Abstract
Metabolic health is highly dependent on intestinal and hepatic handling of dietary and endogenous lipids and lipoproteins. Disorders of lipid and lipoprotein metabolism are commonly observed in patients with insulin resistant states such as obesity, metabolic syndrome, and type 2 diabetes. Evidence from both animal models and human studies indicates that a major underlying factor in metabolic or diabetic dyslipidemia is the overproduction of hepatic and intestinal apolipoprotein (apo)B-containing lipoprotein particles. These particles are catabolized down into highly proatherogenic remnants, which can be taken up into the arterial intima and promote plaque development. Several gut-derived peptides have been identified as key regulators of energy metabolism; one such peptide is the incretin hormone glucagon-like peptide (GLP)-1. Our laboratory has previously demonstrated that GLP-1 can signal both centrally and peripherally to reduce postprandial and fasting lipoprotein secretion. Moreover, we have demonstrated that GLP-1 receptor (GLP-1R) agonists can ameliorate diet-induced dyslipidemia. Recently, we published evidence for a novel vagal neuroendocrine signalling pathway by which native GLP-1 may exert its anti-lipemic effects. Furthermore, we demonstrated a novel role for other gut-derived peptides in regulating intestinal lipoprotein production. Overall, ample evidence supports a key role for GLP-1R on the portal vein afferent neurons and nodose ganglion in modulating intestinal fat absorption and lipoprotein production and identifies other gut-derived peptides as novel regulators of postprandial lipemia. Insights from these data may support identification of potential drug targets and the development of new therapeutics targeting treatment of diabetic dyslipidemia.
Introduction
The intestine regulates several aspects of metabolism via secretion of enteroendocrine hormones from a variety of specialized cells in the intestinal epithelium. These peptide hormones have key roles in modulating components of gastrointestinal function such as gastric acid secretion, intestinal motility, and gall bladder contraction. Importantly, several of these hormones have also been implicated in the regulation of metabolism on a global scale, influencing satiety, glucose homeostasis, thermogenesis, and nutrient mobilization [1]. Indeed, some of these peptides have already been shown to influence lipoprotein metabolism, such as glucagon-like peptide (GLP)-1 (discussed below) [2]. Importantly, these peptides can signal both locally within the GI tract and to peripheral and central nervous centers to regulate components of metabolism [3].
Glucagon-like peptides (GLPs)
GLP-1 is a 31 amino acid peptide that acts as an incretin hormone, potentiating the release of insulin. It is 50 % homologous with glucagon [4], and the majority of its presence in the circulation originates from the intestinal L-cell [5]. GLP-1 has been the focus of several pharmacological interventions for the treatment of metabolic disease due to its ability to normalize body weight, blood glucose, and blood lipids in obese and type 2 diabetic (T2D) individuals [6]. GLP-2 on the other hand is used as an intestinotrophic drug for the treatment of short bowel disease due to its ability to stimulate enterocyte growth and improve intestinal barrier function [7]. GLPs bind to their eponymous G-protein coupled receptors GLP-1 receptor (GLP-1R) and GLP-2 receptor (GLP-2R). The focus of this review will center on GLP-1 and its effects on postprandial and fasting lipoprotein production.
GLP-1 and GLP-2 are derived from the proglucagon gene, present in the pancreatic alpha cells, intestinal endocrine L-cells, and in neurons of the nucleus tractus solitarius (NTS) in the caudal brainstem [4, 8]. Post-translational processing of the 158 amino acid proglucagon polypeptide will yield a tissue-specific profile of metabolic hormones. In the intestine and brainstem, prohormone convertase (PC)-1/3 cleaves proglucagon into GLP-1, GLP-2, intervening peptide (IP)-2, oxyntomodulin, and glicentin; whereas, in the pancreatic alpha cells, PC-2 will cleave proglucagon to produce glucagon, IPs, glicentin-related polypeptide (GRPP), and major proglucagon fragment (MPFG). Importantly, GLP-1 and GLP-2 are produced at an equimolar concentration, however, have diverging metabolic effects [2]. Notably, both GLP-1 and GLP-2 are still present in the fasting state with a circulating concentration of 5–10 pmol/L of GLP-1 (in a study conducted in 2009 on children during neonatal period) [9] and 116±22 pg/mL of GLP-2 (in a study conducted in 1997 in adults (6 adults, male and female, aged 23–32) [10]. GLPs are primarily released in response to nutrient consumption and display a biphasic pattern of secretion [11]. Direct contact of nutrients with intestinal L-cells on the apical intestinal lumen produces an initial secretory peak as early as 10–15 min after consumption of a meal, followed by a secondary peak around 30–60 min post-consumption [11]. Secretion is prompted by several macronutrients, including glucose, fatty acids, and amino acids [11, 12]. Fatty acids in particular produce a more sustained elevation in GLP-1 secretion compared to glucose, mediated by intestinal L-cell expression of the long chain fatty acid receptors GPR40 and GPR120 [13] which have been shown to stimulate GLP-1 secretion [14, 15]. This is in line with evidence that intraduodenal lipid emulsion in rats increases GLP-1 secretion in a dose dependent manner [16]. However, the initial peak in GLP-1’s biphasic secretion is likely mediated not by direct nutrient contact but via a more rapid mechanism such as neural signaling, since the majority of intestinal L-cells are located in the distal ileum, and small intestinal transit time can exceed 3 h [17, 18]. This is supported by evidence that stimulation of the vagus nerve – which provides parasympathetic innervation to the gut – with a bipolar electrode potentiates GLP-1 secretion in an in-situ rat model. In turn, subdiaphragmatic truncal vagotomy has the opposite effect, abrogating early rises in GLP-1 induced by lipid load [19]. Similarly, in vivo parasympathetic blockade in humans and rats has been demonstrated to impair early GLP-1 secretion [20, 21].
GLP-1R
GLP-1 binds to the GLP-1R, a class B heterotrimeric G protein-coupled receptor (GPCR) with 463 amino acid residues that spans seven transmembrane domains [22]. The receptor exhibits diverse tissue expression but is most notably found in the pancreatic beta-cells, stomach, duodenum, vagal afferent nerves, lungs and in the CNS [23, 24]. The extracellular domain of the GLP-1R binds with the C-terminal half of GLP-1, and the third transmembrane domain interacts with the N-terminal half of GLP-1 [25]. Transmembrane topology is common to all class B GPCRs, with the N-terminal binding of G-proteins in this domain crucial for the selective recognition of peptide ligands [26]. Thus, peptides that have an N-terminal truncation such as exendin 9–39 will act as GLP-1R antagonists [27]. Upon successful binding of GLP-1, receptor-bound adenylyl cyclase will catalyze the conversion of ATP to cyclic AMP (cAMP), leading to subsequent activation of protein kinase A (PKA) and the exchange protein directly activated by cAMP (EPAC) family. In the beta-cell this increase in PKA causes ADP-dependent phosphorylation of the SUR1 K(ATP) channel subunit, ultimately triggering the insulin secretory pathway via changes in membrane depolarization [28]. Moreover, there is evidence that in addition to inhibition of ATP-regulated potassium channels PKA and EPAC can increase the activity of L-type voltage gated calcium channels (VGCCs) and opening of non-specific cation channels [28], [29], [30]. Increased phosphoinositol turnover results in additional release of intracellular Ca2+ via phospholipase-C-mediated production of inositol triphosphate (IP)3 [31]. Together leading to increased calcium influx to the cell, and calcium induced insulin secretion. Diacylglycerol generation from this pathway and elevated intracellular Ca2+ will also result in the activation of protein kinase C (PKC). Elevated PCK will phosphorylate extracellular signal-regulated kinase (ERK)1/2, which in turn phosphorylates the C-terminus of the GLP-1R [32]. Importantly, spatiotemporal control of GPCR signaling is classically mediated by receptor desensitization and cellular internalization. Wherein, the phosphorylation of the C-terminus by ERK1/2 recruits β-arrestins to sterically hinder interactions with GPCR kinases, initiating endocytosis likely via the assembly of clathrin-coated pits [24, 33]. However, there is emerging evidence that internalized GPCRs such as GLP-1R molecules may continue to signal within endosomes [34].
While there is notable overlap with the periphery, less is known about GLP-1R signaling in the CNS. It is hypothesized that GLP-1 works primarily through enhanced calcium influx through VGCCs. In the hindbrain VGCC activation is mediated by PKA, whereby, calcium influx activates mitogen-activated protein kinase (MAPK) and suppresses AMP-activated protein kinase (AMPK) [35]. Similarly, in midbrain structures such as the hypothalamus and hippocampus GLP-1R activation has been shown to increase cAMP and activate L-type VGCCs via cAMP response element binding protein (CREB) [35, 36]. PKA also has been shown to enhance glutamatergic transmission via phosphorylation of the α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor glutamate receptor (GluR)1 subunit in the paraventricular nucleus of the hypothalamus [37].
Biological functions of GLP-1
GLP-1 influences several aspects of metabolism (Figure 1). In the periphery it stimulates insulin secretion in a glucose-dependent manner, and in the brain it enhances satiety and reduces food consumption [38]. It has also been shown to have important glucoregulatory effects, with chronic treatment in T2D individuals resulting in attenuated fasting plasma glucose levels and improved hemoglobin A1c levels [39]. Moreover, patients demonstrate improved insulin sensitivity and enhanced beta cell function. Whereas, the reverse was seen when the GLP-1R antagonist exendin 9–39 was administered to healthy men, which resulted in elevations in fasting plasma glucose levels [40]. Similarly, portal vein infusion of exendin 9–39 in rats has been shown to worsen glucose tolerance [41]. Direct effects of GLP-1 include the inhibition of glucagon and somatostatin secretion, resulting in suppressed hepatic glucose production and potentiated insulin release, respectively [42], [43], [44]. In humans exenatide monotherapy has been shown to reduce plasma TG levels [45] and postprandial ApoB48 biosynthesis [46]. These observations are consistent in individuals with impaired glucose tolerance, where a single dose of the GLP-1R agonist exendin-4 reduced postprandial plasma TG, ApoB48, and remnant lipoprotein cholesterol and TG [47].
