Abstract
C12H14N4O6Zn, triclinic, P
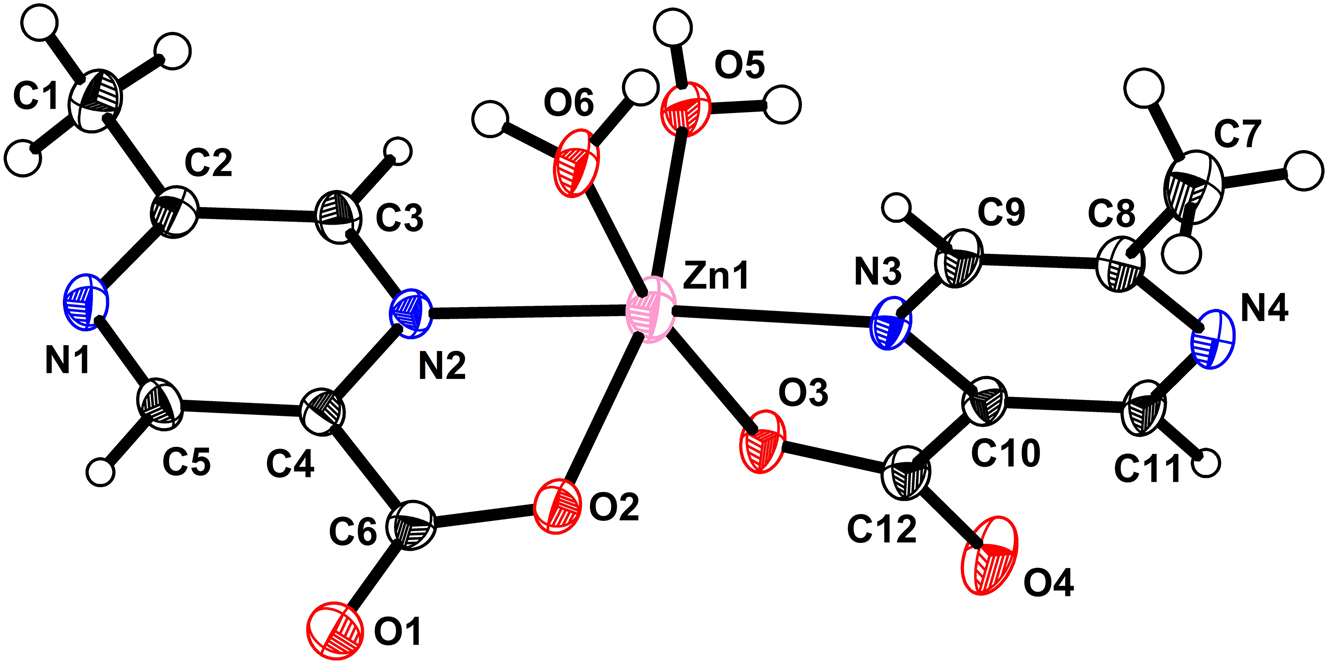
The molecular structure is shown in the figure. Table 1 contains the crystallographic data and the list of the atoms including atomic coordinates and displacement parameters can be found in the cif-file attached to this article.
Data collection and handling.
| Crystal: | Clear light colourless block |
| Size: | 0.15 × 0.12 × 0.10 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 1.71 mm−1 |
| Diffractometer, scan mode: | XtaLAB, ω scans |
| θmax, completeness: | 25.0°, 99 % |
| N(hkl)measured, N(hkl)unique, Rint: | 8220, 2573, 0.050 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 2325 |
| N(param)refined: | 212 |
| Programs: | Rigaku, 1 Olex2, 2 SHELX 3 , 4 |
1 Source of materials
All chemicals were obtained commercially. A mixture of 5-methyl-2-pyrazinecarboxylic acid (MPCA) (1 mmol) and zinc acetate (0.5 mmol) was dissolved in 5 mL of ultrapure water. After stirring approximately 10 minutes, a clear solution was obtained; it was subsequently filtered and allowed to evaporate at ambient temperature. After several days, colorless block crystals were obtained.
2 Experimental details
The crystal data were collected on Rigaku diffractometer. 1 Using Olex2, the structure was solved by SHELXT and refined with SHELXT. 2 , 3 , 4 The H atoms were constrained to ride on their parent atoms.
