Abstract
C22H20ClNO3, monoclinic, P21/n (no. 14), a = 9.3891(4) Å, b = 11.0821(4) Å, c = 17.4662(5) Å, β = 98.361(3)°; V = 1798.06 Å3, Z = 4, R gt (F) = 0.0361, wR ref (F 2) = 0.0911, T = 100 K.
Table 1 contains the crystallographic data and the list of the atoms including atomic coordinates and displacement parameters can be found in the cif-file attached to this article.
Data collection and handling.
| Crystal: | Colourless block |
| Size: | 0.20 × 0.17 × 0.15 mm |
| Wavelength: μ: |
Mo Kα radiation (0.71073 Å) 0.24 mm−1 |
| Diffractometer, scan mode: θ max, completeness: |
Synergy R, ω scans 30.6°, 100 % |
| N(hkl)measured, N(hkl)unique, R int: | 12377, 4515, 0.029 |
| Criterion for I obs, N(hkl)gt: | I obs > 2σ(I obs), 3,778 |
| N(param)refined: | 246 |
| Programs: | Rigaku, 1 Olex2, 2 SHELX, 3 , 4 Diamond 5 |
1 Source of material
The title compound, (E)-4-chloro-7-(diethylamino)-3-(3-oxo-3-phenylprop-1-en-1-yl)-2H-chromen-2-one, was synthesized according to the literature method with a slight modification. 6 4-Chloro-7-(diethylamino)-3-formyl-2H-chromen-2-one (1.4 g, 5 mmol) and 2-(triphenylphosphoranylidene)aceto-phenone (2.0 g, 5.3 mmol) were dissolved in anhydrous dichloromethane (60 mL) and stirred for 24 h. The reaction progress was monitored by thin-layer chromatography (TLC). When the TLC analysis indicated the complete consumption of 4-chloro-7-(diethylamino)-3-formyl-2H-chromen-2-one, the reaction was quenched with distilled water (50 mL) and then it was extracted with dichloromethane (50 mL × 3), washed with distilled water (50 mL × 3). The collected organic phase was dried over MgSO4. The volatile component was evaporated under reduced pressure. The residue was purified by column chromatography on silica gel (200–300 mesh), using dichloromethane as the eluent. The single crystal growth was carried in saturated ethanol and the solution was sealed with parafilm in a 20 mL vial. The holes on the parafilm cover was used to control a slow solvent evaporation speed. Finally, regular shaped crystals can be found for X-ray diffraction data collection.
2 Experimental details
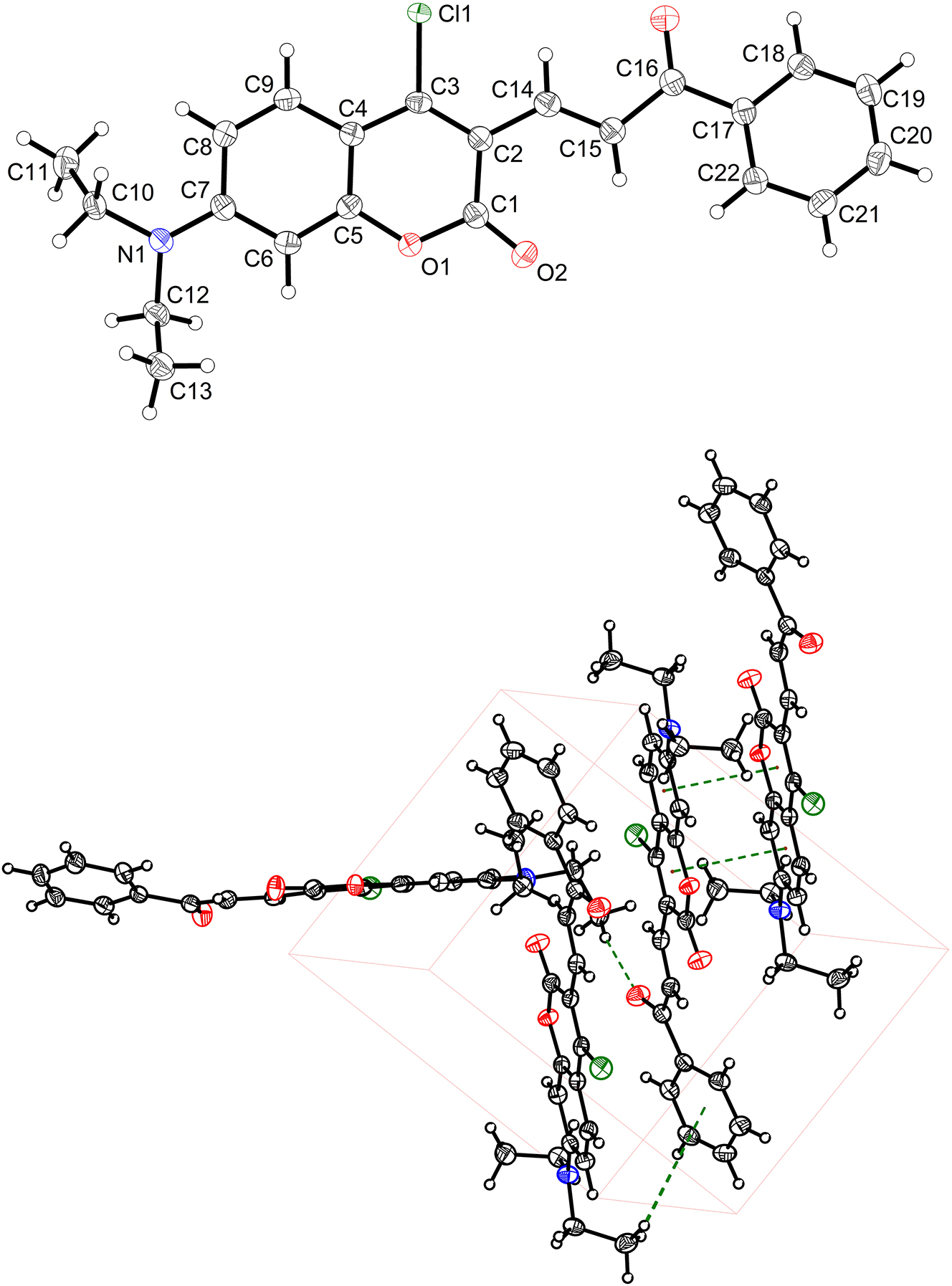
The single-crystal X-ray diffraction measurement was carried out on a XtaLAB Synergy R, DW system, HyPix diffractometer. Using Olex2, 2 the structure was solved with the SHELXT 3 structure solution program and refined with the SHELXL 4 refinement package. Hydrogen atoms attached to C atoms were placed geometrically and refined using a riding model approximation, assigned isotropic thermal parameters with d (C–H) = 0.93, 0.96 or 0.97 Å (–CH, –CH 3, and –CH 2). U iso(H) = 1.2 U eq(C) for CH and U iso(H) = 1.5 U eq(C) for CH3 and CH2 groups. The structure was examined using the ADDSYM subroutine of PLATON to ensure that no additional symmetry could be applied to the models. The molecular graphics were drawn using DIAMOND software with 50 % probability ellipsoids in the figure (top). 5
