Abstract
C19H15N3O2, orthorhombic, P212121 (no. 19), a = 8.8676(14) Å, b = 10.8842(17) Å, c = 16.167(3) Å, V = 1560.3(4) Å3, Z = 4, Rgt(F) = 0.0305, wR ref (F2) = 0.0828, T = 296(2) K.
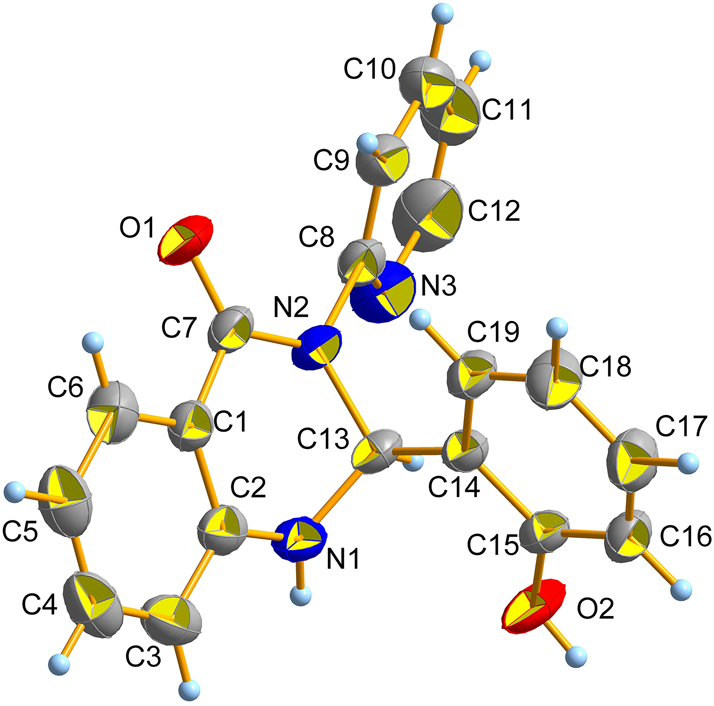
The molecular structure is shown in the figure. Table 1 contains the crystallographic data. The list of the atoms including atomic coordinates and displacement parameters can be found in the cif-file attached to this article.
Data collection and handling.
| Crystal: | Colorless block |
| Size: | 0.46 × 0.41 × 0.35 mm |
| Wavelength: | MoKα radiation (0.71073 Å) |
| μ: | 0.09 mm−1 |
| Diffractometer, scan mode: | Bruker Apex-II, φ and ω-scans |
| θmax, completeness: | 25°, >99 % |
| N(hkl)measured, N(hkl)unique, Rint: | 14903, 2753, 0.016 |
| Criterion for Iobs, N(hkl)gt: | Iobs°>°2σ(Iobs), 2,662 |
| N(param)refined: | 219 |
| Programs: | Bruker programs, 1 Shelx, 2 , 3 Olex2 4 |
1 Source of material
Salicylaldehyde (0.244 g, 2 mmol) and 2-amino-N-(pyridin-2-yl)benzamide (0.426 g, 2 mmol) were dissolved in ethanol (20 mL) and the mixture was refluxed for 6 h. The reaction mixture was concentrated in vacuum, and the title compound was isolated through a silica gel column chromatography (PE:EtOAc = 4:1) as a white solid (0.349 g, 55 % yield). The single crystal of the product was obtained by recrystallization from ethanol through slow evaporation within one week.
2 Experimental details
All hydrogen atoms were identified in difference Fourier syntheses. The Uiso values of the phenolic hydroxyl were set to 1.5 Ueq (C), and the Uiso values of all other hydrogen atoms were set to 1.2 Ueq (C).