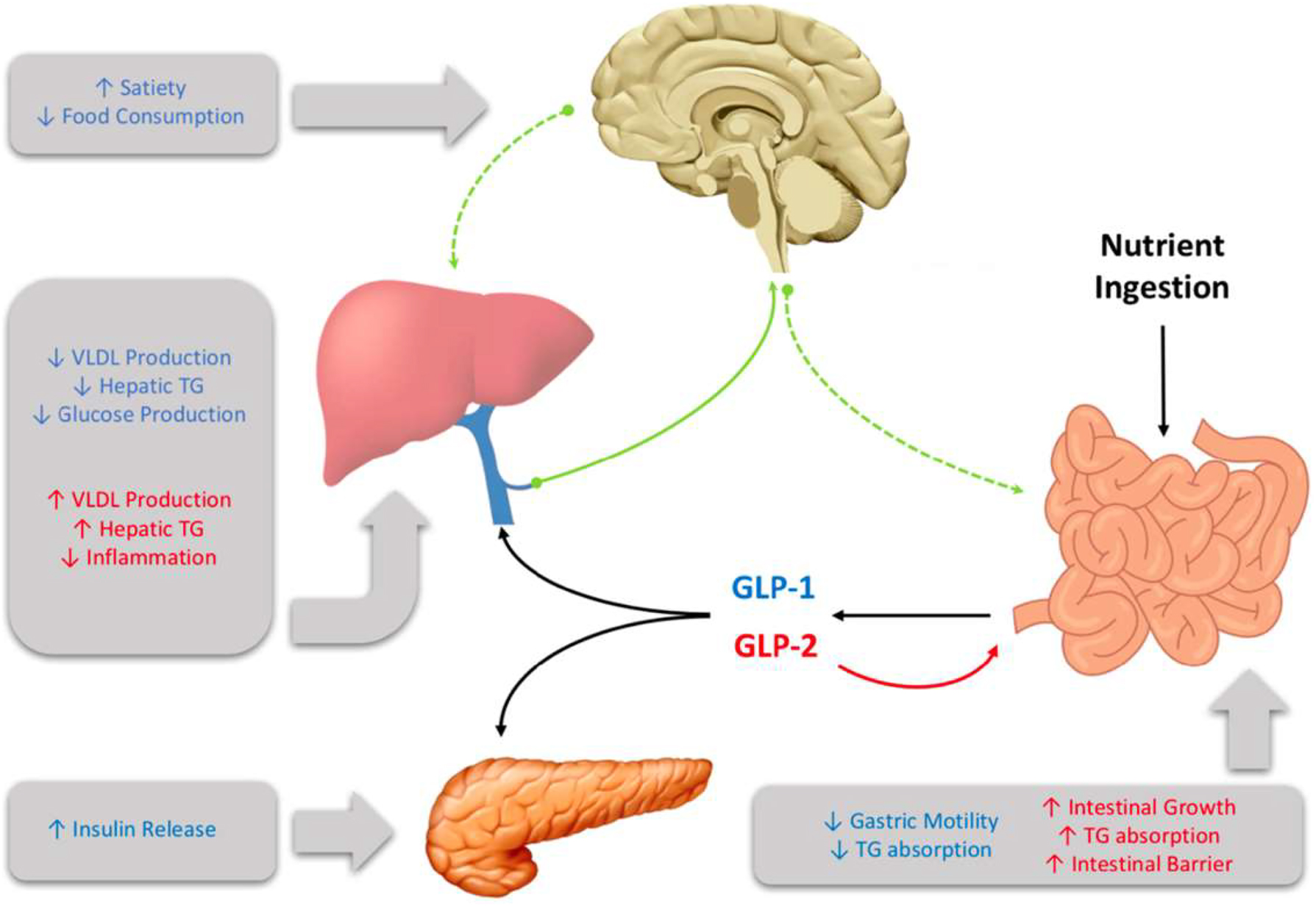
Glucagon-like peptide (GLP)-1 and GLP-2 are multi-organ hormones that exert their effects through both central and peripheral signalling.
Direct impact of GLP-1 on hepatic functions
The complete understanding of the metabolic repercussions of GLP-1 and GLP-2 on hepatic lipid and lipoprotein metabolism is still pending (Figure 2). Nonetheless, both play a significant role in influencing hepatic health. When primary hepatocytes and immortalized cell lines are exposed to GLP-1R agonists, the outcomes appear to be diverse. Despite reports indicating the expression and functional activity of GLP-1Rs in immortalized hepatocyte cell lines like HuH7 and HepG2, as well as in primary human hepatocytes [48], the effects vary. Upon treatment with the GLP-1R agonist exendin-4, steatotic HuH7 and HepG2 cells exhibited reduced lipid accumulation compared to vehicle controls, as evidenced by oil red O staining [48]. Furthermore, HepG2 cells treated with liraglutide demonstrated a dose-dependent decrease in protein and mRNA levels of proprotein convertase subtilisin/kexin type 9 (PCSK9), a pro-hypercholesterolemic factor [49]. In contrast, McArdle cells treated with palmitic acid displayed increased expression of genes involved in de novo lipogenesis (SREBP-1c, FAS, SCD1, and ACC). Co-incubation with exendin-4, however, mitigated this steatotic phenotype [50]. Surprisingly, ex vivo treatment of primary hamster hepatocytes with exendin-4 did not alter cAMP production, an indicator of GLP-1R signaling [51]. This is unexpected since GLP-1R activation is anticipated to elevate cAMP within hepatocytes, leading to increased phosphorylation of AMPK, a key enzymatic suppressor of lipogenesis [52]. Consequently, conflicting evidence exists regarding the presence of GLP-1Rs on hepatocytes [53], [54], [55], [56]. Interestingly, however, a direct effect of GLP-1 infusion on endogenous glucose production has been demonstrated in humans under conditions where plasma insulin and glucagon are not allowed to change and glucose concentrations are matched, suggesting a potential direct effect on hepatocytes [57].
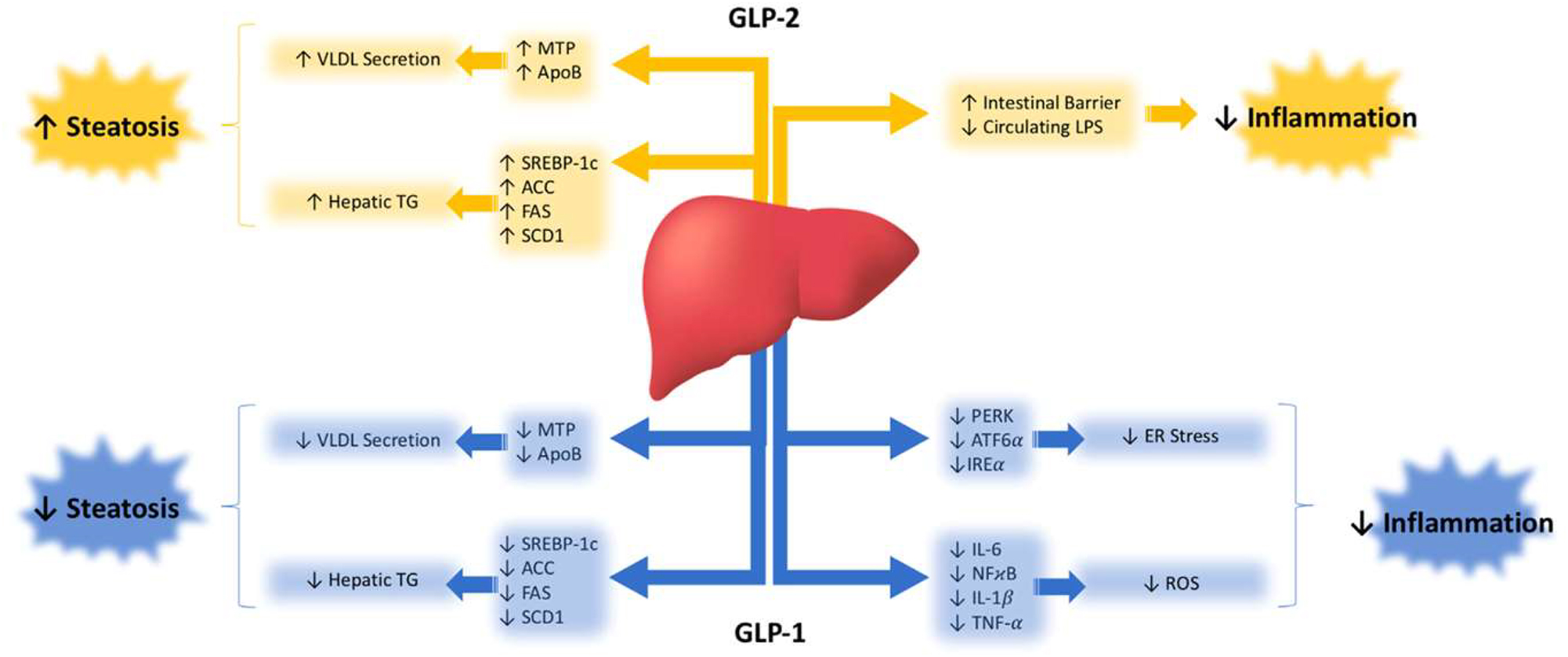
Glucagon-like peptide (GLP)-1 and GLP-2 modulate liver health through various metabolic signalling cascades.
