex tissues. Trends Biotechnol 2013;31:108–15. https://doi.org/10.1016/j.tibtech.2012.12.003.Search in Google Scholar PubMed
37. Huygens, A, Huyghe, D, Bormans, G, Verbruggen, A, Kamuhabwa, AR, Roskams, T, et al.. Accumulation and photocytotoxicity of hypericin and analogs in two- and three-dimensional cultures of transitional cell carcinoma cells. Photochem Photobiol 2003;78:607–14. https://doi.org/10.1562/0031-8655(2003)0780607aapoha2.0.co2.Search in Google Scholar
38. Bigelow, CE, Mitra, S, Knuechel, R, Foster, TH. ALA- and ALA-hexylester-induced protoporphyrin IX fluorescence and distribution in multicell tumour spheroids. Br J Cancer 2001;85:727–34. https://doi.org/10.1054/bjoc.2001.1977.Search in Google Scholar PubMed PubMed Central
39. Kloek, J, van Henegouwen, B. Prodrugs of 5‐aminolevullinic acid for photodynamic therapy. Photochem Photobiol 1996;64:994–1000.10.1111/j.1751-1097.1996.tb01868.xSearch in Google Scholar PubMed
40. Aebisher, D, Przygórzewska, A, Myśliwiec, A, Dynarowicz, K, Krupka-Olek, M, Bożeket, A, et al.. Current photodynamic therapy for glioma treatment: an update. Biomedicines 2024;12:375. https://doi.org/10.3390/biomedicines12020375.Search in Google Scholar PubMed PubMed Central
41. Bartusik-Aebisher, D, Woźnicki, P, Dynarowicz, K, Aebisher, D. Photosensitizers for photodynamic therapy of brain cancers—a review. Brain Sci 2023;13:1299. https://doi.org/10.3390/brainsci13091299.Search in Google Scholar PubMed PubMed Central
42. Dundar, B, Markwell, SM, Sharma, NV, Olson, CL, Mukherjee, S, Brat, DJ. Methods for in vitro modeling of glioma invasion: choosing tools to meet the need. Glia 2020;68:2173–91. https://doi.org/10.1002/glia.23813.Search in Google Scholar PubMed
43. Pollard, SM, Yoshikawa, K, Clarke, ID, Danovi, D, Stricker, S, Russell, R, et al.. Glioma stem cell lines expanded in adherent culture have tumor-specific phenotypes and are suitable for chemical and genetic screens. Cell Stem Cell 2009;4:568–80. https://doi.org/10.1016/j.stem.2009.03.014.Search in Google Scholar PubMed
44. Li, P, Qin, Z, Zhong, Y, Kang, H, Zhang, Z, Hu, Y, et al.. Selective single-cell expansion on a microfluidic chip for studying heterogeneity of glioma stem cells. Anal Chem 2022;94:3245–53. https://doi.org/10.1021/acs.analchem.1c04959.Search in Google Scholar PubMed
45. van Pel, DM, Harada, K, Song, D, Naus, CC, Sin, WC. Modelling glioma invasion using 3D bioprinting and scaffold-free 3D culture. J Cell Commun Signal 2018;12:723–30. https://doi.org/10.1007/s12079-018-0469-z.Search in Google Scholar PubMed PubMed Central
46. Liu, D, Chen, S, Naing, MW. A review of manufacturing capabilities of cell spheroid generation technologies and future development. Biotechnol Bioeng 2021;118:542–54. https://doi.org/10.1002/bit.27620.Search in Google Scholar PubMed
47. Alemany-Ribes, M, García-Díaz, M, Busom, M, Nonell, S, Semino, CE. Toward a 3D cellular model for studying in vitro the outcome of photodynamic treatments: accounting for the effects of tissue complexity. Tissue Eng 2013;19:1665–74. https://doi.org/10.1089/ten.tea.2012.0661.Search in Google Scholar
48. Breslin, S, O’Driscoll, L. Three-dimensional cell culture: the missing link in drug discovery. Drug Discov Today 2013;18:240–9. https://doi.org/10.1016/j.drudis.2012.10.003.Search in Google Scholar PubMed
49. Li, Y, Huang, G, Li, M, Wang, L, Elson, EL, Lu, TJ, et al.. An approach to quantifying 3D responses of cells to extreme strain. Sci Rep 2016;6:19550. https://doi.org/10.1038/srep19550.Search in Google Scholar PubMed PubMed Central
50. Jubelin, C, Muñoz-Garcia, J, Griscom, L, Cochonneau, D, Ollivier, E, Heymann, M-F, et al.. Three-dimensional in vitro culture models in oncology research. Cell Biosci 2022;12:155. https://doi.org/10.1186/s13578-022-00887-3.Search in Google Scholar PubMed PubMed Central