3 Comment
The title compound is a coumarin-containing fluorescent dye, which can emit bright blue fluorescence in crystal state when irradiated with 365 nm UV light. Coumarin, naphthalimide, and BODIPY are of the typical fluorescent moiety in molecular engineering and has been widely used in dye configuration aiming for the practical application in chemsensors and biological labeling. 7 , 8 , 9 , 10 In the title dye structure, the build-in NEt2 and carbonyl serve as electron acceptor and donor, respectively. This intramolecular electron push-pull, together with the π system, could be applied in dye molecular construction. 11 , 12 Based on this molecule designing strategy, highly fluorescent dyes can be developed based on the framework of coumarin for biological purpose or device fabrication. 13 , 14 , 15 , 16
In the title crystal structure, the asymmetric unit contains only one molecule. The coumarin framework (C1/C2/C3/C4/C5/C6/C7/C8/C9/O1/O2) is planar with the root mean square error (RMSD) in distance estimated to be 0.031 Å. The carbonyl (C16=O3) is estimated 1.228 Å, having the typical character of double bond. At the same time, the bond length of C14–C15 (α and β carbon, bond length 1.347 Å) is shorter than the regular C–C single bond, having the partial double bond character. Therefore, the coumarin is conjugated with the carbonyl (C16=O3) in some degree. The two carbonyl groups (C16=O3 and C1=O2) both are electron acceptors in the dye structure. On the one hand, the geometric configuration of (NEt2) does not construct the regular trigonal pyramid geometry. On the contrary, it becomes coplanar with the RMSD 0.001 Å. And the planar (N1/C7/C10/C12) is twisted off the coumarin with the angle 8.6°. On the other hand, the bond length of N1–C7 (1.359 Å) is shorter than that of N1–C10 (1.464 Å) and N1–C12 (1.470 Å). It indicates that the lone pair electron of N1 partially overlapped with the π electron of coumarin, which promotes the electron-donating character towards the coumarin π system. The electron donor (NEt2) and electron acceptor carbonyl group (C16=O3 and C1=O2) configures the strong intramolecular electron “push-pull” effect, which determines the emission performance of fluorescent dyes. The push-pull dye system induced intramolecular charge transfer and excited-state intramolecular proton transfer are commonly employed to modulate the emission wavelength. 17 , 18 , 19 , 20 , 21 In comparison with its analogue, the bond length and geometric configuration of the coumarin and the attached nitrogen atom are identical to each other. In our case, the two ethyl groups anchored to the N twisted in opposite direction, while they were fixed in the same side of coumarin plane, induced by the intermolecular weak interactions. 22 The strong π⋯π interaction assembles the molecules parallel to each other (figure bottom). 