Abstract
C24.5H21.5N2O6.25Ni, monoclinic, I2/a (no. 15), a = 13.8298(5) Å, b = 17.5416(4) Å, c = 19.4291(6) Å, β = 108.974(4)°, V = 4457.3(3) Å3, Z = 8, Rgt(F) = 0.0845, wRref(F2) = 0.0889, T = 305(2) K.
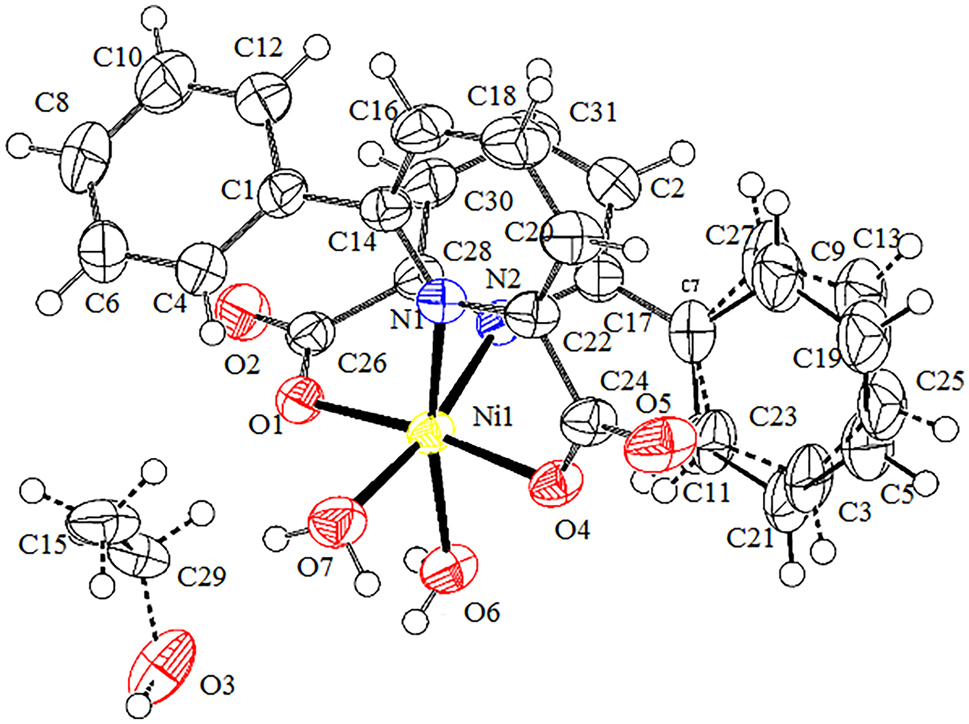
The molecular structure is shown in the figure. Table 1 contains the crystallographic data and the list of the atoms including atomic coordinates and displacement parameters can be found in the cif-file attached to this article.
Data collection and handling.
| Crystal: | Brown block |
| Size: | 0.11 × 0.13 × 0.16 mm |
| Wavelength: | Mo Kα radiation (0.71073 Å) |
| μ: | 0.81 mm−1 |
| Diffractometer, scan mode: | Bruker P4, φ and ω scans |
| θmax, completeness: | 25.1°, 98 % |
| N(hkl)measured, N(hkl)unique, Rint: | 19875, 3907, 0.151 |
| Criterion for Iobs, N(hkl)gt: | Iobs > 2 σ(Iobs), 2,609 |
| N(param)refined: | 307 |
| Programs: | Bruker, 1 Olex2, 2 Shelx, 3 Diamond 4 |
1 Source of materials
All chemicals and solvent in the experiment were used as received. The [bis(6-phenylpyridine-2-carboxylate-κ2N, O)-dihydratenickel(II)] compound was synthesized as following: a mixture of 6-phenylpyridine-2-carboxylic acid (0.25 mmol, 0.0498 g), 2-formylphenoxyacetic acid (0.25 mmol, 0.0451 g), NaOH (0.5 mmol, 0.020 g), and NiCl2·6H2O (0.25 mmol, 0.0594 g) were dissolved in 30 mL 95 % ethanol-water solution (v:v = 2:1) with stirring at room temperature. The mixed solution was stirred at 78 °C for 3 h, after cooling to room temperature, the mixed solution was stirred for 4 h at room temperature. Then the solution was filtered and the filtrate was allowed to stand at room temperature. After slow evaporation of 30 days, the brown crystals of the title compound were obtained. IR (KBr pellet): 3,258 cm−1, 1,603 cm−1 and 1,450 cm−1.
