Abstract
C33H33N3O4, monoclinic, P21/c (no. 14), a = 10.9636(14) Å, b = 9.6450(12) Å, c = 27.328(4) Å, β = 99.637(2)°, V = 2848.9 Å3, Z = 4, R gt (F) = 0.0463, wR ref (F2) = 0.1189, T = 296 K.
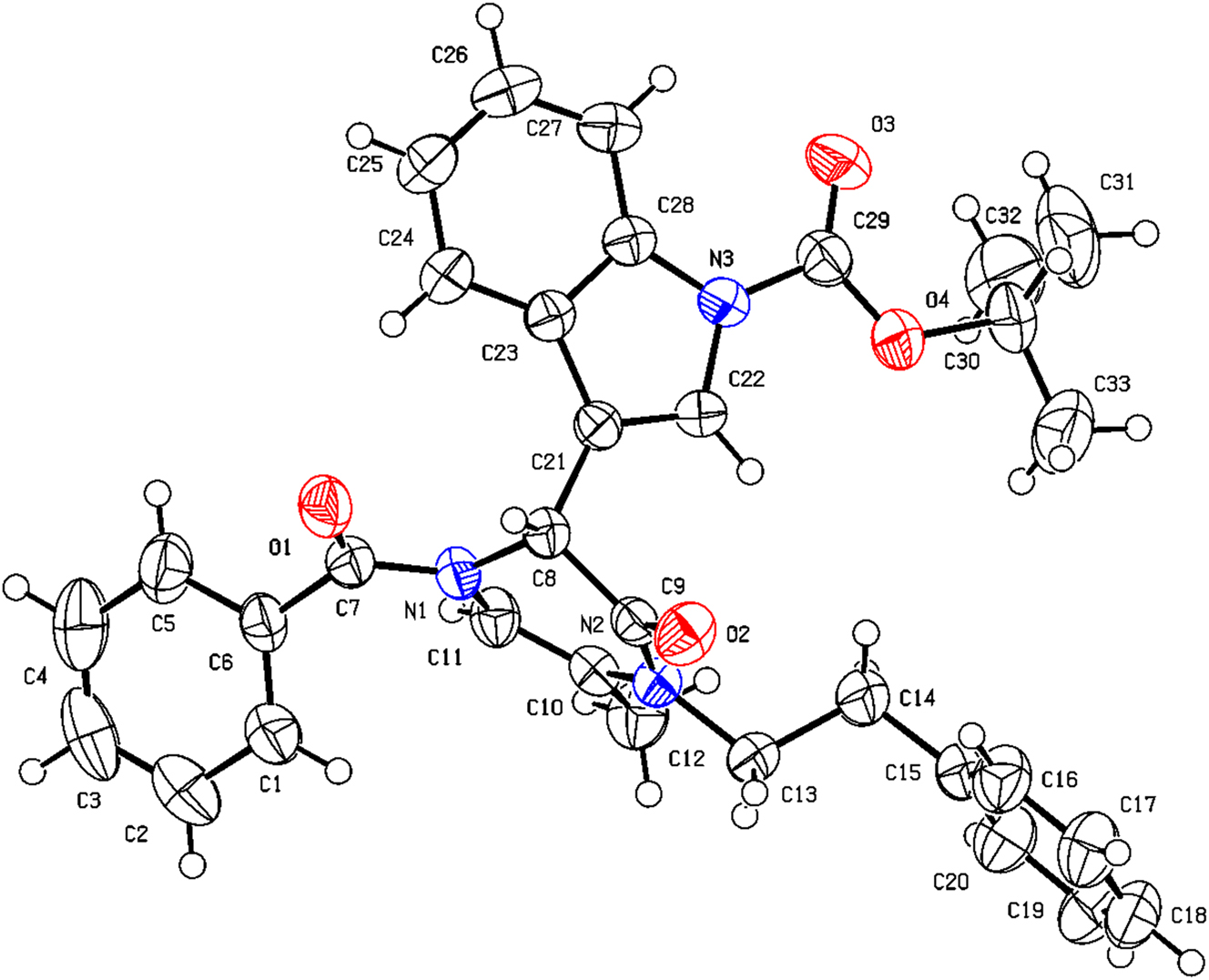
The molecular structure is shown in the figure. Table 1 contains the crystallographic data and the list of the atoms including atomic coordinates and displacement parameters can be found in the cif-file attached to this article.
Data collection and handling.
| Crystal: | Colorless block |
| Size: | 0.26 × 0.24 × 0.20 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.08 mm−1 |
| Diffractometer, scan mode: | Bruker APEX2, φ and ω scans |
| θmax, completeness: | 25.0°, 100 % |
| N(hkl)measured, N(hkl)unique, Rint: | 14044, 5010, 0.022 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 3,824 |
| N(param)refined: | 365 |
| Programs: | Bruker, 1 SHELX 2 , 3 |
1 Source of materials
1-Boc-3-formylindole (1.0 mmol) and prop-2-yn-1-amine (1.0 mmol) were mixed in methanol (1 mL) and stirred for 10 min. Then, benzoic acid (1.0 mmol) and phenethyl isocyanide (1.0 mmol) were added in sequence. The reaction system was stirred overnight at room temperature. After the reaction was completed, the solvent was removed under reduced pressure. Then the crude residue was dissolved in DMF (N,N-dimethylformamide, 5 mL) followed by the addition of DBU (1,8-diazabicyclo[5,4,0]-undec-7-ene, 2.0 mmol). The mixture was stirred under microwave irradiation condition at 120 °C for 10 min. After the microwave vial was cooled to room temperature, the residue was purified by silica gel column chromatograph to obtained the title compound. The title compound was dissolved in a mixture of methanol and dichloromethane. Crystals of the title compound were obtained by slow evaporation.
