Abstract
Cardiovascular research has heavily relied on studies using patient samples and animal models. However, patient studies often miss the data from the crucial early stage of cardiovascular diseases, as obtaining primary tissues at this stage is impracticable. Transgenic animal models can offer some insights into disease mechanisms, although they usually do not fully recapitulate the phenotype of cardiovascular diseases and their progression. In recent years, a promising breakthrough has emerged in the form of in vitro three-dimensional (3D) cardiovascular models utilizing human pluripotent stem cells. These innovative models recreate the intricate 3D structure of the human heart and vessels within a controlled environment. This advancement is pivotal as it addresses the existing gaps in cardiovascular research, allowing scientists to study different stages of cardiovascular diseases and specific drug responses using human-origin models. In this review, we first outline various approaches employed to generate these models. We then comprehensively discuss their applications in studying cardiovascular diseases by providing insights into molecular and cellular changes associated with cardiovascular conditions. Moreover, we highlight the potential of these 3D models serving as a platform for drug testing to assess drug efficacy and safety. Despite their immense potential, challenges persist, particularly in maintaining the complex structure of 3D heart and vessel models and ensuring their function is comparable to real organs. However, overcoming these challenges could revolutionize cardiovascular research. It has the potential to offer comprehensive mechanistic insights into human-specific disease processes, ultimately expediting the development of personalized therapies.
Introduction
Cardiovascular diseases (CVDs) refer to a class of heart and blood vessel disorders and are the leading cause of death globally [1]. Advancements in prevention strategies and interdisciplinary treatments have led to improved outcomes for CVDs [2]. The development of precise molecular targets and effective therapies is further accelerated by the increasing understanding of the etiology, mechanisms, and progression of CVDs. Preclinical animal studies have shed light on demonstrating disease causality and elucidating disease mechanisms, whereas translation to clinical practice is often hampered by genetic, molecular and cellular differences in the cardiovascular system across species [3], [4], [5]. Human primary heart and blood vessel tissues are ideal for studying pathological processes of CVDs, but limited accessibility of primary tissues and difficulties in long-term culture and genetic manipulation hinder their applications [6]. Therefore, in vitro human multicellular models could bridge the gap between preclinical animal studies and clinical patient studies. With negligible ethical concerns and the opportunity to generate patient-specific models, human induced pluripotent stem cells (hiPSCs) hold great potential for applications in disease modeling and drug development [7]. Since hiPSCs were first generated by the research team of Shinya Yamanaka in 2007 [8], a variety of protocols for differentiating cells into the heart and vessels have been reported [9, 10]. The availability of hiPSC-derived cardiomyocytes (hiPSC-CMs) and other cell types provides a versatile toolkit for cardiac regenerative therapy, disease model development, and drug screening [3, 7]. However, conventional two-dimensional (2D) monomorphic cellular models lack cellular heterogeneity and three-dimensional (3D) heart structure. To circumvent this hurdle, multiple 3D models mimicking the human heart have been developed.
In this review, we first summarize the current state-of-the-art of cardiovascular 3D models, including spheroids, organoids, engineered cardiac microtissues, and organ-on-a-chip systems. We then discuss the research and medical applications of different 3D models and highlight their advantages and limitations in the context of translational and innovative cardiovascular research. Lastly, we outline the current challenges and future achievable goals in the field (Figure 1).

Perspectives regarding applications and future directions of in vitro 3D cardiovascular models. Various 3D cardiovascular models have distinct culture platforms, analysis tools, and applications. The future directions should focus on overcoming existing limitations to generate the replica of human primary heart tissues, including but not limited to the integration of vascular and immune cells into the 3D models, as well as the development of chamber-specific organoids to enhance their physiological relevance. The figure was drawn using BioRender.
3D heart models
Overall, scaffold-based and scaffold-free approaches are the two major culture methods for engineering 3D cardiovascular tissues. In scaffold-based methods, hydrogels or other materials mimic the extracellular matrix (ECM) surrounding cells. In contrast, scaffold-free methods rely on the ability of cells to produce their own extracellular matrix and to form 3D structures [11]. Each strategy has its unique advantages and limitations, including cell number requirement, manipulation difficulty, and relative cost, etc (Figure 2), which are outlined in the following sections.
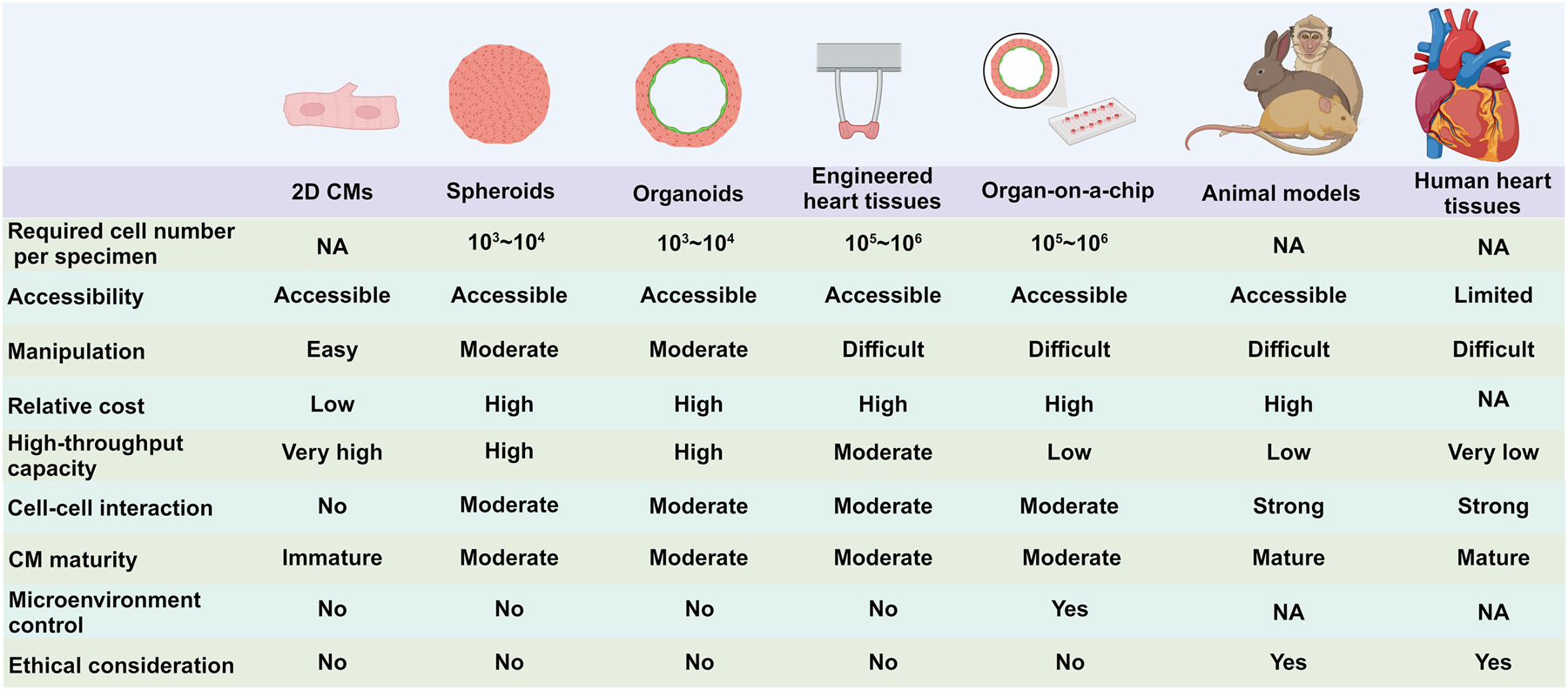
A schematic diagram depicts current models used for cardiovascular research. The array of models include 2D hiPSC-CMs, 3D models, animal models, and human primary tissues. Each model comes with its own set of advantages and limitations. These models differ in various aspects such as the requirement of cell number, manipulation difficulty, relative cost, high-throughput capability, the extent of cell-cell interaction, CM maturity, microenvironment control, and ethical consideration. These factors play a crucial role in determining the suitability and effectiveness of each model for specific research objectives in the field of cardiovascular research. The figure was drawn using BioRender.
