Abstract
Natural products, the most important chemical library with magical structures and unique functions, have long been playing significant roles in contributing to the discovery of novel drugs. The complexity and diversity of natural products present great challenges regarding the exploration of their potential targets. Identifying the targets of natural products not only enhances our understanding of biological functions and molecular mechanisms, but also paves the way for discovering novel lead compounds for disease treatment. Recent advances in technologies like chemical biology, structural biology, and artificial intelligence have provided powerful tools for pinpointing natural product target and unraveling molecular mechanisms. This review aims to comprehensively summarize the innovative strategies employed in recent years to identify natural product targets, and evaluate their impact on biological pathways by modulating target functions for pharmacological effects. Moreover, we also discuss the challenges encountered in this field and outline future research prospects, aiming to offer guidance for researchers in natural product chemical biology.
Introduction
Drug targets are essential in the development of new medications and diseases treatment. Hence, the identification of drug targets is critical for understanding complex pharmacological mechanisms and guiding precision medicine in clinical practice. Natural products have emerged as a significant source of novel drugs due to their structural diversity and unique molecular mechanisms. Approximately half of the small molecule drugs approved by the Food and Drug Administration (FDA) in the United States are either directly or indirectly derived from natural source. In contrast to chemically synthesized small molecules that are specifically designed to target, natural products frequently lack well-defined targets owing to their diverse chemical structures. Thus, the complexity of natural products poses great challenges for exploring the molecular targets and pharmacological mechanisms, thereby hindering the in-depth innovation of drug development.
Recent advancements in chemical biology, molecular biology, structural biology, as well as artificial intelligence have led to the development of diverse methodologies for identifying the targets of natural products. These methods have been widely utilized to discover the targets of natural compounds, as well as medicinal plant extracts or traditional formulations, providing crucial insights for exploration of cellular signaling pathways. For instance, chemical labeling technology was initially employed to investigate the natural product Kongensin A, leading to the identification of the HSP90 as a crucial molecular target responsible for the anti-necroptosis activity [1]. Subsequently, the research demonstrates that the Kongensin A exerts the anti-necroptosis effect through targeting HSP90 in the RIP3-dependent necroptosis signaling pathway. Thus, it indicates that the identification of cellular targets is highly instructive in unraveling the pharmacological mechanisms of natural products. Currently, several reviews have been published on the topic of target identification for natural products. For instance, Zhou et al. investigated methods based on chemical proteomics to identify molecular targets [2]. Dong et al. summarized label-free target identification methods for natural product through proteomics [3]. Furthermore, Pichler et al. presented an overview of natural product target identification using activity-based protein profiling (ABPP) [4]. This review offers a comprehensive summary of recent advancements in methodologies for identifying targets of natural products. These state-of-the-art strategies will greatly enhance the comprehension of the pharmacological mechanisms of natural products. Additionally, our objective is to discuss current research obstacles and offer guidance to researchers in the fields of pharmacology, chemical biology, and natural product chemistry, emphasizing the promotion of further technological advancements.
Methodologies for target identification of natural products
Currently, the methodologies for identifying targets of natural products generally fall into two categories: chemical labeling and label-free strategies. Chemical labeling strategy integrates chemical labels into the structure of natural products, allowing for the selective enrichment of direct binding proteins for target identification via mass spectrometry. In contrast, label-free strategy is primarily dependent on alterations in biophysical characteristics, such as the thermal and enzymatic stability of target proteins following their interaction with natural products. In addition, these two approaches can also be integrated with quantitative proteomic methodologies to broaden their utility and thus identify a wider array of low-abundance target proteins. This section provides a systematic overview of current strategies for identifying the targets of natural products as depicted in Figure 1, including the clarification of principles and illustrative cases.

Methodologies for target identification of natural products.
Chemical labeling-based target identification of natural products
The strategy for target identification based on chemical labeling involves incorporating specific labels (such as biotin, diazide, or alkynyl) into the chemical structure of natural products. Natural products possess a distinctive affinity for target proteins within cellular or tissue lysates, where the labels interact with a solid-phase carrier through biotin-avidin or click chemistry. This process facilitates the enrichment of the target proteins on solid-phase carrier for subsequent identification. Meanwhile, in recent years, there has been a surge in the use of natural products-based proteolysis-targeting chimeras (PROTACs) for target identification. Additionally, ultraviolet light-induced photoaffinity labeling technology has emerged as a promising strategy for elucidating targets of complex natural extracts. These innovative strategies have provided valuable insights for potential targets and molecular mechanisms of natural products, thereby broadening opportunities for disease treatment.
Biotin-labeled strategy for target identification
This approach involves utilizing functional groups (e.g., -OH, -NH2, -COOH) in natural products to synthesize a biotin-labeled probe for target identification. The biotin-labeled probes are immobilized on the surface of streptavidin-coated solid-phase beads, thereby enabling the selective enrichment of binding proteins from cellular or tissue lysates. The captured proteins are subsequently subjected to separation through gel electrophoresis and analyzed by mass spectrometry (Figure 2). Currently, biotin-labeled probes are extensively utilized for the target identification of natural products and have achieved a relatively advanced stage of development.
For instance, biotin-labeled adenanthin has identified the peroxiredoxin I/II (Prdx I/II) as the target proteins for its anti-leukemia activity [5]. Moreover, pull-down assay with biotin-conjugated artesunate (bio-ATS) demonstrates that artesunate improves the phenotype of polycystic ovary syndrome by targeting the mitochondrial protease LONP1 [6]. Likewise, the biotin-conjugated bruceine A suggests that the inhibition of the galectin-1 protein-mediated inflammatory pathway contributes to the therapeutic effect against diabetic kidney disease [7]. Meanwhile, the construction of a biotin-labeled ainsliadimer A facilitates the identification of the target protein IKKα/β for anti-inflammatory properties [8]. Additionally, biotin-labeled probe also contributes to the identification of extracellular-signal-regulated kinase 2 (ERK2) as the molecular target of natural product strictosamide for anti-inflammatory effect [9].
The development of Stable Isotope Labeling with Amino Acids in Cell Culture (SILAC) technology has provided a crucial platform for identifying low-abundance target proteins (Figure 3). This strategy involves the utilization of essential amino acids labeled with natural isotopes (light labeling) and stable isotopes (heavy labeling) to substitute the corresponding amino acids in the cell culture medium. As a result, stable isotope labeled amino acids (e.g., 13C and 15N-labeled arginine or lysine) are integrated into newly synthesized proteins in cells, facilitating accurate quantification through mass spectrometry. For target identification, cell lysates labeled with light isotopes are used to capture the targets using the biotin-labeled probe, while cell lysates labeled with heavy isotopes are employed for competitive analysis by introducing free molecules. Subsequently, the proteins with significant variations are often considered as potential targets by quantitative proteomics analysis [10].

Target identification via biotin-labeled Strategy.
In recent years, the SILAC-based quantitative proteomics technology has been utilized for the target identification of CDK8 and CDK19 for natural compound CCT251545 [11]. Furthermore, the SILAC-coupled with LC-MS plays a crucial role in uncovering anti-silencing factor 1a (ASF1a) as a cellular target of artone [12]. Similarly, the application of this approach leads to the successfully discovery of peroxiredoxin 6 (PRDX6) as a target for withangulatin A (WA) [13].
Biotin-labeled probes are commonly used in conjunction with protein chip technology for target identification. Protein chips, also known as protein microarrays, are effective tools for high-throughput analysis of protein-ligand interactions, such as protein-protein, protein-nucleic acid, or protein-small molecule. The procedure involves immobilizing recombinant proteins onto the surface of a microarray, followed by co-incubation with biotin-labeled probes. Subsequently, avidin-labeled fluorescence markers are attached to the probes, enabling the tracking of target protein location on the chip for target identification (Figure 4). For instance, hexokinase 2 (HK2) has been identified as a critical target for leukemia therapy using a biotin-labeled arsenic probe and a human protein array chip [14]. Furthermore, the integration of protein chip with surface plasmon resonance strategy has elucidated that geniposide inhibits the progression of hepatocellular carcinoma by specifically targeting TLR4 [15]. Similarly, protein chip technology has also been utilized to identify peroxiredoxin 2 as a potential target of celastrol against gastric cancer [16].

Target identification via SILAC labeling Strategy.
Alkyne/azide or diazirine-labeled strategy for target identification
In recent years, bioorthogonal reactions have been incorporated in the identification of targets for natural products. Bioorthogonal reactions are highly specific chemical reactions within biological systems that do not interfere with normal biochemical processes [17], 18]. Similar to the biotin-labeled strategy, bioorthogonal reactions involve introducing alkyne or azide labels into the functional groups (-COOH, -NH2, -OH) of natural products to synthesize probes. Subsequently, the proteins captured by the probe are enriched through the copper(I)-catalyzed Alkyne-Azide cycloaddition (CuAAC) reaction (Figure 5). In recent years, technological advancements have facilitated the expansion of this approach through the integration of photoaffinity labeling (PAL) to investigate transient or weak interactions between ligands and target proteins. Notably, the diazirine is widely employed as the primary photoreactive moiety in PAL. Diazirine undergoes a photochemical reaction upon ultraviolet light, forming a carbene intermediate that enables the covalent crosslinking of target proteins for subsequent affinity purification and mass spectrometry analysis.

Target identification via human protein Chip.
Currently, bioorthogonal strategies, such as the CuAAC reaction, are extensively employed in the identification of targets for natural products. For instance, a clickable photoaffinity probe of chloroquine (CQ) containing the azide-diazirine bifunctional group has been developed for identifying targets including PfLDH, PfOAT, PfPyrK, PfPGK, and PfTPI. Further investigations have revealed that CQ disrupts the glycolysis and energy metabolism of malaria parasites by directly binding to these target proteins, thereby exerting the antimalarial effect [19]. Similarly, a dual-functional probe derived from benzoxepane derivative 10i has contributed to identifying pyruvate kinase isozyme type M2 (PKM2) as the target protein responsible for anti-inflammatory effect by modulating the glycolysis and NLRP3 signaling pathway [20]. Moreover, alkyne-diazirine photosensitive group also facilitates the identification of retinoblastoma binding protein 4 (RBBP4) as the target protein mediating the anti-tumor properties of protopanaxadiol (PPD) [21]. Additionally, the bioorthogonal approach has been employed to identify disulfide isomerase as the target protein of the diterpenoid compound vinigrol for anti-arthritis effect [22]. Further, the diazirine-labeled metformin bifunctional probe directly binds to PEN2 for the activation of AMP-activated protein kinase (AMPK) to achieve a hypoglycemic effect [23].
Benzophenone-crosslinking strategy for target identification
Benzophenone is a photosensitive group that undergoes carbene formation upon ultraviolet light excitation. The carbene of the activated benzophenone subsequently forms a covalent bond with the carbon-hydrogen bonds of ligands, thereby immobilizing natural products onto the surface of solid-phase beads for target identification (Figure 6). Given that natural products mainly comprise organic molecules, the benzophenone labeling strategy proves effective in solid-phase immobilization of a wide range of natural products for target identification. For example, schisandrol A (SolA), a naturally derived neuroprotective agent, is immobilized onto a solid-phase beads using the benzophenone labeling strategy. Subsequently, ATP6V0d1 has been identified as the crucial target protein responsible for the neuroprotective effects of SolA in neuronal cells [24]. Moreover, the benzophenone-crosslinking strategy has facilitated the identification of the target protein histone H2B for the anti-neuroinflammatory effect of the natural product epoxymicheliolide [25]. Additionally, the targets of natural products such as vancomycin and cyclosporin are also revealed using a similar method based on benzophenone [26], 27]. Meanwhile, benzophenone-crosslinking strategy also aids in identifying target proteins in complex chemical systems, such as herbal extracts, including the extracts of Chrysanthemum indicum L., Caesalpinia sappan L., Albizia julibrissin Durazz., Cistanche deserticola Y.C.Ma, as well as traditional Chinese herbal formula such as Shouhui Laxative Capsule and Jingfang Granules [28], [29], [30], [31].

Target identification via alkyne/azide or diazirine photoaffinity labeling Strategy.

Target identification via benzophenone-crosslinking Strategy.
Epoxy-crosslinking strategy for target identification
Epoxy is a reactive group that can undergo covalent bonding with active groups (such as hydroxyl groups, amino groups, thiol groups) in natural products. Therefore, epoxy is an effective chemical tool that can be used to immobilize natural products onto solid microspheres for target identification. Currently, epoxy-activated Sepharose 6 B beads/Sepharose 4 B beads are among the commonly used commercial carriers. Following the immobilization of small molecules onto Sepharose 6 B beads, a blocking agent, such as ethanolamine, is typically employed to neutralize the residual epoxy groups and minimize nonspecific interference. Thus, the epoxy-crosslinking strategy proves useful in uncovering targets for natural products. For example, previously investigation has revealed CRAF and MEK1/2 as the direct targets for the anti-tumor effect of natural product erianin from Drumstick Dendrobium through activated epoxy groups [32]. Similarly, 1β-hydroxyalantolactone, termed as IJ-5 derived from I. japonica Thunb, undergoes chemical crosslinking with epoxy groups-modified beads, leading to the identification of UbcH5 as the crucial target in inhibiting inflammatory gene transcription [33]. Furthermore, sepharose 4 B beads-coupled with gingerenone A indicates the alleviation of ulcerative colitis by targeting IL-17RA [34]. Additionally, Bax is identified as the neuroprotective target of icariin through a similar approach, indicating a promising direction for drug development in diabetic encephalopathy [35].
