Abstract
Objectives
Alternative medicines commonly supplement or, at times, replace standard medical treatment. One area of increasing attention is disease-modifying medicines for neurodegenerative diseases. However, few such alternatives have been investigated thoroughly with an eye towards understanding mechanisms of action for clinical use. Medicinal mushrooms have important health benefits and pharmacological activities with anti-inflammatory, antioxidant, antibacterial, antiviral, immunomodulatory, digestive, cytoprotective, homeostatic, and neuroprotective activities. Edible mushrooms are known to play roles in preventing age-related diseases. Several studies have revealed that polysaccharides, terpenes, and phenolic compounds are chemical components derived from mushrooms with pharmacological activities. Due to limited effective protocols for mushroom protein extraction for proteomic studies, information about these medicinally related proteins and their biological functions remains enigmatic.
Methods
Herein, we have performed proteomic studies of two mushroom species Laricifomes officinalis (agarikon) and Grifola frondosa (maitake).
Results
These studies serve to uncover a foundation for putative proteome-associated neuroprotective processes. The recovered proteins from both species show multiple cell-specific signaling pathways including unfolded protein response, and mitochondrial protein import as well as those linked to BAG2, ubiquitination, apoptosis, microautophagy, glycolysis, SNARE, and immunogenic cell signaling pathways.
Conclusions
This study uncovered mushroom proteome-associated proteins which serve to better understand the structural and functional properties of mushrooms used as alternative medicines for broad potential health benefits.
Introduction
Neurodegenerative diseases include, but are not limited to, Alzheimer’s and Parkinson’s diseases (AD and PD), Huntington’s disease (HD), multiple sclerosis (MS), and amyotrophic lateral sclerosis (ALS). Neurological disorders are the leading cause of global physical and cognitive disability, and currently affect approximately 15 % of the worldwide population [1]. In addition to direct costs associated with medical management of neurodegenerative diseases, indirect costs are associated with job loss, caregivers, medical equipment, and home safety [2]. Currently, available medications are palliative. No current therapies can halt or reverse cognitive or motor dysfunctions.
While multiple natural materials have been touted to positively affect the course of disease by possessing bioactive components, there is limited to no evidence that any protects or ameliorates disease. One example is edible mushrooms that are a known source of bio-compounds that have nutritional, pharmacological, and medicinal values [3]. For example, the fruiting body and dried mycelium extract of different mushrooms contain potential therapeutic components [4]. Moreover, prior studies showed that mushrooms do have antioxidative, anti-inflammatory, immunomodulatory, antimicrobial, and anticancer properties [5–7]. Thus, the consumption of mushrooms could potentially boost immune responses or affect the signs and symptoms of disease [8]. Notably, mycelium extracts and isolated bioactive compounds (melatonin, ergosterol, terpenoids, and phenolic compounds) extracted from mushrooms have been observed to demonstrate a spectrum of ameliorating effects on AD. These include, but are not limited to, neuroprotective and anti-inflammatory activities. Mushrooms from fungi such as Grifola frondosa, Lignosus rhinocerotis, Hericium erinaceus, may positively affect cognitive functions [9]. The aqueous extract of Ganoderma lucidum (500 mg/mL) was found to recover the synaptic dysfunction in rat cortical neurons via c-Jun N-terminal kinase (JNK) and P38 kinase pathway [10]. Furthermore, microglial activation has been significantly inhibited by G. lucidum extract, and consequently, reduced the dopaminergic neuron death in PD [11]. Mushrooms’ diverse biological functions that help to prevent age-based neuronal dysfunctions have received widespread attention. Studies have begun to identify mushroom’s active compounds [9, 12, 13]. This may be only a beginning in discovery toward implementation as research continues to seek alternative remedies through mycotic medicines.
Over the past decades, proteomic studies have been developed and used to study protein content amongst a broad variety of cells, tissues, and organisms, and notably have achieved relevancy in works to discover mycotic medicines [3]. These studies were performed to identify mushroom proteins with potential as antioxidant, anti-inflammatory, anticancer, anti-diabetic, antimicrobial, and apoptotic effectors. The use of proteomics for identification of fungal homologs with relevance to human function remains a challenge due to limitations in precisely identifying human proteins and extended efforts to obtain a complete proteome [14]. In our efforts to uncover the proteomic signature of mushrooms and putative activities, we utilized label-free quantification (LFQ) mass spectrometry-based methods to identify the proteins in the fruiting bodies of both Laricifomes officinalis (agarikon) and G. frondosa (maitake). We performed functional and pathway enrichment analyses of the relevant identified proteins. The data began to unravel a spectrum of linked biological processes, molecular functions, and immune responses that point to novel neuroprotective mushroom biological activities.
Materials and methods
Materials
Fruiting bodies of the fungal species, L. officinalis (agarikon) and G. frondosa (maitake), used in this research were obtained from Mushroom Design (US) and Beond Ibogaine Treatment for Trauma and Addiction (Mexico). All reagents used for proteomic analyses were high-performance liquid chromatography (HPLC), or liquid chromatography – mass spectrometry (LC-MS) grade reagents.
Methods
Protein extraction
Ten grams of powdered fruiting bodies were used for each protein extraction and were performed in triplicate for each species. Trichloroacetic acid (TCA)/acetone precipitation method was performed as previously described [14]. The freeze-dried fresh sample was put into a mortar and ground into fine powder in the presence of liquid nitrogen. The fine powder was then homogenized in extraction buffer containing 0.1 M Tris-HCl (Fisher BioReagents, BP1758), 10 mM ethylenediaminetetraacetic acid (EDTA, Sigma-Aldrich, 79884), 1 % polyvinylpolypyrrolidone (PVPP, Sigma-Aldrich, 77627), 0.4 % β-mercaptoethanol (Sigma-Aldrich, M-3148) for 30 min (min). Afterwards, mixtures were centrifuged at 20,000 g for 20 min at 4 °C using Sorvall LYNX 4000 Superspeed Centrifuge (Thermo Fisher Scientific, 75006580). Following centrifugation, the liquid phase was precipitated with 4 volumes of 20 % TCA (Sigma-Aldrich, T0699) for 20 min at −20 °C, then centrifuged at 20,000 g for 20 min at 4 °C. The precipitate was then collected, suspended in 80 % acetone (Sigma-Aldrich, 1.00020), and centrifuged at 20,000 g for 20 min at 4 °C. Precipitation, collection, suspension, and centrifugation were repeated 7 times. The acetone was volatilized after the last centrifugation inside a chemical safety hood, and protein extract was stored at −80 °C.
