Abstract
The explosive growth of nanotechnology has brought challenging innovations in the synthesis of multifunctional nano-objects able to revolutionize the field of diagnosis and therapy in medicine. Furthermore, one important input of today’s nanotechnology in biology is that their design will also allow real progress to achieve temporal and spatial site local therapy and imaging. Such a breakthrough is made possible by the development of multifunctional biocompatible nanosystems resulting from cutting-edge researches based on pluridisciplinary approaches. Among the challenges are the design of the organic coating and its grafting at the surface of NPs while preserving the properties of both NPs and molecules. The molecules should ensure the colloidal stability of NPs in physiological media, their biocompatibility and biodistribution, and may bear functions to couple bioactive groups. This paper aims at providing challenges in functionalization of iron oxide nanoparticles for biomedical applications.
1 Introduction
The development of functionalized iron oxide NPs is of great interest due their wide range of applications in the biomedical field. The composition, size, and morphology of the NP inorganic core are now modulated to combine properties in therapy (magnetic hyperthermia) and diagnosis (MRI) and to develop theranostic agents [1–8]. However, regarding biomedical applications, these NPs should always be functionalized with organic molecules to ensure at first their biocompatibility and biodistribution. Indeed, there is a set of requirements that the NP functionalization needs to meet in the case of application in the biomedical field: i) to provide hydrophilic and biocompatible NPs, ii) to ensure stealth NPs, iii) to allow their colloidal stability in water and protein-rich physiological media, iv) to lead to mean hydrodynamic size after functionalization lower than 100 nm, v) to add functional groups for coupling of bioactive molecules (drug, fluorophore, targeting ligand).
An optimal biodistribution implies that the NPs are not captured by the reticulo-endothelial system (RES) (low internalization by nonspecific tissues) and are eliminated. This biodistribution depends on the nature of the molecule, but also strongly on the colloidal stability and mean hydrodynamic size of functionalized NPs, which depend on the NP core size, the molecule size, and the grafting strategy. Indeed, the coating must favor the NP colloidal stabilization by preventing their aggregation in physiological media. The absence of aggregates is essential in the case of intravenous injection because aggregates may cause pulmonary embolism (macroscopic aggregates) or coagulation disorders (microscopic aggregates), inducing the death of animals. Furthermore, functionalized NPs are subject to opsonization when entering the bloodstream. This process is the nonspecific adhesion of plasma proteins on the surface of the NPs and then internalization by the RES [9]. In order to prevent NPs from opsonization and to increase their ability to escape the RES, the nature of the organic coating, its anchoring to the surface of the NP, and the functionalized NP size distribution must be optimized. Indeed NP suspensions with mean hydrodynamic size in the 10–100 nm range is optimal for a good biodistribution and in vivo delivery, while the smaller (<10 nm) are rapidly cleared by the renal system, and the larger (>200 nm) are rapidly sequestered by the RES [10, 11]. Furthermore, molecules grafted onto the surface of the NPs may bring new functions such as fluorophores for optical imaging, targeting ligands to reach specific tissues and cells, or therapeutic agents to deliver locally.
The nature and the size of the organic coating and the grafting strategy are very important parameters, and the grafting strategy must be able to preserve the properties of both the coating and the NP. Indeed, they will determine the above-mentioned requirements/parameters [12]. The functionalization strategy depends on the NP nature and synthesis method. Thus, functionalization is an important issue. This paper presents the necessary considerations to be taken into account in the NP functionalization for their use in the biomedical field. The main synthesis methods will be presented, and then, the functionalization strategies will be described.
2 Main NP synthesis methods
Many methods are used to synthesize iron oxide NPs [10, 13–16]. The advantages and disadvantages of the main techniques are presented in Table 1.
Advantages and disadvantages of the main synthesis methods used to prepare NPs.
| Synthesis methods | Synthesis conditions | T (°C) | Reaction time | Solvent | Size (nm) | Size distribution | Morphology control | Yield |
|---|---|---|---|---|---|---|---|---|
| Co-precipitation | Very easy | 20–90 | Minutes | Water | <40 | Average | Average | Very high |
| Microemulsion | Complex | 20–50 | 10 min | Water/organic | <50 | Quite small | Good | Low |
| Polyol method | Very easy | >180 | 10 min | Organic | <10 | Quite small | Very good | Average |
| Hydrothermal method | Simple but under high pressure | >200 | Hours | Water/ethanol | <1000 | Quite small | Very good | Average |
| Thermal decomposition | Complex | 200–400 | Hours | Organic | <20 | Small | Very good | High |
The coprecipitation method is the most and easiest method used. The synthesis is carried out by adding a base in aqueous solutions of acid salts of ferrous ions Fe2+ and Fe3+. The ferrous and ferric ions are soluble in an acid medium and precipitate when the basicity of the medium increases. It has the advantage of leading to a large quantity of powder in water. However, the size of NPs is polydispersed, and the NPs tend to aggregate together as they are “naked”, i.e. without organic ligands on their surface, in water. Other methods such as the synthesis by microemulsion [17–21], the hydrothermal synthesis [22–26], or the polyol method [27–30] have been developed and allow better NP size and morphology control. Since 2000 [31–33], the synthesis of various NPs by thermal decomposition is highly developed. It consists in the decomposition of a metal complex in a high boiling temperature organic solvent in the presence of surface-active agents, acting as stabilizers. This technique allows obtaining well-crystallized NPs in situ functionalized with an organic hydrophobic layer ensuring their high colloidal stability in suspension in organic solvents [34]. The NP surfactants mainly used are oleic acid and oleylamine.
Thus, it appears that according to NP synthesis methods, the NP surface is different (“naked” or covered by an organic ligand), and therefore, the functionalization method will defer depending on the surface state of the NPs. Furthermore, the NP surface state will also define which stabilization strategy (electrostatic or steric stabilization) needs to be implemented.
3 Functionalization of NPs
3.1 Colloidal stability of NPs
Functionalization of iron oxide NPs is essential to obtain stable suspensions in physiological media. The well-known Derjaguin-Landau-Verwey-Overbeek (DLVO) theory is used to calculate the total interaction potential around a particle surface [35, 36]. The colloidal stability of NPs is the result of a balance between attractive forces and repulsive forces. In the case of iron oxide NPs, the attractive forces are mainly due to van der Waals interactions and magnetic interactions between NPs acting as magnetic dipoles. These interactions depend on the nature of the material and the distance between two NPs. The van der Waals interactions energy varies as 1/r2 and dipole-dipole interactions energy as 1/r3, where r is the distance between NPs. As iron oxide NPs are superparamagnetic, magnetic interactions can be neglected, and the main attractive interactions are the van der Waals ones. To ensure a colloidal stability of NPs in suspension, repulsive forces are needed and are mainly induced by electrostatic interactions or/and steric repulsions.
The attractive and repulsive contributions to the total energy forces are represented in Figure 1. The attractive forces dominate when the distance is short between two NPs. A first minimum corresponding to the aggregation of a group of NPs is observed on the energy curve. This aggregation state is irreversible due to the large energy barrier to overcome. However, when the system is located at the second minimum, a reversible flocculation can take place, resulting in a stable suspension.
![Figure 1: Schematic representation of the attractive and repulsive force contributions to the total energy according to Derjaguin et al. [37].](/document/doi/10.1515/ntrev-2015-0014/asset/graphic/j_ntrev-2015-0014_fig_001.jpg)
Schematic representation of the attractive and repulsive force contributions to the total energy according to Derjaguin et al. [37].
To ensure either electrostatic and/or steric stabilization to obtain stable NP suspensions at physiological pH, the iron oxide NPs have to be functionalized by organic molecules. Indeed, “naked” iron oxide NPs can be “naturally” stabilized by electrostatic interactions by adjusting the pH but are not stable by this way at physiological pH (~7). As for all oxides, the surface of iron oxide is covered by hydroxyl groups due to water chemisorption (Figure 2). Part of these surface -OH groups are amphoteric, and the surface charge can be changed by varying the pH. Thus, depending on the pH of the solution, the surface of the iron oxide NPs can be positively or negatively charged (Figure 2). The isoelectric point (IEP) is the pH at which the surface has as many positive charges as negative charges. IEP for iron oxides is around 6.8 [38]. Iron oxide NPs are thus stable in suspension by electrostatic interactions at a pH far from their IEP: at acidic pH, the NPs are positively charged and at basic pH, negatively charged (Figure 2). However, the IEP of iron oxide NPs is exactly in the pH range of physiological media, and they are not stable in suspension in this environment.
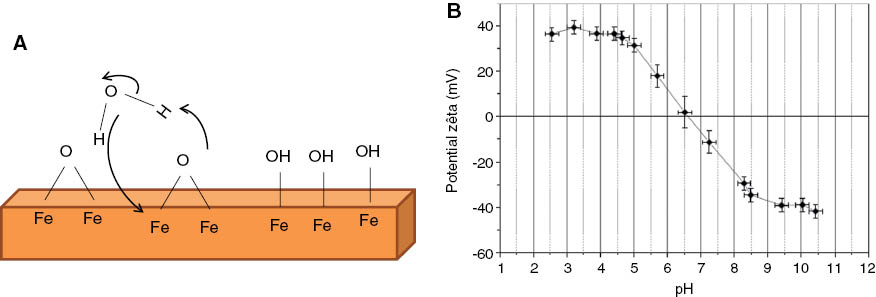
(A) Schematic representation of the iron oxide surface, (B) surface charge and ζ potential evolution according to the pH in the case of “naked” iron oxide NPs synthesized by the co-precipitation method.