51. Habanjar, O, Diab-Assaf, M, Caldefie-Chezet, F, Delort, L. 3D cell culture systems: tumor application, advantages, and disadvantages. Int J Mol Sci 2021;22:12200. https://doi.org/10.3390/ijms222212200.Search in Google Scholar PubMed PubMed Central
52. Kucinska, M, Plewinski, A, Szczolko, W, Kaczmarek, M, Goslinski, T, Murias, M. Modeling the photodynamic effect in 2D versus 3D cell culture under normoxic and hypoxic conditions. Free Radic Biol Med 2021;162:309–26. https://doi.org/10.1016/j.freeradbiomed.2020.10.304.Search in Google Scholar PubMed
53. Nkune, NW, Simelane, NWN, Montaseri, H, Abrahamse, H. Photodynamic therapy-mediated immune responses in three-dimensional tumor models. Int J Mol Sci 2021;22:12618. https://doi.org/10.3390/ijms222312618.Search in Google Scholar PubMed PubMed Central
54. Chen, Q, Chopp, M, Madigan, L, Dereski, MO, Hetzel, FW. Damage threshold of normal rat brain in photodynamic therapy. Photochem Photobiol 1996;64:163–7. https://doi.org/10.1111/j.1751-1097.1996.tb02437.x.Search in Google Scholar
55. Lilge, L, Portnoy, M, Wilson, BC. Apoptosis induced in vivo by photodynamic therapy in normal brain and intracranial tumour tissue. Br J Cancer 2000;83:1110–7. https://doi.org/10.1054/bjoc.2000.1426.Search in Google Scholar
56. Hirschberg, H, Sørensen, DR, Angell-Petersen, E, Peng, Q, Tromberg, B, Sun, CH, et al.. Repetitive photodynamic therapy of malignant brain tumors. J Environ Pathol Toxicol Oncol 2006;25:261–80. https://doi.org/10.1615/jenvironpatholtoxicoloncol.v25.i1-2.170.Search in Google Scholar
57. Olzowy, B, Hundt, CS, Stocker, S, Bise, K, Reulen, HJ, Stummer, W. Photoirradiation therapy of experimental malignant glioma with 5-aminolevulinic acid. J Neurosurg 2002;97:970–6. https://doi.org/10.3171/jns.2002.97.4.0970.Search in Google Scholar
58. Tsai, JC, Hsiao, YY, Teng, LJ, Chen, CT, Kao, MC. Comparative study on the ALA photodynamic effects of human glioma and meningioma cells. Laser Surg Med 1999;24:296–305. https://doi.org/10.1002/(sici)1096-9101(1999)24:4<296::aid-lsm7>3.3.co;2-6.10.1002/(SICI)1096-9101(1999)24:4<296::AID-LSM7>3.3.CO;2-6Search in Google Scholar
59. Etminan, N, Peters, C, Lakbir, D, Bünemann, E, Börger, V, Sabel, MC, et al.. Heat-shock protein 70-dependent dendritic cell activation by 5-aminolevulinic acid-mediated photodynamic treatment of human glioblastoma spheroids in vitro. Br J Cancer 2011;105:961–9. https://doi.org/10.1038/bjc.2011.327.Search in Google Scholar
60. Chakrabarti, M, Banik, NL, Ray, SK. Photofrin based photodynamic therapy and miR-99a transfection inhibited FGFR3 and PI3K/akt signaling mechanisms to control growth of human glioblastoma in vitro and in vivo. PLoS One 2013;8:e55652. https://doi.org/10.1371/journal.pone.0055652.Search in Google Scholar
61. Reburn, C, Gawthorpe, G, Perry, A, Wood, M, Curnow, A. Novel iron-chelating prodrug significantly enhanced fluorescence-mediated detection of glioma cells experimentally in vitro. Pharmaceutics 2023;15:2668. https://doi.org/10.3390/pharmaceutics15122668.Search in Google Scholar
62. Kaundal, B, Srivastava, AK, Sardoiwala, MN, Karmakar, S, Choudhury, SR. A NIR-responsive indocyanine green-genistein nanoformulation to control the polycomb epigenetic machinery for the efficient combinatorial photo/chemotherapy of glioblastoma. Nanoscale Adv 2019;1:2188–207. https://doi.org/10.1039/c9na00212j.Search in Google Scholar
63. Teng, L, Nakada, M, Zhao, S-G, Endo, Y, Furuyama, N, Nambu, E, et al.. Silencing of ferrochelatase enhances 5-aminolevulinic acid-based fluorescence and photodynamic therapy efficacy. Br J Cancer 2011;104:798–807. https://doi.org/10.1038/bjc.2011.12.Search in Google Scholar