Proteomic analysis
Protein extracts were suspended in lysis buffer of 0.1 M Tris-HCL, 6 M urea (Avantor, 97063-802), 3 M thiourea (Sigma-Aldrich, T7875), 4 % 3-[(3-cholamidopropyl) dimethylammonio] propanesulfonate (CHAPS, Thermo Fisher Scientific, 28300), 100 mM dithiothreitol (DTT, Promega, V3151), 2 % Pharmalyte (Sigma-Aldrich, GE17-0456-01), and protease and phosphatase inhibitor cocktail (Thermo Fisher Scientific, 0078441), and the pH of buffer was adjusted to 8.0. Protein concentration was determined using Pierce 660 Protein Assay kit (Thermo Fisher Scientific, 22662) according to the manufacturer’s instructions. Afterward, samples were processed as previously described (29) using filter-aided sample preparation (FASP, Pall Life Sciences, OD010C34) to digest 60 μg per column (3 columns/sample). Following trypsin digestion overnight, samples were cleaned using the Oasis MCX column (Waters, 186000252). Cleaned peptides were then quantitated using NanoDrop2000 at λ = 205 nm. Following resuspension in 0.1 % formic acid, 10 μL of each sample (1 μg/μL) was used for label-free quantification LFQ in the Mass Spectrometry and Proteomics Core Facility in the University of Nebraska Medical Center (UNMC) as previously described [15].
Protein identification was performed by searching MS/MS data against the Swiss-Prot Homo sapiens protein database downloaded in October 2023 using the in-house PEAKS X + DB search engine. H. sapiens protein database was used in this study to be able to identify mushroom proteins having similar structures and functions to those present in humans. The search was set for full tryptic peptides with a maximum of 2 missed cleavage sites. Variable modifications were acetylation of protein N-terminus and oxidized methionine, while carbamidomethylation of cysteine was set as a fixed modification. The precursor mass tolerance threshold was set at 10 ppm and the maximum fragment mass error was 0.02 Da. The significance threshold of the ion score was calculated based on a false discovery rate (FDR) of ≤1 %. Quantitative data analysis was performed to determine differentially expressed proteins between 2 different mushroom species using Progenesis QI Proteomics 4.2 (Nonlinear Dynamics).
Statistical analysis was performed using Student’s t-test and FDR and controlled using the Benjamini-Hochberg (BH) method [16]. p value (computed from the proteomics core) and absolute fold changes were used to identify differentially expressed proteins. A protein was differentially expressed if the p value ≤0.05 and the absolute fold change ≥2. Unique proteins of each species were identified. We used Ingenuity Pathway Analysis (IPA, Qiagen) to identify the pathways and networks in maitake compared to agarikon. We also conducted functional and pathway enrichment analyses using Cytoscape in conjunction with the plug-in ClueGO to identify the enriched biological processes, cellular components, molecular functions, immune responses, Reactome pathways, and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways. To assess the protein-protein interactions (PPIs), the search tool for the retrieval of interacting genes/proteins (STRING) local network cluster enrichment was conducted using Cytoscape in conjunction with the plug-in STRING Enrichment.
Statistical analysis
For volcano plot and differential expression analysis summary, the p value threshold was 0.05 and absolute fold change threshold was 2. The IPA was performed using the fold change cut-off p value of 0.05. The p value cut-off of 0.05 was also used to select the significantly enriched pathways in both IPA and ClueGO analyses.
Results
Proteins were identified based on the human proteome database. Proteins were considered unique when expressed in one species but not the other. The proteome of agarikon included 117 unique proteins, while that of maitake included 97 proteins (Supplementary file 1). To identify the biological processes and molecular functions related to unique proteins, we performed ClueGO gene ontology (GO) which showed enrichment of beta-tubulin:GTP cofactors, cornified envelope-bound lamellar bodies, aldehyde dehydrogenase, and vascular endothelial growth factor receptor signaling pathways in agarikon (Figure 1A and Supplementary file 2). The protein repertoire unique to maitake showed enrichment of multiple signaling pathways such as phosphorylated juxtamembrane, extracellular and kinase domain KIT mutants, KIT in disease, ERBB2, non-receptor tyrosine kinases, tyrosine-protein kinase 6 (PTK6), mitogen-activated protein kinase 1 (MAPK1)/MAPK3 (Figure 1B and Supplementary file 2).