2 Experimental details
The hydrogen atoms were positioned geometrically (C–H = 0.93–0.96 Å, and O–H = 0.85–0.86 Å). Their Uiso values were set to 1.2Ueq or 1.5Ueq of the parent atoms.
3 Comment
The design and construction of metal complexes have attracted interest because of their novel structures 5 as well as wide potential applications as functional materials such as cancer therapy activities, 6 , 7 efficient photocatalysts for organic transformations, 8 electrochemical properties, 9 anticonvulsant and antianxiety activities, 10 magnetic properties, 11 and so on. The choice of the organic ligand is one of the key factors in the synthesis of metal complexes. 6-Phenylpyridine-2-carboxylic acid, as a bifunctional ligand containing pyridine and carboxylate groups, exhibits strong coordination ability and coordination diversity. For example, our group has reported Pb(II), Co(II), Zn(II), Mn(II) and Cd (II) complexes of it alone or with other N-donor ligands (1,10-phenanthroline-1H-pyrazolo[3,4-b]pyridine-3-amine-2,2′-bipyridine). 12 , 13 , 14 , 15 , 16 , 17 In order to continue the study of the coordination behavior of 6-phenylpyridine-2-carboxylic acid ligand, we synthesized and structurally analyzed a new Ni(II) complex, obtained from with NiCl2·6H2O, 2-formylphenoxyacetic acid, 6-phenylpyridine-2-carboxylic acid and NaOH as the starting material. At the beginning, our goal was to synthesize a ternary nickel complex from NiCl2·6H2O, 2-formylphenoxyacetic acid and 6-phenylpyridine-2-carboxylic acid as the raw material. Unfortunately, the 2-formylphenoxyacetic acid ligand did not participate in the coordination. The IR spectrum of Ni(II) complex shows the –OH band at 3258 cm−1, showing that the Ni(II) complex contains water molecules, which is consistent with the results of single crystal analysis. The bands at 1603 cm−1 and 1450 cm−1, show that the O atom of 6-phenylpyridine-2-carboxylate ligand is coordinated to Ni(II) ion. The molecular structure of Ni(II) complex is shown in Figure 1. The structural analysis reveals that the Ni(II) complex consists of one Ni(II) ion, two 6-phenylpyridine-2-carboxylate ligands, two coordinated water molecules, and uncoordinated ethanol molecule (25 % occupied). The Ni(II) ion is six coordinated with two O atoms (Ni1–O1 2.0001(13) Å, and Ni1–O4 2.0194(13) Å) and two N atoms (Ni1–N1 2.1825(15) Å and Ni1–N2 2.1651(15) Å) from two different 6-phenylpyridine-2-carboxylate ligands, two O atoms (Ni1–O6 2.0795(14) Å and Ni1–O7 2.1093(14) Å) from two different coordinated water molecules. The dihedral angles of the pyridine ring 1 (N1–C22–C20–C18–C16–C14–N1) and the benzene ring 1 (C6–C4–C1–C8–C10–C12–C6), and the pyridine ring 2 (N2–C17–C2–C31–C30–C28–N2) and the benzene ring 2 (C7–C11–C21–C5–C19–C9–C7) of 6-phenylpyridine-2-carboxylate ligands are 51.90° and 42.03°, respectively, indicating that the 6-phenylpyridine-2-carboxylate ligands in Ni(II) complex are not coplanar. The Ni(II) ion displays a distorted octahedral coordination geometry, in which the equatorial position is occupied by N1, N2, O6 and O7 atoms, while O1 and O4 atoms occupy the axial position (O1–Ni1–O4 172.84(5)°). The distances of Ni1–N and Ni1–O are in agreement with those of ref. 18]. The Ni(II) complex molecules form a 3D network structure by the hydrogen bonds, and the O⋯H distances are 1.84 Å (O2⋯H6A), 2.01 Å (O5⋯H6B), 2.07 Å (O4⋯H7A), 2.54 Å (O5⋯H7A), and 2.15 Å (O3⋯H7B).