Thus, for applications in the biomedical field, the iron oxide NPs have to functionalize with molecules ensuring steric or electrostatic interactions. The functionalization of iron oxide NPs with molecules bearing peripheral charged groups such as carboxylate or ammonium will allow moving the IEP compared to bare NPs and ensuring electrostatic interactions. The NP colloidal stability by electrostatic interactions in water is generally assessed by ζ potential measurements. A water suspension of NPs is considered stable by electrostatic interaction at a given pH, when the absolute value of its ζ potential is greater than or equal to 30. The particle size distribution in suspension is generally determined by granulometric measurements, which gives the mean hydrodynamic diameter of functionalized NPs (diameter of coated NPs) in suspension if the size distribution is monomodale.
Another way of reducing the attractive forces is to induce steric hindrance by grafting large organic molecules at the NP surface. Polymer molecules are often used. These molecules repel each other NPs and weaken the attraction forces, which vary as 1/r3.
Grafting molecules that allow both electrostatic and steric stabilization on the surface of NPs is more and more used. These molecules are large enough to induce a steric repulsion and present charged groups providing electrostatic repulsion. We may talk about “electrosteric” stabilization. Recently, dendron molecules, which display an arborescent architecture (small branched molecule), have been shown to induce such electrosteric repulsion [39].
Hydrophilic oligoethyleneglycol-derivatized dendrons bearing a long and functional octaethylene glycol chain in para position with a carboxylate function (Figure 5 top) have been grafted at the surface of 10-nm-sized iron oxide NPs. The ζ potential value at pH 7.4 is around -22 mV confirming the grafting of the dendron and the presence of electrostatic interactions induced by the peripheral carboxylate functions, which are deprotonated at the working pH. The IEP is around 3.5. In addition, granulometric measurements performed as a function of pH demonstrated the preservation of a monomodal size distribution and of a mean hydrodynamic size (18±3) on a large pH range (2.5–12) even below the pKa of the peripheral carboxylate groups (~4). These results demonstrate that, with the dendron molecules, the colloidal stability is not only due to the electrostatic interactions but also to steric hindrance induced by the dendron architecture. The dendron used displays a conical-like architecture, which improves steric resistance to macromolecules such as proteins while preventing particle agglomeration more effectively by comparison with their linear counterparts [39, 40]. We may advance here that dendrons favor a colloidal stability by electrosteric interactions.
To obtain colloidal suspensions of iron oxide NPs suitable for biomedical application, one request is the NP functionalization by organic molecules. Two methods are mainly developed to graft these molecules at the NP surface according to their surface state after synthesis: an in situ functionalization method (during the NPs synthesis) and a post-synthesis functionalization process.
3.2 Functionalization methods
In situ functionalization consists in mixing precursors that will allow synthesizing the magnetic NPs and molecules to be grafted in the same solution. NP nucleation occurs within the functionalization material [41–43]. Such a strategy is mainly used with polymeric molecules.
In the case of post-synthesis functionalization, which is currently the most functionalization strategy used, NPs and organic molecules are synthesized separately. However, according to their preparation method, NPs may be naked (as in co-precipitation) or coated with a surfactant (case of thermal decomposition). The functionalization then takes place by direct grafting or by direct grafting by ligand exchange or by ligand exchange and phase transfer (Figure 3) [44].
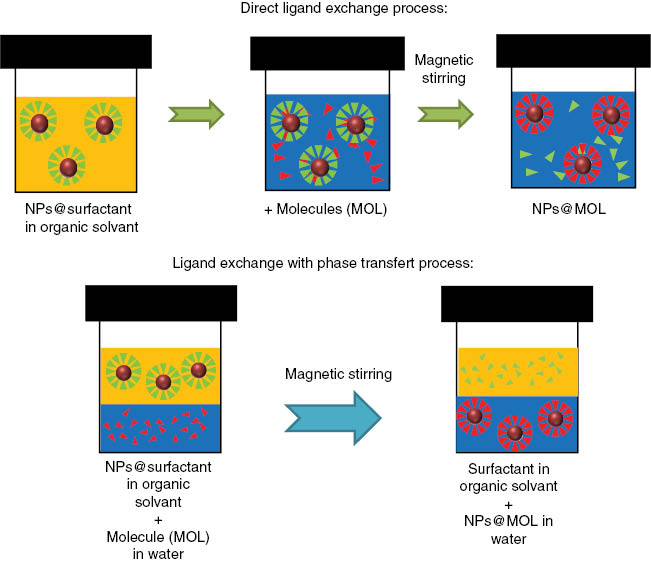
Functionalization of NPs covered with a surfactant molecule: top: direct ligand exchange process, bottom: ligand exchange with phase transfer process.
Direct grafting is mainly used in the case of bare NPs by introducing the molecules in the suspension of NPs. In the case of direct ligand exchange, the NPs and the molecule to be grafted are mixed in an organic solvent (Figure 3). The molecules have to be soluble in the organic solvent, which is generally quite polar. They present generally an anchoring group displaying a high affinity with the surface to be functionalized and replace the former ligand. NPs are then transferred into water after functionalization. In the case of ligand exchange with a phase transfer, molecules and NPs are in two separate phases. Molecules are dissolved in the aqueous phase, and the NPs are dispersed in a non-water-miscible organic solvent. Under stirring, the two phases come into contact, and the NP transfer from the organic phase to the aqueous phase is observed due to the molecule grafting at the NP surface.
The molecules grafted onto the surface of the NPs can interact in various ways with the surface of the NP. They can be physisorbed at the iron oxide surface in water suspension: the molecules bear functional groups (carboxylate, ammonium...) that interact through electrostatic interactions with the protonated or deprotonated OH groups at the surface of the NPs, or the molecules can react with the -OH groups of the NP surface to form a chemical bond between the surface and the molecule. Purely electrostatic interactions are not strong enough for applications in the biomedical field. Indeed, it is important that the molecule does not desorb during injection or over time. A weak bond between molecules and the iron oxide surface makes that the molecules may be replaced by blood proteins and causes NP capture by the RES. Therefore, grafting methods promoting a strong bond with the surface of the NPs were developed using anchoring group present on the molecules.
3.3 Anchoring groups
The anchoring group used depends primarily on the chemical nature of the NP and on the nature of the surfactant present on the surface prior to functionalization and which will be exchanged [45]. A silane function or groups such as sulfates, carboxylates, phosphonates, or catechols (Figure 4) can be used as coupling or anchoring agents. More or less strong interactions between these groups and the surface of iron oxides (OH surface) have been reported, and such discrepancies on the strength of the bond were due to the fact that depending on the NP synthesis method, the nature of molecules, and the grafting method, different types of complex may be observed. The strength of the anchoring influences further the NP stability in physiological media.
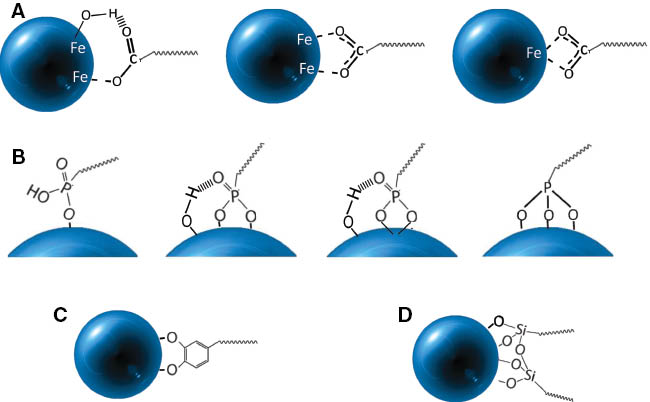
Possible surface complexes according to the coupling agent: (A) carboxylate, (B) phosphonate, (C) catechol, (D) silane.
The silanization process is based on the formation of a covalent network of silane molecules at the surface of the NPs. The silane groups are covalently bound to the NP by reaction between the surface hydroxyl groups and the alkoxysilane function (-Si-OR). Aminosilanes are mainly used for the silanization of iron oxide NPs [46–50], but organosilanes, namely, aminopropyltriethoxysilane (APTS) or aminopropyltrimethoxysilane (APTMS), do not lead to stable iron oxide NPs in water at pH 7 (the pH has to be decreased down to pH 5 to protonate the amine moieties). Therefore, these aminosilanes are most useful as intermediate groups for further coupling of other (bioactive) molecules. The alkaline hydrolysis of tetraethyl orthosilicate (TEOS) known as the Stöber synthesis is widely used for the formation of silica shell on the particles [51–53]. This method allows to obtain iron oxide NPs that are very stable in water as the silica isoelectric point is around 2–3, which is much lower than the physiologic pH. This shell presents a high density of -OH-reactive groups on their surface, which can be used to couple other bioactive molecules. The silica shell can also be further silanized with functional organosilanes [54].
The carboxylic acid group is another function widely used to modify the surface of the NPs. The most commonly used carboxylic acids are citric acid and dimercaptosuccinic acid (DMSA) [55–59]. These polyacids lead to stable suspensions, thanks to their good affinity to the NP surface. However, it was observed that the coordination bond -COOH/NPs can be labile and break as the temperature rises or be replaced by interactions with another carboxylic acid group or another stronger interacting group [60].
Catechols, which are dopamine derivatives, have also been grafted onto NPs [61–63]. However, some instability of this bond in water and in biological fluids has been reported [64].
