The crystal structure of (E)-4-(2-methoxyphenoxy)-3-(2-(-1,3,3,6a-tetramethyl-8-methylenedecahydro-1H-naphtho[2,1-d][1,3]dioxin-7-yl)ethylidene)dihydrofuran-2(3H)-one, C30H40O6
Abstract
C30H40O6, monoclinic, C2 (no. 5), a = 25.932(5) Å, b = 7.2230(14) Å, c = 18.458(4) Å, β = 127.75(3)°, V = 2,733.7(9) Å3, Z = 4, Rgt(F) = 0.0556, wRref(F2) = 0.0951, T = 293(2) K.
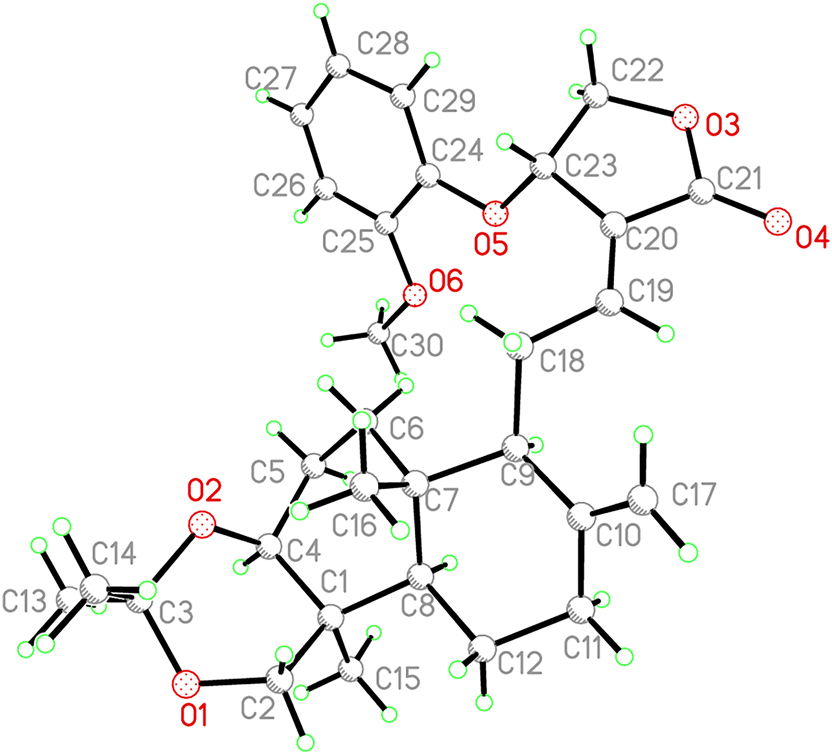
The molecular structure is shown in the figure. Table 1 contains the crystallographic data and the list of the atoms including atomic coordinates and displacement parameters can be found in the cif-file attached to this article.
Data collection and handling.
| Crystal: | Clear light colourless block |
| Size: | 0.20 × 0.10 × 0.10 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.08 mm−1 |
| Diffractometer, scan mode: | Bruker APEX-II, φ and ω scans |
| θmax, completeness: | 25.4°, 100 % |
| N(hkl)measured, N(hkl)unique | 2735a, 2735 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 1394 |
| N(param)refined: | 325 |
| Programs: | Bruker 1 , Olex2, 2 SHELX 3 , 4 |
-
aMerged data.
1 Source of materials
To the solution of andrographolide (9.95 g, 28.4 mmol) and 2,2-dimethoxypropane (24 mL, 195.3 mmol) in 20 mL of anhydrous dichloromethane, PPTS (0.71 g, 2.8 mmol) was added and the reaction mixture was heated at 40 °C. The reaction was monitored by TLC and then treated with ethyl acetate and sat. NaHCO3 after the reaction was complete. The organic phase was washed with brine, dried over anhydrous Na2SO4, and then filtered organic solution was evaporated to dryness. The residue was purified by silica gel column chromatography (petroleum ether/ethyl acetate = 1:1) to produce (S,E)-4-hydroxy-3-(2-((4aR,6aS,7R,10aS,10bR)-3,3,6a,10b-tetramethyl-8-methylenedecahydro-1H-naphtho[2,1-d][1,3]dioxin-7-yl)ethylidene)dihydrofuran-2(3H)-one (10.01 g, 24.7 mmol) as a white solid. 5 Under N2 atmosphere, compound (S,E)-4-hydroxy-3-(2-((4aR,6aS,7R,10aS,10bR)-3,3,6a,10b-tetramethyl-8-methylenedecahydro-1H-naphtho[2,1-d][1,3]dioxin-7-yl)ethylidene)dihydrofuran-2(3H)-one (3.94 g, 10.1 mmol), PPh3 (3.97 g, 15.1 mmol) and 2-methoxyphenol (1.87 g, 15.1 mmol) were dissolved in 30 mL of anhydrous THF. The solution was cooled to 0 °C and then treated with DIAD (3 mL, 15.1 mmol) in 5 mL of anhydrous THF. The reaction was stirred overnight at room temperature after being stirred at 0 °C for 1 h. After distilling off the volatile solvents, the residue was dissolved in ethyl acetate and washed with brine about 5 times and dried over anhydrous Na2SO4. The filtered organic solution was evaporated to dryness and the residue was purified by silica gel column chromatography (petroleum ether/ethyl acetate = 5:1) to give (R,E)-4-(2-methoxyphenoxy)-3-(2-((4aR,6aS,7R,10aS,10bR))-3,3,6a,10b-tetramethyl-8-methylenedecahydro-1H-naphtho[2,1-d][1,3]dioxin-7-yl)ethylidene)dihydrofuran-2(3H)-one (4.48 g, 9.0 mmol) as a white solid. 5
2 Experimental details
X-ray reflections on compound cocrystal were collected on a Bruker. 1 MoKα radiation (λ = 0.71073) was used to collect X-ray reflections on the single crystal at 293(2) K. All H atoms attached to carbon were included using a riding-model using Olex2, 2 with C–H = 0.93–0.98 Å, and their Uiso values were set to 1.2Ueq, the structure was solved with the SHELXT structure solution program and refined with the SHELXL refinement package. 3 , 4
3 Comment