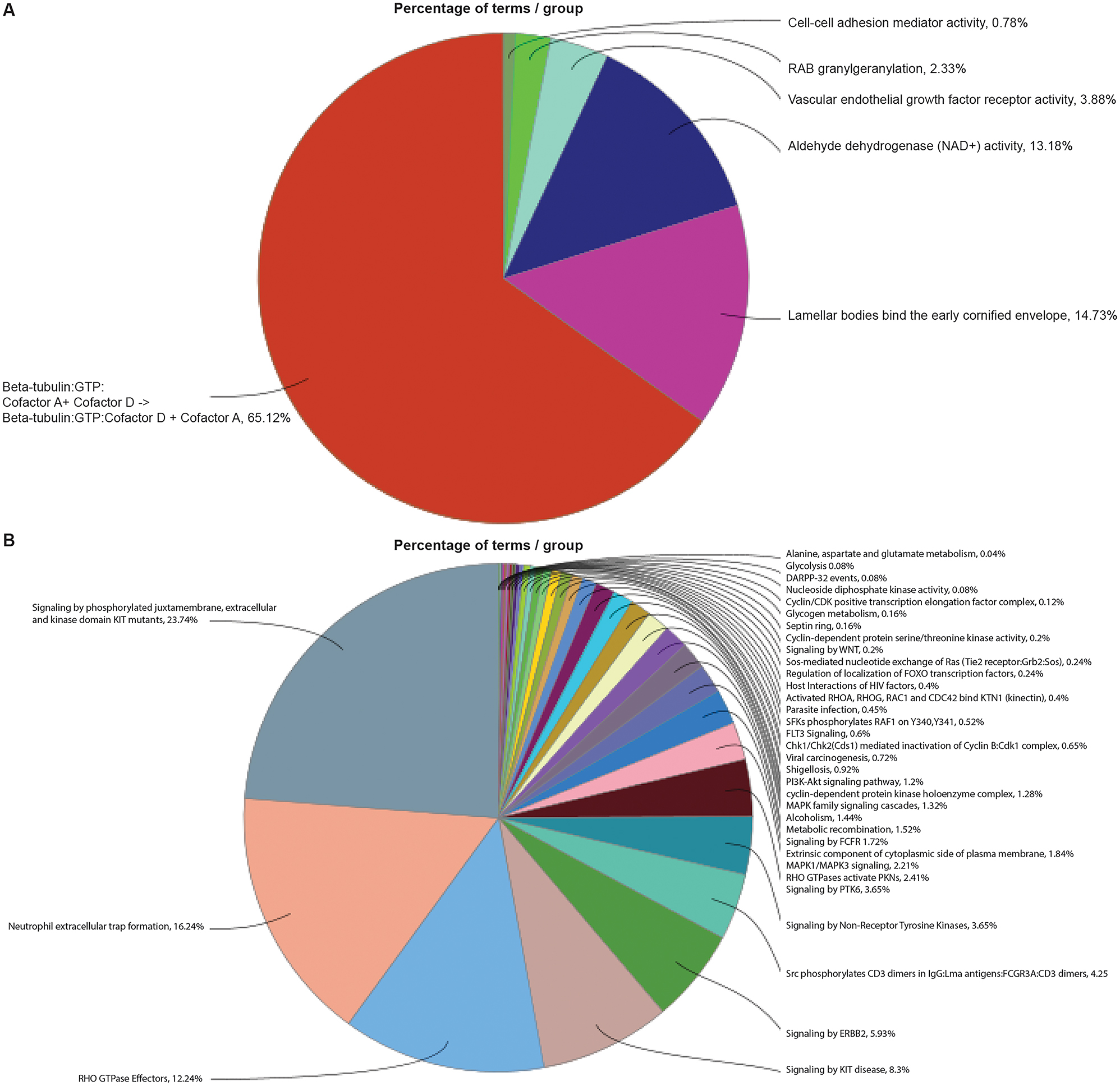
Pathway enrichment of differentially expressed unique proteins in agarikon and maitake mushrooms. Gene ontology (GO)-term functional enrichment by 5 categories (immune response, biological process, cellular component, KEGG, and Reactome) performed using Cytoscape in conjunction with the plug-in ClueGO. Enriched signaling pathways of unique proteins for (A) agarikon and (B) maitake.
Principal component analysis (PCA) of samples of both species is shown in Figure 2A. Volcano plot and heatmap depicting differentially expressed proteins in maitake compared to agarikon are shown in Figure 2B and C. Out of 134 proteins common to agarikon and maitake, 58 proteins (55 upregulated proteins and 3 downregulated proteins) were significantly changed between the two species (Supplementary file 3). To elucidate the potential protein protective activities, we performed functional and pathway enrichment analysis of maitake against agarikon using IPA. Multiple signaling pathways were found enriched, including BAG co-chaperone 2 (BAG2) (p=7.94 × 10−12), unfolded protein response (p=2.24 × 10−4), mitochondrial protein import (p=1.29 × 10−3), protein ubiquitination (p=7.94 × 10−11), regulation of apoptosis (p=2.29 × 10−8), microautophagy (p=9.55 × 10−7), glycolysis I (p=1.15 × 10−6), SNAP receptor (SNARE) (p=8.13 × 10−5), and immunogenic cell death (p=1.82 × 10−4) (Figure 3 and Supplementary file 4). These results show proteins that delineate immune functions related to potential neuroprotective mechanisms such as autophagy, apoptosis, and glycolysis [17–21]. In Cytoscape ClueGo analysis, the proteins were divided into upregulated and downregulated clusters. The upregulated protein cluster showed enrichment of multiple biological and molecular functions with 50 % relationship to Parkinson’s disease (PD) and prion disease (Figure 4A and Supplementary file 5). This suggests that the proteome of agarikon and maitake share common proteins that are related to different biological functions involved in PD and other prion disease processes. In stark contrast, the downregulated protein cluster showed a dominance of Reactome reactions associated with keratin type I and type II interactions (Figure 4B and Supplementary file 5). PPIs were identified as related to five categories including biological processes, cellular components, molecular functions, KEGG pathways, and Reactome pathways, and were validated using STRING analysis for interactive networks for both upregulated and downregulated common proteins (Supplementary file 6). Complex interactions of genes involved in biological processes (such as substantia nigra development, protein refolding, and cellular response to unfolded protein); sub-cellular components (such as proteasome complex); molecular functions (such as unfolded protein binding and misfolded protein binding); and KEGG pathways of different neurodegenerative diseases (NDs) including AD, PD, prion disease, and ALS are shown in Supplementary file 6.

Differential proteomic analysis of agarikon and maitake common proteins. (A) Principal component analysis (PCA) showing the three top components used for proteomic analysis. (B) Volcano plot showing the fold change plotted against the p value highlighting significantly changed proteins in maitake compared to agarikon. Red – upregulation and green – downregulation; p≤0.05 and an absolute fold change ≥2. (C) Heatmap showing hierarchical clustering of agarikon and maitake FDR-adjusted proteins. Red – upregulation and green – downregulation. Sample_A, agarikon; sample_M, maitake.
