Abstract
C7H11N7O, monoclinic, P21/c (no. 14), a = 8.805(3) Å, b = 7.483(3) Å, c = 14.741(6) Å, β = 105.719(6)°, V = 934.9(6) Å3, Z = 4, R gt (F) = 0.0646, wR ref (F2) = 0.1603, T = 296 K.
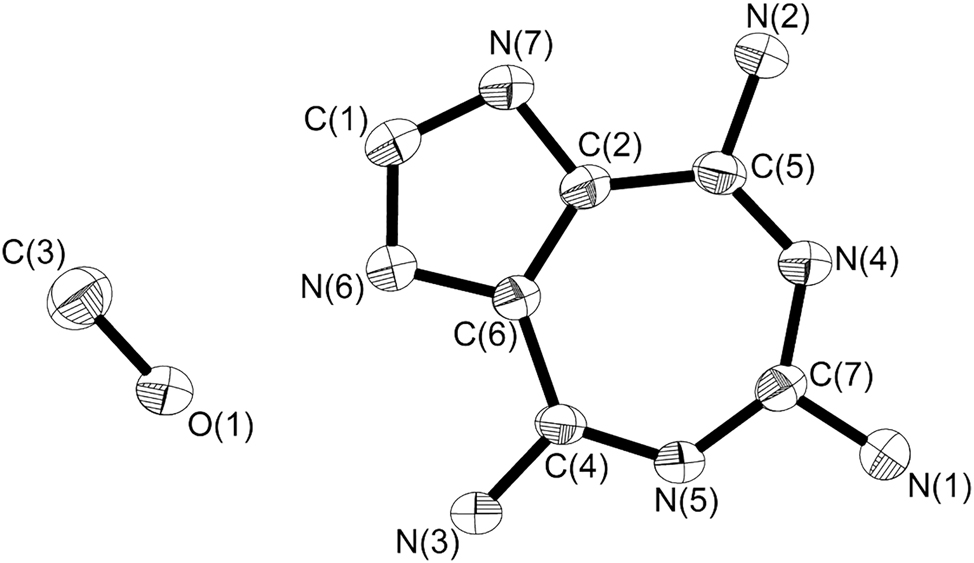
The molecular structure is shown in the figure. Table 1 contains the crystallographic data. The list of the atoms including atomic coordinates and displacement parameters can be found in the cif-file attached to this article.
Data collection and handling.
| Crystal: | Block, colourless |
| Size: | 0.11 × 0.08 × 0.05 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.11 mm−1 |
| Diffractometer, scan mode: | Bruker APEX-II, φ and ω-scans |
| θmax, completeness: | 27.5°, >99 % |
| N(hkl)measured, N(hkl)unique, Rint: | 8523, 2148, 0.106 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 1081 |
| N(param)refined: | 155 |
| Programs: | Bruker programs, 1 SHELX, 2 , 3 OLEX2, 4 |
1 Source of material
2-Amino-4,5-dicyanoimidazole (133.0 mg, 1.0 mmol) was dissolved in 15 mL of ethanol. A methanol solution of guanidine (64.9 mg, 1.1 mmol) was added to the mixture at room temperature, and the reaction was then heated to 80 °C and stirred for 10 h. After completion, the reaction mixture was cooled to room temperature, filtered, and recrystallized with methanol to afford the target compound 1 (117.1 mg, 61 % yield).
2 Experimental details
Hydrogen atoms were placed in their geometrically idealized positions and constrained to ride on their parent atoms. All the non-hydrogen atoms were refined anisotropically.
3 Discussion
Energetic materials, as energy materials critical to national strategic security, serve as the energy source for the propulsion, launch, and damage of weapon systems. With the continuous changes in the international landscape and the evolution of the times, the requirements for energetic materials are becoming increasingly stringent, particularly regarding safety performance to address factors during manufacturing, transportation, use, and storage. Nitrogen-containing fused ring skeletons, which consist of two or more nitrogen-containing rings fused together, are widely used in low-sensitivity high-energy energetic materials. Novel energetic materials based on nitrogen-containing fused ring skeletons have become a current research hotspot. However, at present, nitrogen-containing fused ring skeletons are primarily limited to three types: five-membered rings fused with five-membered rings, five-membered rings fused with six-membered rings, and six-membered rings fused with six-membered rings. The varieties of such fused ring skeletons are not only limited, but the scarcity of modification sites on these skeletons often necessary for attaching high-energy functional groups also restricts the development of new low-sensitivity high-energy energetic materials to some extent. Therefore, given the current lack of diversity in nitrogen-containing fused ring skeletons, developing novel nitrogen-containing fused ring skeletons is of great importance to the field of new energetic materials. 5 , 6 , 7 , 8 , 9 , 10
The crystal structure of the title compound features a unique penta-fused heptacyclic nitrogen-containing condensed ring structure, with its core skeleton comprising two heterocyclic systems: (1) a seven-membered heterocycle (formed by atoms C2, C4, C5, C6, C7, N4, and N5), in which the C4, C5, and C7 positions are modified with amino functional groups. The introduction of these amino groups significantly enhances the compound’s reactivity and molecular diversity; (2) a five-membered heterocycle (containing atoms C1, C2, C6, N6, and N7), where the C1 position is substituted with a methyl group.