Similar uncertainties surround GLP-2R, which is acknowledged to be expressed in the nervous system and on enteric neurons, myofibroblasts, and enteroendocrine cells of the gut. However, its presence in the liver is contentious, with some studies suggesting its presence [58] and others indicating its absence [59] in hepatocytes. A study investigating the localization of intravenously injected radiolabeled 125 I-GLP-2 (1–33) proposed that the primary site of GLP-2R-specific binding is within the small intestine. The liver and kidney were proposed to metabolize GLP-2 through a non-specific metabolic pathway [60]. Consequently, further research is imperative to definitively establish whether GLP-1 and GLP-2 directly exert their metabolic effects on the liver.
Direct intestinal effects of GLP-1
In humans GLP-1 has been shown to slow digestion by suppressing gastric acid secretion [61] and slowing gastric emptying [62, 63]. In rats GLP-1 has been shown to lower plasma lipid accumulation after intraduodenal fat load by suppressing intestinal lymph flow, triglyceride absorption, and ApoB production [64]. Similarly, GLP-1R agonist treatment in Syrian golden hamsters suppressed hepatic very-low density lipoprotein (VLDL) and TRL TG accumulation, with tandem reductions in TRL ApoB48 [65]. Peripheral exendin-4 treatment has also been shown to depress intestinal microsomal triglyceride transfer protein (MTP) activity, decreasing the lipidation and secretion of ApoB48 particles after lipid load [66]. Recently, the GLP-1R has been found in human colon cell culture models, and in the colonic epithelium [67]. Similarly, in CD1 mice GLP-1R expression was found by immunohistochemical staining to be localized to the mucosal villi layer of the ileum and colon [68]. Interestingly, while GLP-1Rs are not found on Villin+enterocytes and are predominantly localized to intraepithelial lymphocytes (IELs) [69]. Regardless, GLP-1R agonist treatment has been shown to reduce ApoB48 secretion from primary hamster enterocyte cultures [65]. Similarly, exendin-4 has been shown to directly increase cAMP activity in these IELs, however, their role in modulating enterocyte function has yet to be fully explored [69]. GLP-1R mRNA has also been found in acid secreting parietal cells [70] where they may block gastric acid secretion and in specialized mucus secreting cells in the proximal duodenum called Brunners glands [71].
GLP-1R expression is also prominent in the enteric nervous system (ENS). In reporter mice expressing yellow fluorescent protein (YFP) under a GLP-1R-Cre, expression was found in a subpopulation of enteric neurons, the action potential frequency of which could be modulated ex vivo by GLP-1 administration [23]. Interestingly, a significant proportion of these ENS neurons were positive for neuronal nitric oxide synthase (nNOS) expression, which has been linked to the pro-lipemic properties of GLP-2 [72]. RNA seq data suggests that these neurons may be secretomotor/vasodilatory in nature [73]. However, their relative importance to GLP-1s anti-lipemic effects may be minimal as GLP-1R KO in the enteric neurons of mice did not affect plasma TG accumulation after an oral fat load [74].
Gut-brain axis
Recent data has demonstrated a strong role for bidirectional neuronal governance over intestinal and hepatic lipid metabolism. The intestine itself boasts its own independent neural network called the enteric plexus, which is comprised of over 100 million neurons that can operate autonomously, or in tandem with afferent and efferent sensory feedback from the CNS [75]. To complicate this, the intestine is the largest endocrine organ in the body, secreting over 100 bioactive peptides, which can act in an autocrine, paracrine, and neuroendocrine manner [76]. These hormonal signals when released upon nutrient consumption can signal through binding to vagal or somatosensory afferent nerve fibers which project to the dorsal vagal complex in the brainstem, in turn relaying sensory information to key metabolic regulatory nuclei in the hypothalamus. Alternatively, some hormones may be able to circulate and directly bind to receptors in hypothalamic nuclei, either by active transport through the blood brain barrier, or via access to circumventricular organs [77], [78], [79], [80]. This gut-brain axis has already been implicated in the signaling of several key hormones released by the gut such as GLP-1, CCK, and PYY. These hormones act as intermediate messengers to signal information via peripheral nerves about meal size and composition to the brain [1, 81]. Hypothalamic centers in the brain then act on these signals to alter key components of metabolism such as energy expenditure, food intake, and gastrointestinal function [82, 83]. Many have demonstrated the integral role of the vagus nerve in mediating this system, as CCK-related satiation is dependent on intact gastric and duodenal vagal afferent signaling [84, 85]. Similarly, the inhibitory effects of PYY and GLP-1 and the stimulatory effects of ghrelin on feeding, were lost after vagotomy [86], [87], [88]. In the same vein, the anorectic effects of GLP-1 by intraperitoneal exendin-4 administration were also lost after capsaicin-mediated vagal denervation [89]. Loss of certain subpopulations of vagal nerves shows similar results, where selective denervation of CCK receptor-containing vagal afferents abolished the satiating effects of GLP-1 and CCK, as well as feeding-induced c-fos expression in the NTS [90]. The relative contributions of the peripheral and central nervous systems are discussed further below.
Peripheral neural control of metabolism
The peripheral nervous system consists of nerves and associates ganglia which lie outside the brain and spinal cord. Autonomic signals from these nerves deliver sympathetic and parasympathetic drive to the body wall and viscera. The sympathetic system provides the “fight or flight” response, and its effects are mediated through stimulatory neurotransmitters such as norepinephrine and epinephrine. Pre-ganglionic sympathetic motor neurons originate from the ventral horn of the spinal cord, they then travel through ventral rootlets to the white rami communicantes of spinal nerves, where they synapse on post-ganglionic sympathetic neurons in sympathetic chain ganglia. Contributions of several spinal levels spanning thoracic spinal nerve 5 (T5) to T12 form sympathetic splanchnic nerves which innervate the viscera [91]. In contrast, the parasympathetic system provides the “rest and digest” response, mediated by release of the neurotransmitter acetylcholine. Parasympathetic neurons which innervate the viscera do not originate from the spinal cord, but from cranial nerves which project directly from the brainstem. Together, the sympathetic greater and lesser splanchnic nerves, and parasympathetic anterior and posterior vagal trunks coalesce in the celiac plexus (solar plexus), which is a network of interconnecting fibers that innervate the abdominal contents, both uniquely influencing metabolism and lipoprotein production [87].
Neural control of hepatic lipoprotein metabolism by GLP-1
It has long been known that GLP-1 and GLP-2 can act as neurotransmitters in the brain [92]. Early studies have shown that hindbrain pre-proglucagon (PPG) producing neurons in the NTS can be activated even without gut-associated hormone release, just by simple mechanical distention of the stomach [93]. This, paired with the observation that GLP-1 can act as a neuroendocrine signalling peptide in the circulation tightly links central and peripheral GLP-1 signalling to the regulation of energy homeostasis and satiety. Although the effect of GLP-1 in regulating satiety has been known for some time, the relative contribution of GLP-1 to hepatic and intestinal lipid homeostasis is only recently emerging. Intracerebroventricular (ICV) injection of active GLP-1(7–37) peptide into the brains of HFD-fed mice resulted in enhanced Akt-mediated hepatic insulin signalling during hyperinsulinemic-euglycemic clamp experiments. This was associated with elevations in insulin secretion, improved glucose tolerance, and decreased hepatic TG accumulation in the livers of HFD-fed mice. In contrast, ICV injection of the GLP-1R antagonist exendin 9–39 impaired the suppressive effects of insulin on hepatic glucose production, suggesting that central GLP-1R inhibition deteriorates hepatic insulin signalling. Moreover, central GLP-1R agonism selectively attenuated hepatic TG accumulation in HFD-fed mice, with no observed change in muscle, white adipose tissue (WAT), or plasma TG during hyperinsulinemia [93]. ICV injections of exendin-4 have also been shown to lower fasting hypertriglyceridemia and hepatic VLDL production in a dietary fructose-induced dyslipidemic hamster model [50]. Similarly, both acute and chronic treatment with ICV exendin-4 has been shown to reduce circulating plasma TG, cholesterol, and low-density lipoprotein cholesterol (LDLc) in addition to hepatic lipids. This was further associated with reductions in hepatic expression of sterol regulatory element binding protein (SREBP)-1c and elevated LDL receptor expression, which occurred independent of food consumption [94].
Separate experiments have shown that bilateral injection of active GLP-1(7–37) peptide into the dorsomedial hypothalamus (DMH) of mice results in increased TG mobilization from the liver. Alternatively, GLP-1R knockdown in the DMH induced hepatic steatosis, coupled with elevated de novo lipogenesis, and the development of insulin resistance [95]. This is in line with previous observations that ICV administration of exendin-4 increases sympathetic outflow to brown adipose tissue (BAT) and WAT depots, resulting in increased thermogenesis and uptake of fatty acid (FA) to these tissues. Interestingly, this effect on WAT was seen to be blunted in a diet-induced obese mouse model [96]. Recently, chronic ICV infusion of a GLP-1R and glucagon receptor co-agonist has been shown to significantly decrease plasma and liver lipids in a cholesterol-fed hamster model of dyslipidemia. Co-agonist treated hamsters also showed increased hepatic TG excursion, and depressed expression of hepatic lipogenic factors such as SREBP-1c, 3-hydroxy-3-methyl-glutaryl (HMG)-CoA reductase, stearoyl-CoA desaturase (SCD)-1, FA synthase, and acetyl-CoA carboxylase. Interestingly, these effects could be partially blocked by co-administration of the GLP-1R antagonist exendin 9–39, and completely abrogated by surgical vagotomy [97] - further reinforcing the importance of central GLP-1R activity, and its influence on hepatic lipid metabolism.
