Abstract
C13H13O3N3S, monoclinic, Pn (no. 7), a = 6.9318(5) Å, b = 12.0202(8) Å, c = 7.8080(6) Å, β = 98.469(3)°, V = 643.50(8) Å3, Z = 2, Rgt(F) = 0.0906, wRref(F2) = 0.0914, T = 100 K.
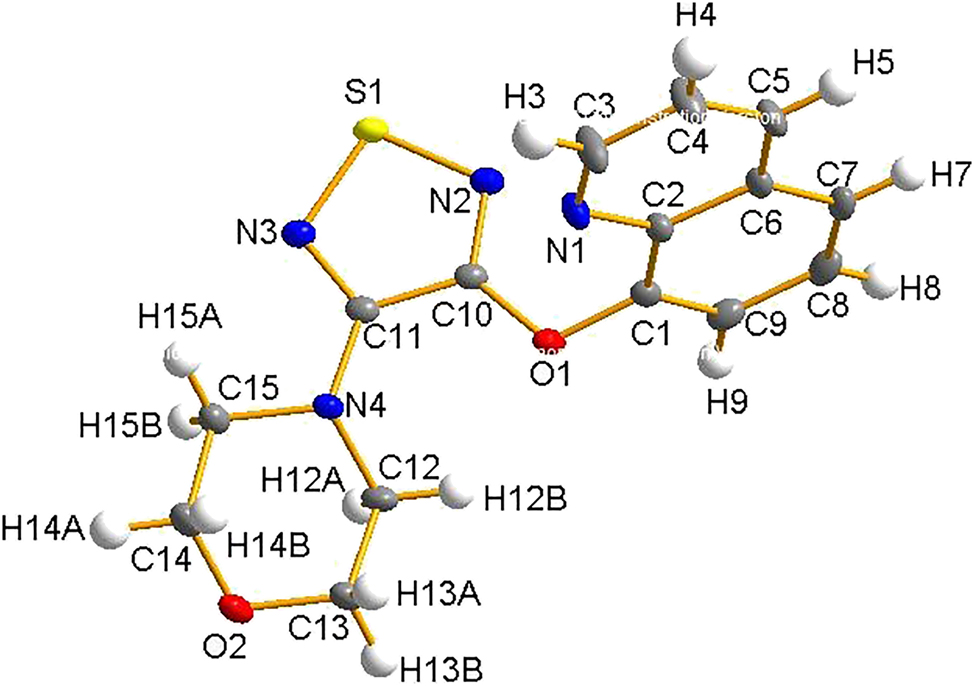
The molecular structure is shown in the figure. Table 1 contains the crystallographic data and the list of the atoms including atomic coordinates and displacement parameters can be found in the cif-file attached to this article.
Data collection and handling.
| Crystal: | Yellow block |
| Size: | 0.18 × 0.13 × 0.07 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.26 mm−1 |
| Diffractometer, scan mode: | Bruker APEX-II, φ and ω scans |
| θmax, completeness: | 28.4°, 100 % |
| N(hkl)measured, N(hkl)unique, Rint: | 14223, 3194, 0.049 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 3113 |
| N(param)refined: | 181 |
| Programs: | SHELX, 1 Bruker, 2 Olex2, 3 Diamond 4 |
1 Source of materials
The title compound was synthesized by dissolving (200 mg, 1.31 mmol) of salicylaldehyde and (264 mg, 1.44 mmol, 1.1 equiv.) of 4-(4-chloro-1,2,5-thiadiazol-3-yl) morpholine in 10 mL of anhydrous DMF under nitrogen. Then 641 mg (1.97 mmol, 1.5 equiv) of potassium carbonate was added to the reaction mixture, and the reaction was stirred at 120 °C for 18–24 h, monitored by TLC. Upon completion, the reaction was cooled, poured into 20 mL of water, and extracted with ethyl acetate (3 × 20 mL). The combined organic layers were washed with brine, dried over anhydrous sodium sulfate (Na2SO4), filtered, and concentrated under reduced pressure. The crude product was purified by silica gel column chromatography using ethyl acetate:hexane (2:8), and the product was recovered as a light-yellow solid. The product was dissolved in 3 mL of acetone and left to crystallize, resulting in the formation of light-yellow crystals.
2 Experimental details
All H-atoms were positioned on geometrically idealized positions and refined using the riding model with fixed C–H distances for aromatic C–H of 0.95 Å (C–H) [Uiso (H) = 1.2 Ueq]. The graphics were obtained using the DIAMOND 4 program with 50 % probability ellipsoids.