Cardiac spheroids and organoids
Cardiac spheroids broadly refer to scaffold-free 3D aggregates of differentiated or primary adult cells [12]. In general, cardiac spheroids are formed by cardiomyocytes (CMs) alone or in combination with stromal cells in a defined ratio, giving rise to tissue-like structures [13]. Different from spheroids, organoids are typically defined as “3D structures derived from stem cells and consisting of organ-specific cell types that self-organize through cell sorting and spatially restricted lineage commitment” [14]. Organoids often exhibit a lineage-dependent spatial organization, making them resemble native tissue more closely than spheroids [15]. Both spheroids and organoids are typically formed in low adhesion multi-well plates or bioreactors, and their fabrication requires low cell number (1,000–10,000 cells per specimen), allowing for simultaneously generating hundreds to thousands of replicates in an array [12]. To date, cardiac spheroids and organoids have been shown to serve as useful tools for disease modeling, cardiotoxicity studies, drug discovery, as well as regenerative research [16]. The distinct advantage of cardiac organoids over other 3D heart models lies in their crucial role in exploring developmental biology and in modeling congenital heart disease [17].
Since 2021, there have been multiple studies describing the generation of cardiac organoids to validate the mimicry of cardiac structures such as chambers, epicardium/myocardium, and atrium/ventricle-reminiscent regions (see reviews for more details [18, 19]). In this section, we will highlight the recent major advances and perspectives in this field.
Drakhlis et al. encapsulated hiPSC aggregates in Matrigel followed by directed cardiac differentiation via Wnt pathway modulation with small molecules to generate multi-lineage and highly organized heart-forming organoids (HFOs) [20]. During differentiation, HFOs developed three layers: the inner layer containing endothelial cells (ECs) and foregut-like cells with microvilli; the middle layer predominantly consisting of CMs and some epicardial cells; the outer layer containing mesenchymal cells and liver cells. These structures resembled the aspects of early heart and foregut anlagen in the developing embryo. Additionally, the appearance of liver cells within the HFOs presented a unique platform to study multi-organ interactions during cardiac development. Regarding the generation of multiple cell types and structurally relevant self-assembling cardiac organoids, other studies like cardiac-gut organoids from hiPSCs have also been reported, with co-emergence of cardiac tissues and endodermal-derived tissues (e.g., gut and intestine) [21]. Another cardiac organoid model termed “Cardioid” contained a large internal chamber surrounded by CMs, ECs, and epicardial cells [22]. These approaches yield multi-cellular and structural cardiac organoids, but they can only mimic a certain degree of the physiological and metabolic functions of the human heart. To overcome this limitation, more complex and physiologically relevant cardiac organoids were generated by the sequential modulation of Wnt signaling (activation/inhibition/activation) [23]. These cardiac organoids are comparable to human fetal cardiac tissues at the transcriptomic, cellular, and structural levels. Importantly, these organoids displayed robust beating and electrophysiological activity with well-defined action potential (AP) waves reminiscent of QRS complexes, T, and P waves detected by a multielectrode array (MEA) system. Additionally, this type of organoid was successfully used to model pregestational diabetes (PGD) -induced congenital heart defects, showing structural, metabolic, and functional changes in organoids under diabetic conditions [17, 24]. Another recent study developed an “epicardioid” with an inner core of ventricular CMs and a thick envelope containing epicardial cells [25]. Interestingly, in epicardioid slices, the AP duration was significantly shorter in CMs closest to the epicardial layer than in the middle of the ventricular layer. This is consistent with a conserved feature known as the transmural voltage gradient in vivo to increase the ventricular contraction efficiency [26]. These studies strongly support the idea that these structurally and functionally relevant cardiac organoids will likely serve as a powerful tool for studying heart development, disease, and regeneration.
Despite the fact that some studies have reported high reproducibility of the cardiac organoids across multiple hiPSC lines, all of which displayed similar differentiation efficiency, growth rate, and size [22, 23], variations still exist in the cardiac organoid shape, architecture, cellular composition, as well as chamber formation (i.e., the number and size of the cavities per organoid). These variations may affect modeling cardiogenesis and heart disease, as well as drug testing. Therefore, improvement in the reproducibility of the generation of cardiac organoids remains a crucial area that needs significant enhancement.
Engineered heart tissues (EHTs)
While cardiac spheroids and organoids benefit from the requirement of small cell number, making them suitable for high-throughput studies, researchers face limitations in controlling the tissues’ morphology as well as functional assays available for characterization [15]. EHTs provide an attractive complementary strategy by integrating customized devices and engineered biomaterials to achieve a tissue-level structure with a defined size and geometry [27]. The most common method used to generate EHTs is a hydrogel-based approach. In general, hPSC-CMs are mixed with fibroblasts (and/or other cardiac cells) in hydrogels and then poured into casting molds [27, 28]. Since the development of the first EHTs two decades ago, a series of tissue models have been produced with diverse geometries and formats such as cardiac biowires [29, 30], microwires [31], patches [32], tube-shaped EHTs [33], ring-shaped EHTs [34, 35], and chamber-forming ventricle models [36, 37]. Many of these EHT models can incorporate genetically encoded calcium and voltage indicators with laser-confocal microscopy and optical mapping technology to enable simultaneous analyses of contractile force, electrophysiological properties, and calcium dynamics [27]. Aside from functional analyses, an important advantage of EHTs for regular use in studying cardiotoxicity is their high reproducibility. In a recent blinded multi-center study, 36 compounds with known effects on cardiac physiology were utilized to evaluate the capability of EHTs to predict cardiotoxic effects across different institutions [38]. They reported up to 93 % accuracy in prediction when using EHTs, and 85 % for monolayers, whereas animal models only displayed 78 % accuracy. A similar study using EHTs to test several compounds with known cardiotoxic effects was published recently [39]. Consistent with previous studies, EHTs again faithfully exhibited the expected drug responses.
One of the major challenges associated with EHTs is their limited extent of maturation. Although the structure of EHTs closely resembles that of neonatal heart tissue, the contractile force of EHTs (0.05–2.00 mN/mm2 [40]) is still smaller than the force generated by native tissues (about 50 mN/mm2 [41]), and the stiffness of EHTs is approximately half that of native tissue [42]. This may be caused by the insufficient mechanical properties of the matrix and by the casting-based fabrication approach [43]. In the future, the development of new materials suitable for EHTs will be necessary. Despite this limitation, EHTs are expected to be a promising tool for disease modeling and pharmaceutical drug/toxicity screening.
Heart-on-a-chip
Although 3D organoids hold great potential as valuable tools in disease modeling and drug screening, most of these studies are conducted in static culture, which hinders oxygen and nutrient perfusion to the core of the organoids, leading to necrosis [44]. This limitation not only restricts the long-term culture of organoids but also affects studies in the context of perfusion-related diseases, such as ischemia-reperfusion injury. To overcome this limitation, the concept of “organ-on-a-chip” has emerged by integrating organoids with the microfluidic system [44]. This platform offers the following advantages: (1) The organ-on-a-chip system provides a controlled environment, ensuring optimal conditions such as temperature, pH, nutrient, oxygen supply, and waste removal through a continuous flow of fresh culture medium [45]; (2) It provides a perfusion condition which may mimic blood flow and the microenvironment. For example, Arslan et al. established a vascularized cardiac microtissue on-chip (VMToC) by integrating pre-vascularized cardiac MTs with a microfluidic system [46]. Within days, the VMToC formed lumenized and perfusable vascular networks; (3) Incorporating biosensors into microfluidic organ-on-a-chip devices using minimally- or non-invasive techniques facilitates the real-time monitoring of culture conditions and functional parameters [47]. Some of these capabilities have already been demonstrated. A recent study reported a bioelectronic heart-on-a-chip model [48]. This innovative model enables control of the oxygen concentration in the microfluidic channel as well as the continuous monitoring of the cardiac electrophysiological responses to acute hypoxia; (4) More importantly, the microfluidic system allows for the integration of multiple organ compartments within a single microfluidic circuitry for the study of interactions between different tissues, such as heart-breast cancer on-a-chip [49] and heart/liver-on-a-chip [50].