Genetic ablation of specific neuronal GLP-1R-containing populations has recently been achieved in mice. Ablation in Wnt1 expressing neurons, representing neurons in the hypothalamus, brainstem, and ENS was compared to ablation in Phox2b-expressing neurons, representing peripheral autonomic nerves. Strikingly, plasma TG following an oral fat load was unaffected in either model, nor were the anti-lipemic effects of several GLP-1R agonists [74]. While lipid tolerance was unaffected, the distinct kinetics of excursion and clearance were not assessed; nor were intestinal and hepatic lipoproteins delineated. Thus, the exact signaling cascade resulting in central modulation of lipoprotein secretion by GLP-1 has yet to be fully elucidated.
The most compelling data for central control over intestinal lipoprotein metabolism comes from animal studies examining the effects of central GLP-1R activation. In the Syrian golden hamster ICV injections of exendin 9–39 into the third ventricle acutely depressed postprandial TRL-TG and ApoB48 secretion by approximately 55 %. Antagonism of central GLP-1R with exendin 9–39 did not completely abrogate the effects of peripheral exendin-4 administration, suggesting at least partial independence of these systems under conditions of prolonged GLP-1 activation. Importantly, depressions in postprandial lipoprotein metabolism via central GLP-1R activation were mediated via increased sympathetic outflow, as pharmacological blockade abolished the effects of ICV GLP-1. While no changes were seen in the activity of lipogenic genes, jejunal MTP activity was significantly reduced, explaining how sympathetic outflow may exert rapid temporal control over chylomicron lipidation and secretion [66].
However, endogenous GLP-1 is rapidly cleaved in the circulation, leading to recent doubts regarding its endocrine potential to signal central metabolic regulatory nuclei and peripheral organs [98]. That said, native GLP-1 is secreted into the portal vein, which is richly innervated with vagal afferent nerve terminals containing GLP-1Rs [41]; constituting a rapid mechanism by which GLP-1 may exert its anti-lipemic effects within its short life-span. The GLP-1R containing vagal afferents overlying the portal bed house their cell bodies in the nodose ganglia where GLP-1R expression has also been observed [99]. Moreover, primary isolated nodose neurons show action potential generation, coupled with increases in intracellular Ca2+ when exposed to GLP-1 [100]. The role of these GLP-1R-containing NG neurons in regulating peripheral metabolism has been evidenced previously by GLP-1R knockdown in the vagal afferent nerves of rats, which display increased food consumption, accelerated gastric emptying, and post-meal glycemia coupled with depressed insulin secretion [101, 102]. Moreover, vagal afferent neurons project to GLP-1 producing neurons in the caudal brainstem which secrete GLP-1 into hypothalamic nuclei which regulate energy metabolism [103]. Importantly, activation of GLP-1Rs in the CNS shows similar anti-lipemic actions as peripheral administration, where ICV injection of exendin-4 markedly suppressed chylomicron excursion [66]. Together, suggesting that a portal-vagal axis may explain how endogenous GLP-1 modulates lipoprotein production. Indeed, recently we have demonstrated the importance of this portal-vagal axis in lipid homeostasis in Syrian golden hamsters (Figure 3). Wherein, portal but not jugular or caval administration of active GLP-1(7–37) caused significant reductions in postprandial lipids [104]. This reduction was lost upon surgical or pharmacological vagal deafferentation, or under conditions of adrenergic blockade – corroborating previous reports that GLP-1 both signals via the vagus and reduces plasma lipids via sympathetic signalling [66, 101]. Strikingly, this axis was sensitive to diet-induced insulin resistance, and portal GLP-1 resistance rapidly developed under high-fructose diet feeding [104]. Suggesting that loss of endogenous GLP-1 signalling may be a contributing or initiating factor in the development of hypertriglyceridemia.
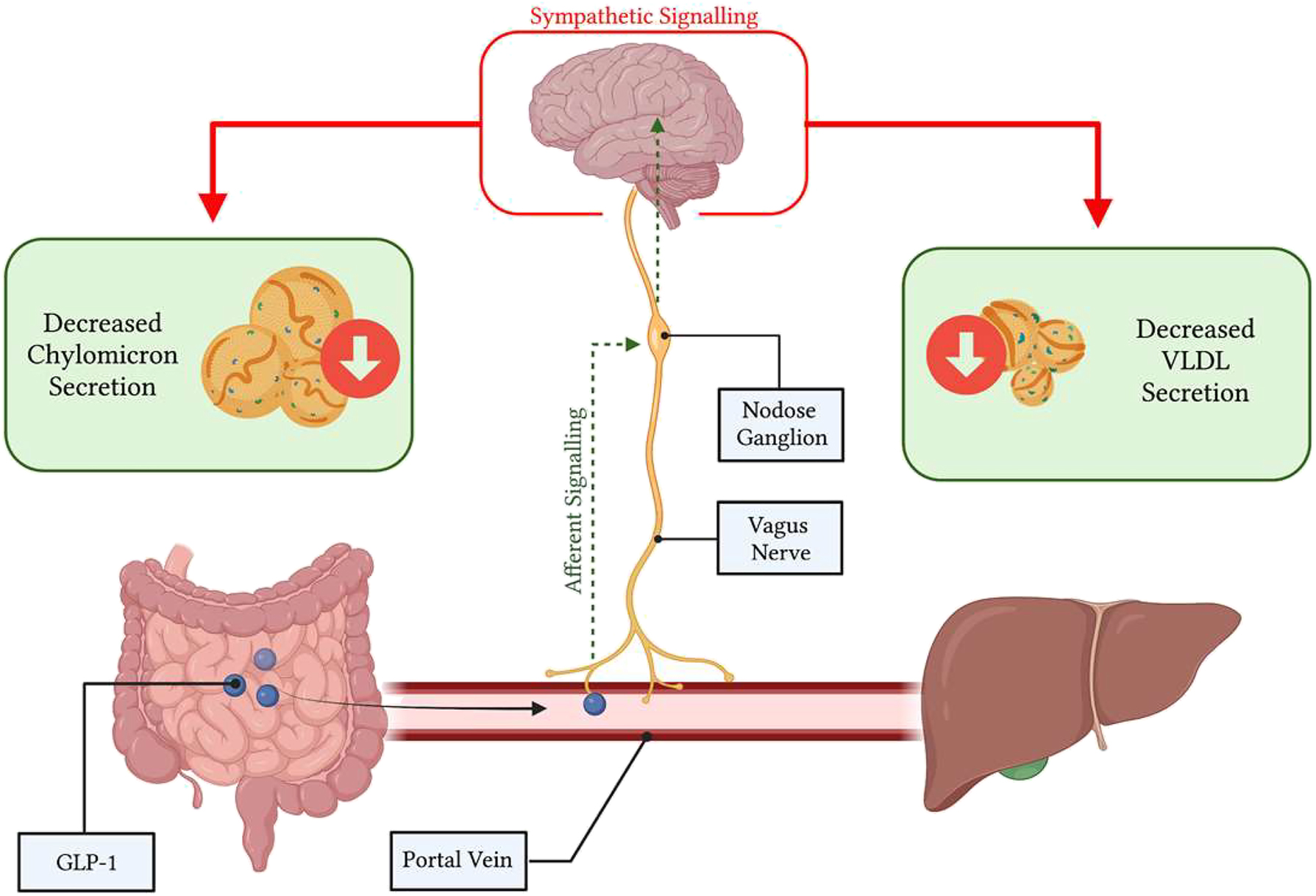
Glucagon-like peptide (GLP)-1 works through a portal-vagal signalling axis to modulate postprandial and fasting lipids. Native GLP-1 works in a site-specific manner within the portal vein, binding to GLP-1R on vagal afferent nerves. Vagal afferents project to the nodose ganglion to integrate viscerosensory data, then impulses are propagated to the brainstem, and central metabolic regulator nuclei. Changes in efferent sympathetic tone alter postprandial and fasting lipoprotein secretion and lipemia. Image created with BioRender.com.
Concluding remarks
Dyslipidemia is a common co-morbidity in insulin resistant states, resulting in the overproduction of ApoB-containing chylomicron particles, VLDL particles, and elevated plasma TG levels [100]. This leads to the generation of atherogenic remnant particles, precursors to the development of atherosclerosis and CVD, the leading cause of death in T2D [105, 106]. Several hormones have been shown to regulate intestinal and hepatic lipid metabolism such as insulin and gut-derived incretin hormones released upon nutrient consumption [50, 107]. Key viscerosensory peptides released from the gut are the incretin hormones GLP-1 and gastric inhibitory peptide (GIP), CCK, and PYY [74, 84, 108], [109], [110]. Notably, GLP-1 has been shown by our laboratory and others to modulate intestinal and hepatic lipoprotein production through a complex gut-brain-liver axis [66, 111], [112], [113].
Funding source: Canadian Institutes of Health Research
Award Identifier / Grant number: FDN-148396
-
Research ethics: This is a review article and does not require ethical approval.
-
Informed consent: Not applicable.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Competing interests: Authors state no conflict of interest.
-
Research funding: Canadian Institutes of Health Research (CIHR) (FDN-148396).
-
Data availability: Not applicable.
