Abstract
C4H8O6N4, orthorhombic, Pca21 (no. 29), a = 8.22531(7) Å, b = 8.43724(8) Å, c = 11.23430(9) Å, V = 779.648(12) Å3, Z = 4, R gt (F) = 0.0229, wR ref (F2) = 0.0617, T = 100 K.
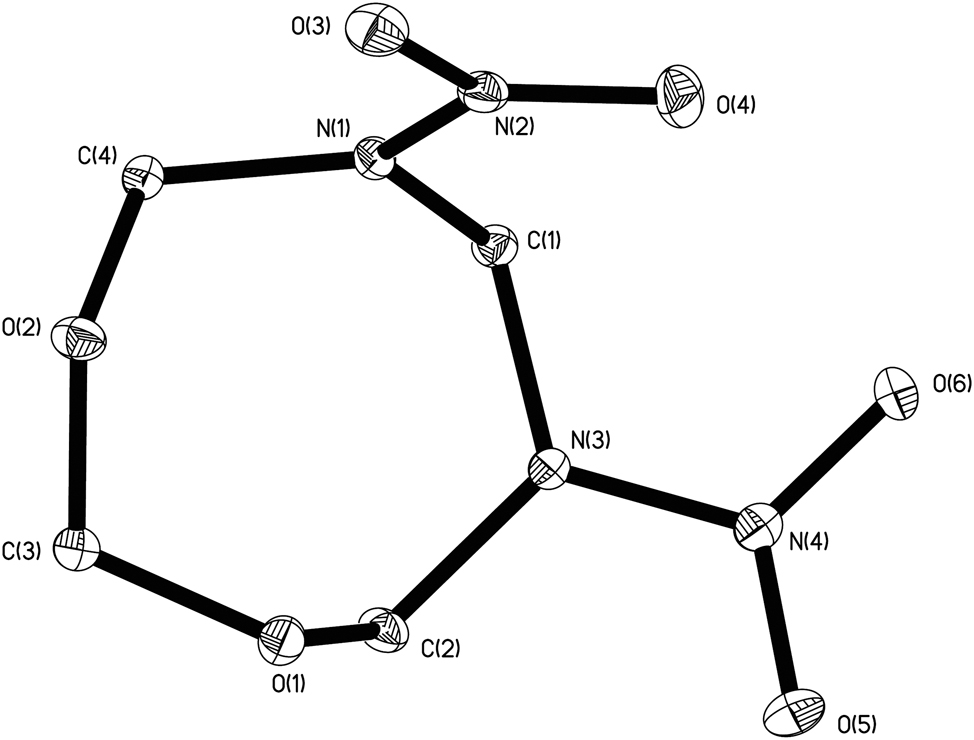
The molecular structure is shown in the figure (hydrogen atoms are omitted for clarity). Table 1 contains the crystallographic data and the list of the atoms including atomic coordinates and displacement parameters can be found in the cif-file attached to this article.
Data collection and handling.
| Crystal: | Clear light colourless block |
| Size: | 0.14 × 0.12 × 0.10 mm |
| Wavelength: | Cu Kα radiation (1.54184 Å) |
| μ: | 1.48 mm−1 |
| Diffractometer, scan mode: | Bruker Apex2, φ and ω scans |
| θmax, completeness: | 72.7°, 99 % |
| N(hkl)measured, N(hkl)unique, Rint: | 6780, 1442, 0.017 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 1,442 |
| N(param)refined: | 135 |
| Programs: | Olex2, 1 SHELX, 2 , 3 Bruker 4 |
1 Source of material
The waste liquid from the synthesis of RDX using the acetic anhydride enrichment method was extracted with ethyl acetate. Extract the wastewater with ethyl acetate. Evaporate the extract under reduced pressure to obtain a yellow powder. Take 0.5 g of the yellow powder and perform initial separation using column chromatography with petroleum ether and ethyl acetate as eluents, yielding a mixture of the target compound and RDX as a solid. The resulting mixture was further purified to yield the title compound as a pure solid. Crystals of the title compound were obtained by recrystallization from a mixed solvent of petroleum ether and dichloromethane to produce colorless plate crystals.