3 Discussion
2,3-Dihydroquinazolin-4(1H)-ones have attracted the attention of pharmaceutical chemists due to their diverse biological activities. 5 , 6 Because their structures contain chiral carbon atoms, their structures are also the focus of research. 7 , 8 , 9
Herein, a new 2,3-dihydroquinazolin-4(1H)-one compound was synthesized and characterized by single-crystal X-ray diffraction. 1 , 2 , 3 , 4 The crystal structure reveals that C(13) atom is sp3 hybridized and chiral carbon. Plane I (C1–C6) forms a dihedral angle of 31.813(1)° with plane II (C8–N3), and 89.133(2)° with plane III (C14–C19). The dihedral angle between plane II and plane III is 79.387(2)°. The bond lengths of O(1)–C(7), C(1)–C(7) and C(15)–O(2) are 1.233(2) Å, 1.481(3) Å and 1.361(3) Å. The C(13)–N(1), C(13)–N(2) and C(8)–N(2) bond lengths are 1.446(3) Å, 1.473(2) Å and 1.435(3) Å, which are longer than (C2)–N(1), C(7)–N(2), C(8)–N(3), C(12)–N(3) bond lengths of 1.380(3) Å, 1.359(3) Å, 1.322(3) Å, 1.346(4) Å, respectively.
All geometric parameters are in the xpected ranges. 8 , 9 , 10
-
Research funding: This work was financially supported by the construct program of applied characteristic discipline in Hunan Province.
References
1. Bruker. Apex3, Saint-Plus, Xprep; Bruker AXS Inc.: Madison, Wisconsin, USA, 2016.Search in Google Scholar
2. Sheldrick, G. M. Shelxt – Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. 2015, A71, 3–8; https://doi.org/10.1107/s2053273314026370.Search in Google Scholar PubMed PubMed Central
3. Sheldrick, G. M. Crystal Structure Refinement with Shelxl. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Search in Google Scholar
4. Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A.; Puschmann, H. Olex2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341; https://doi.org/10.1107/s0021889808042726.Search in Google Scholar
5. Shi, D.; Li, Z.; Shi, C.; Zhuang, Q.; Zhang, Y. 3-(4-Bromo-phenyl)-2,2-di-methyl-2,3-Dhydro-quinazolin-4(1H)-one. Acta Cryst. 2004, E60, o1992–o1994; https://doi.org/10.1107/s1600536804025115.Search in Google Scholar
6. Patel, N. B.; Patel, J. C. Synthesis and Antimicrobial Activities of 2-Azetidinyl-4-Quinazolinone Derivatives of Diclofenac Analogue. Med. Chem. Res. 2001, 20, 511–521; https://doi.org/10.1007/s00044-010-9345-y.Search in Google Scholar
7. Amnerkar, N. D.; Bhusari, K. P. Synthesis, Anticonvulsant Activity and 3D-QSAR Study of Some Prop-2-Eneamido and 1-Acetyl-pyrazolin Derivatives of Aminobenzothiazole. Eur. J. Med. Chem. 2010, 45, 149–159; https://doi.org/10.1016/j.ejmech.2009.09.037.Search in Google Scholar PubMed
8. Liu, S.-J.; Zhang, Y.; Yuan, L.; Chen, X.-M. The Crystal Structure of 2,3-Di(pyridin-2-yl)-2,3-dihydroquinazolin-4(1H)-one, C18H14N4O. Z. Kristallogr. NCS 2024, 239, 1001–1002; https://doi.org/10.1515/ncrs-2024-0257.Search in Google Scholar
9. Butcher, R. J.; Jasinski, J. P.; Narayana, B.; Sunil, K.; Yathirajan, H. S. 2-(4-Chlorophenyl)-3-{[(1E)-(4-chlorophenyl) methylene]amino}-2,3-dihydroquinazolin-4(1H)-one. Acta Cryst. 2007, E63, o4025–o4026; https://doi.org/10.1107/s1600536807043632.Search in Google Scholar
10. Memarian, H. R.; Ebrahimi, S.; Rudbari, H. A.; Sabzyan, H.; Nardo, V. M. Inter- and Intramolecular Interactions in 2,3-Dihydroquinazolin-4(1H)-Ones: Molecular Structure and Conformational Analysis. J. Iran. Chem. Soc. 2016, 13, 1395–1404; https://doi.org/10.1007/s13738-016-0854-6.Search in Google Scholar
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.

