Abstract
C16H25NO2S2, monoclinic, P21 (no. 4), a = 10.8352(3) Å, b = 6.3694(2) Å, c = 13.3196(4) Å, β = 107.681(4)°, Z = 2, V = 875.81(5) Å3, R gt (F) = 0.0233, wR ref = 0.0525, Flack–Parsons-parameter: −0.002(6) using 1518 quotients [(I+)−(I−)]/[(I+)+(I−)] (Parsons, S.; Flack, H. D.; Wagner, T. Use of Intensity Quotients and Differences in Absolute Structure Refinement. Acta Crystallogr. 2013, B69, 249–259), T = 150(2) K.
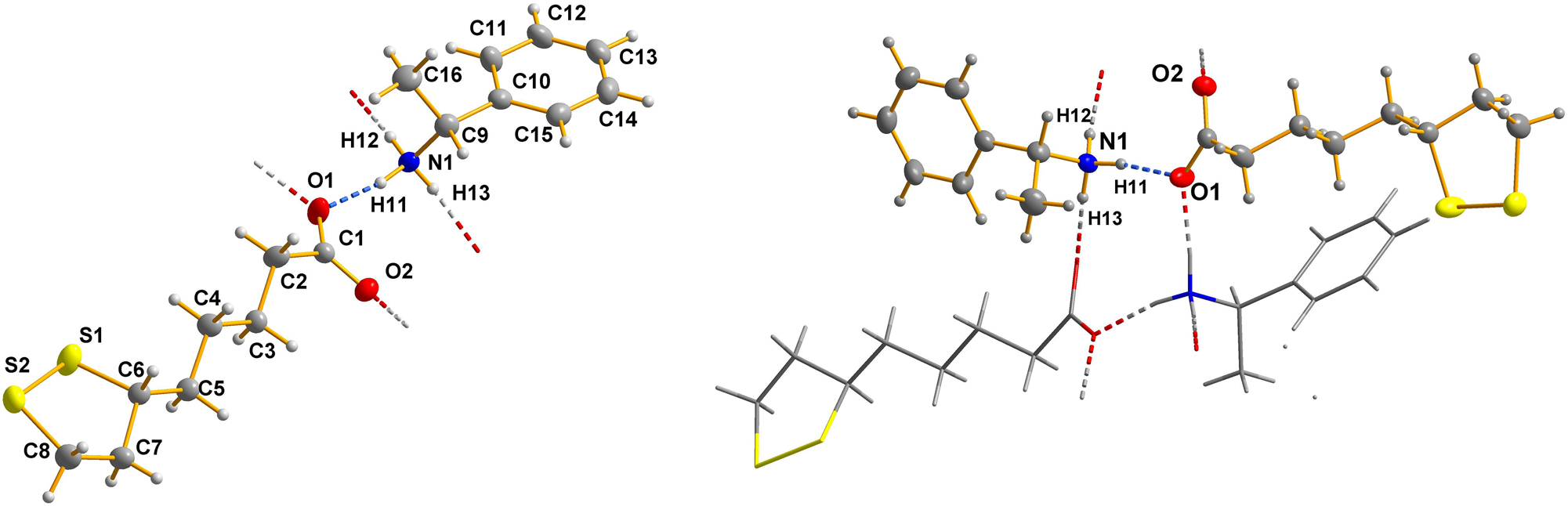
The left part of the figure shows the asymmteric unit of the title salt; the right part of the figure shows a selected region of the hydrogen bonded chain – running along [010] – within the title crystal structure.
Table 1 containing crystallographic data and the list of the atoms including atomic coordinates and displacement parameters can be found in the cif-file attached to this article.