23 , 24 The distance between neighboring parallel coumarin planes is determined to be 3.556 Å and the parallel shift is 0.671 Å, corresponding to the parallel-displaced geometry. 25 , 26 The C–H⋯π interactions also contribute to the crystal formation. The hydrogen atom H11A i1 (i1: 1 − x, 1 − y, 1 − z) locates above the carbon ring (C17/C18/C19/C20/C21/C22) with the H11A-centroid distance 3.737 Å. Also, there exists multiple hydrogen bonds in the crystal. 27 , 28 The typical one is the C11–H11c i2⋯O3 (i2: 1/2 + x, 1/2 − y, −1/2 + z) with the C⋯O distance being 2.535 Å and the C–H⋯O angle 167.8°. Introducing reasonable intermolecular interactions can be effectively modulating the crystal emission behavior. 29 , 30 , 31 , 32 Super srong intermolecular interactions will quench the emission in solid state. 33 , 34 , 35 , 36
In the crystal lattice, the dye molecules are regularly packed by the intermolecular force, including C–H⋯π, π⋯π interactions and multiple hydrogen bonods. Both the bond lengths and the angles are in the expected ranges. The driving force that packs the dye molecules parallel to each other is based on the stronger π⋯π interaction. 37 , 38 The hydrogen bonds and C–H⋯π join the paralleled molecules side by side to form the network. 36 , 39 , 40
Acknowledgments
The authors appreciates the finical supporting by the Application Research Program for Key Scientific Research Projects in Henan Provincial Higher Education Institutions (No. 25B150014).
References
1. Oxford Diffraction Ltd. CrysAlisPRO: Abingdon, Oxfordshire, England, 2006.Search in Google Scholar
2. Dolomanov, O. V.; Bourhis, L. J.; Gildea, R. J.; Howard, J. A. K.; Puschmann, H. Olex2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341; https://doi.org/10.1107/s0021889808042726.Search in Google Scholar
3. Sheldrick, G. M. Shelxtl–Integrated Space–Group and Crystal–Structure Determination. Acta Crystallogr. 2015, A71, 3–8; https://doi.org/10.1107/S2053273314026370.Search in Google Scholar PubMed PubMed Central
4. Sheldrick, G. M. Crystal Structure Refinement with Shelxl. Acta Crystallogr. 2015, C71, 3–8; https://doi.org/10.1107/s2053229614024218.Search in Google Scholar
5. Brandenburg, K. DIAMOND. Visual Crystal Structure Information System. Ver. 4.0; Crystal Impact: Bonn, Germany, 2015.Search in Google Scholar
6. Bochkov, A. Y.; Akchurin, I. O. A.; Dyachenko, O. A.; Traven, V. F. NIR–Fluorescent Coumarin–Fused BODIPY Dyes with Large Stokes Shifts. Chem. Commun. 2013, 49, 11653–11655; https://doi.org/10.1039/c3cc46498a.Search in Google Scholar PubMed
7. Li, X.; Han, Y.; Min, K.; Son, Y. A. Configuration of White Light Emission by Courmarin and Naphthalimide. Mol. Cryst. Liq. Cryst. 2018, 660, 10–16; https://doi.org/10.1080/15421406.2018.1452861.Search in Google Scholar
8. Liu, Y.; Li, X.; Min, K. S.; Son, Y. A. Emission Behavior of Naphthalimide-Coumarin Cassette. Mol. Cryst. Liq. Cryst. 2018, 662, 139–146; https://doi.org/10.1080/15421406.2018.1466533.Search in Google Scholar