In the recent years, functionalization of iron oxide NPs by phosphonate groups has grown because of the strong complexing ability of the -PO (OH)2 group with iron ions. The coupling of the phosphonate group gives rise to a greater grafting rate than the carboxylate group, and the bonds formed are more stable than those formed with carboxylic acids [65–67]. In addition, phosphating the surface of the NP allows to protect them from oxidation and to preserve the magnetic properties of NPs [65, 66, 68–70].
The various anchoring groups may interact more or less strongly with the surface (Figure 4). The anchor may be mono or bidentate like for carboxylates and phosphonates [71]: more or less hydrogen interactions may exist. The type of surface complex depends strongly on the NP surface (hydroxylation rate), the type of molecule to be grafted, and the grafting conditions. However, it is relatively difficult to characterize the type of surface complex. Infrared spectroscopy and, more recently, X-ray photoemission (XPS) have been mainly used to determine the coordination mode of the surface complex.
For carboxylates, either monodentate or bridging bidentate or chelating bidentate complexes may be observed: the difference Δ between the carboxylate groups asymmetric and symmetric vibration position is directly related to interaction between the carboxylate and the oxides [72, 73] or metal [74–76] surface. A large difference (200–300 cm-1) corresponds to a monodentate interaction, a difference in the range 140–200 cm-1 indicates a bridging bidentate interaction and a smaller difference (<110 cm-1) a bidentate chelate interaction [77]. However, molecules display generally a lot of IR bands, which overlap and makes the IR bands’ position difficult to ascertain. Most reported surface complexes are either bridging bidentate or chelate bidentate [78].
In the case of phosphonates, IR bands of the molecules before and after grafting onto iron oxide NPs showed the formation of surface complex [79] with the disappearance of the P-OH bands and sometimes of the P=O band. The disappearance of this last band is not obvious, and often a slight shift and decrease in its intensity are observed suggesting the formation of a bidentate complex (disappearance of P-OH bands). Previously, it has been shown that the functionalization of NPs synthesized by co-precipitation would lead to a trinuclear surface complex, while a binuclear complex is formed in the case of NPs synthesized by thermal decomposition [79]. Borggaard et al. [80] found a high adsorption affinity of phosphate ions on iron oxides such as goethite (α-FeOOH), which has been linked to the formation of a phosphate binuclear complex on the surface of the goethite by substitution of the OH groups.
In fact, the unambiguous identification of the surface complex is difficult by IR spectroscopy due to the overlapping of IR bands. Photoemission spectroscopy (XPS) may allow evaluating the strength of bonds [69, 81]. For example, the phosphorus 2p bands of a dendron are observed to shift after the grafting confirming the anchoring of the dendron through this group. However, the shift depends also on the grafting method. A higher shift is observed after direct ligand exchange than after ligand exchange and phase transfer (Figure 5). This suggests that the strength of the bond is higher with the former grafting method.
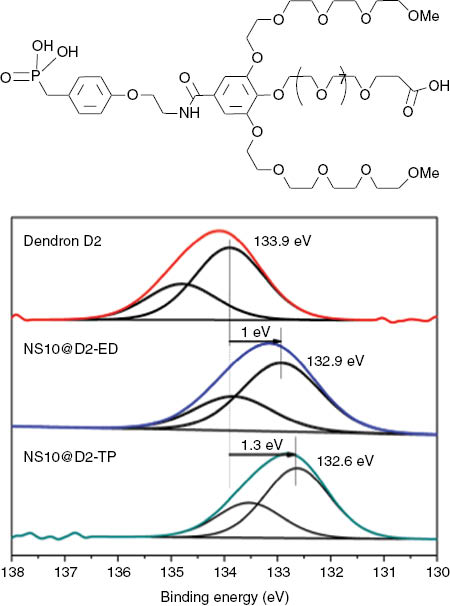
Top: schema of a dendron, bottom: P 2p XPS bands of the dendron (A), of the NPs grafted with dendron after direct ligand exchange (B), or after ligand exchange and phase transfer (C).
The colloidal stability in physiological media appears to be greatly improved when molecules bearing several anchoring groups are grafted [82]. Bis [83–87] or tri-phosphonates [81] as well as multi-catechols [88–90] have been recently reported, and stability studies under various conditions have shown that NPs that functionalized with such molecules (carboxylic acid [82] or catechol [89]) are more stable than the NPs that functionalized with only one anchoring group. Because of their excellent complexing properties, bisphosphonates were evaluated as coupling agents to iron oxide NPs [85]. Portet et al. [91] have studied stabilizing compounds having different anchoring groups such as sulfates, carboxylates, and showed that the functionalization using bisphosphonates provides stable colloidal suspensions of NPs over a long period (4 weeks). Torres et al. have coupled a radioactive label to a bisphosphonate molecule and have functionalized iron oxide NPs. The co-location of the magnetic NPs by magnetic resonance imaging and of the radiotracer has highlighted the strong anchoring of bisphosphonates also in vivo [92, 93].
3.4 Main grafted molecules
Besides the anchoring group allowing the grafting of the molecule and described above, molecules generally present a hydrophilic part providing biocompatibility and stealth and also functions allowing coupling of bioactive molecules (drug, fluorophores, radiomarker, etc.) [94, 95]. This part is called “spacer” and can have rather a linear architecture [83, 90, 96] or a hyperbranched architecture [83, 97–100] (Figure 6) The linear or hyperbranched “spacers” can be of different types. Thus, various types of molecules have been grafted to iron oxide NP surface for applications in the biomedical field [10, 13–16, 45]. Synthetic or natural polymers have been widely used to cover the surface of the NPs. Among the natural polymers, dextran [101–103] and chitosan [104, 105] have been widely studied because they are biocompatible and hydrophilic.
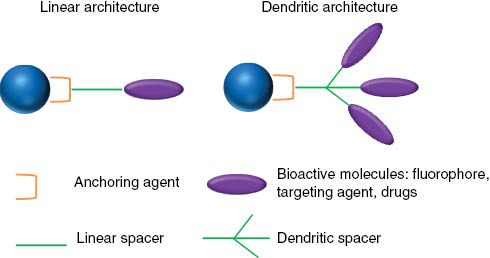
Main molecule architectures used to functionalize iron oxide NPs.
Many synthetic polymers such as polyethylene glycol (PEG) [96, 106, 107], polyvinyl alcohol (PVA) [108, 109], or polyvinylpyrrolidone (PVP) [110, 111] have been grafted onto NPs. All these polymers are biocompatible and facilitate NP dispersion in water. The different types of polymers, their advantages and disadvantages in the biomedical field are summarized in Table 2. For the use of NPs as a therapeutic agent, it is important to avoid the NP capture by the RES and promote their biodistribution and elimination. This is achieved by forming a protective layer around the NPs, which prevents adhesion proteins responsible for uptake by the RES. Currently, polyethylene glycol (PEG) is considered as the more hydrophilic polymer and the best way to achieve biodistribution of NPs injected in vivo [9, 107, 112] and reduce protein adsorption on their surface: increasing the length of the PEG chain or the PEG chain density reduces protein adsorption and uptake by macrophages [113, 114], increases the NP suspension stability [107], and increases the blood circulation time [96, 115]. Therefore, it is widely used as a “spacer” group between the anchor and a bioactive molecule.
Main polymers used to functionalize iron oxide NPs.
| Synthetic polymers | PEG | Improves the biocompatibility, blood circulation time, and internalization efficiency of the NPs |
| PVP | Enhances blood circulation time and stabilizes colloidal suspensions | |
| PVA | Prevents particles’ coagulation, giving rise to monodisperse NPs | |
| PAA | Increases NP stability and biocompatibility and helps in bioadhesion | |
| Poly(N-isopropylacryl-amide) | Allows thermosensitive drug delivery | |
| Natural polymers | Dextran | Enhances blood circulation lifetime and stabilizes colloidal suspensions |
| Gelatin | Is used as a gelling agent, hydrophilic, and biocompatible emulsifier | |
| Chitosan | Is used as non-viral gene delivery system, biocompatible, and hydrophilic |
The end group of the “spacer” can be a carboxylic group, a thiol, or an amine group to ensure the colloidal stability but also to allow the conjugation of fluorophores, drugs, or targeting molecules. Many bioactive molecules have been used in order to accumulate nanoparticles in a specific area (tumor cells or tumoral tissues) for a better diagnosis or therapy [9, 13, 14, 116]. Small molecules like folic acid [117–119] or aptamers [120–122] and bigger molecules as peptides [123, 124], proteins [125], or antibodies [126] can bind to many cell receptors and are used as targeting agents.
3.5 Dendronized iron oxide NPs
Among the currently developed organic coatings, a dendritic approach appears particularly promising in the field of cancer diagnosis. The use of dendrimers or dendrons in the biomedical field has been motivated by the many benefits that the arborescent structure of these molecules can bring: i) conformation and size can be easily controlled by varying the generation (G) of the dendrimer (or dendron) or the nature and the number of peripheral chains. It is then possible to control the pharmacokinetics and biodistribution. ii) Their biological properties may be attributed to a single molecular entity rather than a random distribution as in the case of polymers. iii) The dendron generation can be adapted to the nanometer size of the NP and minimize the thickness of the organic layer. iv) The outlying chains or the core can be functionalized with drugs, chromophores, or other ligands, which can provide additional functionality to the dendron. Since the early work of Vögtle et al. and innovative studies of Fréchet et al. in the field of connected molecules, dendrimers/dendrons have attracted the attention of many scientists in different fields such as catalysis and biomedical. In this way, dendrons were used to functionalize gold NPs [124, 127–129], quantum dots [130], polymeric NPs [131], carbon nanotubes [132, 133], surfaces [134], and in particular iron oxide NPs [68, 69, 79, 85, 89, 97, 99, 131, 135–140].