References
1. Gribble, FM, Reimann, F. Function and mechanisms of enteroendocrine cells and gut hormones in metabolism. Nat Rev Endocrinol 2019;15:226–37. https://doi.org/10.1038/s41574-019-0168-8.Search in Google Scholar PubMed
2. Hein, GJ, Baker, C, Hsieh, J, Farr, S, Adeli, K. GLP-1 and GLP-2 as yin and yang of intestinal lipoprotein production: evidence for predominance of GLP-2-stimulated postprandial lipemia in normal and insulin-resistant states. Diabetes 2013;62:373–81. https://doi.org/10.2337/db12-0202.Search in Google Scholar PubMed PubMed Central
3. Murphy, KG, Bloom, SR. Gut hormones and the regulation of energy homeostasis. Nature 2006;444:854–9. https://doi.org/10.1038/nature05484.Search in Google Scholar PubMed
4. Mojsov, S, Heinrich, G, Wilson, IB, Ravazzola, M, Orci, L, Habener, JF. Preproglucagon gene expression in pancreas and intestine diversifies at the level of post-translational processing. J Biol Chem 1986;261:11880–9. https://doi.org/10.1016/s0021-9258(18)67324-7.Search in Google Scholar
5. Glucagon like peptide – an overview. Science Direct Topics [Internet]. 2023 Available from: https://www.sciencedirect.com/topics/medicine-and-dentistry/glucagon-like-peptide [Accessed 30 Jan 2024].Search in Google Scholar
6. Nauck, MA, Quast, DR, Wefers, J, Meier, JJ. GLP-1 receptor agonists in the treatment of type 2 diabetes – state-of-the-art. Mol Metabol 2021;46:101102. https://doi.org/10.1016/j.molmet.2020.101102.Search in Google Scholar PubMed PubMed Central
7. Kounatidis, D, Vallianou, NG, Tsilingiris, D, Christodoulatos, GS, Geladari, E, Stratigou, T, et al.. Therapeutic potential of GLP-2 analogs in gastrointestinal disorders: current knowledge, nutritional aspects, and future perspectives. Curr Nutr Rep 2022;11:618–42. https://doi.org/10.1007/s13668-022-00433-0.Search in Google Scholar PubMed
8. Larsen, PJ, Tang-Christensen, M, Holst, JJ, Orskov, C. Distribution of glucagon-like peptide-1 and other preproglucagon-derived peptides in the rat hypothalamus and brainstem. Neuroscience 1997;77:257–70. https://doi.org/10.1016/s0306-4522(96)00434-4.Search in Google Scholar PubMed
9. Padidela, R, Patterson, M, Sharief, N, Ghatei, M, Hussain, K. Elevated basal and post-feed glucagon-like peptide 1 (GLP-1) concentrations in the neonatal period. Eur J Endocrinol 2009;160:53–8. https://doi.org/10.1530/eje-08-0807.Search in Google Scholar PubMed
10. Brubaker, PL, Crivici, A, Izzo, A, Ehrlich, P, Tsai, CH, Drucker, DJ. Circulating and tissue forms of the intestinal growth factor, glucagon-like peptide-2. Endocrinology 1997;138:4837–43. https://doi.org/10.1210/endo.138.11.5482.Search in Google Scholar PubMed
11. Herrmann, C, Göke, R, Richter, G, Fehmann, HC, Arnold, R, Göke, B. Glucagon-like peptide-1 and glucose-dependent insulin-releasing polypeptide plasma levels in response to nutrients. Digestion 1995;56:117–26. https://doi.org/10.1159/000201231.Search in Google Scholar PubMed
12. Tarini, J, Wolever, TMS. The fermentable fibre inulin increases postprandial serum short-chain fatty acids and reduces free-fatty acids and ghrelin in healthy subjects. Appl Physiol Nutr Metab Physiol Appl Nutr Metab 2010;35:9–16. https://doi.org/10.1139/h09-119.Search in Google Scholar PubMed
13. Hansen, KB, Rosenkilde, MM, Knop, FK, Wellner, N, Diep, TA, Rehfeld, JF, et al.. 2-Oleoyl glycerol is a GPR119 agonist and signals GLP-1 release in humans. J Clin Endocrinol Metab 2011;96:E1409–1417. https://doi.org/10.1210/jc.2011-0647.Search in Google Scholar PubMed
14. Edfalk, S, Steneberg, P, Edlund, H. Gpr40 is expressed in enteroendocrine cells and mediates free fatty acid stimulation of incretin secretion. Diabetes 2008;57:2280–7. https://doi.org/10.2337/db08-0307.Search in Google Scholar PubMed PubMed Central
15. Hirasawa, A, Tsumaya, K, Awaji, T, Katsuma, S, Adachi, T, Yamada, M, et al.. Free fatty acids regulate gut incretin glucagon-like peptide-1 secretion through GPR120. Nat Med 2005;11:90–4. https://doi.org/10.1038/nm1168.Search in Google Scholar PubMed
16. Yoder, SM, Yang, Q, Kindel, TL, Tso, P. Stimulation of incretin secretion by dietary lipid: is it dose dependent? Am J Physiol Gastrointest Liver Physiol 2009;297:G299–305. https://doi.org/10.1152/ajpgi.90601.2008.Search in Google Scholar PubMed PubMed Central
17. Sadik, R, Abrahamsson, H, Stotzer, PO. Gender differences in gut transit shown with a newly developed radiological procedure. Scand J Gastroenterol 2003;38:36–42. https://doi.org/10.1080/00365520310000410.Search in Google Scholar PubMed
18. Kim, SK. Small intestine transit time in the normal small bowel study. Am J Roentgenol Radium Ther Nucl Med 1968;104:522–4. https://doi.org/10.2214/ajr.104.3.522.Search in Google Scholar PubMed
19. Rocca, AS, Brubaker, PL. Role of the vagus nerve in mediating proximal nutrient-induced glucagon-like peptide-1 secretion. Endocrinology 1999;140:1687–94. https://doi.org/10.1210/endo.140.4.6643.Search in Google Scholar PubMed
20. Anini, Y, Hansotia, T, Brubaker, PL. Muscarinic receptors control postprandial release of glucagon-like peptide-1: in vivo and in vitro studies in rats. Endocrinology 2002;143:2420–6. https://doi.org/10.1210/en.143.6.2420.Search in Google Scholar
21. Balks, HJ, Holst, JJ, von zur Mühlen, A, Brabant, G. Rapid oscillations in plasma glucagon-like peptide-1 (GLP-1) in humans: cholinergic control of GLP-1 secretion via muscarinic receptors. J Clin Endocrinol Metab 1997;82:786–90. https://doi.org/10.1210/jc.82.3.786.Search in Google Scholar
22. Lagerström, MC, Schiöth, HB. Structural diversity of G protein-coupled receptors and significance for drug discovery. Nat Rev Drug Discov 2008;7:339–57. https://doi.org/10.1038/nrd2518.Search in Google Scholar PubMed
23. Richards, P, Parker, HE, Adriaenssens, AE, Hodgson, JM, Cork, SC, Trapp, S, et al.. Identification and characterization of GLP-1 receptor-expressing cells using a new transgenic mouse model. Diabetes 2014;63:1224–33. https://doi.org/10.2337/db13-1440.Search in Google Scholar PubMed PubMed Central
24. Manchanda, Y, Bitsi, S, Kang, Y, Jones, B, Tomas, A. Spatiotemporal control of GLP-1 receptor activity. Curr Opin Endocr Metab Res 2021;16:19–27. https://doi.org/10.1016/j.coemr.2020.07.003.Search in Google Scholar
25. Hällbrink, M, Holmqvist, T, Olsson, M, Ostenson, CG, Efendic, S, Langel, U. Different domains in the third intracellular loop of the GLP-1 receptor are responsible for Galpha(s) and Galpha(i)/Galpha(o) activation. Biochim Biophys Acta 2001;1546:79–86. https://doi.org/10.1016/s0167-4838(00)00270-3.Search in Google Scholar PubMed
26. Couvineau, A, Laburthe, M. The family B1 GPCR: structural aspects and interaction with accessory proteins. Curr Drug Targets 2012;13:103–15. https://doi.org/10.2174/138945012798868434.Search in Google Scholar PubMed
27. Wootten, D, Reynolds, CA, Smith, KJ, Mobarec, JC, Koole, C, Savage, EE, et al.. The extracellular surface of the GLP-1 receptor is a molecular trigger for biased agonism. Cell 2016;165:1632–43. https://doi.org/10.1016/j.cell.2016.05.023.Search in Google Scholar PubMed PubMed Central
28. Light, PE, Manning Fox, JE, Riedel, MJ, Wheeler, MB. Glucagon-like peptide-1 inhibits pancreatic ATP-sensitive potassium channels via a protein kinase A- and ADP-dependent mechanism. Mol Endocrinol Baltim Md 2002;16:2135–44. https://doi.org/10.1210/me.2002-0084.Search in Google Scholar PubMed
29. Britsch, S, Krippeit-Drews, P, Lang, F, Gregor, M, Drews, G. Glucagon-like peptide-1 modulates Ca2+ current but not K+ATP current in intact mouse pancreatic B-cells. Biochem Biophys Res Commun 1995;207:33–9. https://doi.org/10.1006/bbrc.1995.1149.Search in Google Scholar PubMed
30. Leech, CA, Habener, JF. Insulinotropic glucagon-like peptide-1-mediated activation of non-selective cation currents in insulinoma cells is mimicked by maitotoxin. J Biol Chem 1997;272:17987–93. https://doi.org/10.1074/jbc.272.29.17987.Search in Google Scholar PubMed
31. Wheeler, MB, Lu, M, Dillon, JS, Leng, XH, Chen, C, Boyd, AE. Functional expression of the rat glucagon-like peptide-I receptor, evidence for coupling to both adenylyl cyclase and phospholipase-C. Endocrinology 1993;133:57–62. https://doi.org/10.1210/endo.133.1.8391428.Search in Google Scholar PubMed