Advancements in organoid-on-a-chip technology could largely reduce the cost and duration of drug discovery [51]. In August 2022, the world’s first investigational new drug (IND) application entirely based on organoid-on-a-chip data was approved (NCT04658472) [52]. Following this, the US president signed a bill in December 2022 that allows the United States Food and Drug Administration (FDA) to approve new drugs without them being tested on animals and specifically states that alternative testing methods should be included, such as cell-based assays, organ chips, and micro-physiological systems [53]. This milestone indicates that incorporation of the organoid-on-chip platform in drug development pipelines is feasible and promising, suggesting that this innovative platform will be increasingly used in the future.
Applications of 3D models in cardiovascular research
The complexity and limited accessibility of the human heart and blood vessel tissues make it difficult to study molecular mechanisms and the pathophysiology of cardiovascular diseases. With the advancement of human in vitro 3D cardiovascular models, these are now extensively utilized in disease modeling, regenerative medicine, and cardiotoxicity testing. In this section, we will discuss the various applications using 3D cardiovascular models (Table 1).
Applications of 3D cardiovascular models.
| Disease classes | Diseases | Mutations/stress | 3D models | Ref. |
|---|---|---|---|---|
| Congenital heart diseases (CHD) | Cardiac malformations | NKX2-5 knockout | Cardiac organoids | [20] |
| Hypoplastic left heart syndrome | HAND1 knockout | Cardiac organoids | [22] | |
| Ebstein’s anomaly | NKX2-5 mutation | Cardiac organoids | [60] | |
| Noonan syndrome | PTPN11 mutation | Cardiac organoids | [25] | |
| Pregestational diabetes-induced CHD | High insulin and high glucose | Cardiac organoids | [17, 23, 24] | |
| Inherited Cardiomyopathy | Hypertrophic cardiomyopathy | ACTN2 mutation | EHTs | [70] |
| MYH7 mutation | EHTs | [71, 73] | ||
| MYBPC3 mutation | EHTs | [72, 74, 75] | ||
| FLNC mutation | EHTs | [76] | ||
| Restrictive cardiomyopathy | SCN5A mutation | Heart-on-a-chip | [86] | |
| Dilated cardiomyopathy | KLHL24 mutation | EHTs | [77] | |
| PLN mutation | EHTs | [78] | ||
| Barth syndrome | TAZ mutation | Heart-on-a-chip | [87] | |
| Duchenne muscular dystrophy | DMD mutation | Cardiac organoids | [89] | |
| DMD mutation | EHTs | [79] | ||
| Cardiac fibrosis | Hypoxia and norepinephrine | Cardiac spheroids | [95] | |
| TGF-β | Heart-on-a-chip | [96, 97] | ||
| Infectious diseases | SARS-Cov-2 infection | Cardiac spheroids | [106, 107] | |
| EHTs | [108] | |||
| Heart-on-a-chip | [101] | |||
| Blood vessel organoids | [111, 163, 164] | |||
| Cardiac arrhythmias | Atrial or ventricular arrhythmias | Lidocaine/Vernakalant | EHTs | [34] |
| Atrial fibrillation | Metabolites (e.g., C18:1AC) | EHTs | [120] | |
| Cardiotoxicity | Doxorubicin | Cardiac spheroids | [126] | |
| Heart-on-a-chip | [49, 50] | |||
| Cadmium | Cardiac organoids | [127] | ||
| Polystyrene microplastics | Cardiac organoids | [128] | ||
| Bisphenol A | Cardiac organoids | [129] | ||
| Regenerative medicine | Cardiac spheroids | [148– 150, 154, 155] | ||
| Vascular cell spheroids | [156] | |||
| Exosomes from cardiac spheroids | [157, 158] | |||
| Atherosclerosis | Vessel-on-a-chip | [172, 173] | ||
| Diabetic vasculopathy | Vessel organoids were cultured in diabetic medium (high glucose) or transplanted into streptozotocin-induced diabetic mice | Vessel organoids | [160] |
Congenital heart disease
Congenital heart disease (CHD) is one of the most common types of birth defects, affecting approximately 1 in 100 live births worldwide [54, 55]. Generally, CHD is defined as a structural abnormality of the heart and/or great vessels [56]. Although the etiology of CHD is largely unknown, studies have suggested that the cause of CHD is multifactorial, and both genetic and environmental factors contribute to the development of CHD [57, 58]. The combination of genetic technologies (such as CRISPR/Cas9) and organoid modeling allows studies of disease mechanisms, as the process of differentiating cardiac organoids can recapitulate the key steps of heart development [59]. Drakhlis et al. demonstrated that NKX2-5-knockout cardiac organoids showed a phenotype reminiscent of cardiac malformations including decreased cardiomyocyte adhesion, disorganized sarcomeres, and altered gene expression as previously observed in transgenic mice [20]. Owing to the rapid pace of advances in the development of cardiac organoids in recent years, other CHD syndromes like hypoplastic left heart syndrome (HLHS), Ebstein’s anomaly, and Noonan syndrome have also been successfully modeled using cardiac organoids [22, 25, 60]. Additionally, environmental factors such as PGD-induced CHD can also be mimicked in organoids under defined conditions [17, 23, 24]. As one of the most common non-genetic factors causing CHD, PGD results in a 12-fold increase in the occurrence of CHD, fetal hypertrophic cardiomyopathy, and impaired cardiac function in infants [61, 62]. Aguirre’s group cultured cardiac organoids under PGD-like conditions (high insulin and glucose) and found that the organoids had decreased oxygen consumption, increased glycolysis, accumulation of lipid droplets, and an increased propensity for arrhythmias, suggesting altered glucose and lipid metabolism [17, 23]. The same research group recently used this model to further elucidate the underlying mechanisms of PGD-induced CHDs [24]. Interestingly, they found that endoplasmic reticulum (ER) stress and very long chain fatty acid (VLCFA) lipid metabolism imbalance were two critical factors that gave rise to cardiac defects. Therefore, restoring VLCFA levels either by inhibiting ER stress or through the dietary administration of VLCFAs has the potential to significantly mitigate the harmful effects of PGD. This study paves the way for novel clinical strategies to prevent and treat PGD-associated CHDs.
Overall, these studies demonstrate the basic foundation for developing cardiac organoids as a promising tool for studying the molecular pathology of CHDs. However, current cardiac organoid models are not suitable for studying common CHDs such as atrial septal defects and some valvular diseases due to the lack of key structural components of the heart. Moreover, some aspects of heart physiology such as blood flow cannot be mimicked in cardiac organoids, which limits their applications in CHDs. Structural defects in CHD patients are frequently linked to mutations in genes involved in early cardiogenesis, including transcription factors, chromatin remodeling factors, and developmental signaling pathways [63]. These genetic regulators have been widely studied using hiPSC-CMs, but there is a paucity of studies using 3D heart models. A recent study investigated NOTCH1 deficiency-induced HLHS in hiPSC-CMs [64], using cardiac organoids to elucidate detailed mechanisms of cardiac cell lineage commitment under the NOTCH1 deficiency condition is expected to be a future research direction. Additionally, the combination of cardiac organoids with lineage tracing tools, such as TBX5/MYL2 [65] or TBX5/NKX2-5 [66] fluorescent reporter systems, will be useful for tracing cell fate under both normal and diseased conditions.