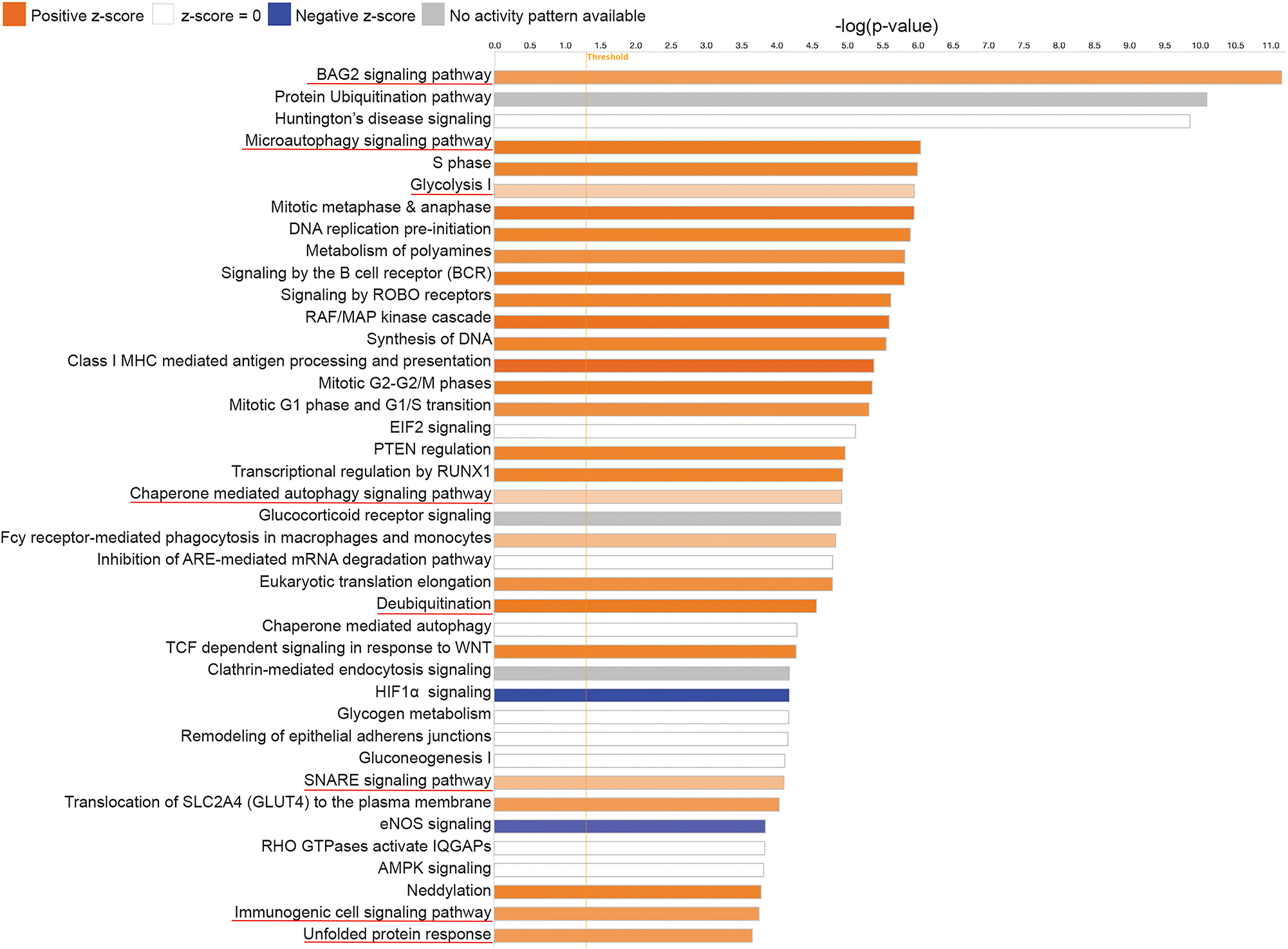
Pathway enrichment of differentially expressed common proteins in agarikon and maitake mushrooms. Canonical pathway enrichment analysis of maitake proteome compared to agarikon proteome was performed using IPA (Qiagen). Orange color (activation of pathway), blue color (inhibition of pathway), and grey color (no activity pattern of pathway). The p value ≤0.05.

Pathway enrichment of up- and down-regulated common proteins in agarikon and maitake mushrooms. Enriched signaling pathways of common mushroom proteins that were (A) upregulated or (B) downregulated. Gene ontology (GO)-term functional enrichment by 5 categories (immune response, biological process, cellular component, KEGG, and Reactome) performed using Cytoscape in conjunction with the plug-in ClueGO.
Discussion
Prior studies showed that natural product consumption affects NDs due to protective functions of bioactive molecules in these products [22–24]. Mushrooms possess potent medicinal properties with antioxidant, anti-inflammatory, immunomodulatory, and antimicrobial properties [9, 25]. To our knowledge, the current study is the first to uncover the proteomic signature of mushrooms and their related putative neuroprotective processes. We identified proteins in the fruiting bodies of agarikon and maitake mushrooms. Functional and pathway enrichment analyses of identified proteins in both species uncovered significant enrichment of different signaling pathways related to BAG2, unfolded protein response (UPR), mitochondrial protein import, protein ubiquitination, microautophagy, glycolysis I, SNARE, regulation of apoptosis, and immunogenic cell death. Each alone or together support prior works where polysaccharides, terpenes, phenolic compounds, and vitamins from mushrooms have been shown to potentially affect the onset and progression of NDs [13]. While decades of research have shown that natural compounds produced by living organisms can be at the forefront of new therapeutic discoveries, few have reached basic and clinical affirmations. Mushrooms are well-established to have nutritional and medicinal properties, but have not yet been studied for their potential use in disease treatments, most notably for those linked to AD. However, products extracted from mushrooms may have beneficial outcomes in AD-related processes including the inhibition of acetylcholinesterase and beta-secretase (β-secretase); and the prevention of amyloid beta (Aβ) aggregation and neurotoxicity with linked antioxidant and anti-inflammatory activities [25].