Targeted protein degradation strategy for target identification
Proteolysis-targeting chimeras (PROTAC) represents a novel strategy utilizing chemical molecules to induce the degradation of specific proteins via the ubiquitin-proteasome system (UPS) [36]. PROTAC molecule comprises an E3 ubiquitin ligase ligand, a target protein ligand, and a linker. The degradation process typically involves ligand binding to target protein, the E3 ligand binding to E3 ubiquitin ligase, and the linker facilitating the proximity of the target protein to the E3 ubiquitin ligase. This process ultimately leads to the degradation of the target protein via a UPS-dependent mechanism.
The conventional PROTAC strategy involves the chemical synthesis of PROTAC molecules to target-specific protein degradation in cells. In recent years, this approach has been employed to explore the target proteins of natural products. Furthermore, targeted degradomics (TGDO), utilizing PROTAC molecular probes from natural compounds for target identification through quantitative proteomics analysis has been proposed (Figure 7).
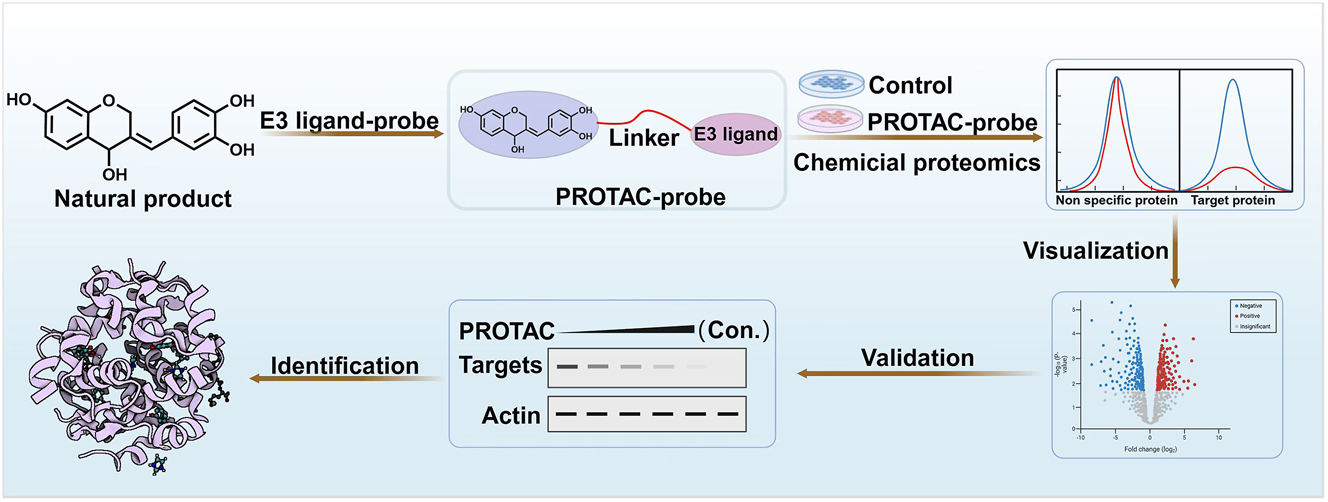
Target identification via targeted protein degradation Strategy.
Unlike traditional affinity-based methods, TGDO does not require a strong interaction between the compound and the target protein. Instead, TGDO is dependent on the formation of a ternary complex comprising the target protein, PROTAC, and E3 ligase, thereby ensuring high ligand selectivity. For example, researchers have synthesized the ZCY-PROTAC by conjugating lathyrol with thalidomide to investigate the specific target [37]. Quantitative proteomics analysis demonstrates that MAFF is the protein most efficiently degraded following ZCY-PROTAC treatment. Subsequent investigation indicates that lathyrol facilitates the formation of MAFF-Nrf2 heterodimers while inhibiting MAFF homodimers, thereby leading to the regulation of HO-1 downstream expression for antioxidant and anti-inflammatory effects. Recently, AD4, an artemisinin-based PROTAC, has been synthesized to reveal PCLAF as a crucial target for antitumor activity [38]. Moreover, the anti-tumor target of natural product evodiamine is determined to be REXO4 using TGDO strategy [39]. Notably, an innovative degradation-based protein profiling (DBPP) strategy has been proposed by integrating the PROTAC approach with quantitative proteomics and immunoprecipitation-mass spectrometry. DBPP approach enables the identification of the targets for natural product celastrol, including IKKβ, PI3Kα, and CIP2A, as well as the discovery of potential new targets such as CHK1, OGA, and ERCC6L [40].
Significant progress has been made in the target identification of natural products using chemical labeling strategies. This advancement provides a powerful tool for elucidating the direct targets and pharmacological mechanisms of these compounds. However, many natural products lack available functional groups for chemical modification, greatly limiting the application of these methodologies. To address these constraints, researchers have developed a range of label-free methods for target identification, presenting novel avenues for exploring pharmacological mechanisms.
Label-free-based target identification of natural products
The label-free target identification strategies for natural products rely on the concept that ligand binding affects the biophysical characteristics of target proteins, including thermal stability, enzymatic stability, and oxidation rate. Label-free strategies distinguish themselves by avoiding chemical modifications to natural products, thereby preserving their structural integrity and biological activity. Therefore, this approach facilitates the rapid target identification of bioactive natural products. Currently, label-free methodologies have evolved into several approaches, such as drug affinity responsive target stability analysis (DARTS), cellular thermal shift assay (CETSA), thermal proteome profiling (TPP), limited proteolysis-mass spectrometry (LiP-MS) and pulse proteolysis (PP).
CETSA
CETSA represents the foremost label-free approach for the identification of drug targets. CETSA operates on the principle that drug binding enhances the stability of protein spatial conformation, thereby distinguishing targets proteins from non-specific targets proteins [41]. In CETSA, cellular or tissue lysates are treated with small-molecule, and then exposed to temperature gradient to induce protein thermal denaturation. Subsequently, the samples are centrifuged to separate soluble components from precipitated proteins. The levels of target proteins in soluble fraction are quantified using western blotting, and the thermal stability is assessed by analyzing their melting curves with mass spectrometry.
Currently, the CETSA strategy is frequently utilized in the identification of target proteins for natural products. For instance, CETSA assay highlights that SHP-2 tyrosine phosphatase plays a crucial role in the anti-tumor activity of geranylnaringenin (CG902), a bioactive ingredient in Artocarpus heterophyllus Lam [42]. Using CETSA strategy, researchers demonstrates that nuciferine directly binds to HBXIP, ameliorating hepatic steatosis and insulin resistance [43]. Similarly, CETSA coupled with mass spectrometry indicates that the podophyllotoxin derivative SU056 targets Y-box binding protein-1 (YB-1) to regulate protein translation, thereby inhibiting triple-negative breast cancer progression [44]. Moreover, CETSA has effectively elucidated the target protein WD Repeat Domain 1 (WDR1) for the glioma inhibitory compound gambogic amide [45]. Importantly, CETSA is used to identify the target protein purine nucleoside phosphorylase for the antimalarial natural product quinine [46]. Additionally, investigations have demonstrated that TLR4 and the tumor-associated NADH oxidase as the direct targets of cucurbitacin B and capsaicin through the CETSA [47], 48].
TPP
TPP strategy represents an advanced version of CETSA, facilitating the effective discovery of low-abundance target proteins (Figure 8) [49]. TPP enables the identification of proteins that show changes in thermal stability in response to ligand binding at different temperatures on a proteomic scale. In this strategy, cells are treated with drugs at varying concentrations and then exposed to heat treatment. Subsequently, the soluble protein components are extracted and labeled with stable isotope labeling reagents tandem mass tags for mass spectrometry analysis, subsequently determining the specific melting temperature of each protein. Thus, TPP strategy aids in identifying proteins that display fluctuations in stability.
At present, TPP has been extensively utilized for the target identification of natural products. For instance, TPP reveals transcription factor specificity protein 1 (Sp1) as a direct target of the natural product murrayafoline A, providing valuable insights for directing therapeutic interventions toward Sp1 in for neuroinflammation [50]. Similarly, TPP facilitates the identification of kurarinone as targeting soluble epoxide hydrolase (sEH), linked to neuroinflammation in Parkinson’s disease [51]. Additionally, TPP has confirmed that oleanolic acid nanomicelles specifically target the 20 S proteasome subunit alpha 6 (PSMA6) to exert antitumor activity and vioprolide A targets nucleolar protein 14 for the treatment of lymphoblastic leukemia [52], 53].
Drug affinity-responsive target stability analysis (DARTS)
In 2009, Lomenick introduced the fundamental concept of DARTS, which is based on the principle that the spatial conformation of the target protein stabilizes upon ligand binding, rendering target protein less susceptible to protease degradation (Figure 9) [54]. Thus, the effect of enzymatic degradation on proteins can be discerned through SDS-PAGE, as well as mass spectrometry analysis. The convenience and efficiency of DARTS assays have facilitated their widespread application in the identification of targets for natural products. For instance, DARTS assay has facilitated the discovery that natural product oridonin exerts anticancer activity by targeting nucleolin [55]. Similarly, investigation suggests that cholesterol 25-hydroxylase binds directly with ARF4, thereby enhancing its activity to improve DKD [56]. Furthermore, DARTS elucidates that diosgenin ameliorates Alzheimer’s disease by targeting the 1,25D3-membrane-associated, rapid response steroid-binding protein (1,25D3-MARRS) [57]. Notably, DARTS has revealed that betulinic acid targets glucose-regulated protein 78 (GRP78) to induce endoplasmic reticulum stress-mediated apoptosis in breast cancer cells [58]. DARTS has also played a key role in elucidating that tubocapsenolide A exerts anti-osteosarcoma activity by targeting Src homology two phosphatase 2 [59].

Target identification via TPP Strategy.
The LiP-MS strategy represents an advanced version of the DARTS assay. LiP-MS provides a proteomics approach that enables the detection of protein structural alterations within complex biological contexts at a proteome-wide scale. LiP-MS analyzes protein hydrolysis patterns to discern the impact of drugs on protein structures. This enables the identification of target proteins and the precise localization of drug-binding sites by comparing the mass spectra of hydrolyzed peptides [60], 61]. For instance, LiP-MS discovers dihydrolipoamide S-acetyltransferase as the target protein of hyperforin, a natural product known to promote energy expenditure for obesity [62].
Likewise, PP is a strategy that capitalizes on the differential sensitivities of folded and unfolded proteins to proteases, allowing for the determination of ligand affinity for protein targets [63]. PP works by selectively digesting unfolded proteins within a mixture that contains both folded and unfolded proteins. This is achieved by exposing protein samples to varying concentrations of chemical denaturants (e.g., urea, guanidine hydrochloride), which establishes an equilibrium between the folded and unfolded states. Subsequently, a brief “pulse” with an excess of nonspecific protease treatment is applied to preferentially digest the unfolded proteins. The resulting data helps to define a functional relationship that links the proportion of folded proteins to the denaturant concentration [64]. Utilizing PP, investigations have successfully monitored the interaction between maltose and maltose-binding protein, obtaining a dissociation constant that aligns with those established through conventional biophysical analyses [63].
Other label-free target identification methodologies
In recent years, advancement in molecular biology, structural biology, and artificial intelligence technologies have driven the development of diverse label-free methodologies. These strategies, based on divergent principles, demonstrate unique capabilities for the discovery of the target proteins and biological mechanisms of natural products. For instance, the stability of proteins from rates of oxidation (SPROX) target identification strategy determines the protein-ligand binding mechanism by detecting the oxidation levels of methionine in target proteins [65]. The SPR provides kinetic information, such as the KD value of target molecule-ligand binding, by detecting changes in the refractive index on the surface of SPR biosensors and the SPR angle caused by the binding of target molecules to ligands, thus clarifying the mechanism of protein-ligand binding [66].
Moreover, a variety of label-free target identification approaches have been continuously emerged, including chemical denaturation and protein precipitation (CPP) [67], solvent-induced protein precipitation (SIP) [68], mechanical stress-induced protein precipitation (MSIPP) [69], and biolayer interferometry (BLI) [70]. These approaches significantly facilitate the identification of target proteins by assessing changes in mechanical stress and organic solvent tolerance exhibited by proteins upon drug binding.

Target identification via DARTS Strategy.
Furthermore, connectivity Map (cMap)-based target identification strategy uses gene expression patterns and pattern-matching software to reveal drug action mechanisms [71]. In addition, molecular docking techniques, such as cutting-edge computational methods or artificial intelligence programs like AlphaFold, are employed to model the binding interactions between numerous proteins and drugs to explore potential drug targets [72]. Despite the high-throughput and rapid features offered by these approaches, extensive experiments are still required to validate their applicability and reliability.
Currently, the application of these label-free strategies has garnered widespread attentions, leading to the increasing cases in natural products target identification. For example, the utilization of SPROX indicates Rab1a as the target protein of the N-arylbenzimidazole NAB2, demonstrating its therapeutic potential for neurodegenerative diseases [73]. Nicotine and its metabolite, cotinine, directly target myeloid differentiation protein two to inhibit toll-like receptor four signaling, thereby ameliorating neuroinflammation [74]. Notably, SIP has been successfully applied in the target identification of methotrexate, SNS-032, and staurosporine [75]. Meanwhile, the application of cMap database shows that celastrol and gedunin inhibit the HSP90 signaling pathway for prostate cancer therapy [76]. Specifically, molecular docking analysis demonstrates the interaction between celastrol and STAT3, leading to the mitigation of cardiac dysfunction [77]. In addition, BLI analysis suggests that capsaicin directly binds to KEAP1 to activate NRF2 function, which potentially ameliorates oxidative stress-related diseases [78]. In summary, it is noteworthy that label-free strategies will continue to evolve into diverse avenues and maintain their critical involvement in the discovery of targets for natural products.
