Abstract
C10H12Br2, monoclinic, P21/c (no. 14), a = 8.1816(14) Å, b = 4.4288(7) Å, c = 15.2809(19) Å, β = 93.126(2)°, V = 516.10(14) Å3, Z = 2, R gt (F) = 0.0403, wR ref (F2) = 0.1011, T = 170 K.
Table 1 contains the crystallographic data. The list of the atoms including atomic coordinates and displacement parameters can be found in the cif-file attached to this article.
Data collection and handling.
| Crystal: | Needle, greenish |
| Size: | 0.22 × 0.12 × 0.1 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 7.8 mm−1 |
| Diffractometer, scan mode: | Bruker APEX-II, φ and ω-scans |
| θmax, completeness: | 26.4°, >99 % |
| N(hkl)measured, N(hkl)unique, Rint: | 1947, 1059, 0.047 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 781 |
| N(param)refined: | 56 |
| Programs: | CrysAlisPRO, 1 OLEX2, 2 SHELX 3 , 4 |
1 Source of materials
A mixture of 48 % hydrobromic acid (100 mL), paraformaldehyde (7.7 g), and p-xylene (15.5 mL) in a 500 mL round-bottom flask was stirred under reflux for 48 h. The white precipitate was collected by filtration to afford approximately 35 g of the crude title compound. Recrystallisation from carbon tetrachloride yielded pure product as white needles, suitable for single-crystal X-ray diffraction analysis.
2 Experimental details
The hydrogen atoms of the methyl group were located in difference Fourier maps and then placed in idealized positions with Uiso(H) = 1.5Ueq(C) and C–H distances constrained to 0.96 Å. The remaining hydrogen atoms were refined using a riding model with Uiso(H) = 1.2Ueq(C). Crystal data, data collection and structure refinement details are summarized in Table 1.
3 Comment
1,4-Bis(bromomethyl)-2,5-dimethylbenzene is widely used as a key intermediate in the organic synthesis of s-indacenes 5 and as a bridging building block for the preparation of novel macrocycles. 6 Its utility stems from its straightforward and high-yielding synthesis, exemplified by the bromomethylation of 1,4-dimethylbenzene, which unexpectedly affords the corresponding dibromo compound in nearly quantitative yield, significantly outperforming the typically low-yielding chloromethylation reaction. 5
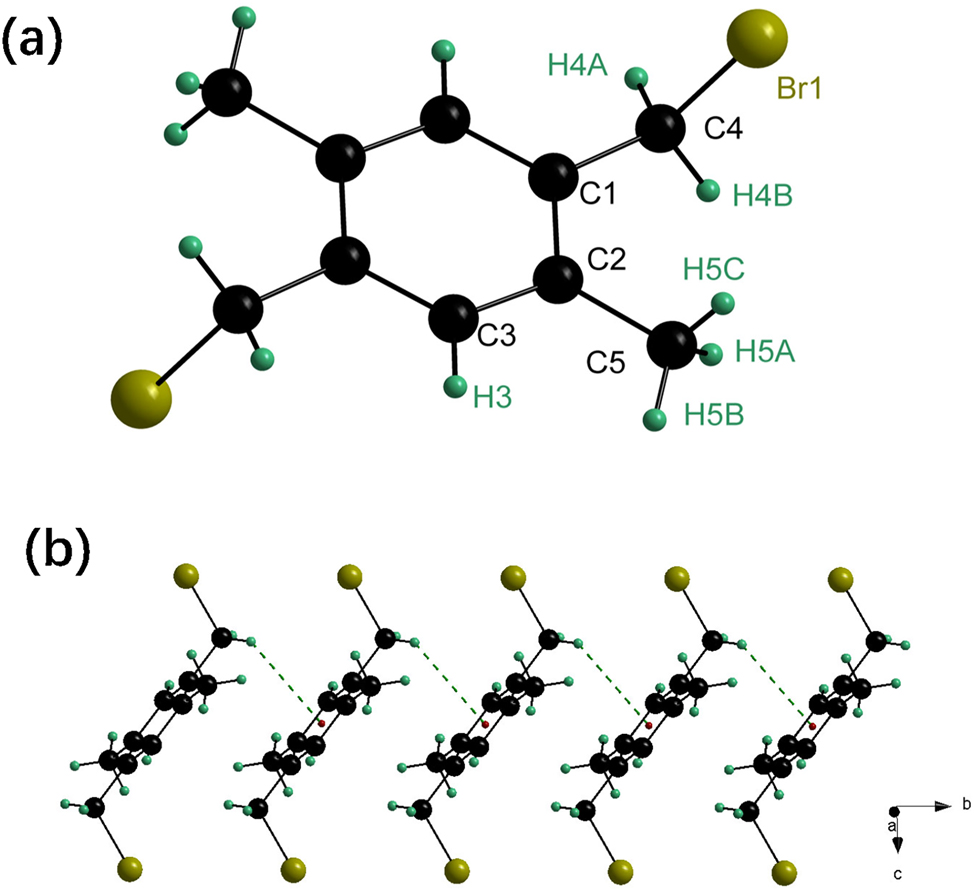
The title compound, C10H12Br2, features a center of inversion situated at the centroid of the benzene ring. The asymmetric unit consists of one half of the molecule positioned on an inversion center, with its labeling shown in Figure (part a). Two bromomethyl groups are located on opposite sides of the benzene ring and are nearly perpendicular to it, as indicated by the Br1–C4–C1–C3 and Br1–C4–C1–C2 torsion angles of 98.4(4)° and 82.3(5)°, respectively. The carbon atom C4 is nearly coplanar with the benzene ring, supported by the C4–C1–C2–C3 and C4–C1–C2–C5 torsion angles of 179.4(4)° and 179.3(4)°, respectively. A comparison with the crystal structures of 1,1-bis(bromomethyl)benzene, 7 1,4-bis(bromomethyl)benzene, 8 and 1,2-bis(bromomethyl)benzene 9 reveals no unusual bond lengths or angles. However, the C–Br bond length in the title compound (1.988 Å) is slightly longer than those in 1,1-bis(bromomethyl)benzene (1.967 Å), 1,4-bis(bromomethyl)benzene (1.965 Å), 1,2-bis(bromomethyl)benzene (1.975 Å), and other bromomethyl-substituted benzenes (1.974–1.985 Å). 3 , 4 , 5 , 6 , 7 , 8 , 9 , 10