Although dendric molecules are used to functionalize various supports, the contribution of dendron versus linear molecules is not clearly demonstrated in the literature. Recently, Marcus Textor et al. [97] brought an interesting contribution. By comparing the colloidal stability, iron oxide NPs functionalized with linear molecules and dendrons with PEG chains of various molecular weights, they demonstrated the reversible aggregation of NPs functionalized with dendrons at increased temperature, while NPs functionalized with linear molecules aggregate and sediment irreversibly. Na et al. [89] have compared two branched structures and two linear structures, carrying carboxylic acids or catechols as a coupling agent. They showed that the introduction of PEG chain increases the colloidal stability in water, in a 1-m NaCl solution and a medium used for cell culture. The influence of the dendron generation has been the subject of several articles. In particular, Chaung Duanmu et al. [135] showed the influence of dendron generation on MRI properties of iron oxide NPs: r2 increases as NPs are NP functionalized with a higher generation of dendrons. The generation increase also increases the functionality of the NPs [99, 131]. In 2012, Kenji Kono team’s [141] showed that transfection activity of PAMAM dendron bearing lipids depends on the generation of the dendron. Huang et al. [142] studied six generations of PAMAM dendrimers grafted onto NP iron oxide polyplexes with polyethyleneimine (PEI)/DNA to increase the PEI transfection using a magnetic field. Besides magnetic transfection, the use of dendronized NPs has been considered in many other applications. Gene delivery [136], delivery of doxurubicine [137, 138], and streptavidin [143] with iron oxide NPs with dendrons was also studied. Recently, Walter et al. have compared the linear PEG molecule and the dendron of different generations grafted at the surface of 10-nm NPs and showed that dendrons provide an electrosteric stabilization of NPs in suspension and ensure a high MRI contrast [39].
Indeed, the main reported application of dendronized NPs is their use as a contrast agent for MRI [68, 69, 79, 85, 139, 140]. Iron oxide nanoparticles were coated with the dendron displayed in Figure 5. In vivo and in vitro MRI measurements showed that the contrast enhancement properties of the dendronized NPs were higher than those obtained with commercial polymer-coated NPs. They were demonstrated to induce any cytotoxicity. Moreover, dendronized NPs were eliminated by urinary and hepatobiliary pathways without unspecific uptake especially in the RES organs and in the lungs. The design of dendronized NPs was further improved to obtain theranostic nano-objects (which can both identify disease states and simultaneously deliver therapy) by adjusting the morphology and the composition of the inorganic magnetic core [144] and by designing multifunctionalized dendrons [39]. Thus, different types of iron oxide NPs having different sizes, morphologies (spheres, cubes, octopodes), and internal structures (core shell) were then synthesized and functionalized with a dendron molecule. The MRI and magnetic hyperthermia properties were studied according to the morphology and composition of the inorganic core of dendronized NPs. The significant effects of contrast enhancement in MRI and high heating power were recorded at low concentrations, especially for cubic NPs. These NPs were found suitable to combine imaging and therapy by hyperthermia.
4 Conclusion
In the field of the synthesis and functionalization of inorganic NPs for biomedical applications, most researches aim at developing multifunctional theranostic NPs, which can both identify disease states and deliver therapy and, thus, allow following the therapy effect by imaging. To develop such theranostic nano-objects, several challenges are still to be overcome among which are the designs of the organic coating and its grafting at the surface of NPs, while preserving the properties of both the NPs and molecules. Indeed, the molecules anchored at the surface of the NPs should bring different functions such as dyes for optical imaging, targeting ligands to reach target tissue or cells, or therapeutic agents (drug delivery). There must also be functions preventing NPs from agglomeration in a physiological environment and favoring their biodistribution and bioelimination.
However, besides the design of the bioactive molecules, their grafting has to be performed by ensuring hydrodynamic sizes between 10 and 50 nm to favor a good biodistribution and by ensuring a strong bond between the molecules and NPs to avoid their desorption after in vivo injection. These requirements strongly depend on the size of molecules and NPs, the choice of anchoring group, and the grafting strategy.
References
[1] Liong M, Lu J, Kovochich M, Xia T, Ruehm SG, Nel AE, Tamanoi F, Zink JI. Multifunctional inorganic nanoparticles for imaging, targeting, and drug delivery. ACS Nano 2008, 2, 889–896.10.1021/nn800072tSearch in Google Scholar
[2] Kim J, Piao Y, Hyeon T. Multifunctional nanostructured materials for multimodal imaging, and simultaneous imaging and therapy. Chem. Soc. Rev. 2009, 38, 372–390.Search in Google Scholar
[3] De M, Ghosh PS, Rotello VM. Applications of nanoparticles in biology. Adv. Mater. 2008, 20, 4225–4241.Search in Google Scholar
[4] Fang C, Zhang M. Multifunctional magnetic nanoparticles for medical imaging applications. J. Mater. Chem. 2009, 19, 6258–6266.Search in Google Scholar
[5] Pankhurst QA, Connolly J, Jones SK, Dobson J. Applications of magnetic nanoparticles in biomedicine. J. Phys. Appl. Phys. 2003, 36, R167.Search in Google Scholar
[6] Mura S, Couvreur P. Nanotheranostics for personalized medicine. Adv. Drug Deliv. Rev. 2012, 64, 1394–1416.Search in Google Scholar
[7] Choi KY, Liu G, Lee S, Chen X. Theranostic nanoplatforms for simultaneous cancer imaging and therapy: current approaches and future perspectives. Nanoscale 2012, 4, 330–342.10.1039/C1NR11277ESearch in Google Scholar
[8] Barick KC, Singh S, Jadhav NV, Bahadur D, Pandey BN, Hassan PA. pH-responsive peptide mimic shell cross-linked magnetic nanocarriers for combination therapy. Adv. Funct. Mater. 2012, 22, 4975–4984.Search in Google Scholar
[9] Reddy LH, Arias JL, Nicolas J, Couvreur P. Magnetic nanoparticles: design and characterization, toxicity and biocompatibility, pharmaceutical and biomedical applications. Chem. Rev. 2012, 112, 5818–5878.Search in Google Scholar
[10] Colombo M, Carregal-Romero S, Casula MF, Gutierrez L, Morales MP, Bohm IB, Heverhagen JT, Prosperi D, Parak WJ. Biological applications of magnetic nanoparticles. Chem. Soc. Rev. 2012, 41, 4306–4334.Search in Google Scholar
[11] Xie J, Liu G, Eden HS, Ai H, Chen X. Surface-engineered magnetic nanoparticle platforms for cancer imaging and therapy. Acc. Chem. Res. 2011, 44, 883–892.Search in Google Scholar
[12] Nguyen NTK, Ed., Magnetic Nanoparticles: From Fabrication to Clinical Applications, CRC Press, Taylor and Francis: Boca Raton, London, New York, 2012.Search in Google Scholar
[13] Laurent S, Forge D, Port M, Roch A, Robic C, Elst LV, Muller RN. Magnetic iron oxide nanoparticles: synthesis, stabilization, vectorization, physicochemical characterizations, and biological applications. Chem. Rev. 2008, 108, 2064–2110.Search in Google Scholar
[14] Gupta AK, Gupta M. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials 2005, 26, 3995–4021.10.1016/j.biomaterials.2004.10.012Search in Google Scholar
[15] Figuerola A, Di Corato R, Manna L, Pellegrino T. From iron oxide nanoparticles towards advanced iron-based inorganic materials designed for biomedical applications. Pharmacol. Res. 2010, 62, 126–143.Search in Google Scholar
[16] Mornet S, Vasseur S, Grasseta F, Duguet E. Magnetic nanoparticle design for medical diagnosis and therapy. J. Mater. Chem. 2004, 14, 2161–2175.Search in Google Scholar
[17] Lu A-H, Salabas EL, Schüth F. Magnetic nanoparticles: synthesis, protection, functionalization, and application. Angew. Chem. Int. Ed. 2007, 46, 1222–1244.Search in Google Scholar