Prions are the hallmarks of degenerative diseases and include alpha-synuclein (α-syn) in PD, and Aβ and Tau in AD. Quality control of proteins plays an important role in protein homeostasis and depends on specific chaperones that regulate the balance between protein folding, assembly, disassembly, translocation, differentiation, and degradation [26]. The co-chaperone BAG2 delivers phosphorylated Tau for degradation to the proteasome in a ubiquitin-independent manner in AD models, thus playing a fundamental role in the maintenance of neuronal structure [27]. In addition, stabilization of PTEN-induced kinase 1 (PINK1) by BAG2 triggers Parkin-mediated mitophagy and protects neurons against 1-methyl-4-phenylpyridinium (MPP+)-induced oxidative stress in an in vitro cell model of PD [28]. Agarikon and maitake proteomes were enriched in BAG2 pathway activity with neuroprotective functions.
Defects of mitochondria and endoplasmic reticulum (ER) were reported in different NDs [29, 30]. The main functions of the ER are synthesis and folding of proteins along the secretory pathway, a process mediated by luminal resident chaperones and foldases. The UPR provides a coordinated response that is initiated in the ER and allows the correct folding of proteins [20]. Alterations in ER functions, such as altered calcium levels, increase oxidative stress and/or dysfunction of protein N-glycosylation causing the accumulation of misfolded proteins in the ER, which triggers the ER stress that activates the UPR to augment the ER folding capacity [31]. Two major degradation pathways, the ubiquitin-proteasome system and the autophagy-lysosomal pathway, remove damaged or misfolded proteins to prevent accumulation and maintain homeostasis of cellular proteins [32]. Aging, a primary risk factor of many NDs, is associated with a general reduction in proteasomal degradation and autophagy with the consequent accumulation of potentially neurotoxic protein aggregates of Aβ, Tau, and α-syn [32]. In concert with proteasomal degradation, ubiquitination is associated with degradation signals to process aberrant proteins which form aggregates and lead to neuronal cell damage and death if not chaperoned to the proteasome. Furthermore, autophagy is one of the major intracellular mechanisms to eliminate misfolded proteins and maintain proteostasis [33]. Dysregulated autophagy is increasingly considered to play key roles in most NDs, thus the regulation of autophagy is proposed as a potential therapeutic avenue for these diseases [34, 35]. Coincidentally, agarikon and maitake proteome exhibit proteome pathways that are enriched for protein homeostasis and autophagolysosomal activities.
SNARE proteins are small proteins of around 100–300 amino acids that mediate the fusion of biological membranes by localizing both at the vesicular membrane and on the target membrane [36]. SNARE proteins have fundamental roles in neuronal functions such as neurite initiation and outgrowth, axon specification, axon extension, synaptogenesis, and synaptic transmission [37]. The formation of the SNARE complex was found to be fundamental for vesicle fusion, vesicle recycling, and neurotransmitter release [38]. Inhibition of the formation of the SNARE complex, defects in the SNARE-dependent exocytosis, and altered regulation of SNARE-mediated vesicle fusion were found to be associated with neurodegeneration [39–41]. Agarikon and maitake protein-enriched SNARE signaling pathways support mushroom neuroprotective functions.
The brain depends mainly on glucose as a source of energy to maintain normal functions. Aging impairs cerebral glucose metabolism, reduces mitochondrial biogenesis, and decreases ATP levels [42]. Glycolysis and mitochondrial functions are dysregulated in individuals with NDs [43–45]. Decreased glycolytic flux has been shown to correlate with the severity of amyloid and Tau pathology in AD patients [46, 47]. Glycolytic dysfunction has also been observed in PD [18], HD [48], and ALS [49], thus identifying glycolytic dysfunction as a common pathway contributing to neurodegeneration. In general, glycolysis upregulation was found to be neuroprotective in several different NDs [50, 51]. In that vein, agarikon and maitake protein repertoires showed that upregulation of glycolysis offered another possible neuroprotective role.
Immunogenic cell death plays an important role in immunosurveillance by which immune cells look for and recognize foreign pathogens such as bacteria, viruses, or pre-cancerous and cancerous cells in the body [19]. NDs are characterized by progressive loss of particular populations of neurons whereby programmed cell death (apoptosis) has been largely implicated in their pathobiology [52]. The homeostatic maintenance of most organs and tissues is ensured by a controlled balance of cell proliferation and elimination via apoptosis throughout the life of an organism. However, the nervous system is different from most organs because neuronal cell division and proliferation are primarily restricted to the embryonic development stage. The threshold required to induce apoptosis becomes much greater once the mature nervous system is established, resulting in markedly less neuronal cell death. Indeed, mature neurons strikingly restrict the apoptosis pathway after development to ensure long-term survival and maintenance throughout life [53]. The protein repertoires of agarikon and maitake comprised proteins linked regulation of immunogenic cell death and apoptosis which have been shown to serve protective functions for neurons and the entire nervous system. Altogether, with the evaluation of common and unique proteins amongst the agarikon and maitake proteomes, we uncovered signaling pathways and molecular functions that are related to neuroprotective activities in the realm of human signaling pathways. To our knowledge, this study is the first to unravel the mushroom proteome of fungal species and expose proteins involved in mechanisms associated with neuroprotective activities for various NDs.
Funding source: Howard Cooper
Award Identifier / Grant number: Private Donor
Acknowledgments
The authors thank Zachary Petrover, CSO of Mushroom design (US), and Talia Eisenberg, Director of Beond Ibogaine treatment for trauma and addiction (Mexico), for providing the mushroom species used in this study. The authors would also like to thank the UNMC Proteomics Core Facility for assistance with the proteomic analysis. The authors would also like to thank the UNMC Bioinformatics and Systems Biology Core Facility for bioinformatic analysis. The authors would also like to thank the Vice Chancellor’s Office of the UNMC for Core Facility support.
-
Research ethics: Not applicable.
-
Informed consent: Not applicable.
-
Author contributions: MMA: investigation, methodology, validation, visualization, data analysis, data curation, writing-original draft, review and editing; RK: sample preparation, writing-review and editing; RLM: conceptualization, writing-review and editing; HEG: conceptualization, funding, acquisition, supervision, writing-review and editing; All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Competing interests: No conflicts of interest.