64. Vasilev, A, Sofi, R, Smith, SJ, Rahman, R, Teschemacher, AG, Kasparov, S. Feasibility of photodynamic therapy for glioblastoma with the mitochondria-targeted photosensitizer tetramethylrhodamine methyl ester (TMRM). Biomedicines 2021;9:1453. https://doi.org/10.3390/biomedicines9101453.Search in Google Scholar PubMed PubMed Central
65. Turubanova, VD, Balalaeva, IV, Mishchenko, TA, Catanzaro, E, Alzeibak, R, Peskova, NN, et al.. Immunogenic cell death induced by a new photodynamic therapy based on photosens and photodithazine. J Immunother Cancer 2019;7:350. https://doi.org/10.1186/s40425-019-0826-3.Search in Google Scholar PubMed PubMed Central
66. Hartl, BA, Hirschberg, H, Marcu, L, Cherry, SR. Activating photodynamic therapy in vitro with Cerenkov radiation generated from yttrium-90. J Environ Pathol Toxicol Oncol 2016;35:185–92. https://doi.org/10.1615/jenvironpatholtoxicoloncol.2016016903.Search in Google Scholar
67. Chen, M-H, Jenh, Y-J, Wu, S-K, Chen, Y-S, Hanagata, N, Lin, F-H. Non-invasive photodynamic therapy in brain cancer by use of Tb3+-doped LaF3 nanoparticles in combination with photosensitizer through X-ray irradiation: a proof-of-concept study. Nanoscale Res Lett 2017;12:62. https://doi.org/10.1186/s11671-017-1840-3.Search in Google Scholar PubMed PubMed Central
68. Pedrosa, L, Bedia, C, Diao, D, Mosteiro, A, Ferrés, A, Stanzani, E, et al.. Preclinical studies with glioblastoma brain organoid co-cultures show efficient 5-ALA photodynamic therapy. Cells 2023;12:1125. https://doi.org/10.3390/cells12081125.Search in Google Scholar PubMed PubMed Central
69. Omura, N, Nonoguchi, N, Fujishiro, T, Park, Y, Ikeda, N, Kajimoto, Y, et al.. Ablation efficacy of 5-aminolevulinic acid-mediated photodynamic therapy on human glioma stem cells. Photodiagnosis Photodyn Ther 2023;41:103119. https://doi.org/10.1016/j.pdpdt.2022.103119.Search in Google Scholar PubMed
70. Wang, W, Tabu, K, Hagiya, Y, Sugiyama, Y, Kokubu, Y, Murota, Y, et al.. Enhancement of 5-aminolevulinic acid-based fluorescence detection of side population-defined glioma stem cells by iron chelation. Sci Rep 2017;7:42070. https://doi.org/10.1038/srep42070.Search in Google Scholar PubMed PubMed Central
71. Golla, C, Bilal, M, Dwucet, A, Bader, N, Anthonymuthu, J, Heiland, T, et al.. Photodynamic therapy combined with Bcl-2/Bcl-xL inhibition increases the Noxa/Mcl-1 ratio independent of Usp9X and synergistically enhances apoptosis in glioblastoma. Cancers 2021;13:4123. https://doi.org/10.3390/cancers13164123.Search in Google Scholar PubMed PubMed Central
72. Tetard, M-C, Vermandel, M, Mordon, S, Lejeune, J-P, Reyns, N. Experimental use of photodynamic therapy in high grade gliomas: a review focused on 5-aminolevulinic acid. Photodiagnosis Photodyn Ther 2014;11:319–30. https://doi.org/10.1016/j.pdpdt.2014.04.004.Search in Google Scholar PubMed
73. Stylli, SS, Kaye, AH. Photodynamic therapy of cerebral glioma – a review Part I – a biological basis. J Clin Neurosci 2006;13:615–25. https://doi.org/10.1016/j.jocn.2005.11.014.Search in Google Scholar PubMed
74. Ihata, T, Nonoguchi, N, Fujishiro, T, Omura, N, Kawabata, S, Kajimoto, Y, et al.. The effect of hypoxia on photodynamic therapy with 5-aminolevulinic acid in malignant gliomas. Photodiagnosis Photodyn Ther 2022;40:103056. https://doi.org/10.1016/j.pdpdt.2022.103056.Search in Google Scholar PubMed
75. Udomsak, W, Kucinska, M, Pospieszna, J, Dams-Kozlowska, H, Chatuphonprasert, W, Murias, M. Antioxidant enzymes in cancer cells: their role in photodynamic therapy resistance and potential as targets for improved treatment outcomes. Int J Mol Sci 2024;25:3164. https://doi.org/10.3390/ijms25063164.Search in Google Scholar PubMed PubMed Central