Target-driven pharmacological mechanisms elucidation of natural products
At present, more than 50 % of small molecule drugs utilized in clinical practice are either directly or indirectly sourced from natural origins, exemplified by compounds like paclitaxel, vincristine, and artemisinin. Although several well-established compounds are acknowledged, the precise cellular targets of the majority of natural-derived compounds remain unclear. The unknown cellular targets hinder the comprehensive understanding of their pharmacological mechanisms, especially in intricate molecular signaling pathways, consequently limiting their potential applications. In recent years, the rapid advancement of chemical biology has greatly enhanced the identification of targets for natural products, providing valuable tools to elucidate intricate biological regulatory mechanisms. In particular, the discovery of targets provides crucial indications for uncovering novel pharmacological signaling pathways regulated by natural products (Table 1). This section aims to highlight representative cases related to the elucidation of novel cellular signaling pathways targeted by bioactive natural products.
Representative cases Illustrating the Molecular target of natural products.
| Year | Strategy | Natural products | Targets | Ref. |
|---|---|---|---|---|
| 2020 | Biotin labeling strategy combined with SILAC technology | Artone | ASF1a | [12] |
| Biotin labeling strategy combined with protein chip technology | Geniposide | TLR4 | [15] | |
| Celastrol | PRDX2 | [16] | ||
| Alkyne/Azide or diazirine-labeled strategy | 10i | PKM2 | [20] | |
| TPP | Vioprolide A | NOP14 | [53] | |
| CETSA, DARTS and SPR | Protosappanin A | 14-3-3ζ | [79] | |
| SIP | Methotrexate | DHFR | [75] | |
| Molecular docking | Celastrol | STAT3 | [77] | |
| Ginsenoside Rg3 | FoxO3a | [80] | ||
| 2021 | Alkyne/Azide or diazirine-labeled strategy combined with SILAC technology | Withangulatin A | PRDX6 | [13] |
| Alkyne/Azide-labeled strategy | Withangulatin A | PHGDH | [81] | |
| Curcusone D | BRAT1 | [82] | ||
| Benzophenone-crosslinking strategy | Dendranthema indicum extracts | UBE2I,PRDX6,RHOA, PRKCH,CREBBP and EP300 | [29] | |
| Epoxy-crosslinking strategy | Echinacoside | CK2 | [83] | |
| J13 | MYH9/actin | [84] | ||
| DARTS | Tubocapsenolide A | SHP2 | [59] | |
| LiP-MS | Hyperforin | DLAT | [62] | |
| SPR | Nicotine and its metabolite cotinine | MD-2 | [74] | |
| CETSA、DARTS and SPR | Bufalin | SDC4 | [85] | |
| DARTS, CETSA and BLI | Tubocapsenolide A | SHP-2 | [59] | |
| Molecular docking | Norlichexanthone | ERα | [86] | |
| Molecular docking and CETSA | Cucurbitacin B | TLR4 | [47] | |
| Molecular docking, CETSA and DARTS | Bruceine D | ICAT | [87] | |
| 2022 | Biotin-labeled strategy | Bruceine A | Galectin-1 | [7] |
| Paeoniflorin | C1qa | [88] | ||
| Biotin labeling strategy combined with protein chip technology | Bufalin | E2F2 | [89] | |
| Eupalinolide B | USP7 | [90] | ||
| Alkyne/Azide or diazirine-labeled strategy | Chloroquine | PfLDH, PfOAT, PfPyrK, PfPGK, and PfTPI | [19] | |
| Protopanaxadiol | RBBP4 | [31] | ||
| Metformin | PEN2 | [23] | ||
| Alkyne/Azide-labeled strategy | Cucurbitacin B | GRP78 | [91] | |
| Benzophenone-crosslinking strategy | Xiaoer xiaoji zhike oral liquid | SF3b4 and other 46 targets | [30] | |
| Schisandrol A | ATP6V0d1 | [24] | ||
| Epoxymicheliolide | H2B | [25] | ||
| Metformin | PEN2 | [23] | ||
| Epoxymicheliolide | H2B | [25] | ||
| Targeted protein degradation strategy | Lathyrol | MAFF | [37] | |
| Biotin-labeled strategy, SPR, CETSA and DARTS | Cucurbitacin B | IGF2BP1 | [92] | |
| CETSA | Nuciferine | HBXIP | [43] | |
| TPP | Murrayafoline A | Sp1 | [50] | |
| Kurarinone | sEH | [51] | ||
| TPP and SIP | Shikonin | NEMO/IKKβ | [93] | |
| Molecular docking | Ruscogenin | NMMHC IIA | [94] | |
| Equisetin | 11β-HSD1 | [95] | ||
| Corylin | Gtr1 | [96] | ||
| Ailanthone | c-Jun | [97] | ||
| Nitidine chloride | PI3K | [98] | ||
| Herbacetin | HBT | [99] | ||
| Justicia adhatoda L. leaf extract | NF-κB | [100] | ||
| SPROX | N-arylbenzimidazole 2 | Rab1a | [73] | |
| SPR | Artepillin C | CREB | [101] | |
| SPR, molecular docking and CETSA | Gentiopicroside | PAQR3 | [102] | |
| 2023 | Biotin-labeled strategy | P57 | PDXK | [103] |
| Quercetin | G6PD | [104] | ||
| Atractylenolide II | DGKQ | [105] | ||
| 6 k | EWSR1 | [106] | ||
| Alkyne/Azide or diazirine-labeled strategy | Forskolin | TGM2 | [107] | |
| ethyl gallate | PEPB1 | [108] | ||
| Vinigrol | PDIA1 | [109] | ||
| Benzophenone-crosslinking strategy | Jingfang Granules | TR150 and other 186 targets | [31] | |
| Berberine | EIF2AK2 | [110] | ||
| Epoxy-crosslinking strategy | Erianin | CRAF and MEK1/2 | [32] | |
| usenamineA | Myosin-9 | [111] | ||
| Targeted protein degradation strategy | Artemisinin | PCLAF | [38] | |
| Evodiamine | REXO4 | [39] | ||
| CETSA | Gambogic amide | WDR1 | [45] | |
| TPP | Lycorine | IDH1 | [112] | |
| TPP, SPR, CETSA and DARTS | Oleanolic acid nanomicelles | PSMA6 | [52] | |
| Molecular docking | Glycyrrhisoflavone and licoisoflavone A | RdRp | [113] | |
| Martynoside | RPL27A | [114] | ||
| Molecular docking, CETSA and DARTS | Scutellarin | PDK2 | [115] | |
| Arbutin | FTO | [116] | ||
| Molecular docking and SPR | XYA-2 | MYC and SLC39A10 | [117] | |
| Polyphyllin I | SQLE | [118] | ||
| SPR | Salvianolic acid B | NEU1 | [114] | |
| SPR, CETSA and DARTS | Cucurbitacin B | PCK2 | [119] | |
| cMap, DARTS and TPP | Ainsliadimer A | PRDX1 and PRDX2 | [120] | |
| 2024 | Biotin-labeled strategy | Artemisinins | LONP1 | [6] |
| Strictosamide | ERK2 | [9] | ||
| Celastrol | EPAC-1 | [121] | ||
| Alkyne/Azide or diazirine-labeled strategy | Vinigrol | Disulfide isomerase | [32] | |
| Epoxy-crosslinking strategy | Gingerenone A | IL-17RA | [34] | |
| Targeted protein degradation strategy | Celastrol | CHK1, OGA, and ERCC6L | [40] | |
| CETSA | SU056 | YB-1 | [44] | |
| DARTS | Cholesterol 25-hydroxylase | ARF4 | [56] | |
| Pristimerin | AIM2-PYCARD/ASC | [122] | ||
| (+)-6-Br-JP18 and (+)-6-Cl-JP18 | β-tubulin | [123] | ||
| CETSA and DARTS | Vanillic acid | CA9 | [124] | |
| TPP, CETSA and DARTS | Artemether | PKCδ | [125] | |
| TPP, molecular docking and CETSA | Daphnepedunin A | Cdc42 | [126] | |
| BLI | Capsaicin | KEAP1 | [78] | |
| SPR | Coptisine chloride | METTL3 | [127] | |
| SPR and CETSA | nordihydroguaiaretic acid | RARγ | [128] | |
| Molecular docking | DHPO | USP7 | [129] | |
| Aimosaponin AIII | A1R | [130] | ||
| Molecular docking and CETSA | Ellagic acid | β-catenin | [131] | |
| Molecular docking and SPR | 2,3,5,4′-tetrahydroxystilbene-2-O-β-D-glucoside | AMPK | [132] | |
| Molecular docking, SPR and CETSA | 2-APQC | SIRT3 | [133] | |
| Molecular docking, SPR, CETSA and DARTS | A3–6 | PC | [134] |
Target and molecular mechanism of natural compounds
Mechanisms of anti-inflammatory natural products
Natural products are known to contain a plethora of compounds with substantial anti-inflammatory and immunosuppressive activities. The identification of targets is crucial for elucidating the underlying mechanisms of action of these natural products. Sappanone A (SA) derived from C. sappan exihibits noteworthy anti-neuroinflammatory properties. A biotin-labeled SA probe is employed to uncover inosine monophosphate dehydrogenase 2 (IMPDH2) as the pivotal target accountable for the anti-neuroinflammatory effects in microglial cells. Subsequent studies revealed that SA covalently binds to the Cys140 in IMPDH2, thereby impeding the neuroinflammatory response through inhibiting nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) and p38 mitogen-activated protein kinase (p38 MAPK) signaling pathways [135]. Moreover, benzoxepane derivative compound 10i exerts anti-neuroinflammatory effects by targeting the M2 isoform of pyruvate kinase (PKM2), thereby inhibiting PKM2-mediated glycolysis and nod-like receptor protein 3 (NLRP3) inflammasome activation [20]. Notably, coptisine chloride alleviates NLRP3 inflammasome activation by directly inhibiting the enzymatic activity of methyltransferase-like 3 [127]. Similarly, berberine exhibits anti-inflammatory properties through the direct inhibition of the eukaryotic translation initiation factor 2 alpha kinase two dimerization, subsequently regulating multiple inflammatory signaling pathways such as c-Jun N-terminal kinase (JNK), NF-κB, protein kinase B (AKT), and NLRP3 [110]. As an effective agent ameliorating ulcerative colitis, vanillic acid binds to carbonic anhydrase IX (CA9) to inhibit CA9/stromal interaction molecule 1-mediated ferroptosis for restoration of intestinal epithelium homeostasis [124]. In addition, the derivative A3-6 of anemoside B4 modulates macrophage polarization by specifically inhibiting the activity of pyruvate carboxylase (PC), thereby exerting a therapeutic effect on colitis [134].
Moreover, ruscogenin exhibits the capability to attenuate LPS-induced pulmonary endothelial barrier dysfunction by directly targeting non-muscle myosin heavy chain IIA for the regulation of toll-like receptor four signaling [94]. Likewise, pristimerin, a quinone methide triterpenoid, alleviates the progression of tendinopathy by modulating absent in melanoma 2-PYD and CARD domain containing (AIM2-PYCARD/ASC) stability via sequestosome 1/p62-mediated autophagic degradation, thus providing a promising autophagy-based therapeutic for tendinopathy [122]. In particular, glycyrrhisoflavone and licoisoflavone A from Huashi Baidu decoction demonstrate potent anti-inflammatory effects by directly inhibiting the cAMP-specific 3′,5′-cyclic phosphodiesterase 4 [113]. Taken together, there may be a heightened emphasis on exploring the potential therapeutic targets and molecular mechanisms of natural products for anti-inflammatory application in the future.
Mechanisms of neuroprotective natural products
Numerous natural products demonstrate neuroprotective properties and serve as a key source for developing potential drugs in brain ischemia and neurodegeneration. Therefore, exploring the targets and molecular mechanisms of natural products contributes to a more understanding of the pathogenesis of these diseases. For example, echinacoside (ECH) is a bioactive compound with potent neuroprotective effects. By using a chemical crosslinking strategy, recent study demonstrates that ECH directly interacts with the casein kinase 2 α′ subunit, subsequently activating the Wnt signaling pathway to promote mitochondrial fusion for a protective effect against brain ischemic damage [83]. Meanwhile, celastrol has the potential to alleviate mitochondrial dysfunction in neurons by interacting with cAMP-activated exchange protein-1 (EPAC-1), thus potentially enhancing the outcome of secondary brain injury resulting from intracerebral hemorrhage [121]. Similarly, scutellarin (SG) mitigates mitochondrial damage by selectively inhibiting the enzymatic activity of pyruvate dehydrogenase kinase 2 (PDK2), subsequently regulating mitochondrial glucose oxidation via the Pdk/pyruvate dehydrogenase complex axis [115]. This offers a promising strategy for neurological disease therapy by ameliorating mitochondrial bioenergetic deficits. Furthermore, cordycepin promotes neuronal survival and plays the key role in combating Alzheimer’s disease by dual-targeting hexokinase II (HKII) and PDK2 to modulate microglial polarization coupled with mitochondrial metabolic reprogramming [136]. Additionally, the natural product P57 induces hypothermia by inhibiting the activity of pyridoxal kinase (PDXK) to promote the accumulation of pyridoxal in hypothalamus, thus exhibiting neuroprotective effect in the ischemic stroke model [103]. Thus, these cases provide a novel perspective for the discovery of target-based neuroprotective agents from natural products.
Mechanisms of anti-tumor natural products
Natural products offer valuable insights for the development of anticancer drugs, thus elucidating the targets and molecular mechanisms that hold great biological significance. Nordihydroguaiaretic acid, a naturally occurring lignan from Larrea tridentata, exhibits potent anti-tumor effects against inflammation-associated colorectal cancer by specifically targeting retinoic acid receptor γ (RARγ). Further investigation has elucidated that nordihydroguaiaretic acid enhances the interaction between RARγ and tumor necrosis factor receptor-associated factor 6 (TRAF6), leading to the suppression of TRAF6-mediated NF-kB and interleukin-6-signal transducer and activator of transcription 3 (IL-6-STAT3) activation, thereby inhibiting tumor proliferation [128]. Meanwhile, cucurbitacin B (CuB) induces a conformational change in the insulin-like growth factor-2 mRNA-binding protein 1 (IGF2BP1) via covalent binding to Cys253, disrupting the recognition of downstream m6A target genes for anti-tumor effects [92]. Furthermore, studies suggest the potential of bruceine D (BD) as a modulator of the β-catenin/hypoxia-inducible factor-1 alpha axis by targeting inhibitor of β-catenin and T-cell factor for the treatment of hepatocellular carcinoma [87].