Inherited cardiomyopathy
Cardiomyopathies are a group of disorders characterized by heart muscle damage, resulting in myocardial disorders, diminished cardiac function, heart failure, and even sudden death [67]. Based on morphological and structural phenotype, cardiomyopathies can be divided into five types: hypertrophic cardiomyopathy (HCM), dilated cardiomyopathy (DCM), restrictive cardiomyopathy (RCM), arrhythmogenic right ventricular cardiomyopathy, and unclassified cardiomyopathy [68]. EHTs are a suitable miniaturized platform to assess CM contractility, making them useful in modeling various cardiomyopathies, such as HCM [69], [70], [71], [72], [73], [74], [75], RCM [76], DCM [77, 78], and Duchenne muscular dystrophy (DMD) [79]. Prondzynski et al. demonstrated that EHTs generated from hiPSCs harboring an alpha-actinin 2 (ACTN2) mutation (p.T247M) recapitulated several hallmarks of HCM, such as hypertrophy, hypercontractility, sarcomere disarray, prolonged AP duration, and enhanced L-type calcium current [70]. In a following study, the same group found that microtubule tyrosination and detyrosination played an important role in HCM progression by modulating CM contraction [69]. A well-balanced level of detyrosination in microtubules is important for proper CM contraction as microtubules bind to sarcomeres and form a physiological resistance to contraction [80]. Decreasing microtubule detyrosination improved contractility and reduced stiffness in both HCM mice and EHTs, providing cues that targeting the regulatory mechanism controlling microtubule detyrosination could be a new inotropic strategy for improving cardiac function in HCM [69]. In addition, EHTs were used to model RCM, which is defined functionally as restricted ventricular filling characterized by increased myocardial wall tension and failure to relax during diastole [81]. However, measuring these parameters in 2D cells is challenging due to their adherence to a plastic substrate. In this study, EHTs generated using hiPSC-CMs from patients carrying a mutation in filamin C exhibited RCM-like phenotypes including sarcomere disorganization, decreased active force, and increased passive tension [76]. Following small molecule drug screening, trequinsin, a phosphodiesterase 3 inhibitor, was identified as a promising candidate for alleviating the phenotypes associated with RCM. These studies indicated that EHTs have great potential in revealing the biological mechanism of and developing new drugs for cardiomyopathy.
In contrast to HCM, DCM is characterized by left ventricular dilatation and impaired contractility, and ventricular arrhythmias [82]. Mutations in the SCN5A gene, encoding the cardiac voltage-gated sodium channel NaV1.5, are reported in 2–4% of patients with DCM, often in association with arrhythmias [83]. One of the mutations (R222Q) is exclusively found in adult splice variants of the SCN5A gene [84, 85], making it difficult to study in hiPSC-CMs due to their fetal-like phenotype. To address this, a 3D heart-on-a-chip was engineered using hiPSCs carrying the R222Q mutation [86]. In accordance with the phenotypic characteristics observed in some DCM patients, the disease-specific heart-on-a-chip exhibited arrhythmogenic features, including shortened AP duration, reduced Na+ current, and decreased contractility. Furthermore, the R222Q heart-on-a-chip displayed reduced expression levels of sarcomeric proteins, accompanied by severe sarcomere disarray compared with isogenic control. Notably, these phenotypes were not observed in 2D hiPSC-CMs, suggesting that the differential manifestations might be attributed to the enhanced alignment and maturation of CMs in the heart-on-a-chip. In addition to DCM, a heart-on-a-chip was used to study Barth syndrome, a mitochondrial cardiomyopathy caused by a mutation of TAZ gene encoding tafazzin [87]. hiPSC-CMs carrying the TAZ mutation in the heart-on-a-chip showed irregular sarcomere assembly and weakened contraction strength, recapitulating the Barth syndrome phenotype in patients, while the disease phenotype could be rescued by treatment with isoproterenol, verapamil, metoprolol, or E4031 (hERG blocker) [87]. These studies highlight the ability of heart-on-a-chip models to recapitulate features of cardiomyopathies and to facilitate mechanistic investigation.
Compared with EHTs and heart-on-a-chip, fewer studies have employed organoids to model cardiomyopathies. DMD, an X-linked neuromuscular disorder caused by mutations in the dystrophin gene, leads to severe reduction of sarcoglycans and is not curable [88]. Until recently, cardiac organoids generated from DMD patients’ hiPSCs were used to study DMD-related cardiomyopathy [89]. Cardiac organoids exhibited manifestations of DMD-related cardiomyopathy and disease progression during long-term culture (up to 93 days). RNA sequencing analysis showed a distinct transcriptomic profile in DMD cardiac organoids, characterized by enrichment in pathways related to hypertrophy, arrhythmias, adipogenesis, and fibrosis. Notably, DMD cardiac organoids exhibited a loss of α, β, γ, and δ-sarcoglycan expression over time, likely due to the lack of connection to dystrophin. After correcting the genetic mutation using CRISPR/Cas9, the function of isogenic cardiac organoids was restored. The advantages of 3D models over 2D hiPSC-CMs were further underscored by a previous study from the same group [90], which demonstrated the immaturity of 2D hiPSC-CMs because they lacked α-, γ-, and δ-sarcoglycan protein expression, making it challenging to use these cells to replicate the DMD-associated phenotype. In this case, the 3D cardiac organoids mimicked some of the pathological findings in DMD cardiomyopathy [89]. Although studies involving cardiac organoid modeling of cardiomyopathies remain limited, cardiac organoids hold great potential for future disease modeling. As mentioned earlier, epicardioids have epicardial and ventricular layers, and the latter can be further subdivided into compact and trabecular layers due to different CM densities [25]. Importantly, the thickness of the two distinct layers is altered under exogenous simulations, which makes the epicardioid an ideal model to mimic trabeculation- and compaction-related diseases such as left ventricular noncompaction (LVNC). The distinct features of LVNC are challenging to replicate in EHTs and heart-on-a-chip models, pointing to a future direction for cardiac organoids in cardiomyopathy modeling.
Cardiac fibrosis
Cardiac fibrosis is a common condition in various heart diseases and is characterized by the excessive deposition of ECM [91]. Cardiac fibroblasts (CFs) are the essential cell type responsible for myocardial ECM homeostasis and secrete paracrine factors that regulate the function of CMs, ECs, and immune cells in the heart [92]. During fibrosis, myofibroblast activation and excessive collagen deposition by CFs cause increased ventricular stiffness and decreased chamber compliance, leading to diastolic dysfunction [91, 93].
Cardiac fibrosis can be triggered by myocardial injury, mechanical stretch, and inflammatory stimuli [94]. Cardiac spheroids have been used for modeling cardiac fibrosis subject to an infarction injury [95]. Cardiac spheroids composed of hiPSC-CMs, CFs, human umbilical vein endothelial cells (HUVECs), and human adipose-derived stem cells were cultured under hypoxia and norepinephrine stimulation to mimic myocardial infarction (MI) in vitro. After 10 days of stimulation with noradrenaline, cardiac spheroids showed an up-regulation of the fibrosis-related genes and the presence of myofibroblast-like cells, as well as changes in stiffness and calcium handling [95]. Moreover, by incorporating a human cardiac fibrosis-on-chip (hCF-on-a-chip) model with a live imaging modality, researchers can monitor organoid contraction in real-time [96]. Under the stimulation of transforming growth factor-β (TGF-β), the hCF-on-a-chip displayed hallmarks of cardiac fibrosis, including increased collagen deposition, higher tissue stiffness, and loss of contractile function. Interestingly, treatment of hCF-on-a-chip with pirfenidone, an FDA-approved medication for the treatment of idiopathic pulmonary fibrosis, significantly decreased tissue stiffness and B-type natriuretic peptide (BNP) secretion, suggesting a potential application in cardiac fibrosis. Besides phenotypic switching of CFs, the change in the ratio of hiPSC-CMs to CFs from 3:1 to 1:3 in a biowire model mimicked a transition from the healthy state to the fibrotic condition [97]. Increased passive tension and diminished active contractile force were observed in the fibrotic biowires, accompanied by a significant accumulation of fibrillar collagen, along with abnormal calcium transients and altered electrophysiological properties.
One key limitation of the aforementioned fibrosis studies is the absence of immune cells. Immune cells play an important role in regulating cardiac tissue homeostasis and the fibrotic process [98]. Macrophage proliferation is stimulated by increased tissue stiffness, thus promoting a continuous positive feedback loop which further exacerbates cardiac fibrosis [99]. Given the importance of immune cells, the incorporation of immune cells such as macrophages or peripheral blood mononuclear cells (PBMC) into EHTs [100] or heart-on-a-chip [101] could represent a great improvement in the ability to recapitulate the complexity of the tissue microenvironment when modeling cardiac fibrosis.