76. Hefti, M, Albert, I, Luginbuehl, V. Phenytoin reduces 5-aminolevulinic acid-induced protoporphyrin IX accumulation in malignant glioma cells. J Neuro Oncol 2012;108:443–50. https://doi.org/10.1007/s11060-012-0857-9.Search in Google Scholar PubMed
77. Hirschberg, H, Sun, C-H, Tromberg, BJ, Yeh, AT, Madsen, SJ. Enhanced cytotoxic effects of 5-aminolevulinic acid-mediated photodynamic therapy by concurrent hyperthermia in glioma spheroids. J Neuro Oncol 2004;70:289–99. https://doi.org/10.1007/s11060-004-9161-7.Search in Google Scholar PubMed
78. Shinoda, Y, Kato, D, Ando, R, Endo, H, Takahashi, T, Tsuneoka, Y, et al.. Systematic review and meta-analysis of in vitro anti-human cancer experiments investigating the use of 5-Aminolevulinic acid (5-ALA) for photodynamic therapy. Pharmaceuticals 2021;14:229. https://doi.org/10.3390/ph14030229.Search in Google Scholar PubMed PubMed Central
79. Müller, P, Abdel Gaber, SA, Zimmermann, W, Wittig, R, Stepp, H. ABCG2 influence on the efficiency of photodynamic therapy in glioblastoma cells. J Photochem Photobiol, B 2020;210:111963. https://doi.org/10.1016/j.jphotobiol.2020.111963.Search in Google Scholar PubMed
80. Mansi, M, Howley, R, Chandratre, S, Chen, B. Inhibition of ABCG2 transporter by lapatinib enhances 5-aminolevulinic acid-mediated protoporphyrin IX fluorescence and photodynamic therapy response in human glioma cell lines. Biochem Pharmacol 2022;200:115031. https://doi.org/10.1016/j.bcp.2022.115031.Search in Google Scholar PubMed PubMed Central
81. Sun, W, Kajimoto, Y, Inoue, H, Miyatake, S-I, Ishikawa, T, Kuroiwa, T. Gefitinib enhances the efficacy of photodynamic therapy using 5-aminolevulinic acid in malignant brain tumor cells. Photodiagnosis Photodyn Ther 2013;10:42–50. https://doi.org/10.1016/j.pdpdt.2012.06.003.Search in Google Scholar PubMed
82. Zhao, S-G, Chen, X-F, Wang, L-G, Yang, G, Han, D-Y, Teng, L, et al.. Increased expression of ABCB6 enhances protoporphyrin IX accumulation and photodynamic effect in human glioma. Ann Surg Oncol 2013;20:4379–88. https://doi.org/10.1245/s10434-011-2201-6.Search in Google Scholar PubMed
83. Chen, X, Wang, C, Teng, L, Liu, Y, Chen, X, Yang, G, et al.. Calcitriol enhances 5-aminolevulinic acid-induced fluorescence and the effect of photodynamic therapy in human glioma. Acta Oncol 2014;53:405–13. https://doi.org/10.3109/0284186x.2013.819993.Search in Google Scholar
84. Wang, C, Chen, X, Wu, J, Liu, H, Ji, Z, Shi, H, et al.. Low-dose arsenic trioxide enhances 5-aminolevulinic acid-induced PpIX accumulation and efficacy of photodynamic therapy in human glioma. J Photochem Photobiol, B 2013;127:61–7. https://doi.org/10.1016/j.jphotobiol.2013.06.001.Search in Google Scholar PubMed
85. Shi, L, Buchner, A, Pohla, H, Pongratz, T, Rühm, A, Zimmermann, W, et al.. Methadone enhances the effectiveness of 5‐aminolevulinic acid‐based photodynamic therapy for squamous cell carcinoma and glioblastoma in vitro. J Biophot 2019;12:e201800468. https://doi.org/10.1002/jbio.201800468.Search in Google Scholar PubMed
86. Madsen, SJ, Mathews, MS, Angell-Petersen, E, Sun, C-H, Vo, V, Sanchez, R, et al.. Motexafin gadolinium enhances the efficacy of aminolevulinic acid mediated-photodynamic therapy in human glioma spheroids. J Neuro Oncol 2009;91:141–9. https://doi.org/10.1007/s11060-008-9692-4.Search in Google Scholar PubMed PubMed Central
87. Werner, M, Lyu, C, Stadlbauer, B, Schrader, I, Buchner, A, Stepp, H, et al.. The role of Shikonin in improving 5-aminolevulinic acid-based photodynamic therapy and chemotherapy on glioblastoma stem cells. Photodiagnosis Photodyn Ther 2022;39:102987. https://doi.org/10.1016/j.pdpdt.2022.102987.Search in Google Scholar PubMed