Most recently, the anti-tumor compound DHPO induces ferroptosis in gastric cancer by targeting ubiquitin-specific protease 7 (USP7), promoting the ubiquitination and subsequent proteasomal degradation of stearoyl-CoA desaturase [129]. Indole terpenoid derivates (+)-6-Br-JP18 and (+)-6-Cl-JP18, disrupt the spindle microtubule dynamics by specifically binding to the colchicine-binding site of β-tubulin, thereby exerting the anti-tumor effect [123]. Moreover, bufalin functions as a molecular glue that facilitates the assembly of a ternary complex comprising the transcription factor E2 factor 2 (E2F2) and the unconventional ubiquitin ligase ZFP91. This complex promotes the ubiquitination degradation of E2F2, thus offering a potential treatment for hepatocellular carcinoma [89]. Recent study has highlighted the potential implication of quercetin in overcoming T790 M mutation in epidermal growth factor receptor (EGFRT790M)-driven tyrosine kinase inhibitors resistance by directly targeting glucose-6-phosphate dehydrogenase [104]. In addition, a natural product derived from Chaetomium globosum, XYA-2 specifically binds to the SH2 domain of STAT3, leading to a synergistic inhibition of myelocytomatosis and solute carrier family 39 member 10, ultimately suppressing tumor growth and metastasis in gastric cancer [117]. Therefore, the elucidation of the anti-tumor targets and signaling pathways of these natural products holds practical significance for the subsequent in-depth exploration of novel anti-tumor treatment strategies.
Mechanisms of lipid-lowering natural products
Natural products are an important source of lipid metabolism-regulating drugs. The target identification for natural products greatly contributes to the elucidation of the lipid-lowering mechanisms. For example, artemether (ART), a derivative of artemisinin, specifically targets protein kinase Cδ (PKCδ) to inhibit the palmitoylation modification by blocking the interaction of zinc finger DHHC-type palmitoyltransferase 5. This disruption results in the suppression of subsequent neuroinflammation signaling in the hypothalamus, subsequently activating the neuroendocrine axis, thereby presenting a promising therapeutic target for fatty liver diseases [125]. Furthermore, natural product arbutin (ARB) has been shown to inhibit the demethylase activity of fat mass and obesity-related protein (FTO), resulting in the accumulation of m6A methylation in the solute carrier family seven member 11 mRNA and consequently impeding ferroptosis [116]. Concurrently, equisetin, a bioactive compound derived from marine fungus, has been found to directly binds to 11β-hydroxysteroid dehydrogenase 1 (11β-HSD1) and presents remarkable inhibition on 11β-HSD1 for anti-obesity effect [95]. In addition, baicalin, a flavonoid from Scutellaria baicalensis, demonstrates unique anti-steatosis activity through directly allosteric activation of carnitine palmitoyltransferase 1, opening new opportunities for pharmacological treatment of diet-induced obesity and associated sequelae [137].
Meanwhile, investigators have discovered that artepillin C (APC) from Brazilian green propolis serves as an inhibitor of cAMP-response element binding protein-CREB regulated transcriptional coactivator 2 (CREB-CRTC2) interaction. APC suppresses the transcription of gluconeogenic and sterol-regulatory element binding protein genes mediated by CREB/CTRC2, resulting in decreased fasting glucose levels, enhanced insulin sensitivity, and reduced lipid levels in both serum and liver [101]. In a recent study, gentiopicroside (GPS), the bioactive secoiridoid glycoside present in Gentiana manshurica Kitagawa, has been determined to decrease lipid synthesis and restore the insulin signaling pathway through direct interaction with the progestin and adipoQ receptor 3 (PAQR3), thereby facilitating the activation of the phosphatidylinositol-3-kinase/AKT axis [102]. Moreover, atractylenolide II (AT II) is a sesquiterpene compound from Atractylodes macrocephala, demonstrating strong lipid-lowering effects by decreasing hepatic sn-1,2-diacylglycerol levels and ameliorating obesity-induced hyperlipidemia, hepatosteatosis, and insulin resistance via targeting diacylglycerol kinase family member DGKQ [105]. Also, aimosaponin AIII contributes to ameliorating diet-induced metabolic dysfunction-associated fatty liver and metabolic dysfunction-associated steatohepatitis in mice by directly targeting to activate hepatic adenosine A1 receptor (A1R) [130]. Puerarin (PU) may provide therapeutic benefits in combating atherosclerosis by modulating the gut microbiota [138]. Future advancements in characterizing drug targets and pharmacological mechanisms in lipid metabolism are expected to propel the field forward and enhance our comprehension of the complex mechanisms governing metabolic syndrome.
Mechanisms of anti-fibrosis natural products
Natural products are increasingly vital for drug discovery targeting fibrotic diseases including liver fibrosis, kidney fibrosis, pulmonary fibrosis, and others. For example, daphnepedunin A (DA) has emerged as a promising candidate for the treatment of renal fibrosis. Mechanistically, DA specially decreases cell division cycle 42 (Cdc42) activity to down-regulate its downstream phosphor-protein kinase Cζ/phosphor-glycogen synthase kinase-3β, thereby promoting β-catenin proteolysis to block classical pro-fibrotic β-catenin signaling [126]. Salvianolic acid B, a component of Salvia miltiorrhiza, also demonstrates a robust affinity to neuraminidase 1 (NEU1) and exhibiting significant protective effects against renal fibrosis [139]. Meanwhile, 2-APQC, analogues derived from camptothecin, is a specific sirtuin 3 (SIRT3) activator that alleviates myocardial hypertrophy and fibrosis by regulating mitochondrial homeostasis [133]. Similarly, ailanthone ameliorates pulmonary fibrosis by directly targeting mesenchymal homobox 1 (MEOX1) [140]. The matrine-derived compound 6 k regulates liver fibrosis by inhibiting the function of Ewing sarcoma breakpoint region 1 (EWSR1) to affect the expression of liver fibrosis-related genes [106]. In summary, tissue fibrosis is a significant contributor to disability in various diseases. Consequently, the identification of anti-fibrotic targets allows for an in-depth exploration of pharmacological mechanisms and promotes the development of anti-fibrotic agents.
Other pharmacological mechanisms of natural products
The structural variability of natural products contributes to a broad spectrum of biological activities, which are regulated by signal pathways through various functional proteins. Therefore, elucidating the signaling pathways behind the diverse pharmacological activities is crucial for understanding the mechanisms of natural products. For example, forskolin, a natural diterpene from Coleus forskohlii Briq, has demonstrated to promote osteogenesis by targeting transglutaminase 2 (TGM2). Further investigations have revealed that forskolin interacts with amino acid residues, specifically Asn299 and Thr368, in TGM2 via hydrogen bonding, consequently enhancing cell energy metabolism through mitochondrial-associated signaling pathway [107]. Similarly, natural product norlichexanthone (NOR) prevents postmenopausal osteoporosis by targeting estrogen receptor-α (Erα) to inhibit receptor activator of nuclear factor-kappa B ligand signal [86]. Moreover, the flavonoid compound morusin activates the nuclear factor erythroid 2-related factor 2-mediated anti-aging signaling pathway by downregulating cyclin D1 expression and promoting kelch-like ECH-associated protein 1 degradation through targeting tripartite motif-containing 25 (TRIM25) [141]. In addition, 2,3,5,4′-tetrahydroxystilbene-2-O-β-D-glucoside facilitates epigenetic reprogramming of DNA methylation patterns that regulate hematopoietic stem cells by targeting Ten-eleven-translocation 2 (TET2) [132].
Meanwhile, investigations have suggested that corylin derived from Psoralea corylifolia can alleviate the senescence process in vascular endothelial cells by targeting the Gtr1 protein and suppressing the mTOR signaling pathway [96]. Notably, ellagic acid can inhibit dihydrotestosterone-induced ferroptosis, thereby promoting hair regeneration by targeting β-catenin [131]. Martynoside demonstrates the potential to rescue the impairment of ribosome biogenesis induced by 5-fluorouracil by stabilizing the ribosomal protein L27a (RPL27A) [114]. Additionally, metformin initiates the AMP-activated protein kinase signaling pathway by directly binding to a γ-secretase subunit, PEN2, and subsequently inhibiting the lysosomal proton pump v-ATPase. This mechanism enables metformin to exert its antidiabetic activity [23].
In conclusion, natural products possess a wide range of biological activities and potential medicinal value. Thus, identifying target proteins to elucidate downstream pharmacological signaling pathways is critical for comprehensively understanding how natural products modulate human disease occurrence and beneficial for precise clinical medication.
Target and molecular mechanism of complex natural extracts
Natural extracts are also an important source for the development of clinical drugs due to their potent pharmacological activity. Although the chemical composition is complex, natural extracts often exhibit higher efficacy compared to single compounds, rendering them valuable for studying target networks and complex molecular signaling pathways. For instance, the C. sappan L ethanolic extract (CEE) has shown promising therapeutic potential for ischemic stroke.The neuroprotective effect of CEE involves 150 potential target proteins that are associated with six biological processes and 10 pathways, including the janus tyrosine kinase-STAT (JAK-STAT), heat shock protein-90 (HSP90) and DNA damage/telomere stress. Specifically, 52 effective targets including tumor necrosis factor alpha (TNF-α), caspase-3 (CASP3), mitogen-activated protein kinase kinase kinase 1 (MAP3K1),JNK, activator protein-1 (AP1), interleukin-1 beta (IL-1β) and interleukin-6 (IL-6) have been identified for safflower in the treatment of cerebral infarction (CI). Further study demonstrates that safflower could treat CI by regulating the TNF-α/MAPK pathway via CASP3 [142].
Likewise, the network pharmacology analysis has discovered 109 targets of Platycladi semen extracts which play an anti-anxiety role. The study further illustrates that the primary constituents in Platycladi Semen can interact with multiple targets, including peroxisome proliferator-activated receptor delta (PPARδ), PPARα, and fatty acid binding protein 5 (FABP5), thereby eliciting an anxiolytic effect through the regulation of lipid metabolism and neuroactive ligand-receptor interaction [143]. Furthermore, Carpesium cernuum extract blocks breast cancer metastasis by targeting tissue inhibitor of metalloproteinase 1 (TIMP1), matrix metalloproteinase-9 (MMP9), cluster of differentiation 44 (CD44) and collagen type IV alpha 2 (COL4A2) chain and downstream signaling pathways [144]. Additionally, exportin-2, β-actin-like protein 2, myosin-9, protein transport protein Sec61 subunit β, and cytochrome c oxidase copper chaperone have been identified as the principal targets of total saponins from A. julibrissin bark in the suppression of tumor cell proliferation and migration, employing solid-phase affinity purification technique [145].
A variety of medicinal plant extracts are commonly mixed to prepare formulations for treating diseases. These intricate formulations, originating from natural sources, possess distinct clinical efficacy. However, the intricate chemical composition of these formulations hinders the exploration of underlying molecular mechanisms. It is worth noting that target identification strategies have greatly assisted in addressing the molecular mechanisms of these complex natural products derived from formulations. For example, Xiao’er Xiaoji Zhike Oral Liquid, which is composed of extracts from 10 medicinal plants, is a frequently used in clinical settings for its anti-pneumonia properties. Researchers have discovered 62 potential target proteins, primarily linked to pathways such as Ras, leukocyte transendothelial migration, and mitochondrial autophagy, which are essential in processes of infectious pneumoni such as inflammation, microcirculation, fibrosis, and energy metabolism [30]. Additionally, Kang-fuxin (PEEPA) comprises a range of bioactive peptides that have been shown to stimulate the growth of gastric organoids and facilitate mucosal recovery. Previous study has confirmed that PEEPA may exert its therapeutic effects on chronic atrophic gastritis by potentially targeting the EGF-EGFR complex to activate the downstream signaling pathways of extracellular regulated protein kinases and STAT1 [146]. Notably, PPARγ has been identified as a key target of Yin-xing-tong-mai decoction in the activation of PPARγ-liver X receptor alpha-ATP-binding cassette transporter A1/ATP-binding cassette transporter G1 (PPARγ-LXRα-ABCA1/ABCG1) axis for the treatment of atherosclerosis [147]. Additionally, Baoyuan decoction has been found to interact with 46 potential target proteins, effectively enhancing mitochondrial function and ATP energy production [28]. In summary, the discovery of the targets and pharmacological pathways is beneficial for comprehending the molecular mechanisms of herbal formulas and establishing the foundation for their clinical application.
Research challenges and prospects
Remarkable advancements have been achieved in the methodology of identifying targets and elucidating pharmacological mechanisms of natural products, providing valuable insights for the development of new drugs and their clinical applications. Nevertheless, it is crucial to acknowledge that both chemical labeling and label-free strategies often result in the identification of numerous potential drug targets. Therefore, it is essential to establish expert consensus and standards for target screening in order to focus on key functional targets and conduct in-depth molecular mechanism research. Simultaneously, the advancement of multi-target identification methodology for highly complex natural products system will revolutionize the molecular mechanism research of traditional medicines. Furthermore, the future potential of live-cell super-resolution imaging techniques and dynamic real-time monitoring technologies may provide substantial assistance in the accurate identification and dynamic tracking of drug targets. Importantly, current investigation on target identification and mechanism elucidation often relies on in vitro cell models or ex vivo tissue organs with the absence of live animal indicators. These approaches may fail to accurately reflect the real interaction between drugs and targets in vivo within living biological system and has the potential to introduce biases in proposed drug mechanisms. Consequently, there is an urgent requirement to develop more cutting-edge technologies such as novel chemical probes to effectively explore the drug targets of natural products in real-time within animal models.