2 Experimental details
The data were collected and processed using Bruker SMART. 1 And the structures was solved by Direct Methods using Olex2 software 2 and refined with the SHELXL. 3
3 Comment
As one of the most important heterocycles, piperazine derivatives are of great significance in organic chemistry and medicinal application. Many bioactive compounds contain this main core structure. 4 On the other hand, due to the medicinal importance of indole, indole-containing scafolds have received remarkable attention in recent years. Therefore, the synthesis of such compounds is of great significance to medicinal chemistry. 5 Multicomponent reactions (MCRs) 6 , 7 have emerged as a pivotal synthetic tool for the rapid and convergent assembly of diverse complex molecules. As one of the most important MCRs, the Ugi reaction is often used in the synthesis of important heterocycles and bioactive molecules. 8 , 9 We previously reported the use of this strategy to achieve the construction of various heterocyclic molecules with potent anticancer activity. 10 , 11 As show in the figure, the title compound contained one tetrahydropyrazin heterocyclic ring and one indole heterocyclic ring in one molecule. The bond lengths and angles in both crystallographically independent molecules are in the normal ranges. In conclusion, we have developed a facile process for the synthesis of tert-butyl 3-(1-benzoyl-5-methyl-3-oxo-4-phenethyl-1,2,3,4-tetrahydropyrazin -2-yl)-1H-indole-1-carboxylate, which contains two important heterocyclic cores, using Ugi reaction followed by intramolecular cyclization reaction.
Acknowledgements
This work was financially supported by the Natural Science Foundation of Chongqing Science and Technology Bureau (Grant No. CSTB2022NSCQ-MSX0236).
References
1. BRUKER. Saint, Apex2 and Sadabs; Bruker AXS Inc.: Madison, WI, USA, 2019.Search in Google Scholar
2. Sheldrick, G. M. Shelxtl – Integrated Space-Group and Crystal structure Determination. Acta Crystallogr. 2015, A71, 3–8.10.1107/S2053273314026370Search in Google Scholar PubMed PubMed Central
3. Sheldrick, G. M. Crystal Structure Refinement with Shelxl. Acta Crystallogr. 2015, C71, 3–8, https://doi.org/10.1107/s2053229614024218.Search in Google Scholar
4. Zhang, W.; Guo, S.; Yu, L.; Wang, Y.; Chi, Y. R.; Wu, J. Piperazine: Its Role in the Discovery of Pesticides. Chin. Chem. Lett. 2023, 34, 108123; https://doi.org/10.1016/j.cclet.2022.108123.Search in Google Scholar
5. Zeng, W.; Han, C.; Mohammed, S.; Li, S.; Song, Y.; Sun, F.; Du, Y. Indole-Containing Pharmaceuticals: Targets, Pharmacological Activities, and SAR Studies. RSC Med. Chem. 2024, 15, 788–808; https://doi.org/10.1039/d3md00677h.Search in Google Scholar PubMed PubMed Central
6. Reguera, L.; Méndez, Y.; Humpierre, A. R.; Valdés, O.; Rivera, D. G. Multicomponent Reactions in Ligation and Bio Conjugation Chemistry. Acc. Chem. Res. 2018, 51, 1475–1486; https://doi.org/10.1021/acs.accounts.8b00126.Search in Google Scholar PubMed
7. Cimarelli, C. Multicomponent Reactions. Molecules 2019, 24, 2372; https://doi.org/10.3390/molecules24132372.Search in Google Scholar PubMed PubMed Central
8. Lei, J.; Meng, J. P.; Tang, D. Y.; Frett, B.; Chen, Z. Z.; Xu, Z. G. Recent Advances in the Development of Polycyclic Skeletons via Ugi Reaction Cascades. Mol. Divers. 2018, 22, 503–516; https://doi.org/10.1007/s11030-017-9811-2.Search in Google Scholar PubMed
9. Kalhans, P.; Singh, A.; Mishra, S.; Pandey, S. Recent Advances in Ugi Reaction and its Post-transformation Strategies for Crafting Diverse Indole Frameworks. Org. Biomol. Chem. 2025, 23, 3243–3269; https://doi.org/10.1039/d4ob02094d.Search in Google Scholar PubMed
10. Li, Y.; Xu, J.; He, L.-J.; Luo, Y.-F.; Meng, J.-P.; Tang, D.-Y.; Li, H.-Y.; Chen, Z.-Z.; Xu, Z.-G. Dieckmann Condensation of Ugi N-Acylamino Amide Product: Facile Access to Functionalized 2,2-Disubstituted Indolin-3-ones. J. Org. Chem. 2022, 87, 823–834; https://doi.org/10.1021/acs.joc.1c02501.Search in Google Scholar PubMed
11. Li, Y.; He, L.; Qin, H.; Liu, Y.; Yang, B.; Xu, Z.; Yang, D. A Facile Ugi/Ullmann Cascade Reaction to Access Fused Indazolo-Quinoxaline Derivatives with Potent Anticancer Activity. Molecules 2024, 29, 464; https://doi.org/10.3390/molecules29020464.Search in Google Scholar PubMed PubMed Central
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.