[18] Lopez-Quintel MA. Synthesis of nanomaterials in microemulsions: formation mechanisms and growth control. Curr. Opin. Colloid Interface Sci. 2003, 8, 137–144.10.1016/S1359-0294(03)00019-0Search in Google Scholar
[19] Wongwailikhit K, Horwongsakul S. The preparation of iron (III) oxide nanoparticles using W/O microemulsion. Mater. Lett. 2011, 65, 2820–2822.Search in Google Scholar
[20] Lu L, Wang J, Yin J, Wang A, Wang X, Zhang T. Surfactant effects on the microstructures of Fe3O4 nanoparticles synthesized by microemulsion method. Colloids Surf. A: Physicochem. Eng. Asp. 2013, 436, 675–683.Search in Google Scholar
[21] Han L-H, Liu H, Wei Y. In situ synthesis of hematite nanoparticles using a low-temperature microemulsion method. Powder Technol. 2011, 207, 42–46.Search in Google Scholar
[22] Chen D, Xu R. Hydrothermal synthesis and characterization of nanocrystalline Fe3O4 powders. Mater. Res. Bull. 1998, 33, 1015–1021.Search in Google Scholar
[23] Daou TJ, Pourroy G, Bégin-Colin S, Grenèche JM, Ulhaq-Bouillet C, Legaré P, Bernhardt P, Leuvrey C, Rogez G. Hydrothermal synthesis of monodisperse magnetite nanoparticles. Chem. Mater. 2006, 18, 4399–4404.Search in Google Scholar
[24] Ge S, Shi X, Sun K, Li C, Uher C, Baker JR, Banaszak Holl MM, Orr BG. Facile hydrothermal synthesis of iron oxide nanoparticles with tunable magnetic properties. J. Phys. Chem. C 2009, 113, 13593–13599.10.1021/jp902953tSearch in Google Scholar PubMed PubMed Central
[25] Gao G, Qiu P, Qian Q, Zhou N, Wang K, Song H, Fu H, Cui D. PEG-200-assisted hydrothermal method for the controlled-synthesis of highly dispersed hollow Fe3O4 nanoparticles. J. Alloys Compd. 2013, 574, 340–344.Search in Google Scholar
[26] Cheng Z, Chu XZ, Yin J, Zhong H, Xu J. Surfactantless synthesis of Fe3O4 magnetic nanobelts by a simple hydrothermal process. Mater. Lett. 2012, 75, 172–174.Search in Google Scholar
[27] Wan J, Cai W, Meng X, Liu E. Monodisperse water-soluble magnetite nanoparticles prepared by polyol process for high-performance magnetic resonance imaging. Chem. Commun. 2007, 5004–5006.10.1039/b712795bSearch in Google Scholar PubMed
[28] Zhang B, Tu Z, Zhao F, Wang J. Superparamagnetic iron oxide nanoparticles prepared by using an improved polyol method. Appl. Surf. Sci. 2013, 266, 375–337.Search in Google Scholar
[29] Abbas M, Rao BP, Naga SM, Takahashi M, Kim C. Synthesis of high magnetization hydrophilic magnetite (Fe3O4) nanoparticles in single reaction – surfactantless polyol process. Ceram. Int. 2013, 39, 7605–7611.Search in Google Scholar
[30] Kishimoto M, Isaka D, Horiuchi A, Yanagihara H, Kita E. Magnetic properties in oriented platelet Fe3O4 particles prepared by the polyol method using α-FeOOH as precursors. J. Magn. Magn. Mater. 2014, 352, 13–16.Search in Google Scholar
[31] Park J, An K, Hwang Y, Park J-G, Noh H-J, Kim J-Y, Park J-H, Hwang N-M, Hyeon T. Ultra-large-scale syntheses of monodisperse nanocrystals. Nat. Mater. 2004, 3, 891–895.Search in Google Scholar
[32] Sun S, Zeng H. Size-controlled synthesis of magnetite nanoparticles. J. Am. Chem. Soc. 2002, 124, 8204–8205.Search in Google Scholar
[33] Sun S, Zeng H, Robinson DB, Raoux S, Rice PM, Wang SX, Li G. Monodisperse MFe2O4 (M = Fe, Co, Mn) nanoparticles. J. Am. Chem. Soc. 2003, 126, 273–279.Search in Google Scholar
[34] Jeong U, Teng X, Wang Y, Yang H, Xia Y. Superparamagnetic colloids: controlled synthesis and niche applications. Adv. Mater. 2007, 19, 33–60.Search in Google Scholar
[35] Verwey EJW, Overbeek TG. Theory of the Stability of Lyophobic Colloids, Elsevier: Amsterdam, The Netherlands, 1948.Search in Google Scholar
[36] Derjaguin BV, Landau LD. A theory of the stability of strongly charged lyophobic sols and the coalescence of strongly charged particles in electrolytic solution. Acta Phys. URSS 1941, 14, 633.Search in Google Scholar
[37] Derjaguin BV, Voropayeva TN, Kabanov BN, Titiyevskaya AS. Surface forces and the stability of colloids and disperse systems. Progr. Surf. Sci. 1993, 43, 83–105.Search in Google Scholar
[38] Bacri J-C, Perzynski R, Salin D, Cabuil V, Massart R. Ionic ferrofluids: a crossing of chemistry and physics. J. Magn. Magn. Mater. 1990, 85, 27–32.Search in Google Scholar
[39] Walter A, Garofalo A, Bonazza P, Parat A, Taleb J, Jouhannaud J, Pourroy G, Voirin E, Laurent S, Vander Elst L, Muller RN, Billotey C, Begin-Colin S, Felder-Flesch D. Validation of a dendron concept to tune colloidal stability, MRI relaxivity and bioelimination of functional nanoparticles. J. Mater. Chem. B 2015, 3, 1484–1494.10.1039/C4TB01954GSearch in Google Scholar PubMed
[40] Gillich T, Acikgöz C, Isa L, Schluter AD, Spencer ND, Textor M. PEG-stabilized core-shell nanoparticles: impact of linear versus dendritic polymer shell architecture on colloidal properties and the reversibility of temperature-induced aggregation. ACS Nano 2013, 7, 316–329.10.1021/nn304045qSearch in Google Scholar PubMed
[41] Steitz B, Salaklang J, Finka A, O’Neil C, Hofmann H, Petri-Fink A. Fixed bed reactor for solid-phase surface derivatization of superparamagnetic nanoparticles. Bioconjug. Chem. 2007, 18, 1684–1690.Search in Google Scholar
[42] Tsai Z-T, Wang J-F, Kuo H-Y, Shen C-R, Wang J-J, Yen T-C. In situ preparation of high relaxivity iron oxide nanoparticles by coating with chitosan: a potential MRI contrast agent useful for cell tracking. J. Magn. Magn. Mater. 2010, 322, 208–213.Search in Google Scholar
[43] Dai L, Liu Y, Wang Z, Guo F, Shi D, Zhang B. One-pot facile synthesis of PEGylated superparamagnetic iron oxide nanoparticles for MRI contrast enhancement. Mater. Sci. Eng. C 2014, 41, 161–167.10.1016/j.msec.2014.04.041Search in Google Scholar PubMed
[44] Basly B, Popa G, Fleutot S, Pichon B, Garofalo A, Ghobril C, Billotey C, Berniard A, Bonazza P, Martinez H, Felder-Flesch D, Begin-Colin S. Effect of the nanoparticle synthesis way on dendronized iron oxides as MRI contrast agents. Dalton Trans. 2013, 42, 2146–2157.Search in Google Scholar
[45] Nam J, Won N, Bang J, Jin H, Park J, Jung S, Jung S, Park Y, Kim S. Surface engineering of inorganic nanoparticles for imaging and therapy. Adv. Drug Deliv. Rev. 2013, 65, 622–648.Search in Google Scholar
[46] Yamaura M, Camilo RL, Sampaio LC, Macêdo MA, Nakamura M, Toma HE. Preparation and characterization of (3-aminopropyl)triethoxysilane-coated magnetite nanoparticles. J. Magn. Magn. Mater. 2004, 279, 210–217.Search in Google Scholar
[47] Shen M, Cai H, Wang X, Cao X, Li K, Wang SH, Guo R, Zheng L, Zhang G, Shi X. Facile one-pot preparation, surface functionalization, and toxicity assay of APTS-coated iron oxide nanoparticles. Nanotechnology, 2012, 23, 105601 (10pp).10.1088/0957-4484/23/10/105601Search in Google Scholar PubMed
[48] Yathindranath V, Sun Z, Worden M, Donald LJ, Thliveris JA, Miller DW, Hegmann T. One-pot synthesis of iron oxide nanoparticles with functional silane shells: a versatile general precursor for conjugations and biomedical applications. Langmuir 2013, 29, 10850–10858.10.1021/la402007dSearch in Google Scholar PubMed
[49] Liu Y, Li Y, Li X-M, He T. Kinetics of (3-aminopropyl)triethoxylsilane (APTES) silanization of superparamagnetic iron oxide nanoparticles. Langmuir 2013, 29, 15275–15282.10.1021/la403269uSearch in Google Scholar PubMed
[50] Herrmann IK, Grass RN, Mazunin D, Stark WJ. Synthesis and covalent surface functionalization of nonoxidic iron core-shell nanomagnets. Chem. Mater. 2009, 21, 3275.Search in Google Scholar
[51] Lee H, Yu MK, Park S, Moon S, Min JJ, Jeong YY, Kang H-W, Jon S. Thermally cross-linked superparamagnetic iron oxide nanoparticles: synthesis and application as a dual imaging probe for cancer in vivo. J. Am. Chem. Soc. 2007, 129, 12739–12745.Search in Google Scholar
[52] Selvan ST, Tan TTY, Yi DK, Jana NR. Functional and multifunctional nanoparticles for bioimaging and biosensing. Langmuir 2010, 26, 11631–11641.10.1021/la903512mSearch in Google Scholar PubMed