Data collection and handling.
| Crystal: | Colorless block |
| Size: | 0.36 × 0.20 × 0.11 mm |
| Wavelength: | CuKα radiation (1.54184 Å) |
| μ: | 2.78 mm−1 |
| Diffractometer, scan mode: | XtaLAB Synergy, ω scans |
| θ max, completeness: | 78.7°, 100 % |
| N(hkl)measured, N(hkl)unique, R int: | 11582, 3540, 0.017 |
| Criterion for I obs, N(hkl)gt: | I obs > 2 σ(I obs), 3521 |
| N(param)refined: | 204 |
| Programs: | CrysAlisPRO, 1 SHELX, 2 , 3 ShelXLe, 4 Diamond 5 |
1 Source of material
The crystalline title compound (systematic name: (R)-1-phenylethan-1-aminium (R)-5-(1,2-dithiolan-3-yl)pentanoate) has been obtained from the Degussa Company (Frankfurt). According to the documentation of Degussa the provided crystals were grown fom methanol. No further purification or recrystallisation is needed.
2 Experimental details
A crystal of the title compound was directly selected from the delivered batch and transferred to the RIGAKU-Synergy diffractometer. An absorption correction (Multi-Scan method) was applied. 1 The structure solution and the refinement were successfully carried out using the SHELX program system. 2 , 3 , 4 All hydrogen atoms were seen in the difference electron density map after all non-hydrogen atoms were located. The coordinates and the isotropic displacement parametes of the three hydrogen atoms of the aminium group were refined with a N–H DFIX option of the SHELXL system. All other hydrogen atoms were included in the refinement using the corresponding AFIX options of the SHELXL system. 3 The absolute structure has been verified by the structure refinement. 6 The figure was created using the Diamond software. 5
3 Introduction
The α-lipoic acid ((+)-lipoic acid; trivial name: thioctic acid; systematic name: (R)-5-(1,2-dithiolan-3-yl)pentanoic acid) is a naturally occurring compound 7 and a pharmacologically active agent that has been studied and used for decades. 8 , 9 A recent survey of the Cambridge Structural Database (CSD) 10 identified approximately 30 crystal structures containing the (1,2-dithiolan-3-yl)pentanyl moiety. In some structures, neutral α-lipoic acid serves as a component of a co-crystal, 11 while in others the mono-deprotonated analogue acts as an anionic ligand. 12 To date, there is only one comparable salt structure 13 listed in the CSD 10 featuring a (R)-5-(1,2-dithiolan-3-yl)pentanoate anion, which engages in hydrogen bonding and weak intermolecular interactions in its vicinity. The 1-phenylethan-1-aminium cation is widely used as the counterion for various systems. In the CSD, 10 there are more than 700 corresponding entries (e.g., refs. 14] and 15]).
This work forms part of our long-standing program to investigate the synthesis, structural characterization, and hydrogen-bonding patterns of natural products and dayly drugs with clinical relevance, such as theophylline 16 or nicotine. 17
4 Molecular structure description
The asymmetric unit of the reported crystal structure contains one (R)-1-phenylethan-1-aminium cation and one (R)-5-(1,2-dithiolan-3-yl)pentanoate anion (see the left part of the figure). Bond lengths and angles are all in the expected ranges. The cation shows conformational flexibility about the C–C bond connecting the phenyl group with the 1-ammonio-ethyl moiety. This conformation is best characterized by the angle between the plane of the phenyl ring and the plane defined by H9–C9–C10–C11, which should be near 0° for the estimated most stable conformation, as the steric hindrance between the phenyl, the methyl, and the aminium group, respectively, is minimized. In the title structure this parameter is found to be 1.2(2)°.
The question of conformation arises also in the anion. Within the 1,2-dithiolan-3-yl moiety there is a limited conformational flexibility, but the alkyl moiety can more easily conform to different geometries. In the literature the only comparable hydrogen-bonded 5-(1,2-dithiolan-3-yl)pentanoate anion shows a hook-type arrangement. In contrast to this, in the title structure the alkyl subunit as well as the C3H5 moiety of the 1,2-dithiolan-3-yl ring are in an all-trans conformation (see left side of the figure). The all-trans conformation is documented by dihedral angles within the hydrocarbon backbone of the anion as follows: C1–C2–C3–C4 = 178.49(17)°; C2–C3–C4–C5 178.19(17)°; C3–C4–C5–C6 −177.38(16)°; C4–C5–C6–C7 −173.52(17)°; C5–C6–C7–C8 −173.50(18)°, respectively.