COVID-19-associated myocarditis
Coronavirus disease 2019 (COVID-19) is an infectious disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) which mainly affects the respiratory system, causing interstitial pneumonia and acute respiratory distress syndrome [102]. Although the lungs are the first organ affected by SARS-CoV-2, accumulating evidence indicates that the virus can infect other organs, including the heart, blood vessels, kidneys, gut, and brain [103]. The most common cardiovascular complications are myocarditis, myocardial injuries, arrhythmias (ventricular tachycardia and atrial fibrillation), coagulopathies, and heart failure [104]. Cardiac spheroids [105], [106], [107], heart-on-a-chip [101], and EHTs [108] have been used to study SARS-CoV-2 infection in the cardiovascular system. Upon exposure to signature cytokines in response to SARS-CoV-2, the proportion of apoptotic hPSC-CMs in cardiac spheroids significantly increased, along with the decreased contractility and altered electrophysiological function, calcium handling, and sarcomere organization [106, 107]. In a recent study, bromodomain and extraterminal domain family inhibitors were demonstrated to alleviate the cardiac dysfunction caused by SARS-CoV-2-induced cytokine storm [106]. However, whether cardiac manifestations of SARS-CoV-2 are a result of direct viral infection or systemic inflammation and/or microvascular thrombosis remains unclear [108]. Some studies have highlighted the dysfunction of monocytes and macrophages in COVID-19 and suggested that the inflammatory response is a major contributor to SARS-CoV-2-induced cytokine storm [109]. To understand which types of cells in the heart are being targeted by SARS-CoV-2, a recombinant SARS-CoV-2 virus expressing a NeonGreen fluorescent reporter was incubated with hiPSC-CMs, fibroblasts, macrophages, and ECs [108]. After 3 days of infection, flow cytometry analysis revealed NeonGreen only in CMs, indicating that only hiPSC-CMs were susceptible to infection. To assess the functional impact on CMs, researchers generated novel EHTs composed of CMs, fibroblasts, and macrophages, infected EHTs with SARS-CoV-2 virus, and showed that they displayed reduced contraction amplitude, slower contraction, sarcomere disassembly, and cell death. Consistently, flow cytometry analysis showed that only hiPSC-CMs were infected by the virus. This study provides evidence that SARS-CoV-2 is capable of directly infecting CMs, partly because CMs express angiotensin-converting enzyme 2 (ACE2) through which SARS-CoV-2 can enter CMs. However, since ACE2 is also expressed in other cardiac cell types such as ECs [110] and pericytes [111], the reason why these two types of cells are not infected remains unclear. In contrast to direct infection, another study proposed that systemic inflammation secondary to SARS-CoV-2 infection led to cardiac complications [101]. To mimic systemic inflammation in vitro, the author engineered a vascularized heart-on-a-chip by integrating hiPSC-CMs and HUVECs with PBMCs to model SARS-CoV-2-induced acute myocarditis. After SARS-CoV-2 infection, PBMCs infiltrated the cardiac tissue from the vascular compartment, creating a hyper-inflammatory microenvironment that resulted in myocarditis. Importantly, they found that extracellular vesicles (EVs) derived from HUVECs substantially attenuated the pro-inflammatory response and mitochondrial impairment through the toll-like receptor-NF-κB axis, suggesting the potential of EVs as a promising therapeutic agent.
Cardiac arrhythmias
Inherited cardiac arrhythmias are caused by pathogenic variants in genes encoding components or accessory elements of ion channels or desmosomes [112]. Despite notable advances in using hiPSC-CMs to study cardiac arrhythmias over the past two decades, their immature phenotype in terms of lower ion channel expression highlights the necessity of 3D models for studying cardiac arrhythmias [113].
Atrial and ventricular CMs in the human heart have different electrophysiological properties due to different expression levels of ion channels [114]. Thus, the specific disease mechanisms and therapeutic approaches for atrial and ventricular arrhythmias are different. To better mimic the chamber-specific tissues, ring-shaped chamber-specific EHTs were generated and displayed distinct atrial vs. ventricular phenotypes and drug responses [34]. When EHTs were treated with the anti-arrhythmic drug lidocaine (a sodium channel blocker), only ventricular EHTs, not atrial EHTs, displayed a slower electrical conduction velocity. In contrast, when EHTs were treated with vernakalant (a drug used for treating atrial fibrillation), only atrial EHTs showed increased AP duration. In addition to different drug responses, the ventricular EHTs generated a higher contractile force compared to the atrial EHTs, similar to the human heart [115]. Therefore, these chamber-specific EHTs recapitulate structural and functional properties ascribed to specific cardiac chambers and provide a proof-of-concept for their potential in modeling diseases such as atrial fibrillation (AF).
AF affects up to 2 % of the general population and causes a considerable public health burden affecting millions of people worldwide [116, 117]. The pathology of AF is complex and is still not completely understood. Some evidence suggests that metabolic alterations contribute to the pathogenesis of AF [118, 119]. In the heart, long-chain fatty acids (FA) are the major energy source. To investigate the impact of long-chain acyl-carnitine (AC) C18:1AC on AF, Krause et al. developed atrial EHTs and observed that C18:1AC led to impaired contractile force, disrupted calcium handling, and altered mitochondrial metabolism in the atrial EHTs [120]. Moreover, high serum concentrations of C18:1AC were linked to the onset and prevalence of AF, suggesting C18:1AC’s potential role as a novel circulating biomarker for AF prediction. These studies position EHTs as suitable and highly sensitive screening tools for anti-arrhythmic compounds.
Cardiotoxicity
Since cardiotoxicity is one of the leading causes of post-approval withdrawal of drugs [121], the prediction of cardiotoxicity is of utmost importance. Additionally, evaluating the cardiotoxicity of chemotherapeutic agents, including some anthracyclines, tyrosine kinase inhibitors (TKIs), and monoclonal antibodies can significantly assist healthcare providers in choosing the most appropriate medications for their patients, thus potentially reducing adverse effects and enhancing treatment outcomes [122]. While determining toxicity profiles during pharmaceutical development is costly and requires animal studies and clinical trials, utilizing hiPSC-CMs for early detection not only enhances the predictive accuracy but also reduces in both financial and time costs [123]. In one of the studies utilizing hiPSC-CMs for cardiotoxicity testing, a high-throughput toxicity screen was performed to assess the cardiotoxicity of TKIs by establishing a “cardiac safety index” (CSI) using cell survival and contractility parameters [124]. Notably, they found that the in vitro CSI was consistent with the drugs’ clinical cardiotoxicity profiles. Moreover, hiPSC-CMs from breast cancer patients with and without doxorubicin-induced cardiotoxicity in clinics can recapitulate the patient-specific phenotype of cardiotoxicity [125]. Due to the severe doxorubicin-induced cardiotoxicity, targeted delivery of doxorubicin to tumors may reduce cardiac side effects. Arzt et al. developed a non-cardiotoxic doxorubicin delivery system – single protein encapsulated doxorubicin (SPEDOX-6) [126]. This innovative approach minimized cardiotoxicity in both hiPSC-CMs and cardiac spheroids while preserving doxorubicin’s anti-cancer effect. In addition to investigating the cardiotoxic effects induced by anti-cancer drugs, cardiac organoids have also recently become a vital tool in exploring the cardiotoxicity arising from environmental pollutants [127], [128], [129]. Following exposure to polystyrene microplastics with a dose equivalent to the exposure level in humans, cardiac organoids exhibited increased oxidative stress, inflammatory response, apoptosis, and collagen accumulation which were consistent with an in vivo study [128]. These studies demonstrated that the 3D heart models can fully recapitulate the manifestations of cardiotoxicity in vitro.