[53] Piao Y, Burns A, Kim J, Wiesner U Hyeon T. Designed fabrication of silica-based nanostructured particle systems for nanomedicine applications. Adv. Funct. Mater. 2008, 18, 3745–3758.Search in Google Scholar
[54] Zhang C, Wängler B, Morgenstern B, Zentgraf H, Eisenhut M, Untenecker H, Krüger R, Huss R, Seliger C, Semmler W, Kiessling F. Silica- and alkoxysilane-coated ultrasmall superparamagnetic iron oxide particles: a promising tool to label cells for magnetic resonance imaging. Langmuir 2007, 23, 1427–1434.10.1021/la061879kSearch in Google Scholar PubMed
[55] Fauconnier N, Pons JN, Roger J, Bee A. Thiolation of maghemite nanoparticles by dimercaptosuccinic acid. J. Colloid Interface Sci. 1997, 194, 427–433.Search in Google Scholar
[56] Lattuada M, Hatton TA. Functionalization of monodisperse magnetic nanoparticles. Langmuir 2006, 23, 2158–2168.10.1021/la062092xSearch in Google Scholar PubMed
[57] Maurizi L, Bisht H, Bouyer F, Millot N. Easy route to functionalize iron oxide nanoparticles via long-term stable thiol groups. Langmuir 2009, 25, 8857–8859.10.1021/la901602wSearch in Google Scholar PubMed
[58] Miguel-Sancho N, Bomatí-Miguel O, Colom G, Salvador J-P, Marco M-P, Santamaría J. Development of stable, water-dispersible, and biofunctionalizable superparamagnetic iron oxide nanoparticles. Chem. Mater. 2011, 23, 2795–2802.Search in Google Scholar
[59] Racuciu M, Creanga DE, Airinei A. Citric-acid-coated magnetite nanoparticles for biological applications. Eur. Phys. J. E 2006, 21, 117–121.10.1140/epje/i2006-10051-ySearch in Google Scholar PubMed
[60] Turcheniuk K, Tarasevych AV, Kukhar VP, Boukherroub R, Szunerits S. Recent advances in surface chemistry strategies for the fabrication of functional iron oxide based magnetic nanoparticles. Nanoscale 2013, 5, 10729–10752.10.1039/c3nr04131jSearch in Google Scholar PubMed
[61] Amstad E, Zurcher S, Mashaghi A, Wong JY, Textor M, Reimhult E. Surface functionalization of single superparamagnetic iron oxide nanoparticles for targeted magnetic resonance imaging. Small 2009, 5, 1334–1342.10.1002/smll.200801328Search in Google Scholar PubMed
[62] Frey NA, Peng S, Cheng K, Sun S. Magnetic nanoparticles: synthesis, functionalization, and applications in bioimaging and magnetic energy storage. Chem. Soc. Rev. 2009, 38, 2532–2542.Search in Google Scholar
[63] Xu C, Xu C, Xu K, Gu H, Zheng R, Liu H, Zhang X, Guo Z, Xu B. Dopamine as a robust anchor to immobilize functional molecules on the iron oxide shell of magnetic nanoparticles. J. Am. Chem. Soc. 2004, 126, 9938–9939.Search in Google Scholar
[64] Shultz MD, Reveles JU, Khanna SN, Carpenter EE. Reactive nature of dopamine as a surface functionalization agent in iron oxide nanoparticles. J. Am. Chem. Soc. 2007, 129, 2482–2487.Search in Google Scholar
[65] Daou TJ, Grenèche JM, Pourroy G, Buathong S, Derory A, Ulhaq-Bouillet C, Donnio B, Guillon D, Begin-Colin S. Coupling agent effect on magnetic properties of functionalized magnetite-based nanoparticles. Chem. Mater. 2008, 20, 5869–5875.Search in Google Scholar
[66] Daou TJ, Buathong S, Ung D, Donnio B, Pourroy G, Guillon D, Bégin S. Investigation of the grafting rate of organic molecules on the surface of magnetite nanoparticles as a function of the coupling agent. Sens. Actuators B Chem. 2007, 126, 159–162.Search in Google Scholar
[67] Sahoo Y, Pizem H, Fried T, Golodnitsky D, Burstein L, Sukenik CN, Markovich G. Alkyl phosphonate/phosphate coating on magnetite nanoparticles: a comparison with fatty acids. Langmuir 2001, 17, 7907–7911.10.1021/la010703+Search in Google Scholar
[68] Basly B, Felder-Flesch D, Perriat P, Pourroy G, Bégin-Colin S. Properties and suspension stability of dendronized iron oxide nanoparticles for MRI applications. Contrast Media Mol. Imaging 2011, 6, 132–138.10.1002/cmmi.416Search in Google Scholar PubMed
[69] Basly B, Felder-Flesch D, Perriat P, Billotey C, Taleb J, Pourroy G, Begin-Colin S. Dendronized iron oxide nanoparticles as contrast agents for MRI. Chem. Commun. 2010, 46, 985–987.Search in Google Scholar
[70] Smolensky ED, Park HY, Berquó TS, Pierre VC. Surface functionalization of magnetic iron oxide nanoparticles for MRI applications – effect of anchoring group and ligand exchange protocol. Contrast Media Mol. Imaging 2011, 6, 189–199.10.1002/cmmi.417Search in Google Scholar PubMed PubMed Central
[71] Queffelec C, Petit M, Janvier P, Knight DA, Bujoli B. Surface modification using phosphonic acids and esters. Chem. Rev. 2012, 112, 3777–3807.Search in Google Scholar
[72] Söderlind F, Pedersen H, Petoral RM, Käll P-O, Uvdal K. Synthesis and characterisation of Gd2O3 nanocrystals functionalised by organic acids. J. Colloid Interface Sci. 2005, 288, 140–148.Search in Google Scholar
[73] Egerton TA, Everall NJ, Tooley IR. Characterization of TiO2 nanoparticles surface modified with aluminum stearate. Langmuir 2005, 21, 3172–3178.10.1021/la047390dSearch in Google Scholar PubMed
[74] Ren Y, Kato T. Polarization modulation infrared reflection absorption spectroscopy of a cadmium arachidate monolayer at the air/water interface. Langmuir 2002, 18, 8560–8565.10.1021/la020184qSearch in Google Scholar
[75] Kariuki NN, Luo J, Hassan SA, Lim I-IS, Wang L, Zhong CJ. Assembly of bimetallic gold-silver nanoparticles via selective interparticle dicarboxylate-silver linkages. Chem. Mater. 2005, 18, 123–132.Search in Google Scholar
[76] Seo YU, Lee SJ, Kim K. Thermal/photo conversion of silver 4-nitrobenzoate to nitro/amine-terminated silver nanoparticles. J. Phys. Chem. B 2004, 108, 4000–4007.10.1021/jp0364250Search in Google Scholar
[77] Bronstein LM, Huang X, Retrum J, Schmucker A, Pink M, Stein BD, Dragnea B. Influence of iron oleate complex structure on iron oxide nanoparticle formation. Chem. Mater. 2007, 19, 3624–3632.Search in Google Scholar
[78] Hofmann A, Thierbach S, Semisch A, Hartwig A, Taupiz M, Rühl E, Graf C. Highly monodisperse water-dispersible iron oxide nanoparticles for biomedical applications. J. Mater. Chem. 2010, 20, 7842–7853.Search in Google Scholar
[79] Basly B, Popa G, Fleutot S, Pichon BP, Garofalo A, Ghobril C, Billotey C, Berniard A, Bonazza P, Martinez H, Felder-Flesch D, Begin-Colin S. Effect of the nanoparticle synthesis method on dendronized iron oxides as MRI contrast agents. Dalton Trans. 2012, 42, 2146–2157.Search in Google Scholar
[80] Borggaard OK, Raben-Lange B, Gimsing AL, Strobel BW. Influence of humic substances on phosphate adsorption by aluminium and iron oxides. Geoderma 2005, 127, 270–279.10.1016/j.geoderma.2004.12.011Search in Google Scholar
[81] Tudisco C, Bertani F, Cambria MT, Sinatra F, Fantechi E, Innocenti C, Sangregorio C, Dalcanale E, Condorelli GG. Functionalization of PEGylated Fe3O4 magnetic nanoparticles with tetraphosphonate cavitand for biomedical application. Nanoscale 2013, 5, 11438–11446.10.1039/c3nr02188bSearch in Google Scholar PubMed
[82] Lee N, Hyeon T. Designed synthesis of uniformly sized iron oxide nanoparticles for efficient magnetic resonance imaging contrast agents. Chem. Soc. Rev. 2012, 41, 2575–2589.Search in Google Scholar
[83] Sandiford L, Phinikaridou A, Protti A, Meszaros LK, Cui X, Yan Y, Frodsham G, Williamson PA, Gaddum N, Botnar RM, Blower PJ, Green MA, de Rosales RTM. Bisphosphonate-anchored PEGylation and radiolabeling of superparamagnetic iron oxide: long-circulating nanoparticles for in vivo multimodal (T1 MRI-SPECT) imaging. ACS Nano 2012, 7, 500–512.10.1021/nn3046055Search in Google Scholar PubMed PubMed Central
[84] Karimi A, Denizot B, Passirani C, Hindré F, Roux J, Legras P, Le Jeune JJ. In vitro and in vivo evaluation of superparamagnetic iron oxide nanoparticles coated by bisphosphonates: the effects of electrical charge and molecule length. Eur. J. Pharm. Sci. 2013, 49, 101–108.Search in Google Scholar
[85] Ghobril C, Popa G, Parat A, Billotey C, Taleb J, Bonazza P, Begin-Colin S, Felder-Flesch D. A bisphosphonate tweezers and clickable PEGylated PAMAM dendrons for the preparation of functional iron oxide nanoparticles displaying renal and hepatobiliary elimination. Chem. Commun. 2013, 49, 9158–9160.Search in Google Scholar