9. Li, X.; Zou, Y.; Heo, G.; Son, Y. A. Emission Shift of an Imidazole Bridged Diethylaminocoumarin and Diphenyl. Mol. Cryst. Liq. Cryst. 2020, 704, 48–56; https://doi.org/10.1080/15421406.2020.1741801.Search in Google Scholar
10. Li, X.; Liu, X.; Li, F. Configuration of Super-Fast Cu2+-Responsive Chemosensor by Attaching Diaminomaleonitrile to BODIPY Scaffold for High-Contrast Fluorescence Imaging of Living Cells. Spectrochim. Acta, Part A 2024, 304, 123377; https://doi.org/10.1016/j.saa.2023.123377.Search in Google Scholar PubMed
11. Li, X.; Han, Y.; Kim, M. J.; Son, Y. A. A BODIPY-Based Highly Emissive Dye with Thiophene-Based Branch Harvesting the Light. Mol. Cryst. Liq. Cryst. 2018, 662, 157–164; https://doi.org/10.1080/15421406.2018.1467613.Search in Google Scholar
12. Li, X.; Liao, M.; Sun, J.; Heo, G.; Son, Y. A. Thiophene Modulated BODIPY Dye as a Light Harvester. Mol. Cryst. Liq. Cryst. 2019, 679, 127–136; https://doi.org/10.1080/15421406.2019.1597557.Search in Google Scholar
13. Zheng, X.; Liu, X.; Liu, L.; Li, X.; Jiang, S.; Niu, C.; Xie, P.; Liu, G.; Cao, Z.; Ren, Y.; Qin, Y.; Wang, J. Multi-Stimuli-Induced Mechanical Bending and Reversible Fluorescence Switching in a Single Organic Crystal. Angew. Chem., Int. Ed. 2022, 61, e202113073; https://doi.org/10.1002/anie.202113073.Search in Google Scholar PubMed
14. Li, X.; Han, Y.; Sun, S.; Shan, D.; Ma, X.; He, G.; Mergu, N.; Park, J.-S.; Kim, C.-H.; Son, Y.-A. A Diaminomaleonitrile-Appended BODIPY Chemosensor for the Selective Detection of Cu2+ via Oxidative Cyclization and Imaging in SiHa Cells and Zebrafish. Spectrochim. Acta, Part A 2020, 233, 118179; https://doi.org/10.1016/j.saa.2020.118179.Search in Google Scholar PubMed
15. Li, X.; Li, F.; Ji, G. A Fluorescent Turn-On Sensor Toward Multiple Heavy Metal Ions Based on meso-Anisole Modified BODIPY Scaffold. J. Fluoresc. 2023, 33, 631–637; https://doi.org/10.1007/s10895-022-03110-1.Search in Google Scholar PubMed
16. Li, X.; Liu, X. A Sensitive Probe of Meso-cyanophenyl Substituted BODIPY Derivative as Fluorescent Chemosensor for the Detection of Multiple Heavy Metal Ions. J. Fluoresc. 2025, 35, 1089–1098; https://doi.org/10.1007/s10895-024-03581-4.Search in Google Scholar PubMed
17. Xie, P.; Zhou, Y.; Li, X.; Liu, X.; Liu, L.; Cao, Z.; Wang, J.; Zheng, X. Strong Dual-State Emission of Unsymmetrical and Symmetrical Thiazolothiazole-Bridged Imidazolium Salts. Chin. Chem. Lett. 2023, 34, 107582; https://doi.org/10.1016/j.cclet.2022.06.005.Search in Google Scholar
18. Li, X.; Yao, C.; Jiang, W. Emission and Energy Transfer Investigation of Non-conjugated Total Carbon Configuration Between BODIPY and Naphthalimide. J. Chem. Sci. 2023, 135, 65; https://doi.org/10.1007/s12039-023-02181-2.Search in Google Scholar
19. Li, X.; Zhou, Q.; Heo, G.; Son, Y. A. 2,4-Dimethylpyrrole Configured Fluorine-Boron Complexes. Mol. Cryst. Liq. Cryst. 2018, 677, 34–41; https://doi.org/10.1080/15421406.2019.1597509.Search in Google Scholar