Acknowledgments
This project was supported by the National Natural Science Foundation of China (No. 21171132, https://doi.org/10.13039/501100001809), the Natural Science Foundation of Shandong (ZR2014BL003, https://doi.org/10.13039/501100007129), the Project of Shandong Province Higher Educational Science and Technology Program (J14LC01, https://doi.org/10.13039/501100015642) and Science Foundation of Weifang (2020ZJ1054).
References
1. Bruker. Saint and Sadabs; Bruker AXS Inc.: Madison, Wisconsin, USA, 2000.Search in Google Scholar
2. Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A K.; Puschmann, H. Olex2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Cryst. 2009, 42, 339–341; https://doi.org/10.1107/s0021889808042726.Search in Google Scholar
3. Sheldrick, G. M. Crystal Structure Refinement with Shelxl. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Search in Google Scholar
4. Brandenburg, K. Diamond. Visual Crystal Structure Information System. Ver. 3.2. Crystal Impact: Bonn, Germany, 2012.Search in Google Scholar
5. Xia, Y. P.; Huang, Y. X.; Li, H. H.; Li, X. T.; Zheng, Z. P.; Zhou, F. Mechanochemical-Assisted Synthesis of a Novel Sandwichtructured Coronene/Trinuclear Silver(I) Pyrazolate Donor-Acceptor Cocrystal: Structure, Spectroscopic, Hirshfeld Surfaces and DFT. J. Mol. Struct. 2025, 1340, 142579; https://doi.org/10.1016/j.molstruc.2025.142579.Search in Google Scholar
6. Cao, S. H.; Li, X. Z.; Gao, Y.; Li, F. H.; Li, K. X.; Cao, X. X.; Dai, Y. W.; Mao, L. R.; Wang, S. S.; Tai, X. S. A Simultaneously GSH-Depleted Bimetallic Cu(II) Complex for Enhanced Chemodynamic Cancer Therapy. Dalton T. 2020, 49, 11851–11858; https://doi.org/10.1039/d0dt01742f.Search in Google Scholar PubMed
7. Wang, L. H.; Tai, X. S.; Azam, M.; Sui, B. L.; Wang, A. L. Synthesis, Structural Characterization, and Hirschfeld Surface Analysis of a Novel Mn(II) Complex Based on N-Acetyl-L-phenylalanine Ligand and its Evaluation as a Cytotoxic. Polyhedron 2025, 279, 117659; https://doi.org/10.1016/j.poly.2025.117659.Search in Google Scholar
8. Zeng, Y.; Zhang, Y. J.; Luo, J. W.; Nie, J. Q. 2,6-Bis (1,2,3-Triazol-4-Yl) Pyridine Ruthenium(II) Complex Embedded Porous Organic Polymers as Efficient Photocatalysts for Organic Transformations.New J. Chem. 2024, 48, 8827–8833; https://doi.org/10.1039/d4nj00810c.Search in Google Scholar
9. Balch, A.-L.; Winkler, K. Electrochemistry of Fullerene/Transition Metal Complexes: Three Decades of Progress. Coord. Chem. Rev. 2021, 438, 213623; https://doi.org/10.1016/j.ccr.2020.213623.Search in Google Scholar