While the human body comprises a complex, multi-systemic entity, it is imperative to acknowledge that replicating a single organ may not adequately capture the systemic effects of drugs [130]. In this case, organ-on-a-chip models have a distinct advantage in mimicking multi-organ interactions in response to drugs. For example, a heart-breast cancer on-a-chip containing hiPSC-derived cardiac tissues and breast cancer SK-BR-3 spheroids was designed to mimic doxorubicin-induced cardiotoxicity in vivo [49]. This platform was combined with electrochemical immune-aptasensors, enabling the monitoring of the secretion of biomarkers including troponin T, creatine kinase-MB, and human epidermal growth factor receptor 2 over time. Upon doxorubicin treatment, biomarkers for cardiotoxicity and cancer simultaneously provide evidence for both chemotherapy-induced cardiotoxicity (CIC) and breast cancer progression. This platform will allow early detection and prediction of CIC in patients in the future. While many drugs are metabolized by the liver, replicating this process in vitro remains a challenge. To address this, a heart/liver-on-a-chip (HLC) microfluidic device was designed, specifically utilizing HepG2 hepatocellular carcinoma cells and H9c2 rat CMs to study doxorubicin-induced cardiotoxicity [50]. As a result, the HLC exhibited more damage to CMs compared to conventional static 3D culture, primarily due to the exposure of cells to both doxorubicin and its cardiotoxic metabolite, doxorubicinol. Moreover, the organ-on-a-chip can be further improved by the integration of sensors and robotics. To facilitate long-term monitoring of contractile forces and the beating frequency of CMs, a living anisotropic structural color hydrogel has been generated and integrated into the heart-on-a-chip [131]. These hydrogels exhibited synchronous structural alterations with the color transitioning from green to blue, corresponding to the contraction and relaxation of hiPSC-CMs, respectively. This provides a real-time and visualizable platform for monitoring cardiotoxicity screening.
In summary, 3D heart models have emerged as a pivotal tool for cardiotoxicity research. However, accurately replicating the crucial influence of the vascular endothelial layer on drug effects within the myocardium remains a significant challenge.
Regenerative medicine
Ischemic heart disease continues to be the leading cause of death globally, with an estimated 8.9 million deaths each year [132]. Due to the limited regenerative capacity of adult human hearts after injury [133], hPSC-CMs represent an attractive cell source to promote cardiac repair [134]. Currently, hPSC-CMs have been proven to remuscularize infarcted hearts and restore contractile function in mice [135], rats [136], guinea pigs [137], and non-human primates [138, 139]. However, preclinical studies have shown that the low survival and engraftment rates of injected hPSC-CMs have hindered their clinical applications [140, 141]. Thereby, the fabrication of cardiac grafts offers a promising strategy for therapy. In recent years, EHTs and cardiac patches have been successfully developed by several groups to treat MI in rats [142], [143], [144] and pigs [137, 145]. More recently, a clinical trial at Osaka University reported the implantation of allogeneic hiPSC-CM patches in a patient with ischemic cardiomyopathy (ClinicalTrials.gov Identifier: NCT04696328) [146]. hiPSC-CM patches were implanted via thoracotomy into the left ventricular epicardium of the patient and showed improved wall motion 6 months post-implantation without any major adverse events, suggesting that the patches were well-tolerated. However, cardiac patches often require complicated fabrication and the delivery requires invasive surgical procedures, which could trigger an undesirable inflammatory response [147]. Alternatively, 3D cardiac microtissues like cardiac spheroids may overcome this hurdle as they can be delivered through minimally invasive approaches (e.g., catheters) due to their injectable size [148, 149]. Transplantation of cardiac spheroids in combination with gelatin hydrogel led to an overall improved cell survival, engraftment, and cardiac function in cryo-injured rat and swine hearts [150]. Moreover, transplanted spheroids mixed with gelatin hydrogel into beating porcine hearts using a transplant injection device resulted in better distribution and retention of the transplanted spheroids than conventional needle-based intramyocardial injection procedures [151]. While cardiac spheroids offer significant potential for restoring the cardiac function of infarcted hearts, the relatively immature phenotype of hiPSC-CMs usually results in unsynchronized contraction, potentially increasing the risk of arrhythmias [152, 153]. To overcome this challenge, electrically conductive materials and multiple cell types can be incorporated into cardiac spheroids [154, 155]. For example, incorporating silicon nanowires into cardiac spheroids containing hPSC-CMs, CFs, ECs, and stromal cells markedly improves therapeutic angiogenesis in ischemic tissues, leading to a significant increase in cell retention and survival [154]. In addition to CMs, vascular cells also have a pivotal role in cell regeneration. Transplanting vascular cell spheroids has been reported to not only enhance cardiac function but also ameliorate cardiac fibrosis following MI [156].
In recent years, exosomes isolated from cardiac spheroids have been explored as a cell-free therapy in cardiac regeneration [157]. One of the potential advantages of exosomes over cardiac spheroids is that they are acellular and therefore are likely to be less immunogenic than cardiac spheroids [157]. Gallet and coworkers indicated that exosomes from cardiosphere-derived cells delivered by intramyocardial injection decreased acute ischemia-reperfusion injury, attenuated left ventricular remodeling, and improved cardiac function in pig MI models, suggesting that the therapeutic effect is mediated via paracrine effects instead of direct remuscularization [158]. Generally, these studies provide evidence of the therapeutic potential of exosomes, but the exact constituents of exosomes and the mechanisms through which exosomes exert their therapeutic effects, remain unclear.
Vessel organoids and vessel-on-a-chip and their applications
In 2019, Wimmer et al. developed a protocol for the directed differentiation of hPSCs into blood vessel organoids by embedding hPSC aggregates into collagen I/Matrigel substrate followed by the step-wise modulation of bone morphogenetic protein, vascular endothelial growth factor, and fibroblast growth factor signaling pathway activities [159, 160]. These organoids comprised networks of ECs surrounded by pericytes, mesenchymal stem-like cells, and hematopoietic cells [159]. Building on this method, a YAP inhibitor, vestigial-like family member 4 (VGLL4) has been demonstrated to promote vessel organoid formation [161]. This is particularly noteworthy given that the Hippo-YAP signaling pathway has been previously established as a critical mediator in cardiovascular development and regeneration [162]. To date, blood vessel organoids have been widely used in disease modeling (e.g., diabetic vasculopathy, COVID-19) [111, 160, 163, 164]. Moreover, the multicellular feature of vessel organoids makes them a valuable tool for investigating the mechanisms of diseases [165]. For instance, one study demonstrated that SARS-CoV-2 can directly infect kidney and blood vessel organoids and the infection can be prevented by soluble recombinant human ACE2 (hrsACE2) antibodies, although which specific cell types are susceptible to SARS-CoV-2 infection remains unclear [163]. Another recent study provides evidence that stimulation of vascular organoids with SARS-CoV-2 antigen could increase pericyte uptake of SARS-CoV-2 over ECs, leading to pericyte apoptosis [111]. Coupled with the greater loss of pericytes in vessel organoids, subsequent in vitro studies revealed that the SARS-CoV-2 spike glycoprotein can trigger pericyte dysfunction and thereby promote EC death [166]. This ultimately leads to the disruption of the vascular network and alterations in vascular permeability, thereby contributing to vasculopathy. These studies suggest that severe microvascular endothelial injury in COVID-19 might be a bystander effect of pericyte injury, and provide evidence for further clinical therapeutic targeting of pericytes in treating COVID-19.
Although vessel organoids provide a promising platform for disease modeling and mechanistic studies, they face a limitation in terms of nutrient and oxygen diffusion, because as organoids grow, extensive cell death occurs in the core of organoids [167, 168]. One of the methods to circumvent this hurdle is to transplant organoids into live organs. Following transplantation into the renal capsule of immunodeficient mice, the vessel organoids were capable of anastomosing with the host vasculature and became fully perfusable [160]. However, this method is not suitable for high throughput studies. To overcome this issue, a vessel-on-a-chip has been developed [169]. The incorporation of flow provides precise mechanical stimulation through fluid flow, enhancing our comprehensive understanding of shear stress and its impact on vascular biology and function [170]. Accurately modeling the various stages of atherosclerosis progression is crucial and facilitated by the advancements in vessel-on-a-chip technology. To model atherosclerosis progression, a shear gradient-activated microfluidic device has been created [171, 172]. This device is capable of inducing lumen occlusion or stenosis, effectively replicating the increased shear stress regions typically observed in developing atherosclerotic plaques. As such, these vessel-on-a-chip platforms designed for modeling atherosclerosis are poised to play a pivotal role in advancing our understanding of the disease and in the discovery of novel drug candidates for future treatments.