The dihedral angle between the plane formed by the seven-membered heterocycle and the plane formed by the five-membered heterocycle is 3.6°. The dihedral angle between the plane of the amino (N1) group and the plane of the seven-membered ring is 6.911°. The dihedral angle between the plane of the amino (N2) group and the plane of the seven-membered ring is 2.9°. The dihedral angle between the plane of the amino (N3) group and the plane of the seven-membered ring is 6.3°.
Funding source: National Natural Science Foundation of China
Award Identifier / Grant number: 22105023
Acknowledgements
This work was supported by National Natural Science Foundation of China No. [22105023].
References
1. BRUKER. Saint, Apex2 and Sadabs; Bruker AXS Inc.: Madison, WI, USA, 2016.Search in Google Scholar
2. Sheldrick, G. M. Shelxt–Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. 2015, 71, 3–8; https://doi.org/10.1107/s2053273314026370.Search in Google Scholar PubMed PubMed Central
3. Sheldrick, G. M. Crystal Structure Refinement with Shelxl. Acta Crystallogr. 2015, 71, 3–8; https://doi.org/10.1107/s2053229614024218.Search in Google Scholar PubMed PubMed Central
4. Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A. K.; Puschmann, H. Olex2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Cryst. 2009, 42, 339–341; https://doi.org/10.1107/s0021889808042726.Search in Google Scholar
5. Dai, Y.; Zhang, T.; Piao, Y. A.; Zhang, X.; Hu, Y.; Zhang, L.; He, H. Computational Study on Fused Five Membered Heterocyclic Compounds Containing Tertiary Oxygen. J. Mol. Struct. 2017, 1129, 98–104; https://doi.org/10.1016/j.molstruc.2016.09.058.Search in Google Scholar
6. Foldesi, T.; Volk, B.; Milen, M. A Review of 2,3-Benzodiazepine-Related Compounds: Diazepines and 1,2,5-Triazepines Fused with Five-Membered Nitrogen Heterocycles. Curr. Org. Synth. 2018, 15, 729–754; https://doi.org/10.2174/1570179415666180601101856.Search in Google Scholar
7. Karimian, A.; Karimi, Z. Synthesis of A Novel Heterocyclic System of 3,8-Disubstituted-5H-Pyrimido[5′,4′:5,6][1,4]thiazino[3,2-e][1,2,4]triazine. Polycyclic Aromat. Compd. 2022, 42, 1516–1523; https://doi.org/10.1080/10406638.2020.1784242.Search in Google Scholar
8. Gu, Z.; Bo, M.; Gao, Z.; Xu, J.; Chen, J.; Xiao, T.; Ma, P. Theoretical Study on Different Substituent-Modified Derivatives of 6-Dinitrophenyl-5,6,7,8-tetrahydro-4-imidazo[4,5-e]furazano[3,4-b]pyrazine. J. Mol. Model. 2024, 30, 192; https://doi.org/10.1007/s00894-024-05993-2.Search in Google Scholar PubMed
9. Cilibrasi, V.; Spano, V.; Bortolozzi, R.; Barreca, M.; Raimondi, M. V.; Rocca, R.; Barraja, P.; Montalbano, A.; Alcaro, S.; Ronca, R.; Viola, G. Synthesis of 2H-Imidazo[2′,1′:2,3][1,3]thiazolo[4,5-e]isoindol-8-Yl-phenylureas with Promising Therapeutic Features for the Treatment of Acute Myeloid Leukemia (AML) with FLT3/ITD Mutations. Eur. J. Med. Chem. 2022, 235, 114292; https://doi.org/10.1016/j.ejmech.2022.114292.Search in Google Scholar PubMed
10. Takhti, S.; Pordel, M.; Davoodnia, A.; Bozorgmehr, M. R. Imidazo[4′,5′:3,4]benzo[1,2-e][1,4]diazepins as New Heterocyclic Systems: Synthesis, Characterization and Their In Vitro Interactions with Benzodiazepine Receptors. Polycyclic Aromat. Compd. 2023, 43, 6927–6933; https://doi.org/10.1080/10406638.2022.2127800.Search in Google Scholar
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.