3 Comment
The molecular structure of 2-((4-morpholino-1,2,5-thiadiazol-3-yl)oxy) benzaldehyde was determined using single-crystal X-ray diffraction. The thiadiazole ring is nearly planar, with S1–N2 and S1–N3 bond distances of 1.655(3) Å and 1.651(3) Å, respectively, and the bond distances indicate delocalized S–N bonds typical of aromatic thiadiazoles, usually ranging from 1.64 to 1.67 Å. 5 The C8–N2 bond distance of 1.290(4) Å and C9–N3 bond distance of 1.321(4) Å suggest a C=N double bond, confirming the conjugated nature of the heterocyclic rings. The oxygen atom O1 connects the benzaldehyde ring to the thiadiazole group through an ether bond, with O1–C8 bond of 1.361(4) Å and O1–C1 bond of 1.395(3) Å, and the values are similar to the average aromatic C–O single bonds reported for related aryl-O-heterocycle systems. 6 The aldehyde group is confirmed by the short C7–O2 bond distance of 1.204(4) Å, which is typical of carbonyl C=O bonds. The morpholine ring shows typical C–O bond distance O3–C11 of 1.428(4) Å and O3–C12 bond distance of 1.427(4) Å and C–N bond distances N1–C9 of 1.379(4) Å and N1–C10 bond distance of 1.472(4) Å, which is consistent with a chair conformation. The C–N bonds indicate partial delocalization within the morpholine-thiadiazole linkage, supported by the N3–C9 bond length of 1.321(4) Å, which falls between a typical single and double bond. Bond angles around the thiadiazole core, such as N3–S1–N2 at 98.69(13)° and C8–N2–S1 at 106.2(2)°, are similar to those seen in other 1,2,5-thiadiazole derivatives, 7 , 8 reflecting ring strain and heteroatom effects in this five-membered system. The benzaldehyde ring is nearly planar, with internal angles like C1–C6–C5 at 119.5(3)° and C3–C4–C5 at 120.0(3)°, consistent with standard aromatic geometry. Overall, the structural parameters closely match related aryl-thiadiazole-morpholine systems, confirming the expected bonding framework and offering insight into the electronic conjugation between the aromatic, heterocyclic, and morpholine groups. All geometric parameters are in general in the expected ranges. 8
Acknowledgments
University of Johannesburg is highly acknowledged for funding.
References
1. Sheldrick, G. M. A Short History of Shelx. Acta Crystallogr. 2008, A64, 112–122; https://doi.org/10.1107/s0108767307043930.Search in Google Scholar PubMed
2. Bruker. Saint-Plus (Version 7.12) and Sadabs (Version 2004/1); Bruker AXS Inc.: Madison, Wisconsin, USA, 2004.Search in Google Scholar
3. Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A. K.; Puschmann, H. Olex2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Cryst. 2009, 42, 339–341; https://doi.org/10.1107/s0021889808042726.Search in Google Scholar
4. Brandenburg, K. Diamond. Visual Crystal Structure Information System. Version 3.0c; Crystal Impact: Bonn, Germany, 2005.Search in Google Scholar
5. Allen, F. H.; Kennard, O.; Watson, D. G.; Brammer, L.; Orpen, A. G.; Taylor, R. Tables of Bond Lengths Determined by X-ray and Neutron Diffraction. Part 1. Bond Lengths in Organic Compounds. J. Chem. Soc., Perkin Trans. 1987, S1–S19; https://doi.org/10.1039/p298700000s1.Search in Google Scholar
6. Ruediger, A.; Rehder, D.; Kuhn, N. Structural Features of Thiadiazole Derivatives. Eur. J. Inorg. Chem. 2001, 1007–1015.Search in Google Scholar
7. Bergman, J.; Engqvist, R.; Staalhandske, C.; Wallin, S. Structure and Reactivity of Thiadiazole-Based Systems. Tetrahedron 2010, 66, 7801–7808.Search in Google Scholar
8. Bhilare, S.; Bandaru, S. S. M.; Shah, J.; Chrysochos, N.; Schulzke, C.; Sanghvi, Y. S., and Kapdi, A. R. Pd/PTABS: Low Temperature Etherification of Chloroheteroarenes. J. Org. Chem. 2018, 83, 13088, https://doi.org/10.1021/acs.joc.8b01840Search in Google Scholar PubMed
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.