88. Abdel Gaber, SA, Müller, P, Zimmermann, W, Hüttenberger, D, Wittig, R, Abdel Kader, MH, et al.. ABCG2-mediated suppression of chlorin e6 accumulation and photodynamic therapy efficiency in glioblastoma cell lines can be reversed by KO143. J Photochem Photobiol, B 2018;178:182–91. https://doi.org/10.1016/j.jphotobiol.2017.10.035.Search in Google Scholar PubMed
89. Fontana, LC, Pinto, JG, Pereira, AHC, Soares, CP, Raniero, LJ, Ferreira-Strixino, J. Photodithazine photodynamic effect on viability of 9L/lacZ gliosarcoma cell line. Laser Med Sci 2017;32:1245–52. https://doi.org/10.1007/s10103-017-2227-5.Search in Google Scholar PubMed
90. De Groof, TWM, Mashayekhi, V, Fan, TS, Bergkamp, ND, Sastre Toraño, J, van Senten, JR, et al.. Nanobody-targeted photodynamic therapy selectively kills viral GPCR-expressing glioblastoma cells. Mol Pharm 2019;16:3145–56. https://doi.org/10.1021/acs.molpharmaceut.9b00360.Search in Google Scholar PubMed PubMed Central
91. Mishchenko, TA, Turubanova, VD, Mitroshina, EV, Alzeibak, R, Peskova, NN, Lermontova, SA, et al.. Effect of novel porphyrazine photosensitizers on normal and tumor brain cells. J Biophot 2020;13:e201960077. https://doi.org/10.1002/jbio.201960077.Search in Google Scholar PubMed
92. Fujishiro, T, Nonoguchi, N, Pavliukov, M, Ohmura, N, Kawabata, S, Park, Y, et al.. 5-Aminolevulinic acid-mediated photodynamic therapy can target human glioma stem-like cells refractory to antineoplastic agents. Photodiagnosis Photodyn Ther 2018;24:58–68. https://doi.org/10.1016/j.pdpdt.2018.07.004.Search in Google Scholar PubMed
93. Christie, C, Madsen, SJ, Peng, Q, Hirschberg, H. Macrophages as a photosensitizer delivery system for photodynamic therapy: potential for the local treatment of resected glioblastoma. Photodiagnosis Photodyn Ther 2024;224:103897. https://doi.org/10.1016/j.pdpdt.2023.103897.Search in Google Scholar PubMed
94. Redkin, TS, Sleptsova, EE, Turubanova, VD, Saviuk, MO, Lermontova, SA, Klapshina, LG, et al.. Dendritic cells pulsed with tumor lysates induced by tetracyanotetra(aryl)porphyrazines-based photodynamic therapy effectively trigger anti-tumor immunity in an orthotopic mouse glioma model. Pharmaceutics 2023;15:2430. https://doi.org/10.3390/pharmaceutics15102430.Search in Google Scholar PubMed PubMed Central
95. Masubuchi, T, Kajimoto, Y, Kawabata, S, Nonoguchi, N, Fujishiro, T, Miyatake, S-I, et al.. Experimental study to understand nonspecific protoporphyrin IX fluorescence in brain tissues near tumors after 5-aminolevulinic acid administration. Photomed Laser Surg 2013;31:428–33. https://doi.org/10.1089/pho.2012.3469.Search in Google Scholar PubMed
96. Simões, JCS, Sarpaki, S, Papadimitroulas, P, Therrien, B, Loudos, G. Conjugated photosensitizers for imaging and PDT in cancer research. J Med Chem 2020;63:14119–50. https://doi.org/10.1021/acs.jmedchem.0c00047.Search in Google Scholar PubMed
97. Abrahamse, H, Hamblin, MR. New photosensitizers for photodynamic therapy. Biochem J 2016;473:347–64. https://doi.org/10.1042/bj20150942.Search in Google Scholar PubMed PubMed Central
98. Dobson, J, de Queiroz, GF, Golding, JP. Photodynamic therapy and diagnosis: principles and comparative aspects. Vet J 2018;233:8–18. https://doi.org/10.1016/j.tvjl.2017.11.012.Search in Google Scholar PubMed
99. Sibata, CH, Colussi, VC, Oleinick, NL, Kinsella, TJ. Photodynamic therapy in oncology. Expet Opin Pharmacother 2001;2:917–27. https://doi.org/10.1517/14656566.2.6.917.Search in Google Scholar PubMed