10. Singh, A.; Kumar, R.; Shiv, K.; Pandey, S. K.; Bharty, M. K.; Butcher, R. J.; PrasadLiang, L. B. Synthesis, Crystal Structure and Screening for Anticonvulsant and Antianxiety Activities of Three New Ni(II), Cu(II), and Zn(II) Dithiocarbamate Complexes. J. Mol. Struct. 2024, 1298, 137052; https://doi.org/10.1016/j.molstruc.2023.137052.Search in Google Scholar
11. Zhang, X. Q.; Zhou, C. C.; Wen, Q.; Che, J. L.; Song, J. F.; Liu, H. F. Synthesis, Crystal Structures and Magnetic Properties of Pyrazole–Carboxylic Acid Complexes. J. Chem. Res. 2023, 47, 1–10; https://doi.org/10.1177/17475198231183323.Search in Google Scholar
12. Tai, X.-S.; Liang, L.; Li, X.-T.; Cao, S.-H.; Wang, L.-H. Crystal Structure of Diaqua-bis(μ2-6-phenylpyridine-2-carboxylate-κ3N,O:O)-Bis(6-Phenylpyridine-2-Carboxylato-k2N,O)Lead(II)-N,N-Dimethylformamide-Water (1/2/4), C54H58N6O16Pb2. Z. Kristallogr.-N. Cryst. Struct. 2021, 236, 1199–1201; https://doi.org/10.1515/ncrs-2021-0277.Search in Google Scholar
13. Tai, X.-S.; Wang, Z.-J.; Ouyang, J.; Li, Y.-F.; Zhang, W.; Jia, W.-L.; Wang, L.-H. The Crystal Structure of [(Phenantroline-κ2N, N)-bis(6-phenylpyridine-2-carboxylate-κ2N,O)cobalt(II)] Monohydrate, C36H26N4O5Co. Z. Kristallogr.-N. Cryst. Struct. 2021, 236, 1309–1311; https://doi.org/10.1515/ncrs-2021-0319.Search in Google Scholar
14. Wang, L.-H.; Liang, L.; Li, X.-T.; Cao, S.-H.; Tai, X.-S. The Crystal Structure of Bis(6-Phenylpyridine-2-carboxylato-κ2N,O)copper(II), C24H16N2O4Cu. Z. Kristallogr.-N. Cryst. Struct. 2021, 236, 1251–1253.10.1515/ncrs-2021-0293Search in Google Scholar
15. Wang, L.-H.; Wang, Z.-J.; Ouyang, J.; Tai, X.-S. The Crystal Structure of Bis(6-Phenylpyridine-2-carboxylate-κ2N,O)-(2,2′-bipyridine-κ2N,N) Zinc(II) Monohydrate, C34H26N4O5Zn. Z. Kristallogr.-N. Cryst. Struct. 2021, 236, 1297–1299; https://doi.org/10.1515/ncrs-2021-0312.Search in Google Scholar
16. Tai, X.-S.; Zhang, L.-L.; Liu, L.-L.; Cao, S.-H.; Wang, L.-H. The Crystal Structure of Bis(ethanol-κ1O)-bis(6-aminopicolinato-κ2N,O)manganese(II), C16H22O6N4Mn. Z. Kristallogr.-N. Cryst. Struct. 2021, 236, 551–552; https://doi.org/10.1515/ncrs-2020-0644.Search in Google Scholar
17. Tai, X. S.; Wang, L. H.; Al–Resayes, S. I.; Azam, M. Synthesis, Structural Characterization, Hirschfeld Surface Analysis and Catalytic Application of a New Cd(II) Complex Bearing 1H-pyrazolo[3,4-b] pyridine-3-amine and Pyridine carboxylic acid. Polyhedron 2025, 279, 117647; https://doi.org/10.1016/j.poly.2025.117647.Search in Google Scholar
18. Zhou, T. T.; Cao, Z. The Crystal Structure of [(2,2-Bipyridine-κ2N,N)-bis(6-phenylpyridine-2-carboxylato-κ2N,O)nickel(II)] Monohydrate, C34H26N4O5Ni. Z. Kristallogr.-N. Cryst. Struct. 2022, 237, 1079–1081.10.1515/ncrs-2022-0361Search in Google Scholar
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.

