Abstract
C13H18N3OCl, monoclinic, P21/c (no. 14), a = 13.5859(4) Å, b = 11.1576(3) Å, c = 8.7933(2) Å, β = 93.2730(10)°, V = 1330.77 Å3, Z = 4, Rgt(F) = 0.0320, wRref(F2) = 0.0804, T = 100 K.
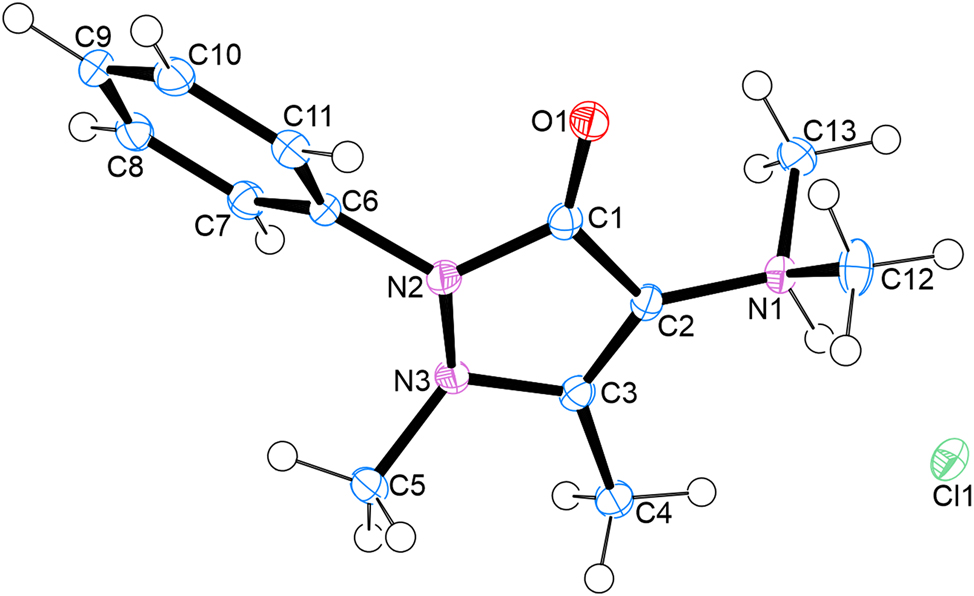
The molecular structure is shown in the figure. Table 1 contains the crystallographic data. The list of the atoms including atomic coordinates and displacement parameters can be found in the cif-file attached to this article.
Data collection and handling.
| Crystal: | Colorless plate |
| Size: | 0.55 × 0.21 × 0.14 mm |
| Wavelength: | MoKα radiation (0.71073 Å) |
| μ: | 0.28 mm−1 |
| Diffractometer, scan mode: | Bruker Apex-II, φ and ω scans |
| θmax, completeness: | 27.5°, 100 % |
| N(hkl)measured, N(hkl)unique, Rint: | 32424, 3071, 0.041 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2σ(Iobs), 2,676 |
| N(param)refined: | 171 |
| Programs: | Bruker, 1 Shelx, 2 , 3 Wingx, 4 , 5 Platon 6 |
1 Source of materials
To synthesize the title compound, 0.10 g (0.435 mmol) of 4-dimethylaminoantipyrine (DMAAP, M = 231.29 g mol−1 was weighed into a glass vial. To this was added 1 mL of 4-heptanone (reagent) and 3 mL of methanol (solvent). The mixture was sonicated for 5 min to promote dissolution. A single drop of concentrated hydrochloric acid (33 %, catalyst) was added to initiate the reaction. The solution was allowed to stand at ambient temperature. A colourless crystal salt formed within 2 days via slow evaporation of the solvent (Table 1).