100. Stummer, W, Pichlmeier, U, Meinel, T, Wiestler, OD, Zanella, F, Reulen, H-J. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: a randomised controlled multicentre phase III trial. Lancet Oncol 2006;7:392–401. https://doi.org/10.1016/s1470-2045(06)70665-9.Search in Google Scholar PubMed
101. Zhao, B, He, Y-Y. Recent advances in the prevention and treatment of skin cancer using photodynamic therapy. Expert Rev Anticancer Ther 2010;10:1797–809. https://doi.org/10.1586/era.10.154.Search in Google Scholar PubMed PubMed Central
102. Traylor, JI, Pernik, MN, Sternisha, AC, McBrayer, SK, Abdullah, KG. Molecular and metabolic mechanisms underlying selective 5-aminolevulinic acid-induced fluorescence in gliomas. Cancers 2021;13:580. https://doi.org/10.3390/cancers13030580.Search in Google Scholar PubMed PubMed Central
103. Schipmann, S, Müther, M, Stögbauer, L, Zimmer, S, Brokinkel, B, Holling, M, et al.. Combination of ALA-induced fluorescence-guided resection and intraoperative open photodynamic therapy for recurrent glioblastoma: case series on a promising dual strategy for local tumor control. J Neurosurg 2021;134:426–36. https://doi.org/10.3171/2019.11.jns192443.Search in Google Scholar
104. Schwake, M, Nemes, A, Dondrop, J, Schroeteler, J, Schipmann, S, Senner, V, et al.. In-vitro use of 5-ALA for photodynamic therapy in pediatric brain tumors. Neurosurgery 2018;83:1328–37. https://doi.org/10.1093/neuros/nyy054.Search in Google Scholar PubMed
105. Cornelius, JF, Eismann, L, Ebbert, L, Senger, B, Petridis, AK, Kamp, MA, et al.. 5-Aminolevulinic acid-based photodynamic therapy of chordoma: in vitro experiments on a human tumor cell line. Photodiagnosis Photodyn Ther 2017;20:111–5. https://doi.org/10.1016/j.pdpdt.2017.09.011.Search in Google Scholar PubMed
106. Schimanski, A, Ebbert, L, Sabel, MC, Finocchiaro, G, Lamszus, K, Ewelt, C, et al.. Human glioblastoma stem-like cells accumulate protoporphyrin IX when subjected to exogenous 5-aminolaevulinic acid, rendering them sensitive to photodynamic treatment. J Photochem Photobiol, B 2016;163:203–10. https://doi.org/10.1016/j.jphotobiol.2016.08.043.Search in Google Scholar PubMed
107. Zelenkov, P, Baumgartner, R, Bise, K, Heide, M, Meier, R, Stocker, S, et al.. Acute morphological sequelae of photodynamic therapy with 5-aminolevulinic acid in the C6 spheroid model. J Neuro Oncol 2007;82:49–60. https://doi.org/10.1007/s11060-006-9252-8.Search in Google Scholar PubMed
108. Madsen, SJ, Sun, CH, Tromberg, BJ, Hirschberg, H. Repetitive 5-aminolevulinic acid-mediated photodynamic therapy on human glioma spheroids. J Neuro Oncol 2003;62:243–50. https://doi.org/10.1023/a:1023362011705.10.1023/A:1023362011705Search in Google Scholar PubMed
109. Hadjipanayis, CG, Stummer, W. 5-ALA and FDA approval for glioma surgery. J Neuro Oncol 2019;141:479–86. https://doi.org/10.1007/s11060-019-03098-y.Search in Google Scholar PubMed PubMed Central
110. de Visscher, SA, Dijkstra, PU, Tan, IB, Roodenburg, JL, Witjes, MJ. mTHPC mediated photodynamic therapy (PDT) of squamous cell carcinoma in the head and neck: a systematic review. Oral Oncol 2013;49:192–210.10.1016/j.oraloncology.2012.09.011Search in Google Scholar PubMed
111. Hirschberg, H, Sun, C-H, Tromberg, BJ, Madsen, SJ. ALA- and ALA-ester-mediated photodynamic therapy of human glioma spheroids. J Neuro Oncol 2002;57:1–7. https://doi.org/10.1023/a:1015784926550.10.1023/A:1015784926550Search in Google Scholar
112. Bartusik-Aebisher, D, Żołyniak, A, Barnaś, E, Machorowska-Pieniążek, A, Oleś, P, Kawczyk-Krupka, A, et al.. The use of photodynamic therapy in the treatment of brain tumors—a review of the literature. Molecules 2022;27:6847. https://doi.org/10.3390/molecules27206847.Search in Google Scholar PubMed PubMed Central