5 Supramolecular aspects
The cation and the anion in the asymmetric unit form one classical hydrogen bond with a donor-acceptor distance of 2.711(2) Å. The ammonium group is connected to two neighboring anions via H12 and H13, with O⋯O donor-acceptor distances of 2.722(2) Å and 2.747(2) Å, respectively. Consequently, the carboxylate function is involved in three hydrogen bonds as an acceptor group (see the right part of the figure). These donor-acceptor distances are all in the expected ranges for charge-supported NH⋯O hydrogen bonds. 18 , 19 In particular, the situation where all three hydrogen atoms of an aminium group form medium-strong hydrogen bonds is not rare. The aforementioned hydrogen bonds form a ladder structure (see right part of the figure) along the crystallographic b-direction. Each rung of the ladder consists of one cation and one anion. The neighboring rungs have a reverse orientation (see the figure).
Funding source: Deutsche Forschungsgemeinschaft (DFG)
Award Identifier / Grant number: INST 208/793
-
Conflict of interest: The author declares no conflicts of interest regarding this article.
-
Research funding: I would like to acknowledge the Deutsche Forschungsgemeinschaft (DFG) for financial support in acquiring the Rigaku Synergy diffractometer (DFG No.: INST 208/793).
References
1. Rigaku CrysAlisPRO; Rigaku Oxford Diffraction Ltd: Yarnton, Oxfordshire, England, 2024.Search in Google Scholar
2. Sheldrick, G. M. A Short History of Shelx. Acta Crystallogr. 2008, A64, 112–122. https://doi.org/10.1107/S0108767307043930.Search in Google Scholar PubMed
3. Sheldrick, G. M. Crystal Structure Refinement with Shelxl. Acta Crystallogr. 2015, C71, 3–8. https://doi.org/10.1107/S2053229614024218.Search in Google Scholar PubMed PubMed Central
4. Hübschle, C. B.; Sheldrick, G. M.; Dittrich, B. ShelXle: A Qt Graphical User Interface for Shelxl. J. Appl. Crystallogr. 2011, 44, 1281–1284. https://doi.org/10.1107/S0021889811043202.Search in Google Scholar PubMed PubMed Central
5. Brandenburg, K. Diamond. Visual Crystal Structure Information System. Ver. 4.5.2; Crystal Impact: Bonn, Germany, 2018.Search in Google Scholar
6. Parsons, S.; Flack, H. D.; Wagner, T. Use of Intensity Quotients and Differences in Absolute Structure Refinement. Acta Crystallogr. 2013, B69, 249–259. https://doi.org/10.1107/S2052519213010014.Search in Google Scholar PubMed PubMed Central
7. Reed, L. J.; DeBusk, B. G.; Gunsalus, I. C.; Schnakenberg, G. H. F. Chemical Nature of α-Lipoic Acid. J. Am. Chem. Soc. 1951, 73, 5920. https://doi.org/10.1021/ja01156a567.Search in Google Scholar
8. Ramachanderan, R.; Schaefer, B. Lipoic Acid. ChemTexts 2019, 5, 18. https://doi.org/10.1007/s40828–019–0091–6.10.1007/s40828-019-0091-6Search in Google Scholar