32. Thompson, A, Kanamarlapudi, V. Agonist-induced internalisation of the glucagon-like peptide-1 receptor is mediated by the Gαq pathway. Biochem Pharmacol 2015;93:72–84. https://doi.org/10.1016/j.bcp.2014.10.015.Search in Google Scholar PubMed
33. Gurevich, VV, Gurevich, EV. GPCR signaling regulation: the role of GRKs and arrestins. Front Pharmacol 2019;10:125. https://doi.org/10.3389/fphar.2019.00125.Search in Google Scholar PubMed PubMed Central
34. Thomsen, ARB, Plouffe, B, Cahill, TJ, Shukla, AK, Tarrasch, JT, Dosey, AM, et al.. GPCR-G protein-β-arrestin super-complex mediates sustained G protein signaling. Cell 2016;166:907–19. https://doi.org/10.1016/j.cell.2016.07.004.Search in Google Scholar PubMed PubMed Central
35. Hayes, MR, Leichner, TM, Zhao, S, Lee, GS, Chowansky, A, Zimmer, D, et al.. Intracellular signals mediating the food intake-suppressive effects of hindbrain glucagon-like peptide-1 receptor activation. Cell Metabol 2011;13:320–30. https://doi.org/10.1016/j.cmet.2011.02.001.Search in Google Scholar PubMed PubMed Central
36. Gilman, CP, Perry, T, Furukawa, K, Grieg, NH, Egan, JM, Mattson, MP. Glucagon-like peptide 1 modulates calcium responses to glutamate and membrane depolarization in hippocampal neurons. J Neurochem 2003;87:1137–44. https://doi.org/10.1046/j.1471-4159.2003.02073.x.Search in Google Scholar PubMed
37. Liu, J, Conde, K, Zhang, P, Lilascharoen, V, Xu, Z, Lim, BK, et al.. Enhanced AMPA receptor trafficking mediates the anorexigenic effect of endogenous glucagon-like peptide-1 in the paraventricular hypothalamus. Neuron 2017;96:897–909.e5. https://doi.org/10.1016/j.neuron.2017.09.042.Search in Google Scholar PubMed PubMed Central
38. Mojsov, S, Weir, GC, Habener, JF. Insulinotropin: glucagon-like peptide I (7-37) co-encoded in the glucagon gene is a potent stimulator of insulin release in the perfused rat pancreas. J Clin Invest 1987;79:616–9. https://doi.org/10.1172/jci112855.Search in Google Scholar
39. Zander, M, Madsbad, S, Madsen, JL, Holst, JJ. Effect of 6-week course of glucagon-like peptide 1 on glycaemic control, insulin sensitivity, and beta-cell function in type 2 diabetes: a parallel-group study. Lancet Lond Engl 2002;359:824–30. https://doi.org/10.1016/s0140-6736(02)07952-7.Search in Google Scholar
40. Schirra, J, Sturm, K, Leicht, P, Arnold, R, Göke, B, Katschinski, M. Exendin(9-39)amide is an antagonist of glucagon-like peptide-1(7-36)amide in humans. J Clin Invest 1998;101:1421–30. https://doi.org/10.1172/jci1349.Search in Google Scholar PubMed PubMed Central
41. Vahl, TP, Tauchi, M, Durler, TS, Elfers, EE, Fernandes, TM, Bitner, RD, et al.. Glucagon-like peptide-1 (GLP-1) receptors expressed on nerve terminals in the portal vein mediate the effects of endogenous GLP-1 on glucose tolerance in rats. Endocrinology 2007;148:4965–73. https://doi.org/10.1210/en.2006-0153.Search in Google Scholar PubMed
42. Fehmann, HC, Habener, JF. Functional receptors for the insulinotropic hormone glucagon-like peptide-I(7-37) on a somatostatin secreting cell line. FEBS Lett 1991;279:335–40. https://doi.org/10.1016/0014-5793(91)80182-3.Search in Google Scholar PubMed
43. Heller, RS, Kieffer, TJ, Habener, JF. Insulinotropic glucagon-like peptide I receptor expression in glucagon-producing alpha-cells of the rat endocrine pancreas. Diabetes 1997;46:785–91. https://doi.org/10.2337/diabetes.46.5.785.Search in Google Scholar
44. Creutzfeldt, WO, Kleine, N, Willms, B, Orskov, C, Holst, JJ, Nauck, MA. Glucagonostatic actions and reduction of fasting hyperglycemia by exogenous glucagon-like peptide I(7-36) amide in type I diabetic patients. Diabetes Care 1996;19:580–6. https://doi.org/10.2337/diacare.19.6.580.Search in Google Scholar PubMed
45. Nauck, MA, Meier, JJ, Cavender, MA, Abd El Aziz, M, Drucker, DJ. Cardiovascular actions and clinical outcomes with glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors. Circulation 2017;136:849–70. https://doi.org/10.1161/circulationaha.117.028136.Search in Google Scholar
46. Xiao, C, Bandsma, RHJ, Dash, S, Szeto, L, Lewis, GF. Exenatide, a glucagon-like peptide-1 receptor agonist, acutely inhibits intestinal lipoprotein production in healthy humans. Arterioscler Thromb Vasc Biol 2012;32:1513–9. https://doi.org/10.1161/atvbaha.112.246207.Search in Google Scholar PubMed
47. Schwartz, EA, Koska, J, Mullin, MP, Syoufi, I, Schwenke, DC, Reaven, PD. Exenatide suppresses postprandial elevations in lipids and lipoproteins in individuals with impaired glucose tolerance and recent onset type 2 diabetes mellitus. Atherosclerosis 2010;212:217–22. https://doi.org/10.1016/j.atherosclerosis.2010.05.028.Search in Google Scholar PubMed
48. Gupta, NA, Kolachala, VL, Jiang, R, Abramowsky, C, Romero, R, Fifadara, N, et al.. The glucagon-like peptide-1 receptor agonist exendin 4 has a protective role in ischemic injury of lean and steatotic liver by inhibiting cell death and stimulating lipolysis. Am J Pathol 2012;181:1693–701. https://doi.org/10.1016/j.ajpath.2012.07.015.Search in Google Scholar PubMed PubMed Central
49. Yang, SH, Xu, RX, Cui, CJ, Wang, Y, Du, Y, Chen, ZG, et al.. Liraglutide downregulates hepatic LDL receptor and PCSK9 expression in HepG2 cells and db/db mice through a HNF-1a dependent mechanism. Cardiovasc Diabetol 2018;17:48. https://doi.org/10.1186/s12933-018-0689-9.Search in Google Scholar PubMed PubMed Central
50. Khound, R, Taher, J, Baker, C, Adeli, K, Su, Q. GLP-1 elicits an intrinsic gut–liver metabolic signal to ameliorate diet-induced VLDL overproduction and insulin resistance. Arterioscler Thromb Vasc Biol 2017;37:2252–9. https://doi.org/10.1161/atvbaha.117.310251.Search in Google Scholar
51. Taher, J, Baker, CL, Cuizon, C, Masoudpour, H, Zhang, R, Farr, S, et al.. GLP-1 receptor agonism ameliorates hepatic VLDL overproduction and de novo lipogenesis in insulin resistance. Mol Metabol 2014;3:823–33. https://doi.org/10.1016/j.molmet.2014.09.005.Search in Google Scholar PubMed PubMed Central
52. Ben-Shlomo, S, Zvibel, I, Shnell, M, Shlomai, A, Chepurko, E, Halpern, Z, et al.. Glucagon-like peptide-1 reduces hepatic lipogenesis via activation of AMP-activated protein kinase. J Hepatol 2011;54:1214–23. https://doi.org/10.1016/j.jhep.2010.09.032.Search in Google Scholar PubMed
53. Panjwani, N, Mulvihill, EE, Longuet, C, Yusta, B, Campbell, JE, Brown, TJ, et al.. GLP-1 receptor activation indirectly reduces hepatic lipid accumulation but does not attenuate development of atherosclerosis in diabetic male ApoE−/− mice. Endocrinology 2013;154:127–39. https://doi.org/10.1210/en.2012-1937.Search in Google Scholar PubMed
54. Baggio, LL, Yusta, B, Mulvihill, EE, Cao, X, Streutker, CJ, Butany, J, et al.. GLP-1 receptor expression within the human heart. Endocrinology 2018;159:1570–84. https://doi.org/10.1210/en.2018-00004.Search in Google Scholar PubMed PubMed Central
55. Bullock, BP, Heller, RS, Habener, JF. Tissue distribution of messenger ribonucleic acid encoding the rat glucagon-like peptide-1 receptor. Endocrinology 1996;137:2968–78. https://doi.org/10.1210/endo.137.7.8770921.Search in Google Scholar PubMed
56. Liu, D, Pang, J, Shao, W, Gu, J, Zeng, Y, He, HH, et al.. Hepatic fibroblast growth factor 21 is involved in mediating functions of liraglutide in mice with dietary challenge. Hepatology 2021;74:2154–69. https://doi.org/10.1002/hep.31856.Search in Google Scholar PubMed
57. Seghieri, M, Rebelos, E, Gastaldelli, A, Astiarraga, BD, Casolaro, A, Barsotti, E, et al.. Direct effect of GLP-1 infusion on endogenous glucose production in humans. Diabetologia 2013;56:156–61. https://doi.org/10.1007/s00125-012-2738-3.Search in Google Scholar PubMed
58. El-Jamal, N, Erdual, E, Neunlist, M, Koriche, D, Dubuquoy, C, Maggiotto, F, et al.. Glugacon-like peptide-2: broad receptor expression, limited therapeutic effect on intestinal inflammation and novel role in liver regeneration. Am J Physiol Gastrointest Liver Physiol 2014;307:G274–85. https://doi.org/10.1152/ajpgi.00389.2012.Search in Google Scholar PubMed