3 Comment
Benefiting from the tunable design of the coordinated metal ions and organic ligands, this compund exhibits considerable potential for applications in catalysis, radiation detection, fluorescence sensing, gas adsorption/separation etc. 5 , 6 , 7
As shown in picture, the asymmetric unit contains one Zn2+ ion, two MPCA ligands and two water molecules. The Zn1 is six-coordinated by two nitrogens and two oxygens coming from two MPCA ligands, two oxygen atoms coming from water molecules. The bond distances of Zn1–N2, Zn1–N3, Zn1–O2, Zn1–O3, Zn1–O5, Zn1–O6 are 2.181, 2.192, 2.079, 2.071, 2.084, 2.115 Å, falling in the normal range reported in the literature. 8 , 9 , 10 The hydrogen bonds of O–H⋯N, O–H⋯O also exist in this structure and its packing structure is zero-dimensional.
-
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
-
Conflict of interest: The authors declare no conflicts of interest regarding this article.
-
Research funding: This work was funded by scientific research project of Jilin Agricultural Science and Technology College (no. X202511439060).
References
1. Rigaku Corporation. CrysAlisPRO; Rigaku Corporation: Yarnton, Oxfordshire, England, 2015.Search in Google Scholar
2. Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A. K.; Puschmann, H. Olex2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341; https://doi.org/10.1107/s0021889808042726.Search in Google Scholar
3. Sheldrick, G. M. Shelxt – Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. 2015, A71, 3–8; https://doi.org/10.1107/s2053273314026370.Search in Google Scholar PubMed PubMed Central
4. Sheldrick, G. M. Crystal Structure Refinement with Shelxl. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Search in Google Scholar PubMed PubMed Central
5. Deng, C. H.; Wang, Z. T.; Zhao, Z. H.; Lin, W. B. Homochiral BINAPO-Based Metal-Organic Frameworks for Luminescence Sensing of Phenylglycinol Enantiomers. Cryst. Growth Des. 2025, 25, 4360–4366; https://doi.org/10.1021/acs.cgd.5c00249.Search in Google Scholar
6. Liang, C. Y.; Zhang, S. T.; Cheng, L. W.; Xie, J.; Zhai, F. W.; He, Y. H.; Wang, Y. X.; Chai, Z. F.; Wang, S. A. Thermoplastic Membranes Incorporating Semiconductive Metal-Organic Frameworks: An Advance on Flexible X-Ray Detectors. Angew. Chem., Int. Ed. 2020, 59, 11856–11860; https://doi.org/10.1002/anie.202004006.Search in Google Scholar PubMed
7. Tang, S. F.; Hou, X. M. A Highly Stable Dual Functional Zinc Phosphite Carboxylate as Luminescent Sensor of Fe3+ and Cr2O2−7. Cryst. Growth Des. 2019, 19, 45–48; https://doi.org/10.1021/acs.cgd.8b01517.Search in Google Scholar
8. Li, G. M.; Liang, Z. G.; Liu, Y.; Li, J. H.; Pan, J.; Han, S. D. Ligand Tailoring and Functional Group Fusion Strategy for Developing Photochromic Compounds: A Case Study for Pyridinedicarboxylic Acid. Cryst. Growth Des. 2023, 23, 2182–2189; https://doi.org/10.1021/acs.cgd.2c01210.Search in Google Scholar
9. Tanase, S.; van Son, M.; van Albada, G. A.; de Gelder, R.; Bouwman, E.; Reedijk, J. Self-Assembly of Extended Structures Through Non-coordination Intermolecular Forces: Synthesis, Crystal Structures, and Properties of Metal Complexes with 5-Methyl-2-Pyrazinecarboxylate. Polyhedron 2006, 25, 2967–2975; https://doi.org/10.1016/j.poly.2006.04.024.Search in Google Scholar
10. Chapman, C. T.; Ciurtin, D. M.; Smith, M. D.; zur Loye, H. C. A New Mixed-Metal Mn Rh Coordination Polymer Assembled from Mn-Containing Molecular Building Blocks and Rh2(OAc)4 Dimers. Solid State Sci. 2002, 4, 1187. https://doi.org/10.1016/S1293–2558(02)01375–4.10.1016/S1293-2558(02)01375-4Search in Google Scholar
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.