Current challenges and future directions
The versatile toolkit offered by hPSC-derived cardiac and vascular tissues holds immense potential for biomedical research. However, the translation of this potential into clinical applications faces significant challenges. One of the major challenges of hPSC-heart models is that the force generated by hPSC-CMs is much smaller than that generated by adult CMs [113]. Therefore, it is imperative to establish standardized criteria for the maturation of hPSC-CMs. These criteria should encompass important factors such as sarcomere organization, maturity markers, electrophysiology, metabolic function, and gene expression profiles. Rigorous attention to these parameters is essential to ensure the consistent and dependable development of hPSC-CMs for both research and clinical applications, as emphasized in Ref. [173]. Furthermore, the field of cardiac and vessel organoids faces a significant challenge due to the inherent variability. This variability stems from different hiPSC lines and differentiation protocols, leading to differences in morphology and developmental progression [174, 175]. Therefore, it is crucial to employ multiple hiPSC lines and optimize protocols to robustly generate cardiac or vessel organoids, addressing the challenges associated with variability and ensuring the reliability of research outcomes and potential clinical applications.
As previously mentioned, the generation of cardiac or vessel organoids requires ECM such as collagen, Matrigel, and gelatin for tissue assembly. However, the current ECM is poorly defined and exhibits batch-to-batch variability, restricting its usefulness in drug screening and other downstream applications. To tackle this issue, researchers are actively working towards creating mechanically and chemically defined, Good Manufacturing Practice (GMP)-grade xeno-free ECM, such as recombinant vitronectin and Laminin-511 [176]. These efforts aim to enhance the consistency and reliability of ECM for various applications, such as culturing hiPSCs [177], but the effectiveness and consistency of the ECM in 3D models still require thorough evaluation and testing before being widely used.
Creating organoids that replicate the complexity of a four-chambered heart is indeed a significant goal in the field. To achieve greater fidelity in modeling heart development and physiology, generating organoids with distinct atrial and ventricular lineages on both left and right sides is crucial. Future advancements may involve refining differentiation methods to allow the formation of atrial-like CMs alongside ventricular ones. This could be achieved by tweaking the environmental cues, growth factors, or genetic manipulations to guide specific cell populations toward simultaneous atrial and ventricular differentiation. Another approach may involve assembling chamber-specific organoids, each differentiated to represent a particular lineage-specific chamber, and then coaxing these specialized organoids to merge or interact in a controlled environment. This strategy could lead to the construction of more anatomically accurate four-chambered organoids, mirroring the complexities of the human heart. Developing these four-chambered organoids will not only advance our understanding of heart development but also provide more accurate models for studying cardiac diseases and testing potential therapies.
Insufficient vascularization prevents the growth and function of 3D heart tissues, which presents a significant obstacle to their clinical applications. Recently, the incorporation of ECs or 3D-printed vessels has shown promising advancements in achieving vascularization-like characteristics within 3D heart tissues. Nevertheless, these approaches have often yielded vascular-like features rather than fully functional vasculatures. Transplanting 3D cardiac or vascular tissues into animals enhances the vascularization of implanted tissues, effectively addressing issues such as nutrient gradient disparities and the accumulation of waste in long-term 3D culture. Moreover, enhancement of angiogenesis by local blood perfusion could drastically improve the survival and size of post-transplanted cardiac organoids. After 75 days of transplantation, the diameter of cardiac organoids remarkably increased to 8 mm, similar to the size of the mouse heart [178]. Besides transplantation, there are several innovative approaches to enhance vascularization in various 3D models. Examples include co-culturing brain organoids with ECs [179], co-culturing brain organoids with vessel organoids [180], and overexpressing the angiogenic transcription factor human ETS variant 2 (ETV2) in brain organoids [181] or kidney organoids [182]. These strategies outlined above can be implemented in cardiac organoid research, pointing toward future directions for advancing this field.
Another significant challenge in current 3D heart and vessel models is the absence of immune and nervous systems. A research group has made progress in addressing this issue by developing neuro-cardiac lineages within a single organoid, referred to as elongating multilineage organized cardiac gastruloid (EMLOC) [183]. These EMLOCs successfully replicate various interconnected developmental aspects of cardiogenesis. Notably, neurons surrounding the EMLOCs are co-developed in a spatially organized pattern, resembling the innervated heart. Therefore, the EMLOC model presents an attractive opportunity to investigate the mechanisms underlying simultaneous neurogenesis and cardiogenesis. In addition, the immune system also plays an important role in the pathogenesis of cardiovascular disease. Single-cell RNA sequencing data indicates that immune cells constitute 10.4 % of all cell types in adult heart atrial tissue [184]. The presence of immune cells is critically important for precise modeling of cardiovascular inflammation and infectious diseases. Despite this importance, most published 3D cardiovascular disease models lack immune cells. Therefore, integrating immune cells into 3D models of cardiovascular diseases emerges as a pivotal research avenue. In other organoid fields, to address the absence of immune cells involves establishing co-culture of retinal organoids with hiPSC-derived macrophage precursor cells [185] or co-differentiation of immune cells during the brain organoid differentiation process [186]. Similar strategies could be applied to cardiac organoids, but with some caution, as immune cells exhibit significant heterogeneity in terms of morphology, gene expression profile, physiological properties, and function, which are determined by their developmental origin and tissue microenvironment [187].
Lastly, advancing 3D cardiovascular models face challenges in 3D imaging and electrical recording techniques, primarily due to the deeper z-plane depth compared to 2D cells. However, recent progress in imaging technologies, including the introduction of tissue-clearing protocols and the creation of multiple fluorescent reporter cell lines, has started to address these challenges [188]. Moreover, the advancement of 3D electrical recording tools like 3D MEA has improved the detection of electrical signals within the core of cardiac organoids [189]. Nevertheless, the current 3D MEA techniques could benefit from refinements. The key hurdle involves inserting electrode arrays into the organoids, risking disruption of their growth and function. Looking ahead, the prospect of developing non-invasive, high-throughput, long-term live-cell imaging alongside electrophysiological recording tools offers significant potential to extend the applications of 3D cardiovascular models.
In conclusion, 3D cardiac and vascular models offer a more accurate and complex in vitro representation of the cardiovascular system, allowing for detailed exploration of disease mechanisms and drug screening. With ongoing technological advancements and further refinement, these models are on the path to becoming standard tools in cardiovascular research. They hold the potential to significantly enhance drug development and therapeutic interventions, promising a brighter future for cardiovascular healthcare.
Funding source: National Natural Science Foundation of China General Program
Award Identifier / Grant number: 82370311
Funding source: Guangdong Province International Science and Technology Cooperation Research Project
Award Identifier / Grant number: 2023A0505050088
-
Research ethics: Not applicable.
-
Informed consent: Informed consent was obtained from all individuals included in this study.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Competing interests: Authors state no conflict of interest.
-
Research funding: This study is funded by National Natural Science Foundation of China General Program (Grant # 82370311) and Guangdong Province International Science and Technology Cooperation Research Project (Grant # 2023A0505050088).
-
Data availability: Not applicable.