113. Howley, R, Chandratre, S, Chen, B. 5-Aminolevulinic acid as a theranostic agent for tumor fluorescence imaging and photodynamic therapy. Bioengineering 2023;10:496. https://doi.org/10.3390/bioengineering10040496.Search in Google Scholar PubMed PubMed Central
114. Stummer, W, Müther, M, Spille, D. Beyond fluorescence-guided resection: 5-ALA-based glioblastoma therapies. Acta Neurochir 2024;166:163. https://doi.org/10.1007/s00701-024-06049-3.Search in Google Scholar PubMed PubMed Central
115. Beck, TJ, Kreth, FW, Beyer, W, Mehrkens, JH, Obermeier, A, Stepp, H, et al.. Interstitial photodynamic therapy of nonresectable malignant glioma recurrences using 5-aminolevulinic acid induced protoporphyrin IX. Laser Surg Med 2007;39:386–93. https://doi.org/10.1002/lsm.20507.Search in Google Scholar PubMed
116. Johansson, A, Faber, F, Kniebuhler, G, Stepp, H, Sroka, R, Egensperger, R, et al.. Protoporphyrin IX fluorescence and photobleaching during interstitial photodynamic therapy of malignant gliomas for early treatment prognosis. Laser Surg Med 2013;45:225–34. https://doi.org/10.1002/lsm.22126.Search in Google Scholar PubMed
117. Schwartz, C, Rühm, A, Tonn, J-C, Kreth, S, Kreth, F-W. SURG-25. Interstitial photodynamic therapy of de-novo glioblastoma multiforme WHO IV [Abstract]. Neuro Oncol 2015;17:v219–20.10.1093/neuonc/nov235.25Search in Google Scholar
118. Vermandel, M, Dupont, C, Quidet, M, Lecomte, F, Lerhun, E, Mordon, S, et al.. Set-up of the first pilot study on intraopertive 5-ALA PDT: INDYGO trial. Photodiagn Photodyn 2017;17:A21. https://doi.org/10.1016/j.pdpdt.2017.01.048.Search in Google Scholar
119. Dupont, C, Vermandel, M, Leroy, HA, Quidet, M, Lecomte, F, Delhem, N, et al.. INtraoperative photoDYnamic therapy for GliOblastomas: study protocol for a phase I clinical trial. Neurosurgery 2019;84:E414–19. https://doi.org/10.1093/neuros/nyy324.Search in Google Scholar PubMed
120. Baglo, Y, Liang, BJ, Robey, RW, Ambudkar, SV, Gottesman, MM, Huang, H-C. Porphyrin-lipid assemblies and nanovesicles overcome ABC transporter-mediated photodynamic therapy resistance in cancer cells. Cancer Lett 2019;457:110–8. https://doi.org/10.1016/j.canlet.2019.04.037.Search in Google Scholar PubMed PubMed Central
121. Brown, JM, Wilson, WR. Exploiting tumour hypoxia in cancer treatment. Nat Rev Cancer 2004;4:437–47. https://doi.org/10.1038/nrc1367.Search in Google Scholar PubMed
122. Trachootham, D, Alexandre, J, Huang, P. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat Rev Drug Discov 2009;8:579–91. https://doi.org/10.1038/nrd2803.Search in Google Scholar PubMed
123. Bobola, MS, Kolstoe, DD, Blank, A, Chamberlain, MC, Silber, JR. Repair of 3-methyladenine and abasic sites by base excision repair mediates glioblastoma resistance to temozolomide. Front Oncol 2012;2:176. https://doi.org/10.3389/fonc.2012.00176.Search in Google Scholar PubMed PubMed Central
124. Thomas, DD, Ridnour, LA, Isenberg, JS, Flores-Santana, W, Switzer, CH, Donzelli, S, et al.. The chemical biology of nitric oxide: implications in cellular signaling. Free Radic Biol Med 2008;45:18–31. https://doi.org/10.1016/j.freeradbiomed.2008.03.020.Search in Google Scholar PubMed PubMed Central
125. Bazak, J, Fahey, JM, Wawak, K, Korytowski, W, Girotti, AW. Enhanced aggressiveness of bystander cells in an anti-tumor photodynamic therapy model: role of nitric oxide produced by targeted cells. Free Radic Biol Med 2017;102:111–21. https://doi.org/10.1016/j.freeradbiomed.2016.11.034.Search in Google Scholar PubMed
126. Chen, T. Unveiling the significance of inducible nitric oxide synthase: its impact on cancer progression and clinical implications. Cancer Lett 2024;592:216931. https://doi.org/10.1016/j.canlet.2024.216931.Search in Google Scholar PubMed