In conclusion, with the continuous development and integration of chemical biology and life science technologies, a series of advanced technical methods will emerge, thus offering new possibilities for elucidating the targets and pharmacological mechanisms of natural products.
Funding source: Natural Science Foundation of Beijing Municipality
Award Identifier / Grant number: 7222265
Award Identifier / Grant number: 7232273
Funding source: National Natural Sciences Foundation of China
Award Identifier / Grant number: 82174008
Award Identifier / Grant number: 82204678
Award Identifier / Grant number: 82325050
Award Identifier / Grant number: U23A20529
-
Research ethics: Not applicable.
-
Informed consent: Not applicable.
-
Author contributions: Not applicable.
-
Use of Large Language Models, AI and Machine Learning Tools: None declared.
-
Conflict of interest: The author states no conflict of interest.
-
Research funding: This work was financially supported by National Natural Sciences Foundation of China (82325050, U23A20529, 82174008, 82204678), Beijing Municipal Natural Science Foundation (7232273, 7222265), the Special Fund for “Tian-Chi Talent Introduction Program”, and the Special Fund for Taishan Scholars Project in Shandong Province (tstp20230633).
-
Data availability: Not applicable.
References
1. Li, D, Li, C, Li, L, Chen, S, Wang, L, Li, Q, et al.. Natural product kongensin A is a non-canonical HSP90 inhibitor that blocks RIP3-dependent necroptosis. Cell Chem Biol 2016;23:257–66. https://doi.org/10.1016/j.chembiol.2015.08.018.Search in Google Scholar PubMed
2. Zhou, LQ, Cui, TR, Hao, N, Zhao, YW, Zhao, B, Liu, YC. Application of chemical proteomics in identifying the molecular targets of natural products. Biol Bull 2023;39:12–26.Search in Google Scholar
3. Dong, RF, Xia, YZ, Kong, LY. Label-free target identification for natural products based on proteomics. Acta Pharm Sin 2023;58:2000–15.Search in Google Scholar
4. Pichler, CM, Krysiak, J, Breinbauer, R. Target identification of covalently binding drugs by activity-based protein profiling (ABPP). Bioorg Med Chem 2016;24:3291–303. https://doi.org/10.1016/j.bmc.2016.03.050.Search in Google Scholar PubMed
5. Liu, CX, Yin, QQ, Zhou, HC, Wu, YL, Pu, JX, Xia, L, et al.. Adenanthin targets peroxiredoxin I and II to induce differentiation of leukemic cells. Nat Chem Biol 2012;8:486–93. https://doi.org/10.1038/nchembio.935.Search in Google Scholar PubMed
6. Liu, Y, Jiang, JJ, Du, SY, Mu, LS, Fan, JJ, Hu, JC, et al.. Artemisinins ameliorate polycystic ovarian syndrome by mediating LONP1-CYP11A1 interaction. Science 2024;384:eadk5382. https://doi.org/10.1126/science.adk5382.Search in Google Scholar PubMed
7. Li, H, Zhao, X, Zheng, L, Wang, X, Lin, S, Shen, J, et al.. Bruceine A protects against diabetic kidney disease via inhibiting galectin-1. Kidney Int 2022;102:521–35. https://doi.org/10.1016/j.kint.2022.04.020.Search in Google Scholar PubMed
8. Dong, T, Li, C, Wang, X, Dian, L, Zhang, X, Li, L, et al.. Ainsliadimer A selectively inhibits IKKα/β by covalently binding a conserved cysteine. Nat Commun 2015;6:6522. https://doi.org/10.1038/ncomms7522.Search in Google Scholar PubMed PubMed Central
9. Geng, Q, Liu, B, Fan, D, Cao, Z, Li, L, Lu, P, et al.. Strictosamide ameliorates LPS-induced acute lung injury by targeting ERK2 and mediating NF-κB signaling pathway. J Ethnopharmacol 2024;322:117593. https://doi.org/10.1016/j.jep.2023.117593.Search in Google Scholar PubMed
10. Zeng, KW, Tu, PF. Recent progress on the methodology for target study of traditional Chinese medicine. Sci China Chem 2018;48:1420–8. https://doi.org/10.1360/n032018-00066.Search in Google Scholar
11. Dale, T, Clarke, PA, Esdar, C, Waalboer, D, Adeniji-Popoola, O, Ortiz-Ruiz, MJ, et al.. A selective chemical probe for exploring the role of CDK8 and CDK19 in human disease. Nat Chem Biol 2015;11:973–80. https://doi.org/10.1038/nchembio.1952.Search in Google Scholar PubMed PubMed Central
12. Zhang, XW, Feng, N, Wang, LC, Liu, D, Hua, YM, Zhang, C, et al.. Small-molecule arone protects from neuroinflammation in LPS-activated microglia BV-2 cells by targeting histone-remodeling chaperone ASF1a. Biochem Pharmacol 2020;177:113932. https://doi.org/10.1016/j.bcp.2020.113932.Search in Google Scholar PubMed
13. Chen, C, Gong, L, Liu, X, Zhu, T, Zhou, W, Kong, L, et al.. Identification of peroxiredoxin 6 as a direct target of withangulatin A by quantitative chemical proteomics in non-small cell lung cancer. Redox Biol 2021;46:102130. https://doi.org/10.1016/j.redox.2021.102130.Search in Google Scholar PubMed PubMed Central
14. Zhang, HN, Yang, L, Ling, JY, Czajkowsky, DM, Wang, JF, Zhang, XW, et al.. Systematic identification of arsenic-binding proteins reveals that hexokinase-2 is inhibited by arsenic. Proc Natl Acad Sci U S A 2015;112:15084–9. https://doi.org/10.1073/pnas.1521316112.Search in Google Scholar PubMed PubMed Central
15. Zhang, C, Wang, N, Tan, HY, Guo, W, Chen, F, Zhong, Z, et al.. Direct inhibition of the TLR4/MyD88 pathway by geniposide suppresses HIF-1α-independent VEGF expression and angiogenesis in hepatocellular carcinoma. Br J Pharmacol 2020;177:3240–57. https://doi.org/10.1111/bph.15046.Search in Google Scholar PubMed PubMed Central
16. Chen, X, Zhao, Y, Luo, W, Chen, S, Lin, F, Zhang, X, et al.. Celastrol induces ROS-mediated apoptosis via directly targeting peroxiredoxin-2 in gastric cancer cells. Theranostics 2020;10:10290–308. https://doi.org/10.7150/thno.46728.Search in Google Scholar PubMed PubMed Central
17. Rostovtsev, VV, Green, LG, Fokin, VV, Sharpless, KB. A stepwise huisgen cycloaddition process: copper(I)-catalyzed regioselective “ligation” of azides and terminal alkynes. Angew Chem Int Ed Engl 2002;41:2596–9. https://doi.org/10.1002/1521-3773(20020715)41:14<2596::aid-anie2596>3.0.co;2-4.10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4Search in Google Scholar
18. Agard, NJ, Prescher, JA, Bertozzi, CR. A strain-promoted [3+2] azide-alkyne cycloaddition for covalent modification of biomolecules in living systems. J Am Chem Soc 2004;126:15046–7. https://doi.org/10.1021/ja044996f.Search in Google Scholar
19. Gao, P, Liu, YQ, Xiao, W, Xia, F, Chen, JY, Gu, LW, et al.. Identification of antimalarial targets of chloroquine by a combined deconvolution strategy of ABPP and MS-CETSA. Mil Med Res 2022;9:30. https://doi.org/10.1186/s40779-022-00390-3.Search in Google Scholar
20. Gao, CL, Hou, GG, Liu, J, Ru, T, Xu, YZ, Zhao, SY, et al.. Synthesis and target identification of benzoxepane derivatives as potential anti-Neuroinflammatory agents for ischemic stroke. Angew Chem Int Ed Engl 2020;59:2429–39. https://doi.org/10.1002/anie.201912489.Search in Google Scholar
21. Zhuo, FF, Guo, Q, Zheng, YZ, Liu, TT, Yang, Z, Xu, QH, et al.. Photoaffinity labeling-based chemoproteomic strategy reveals RBBP4 as a cellular target of protopanaxadiol against colorectal cancer cells. Chembiochem 2022;23:e202200038. https://doi.org/10.1002/cbic.202200038.Search in Google Scholar
22. Zhu, Y, Wang, L, Li, J, Zhao, Y, Yu, X, Liu, P, et al.. Photoaffinity labeling coupled with proteomics identify PDI-ADAM17 module is targeted by (-)-vinigrol to induce TNFR1 shedding and ameliorate rheumatoid arthritis in mice. Cell Chem Biol 2024;31:452–64.e10. https://doi.org/10.1016/j.chembiol.2023.10.003.Search in Google Scholar
23. Ma, T, Tian, X, Zhang, B, Li, M, Wang, Y, Yang, C, et al.. Low-dose metformin targets the lysosomal AMPK pathway through PEN2. Nature 2022;603:159–65. https://doi.org/10.1038/s41586-022-04431-8.Search in Google Scholar PubMed PubMed Central
24. Zhou, X, Zhao, S, Liu, T, Yao, L, Zhao, M, Ye, X, et al.. Schisandrol A protects AGEs-induced neuronal cells death by allosterically targeting ATP6V0d1 subunit of V-ATPase. Acta Pharm Sin B 2022;12:3843–60. https://doi.org/10.1016/j.apsb.2022.06.013.Search in Google Scholar PubMed PubMed Central
25. Zheng, SZ, Zhang, XW, Song, XM, Yang, Z, Yao, L, Tu, PF, et al.. Epoxymicheliolide directly targets histone H2B to inhibit neuroinflammation via recruiting E3 ligase RNF20. Pharmacol Res 2022;177:106093. https://doi.org/10.1016/j.phrs.2022.106093.Search in Google Scholar PubMed
26. Lamos, SM, Krusemark, CJ, McGee, CJ, Scalf, M, Smith, LM, Belshaw, PJ. Mixed isotope photoaffinity reagents for identification of small-molecule targets by mass spectrometry. Angew Chem Int Ed Engl 2006;45:4329–33. https://doi.org/10.1002/anie.200600743.Search in Google Scholar PubMed
27. Eirich, J, Orth, R, Sieber, SA. Unraveling the protein targets of vancomycin in living S. aureus and E. faecalis cells. J Am Chem Soc 2011;133:12144–53. https://doi.org/10.1021/ja2039979.Search in Google Scholar PubMed
28. Wan, YJ, Liao, LX, Liu, YQ, Jiang, Y, Liu, LY, Zeng, KW, et al.. Identification and function analysis of target group for cardioprotection of Baoyuan decoction. China J Chin Mater Med 2017;42:3650–5. https://doi.org/10.19540/j.cnki.cjcmm.2017.0142.Search in Google Scholar PubMed
29. Zhao, M, Yao, L, Zhang, X, Wang, L, Tu, P, Zeng, K. Global identification of the cellular targets for a multi-molecule system by a photochemically-induced coupling reaction. Chem Commun 2021;57:3449–52. https://doi.org/10.1039/d1cc00392e.Search in Google Scholar PubMed
30. Yang, Z, Yao, L, Luo, QW, Yao, JC, Sun, CH, Zhang, GM, et al.. Anti-pneumonia targets and mechanism of xiaoer Xiaoji Zhike oral Liquid. China J Chin Mater Med 2022;47:3007–14. https://doi.org/10.19540/j.cnki.cjcmm.20220128.401.Search in Google Scholar PubMed
31. Zhao, MM, Yao, L, Yao, JC, Sun, CH, Zhang, GM, Zeng, KW. Anti-infectious pneumonia target discovery and molecular mechanism study of Jingfang Granules. China J Chin Mater Med 2023;48:789–96. https://doi.org/10.19540/j.cnki.cjcmm.20220929.402.Search in Google Scholar PubMed
32. Wang, P, Jia, X, Lu, B, Huang, H, Liu, J, Liu, X, et al.. Erianin suppresses constitutive activation of MAPK signaling pathway by inhibition of CRAF and MEK1/2. Signal Transduct Targeted Ther 2023;8:96. https://doi.org/10.1038/s41392-023-01329-3.Search in Google Scholar PubMed PubMed Central
33. Liu, L, Hua, Y, Wang, D, Shan, L, Zhang, Y, Zhu, J, et al.. A sesquiterpene lactone from a medicinal herb inhibits proinflammatory activity of TNF-α by inhibiting ubiquitin-conjugating enzyme UbcH5. Chem Biol 2014;21:1341–50. https://doi.org/10.1016/j.chembiol.2014.10.002.Search in Google Scholar
34. Liang, J, Dai, W, Liu, C, Wen, Y, Chen, C, Xu, Y, et al.. Gingerenone A attenuates ulcerative colitis via targeting IL-17RA to inhibit inflammation and restore intestinal barrier function. Adv Sci 2024:e2400206. https://doi.org/10.1002/advs.202400206.Search in Google Scholar PubMed PubMed Central
35. Zhao, SY, Liao, LX, Tu, PF, Li, WW, Zeng, KW. Icariin inhibits AGE-Induced injury in PC12 cells by directly targeting apoptosis regulator bax. Oxid Med Cell Longev 2019;2019:7940808. https://doi.org/10.1155/2019/7940808.Search in Google Scholar PubMed PubMed Central
36. Sakamoto, KM, Kim, KB, Kumagai, A, Mercurio, F, Crews, CM, Deshaies, RJ. Protacs: chimeric molecules that target proteins to the Skp1-Cullin-F box complex for ubiquitination and degradation. Proc Natl Acad Sci U S A 2001;98:8554–9. https://doi.org/10.1073/pnas.141230798.Search in Google Scholar PubMed PubMed Central