-
Research funding: This work was supported by the Frances and Louie Blumkin and Harriet Singer Research Foundations, the Carol Swarts, MD Emerging Neuroscience Research Laboratory; and the Margaret R. Larson Professorship.
-
Data availability: All data was presented in this manuscript. Proteomic raw data and pathway analyses data are included in supplementary data files.
References
1. Feigin, VL, Vos, T, Nichols, E, Owolabi, MO, Carroll, WM, Dichgans, M, et al.. The global burden of neurological disorders: translating evidence into policy. Lancet Neurol 2020;19:255–65. https://doi.org/10.1016/S1474-4422(19)30411-9.Search in Google Scholar PubMed PubMed Central
2. Deb, A, Thornton, JD, Sambamoorthi, U, Innes, K. Direct and indirect cost of managing alzheimer’s disease and related dementias in the United States. Expert Rev Pharmacoecon Outcomes Res 2017;17:189–202. https://doi.org/10.1080/14737167.2017.1313118.Search in Google Scholar PubMed PubMed Central
3. Al-Obaidi, JR. Proteomics of edible mushrooms: a mini-review. Electrophoresis 2016;37:1257–63. https://doi.org/10.1002/elps.201600031.Search in Google Scholar PubMed
4. Obodai, M, Ferreira, I, Fernandes, Â, Barros, L, Mensah, D, Dzomeku, M, et al.. Evaluation of the chemical and antioxidant properties of wild and cultivated mushrooms of Ghana. Molecules 2014;19:19532–48. https://doi.org/10.3390/molecules191219532.Search in Google Scholar PubMed PubMed Central
5. Muszynska, B, Grzywacz-Kisielewska, A, Kala, K, Gdula-Argasinska, J. Anti-inflammatory properties of edible mushrooms: a review. Food Chem 2018;243:373–81. https://doi.org/10.1016/j.foodchem.2017.09.149.Search in Google Scholar PubMed
6. Nowakowski, P, Markiewicz-Zukowska, R, Bielecka, J, Mielcarek, K, Grabia, M, Socha, K. Treasures from the forest: evaluation of mushroom extracts as anti-cancer agents. Biomed Pharmacother 2021;143:112106. https://doi.org/10.1016/j.biopha.2021.112106.Search in Google Scholar PubMed
7. Uttara, B, Singh, AV, Zamboni, P, Mahajan, RT. Oxidative stress and neurodegenerative diseases: a review of upstream and downstream antioxidant therapeutic options. Curr Neuropharmacol 2009;7:65–74. https://doi.org/10.2174/157015909787602823.Search in Google Scholar PubMed PubMed Central
8. Valverde, ME, Hernandez-Perez, T, Paredes-Lopez, O. Edible mushrooms: improving human health and promoting quality life. Internet J Microbiol 2015;2015:376387. https://doi.org/10.1155/2015/376387.Search in Google Scholar PubMed PubMed Central
9. Rai, SN, Mishra, D, Singh, P, Vamanu, E, Singh, MP. Therapeutic applications of mushrooms and their biomolecules along with a glimpse of in silico approach in neurodegenerative diseases. Biomed Pharmacother 2021;137:111377. https://doi.org/10.1016/j.biopha.2021.111377.Search in Google Scholar PubMed
10. Lai, CS, Yu, MS, Yuen, WH, So, KF, Zee, SY, Chang, RC. Antagonizing beta-amyloid peptide neurotoxicity of the anti-aging fungus Ganoderma lucidum. Brain Res 2008;1190:215–24. https://doi.org/10.1016/j.brainres.2007.10.103.Search in Google Scholar PubMed
11. Zhang, R, Xu, S, Cai, Y, Zhou, M, Zuo, X, Chan, P. Ganoderma lucidum protects dopaminergic neuron degeneration through inhibition of microglial activation. J Evidence-Based Complementary Altern Med 2011;2011:156810. https://doi.org/10.1093/ecam/nep075.Search in Google Scholar PubMed PubMed Central
12. Sabaratnam, V, Kah-Hui, W, Naidu, M, Rosie David, P. Neuronal health – can culinary and medicinal mushrooms help? J Tradit Complementary Med 2013;3:62–8. https://doi.org/10.4103/2225-4110.106549.Search in Google Scholar PubMed PubMed Central
13. Tong, Z, Chu, G, Wan, C, Wang, Q, Yang, J, Meng, Z, et al.. Multiple metabolites derived from mushrooms and their beneficial effect on alzheimer’s diseases. Nutrients 2023;15:1–27. https://doi.org/10.3390/nu15122758.Search in Google Scholar PubMed PubMed Central
14. Li, Z, Luo, R, Zhang, Y, Yan, X, Pang, Q. Effective protein extraction from mycelium and fruiting body of Auricularia auricula for proteomics studies. Int J Food Prop 2018;21:2156–66. https://doi.org/10.1080/10942912.2018.1499111.Search in Google Scholar
15. Gao, L, Kumar, V, Vellichirammal, NN, Park, S, Rudebush, TL, Yu, L, et al.. Functional, proteomic and bioinformatic analyses of Nrf2- and Keap1- null skeletal muscle. J Physiol 2020;598:5427–51. https://doi.org/10.1113/JP280176.Search in Google Scholar PubMed PubMed Central
16. Benjamini, Y, Hochberg, Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Roy Stat Soc B 1995;57:289–300. https://doi.org/10.1111/j.2517-6161.1995.tb02031.x.Search in Google Scholar
17. Lima, RS, Carrettiero, DC, Ferrari, MFR. BAG2 prevents Tau hyperphosphorylation and increases p62/SQSTM1 in cell models of neurodegeneration. Mol Biol Rep 2022;49:7623–35. https://doi.org/10.1007/s11033-022-07577-w.Search in Google Scholar PubMed
18. Cai, R, Zhang, Y, Simmering, JE, Schultz, JL, Li, Y, Fernandez-Carasa, I, et al.. Enhancing glycolysis attenuates Parkinson’s disease progression in models and clinical databases. J Clin Invest 2019;129:4539–49. https://doi.org/10.1172/JCI129987.Search in Google Scholar PubMed PubMed Central