© 2024 the author(s), published by De Gruyter on behalf of Tech Science Press (TSP)
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- Review Articles
- Recent advances in organelle-specific autophagy in melanoma
- Photodynamic therapy in glioma cell culture
- Targeting tumor microenvironments with gold nanoparticles for enhanced photothermal therapy
- Mechanistic insights into traditional Chinese medicine for digestive tract cancers: implications for gastric, hepatic, esophageal, intestinal, and pancreatic tumors
- Effectiveness of supervised combined aerobic and resistance exercise in fatigue of prostate cancer survivors under androgen deprivation therapy: a systematic review and meta-analysis
- Anti-cancer effects of hyperbaric oxygen therapy in mice: a meta-analysis
- Research Articles
- Y27632 induces tongue squamous cell carcinoma cell apoptosis through MAPK-ERK/JNK signal
- FTX promotes esophageal cancer progression and desensitizes esophageal cancer cells to ionizing radiation by microRNA-99a/b-3p/WEE1/ERCC1 axis
- Exosomal microRNA-21-5p from gastric cancer cells promotes angiogenesis by targeting LEMD3 in human endothelial cells
- Sex differences in prognosis of primary bone cancer: a propensity score-matched study
- Real-world analysis of the incidence and risk factors of pneumonitis in non-small cell lung cancer patients treated with combined thoracic radiotherapy and immunotherapy
- Integrating machine learning and multi-omics analysis to develop an immune-derived multiple programmed cell death signature for predicting clinical outcomes in gastric cancer
- Comprehensive analysis of NOTCH pathway with tumor environment in pancreatic adenocarcinoma
- Comprehensive bioinformatics analysis of lncRNA regulation and screening for pathogenic genes in NF2-related schwannomatosis
- Short Commentary
- The role of perioperative treatment in radiation-associated soft tissue sarcomas
Articles in the same Issue
- Frontmatter
- Review Articles
- Recent advances in organelle-specific autophagy in melanoma
- Photodynamic therapy in glioma cell culture
- Targeting tumor microenvironments with gold nanoparticles for enhanced photothermal therapy
- Mechanistic insights into traditional Chinese medicine for digestive tract cancers: implications for gastric, hepatic, esophageal, intestinal, and pancreatic tumors
- Effectiveness of supervised combined aerobic and resistance exercise in fatigue of prostate cancer survivors under androgen deprivation therapy: a systematic review and meta-analysis
- Anti-cancer effects of hyperbaric oxygen therapy in mice: a meta-analysis
- Research Articles
- Y27632 induces tongue squamous cell carcinoma cell apoptosis through MAPK-ERK/JNK signal
- FTX promotes esophageal cancer progression and desensitizes esophageal cancer cells to ionizing radiation by microRNA-99a/b-3p/WEE1/ERCC1 axis
- Exosomal microRNA-21-5p from gastric cancer cells promotes angiogenesis by targeting LEMD3 in human endothelial cells
- Sex differences in prognosis of primary bone cancer: a propensity score-matched study
- Real-world analysis of the incidence and risk factors of pneumonitis in non-small cell lung cancer patients treated with combined thoracic radiotherapy and immunotherapy
- Integrating machine learning and multi-omics analysis to develop an immune-derived multiple programmed cell death signature for predicting clinical outcomes in gastric cancer
- Comprehensive analysis of NOTCH pathway with tumor environment in pancreatic adenocarcinoma
- Comprehensive bioinformatics analysis of lncRNA regulation and screening for pathogenic genes in NF2-related schwannomatosis
- Short Commentary
- The role of perioperative treatment in radiation-associated soft tissue sarcomas