37. Wu, Y, Yang, Y, Wang, W, Sun, D, Liang, J, Zhu, M, et al.. PROTAC technology as a novel tool to identify the target of lathyrane diterpenoids. Acta Pharm Sin B 2022;12:4262. https://doi.org/10.1016/j.apsb.2022.07.007.Search in Google Scholar PubMed PubMed Central
38. Li, Y, Zeng, ZW, Chen, D, Gu, ZC, Yan, WL, Yue, LY, et al.. Facilitated drug repurposing with artemisinin-derived PROTACs: unveiling PCLAF as a therapeutic target. J Med Chem 2023;66:11335. https://doi.org/10.1021/acs.jmedchem.3c00824.Search in Google Scholar PubMed
39. Chen, S, Bi, K, Liang, H, Wu, Z, Huang, M, Chen, X, et al.. PROTAC derivatization of natural products for target identification and drug discovery: design of evodiamine-based PROTACs as novel REXO4 degraders. J Adv Res 2024;63:219–30. https://doi.org/10.1016/j.jare.2023.10.014.Search in Google Scholar PubMed PubMed Central
40. Ni, Z, Shi, Y, Liu, Q, Wang, L, Sun, X, Rao, Y. Degradation-based protein profiling: a case study of celastrol. Adv Sci 2024:e2308186. https://doi.org/10.1002/advs.202308186.Search in Google Scholar PubMed PubMed Central
41. Martinez Molina, D, Jafari, R, Ignatushchenko, M, Seki, T, Larsson, EA, Dan, C, et al.. Monitoring drug target engagement in cells and tissues using the cellular thermal shift assay. Science 2013;341:84–7. https://doi.org/10.1126/science.1233606.Search in Google Scholar PubMed
42. Jin, Y, Yoon, YJ, Jeon, YJ, Choi, J, Lee, YJ, Lee, J, et al.. Geranylnaringenin (CG902) inhibits constitutive and inducible STAT3 activation through the activation of SHP-2 tyrosine phosphatase. Biochem Pharmacol 2017;142:46–57. https://doi.org/10.1016/j.bcp.2017.06.131.Search in Google Scholar PubMed
43. Du, X, Di Malta, C, Fang, Z, Shen, T, Niu, X, Chen, M, et al.. Nuciferine protects against high-fat diet-induced hepatic steatosis and insulin resistance via activating TFEB-mediated autophagy-lysosomal pathway. Acta Pharm Sin B 2022;12:2869–86. https://doi.org/10.1016/j.apsb.2021.12.012.Search in Google Scholar PubMed PubMed Central
44. Dheeraj, A, Garcia Marques, FJ, Tailor, D, Bermudez, A, Resendez, A, Pandrala, M, et al.. Inhibition of protein translational machinery in triple-negative breast cancer as a promising therapeutic strategy. Cell Rep Med 2024;5:101552. https://doi.org/10.1016/j.xcrm.2024.101552.Search in Google Scholar PubMed PubMed Central
45. Qu, J, Qiu, B, Zhang, Y, Hu, Y, Wang, Z, Guan, Z, et al.. The tumor-enriched small molecule gambogic amide suppresses glioma by targeting WDR1-dependent cytoskeleton remodeling. Signal Transduct Targeted Ther 2023;8:424. https://doi.org/10.1038/s41392-023-01666-3.Search in Google Scholar PubMed PubMed Central
46. Dziekan, JM, Yu, H, Chen, D, Dai, L, Wirjanata, G, Larsson, A, et al.. Identifying purine nucleoside phosphorylase as the target of quinine using cellular thermal shift assay. Sci Transl Med 2019;11:eaau3174. https://doi.org/10.1126/scitranslmed.aau3174.Search in Google Scholar PubMed
47. Yuan, R, Zhao, W, Wang, QQ, He, J, Han, S, Gao, H, et al.. Cucurbitacin B inhibits non-small cell lung cancer in vivo and in vitro by triggering TLR4/NLRP3/GSDMD-dependent pyroptosis. Pharmacol Res 2021;170:105748. https://doi.org/10.1016/j.phrs.2021.105748.Search in Google Scholar PubMed
48. Islam, A, Su, AJ, Zeng, ZM, Chueh, PJ, Lin, MH. Capsaicin targets tNOX (ENOX2) to inhibit G1 Cyclin/CDK complex, as assessed by the cellular thermal shift assay (CETSA). Cells 2019;8:1275. https://doi.org/10.3390/cells8101275.Search in Google Scholar PubMed PubMed Central
49. Savitski, MM, Reinhard, FB, Franken, H, Werner, T, Savitski, MF, Eberhard, D, et al.. Tracking cancer drugs in living cells by thermal profiling of the proteome. Science 2014;346:1255784. https://doi.org/10.1126/science.1255784.Search in Google Scholar PubMed
50. Li, CH, Zhou, Y, Tu, PF, Zeng, KW, Jiang, Y. Natural carbazole alkaloid murrayafoline A displays potent anti-neuroinflammatory effect by directly targeting transcription factor Sp1 in LPS-induced microglial cells. Bioorg Chem 2022;129:106178. https://doi.org/10.1016/j.bioorg.2022.106178.Search in Google Scholar PubMed
51. Sun, CP, Zhou, JJ, Yu, ZL, Huo, XK, Zhang, J, Morisseau, C, et al.. Kurarinone alleviated Parkinson’s disease via stabilization of epoxyeicosatrienoic acids in animal model. Proc Natl Acad Sci U S A 2022;119. https://doi.org/10.1073/pnas.2118818119.Search in Google Scholar PubMed PubMed Central
52. Luo, QW, Yao, L, Li, L, Yang, Z, Zhao, MM, Zheng, YZ, et al.. Inherent capability of self-assembling nanostructures in specific proteasome activation for cancer cell pyroptosis. Small 2023;19:e2205531. https://doi.org/10.1002/smll.202205531.Search in Google Scholar PubMed
53. Kirsch, VC, Orgler, C, Braig, S, Jeremias, I, Auerbach, D, Müller, R, et al.. The cytotoxic natural product vioprolide A targets nucleolar protein 14, which is essential for ribosome biogenesis. Angew Chem Int Ed Engl 2020;59:1595–600. https://doi.org/10.1002/anie.201911158.Search in Google Scholar PubMed PubMed Central
54. Lomenick, B, Jung, G, Wohlschlegel, JA, Huang, J. Target identification using drug affinity responsive target stability (DARTS). Curr Protoc Chem Biol 2011;3:163–80. https://doi.org/10.1002/9780470559277.ch110180.Search in Google Scholar PubMed PubMed Central
55. Vasaturo, M, Cotugno, R, Fiengo, L, Vinegoni, C, Dal Piaz, F, De Tommasi, N. The anti-tumor diterpene oridonin is a direct inhibitor of nucleolin in cancer cells. Sci Rep 2018;8:16735. https://doi.org/10.1038/s41598-018-35088-x.Search in Google Scholar PubMed PubMed Central
56. Zhang, L, Fang, Z, Zhu, Q, Yang, S, Fu, J, Sun, Z, et al.. Cholesterol 25-hydroxylase protects against diabetic kidney disease by regulating ADP ribosylation factor 4. Adv Sci 2024;11:e2309642. https://doi.org/10.1002/advs.202309642.Search in Google Scholar PubMed PubMed Central
57. Tohda, C, Urano, T, Umezaki, M, Nemere, I, Kuboyama, T. Diosgenin is an exogenous activator of 1,25D -MARRS/Pdia3/ERp57 and improves Alzheimer’s disease pathologies in 5XFAD mice. Sci Rep 2012;2:535. https://doi.org/10.1038/srep00535.Search in Google Scholar PubMed PubMed Central
58. Cai, Y, Zheng, Y, Gu, J, Wang, S, Wang, N, Yang, B, et al.. Betulinic acid chemosensitizes breast cancer by triggering ER stress-mediated apoptosis by directly targeting GRP78. Cell Death Dis 2018;9:636. https://doi.org/10.1038/s41419-018-0669-8.Search in Google Scholar PubMed PubMed Central
59. Zhu, D, Chen, C, Liu, X, Wang, S, Zhu, J, Zhang, H, et al.. Osteosarcoma cell proliferation suppression via SHP-2-mediated inactivation of the JAK/STAT3 pathway by tubocapsenolide A. J Adv Res 2021;34:79–91. https://doi.org/10.1016/j.jare.2021.06.004.Search in Google Scholar PubMed PubMed Central
60. Feng, Y, De Franceschi, G, Kahraman, A, Soste, M, Melnik, A, Boersema, PJ, et al.. Global analysis of protein structural changes in complex proteomes. Nat Biotechnol 2014;32:1036–44. https://doi.org/10.1038/nbt.2999.Search in Google Scholar PubMed
61. Schopper, S, Kahraman, A, Leuenberger, P, Feng, Y, Piazza, I, Müller, O, et al.. Measuring protein structural changes on a proteome-wide scale using limited proteolysis-coupled mass spectrometry. Nat Protoc 2017;12:2391–410. https://doi.org/10.1038/nprot.2017.100.Search in Google Scholar PubMed
62. Chen, S, Liu, X, Peng, C, Tan, C, Sun, H, Liu, H, et al.. The phytochemical hyperforin triggers thermogenesis in adipose tissue via a Dlat-AMPK signaling axis to curb obesity. Cell Metab 2021;33:565–80.e7. https://doi.org/10.1016/j.cmet.2021.02.007.Search in Google Scholar PubMed
63. Park, C, Marqusee, S. Quantitative determination of protein stability and ligand binding by pulse proteolysis. Curr Protoc Protein Sci 2006;20:20.11.1–20.11.14. https://doi.org/10.1002/0471140864.ps2011s46. Chapter.Search in Google Scholar PubMed
64. Kim, MS, Song, J, Park, C. Determining protein stability in cell lysates by pulse proteolysis and western blotting. Protein Sci 2009;18:1051–9. https://doi.org/10.1002/pro.115.Search in Google Scholar PubMed PubMed Central
65. West, GM, Tang, L, Fitzgerald, MC. Thermodynamic analysis of protein stability and ligand binding using a chemical modification- and mass spectrometry-based strategy. Anal Chem 2008;80:4175–85. https://doi.org/10.1021/ac702610a.Search in Google Scholar PubMed
66. Prabowo, BA, Purwidyantri, A, Liu, KC. Surface plasmon resonance optical sensor: a review on light source technology. Biosensors 2018;8:80. https://doi.org/10.3390/bios8030080.Search in Google Scholar PubMed PubMed Central
67. Meng, H, Ma, R, Fitzgerald, MC. Chemical denaturation and protein precipitation approach for discovery and quantitation of protein-drug interactions. Anal Chem 2018;90:9249–55. https://doi.org/10.1021/acs.analchem.8b01772.Search in Google Scholar PubMed
68. Zhang, X, Wang, Q, Li, Y, Ruan, C, Wang, S, Hu, L, et al.. Solvent-induced protein precipitation for drug target discovery on the proteomic scale. Anal Chem 2020;92:1363–71. https://doi.org/10.1021/acs.analchem.9b04531.Search in Google Scholar PubMed
69. Lyu, J, Wang, Y, Ruan, C, Zhang, X, Li, K, Ye, M. Mechanical stress induced protein precipitation method for drug target screening. Anal Chim Acta 2021;1168:38612. https://doi.org/10.1016/j.aca.2021.338612.Search in Google Scholar PubMed
70. Do, T, Ho, F, Heidecker, B, Witte, K, Chang, L, Lerner, L. A rapid method for determining dynamic binding capacity of resins for the purification of proteins. Protein Expr Purif 2008;60:147–50. https://doi.org/10.1016/j.pep.2008.04.009.Search in Google Scholar PubMed
71. Lamb, J, Crawford, ED, Peck, D, Modell, JW, Blat, IC, Wrobel, MJ, et al.. The connectivity map: using gene-expression signatures to connect small molecules, genes, and disease. Science 2006;313:1929–35. https://doi.org/10.1126/science.1132939.Search in Google Scholar PubMed
72. Kuntz, ID. Structure-based strategies for drug design and discovery. Science 1992;257:1078–82. https://doi.org/10.1126/science.257.5073.1078.Search in Google Scholar PubMed
73. Hatstat, AK, Quan, B, Bailey, MA, Fitzgerald, MC, Reinhart, MC, McCafferty, DG. Chemoproteomic-enabled characterization of small GTPase Rab1a as a target of an N-arylbenzimidazole ligand’s rescue of Parkinson’s-associated cell toxicity. RSC Chem Biol 2022;3:96–111. https://doi.org/10.1039/d1cb00103e.Search in Google Scholar PubMed PubMed Central
74. Li, H, Peng, Y, Lin, C, Zhang, X, Zhang, T, Wang, Y, et al.. Nicotine and its metabolite cotinine target MD2 and inhibit TLR4 signaling. Innovation 2021;2:100111. https://doi.org/10.1016/j.xinn.2021.100111.Search in Google Scholar PubMed PubMed Central
75. Zhang, X, Wang, Q, Li, Y, Ruan, C, Wang, S, Hu, L, et al.. Solvent-Induced protein precipitation for drug target discovery on the proteomic scale. Anal Chem 2020;92:1363–71. https://doi.org/10.1021/acs.analchem.9b04531.Search in Google Scholar PubMed
76. Hieronymus, H, Lamb, J, Ross, KN, Peng, XP, Clement, C, Rodina, A, et al.. Gene expression signature-based chemical genomic prediction identifies a novel class of HSP90 pathway modulators. Cancer Cell 2006;10:321–30. https://doi.org/10.1016/j.ccr.2006.09.005.Search in Google Scholar PubMed