[86] Giger EV, Castagner B, Leroux J-C. Biomedical applications of bisphosphonates. J. Control. Release 2013, 167, 175–188.10.1016/j.jconrel.2013.01.032Search in Google Scholar PubMed
[87] Lalatonne Y, Benyettou F, Bonnin D, Lièvre N, Monod P, Lecouvey M, Weinmann P, Motte L. Characterization of magnetic labels for bioassays. J. Magn. Magn. Mater. 2009, 321, 1653–1657.Search in Google Scholar
[88] Basti H, Tahar LB, Smiri LS, Herbst F, Vaulay M-J, Chau F, Ammar S, Benderbous S. Catechol derivatives-coated Fe3O4 and γ-Fe2O3 nanoparticles as potential MRI contrast agents. J. Colloid Interface Sci. 2010, 341, 248–254.Search in Google Scholar
[89] Na HB, Palui G, Rosenberg JT, Ji X, Grant SC, Mattoussi H. Multidentate catechol-based polyethylene glycol oligomers provide enhanced stability and biocompatibility to iron oxide nanoparticles. ACS Nano 2012, 6, 389–399.10.1021/nn203735bSearch in Google Scholar PubMed
[90] Sara M, Drago C, Ferretti AM, Puglisi A, Ponti A. Colloidal stability of iron oxide nanocrystals coated with a PEG-based tetra-catechol surfactant. Nanotechnology 2013, 24, 105702.10.1088/0957-4484/24/10/105702Search in Google Scholar
[91] Portet D, Denizot B, Rump E, Lejeune J-J, Jallet P. Nonpolymeric coatings of iron oxide colloids for biological use as magnetic resonance imaging contrast agents. J. Colloid Interface Sci. 2001, 238, 37–42.Search in Google Scholar
[92] Torres Martin de Rosales R, Tavaré R, Glaria A, Varma G, Protti A, Blower PJ. 99mTc-bisphosphonate-iron oxide nanoparticle conjugates for dual-modality biomedical imaging. Bioconjug. Chem. 2011, 22, 455–465.Search in Google Scholar
[93] Torres Martin de Rosales R, Tavaré R, Paul RL, Jauregui-Osoro M, Protti A, Glaria A, Varma G, Szanda I, Blower PJ. Synthesis of 64CuII-bis(dithiocarbamatebisphosphonate) and its conjugation with superparamagnetic iron oxide nanoparticles: in vivo evaluation as dual-modality PET-MRI agent. Angew. Chem. Int. Ed. 2011, 50, 5509–5513.Search in Google Scholar
[94] Yiu HH. Engineering the multifunctional surface on magnetic nanoparticles for targeted biomedical applications: a chemical approach. Nanomedicine (Lond) 2011, 6, 1429–1446.10.2217/nnm.11.132Search in Google Scholar
[95] Truong-Dinh Tran T, Van Vo T, Tran PH-L. Design of iron oxide nanoparticles decorated oleic acid and bovine serum albumin for drug delivery. Chem. Eng. Res. Des. 2015, 94, 112–118.Search in Google Scholar
[96] Ruiz A, Salas G, Calero M, Hernández Y, Villanueva A, Herranz F, Veintemillas-Verdaguer S, Martínez E, Barber DF, Morales MP. Short-chain PEG molecules strongly bound to magnetic nanoparticle for MRI long circulating agents. Acta Biomater. 2013, 9, 6421–6430.Search in Google Scholar
[97] Gillich T, Acikgöz C, Isa L, Schlüter AD, Spencer ND, Textor M. PEG-stabilized core-shell nanoparticles: impact of linear versus dendritic polymer shell architecture on colloidal properties and the reversibility of temperature-induced aggregation. ACS Nano 2012, 7, 316–329.10.1021/nn304045qSearch in Google Scholar
[98] Saha I, Chaffee KE, Duanmu C, Woods BM, Stokes AM, Buck LE, Walkup LL, Sattenapally N, Huggenvik J, Gao Y, Goodson BM. pH-sensitive MR responses induced by dendron-functionalized SPIONs. J. Phys. Chem. C 2012, 117, 1893–1903.10.1021/jp306128vSearch in Google Scholar
[99] Heuze K, Rosario-Amorin D, Nlate S, Gaboyard M, Bouter A, Clérac R. Efficient strategy to increase the surface functionalization of core-shell superparamagnetic nanoparticles using dendron grafting. N. J. Chem. 2008, 32, 383.Search in Google Scholar
[100] He X, Wu X, Cai X, Lin S, Xie M, Zhu X, Yan D. Functionalization of magnetic nanoparticles with dendritic-linear-brush-like triblock copolymers and their drug release properties. Langmuir 2012, 28, 11929–11938.10.1021/la302546mSearch in Google Scholar
[101] Tassa C, Shaw SY, Weissleder R. Dextran-coated iron oxide nanoparticles: a versatile platform for targeted molecular imaging, molecular diagnostics, and therapy. Acc. Chem. Res. 2011, 44, 842–852.Search in Google Scholar
[102] Berry CC, Wells S, Charles S, Curtis ASG. Dextran and albumin derivatised iron oxide nanoparticles: influence on fibroblasts in vitro. Biomaterials 2003, 24, 4551–4557.10.1016/S0142-9612(03)00237-0Search in Google Scholar
[103] Huang S, Yan W, Hu G, Wang L. Facile and green synthesis of biocompatible and bioconjugatable magnetite nanofluids for high-Resolution T2 MRI contrast agents. J. Phys. Chem. C 2012, 116, 20558–20563.10.1021/jp305211dSearch in Google Scholar
[104] Bae KH, Park M, Do MJ, Lee N, Ryu JH, Kim GW, Kim C, Park TG, Hyeon T. Chitosan oligosaccharide-stabilized ferrimagnetic iron oxide nanocubes for magnetically modulated cancer hyperthermia. ACS Nano 2012, 6, 5266–5273.10.1021/nn301046wSearch in Google Scholar PubMed
[105] Sanjai C, Kothan S, Gonil P, Saesoo S, Sajomsang W. Chitosan-triphosphate nanoparticles for encapsulation of super-paramagnetic iron oxide as an MRI contrast agent. Carbohydr. Polym. 2014, 104, 231–237.Search in Google Scholar
[106] Cruz LJ, Tacken PJ, Fokkink R, Figdor CG. The influence of PEG chain length and targeting moiety on antibody-mediated delivery of nanoparticle vaccines to human dendritic cells. Biomaterials 2011, 32, 6791–6803.10.1016/j.biomaterials.2011.04.082Search in Google Scholar PubMed
[107] Masoudi A, Hosseini HRM, Shokrgozar MA, Ahmadi R, Oghabian MA. The effect of poly(ethylene glycol) coating on colloidal stability of superparamagnetic iron oxide nanoparticles as potential MRI contrast agent. Int. J. Pharm. 2012, 433, 129–141.Search in Google Scholar
[108] Mahmoudi M, Simchi A, Imani M, Milani AS, Stroeve P. Optimal design and characterization of superparamagnetic iron oxide nanoparticles coated with polyvinyl alcohol for targeted delivery and imaging†. J. Phys. Chem. B 2008, 112, 14470–14481.10.1021/jp803016nSearch in Google Scholar PubMed
[109] Zhou L, He B, Zhang F. Facile one-pot synthesis of iron oxide nanoparticles cross-linked magnetic poly(vinyl alcohol) gel beads for drug delivery. ACS Appl. Mater. Interfaces 2011, 4, 192–199.10.1021/am201649bSearch in Google Scholar PubMed
[110] Bae S-J, Park J-A, Lee J-J, Lee G-H, Kim T-J, Yoo D-S, Chang Y. Ultrasmall iron oxide nanoparticles: synthesis, physicochemical, and magnetic properties. Curr. Appl. Phys. 2009, 9 (1 Suppl), S19–S21.Search in Google Scholar
[111] Lee HY, Lim NH, Seo JA, Yuk SH, Kwak BK, Khang G, Lee HB, Cho SH. Preparation and magnetic resonance imaging effect of polyvinylpyrrolidone-coated iron oxide nanoparticles. J. Biomed. Mater. Res. B: Appl. Biomater. 2006, 79B, 142–150.Search in Google Scholar
[112] Na HB, Palui G, Rosenberg JT, Ji X, Grant SC, Mattoussi H. Multidentate catechol-based polyethylene glycol oligomers provide enhanced stability and biocompatibility to iron oxide nanoparticles. ACS Nano 2012, 6, 389–399.10.1021/nn203735bSearch in Google Scholar PubMed
[113] Walkey CD, Olsen JB, Guo H, Emili A, Chan WCW. Nanoparticle size and surface chemistry determine serum protein adsorption and macrophage uptake. J. Am. Chem. Soc. 2011, 134, 2139–2147.Search in Google Scholar
[114] Huang C, Neoh KG, Wang L, Kang E-T, Shuter B. Magnetic nanoparticles for magnetic resonance imaging: modulation of macrophage uptake by controlled PEGylation of the surface coating. J. Mater. Chem. 2010, 20, 8512–8520.Search in Google Scholar
[115] Ruiz A, Hernández Y, Cabal C, González E, Veintemillas-Verdaguer S, Martínez E, Morales MP. Biodistribution and pharmacokinetics of uniform magnetite nanoparticles chemically modified with polyethylene glycol. Nanoscale 2013, 5, 11400–11408.10.1039/c3nr01412fSearch in Google Scholar PubMed
[116] Berry CC. Progress in functionalization of magnetic nanoparticles for applications in biomedicine. J. Phys. D: Appl.Phys. 2009, 42, 224003, 1–9.10.1088/0022-3727/42/22/224003Search in Google Scholar
[117] Weitman SD, Lark RH, Coney LR, Fort DW, Frasca V, Zurawski VR, Kamen BA. Distribution of the folate receptor GP38 in normal and malignant cell lines and tissues. Cancer Res. 1992, 52, 3396–3401.Search in Google Scholar