20. Kong, Y.; Liu, X.; Jiang, W.; Li, X. Photochromic Properties of Triangle Terthiophene- and Triphenylamine-Configured Diarylethene Type Dye with Propeller-Like Conformation. Chem. Pap. 2025, 79, 2401–2409; https://doi.org/10.1007/s11696-025-03934-8.Search in Google Scholar
21. Li, X.; Guo, X.; Chen, Y.; Cui, T.; Xing, L. Double 3-Ethyl-2,4-dimethylpyrrole Configured Fluorescent Dye with Fluorine-Boron as the Bridge. J. Fluoresc. 2021, 31, 1797–1803; https://doi.org/10.1007/s10895-021-02819-9.Search in Google Scholar PubMed
22. Liu, T.; Yue, Y.; Zhai, Y.; Guo, Z.; Zhao, W.; Yang, X.; Chen, D.; Yin, C. Host–Guest Type Multiple Site Fluorescent Probe for GSH Detection in Living Organisms. Chem. Commun. 2021, 57, 13764–13767; https://doi.org/10.1039/d1cc05494e.Search in Google Scholar PubMed
23. Ji, G.; Hou, Q.; Jiang, W.; Li, X. Investigating the Properties of Double Triangle Terthiophene Configured Dumbbell-Like Photochromic Dye with Ethyne and 1,3-Butadiene Bridge. J. Fluoresc. 2023, 33, 1495–1503; https://doi.org/10.1007/s10895-023-03171-w.Search in Google Scholar PubMed
24. Ji, G.; Hou, Q.; Jiang, W.; Li, X. Investigating the Properties of Triangle Terthiophene and Triphenylamine Configured Propeller‑Like Photochromic Dye with Ethyne Bridge. J. Fluoresc. 2025, 35, 933–941.10.1007/s10895-023-03557-wSearch in Google Scholar PubMed
25. Li, X.; Han, Y.; Kim, M. J.; Son, Y. A. Reversed Photochromism Reactivity of Malononitrile Attached Bisthienylthene. Mol. Cryst. Liq. Cryst. 2018, 662, 147–156; https://doi.org/10.1080/15421406.2018.1466534.Search in Google Scholar
26. Qin, X.; Li, H.; Wang, Y.; Li, Y.; Li, X. Conjugated Iminodibenzyl Dyes Incorporating Phenolic Hydroxyl Group and Strong Electron Donating or Accepting Groups for Facilitating ESIPT and Proton Transfer in Six‑ or Seven‑Membered Cycles. J. Fluoresc. 2025, https://doi.org/10.1007/s10895-025-04285-z, In press.Search in Google Scholar PubMed
27. Li, X.; Cai, Q.; Zhang, J.; Kim, H.; Son, Y. A. An ”Electron Lock” Toward the Photochromic Activity of Phenylacetylene Appended Bisthienylethene. Mol. Cryst. Liq. Cryst. 2020, 706, 141–149; https://doi.org/10.1080/15421406.2020.1743450.Search in Google Scholar
28. Li, X.; Wang, Y.; Jia, C.; Kim, H.; Son, Y. A. Photochromic Reactivity Induced by Electron Distribution: Active or Inactive. Mol. Cryst. Liq. Cryst. 2019, 689, 83–91; https://doi.org/10.1080/15421406.2019.1597556.Search in Google Scholar
29. Zheng, X.; Wang, G.; Liu, L.; Li, X.; Xie, P.; Fan, Y.; Cao, Z.; Niu, C.; Tian, D.; Xie, L. Hydrogen Bonding-Induced Multicolor and Thermochromic Emissions of Triphenylamines. Chem. –Eur. J. 2025, 31, e202500643. https://doi.org/10.1002/chem.202500643.Search in Google Scholar PubMed
30. Liu, Y.; Li, X.; Kim, H.; Son, Y. A. Investigation of Fluorescent Optical Properties of Fluorine-Boron Cored Dye. Mol. Cryst. Liq. Cryst. 2018, 677, 27–33; https://doi.org/10.1080/15421406.2019.1597508.Search in Google Scholar