59. Yusta, B, Huang, L, Munroe, D, Wolff, G, Fantaske, R, Sharma, S, et al.. Enteroendocrine localization of GLP-2 receptor expression in humans and rodents. Gastroenterology 2000;119:744–55. https://doi.org/10.1053/gast.2000.16489.Search in Google Scholar PubMed
60. Thulesen, J, Hartmann, B, Ørskov, C, Jeppesen, PB, Holst, JJ, Poulsen, SS. Potential targets for glucagon-like peptide 2 (GLP-2) in the rat: distribution and binding of i.v. injected 125I-GLP-2. Peptides 2000;21:1511–7. https://doi.org/10.1016/s0196-9781(00)00305-3.Search in Google Scholar PubMed
61. O’Halloran, DJ, Nikou, GC, Kreymann, B, Ghatei, MA, Bloom, SR. Glucagon-like peptide-1 (7-36)-NH2: a physiological inhibitor of gastric acid secretion in man. J Endocrinol 1990;126:169–73. https://doi.org/10.1677/joe.0.1260169.Search in Google Scholar PubMed
62. Schirra, J, Katschinski, M, Weidmann, C, Schäfer, T, Wank, U, Arnold, R, et al.. Gastric emptying and release of incretin hormones after glucose ingestion in humans. J Clin Invest 1996;97:92–103. https://doi.org/10.1172/jci118411.Search in Google Scholar PubMed PubMed Central
63. Schirra, J, Kuwert, P, Wank, U, Leicht, P, Arnold, R, Göke, B, et al.. Differential effects of subcutaneous GLP-1 on gastric emptying, antroduodenal motility, and pancreatic function in men. Proc Assoc Am Phys 1997;109:84–97.Search in Google Scholar
64. Qin, X, Shen, H, Liu, M, Yang, Q, Zheng, S, Sabo, M, et al.. GLP-1 reduces intestinal lymph flow, triglyceride absorption, and apolipoprotein production in rats. Am J Physiol Gastrointest Liver Physiol 2005;288:G943–949. https://doi.org/10.1152/ajpgi.00303.2004.Search in Google Scholar PubMed
65. Hsieh, J, Longuet, C, Baker, CL, Qin, B, Federico, LM, Drucker, DJ, et al.. The glucagon-like peptide 1 receptor is essential for postprandial lipoprotein synthesis and secretion in hamsters and mice. Diabetologia 2010;53:552–61. https://doi.org/10.1007/s00125-009-1611-5.Search in Google Scholar PubMed
66. Farr, S, Baker, C, Naples, M, Taher, J, Iqbal, J, Hussain, M, et al.. Central nervous system regulation of intestinal lipoprotein metabolism by glucagon-like peptide-1 via a brain–gut Axis. Arterioscler Thromb Vasc Biol 2015;35:1092–100. https://doi.org/10.1161/atvbaha.114.304873.Search in Google Scholar PubMed
67. Grau-Bové, C, González-Quilen, C, Cantini, G, Nardini, P, Espina, B, Bani, D, et al.. GLP1 exerts paracrine activity in the intestinal lumen of human colon. Int J Mol Sci 2022;23:3523. https://doi.org/10.3390/ijms23073523.Search in Google Scholar PubMed PubMed Central
68. Kedees, MH, Guz, Y, Grigoryan, M, Teitelman, G. Functional activity of murine intestinal mucosal cells is regulated by the glucagon-like peptide-1 receptor. Peptides 2013;48:36–44. https://doi.org/10.1016/j.peptides.2013.07.022.Search in Google Scholar PubMed
69. Yusta, B, Baggio, LL, Koehler, J, Holland, D, Cao, X, Pinnell, LJ, et al.. GLP-1R agonists modulate enteric immune responses through the intestinal intraepithelial lymphocyte GLP-1R. Diabetes 2015;64:2537–49. https://doi.org/10.2337/db14-1577.Search in Google Scholar PubMed
70. Broide, E, Bloch, O, Ben-Yehudah, G, Cantrell, D, Shirin, H, Rapoport, MJ. GLP-1 receptor is expressed in human stomach mucosa: analysis of its cellular association and distribution within gastric glands. J Histochem Cytochem Off J Histochem Soc. 2013;61:649–58. https://doi.org/10.1369/0022155413497586.Search in Google Scholar PubMed PubMed Central
71. Pyke, C, Heller, RS, Kirk, RK, Ørskov, C, Reedtz-Runge, S, Kaastrup, P, et al.. GLP-1 receptor localization in monkey and human tissue: novel distribution revealed with extensively validated monoclonal antibody. Endocrinology 2014;155:1280–90. https://doi.org/10.1210/en.2013-1934.Search in Google Scholar PubMed
72. Grande, EM, Raka, F, Hoffman, S, Adeli, K. GLP-2 regulation of dietary fat absorption and intestinal chylomicron production via neuronal nitric oxide synthase (nNOS) signaling. Diabetes 2022;71:1388–99. https://doi.org/10.2337/db21-1053.Search in Google Scholar PubMed
73. Williams, EK, Chang, RB, Strochlic, DE, Umans, BD, Lowell, BB, Liberles, SD. Sensory neurons that detect stretch and nutrients in the digestive system. Cell 2016;166:209–21. https://doi.org/10.1016/j.cell.2016.05.011.Search in Google Scholar PubMed PubMed Central
74. Varin, EM, Mulvihill, EE, Baggio, LL, Koehler, JA, Cao, X, Seeley, RJ, et al.. Distinct neural sites of GLP-1R expression mediate physiological versus pharmacological control of incretin action. Cell Rep 2019;27:3371–84.e3. https://doi.org/10.1016/j.celrep.2019.05.055.Search in Google Scholar PubMed
75. Furness, JB. The enteric nervous system and neurogastroenterology. Nat Rev Gastroenterol Hepatol 2012;9:286–94. https://doi.org/10.1038/nrgastro.2012.32.Search in Google Scholar PubMed
76. Ahlman, H, Nilsson, O. The gut as the largest endocrine organ in the body. Ann Oncol Off J Eur Soc Med Oncol 2001;12(2 Suppl):S63–68. https://doi.org/10.1093/annonc/12.suppl_2.s63.Search in Google Scholar PubMed
77. Nonaka, N, Shioda, S, Niehoff, ML, Banks, WA. Characterization of blood-brain barrier permeability to PYY3-36 in the mouse. J Pharmacol Exp Therapeut 2003;306:948–53. https://doi.org/10.1124/jpet.103.051821.Search in Google Scholar PubMed
78. Kastin, AJ, Akerstrom, V, Pan, W. Interactions of glucagon-like peptide-1 (GLP-1) with the blood-brain barrier. J Mol Neurosci 2002;18:7–14. https://doi.org/10.1385/jmn:18:1-2:07.10.1385/JMN:18:1-2:07Search in Google Scholar PubMed
79. Banks, WA, Tschöp, M, Robinson, SM, Heiman, ML. Extent and direction of ghrelin transport across the blood-brain barrier is determined by its unique primary structure. J Pharmacol Exp Therapeut 2002;302:822–7. https://doi.org/10.1124/jpet.102.034827.Search in Google Scholar PubMed
80. Imbernon, M, Saponaro, C, Helms, HCC, Duquenne, M, Fernandois, D, Deligia, E, et al.. Tanycytes control hypothalamic liraglutide uptake and its anti-obesity actions. Cell Metabol 2022;34:1054–63.e7. https://doi.org/10.1016/j.cmet.2022.06.002.Search in Google Scholar PubMed PubMed Central
81. de Lartigue, G, Diepenbroek, C. Novel developments in vagal afferent nutrient sensing and its role in energy homeostasis. Curr Opin Pharmacol 2016;31:38–43. https://doi.org/10.1016/j.coph.2016.08.007.Search in Google Scholar PubMed PubMed Central
82. Schwartz, MW, Woods, SC, Porte, D, Seeley, RJ, Baskin, DG. Central nervous system control of food intake. Nature 2000;404:661–71. https://doi.org/10.1038/35007534.Search in Google Scholar PubMed
83. Lam, CKL, Chari, M, Lam, TKT. CNS regulation of glucose homeostasis. Physiol Bethesda Md 2009;24:159–70. https://doi.org/10.1152/physiol.00003.2009.Search in Google Scholar PubMed
84. Peters, JH, Ritter, RC, Simasko, SM. Leptin and CCK selectively activate vagal afferent neurons innervating the stomach and duodenum. Am J Physiol Regul Integr Comp Physiol 2006;290:R1544–1549. https://doi.org/10.1152/ajpregu.00811.2005.Search in Google Scholar PubMed
85. Smith, GP, Jerome, C, Cushin, BJ, Eterno, R, Simansky, KJ. Abdominal vagotomy blocks the satiety effect of cholecystokinin in the rat. Science 1981;213:1036–7. https://doi.org/10.1126/science.7268408.Search in Google Scholar PubMed
86. Date, Y, Murakami, N, Toshinai, K, Matsukura, S, Niijima, A, Matsuo, H, et al.. The role of the gastric afferent vagal nerve in ghrelin-induced feeding and growth hormone secretion in rats. Gastroenterology 2002;123:1120–8. https://doi.org/10.1053/gast.2002.35954.Search in Google Scholar PubMed
87. Koda, S, Date, Y, Murakami, N, Shimbara, T, Hanada, T, Toshinai, K, et al.. The role of the vagal nerve in peripheral PYY3-36-induced feeding reduction in rats. Endocrinology 2005;146:2369–75. https://doi.org/10.1210/en.2004-1266.Search in Google Scholar PubMed
88. Abbott, CR, Monteiro, M, Small, CJ, Sajedi, A, Smith, KL, Parkinson, JRC, et al.. The inhibitory effects of peripheral administration of peptide YY(3-36) and glucagon-like peptide-1 on food intake are attenuated by ablation of the vagal-brainstem-hypothalamic pathway. Brain Res 2005;1044:127–31. https://doi.org/10.1016/j.brainres.2005.03.011.Search in Google Scholar PubMed