[118] Sun C, Sze R, Zhang M. Folic acid-PEG conjugated superparamagnetic nanoparticles for targeted cellular uptake and detection by MRI. J. Biomed. Mater. Res. A 2006, 78A, 550–557.10.1002/jbm.a.30781Search in Google Scholar PubMed
[119] Coney LR, Tomassetti A, Carayannopoulos L, Frasca V, Kamen BA, Colnaghi MI, Zurawski VR. Cloning of a tumor-associated antigen: MOv18 and MOv19 antibodies recognize a folate-binding protein. Cancer Res. 1991, 51, 6125–6132.Search in Google Scholar
[120] Pilapong C, Sitthichai S, Thongtem S, Thongtem T. Smart magnetic nanoparticle-aptamer probe for targeted imaging and treatment of hepatocellular carcinoma. Int. J. Pharm. 2014, 473, 469–447.Search in Google Scholar
[121] Jalalian SH, Taghdisi SM, Hamedani NS, Kalat SAM, Lavaee P, ZandKarimi M, Ghows N, Jaafari MR, Naghibi S, Danesh NM, Ramezani M, Abnous K. Epirubicin loaded super paramagnetic iron oxide nanoparticle-aptamer bioconjugate for combined colon cancer therapy and imaging in vivo. Eur. J. Pharm. Sci. 2013, 50, 191–197.Search in Google Scholar
[122] Lee JH, Yigit MV, Mazumdar D, Lu Y. Molecular diagnostic and drug delivery agents based on aptamer-nanomaterial conjugates. Adv. Drug Deliv. Rev. 2010, 62, 592–605.Search in Google Scholar
[123] Bolley J. Elaboration et caractérisation d’agent de contraste IRM pour le ciblage des intégrines αvβ3, in Chimie 2014, Université Paris 13.Search in Google Scholar
[124] Montet X, Montet-Abou K, Reynolds F, Weissleder R, Josephson L. Nanoparticle imaging of integrins on tumor cells. Neoplasia 2006, 8, 214–222.10.1593/neo.05769Search in Google Scholar PubMed PubMed Central
[125] Huy TQ, Thuy NT, Blanco-Andujar C, Thanh NTK. Protein A-conjugated iron oxide nanoparticles for separation of Vibriocholerae from water samples. Faraday Discuss. 2014, 175, 73–82.Search in Google Scholar
[126] Xu H, Aguilar ZP, Yang L, Kuang M, Duan H, Xiong Y, Wei H, Wang A. Antibody conjugated magnetic iron oxide nanoparticles for cancer cell separation in fresh whole blood. Biomaterials 2011, 32, 9758–9765.10.1016/j.biomaterials.2011.08.076Search in Google Scholar PubMed PubMed Central
[127] Tomalia DA, Fréchet JMJ. Discovery of dendrimers and dendritic polymers: a brief historical perspective*. J. Polym. Sci. A: Polym. Chem. 2002, 40, 2719–2728.Search in Google Scholar
[128] Love CS, Chechik V, Smith DK, Brennan C. Dendron-stabilised gold nanoparticles: generation dependence of core size and thermal stability. J. Mater. Chem. 2004, 14, 919–923.Search in Google Scholar
[129] Paez JI, Coronado EA, Strumia MC. Preparation of controlled gold nanoparticle aggregates using a dendronization strategy. J. Colloid Interface Sci. 2012, 384, 10–21.Search in Google Scholar
[130] Huang B, Tomalia DA. Dendronization of gold and CdSe/cdS (core-shell) quantum dots with tomalia type, thiol core, functionalized poly(amidoamine) (PAMAM) dendrons. J. Lumin. 2005, 111, 215–223.Search in Google Scholar
[131] Martin AL, Li B, Gillies ER. Surface functionalization of nanomaterials with dendritic groups: toward enhanced binding to biological targets. J. Am. Chem. Soc. 2009, 131, 734–741.Search in Google Scholar
[132] Sun Y-P, Huang W, Lin Y, Fu K, Kitaygorodskiy A, Riddle LA, Yu YJ, Carroll DL. Soluble dendron-functionalized carbon nanotubes: preparation, characterization, and properties. Chem. Mater. 2001, 13, 2864–2869.Search in Google Scholar
[133] Lamanna G, Garofalo A, Popa G, Wilhelm C, Bégin-Colin S, Felder-Flesch D, Bianco A, Gazeau F, Ménard-Moyon C. Endowing carbon nanotubes with superparamagnetic properties: applications for cell labeling, MRI cell tracking and magnetic manipulations. Nanoscale 2013, 5, 4412–4421.10.1039/c3nr00636kSearch in Google Scholar PubMed
[134] Benhabbour SR, Sheardown H, Adronov A. Protein resistance of PEG-functionalized dendronized surfaces: effect of PEG molecular weight and dendron generation. Macromolecules 2008, 41, 4817–4823.10.1021/ma8004586Search in Google Scholar
[135] Duanmu C, Saha I, Zheng Y, Goodson BM, Gao Y. Dendron-functionalized superparamagnetic nanoparticles with switchable solubility in organic and aqueous media: matrices for homogeneous catalysis and potential MRI contrast agents. Chem. Mater. 2006, 18, 5973–5981.Search in Google Scholar
[136] Liu W-M, Xue Y-N, Peng N, He W-T, Zhuo R-X, Huang S-W. Dendrimer modified magnetic iron oxide nanoparticle/DNA/PEI ternary magnetoplexes: a novel strategy for magnetofection. J. Mater. Chem. 2011, 21, 13306–13315.Search in Google Scholar
[137] Chandra S, Mehta S, Nigam S, Bahadur D. Dendritic magnetite nanocarriers for drug delivery applications. New J. Chem. 2010, 34, 648–655.Search in Google Scholar
[138] Rouhollah K, Pelin M, Serap Y, Gozde U, Ufuk G. Doxorubicin loading, release, and stability of polyamidoamine dendrimer-coated magnetic nanoparticles. J. Pharm. Sci. 2013, 102, 1825–1835.Search in Google Scholar
[139] Lamanna G, Kueny-Stotz M, Mamlouk-Chaouachi H, Ghobril C, Basly B, Bertin A, Miladi I, Billotey C, Pourroy G, Begin-Colin S, Felder-Flesch D. Dendronized iron oxide nanoparticles for multimodal imaging. Biomaterials 2011, 32, 8562–8573.10.1016/j.biomaterials.2011.07.026Search in Google Scholar PubMed
[140] Arsalani N, Fattahi H, Laurent S, Burtea C, Elst LV, Muller RN. Polyglycerol-grafted superparamagnetic iron oxide nanoparticles: highly efficient MRI contrast agent for liver and kidney imaging and potential scaffold for cellular and molecular imaging. Contrast Media Mol. Imaging 2012, 7, 185–194.10.1002/cmmi.479Search in Google Scholar PubMed
[141] Kono K, Ikeda R, Tsukamoto K, Yuba E, Kojima C, Harada A. Polyamidoamine dendron-bearing lipids as a nonviral vector: influence of dendron generation. Bioconjug. Chem. 2012, 23, 871–879.Search in Google Scholar
[142] Pan B, Cui D, Sheng Y, Ozkan C, Gao F, He R, Li Q, Xu P, Huang T. Dendrimer-modified magnetic nanoparticles enhance efficiency of gene delivery system. Cancer Res. 2007, 67, 8156–8163.Search in Google Scholar
[143] Gao F, Pan B-F, Zheng W-M, Ao L-M, Gu H-C. Study of streptavidin coated onto PAMAM dendrimer modified magnetite nanoparticles. J. Magn. Magn. Mater. 2005, 293, 48–54.Search in Google Scholar
[144] Walter A, Billotey C, Garofalo A, Ulhaq-Bouillet C, Lefèvre C, Taleb J, Laurent S, Vander Elst L, Muller RN, Lartigue L, Gazeau F, Felder-Flesch D, Begin-Colin S. Mastering shape and composition of dendronized iron oxide nanoparticles to tailor magnetic resonance imaging and hyperthermia. Chem. Mater. 2014, 26, 5252–5264.Search in Google Scholar
©2015 by De Gruyter
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Articles in the same Issue
- Frontmatter
- In this issue
- Editorial
- The interface at the nanoscale level: implications from synthesis to applications
- Reviews
- Creation of interfaces in composite/hybrid nanostructured materials using supercritical fluids
- Nucleation and growth of carbo-nitride nanoparticles in α-Fe-based alloys and associated interfacial process
- Thermal transport across atomic-layer material interfaces
- Hybrid interfaces in layered hydroxides: magnetic and multifunctional superstructures by design
- Functionalization strategies and dendronization of iron oxide nanoparticles
- Colloidal magnetic nanocrystal clusters: variable length-scale interaction mechanisms, synergetic functionalities and technological advantages
Articles in the same Issue
- Frontmatter
- In this issue
- Editorial
- The interface at the nanoscale level: implications from synthesis to applications
- Reviews
- Creation of interfaces in composite/hybrid nanostructured materials using supercritical fluids
- Nucleation and growth of carbo-nitride nanoparticles in α-Fe-based alloys and associated interfacial process
- Thermal transport across atomic-layer material interfaces
- Hybrid interfaces in layered hydroxides: magnetic and multifunctional superstructures by design
- Functionalization strategies and dendronization of iron oxide nanoparticles
- Colloidal magnetic nanocrystal clusters: variable length-scale interaction mechanisms, synergetic functionalities and technological advantages