19. Kroemer, G, Galassi, C, Zitvogel, L, Galluzzi, L. Immunogenic cell stress and death. Nat Immunol 2022;23:487–500. https://doi.org/10.1038/s41590-022-01132-2.Search in Google Scholar PubMed
20. Costa, CAD, Manaa, WE, Duplan, E, Checler, F. The endoplasmic reticulum stress/unfolded protein response and their contributions to Parkinson’s disease physiopathology. Cells 2020;9:1–24. https://doi.org/10.3390/cells9112495.Search in Google Scholar PubMed PubMed Central
21. Margiotta, A. Role of SNAREs in neurodegenerative diseases. Cells 2021;10:1–15. https://doi.org/10.3390/cells10050991.Search in Google Scholar PubMed PubMed Central
22. Feng, J, Zheng, Y, Guo, M, Ares, I, Martínez, M, Lopez-Torres, B, et al.. Oxidative stress, the blood-brain barrier and neurodegenerative diseases: the critical beneficial role of dietary antioxidants. Acta Pharm Sin B 2023;13:3988–4024. https://doi.org/10.1016/j.apsb.2023.07.010.Search in Google Scholar PubMed PubMed Central
23. Pizzino, G, Irrera, N, Cucinotta, M, Pallio, G, Mannino, F, Arcoraci, V, et al.. Oxidative stress: harms and benefits for human health. Oxid Med Cell Longev 2017;2017:8416763. https://doi.org/10.1155/2017/8416763.Search in Google Scholar PubMed PubMed Central
24. Di Paolo, M, Papi, L, Gori, F, Turillazzi, E. Natural products in neurodegenerative diseases: a great promise but an ethical challenge. Int J Mol Sci 2019;20:1–12. https://doi.org/10.3390/ijms20205170.Search in Google Scholar PubMed PubMed Central
25. Trovato Salinaro, A, Pennisi, M, Di Paola, R, Scuto, M, Crupi, R, Cambria, MT, et al.. Neuroinflammation and neurohormesis in the pathogenesis of Alzheimer’s disease and Alzheimer-linked pathologies: modulation by nutritional mushrooms. Immun Ageing 2018;15:8. https://doi.org/10.1186/s12979-017-0108-1.Search in Google Scholar PubMed PubMed Central
26. Qin, L, Guo, J, Zheng, Q, Zhang, H. BAG2 structure, function and involvement in disease. Cell Mol Biol Lett 2016;21:18. https://doi.org/10.1186/s11658-016-0020-2.Search in Google Scholar PubMed PubMed Central
27. Carrettiero, DC, Hernandez, I, Neveu, P, Papagiannakopoulos, T, Kosik, KS. The cochaperone BAG2 sweeps paired helical filament- insoluble tau from the microtubule. J Neurosci 2009;29:2151–61. https://doi.org/10.1523/JNEUROSCI.4660-08.2009.Search in Google Scholar PubMed PubMed Central
28. Qu, D, Hage, A, Don-Carolis, K, Huang, E, Joselin, A, Safarpour, F, et al.. BAG2 gene-mediated regulation of PINK1 protein is critical for mitochondrial translocation of PARKIN and neuronal survival. J Biol Chem 2015;290:30441–52. https://doi.org/10.1074/jbc.M115.677815.Search in Google Scholar PubMed PubMed Central
29. Johri, A, Beal, MF. Mitochondrial dysfunction in neurodegenerative diseases. J Pharmacol Exp Therapeut 2012;342:619–30. https://doi.org/10.1124/jpet.112.192138.Search in Google Scholar PubMed PubMed Central
30. Wilson, EL, Metzakopian, E. ER-mitochondria contact sites in neurodegeneration: genetic screening approaches to investigate novel disease mechanisms. Cell Death Differ 2021;28:1804–21. https://doi.org/10.1038/s41418-020-00705-8.Search in Google Scholar PubMed PubMed Central
31. Harding, HP, Zhang, Y, Ron, D. Protein translation and folding are coupled by an endoplasmic-reticulum-resident kinase. Nature 1999;397:271–4. https://doi.org/10.1038/16729.Search in Google Scholar PubMed
32. Schmidt, MF, Gan, ZY, Komander, D, Dewson, G. Ubiquitin signalling in neurodegeneration: mechanisms and therapeutic opportunities. Cell Death Differ 2021;28:570–90. https://doi.org/10.1038/s41418-020-00706-7.Search in Google Scholar PubMed PubMed Central
33. Le Guerroue, F, Youle, RJ. Ubiquitin signaling in neurodegenerative diseases: an autophagy and proteasome perspective. Cell Death Differ 2021;28:439–54. https://doi.org/10.1038/s41418-020-00667-x.Search in Google Scholar PubMed PubMed Central
34. Menzies, FM, Fleming, A, Rubinsztein, DC. Compromised autophagy and neurodegenerative diseases. Nat Rev Neurosci 2015;16:345–57. https://doi.org/10.1038/nrn3961.Search in Google Scholar PubMed
35. Menzies, FM, Moreau, K, Rubinsztein, DC. Protein misfolding disorders and macroautophagy. Curr Opin Cell Biol 2011;23:190–7. https://doi.org/10.1016/j.ceb.2010.10.010.Search in Google Scholar PubMed PubMed Central
36. Holz, RW, Zimmerberg, J. Dynamic relationship of the SNARE complex with a membrane. Biophys J 2019;117:627–30. https://doi.org/10.1016/j.bpj.2019.07.010.Search in Google Scholar PubMed PubMed Central
37. Urbina, FL, Gupton, SL. SNARE-mediated exocytosis in neuronal development. Front Mol Neurosci 2020;13:133. https://doi.org/10.3389/fnmol.2020.00133.Search in Google Scholar PubMed PubMed Central
38. Han, J, Pluhackova, K, Bockmann, RA. The multifaceted role of SNARE proteins in membrane fusion. Front Physiol 2017;8:5. https://doi.org/10.3389/fphys.2017.00005.Search in Google Scholar PubMed PubMed Central
39. Almandoz-Gil, L, Persson, E, Lindstrom, V, Ingelsson, M, Erlandsson, A, Bergstrom, J. In situ proximity ligation Assay reveals Co-localization of alpha-synuclein and SNARE proteins in murine primary neurons. Front Neurol 2018;9:180. https://doi.org/10.3389/fneur.2018.00180.Search in Google Scholar PubMed PubMed Central