2 Experimental details
First the non-hydrogen atoms were refined isotropically, then anisotropically by full matix least-squares calculations based on F2. Carbon-bound hydrogen atoms were located in the difference map then positioned geometrically and were allowed to ride on their respective parent atoms with thermal displacement parameters 1.2 times of the parent C atom. The coordinates and isotropic displacement parameters of the nitrogen bound H atom was allowed to refine freely. Diagrams and publication material were generated using Ortep-3, 4 Wingx 5 and Platon. 6
3 Discussion
4-Dimethylaminoantipyrine (DMAAP) is a synthetic pyrazolone derivative structurally related to phenazone-based nonsteroidal anti-inflammatory drugs. It has been widely studied for its antipyretic and analgesic properties, but also demonstrates potential as a redox-active scaffold in coordination chemistry and sensor applications. 7 , 8 , 9 Recent pharmaceutical investigations have explored its quantification in drug formulations 7 and its antioxidant reactivity towards reactive oxygen species such as hydroxyl radicals. 8 Furthermore, 4-dimethylaminoantipyrine has gained attention as a useful component in the fabrication of electrochemical sensors due to its broad redox response and compatibility with conductive platforms. 9 , 10
This work forms part of our ongoing investigation into the synthesis and structural characterization of salt and hydrogen-bonded assemblies of bioactive pyrazolone analogues.
The asymmetric unit of the salt contains one organic cation and one chloride anion with the systematic name: (N,N,1,5-tetramethyl-3-oxo-2-phenyl-2,3-dihydro-1H-pyrazol-4-aminium chloride and crystallizes in the monoclinic P21/c space group. A strong N-H···Cl hydrogen bond connects the chloride anions and organic cation. With regard to packing, DMAAP cations interact via π–π stacking of the phenyl rings with a distance of 3.4 Å between them.
Funding source: National Research Foundation
Award Identifier / Grant number: CSUR23042597072
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Conflict of interest: The authors declare no conflicts of interest regarding this article.
-
Research funding: This work was supported by the National Research Foundation (NRF) “Competitive Support for Unrated Researchers” grant Number CSUR23042597072 (Dr MG Smith) and the University of South Africa.
References
1. Bruker Apex3. Saint-Plus and Xprep; Bruker AXS Inc.: Madison, Wisconsin, USA, 2016.Search in Google Scholar
2. Sheldrick, G. M. Crystal Structure Refinement with Shelxl. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Search in Google Scholar
3. Shelxt; Sheldrick, G. M. Shelxtl – Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. 2015, A71, 3–8.10.1107/S2053273314026370Search in Google Scholar
4. Ortep-3; Farrugia, L. J. Wingx and Ortep for Windows: An Update. J. Appl. Crystallogr. 2012, 45, 849–854; https://doi.org/10.1107/s0021889812029111.Search in Google Scholar
5. Farrugia, L. J. Wingx Suite for Small-Molecule Single-Crystal Crystallography. J. Appl. Crystallogr. 1999, 32, 837–838; https://doi.org/10.1107/s0021889899006020.Search in Google Scholar
6. Spek, A. L. Platon. Acta Crystallogr. D 2009, 65, 148–155.10.1107/S090744490804362XSearch in Google Scholar
7. Kalmar, E.; Kormányos, B.; Szakonyi, G.; Dombi, G. Validated HPLC Determination of 4-Dimethylaminoantipyrine in Different Suppository Bases. Indian J. Pharm. Sci. 2014, 76 (1), 31–37.Search in Google Scholar
8. Santos, P. M. P.; Antunes, A. M. M.; Noronha, J.; Fernandes, E.; Vieira, A. J. S. C. Scavenging Activity of Aminoantipyrines Against Hydroxyl Radical. Eur. J. Med. Chem. 2010, 45 (6), 2258–2264; https://doi.org/10.1016/j.ejmech.2010.01.071.Search in Google Scholar
9. Melo, F. C. C.; Alves, R. P.; Valle, A. L.; Santos, C. A.; Goulart, I. M. B.; Oliveira, E. G. A.; Oliveira, G. S.; Rodrigues, L. P.; Goulart, L. R. 4-Dimethylaminoantipyrine as a Broad Electrochemical Indicator for Immunosensors Platform. Sensors 2022, 22 (10), 3681; https://doi.org/10.3390/s22103681.Search in Google Scholar
10. Yang, D.; Wang, R.; Jin, G.; Zhang, B.; Zhang, Li; Lu, Y.; Du, G. Structural and Computational Insights into Cocrystal Interactions: A Case on Cocrystals of Antipyrine and Aminophenazone. Cryst. Growth Design 2019, 19, 6175; https://doi.org/10.1021/acs.cgd.9b00591.Search in Google Scholar
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.