The crystal packing of the title compound features only weak C–H⋯π interactions, with no evidence of strong or weak hydrogen bonds, significant π⋯π interactions, or short Br⋯Br contacts. This behaviour contrasts with that of 1,4-bis(bromomethyl)benzene,
8
which exhibits short Br⋯Br contacts, but resembles the packing mode observed in 1,1-bis(bromomethyl)benzene.
7
Such a packing environment is relatively uncommon among brominated compounds.
9
As shown in Figure, the crystal stability is primarily maintained by C4–H4⋯π interactions between the bromomethyl group and the centroid (C
g
) of a symmetry-related benzene ring [d(C4⋯
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Conflict of interest: The authors declare no conflicts of interest regarding this article.
-
Research funding: This research was funded by the high-level talent research launch project of Zhoukou Normal University (project number: ZKNUC2024023, ZKNUC2023073).
References
1. Rigaku, O. D. CrysAlisPRO; Rigaku Oxford Diffraction Ltd: Yarnton, Oxfordshire, England, 2017.Search in Google Scholar
2. Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A. K.; Puschmann, H. Olex2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341; https://doi.org/10.1107/s0021889808042726.Search in Google Scholar
3. Sheldrick, G. Crystal Structure Refinement with Shelxl. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Search in Google Scholar
4. Shelxt, S. G. M. Integrated Space-Group and Crystal Structure Determination. Acta Crystallogr. 2015, A71, 3–8.10.1107/S2053273314026370Search in Google Scholar PubMed PubMed Central
5. Dahrouch, M. R.; Jara, P.; Mendez, L.; Portilla, Y.; Abril, D.; Alfonso, G.; Chavez, I.; Manriquez, J. M.; Rivière Baudet, M.; Rivière, P.; Castel, A.; Rouzaud, J.; Gornitzka, H. An Effective and Selective Route to 1,5-Dihydropolyalkylated S-Indacenes: Characterization of Their Mono- and Dianions by Silylation. Structure of trans-1,5-Bis(trimethylsilyl)-2,6-diethyl-4,8-dimethyl-s-Indacene. Organometallics 2001, 20, 5591–5597; https://doi.org/10.1021/om010119w.Search in Google Scholar
6. Zhang, G. Giant N-Heterocyclic Carbene-Containing Macrocycles for Cobalt-Catalysed Hydroboration of Alkynes. Chem. Commun. 2022, 58, 8109–8112; https://doi.org/10.1039/d2cc02815h.Search in Google Scholar PubMed
7. Li, Q.-X.; Cai, L.; Wang, X.-F.; Shen, Y.-J. 1,1-Bis(bromomethyl)benzene. Acta Crystallogr. 2006, 62E, o5726–o5727; https://doi.org/10.1107/s1600536806048422.Search in Google Scholar
8. Zhang, M.; Su, P.; Meng, X.-G.; Xu, X.-M. 1,4-Bis(bromomethyl)benzene. Acta Crystallogr. 2007, 63E, o951–o952; https://doi.org/10.1107/s1600536807002954.Search in Google Scholar
9. Jones, P. G.; Kuś, P. Secondary Interactions in Bromomethyl-Substituted Benzenes: Crystal Structures of Three α,α-Bis-Bromoxylenes, 1,2,3,5-Tetrakis(bromomethyl)benzene, and 1,2,4,5-Tetrakis(bromomethyl)benzene. Z. Naturforsch. B 2007, 62, 725–731; https://doi.org/10.1515/znb-2007-0517.Search in Google Scholar
10. Jiang, D.-C.; Zhu, H.-Y. The Crystal Structure of 1,2,4-Tris(bromomethyl)benzene, C9H9Br3. Z. Kristallogr. N. Cryst. Struct. 2024, 239, 429–431; https://doi.org/10.1515/ncrs-2024-0031.Search in Google Scholar
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.