The title compound is an important derivative of natural active substances. To the best of our knowledge, there is no example of an title compound that has been reported so far. The asymmetric unit of the title structure contains one molecule. The bond lengths and angles are unexceptional and lie within the expected ranges. In details, the C–O distances have values between 1.211(9)–1.445(7) Å. The C21–O4 distance is 1.211(9) Å, which suggest a O=C double bond. So esterification is as expected. Andrographolide bioactive compound derived from Andrographis paniculata. 6 Andrographolide can target multiple pathways, exerts a wide range of pharmacological effects, including anti-inflammatory, antiviral, immunostimulatory, and anticancer activities. 5 Both the cyclohexane rings of andrographolide are in chair conformation with the furan ring in twist conformation. The title compound is obtained by linking 2′-methoxy-phenoxy with andrographolide, which is helpful for studying the structure-activity relationship of andrographolide derivatives. 7 , 8 , 9
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Conflict of interest: The authors declare no conflicts of interest regarding this article.
-
Research funding: The work was supported by General Project for Natural Science Research in Jiangsu Province Universities (23KJD350004), and 2023 Jiangsu Province University “Blue Project” Excellent Teaching Team, 2024 Taizhou Vocational Education Federation (Taizhou Vocational Education Group) – Taizhou Polytechnic College Joint Research Project (No. 2024BZD02), and 2024 College Students’ Innovation and Entrepreneurship Cultivation Plan Project (No. TZY2024024), 2024 Taizhou Science and Technology Support Plan (Social Development) Project (No. TN202421).
References
1. Bruker Apex2, Saint-Plus, Xprep; Bruker AXS Inc.: Madison, Wisconsin, USA, 2004.Search in Google Scholar
2. Sheldrick, G. M. Shelxt – Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. 2015, A71, 3–8; https://doi.org/10.1107/s2053273314026370.Search in Google Scholar PubMed PubMed Central
3. Sheldrick, G. M. Crystal Structure Refinement with Shelxl. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Search in Google Scholar PubMed PubMed Central
4. Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A. K.; Puschmann, H. Olex2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Cryst. 2009, 42, 339–341; https://doi.org/10.1107/s0021889808042726.Search in Google Scholar
5. Chen, R.; Zhang, L.; Su, J.; Cheng, Y.; Zhang, G.; Zheng, C.; Xiao, J.; Leung, G.-P.; Li, J.; Zhou, G.-C. Design and Synthesis of Lactam Analogs of Andrographolide and Discovery of Their Anticancer Activity as Dual EGFR and VEGFR2 Inhibitors. Eur. J. Med. Chem. 2025, 299, 118042; https://doi.org/10.1016/j.ejmech.2025.118042.Search in Google Scholar PubMed
6. Phurithap, S.; Jaree, A.; Tangpromphan, P. Purification of Andrographolide, a Bioactive Compound for Relieving COVID-19 Symptoms, from Andrographis Paniculata: Extraction and Separation Using Preparative Pulse-Injection and Adsorption–Desorption Chromatography. Sep. Purif. Technol. 2025, 376, 133928; https://doi.org/10.1016/j.seppur.2025.133928.Search in Google Scholar
7. Sambyal, V. S.; Goswami, K. N. Refined Crystal and Molecular Structure of Andrographolide – Diterpene. Cryst. Res. Technol. 1995, 30, 629–636; https://doi.org/10.1002/crat.2170300508.Search in Google Scholar
8. Liu, Z.; Law, W.-K.; Wang, D.; Nie, X.; Sheng, D.; Song, G.; Guo, K.; Wei, P.; Ouyang, P.; Wong, C.-W.; Zhou, G.-C. Synthesis and Discovery of Andrographolide Derivatives as Non-Steroidal Farnesoid X Receptor (FXR) Antagonists. RSC Adv. 2014, 4, 13533–13545; https://doi.org/10.1039/c3ra46715e.Search in Google Scholar
9. Liu, Z.; Ding, P.; Yang, S.; Zhai, D.; Sha, Y.; Zhang, R.; Wang, L. Crystal Structure of 14-(R)-(2′-Cyano-phenoxy)-3,19-diacetyl Andrographolide, C31H37NO7. Z. Kristallogr. N. Cryst. Struct. 2025, 240, 83–85; https://doi.org/10.1515/ncrs-2024-0374.Search in Google Scholar
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.