77. Ye, S, Luo, W, Khan, ZA, Wu, G, Xuan, L, Shan, P, et al.. Celastrol attenuates angiotensin II-induced cardiac remodeling by targeting STAT3. Circ Res 2020;126:1007–23. https://doi.org/10.1161/circresaha.119.315861.Search in Google Scholar
78. Gao, XN, Guo, WY, Liu, PY, Yuwen, MY, Liu, ZX, Tan, RY, et al.. Capsaicin acts as a novel NRF2 agonist to suppress ethanol induced gastric mucosa oxidative damage by directly disrupting the KEAP1-NRF2 interaction. bioRxiv 2024;01:574490.10.1101/2024.01.06.574490Search in Google Scholar
79. Wan, YJ, Liao, LX, Liu, Y, Yang, H, Song, XM, Wang, LC, et al.. Allosteric regulation of protein 14-3-3ζ scaffold by small-molecule editing modulates histone H3 post-translational modifications. Theranostics 2020;10:797–815. https://doi.org/10.7150/thno.38483.Search in Google Scholar PubMed PubMed Central
80. Li, L, Wang, Y, Guo, R, Li, S, Ni, J, Gao, S, et al.. Ginsenoside Rg3-loaded, reactive oxygen species-responsive polymeric nanoparticles for alleviating myocardial ischemia-reperfusion injury. J Control Release 2020;317:259–72. https://doi.org/10.1016/j.jconrel.2019.11.032.Search in Google Scholar PubMed PubMed Central
81. Chen, C, Zhu, T, Liu, X, Zhu, D, Zhang, Y, Wu, S, et al.. Identification of a novel PHGDH covalent inhibitor by chemical proteomics and phenotypic profiling. Acta Pharm Sin B 2021;12:246–61. https://doi.org/10.1016/j.apsb.2021.06.008.Search in Google Scholar PubMed PubMed Central
82. Cui, C, Dwyer, BG, Liu, C, Abegg, D, Cai, ZJ, Hoch, DG, et al.. Total synthesis and target identification of the curcusone diterpenes. J Am Chem Soc 2021;143:4379–86. https://doi.org/10.1021/jacs.1c00557.Search in Google Scholar PubMed PubMed Central
83. Zeng, KW, Wang, JK, Wang, LC, Guo, Q, Liu, TT, Wang, FJ, et al.. Small molecule induces mitochondrial fusion for neuroprotection via targeting CK2 without affecting its conventional kinase activity. Signal Transduct Targeted Ther 2021;6:71. https://doi.org/10.1038/s41392-020-00447-6.Search in Google Scholar PubMed PubMed Central
84. Qian, Y, Zhao, M, Han, Q, Wang, J, Liao, L, Yang, H, et al.. Pharmacologically targeting molecular motor promotes mitochondrial fission for anti-cancer. Acta Pharm Sin B 2021;11:1853–66. https://doi.org/10.1016/j.apsb.2021.01.011.Search in Google Scholar PubMed PubMed Central
85. Yang, H, Liu, Y, Zhao, MM, Guo, Q, Zheng, XK, Liu, D, et al.. Therapeutic potential of targeting membrane-spanning proteoglycan SDC4 in hepatocellular carcinoma. Cell Death Dis 2021;12:492. https://doi.org/10.1038/s41419-021-03780-y.Search in Google Scholar PubMed PubMed Central
86. Wang, K, Chen, Y, Gao, S, Wang, M, Ge, M, Yang, Q, et al.. Norlichexanthone purified from plant endophyte prevents postmenopausal osteoporosis by targeting ER α to inhibit RANKL signaling. Acta Pharm Sin B 2021;11:442–55. https://doi.org/10.1016/j.apsb.2020.09.012.Search in Google Scholar PubMed PubMed Central
87. Huang, R, Zhang, L, Jin, J, Zhou, Y, Zhang, H, Lv, C, et al.. Bruceine D inhibits HIF-1α-mediated glucose metabolism in hepatocellular carcinoma by blocking ICAT/β-catenin interaction. Acta Pharm Sin B 2021;11:3481–92. https://doi.org/10.1016/j.apsb.2021.05.009.Search in Google Scholar PubMed PubMed Central
88. Wang, Y, You, K, You, Y, Li, Q, Feng, G, Ni, J, et al.. Paeoniflorin prevents aberrant proliferation and differentiation of intestinal stem cells by controlling C1q release from macrophages in chronic colitis. Pharmacol Res 2022;182:106309. https://doi.org/10.1016/j.phrs.2022.106309.Search in Google Scholar PubMed
89. Liu, TT, Yang, H, Zhuo, FF, Yang, Z, Zhao, MM, Guo, Q, et al.. Atypical E3 ligase ZFP91 promotes small-molecule-induced E2F2 transcription factor degradation for cancer therapy. EBioMedicine 2022;86:104353. https://doi.org/10.1016/j.ebiom.2022.104353.Search in Google Scholar PubMed PubMed Central
90. Zhang, XW, Feng, N, Liu, YC, Guo, Q, Wang, JK, Bai, YZ, et al.. Neuroinflammation inhibition by small-molecule targeting USP7 noncatalytic domain for neurodegenerative disease therapy. Sci Adv 2022;8:eabo789. https://doi.org/10.1126/sciadv.abo0789.Search in Google Scholar PubMed PubMed Central
91. Wei, J, Chen, X, Li, Y, Li, R, Bao, K, Liao, L, et al.. Cucurbitacin B-induced G2/M cell cycle arrest of conjunctival melanoma cells mediated by GRP78–FOXM1–KIF20A pathway. Acta Pharm Sin B 2022;12:3861–76. https://doi.org/10.1016/j.apsb.2022.05.021.Search in Google Scholar PubMed PubMed Central
92. Liu, Y, Guo, Q, Yang, H, Zhang, XW, Feng, N, Wang, JK, et al.. Allosteric regulation of IGF2BP1 as a novel strategy for the activation of tumor immune microenvironment. ACS Cent Sci 2022;8:1102–15. https://doi.org/10.1021/acscentsci.2c00107.Search in Google Scholar PubMed PubMed Central
93. Yu, Z, Gao, J, Zhang, X, Peng, Y, Wei, W, Xu, J, et al.. Characterization of a small-molecule inhibitor targeting NEMO/IKKbeta to suppress colorectal cancer growth. Signal Transduct Targeted Ther 2022;7:71. https://doi.org/10.1038/s41392-022-00888-1.Search in Google Scholar PubMed PubMed Central
94. Wu, Y, Yu, X, Wang, Y, Huang, Y, Tang, J, Gong, S, et al.. Ruscogenin alleviates LPS-triggered pulmonary endothelial barrier dysfunction through targeting NMMHC IIA to modulate TLR4 signaling. Acta Pharm Sin B 2022;12:1198–212. https://doi.org/10.1016/j.apsb.2021.09.017.Search in Google Scholar PubMed PubMed Central
95. Xu, Z, Liu, D, Liu, D, Ren, X, Liu, H, Qi, G, et al.. Equisetin is an anti-obesity candidate through targeting 11β-HSD1. Acta Pharm Sin B 2022;12:2358–73. https://doi.org/10.1016/j.apsb.2022.01.006.Search in Google Scholar PubMed PubMed Central
96. Wang, TH, Tseng, WC, Leu, YL, Chen, CY, Lee, WC, Chi, YC, et al.. The flavonoid corylin exhibits lifespan extension properties in mouse. Nat Commun 2022;13:1238. https://doi.org/10.1038/s41467-022-28908-2.Search in Google Scholar PubMed PubMed Central
97. Yu, P, Wei, H, Li, K, Zhu, S, Li, J, Chen, C, et al.. The traditional Chinese medicine monomer ailanthone improves the therapeutic efficacy of anti-PD-L1 in melanoma cells by targeting c-Jun. J Exp Clin Cancer Res 2022;41:346. https://doi.org/10.1186/s13046-022-02559-z.Search in Google Scholar PubMed PubMed Central
98. Yu, F, Tan, W, Chen, Z, Shen, X, Mo, X, Mo, X, et al.. Nitidine chloride induces caspase 3/GSDME-dependent pyroptosis by inhibting PI3K/Akt pathway in lung cancer. Chin Med 2022;17:115. https://doi.org/10.1186/s13020-022-00671-y.Search in Google Scholar PubMed PubMed Central
99. Zhang, S, Wang, Y, Yu, M, Shang, Y, Chang, Y, Zhao, H, et al.. Discovery of herbacetin as a novel SGK1 inhibitor to alleviate myocardial hypertrophy. Adv Sci 2022;9:e2101485. https://doi.org/10.1002/advs.202101485.Search in Google Scholar PubMed PubMed Central
100. Basit, A, Shutian, T, Khan, A, Khan, SM, Shahzad, R, Khan, A, et al.. Anti-inflammatory and analgesic potential of leaf extract of Justicia adhatoda L. (Acanthaceae) in Carrageenan and Formalin-induced models by targeting oxidative stress. Biomed Pharmacother 2022;153:113322. https://doi.org/10.1016/j.biopha.2022.113322.Search in Google Scholar PubMed
101. Chen, Y, Wang, J, Wang, Y, Wang, P, Zhou, Z, Wu, R, et al.. A propolis-derived small molecule ameliorates metabolic syndrome in obese mice by targeting the CREB/CRTC2 transcriptional complex. Nat Commun 2022;13:246. https://doi.org/10.1038/s41467-021-27533-9.Search in Google Scholar PubMed PubMed Central
102. Xiao, H, Sun, X, Lin, Z, Yang, Y, Zhang, M, Xu, Z, et al.. Gentiopicroside targets PAQR3 to activate the PI3K/AKT signaling pathway and ameliorate disordered glucose and lipid metabolism. Acta Pharm Sin B 2022;12:2887–904. https://doi.org/10.1016/j.apsb.2021.12.023.Search in Google Scholar PubMed PubMed Central
103. Wang, R, Xiao, L, Pan, J, Bao, G, Zhu, Y, Zhu, D, et al.. Natural product P57 induces hypothermia through targeting pyridoxal kinase. Nat Commun 2023;14:5984. https://doi.org/10.1038/s41467-023-41435-y.Search in Google Scholar PubMed PubMed Central
104. Ge, Z, Xu, M, Ge, Y, Huang, G, Chen, D, Ye, X, et al.. Inhibiting G6PD by quercetin promotes degradation of EGFR T790M mutation. Cell Rep 2023;42:113417. https://doi.org/10.1016/j.celrep.2023.113417.Search in Google Scholar PubMed
105. Zheng, ZG, Xu, YY, Liu, WP, Zhang, Y, Zhang, C, Liu, HL, et al.. Discovery of a potent allosteric activator of DGKQ that ameliorates obesity-induced insulin resistance via the sn-1,2-DAG-PKCε signaling axis. Cell Metab 2023;35:101–17.e11. https://doi.org/10.1016/j.cmet.2022.11.012.Search in Google Scholar PubMed
106. Bao, Y, Niu, T, Zhu, J, Mei, Y, Shi, Y, Meng, R, et al.. Evolution and discovery of matrin derivatives as a new class of anti-hepatic fibrosis agents targeting ewing sarcoma breakpoint region 1 (EWSR1). J Med Chem 2023;66:7969–87. https://doi.org/10.1021/acs.jmedchem.3c00286.Search in Google Scholar PubMed
107. Yang, Z, Zhang, XW, Zhuo, FF, Liu, TT, Luo, QW, Zheng, YZ, et al.. Allosteric activation of transglutaminase 2 via inducing an “open” conformation for osteoblast differentiation. Adv Sci 2023;10:e2206533. https://doi.org/10.1002/advs.202206533.Search in Google Scholar PubMed PubMed Central
108. Yu, W, Liao, M, Chen, Y, Xue, R, Shi, XM, Liu, D, et al.. Photoaffinity labelling-based chemoproteomic strategy identifies PEBP1 as the target of ethyl gallate against macrophage activation. Chem Commun 2023;59:1022–5. https://doi.org/10.1039/d2cc05440j.Search in Google Scholar PubMed
109. Zhu, Y, Wang, L, Li, J, Zhao, Y, Yu, X, Liu, P, et al.. Photoaffinity labeling coupled with proteomics identify PDI-ADAM17 module is targeted by (−)-vinigrol to induce TNFR1 shedding and ameliorate rheumatoid arthritis in mice. Cell Chem Biol 2024;31:452–64.e10. https://doi.org/10.1016/j.chembiol.2023.10.003.Search in Google Scholar PubMed
110. Wei, W, Zeng, Q, Wang, Y, Guo, X, Fan, T, Li, Y, et al.. Discovery and identification of EIF2AK2 as a direct key target of berberine for anti-inflammatory effects. Acta Pharm Sin B 2023;13:2138–51. https://doi.org/10.1016/j.apsb.2022.12.009.Search in Google Scholar PubMed PubMed Central
111. Yang, A, Zeng, K, Huang, H, Liu, D, Song, X, Qian, Y, et al.. Usenamine A induces apoptosis and autophagic cell death of human hepatoma cells via interference with the Myosin-9/actin-dependent cytoskeleton remodeling. Phytomedicine 2023;116:154895. https://doi.org/10.1016/j.phymed.2023.154895.Search in Google Scholar PubMed
112. Zhuo, FF, Li, L, Liu, TT, Liang, XM, Yang, Z, Zheng, YZ, et al.. Lycorine promotes IDH1 acetylation to induce mitochondrial dynamics imbalance in colorectal cancer cells. Cancer Lett 2023;573:216364. https://doi.org/10.1016/j.canlet.2023.216364.Search in Google Scholar PubMed
113. Xu, H, Li, S, Liu, J, Cheng, J, Kang, L, Li, W, et al.. Bioactive compounds from Huashi Baidu decoction possess both antiviral and anti-inflammatory effects against COVID-19. Proc Natl Acad Sci U S A 2023;120. https://doi.org/10.1073/pnas.2301775120.Search in Google Scholar PubMed PubMed Central
114. Hong, M, Du, Y, Chen, D, Shi, Y, Hu, M, Tang, K, et al.. Martynoside rescues 5-fluorouracil-impaired ribosome biogenesis by stabilizing RPL27A. Sci Bull 2023;68:1662–77. https://doi.org/10.1016/j.scib.2023.07.018.Search in Google Scholar PubMed