40. Law, C, Schaan Profes, M, Levesque, M, Kaltschmidt, JA, Verhage, M, Kania, A. Normal molecular specification and neurodegenerative disease-like death of spinal neurons lacking the SNARE-associated synaptic protein munc18-1. J Neurosci 2016;36:561–76. https://doi.org/10.1523/JNEUROSCI.1964-15.2016.Search in Google Scholar PubMed PubMed Central
41. Pham, E, Crews, L, Ubhi, K, Hansen, L, Adame, A, Cartier, A, et al.. Progressive accumulation of amyloid-beta oligomers in Alzheimer’s disease and in amyloid precursor protein transgenic mice is accompanied by selective alterations in synaptic scaffold proteins. FEBS J 2010;277:3051–67. https://doi.org/10.1111/j.1742-4658.2010.07719.x.Search in Google Scholar PubMed PubMed Central
42. Hoyer, S. Brain glucose and energy metabolism during normal aging. Aging 1990;2:245–58. https://doi.org/10.1007/BF03323925.Search in Google Scholar PubMed
43. Bhatia, S, Rawal, R, Sharma, P, Singh, T, Singh, M, Singh, V. Mitochondrial dysfunction in alzheimer’s disease: opportunities for drug development. Curr Neuropharmacol 2022;20:675–92. https://doi.org/10.2174/1570159X19666210517114016.Search in Google Scholar PubMed PubMed Central
44. Bustamante-Barrientos, FA, Luque-Campos, N, Araya, MJ, Lara-Barba, E, de Solminihac, J, Pradenas, C, et al.. Mitochondrial dysfunction in neurodegenerative disorders: potential therapeutic application of mitochondrial transfer to central nervous system-residing cells. J Transl Med 2023;21:613. https://doi.org/10.1186/s12967-023-04493-w.Search in Google Scholar PubMed PubMed Central
45. Schapira, AH. Mitochondrial complex I deficiency in Parkinson’s disease. Adv Neurol 1993;60:288–91.Search in Google Scholar
46. Querfurth, HW, LaFerla, FM. Alzheimer’s disease. N Engl J Med 2010;362:329–44. https://doi.org/10.1056/NEJMra0909142.Search in Google Scholar PubMed
47. Serrano-Pozo, A, Frosch, MP, Masliah, E, Hyman, BT. Neuropathological alterations in Alzheimer disease. Cold Spring Harbor Perspect Med 2011;1:a006189. https://doi.org/10.1101/cshperspect.a006189.Search in Google Scholar PubMed PubMed Central
48. Dubinsky, JM. Towards an understanding of energy impairment in huntington’s disease brain. J Huntington’s Dis 2017;6:267–302. https://doi.org/10.3233/JHD-170264.Search in Google Scholar PubMed PubMed Central
49. Tefera, TW, Steyn, FJ, Ngo, ST, Borges, K. CNS glucose metabolism in Amyotrophic Lateral Sclerosis: a therapeutic target? Cell Biosci 2021;11:14. https://doi.org/10.1186/s13578-020-00511-2.Search in Google Scholar PubMed PubMed Central
50. Manzo, E, Lorenzini, I, Barrameda, D, O’Conner, AG, Barrows, JM, Starr, A, et al.. Glycolysis upregulation is neuroprotective as a compensatory mechanism in ALS. Elife 2019;8:1–20. https://doi.org/10.7554/eLife.45114.Search in Google Scholar PubMed PubMed Central
51. Takahashi, S. Neuroprotective function of high glycolytic activity in astrocytes: common roles in stroke and neurodegenerative diseases. Int J Mol Sci 2021;22:1–17. https://doi.org/10.3390/ijms22126568.Search in Google Scholar PubMed PubMed Central
52. Erekat, NS. Apoptosis and its therapeutic implications in neurodegenerative diseases. Clin Anat 2022;35:65–78. https://doi.org/10.1002/ca.23792.Search in Google Scholar PubMed
53. Kole, AJ, Annis, RP, Deshmukh, M. Mature neurons: equipped for survival. Cell Death Dis 2013;4:e689. https://doi.org/10.1038/cddis.2013.220.Search in Google Scholar PubMed PubMed Central
Supplementary Material
This article contains supplementary material (https://doi.org/10.1515/nipt-2024-0004).
© 2024 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- Review Article
- X-chromosome linked genes associated with myeloid cell CNS trafficking contributes to female–male differences in the disease outcome for neuroinflammatory diseases
- Short Review Article
- Something to talk about; crosstalk disruption at the neurovascular unit during HIV infection of the CNS
- Research Articles
- Sera from people with HIV and depression induce commensurate metabolic alterations in astrocytes: toward precision diagnoses and therapies
- Neuroprotective mushrooms
- HIV-1 and methamphetamine co-treatment in primary human astrocytes: TAARgeting ER/UPR dysfunction
- Viral-mediated inflammation by Poly I:C induces the chemokine CCL5 in NK cells and its receptors CCR1 and CCR5 in microglia in the neonatal rat cerebellum
Articles in the same Issue
- Frontmatter
- Review Article
- X-chromosome linked genes associated with myeloid cell CNS trafficking contributes to female–male differences in the disease outcome for neuroinflammatory diseases
- Short Review Article
- Something to talk about; crosstalk disruption at the neurovascular unit during HIV infection of the CNS
- Research Articles
- Sera from people with HIV and depression induce commensurate metabolic alterations in astrocytes: toward precision diagnoses and therapies
- Neuroprotective mushrooms
- HIV-1 and methamphetamine co-treatment in primary human astrocytes: TAARgeting ER/UPR dysfunction
- Viral-mediated inflammation by Poly I:C induces the chemokine CCL5 in NK cells and its receptors CCR1 and CCR5 in microglia in the neonatal rat cerebellum