31. He, W.; Li, X.; Kim, H.; Son, Y. A. Shifting the Emission of Proton Transfer Fluorescence with Fluorine-Boron as the Rotation Lock. Mol. Cryst. Liq. Cryst. 2020, 704, 41–47; https://doi.org/10.1080/15421406.2020.1741800.Search in Google Scholar
32. Li, X.; Tian, G.; Shao, D.; Xu, Y.; Wang, Y.; Ji, G.; Ryu, J.; Son, Y. A. A BODIPY Based Emission Signal Turn-On Probe Toward Multiple Heavy Metals. Mol. Cryst. Liq. Cryst. 2020, 706, 38–46; https://doi.org/10.1080/15421406.2020.1743436.Search in Google Scholar
33. Li, X.; Qian, Q.; Jiang, W. Photo-Induced Fluorochromism of a Star-Shaped Photochromic Dye with 2,4-Dimethylthiazole Attaching to Triangle Terthiophene. J. Fluoresc. 2023, 33, 1907–1915; https://doi.org/10.1007/s10895-023-03196-1.Search in Google Scholar PubMed
34. Liu, Y.; Li, X.; Min, K. S.; Son, Y. A. Highly Fluorescent Response of 4-(2,5-Dimethylthiophen-3-yl)-2-hydroxyphenylbenzothiazole Toward BF3٠Et2O and Zn2+. Mol. Cryst. Liq. Cryst. 2018, 662, 132–138; https://doi.org/10.1080/15421406.2018.1466531.Search in Google Scholar
35. Ji, G.; Hou, Q.; Zhang, J.; Li, X. Investigation of Triangle Terthiophene and Hydroxyphenylbenzothiazole Configured Fluorescent Dye with a Triple Bond Bridge. J. Fluoresc. 2023, 33, 153–159; https://doi.org/10.1007/s10895-022-03049-3.Search in Google Scholar PubMed
36. Liu, Y.; Li, X.; Sun, S.; Ji, G.; Son, Y. A. Crystal Structure of 2,7-Diiodo-1,3,6,8-Tetramethyl-bis(difluoroboron)-1,2-bis((1H-pyrrol-2-yl)methylene)hydrazine, C14H14B2F4I2N2. Z. fur Krist. -New Cryst. Struct. 2020, 235, 371–372; https://doi.org/10.1515/ncrs-2019-0678.Search in Google Scholar
37. Li, Y.; Liu, K.; Zhang, W.; Wang, W.; Wang, B.; Wang, Y.; Li, X. Two 3D Ln(III)‑MOFs Based on Phosphineoxide Ligand: Synthesis, Structure Luminescent and Photocatalytic Properties. J. Fluoresc. 2023, 33, 2119–2129; https://doi.org/10.1007/s10895-023-03218-y.Search in Google Scholar PubMed
38. Cao, Z.; Yang, F.; Wu, D.; Wu, L.; Liu, L; Liu, G.; Li, X.; Zheng, X.; Zheng, X.; Qu, D. Supramolecular Aggregates Constructed by Pillar[5]arene-Based Host–Guest Interaction with Aggregation-Induced Emission. Poly. Chem. 2023, 14, 1318–1322; https://doi.org/10.1039/d3py00026e.Search in Google Scholar
39. He, W.; Liu, Y.; Sun, S.; Ji, G.; Li, X. Crystal Structure of 2–Bromo-1,3,6,8-tetramethylBOPHY (BOPHY = bis(difluoroboron)-1,2-bis((1H-pyrrol-2-yl)methylene)hydrazine), C14H15B2BrF4N4. Z. Kristallogr. -New Cryst. Struct. 2021, 236, 949–952; https://doi.org/10.1515/ncrs-2021-0163.Search in Google Scholar
40. Liu, Y.; Sun, S.; Ji, G.; Li, X.; Son, Y. A. Crystal Structure of 2–Phenylethynyl-1,3,6,8-tetramethylBOPHY (BOPHY = bis(difluoroboron)-1,2-bis((1H-pyrrol-2-yl)methylene) Hydrazine), C22H20B2F4N4. Z. Kristallogr. -New Cryst. Struct. 2021, 236, 749–752; https://doi.org/10.1515/ncrs-2021-0074.Search in Google Scholar
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.