115. Sheng, N, Zhang, Z, Zheng, H, Ma, C, Li, M, Wang, Z, et al.. Scutellarin rescued mitochondrial damage through ameliorating mitochondrial glucose qxidation via the Pdk-Pdc axis. Adv Sci 2023;10:e2303584. https://doi.org/10.1002/advs.202303584.Search in Google Scholar PubMed PubMed Central
116. Jiang, T, Xiao, Y, Zhou, J, Luo, Z, Yu, L, Liao, Q, et al.. Arbutin alleviates fatty liver by inhibiting ferroptosis via FTO/SLC7A11 pathway. Redox Biol 2023;68:102963. https://doi.org/10.1016/j.redox.2023.102963.Search in Google Scholar PubMed PubMed Central
117. Guan, X, Yang, J, Wang, W, Zhao, B, Hu, S, Yu, D, et al.. Dual inhibition of MYC and SLC39A10 by a novel natural product STAT3 inhibitor derived from Chaetomium globosum suppresses tumor growth and metastasis in gastric cancer. Pharmacol Res 2023;189:106703. https://doi.org/10.1016/j.phrs.2023.106703.Search in Google Scholar PubMed
118. Li, Z, Fan, Q, Chen, M, Dong, Y, Li, F, Wang, M, et al.. The interaction between polyphyllin I and SQLE protein induces hepatotoxicity through SREBP-2/HMGCR/SQLE/LSS pathway. J Pharm Anal 2023;13:39–54. https://doi.org/10.1016/j.jpha.2022.11.005.Search in Google Scholar PubMed PubMed Central
119. Liu, Y, Li, L, Yang, Z, Liao, LX, Yao, XJ, Tu, PF, et al.. Allosteric regulation of the lid domain of PCK2 as a novel strategy for modulating mitochondrial dynamics. Chem Commun 2023;59:13514–7. https://doi.org/10.1039/d3cc02781c.Search in Google Scholar PubMed
120. Lv, C, Huang, Y, Wang, Q, Wang, C, Hu, H, Zhang, H, et al.. Ainsliadimer A induces ROS-mediated apoptosis in colorectal cancer cells via directly targeting peroxiredoxin 1 and 2. Cell Chem Biol 2023;30:295–307.e5. https://doi.org/10.1016/j.chembiol.2023.02.003.Search in Google Scholar PubMed
121. Li, XS, Liu, W, Jiang, G, Lian, J, Zhong, Y, Zhou, J, et al.. Celastrol ameliorates neuronal mitochondrial dysfunction induced by intracerebral hemorrhage via targeting cAMP-activated exchange Protein-1. Adv Sci 2024;11:e2406154. https://doi.org/10.1002/advs.202307556.Search in Google Scholar PubMed PubMed Central
122. Jiang, H, Xie, Y, Lu, J, Li, H, Zeng, K, Hu, Z, et al.. Pristimerin suppresses AIM2 inflammasome by modulating AIM2-PYCARD/ASC stability via selective autophagy to alleviate tendinopathy. Autophagy 2024;20:76–93. https://doi.org/10.1080/15548627.2023.2249392.Search in Google Scholar PubMed PubMed Central
123. Peng, Y, Zhang, Y, Fang, R, Jiang, H, Lan, G, Xu, Z, et al.. Target identification and mechanistic characterization of indole terpenoid mimics: proper spindle microtubule assembly is essential for Cdh1-mediated proteolysis of CENP-A. Adv Sci 2024;11:e2305593. https://doi.org/10.1002/advs.202305593.Search in Google Scholar PubMed PubMed Central
124. Ni, J, Zhang, L, Feng, G, Bao, W, Wang, Y, Huang, Y, et al.. Vanillic acid restores homeostasis of intestinal epithelium in colitis through inhibiting CA9/STIM1-mediated ferroptosis. Pharmacol Res 2024;202:107128. https://doi.org/10.1016/j.phrs.2024.107128.Search in Google Scholar PubMed
125. Wang, YH, Chen, X, Bai, YZ, Gao, P, Yang, Z, Guo, Q, et al.. Palmitoylation of PKCδ by ZDHHC5 in hypothalamic microglia presents as a therapeutic target for fatty liver disease. Theranostics 2024;14:988–1009. https://doi.org/10.7150/thno.89602.Search in Google Scholar PubMed PubMed Central
126. Hu, X, Gan, L, Tang, Z, Lin, R, Liang, Z, Li, F, et al.. A natural small molecule mitigates kidney fibrosis by targeting Cdc42-mediated GSK-3β/β-catenin signaling. Adv Sci 2024;11:e2307850. https://doi.org/10.1002/advs.202307850.Search in Google Scholar PubMed PubMed Central
127. Zhou, X, Yang, X, Huang, S, Lin, G, Lei, K, Wang, Q, et al.. Inhibition of METTL3 alleviates NLRP3 inflammasome activation via increasing ubiquitination of NEK7. Adv Sci 2024;11:e2308786. https://doi.org/10.1002/advs.202308786.Search in Google Scholar PubMed PubMed Central
128. Li, XM, Yang, Y, Jiang, FQ, Hu, G, Wan, S, Yan, WY, et al.. Histone lactylation inhibits RARγ expression in macrophages to promote colorectal tumorigenesis through activation of TRAF6-IL-6-STAT3 signaling. Cell Rep 2024;43:113688. https://doi.org/10.1016/j.celrep.2024.113688.Search in Google Scholar PubMed
129. Guan, X, Wang, Y, Yu, W, Wei, Y, Lu, Y, Dai, E, et al.. Blocking ubiquitin-specific protease 7 induces ferroptosis in gastric cancer via targeting stearoyl-coA desaturase. Adv Sci 2024;11:e2307899. https://doi.org/10.1002/advs.202307899.Search in Google Scholar PubMed PubMed Central
130. Zhu, W, Hong, Y, Tong, Z, He, X, Li, Y, Wang, H, et al.. Activation of hepatic adenosine A1 receptor ameliorates MASH via inhibiting SREBPs maturation. Cell Rep Med 2024;5:101563. https://doi.org/10.1016/j.xcrm.2024.101477.Search in Google Scholar PubMed PubMed Central
131. Fu, H, Li, W, Liu, J, Tang, Q, Weng, Z, Zhu, L, et al.. Ellagic acid inhibits dihydrotestosterone-induced ferroptosis and promotes hair regeneration by activating the wnt/β-catenin signaling pathway. J Ethnopharmacol 2024;330:118227. https://doi.org/10.1016/j.jep.2024.118227.Search in Google Scholar PubMed
132. Gao, D, Yi, WW, Liu, B, Zhang, CE, Yang, CC, Zeng, L, et al.. Tetrahydroxy stilbene glucoside rejuvenates aging hematopoietic stem cells with predilection for lymphoid differentiation via AMPK and Tet2. J Adv Res 2024;S2090–1232:00170–X. https://doi.org/10.1016/j.jare.2024.04.027.Search in Google Scholar PubMed PubMed Central
133. Peng, F, Liao, M, Jin, W, Liu, W, Li, Z, Fan, Z, et al.. 2-APQC, a small-molecule activator of Sirtuin-3 (SIRT3), alleviates myocardial hypertrophy and fibrosis by regulating mitochondrial homeostasis. Signal Transduct Targeted Ther 2024;9:133. https://doi.org/10.1038/s41392-024-01816-1.Search in Google Scholar PubMed PubMed Central
134. Lv, L, Li, Q, Wang, K, Zhao, J, Deng, K, Zhang, R, et al.. Discovery of a new anti-inflammatory agent from anemoside B4 derivatives and its therapeutic effect on colitis by targeting pyruvate carboxylase. J Med Chem 2024;67:7385–405. https://doi.org/10.1021/acs.jmedchem.4c00222.Search in Google Scholar PubMed
135. Liao, LX, Song, XM, Wang, LC, Lv, HN, Chen, JF, Liu, D, et al.. Highly selective inhibition of IMPDH2 provides the basis of antineuroinflammation therapy. Proc Natl Acad Sci U S A 2017;114:E5986–94. https://doi.org/10.1073/pnas.1706778114.Search in Google Scholar PubMed PubMed Central
136. Zhong, X, Gong, S, Meng, L, Yao, W, Du, K, Jiao, L, et al.. Cordycepin modulates microglial M2 polarization coupled with mitochondrial metabolic reprogramming by targeting HKII and PDK2. Adv Sci 2024;11:e2304687. https://doi.org/10.1002/advs.202304687.Search in Google Scholar PubMed PubMed Central
137. Dai, J, Liang, K, Zhao, S, Jia, W, Liu, Y, Wu, H, et al.. Chemoproteomics reveals baicalin activates hepatic CPT1 to ameliorate diet-induced obesity and hepatic steatosis. Proc Natl Acad Sci U S A 2018;115:E5896–905. https://doi.org/10.1073/pnas.1801745115.Search in Google Scholar PubMed PubMed Central
138. Puerarin alleviates atherosclerosis via the inhibition of Prevotella copri and its trimethylamine production. Gut 2024;73:1934-43.10.1136/gutjnl-2024-331880Search in Google Scholar PubMed
139. Chen, QQ, Liu, K, Shi, N, Ma, G, Wang, P, Xie, HM, et al.. Neuraminidase 1 promotes renal fibrosis development in male mice. Nat Commun 2023;14:1713. https://doi.org/10.1038/s41467-023-37450-8.Search in Google Scholar PubMed PubMed Central
140. Zhao, L, Zhu, Y, Tao, H, Chen, X, Yin, F, Zhang, Y, et al.. Ailanthone ameliorates pulmonary fibrosis by suppressing JUN-dependent MEOX1 activation. Acta Pharm Sin B 2024;14:3543–60. https://doi.org/10.1016/j.apsb.2024.04.013.Search in Google Scholar PubMed PubMed Central
141. Liu, Z, Wang, K, Jiang, C, Chen, Y, Liu, F, Xie, M, et al.. Morusin alleviates aortic valve calcification by inhibiting valve interstitial cell senescence through Ccnd1/Trim25/Nrf2 Axis. Adv Sci 2024;11:e2307319. https://doi.org/10.1002/advs.202307319.Search in Google Scholar PubMed PubMed Central
142. Wang, Y, Shi, Y, Zou, J, Zhang, X, Wang, M, Guo, D, et al.. The intranasal administration of Carthamus tinctorius L. extract/phospholipid complex in the treatment of cerebral infarction via the TNF-α/MAPK pathway. Biomed Pharmacother 2020;130:110563. https://doi.org/10.1016/j.biopha.2020.110563.Search in Google Scholar PubMed
143. Xie, J, Li, Y, Liang, Y, Kui, H, Wang, C, Huang, J. Integration of non-targeted metabolomics with network pharmacology deciphers the anxiolytic mechanisms of Platycladi Semen extracts in CUMS mice. J Ethnopharmacol 2023;315:116571. https://doi.org/10.1016/j.jep.2023.116571.Search in Google Scholar PubMed
144. Dang, H, Li, H, Ma, C, Wang, Y, Tian, J, Deng, L, et al.. Identification of carpesium cernuum extract as a tumor migration inhibitor based on its biological response profiling in breast cancer cells. Phytomedicine 2019;64:153072. https://doi.org/10.1016/j.phymed.2019.153072.Search in Google Scholar PubMed
145. Qian, Y, Han, QH, Liu, D, Tu, PF, Zeng, KW, Liang, H. Anti-tumor target identification and molecular mechanism study of total saponins from Albizia julibrissin. China J Chin Mater Med 2017;42:3661–5. https://doi.org/10.19540/j.cnki.cjcmm.2017.0144.Search in Google Scholar PubMed
146. Li, K, Ma, X, Li, Z, Liu, Y, Shen, G, Luo, Z, et al.. A natural peptide from a traditional Chinese medicine has the potential to treat chronic atrophic gastritis by activating gastric stem cells. Adv Sci 2024;11:e2304326. https://doi.org/10.1002/advs.202304326.Search in Google Scholar PubMed PubMed Central
147. Zheng, S, Huang, H, Li, Y, Wang, Y, Zheng, Y, Liang, J, et al.. Yin-xing-tong-mai decoction attenuates atherosclerosis via activating PPARγ-LXRα-ABCA1/ABCG1 pathway. Pharmacol Res 2021;169:105639. https://doi.org/10.1016/j.phrs.2021.105639.Search in Google Scholar PubMed
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Articles in the same Issue
- Frontmatter
- Reviews
- The potential of generative AI with prostate-specific membrane antigen (PSMA) PET/CT: challenges and future directions
- Target discovery-directed pharmacological mechanism elucidation of bioactive natural products
- Spatio-temporal processes in autophagosome-lysosome fusion
- A review of 3D bioprinting for organoids
- Perspectives
- Can chimeric antigen receptors – based therapy bring a gleam of hope for thyroid-associated ophthalmopathy and other autoimmune diseases?
- Artificial intelligence-powered innovations in radiotherapy: boosting efficiency and efficacy
Articles in the same Issue
- Frontmatter
- Reviews
- The potential of generative AI with prostate-specific membrane antigen (PSMA) PET/CT: challenges and future directions
- Target discovery-directed pharmacological mechanism elucidation of bioactive natural products
- Spatio-temporal processes in autophagosome-lysosome fusion
- A review of 3D bioprinting for organoids
- Perspectives
- Can chimeric antigen receptors – based therapy bring a gleam of hope for thyroid-associated ophthalmopathy and other autoimmune diseases?
- Artificial intelligence-powered innovations in radiotherapy: boosting efficiency and efficacy