89. Talsania, T, Anini, Y, Siu, S, Drucker, DJ, Brubaker, PL. Peripheral exendin-4 and peptide YY(3-36) synergistically reduce food intake through different mechanisms in mice. Endocrinology 2005;146:3748–56. https://doi.org/10.1210/en.2005-0473.Search in Google Scholar PubMed
90. Diepenbroek, C, Quinn, D, Stephens, R, Zollinger, B, Anderson, S, Pan, A, et al.. Validation and characterization of a novel method for selective vagal deafferentation of the gut. Am J Physiol Gastrointest Liver Physiol 2017;313:G342–52. https://doi.org/10.1152/ajpgi.00095.2017.Search in Google Scholar PubMed PubMed Central
91. Netter, M, Frank, H. Atlas of human anatomy, 4th ed. Saunders; 2006.Search in Google Scholar
92. Hoosein, NM, Gurd, RS. Human glucagon-like peptides 1 and 2 activate rat brain adenylate cyclase. FEBS Lett 1984;178:83–6. https://doi.org/10.1016/0014-5793(84)81245-4.Search in Google Scholar PubMed
93. Vrang, N, Phifer, CB, Corkern, MM, Berthoud, HR. Gastric distension induces c-Fos in medullary GLP-1/2-containing neurons. Am J Physiol Regul Integr Comp Physiol 2003;285:R470–478. https://doi.org/10.1152/ajpregu.00732.2002.Search in Google Scholar PubMed
94. Burmeister, MA, Ferre, T, Ayala, JE, King, EM, Holt, RM, Ayala, JE. Acute activation of central GLP-1 receptors enhances hepatic insulin action and insulin secretion in high-fat-fed, insulin resistant mice. Am J Physiol Endocrinol Metab 2012;302:E334–43. https://doi.org/10.1152/ajpendo.00409.2011.Search in Google Scholar PubMed
95. Patel, V, Joharapurkar, AA, Kshirsagar, SG, Patel, KN, Bahekar, R, Shah, G, et al.. Central GLP-1 receptor activation improves cholesterol metabolism partially independent of its effect on food intake. Can J Physiol Pharmacol 2016;94:161–7. https://doi.org/10.1139/cjpp-2014-0457.Search in Google Scholar PubMed
96. Lee, SJ, Sanchez-Watts, G, Krieger, JP, Pignalosa, A, Norell, PN, Cortella, A, et al.. Loss of dorsomedial hypothalamic GLP-1 signaling reduces BAT thermogenesis and increases adiposity. Mol Metabol 2018;11:33–46. https://doi.org/10.1016/j.molmet.2018.03.008.Search in Google Scholar PubMed PubMed Central
97. Patel, V, Joharapurkar, A, Kshirsagar, S, Sutariya, B, Patel, M, Patel, H, et al.. Central administration of coagonist of GLP-1 and glucagon receptors improves dyslipidemia. Biomed Pharmacother 2018;98:364–71. https://doi.org/10.1016/j.biopha.2017.12.068.Search in Google Scholar PubMed
98. Aulinger, BA, Perabo, M, Seeley, RJ, Parhofer, KG, D’Alessio, DA. Rapid hepatic metabolism blunts the endocrine action of portally infused GLP-1 in male rats. Am J Physiol Endocrinol Metab 2020;318:E189–97. https://doi.org/10.1152/ajpendo.00298.2019.Search in Google Scholar PubMed PubMed Central
99. Egerod, KL, Petersen, N, Timshel, PN, Rekling, JC, Wang, Y, Liu, Q, et al.. Profiling of G protein-coupled receptors in vagal afferents reveals novel gut-to-brain sensing mechanisms. Mol Metabol 2018;12:62–75. https://doi.org/10.1016/j.molmet.2018.03.016.Search in Google Scholar PubMed PubMed Central
100. Ridgway, N. Biochemistry of lipids, lipoproteins and membranes. San Diego, CA, USA: Elsevier Science; 2015.Search in Google Scholar
101. Krieger, JP, Arnold, M, Pettersen, KG, Lossel, P, Langhans, W, Lee, SJ. Knockdown of GLP-1 receptors in vagal afferents affects normal food intake and glycemia. Diabetes 2016;65:34–43. https://doi.org/10.2337/db15-0973.Search in Google Scholar PubMed
102. Krieger, JP, Langhans, W, Lee, SJ. Vagal mediation of GLP-1’s effects on food intake and glycemia. Physiol Behav 2015;152:372–80. https://doi.org/10.1016/j.physbeh.2015.06.001.Search in Google Scholar PubMed
103. Singh, I, Wang, L, Xia, B, Liu, J, Tahiri, A, El Ouaamari, A, et al.. Activation of arcuate nucleus glucagon-like peptide-1 receptor-expressing neurons suppresses food intake. Cell Biosci 2022;12:178. https://doi.org/10.1186/s13578-022-00914-3.Search in Google Scholar PubMed PubMed Central
104. Hoffman, S, Alvares, D, Adeli, K. GLP-1 attenuates intestinal fat absorption and chylomicron production via vagal afferent nerves originating in the portal vein. Mol Metabol 2022;65:101590. https://doi.org/10.1016/j.molmet.2022.101590.Search in Google Scholar PubMed PubMed Central
105. Glovaci, D, Fan, W, Wong, ND. Epidemiology of diabetes mellitus and cardiovascular disease. Curr Cardiol Rep 2019;21:21. https://doi.org/10.1007/s11886-019-1107-y.Search in Google Scholar PubMed
106. Langsted, A, Nordestgaard, BG. Nonfasting versus fasting lipid profile for cardiovascular risk prediction. Pathology 2019;51:131–41. https://doi.org/10.1016/j.pathol.2018.09.062.Search in Google Scholar PubMed
107. Taghibiglou, C, Carpentier, A, Van Iderstine, SC, Chen, B, Rudy, D, Aiton, A, et al.. Mechanisms of hepatic very low density lipoprotein overproduction in insulin resistance. Evidence for enhanced lipoprotein assembly, reduced intracellular ApoB degradation, and increased microsomal triglyceride transfer protein in a fructose-fed hamster model. J Biol Chem 2000;275:8416–25. https://doi.org/10.1074/jbc.275.12.8416.Search in Google Scholar PubMed
108. Feltrin, KL, Little, TJ, Meyer, JH, Horowitz, M, Smout, AJPM, Wishart, J, et al.. Effects of intraduodenal fatty acids on appetite, antropyloroduodenal motility, and plasma CCK and GLP-1 in humans vary with their chain length. Am J Physiol Regul Integr Comp Physiol 2004;287:R524–33. https://doi.org/10.1152/ajpregu.00039.2004.Search in Google Scholar PubMed
109. Vrang, N, Madsen, AN, Tang-Christensen, M, Hansen, G, Larsen, PJ. PYY(3-36) reduces food intake and body weight and improves insulin sensitivity in rodent models of diet-induced obesity. Am J Physiol Regul Integr Comp Physiol 2006;291:R367–375. https://doi.org/10.1152/ajpregu.00726.2005.Search in Google Scholar PubMed
110. Lawson, EA. The effects of oxytocin on eating behaviour and metabolism in humans. Nat Rev Endocrinol 2017;13:700–9. https://doi.org/10.1038/nrendo.2017.115.Search in Google Scholar PubMed PubMed Central
111. Farr, S, Taher, J, Adeli, K. Central nervous system regulation of intestinal lipid and lipoprotein metabolism. Curr Opin Lipidol 2016;27:1–7. https://doi.org/10.1097/mol.0000000000000254.Search in Google Scholar
112. Farr, S, Taher, J, Adeli, K. Glucagon-like peptide-1 as a key regulator of lipid and lipoprotein metabolism in fasting and postprandial states. Cardiovasc Hematol Disord: Drug Targets 2014;14:126–36. https://doi.org/10.2174/1871529x14666140505125300.Search in Google Scholar PubMed
113. Taher, J, Farr, S, Adeli, K. Central nervous system regulation of hepatic lipid and lipoprotein metabolism. Curr Opin Lipidol 2017;28:32–8. https://doi.org/10.1097/mol.0000000000000373.Search in Google Scholar
© 2024 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Articles in the same Issue
- Frontmatter
- Editorial
- Hormone based therapy and crosstalk beyond hormones
- Reviews
- Dysregulated bile acid homeostasis: unveiling its role in metabolic diseases
- Beyond reproduction: unraveling the impact of sex hormones on cardiometabolic health
- Glucagon-like peptide (GLP)-1 regulation of lipid and lipoprotein metabolism
- Hepatic function of glucagon-like peptide-1 and its based diabetes drugs
- Biliary fibrosis is an important but neglected pathological feature in hepatobiliary disorders: from molecular mechanisms to clinical implications
Articles in the same Issue
- Frontmatter
- Editorial
- Hormone based therapy and crosstalk beyond hormones
- Reviews
- Dysregulated bile acid homeostasis: unveiling its role in metabolic diseases
- Beyond reproduction: unraveling the impact of sex hormones on cardiometabolic health
- Glucagon-like peptide (GLP)-1 regulation of lipid and lipoprotein metabolism
- Hepatic function of glucagon-like peptide-1 and its based diabetes drugs
- Biliary fibrosis is an important but neglected pathological feature in hepatobiliary disorders: from molecular mechanisms to clinical implications