2 Discussion
High-energy materials possess significant energy potential and are widely utilized in defense, military, and civil aviation sectors. Hexogen (RDX), as a high-energy elemental explosive, offers advantages such as high explosive power, rapid detonation velocity, and excellent chemical stability. It is extensively employed in the production of detonators and detonating cord. 5 The title compound was isolated from the nitration waste liquid obtained during the nitration reaction of urotropine with nitric acid, ammonium nitrate, and acetic anhydride in an acetic acid medium to produce RDX. 6 , 7 , 8 , 9
The crystal structure of the title compound was analyzed and refined by the SHELX program. 1 , 2 , 3 , 4 The asymmetric unit of the title structure a 5,7-dinitro-1,3,5,7-dioxadiazabicycloheptane molecule, forming an eight-membered ring with C1, N1, C4, O2, C3, O1, C2, and N3. The dihedral angle between the plane formed by C1, C2, C3, and C4 and the plane formed by O1, C2, and C3 is 65.32°; the dihedral angle between the plane formed by C1, C2, C3, C4 and the plane formed by O2, C3, C4 is 41.76°; the dihedral angle between the plane formed by C1, C2, C3, C4 and the plane formed by N1, N2, O3, O4 is 71.62°; the dihedral angle between the plane formed by C1, C2, C3, and C4 and the plane formed by N3, N4, O5, and O6 is 36.89°; the dihedral angle between the plane formed by N3, N4, O5, and O6 and the plane formed by N1, N2, O3, and O4 is 84.55°. Compared to 3,5,7-trinitro-1,3,5,7-oxatriazacyclooctane, 10 the title compound features an oxygen atom substituted for one –N–NO2 group on its parent ring. This substitution induces shifts in bond angles, resulting in corresponding alterations to the dihedral angles.
Funding source: Center of Testing and Analysis, Shanghai Institute
Acknowledgments
This work was supported by the Center of Testing and Analysis, Shanghai Institute.
References
1. Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A. K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341; https://doi.org/10.1107/s0021889808042726.Search in Google Scholar
2. Sheldrick, G. M. Shelxt – Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. 2015, 71, 3–8; https://doi.org/10.1107/s2053273314026370.Search in Google Scholar PubMed PubMed Central
3. Sheldrick, G. M. Crystal Structure Refinement with Shelxl. Acta Crystallogr. 2015, 71, 3–8; https://doi.org/10.1107/s2053229614024218.Search in Google Scholar PubMed PubMed Central
4. BRUKER. Saint, Apex2 and Sadabs; Bruker AXS Inc.: Madison, WI, USA, 2016.Search in Google Scholar
5. Yi, W. B.; Cai, C. Synthesis of RDX by Nitrolysis of Hexamethylenetetramine in Fluorous Media. J. Hazard. Mater. 2008, 150, 839–842; https://doi.org/10.1016/j.jhazmat.2007.10.040.Search in Google Scholar PubMed
6. Williams, H. L.; Winkler, C. A. Studies on RDX and Related Compounds: IV. The Bachmann or Combination Process. Can. J. Chem. 1951, 29, 642–645; https://doi.org/10.1139/v51-072.Search in Google Scholar
7. Singh, B.; Chaturvedi, L. K.; Gadhikar, P. N. A Survey on the Cyclotetramethylene Tetranitramine (HMX). Def. Sci. J. 1978, 28, 41–50.Search in Google Scholar
8. Bachmann, W. E.; Horton, W. J.; Jenner, E. L.; MacNaughton, N. W.; Scott, L. B. Cyclic and Linear Nitramines Formed by Nitrolysis of Hexamine. J. Am. Chem. Soc. 1951, 73, 2769–2773; https://doi.org/10.1021/ja01150a099.Search in Google Scholar
9. Bachmann, W. E.; Sheehan, J. C. A New Method of Preparing the High Explosive RDX. J. Am. Chem. Soc. 1949, 71, 1842–1845; https://doi.org/10.1021/ja01173a092.Search in Google Scholar
10. Li, S. J.; Wang, X. J.; Xu, Y. G.; Fan, W.; Lizhen, C.; Jianlong, W. The Crystal Structure of 3,5,7-trinitro-1,3,5,7-oxatriazocane. Z. Kristallogr. N. Cryst. Struct. 2025, 240, 597–598; https://doi.org/10.1515/ncrs-2025-0113.Search in Google Scholar
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.