9. Wang, J.-Q.; Ling, X.; Wang, H.-J.; Chen, F.-E. α-Lipoic Acid Chemistry: The Past 70 Years. RSC Adv. 2023, 13, 36346–36363. https://doi.org/10.1039/D3RA07140E.Search in Google Scholar PubMed PubMed Central
10. Groom, C. R.; Allen, F. H. The Cambridge Structural Database in Retrospect and Prospect. Angew. Chem. Int. Ed. 2014, 53, 662–671. https://doi.org/10.1002/anie.201306438.Search in Google Scholar PubMed
11. Zhao, L.; Raval, V.; Briggs, N. E. B.; Bhardwaj, R. M.; McGlone, T.; Oswald, I. D. H.; Florence, A. J. From Discovery to Scale-Up: α-lipoic Acid:nicotinamide Co-Crystals in a Continuous Oscillatory Baffled Crystalliser. CrystEngComm 2014, 16, 5769–5780. https://doi.org/10.1039/C4CE00154K.Search in Google Scholar
12. Jumabaev, F. R.; Sharipov, A. T.; Mannopova, V. K. K.; Choriyev, O. I.; Ashurov, J. M. Di-Aqua-Bis(DL- +- -Lipoato-κ2O,Oʹ)Manganese(II). IUCrData 2025, 10, x250565; https://doi.org/10.1107/s2414314625005656.Search in Google Scholar PubMed PubMed Central
13. Fulas, O. A.; Laferrire, A.; Ayoub, G.; Gandrath, D.; Mottillo, C.; Titi, H. M.; Stein, R. S.; Friscic, T.; Coderre, T. J. Drug-Nutraceutical Co-Crystal and Salts for Making New and Improved Bi-functional Analgesics. Pharmaceutics 2020, 12, 1144. https://doi.org/10.3390/pharmaceutics12121144.Search in Google Scholar PubMed PubMed Central
14. Wood, M. H.; Clarke, S. M. (S)-(-)-1-Phenyl-Ethanaminium Hexanoate. Acta Crystallogr. E 2012, 68, o3335. https://doi.org/10.1107/S1600536812045746.Search in Google Scholar PubMed PubMed Central
15. Ruan, C.-Y.; Ai, Z.-W.; Liang, G.-Y.; Cao, P.-Y.; Yu, K.-F.; Jiang, C.-J. The Crystal Structure of 1-Phenylethan-1-Aminium 4-Hydroxy-3,5-Dimethoxybenzoate C17H21NO5. Z. Kristallogr. - N. Cryst. Struct. 2025, 240, 271–272. https://doi.org/10.1515/ncrs-2024–0461.10.1515/ncrs-2024-0461Search in Google Scholar
16. Konovalova, I. S.; Shishkina, S. V.; Wyshusek, M.; Patzer, M.; Reiss, G. J. Supramolecular Architecture of Theophylline Polymorphs, Monohydrate and Co-Crystals with Iodine: Study from the Energetic Viewpoint. RSC Adv. 2024, 14, 29774–29788. https://doi.org/10.1039/d4ra04368e.Search in Google Scholar PubMed PubMed Central
17. Reiss, G. J.; Wolke, M. B. Hydrogen Bonding in the Crystal Structure of Nicotin-1,1ʹ-dium Tetrabromidomanganate(II). Z. Kristallogr. – N. Cryst. Struct. 2025, 240, 777–779. https://doi.org/10.1515/ncrs-2025–0247.10.1515/ncrs-2025-0247Search in Google Scholar
18. Heimgert, J.; Neumann, D.; Reiss, G. J. (3-Ammonio-2,2-Dimethyl-Propyl)Carbamate Dihydrate. Molbank 2018, M1015. https://doi.org/10.3390/M1015.Search in Google Scholar
19. Sharma, A.; Thamotharan, S.; Roy, S.; Vijayan, M. X-Ray Studies of Crystalline Complexes Involving Amino Acids and Peptides. XLIII. Adipic Acid Complexes of L- and DL-Lysine. Acta Crystallogr. C 2006, 62, o148. https://doi.org/10.1107/S0108270106003374.Search in Google Scholar PubMed
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.