References
1. Mensah, GA, Roth, GA, Fuster, V. The global burden of cardiovascular diseases and risk factors: 2020 and beyond. J Am Coll Cardiol 2019;74:2529–32. https://doi.org/10.1016/j.jacc.2019.10.009.Search in Google Scholar PubMed
2. Visseren, FLJ, Mach, F, Smulders, YM, Carballo, D, Koskinas, KC, Bäck, M, et al.. 2021 ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur Heart J 2021;42:3227–337. https://doi.org/10.1093/eurheartj/ehab484.Search in Google Scholar PubMed
3. Liu, G, Liu, Z, Cao, N. Human pluripotent stem cell-based cardiovascular disease modeling and drug discovery. Pflügers Archiv 2021;473:1087–97. https://doi.org/10.1007/s00424-021-02542-1.Search in Google Scholar PubMed
4. Lu, HR, Mariën, R, Saels, A, De Clerck, F. Species plays an important role in drug-induced prolongation of action potential duration and early afterdepolarizations in isolated Purkinje fibers. J Cardiovasc Electrophysiol 2001;12:93–102. https://doi.org/10.1046/j.1540-8167.2001.00093.x.Search in Google Scholar PubMed
5. Savoji, H, Mohammadi, MH, Rafatian, N, Toroghi, MK, Wang, EY, Zhao, Y, et al.. Cardiovascular disease models: a game changing paradigm in drug discovery and screening. Biomaterials 2019;198:3–26. https://doi.org/10.1016/j.biomaterials.2018.09.036.Search in Google Scholar PubMed PubMed Central
6. Janssen, PM, Lehnart, SE, Prestle, J, Hasenfuss, G. Preservation of contractile characteristics of human myocardium in multi-day cell culture. J Mol Cell Cardiol 1999;31:1419–27. https://doi.org/10.1006/jmcc.1999.0978.Search in Google Scholar PubMed
7. Mummery, CL. Perspectives on the use of human induced pluripotent stem cell-derived cardiomyocytes in biomedical research. Stem Cell Rep 2018;11:1306–11. https://doi.org/10.1016/j.stemcr.2018.11.011.Search in Google Scholar PubMed PubMed Central
8. Takahashi, K, Tanabe, K, Ohnuki, M, Narita, M, Ichisaka, T, Tomoda, K, et al.. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 2007;131:861–72. https://doi.org/10.1016/j.cell.2007.11.019.Search in Google Scholar PubMed
9. Chen, VC, Ye, J, Shukla, P, Hua, G, Chen, D, Lin, Z, et al.. Development of a scalable suspension culture for cardiac differentiation from human pluripotent stem cells. Stem Cell Res 2015;15:365–75. https://doi.org/10.1016/j.scr.2015.08.002.Search in Google Scholar PubMed PubMed Central
10. Zwi, L, Caspi, O, Arbel, G, Huber, I, Gepstein, A, Park, IH, et al.. Cardiomyocyte differentiation of human induced pluripotent stem cells. Circulation 2009;120:1513–23. https://doi.org/10.1161/circulationaha.109.868885.Search in Google Scholar PubMed
11. Amaral, RLF, Miranda, M, Marcato, PD, Swiech, K. Comparative analysis of 3D bladder tumor spheroids obtained by forced floating and hanging drop methods for drug screening. Front Physiol 2017;8:605. https://doi.org/10.3389/fphys.2017.00605.Search in Google Scholar PubMed PubMed Central
12. Scalise, M, Marino, F, Salerno, L, Cianflone, E, Molinaro, C, Salerno, N, et al.. From spheroids to organoids: the next generation of model systems of human cardiac regeneration in a dish. Int J Mol Sci 2021;22:13180. https://doi.org/10.3390/ijms222413180.Search in Google Scholar PubMed PubMed Central
13. Fennema, E, Rivron, N, Rouwkema, J, van Blitterswijk, C, de Boer, J. Spheroid culture as a tool for creating 3D complex tissues. Trends Biotechnol 2013;31:108–15. https://doi.org/10.1016/j.tibtech.2012.12.003.Search in Google Scholar PubMed
14. Clevers, H. Modeling development and disease with organoids. Cell 2016;165:1586–97. https://doi.org/10.1016/j.cell.2016.05.082.Search in Google Scholar PubMed
15. Augustyniak, J, Bertero, A, Coccini, T, Baderna, D, Buzanska, L, Caloni, F. Organoids are promising tools for species-specific in vitro toxicological studies. J Appl Toxicol 2019;39:1610–22. https://doi.org/10.1002/jat.3815.Search in Google Scholar PubMed
16. Miyamoto, M, Nam, L, Kannan, S, Kwon, C. Heart organoids and tissue models for modeling development and disease. Semin Cell Dev Biol 2021;118:119–28. https://doi.org/10.1016/j.semcdb.2021.03.011.Search in Google Scholar PubMed PubMed Central
17. Lewis-Israeli, YR, Abdelhamid, M, Olomu, I, Aguirre, A. Modeling the effects of maternal diabetes on the developing human heart using pluripotent stem cell-derived heart organoids. Curr Protoc 2022;2:e461. https://doi.org/10.1002/cpz1.461.Search in Google Scholar PubMed PubMed Central
18. Kim, H, Kamm, RD, Vunjak-Novakovic, G, Wu, JC. Progress in multicellular human cardiac organoids for clinical applications. Cell Stem Cell 2022;29:503–14. https://doi.org/10.1016/j.stem.2022.03.012.Search in Google Scholar PubMed PubMed Central
19. Wu, F, He, Q, Li, F, Yang, X. A review of protocols for engineering human cardiac organoids. Heliyon 2023;9:e19938. https://doi.org/10.1016/j.heliyon.2023.e19938.Search in Google Scholar PubMed PubMed Central
20. Drakhlis, L, Biswanath, S, Farr, CM, Lupanow, V, Teske, J, Ritzenhoff, K, et al.. Human heart-forming organoids recapitulate early heart and foregut development. Nat Biotechnol 2021;39:737–46. https://doi.org/10.1038/s41587-021-00815-9.Search in Google Scholar PubMed PubMed Central
21. Silva, AC, Matthys, OB, Joy, DA, Kauss, MA, Natarajan, V, Lai, MH, et al.. Co-emergence of cardiac and gut tissues promotes cardiomyocyte maturation within human iPSC-derived organoids. Cell Stem Cell 2021;28:2137–52. https://doi.org/10.1016/j.stem.2021.11.007.Search in Google Scholar PubMed
22. Hofbauer, P, Jahnel, SM, Papai, N, Giesshammer, M, Deyett, A, Schmidt, C, et al.. Cardioids reveal self-organizing principles of human cardiogenesis. Cell 2021;184:3299–317. https://doi.org/10.1016/j.cell.2021.04.034.Search in Google Scholar PubMed
23. Lewis-Israeli, YR, Wasserman, AH, Gabalski, MA, Volmert, BD, Ming, Y, Ball, KA, et al.. Self-assembling human heart organoids for the modeling of cardiac development and congenital heart disease. Nat Commun 2021;12:5142. https://doi.org/10.1038/s41467-021-25329-5.Search in Google Scholar PubMed PubMed Central
24. Kostina, A, Lewis-Israeli, YR, Abdelhamid, M, Gabalski, MA, Volmert, BD, Lankerd, H, et al.. ER stress and lipid imbalance drive embryonic cardiomyopathy in a human heart organoid model of pregestational diabetes. bioRxiv 2023:06.07.544081. https://doi.org/10.1101/2023.06.07.544081.Search in Google Scholar PubMed PubMed Central
25. Meier, AB, Zawada, D, De Angelis, MT, Martens, LD, Santamaria, G, Zengerle, S, et al.. Epicardioid single-cell genomics uncovers principles of human epicardium biology in heart development and disease. Nat Biotechnol 2023;41:1787–800. https://doi.org/10.1038/s41587-023-01718-7.Search in Google Scholar PubMed PubMed Central
26. Antzelevitch, C. Cardiac repolarization. The long and short of it. Europace 2005;7:3–9. https://doi.org/10.1016/j.eupc.2005.05.010.Search in Google Scholar PubMed PubMed Central
27. Goldfracht, I, Efraim, Y, Shinnawi, R, Kovalev, E, Huber, I, Gepstein, A, et al.. Engineered heart tissue models from hiPSC-derived cardiomyocytes and cardiac ECM for disease modeling and drug testing applications. Acta Biomater 2019;92:145–59. https://doi.org/10.1016/j.actbio.2019.05.016.Search in Google Scholar PubMed
28. Xu, H, Liu, G, Gong, J, Zhang, Y, Gu, S, Wan, Z, et al.. Investigating and resolving cardiotoxicity induced by COVID-19 treatments using human pluripotent stem cell-derived cardiomyocytes and engineered heart tissues. Adv Sci 2022;9:e2203388. https://doi.org/10.1002/advs.202203388.Search in Google Scholar PubMed PubMed Central
29. Zhao, Y, Rafatian, N, Feric, NT, Cox, BJ, Aschar-Sobbi, R, Wang, EY, et al.. A platform for generation of chamber-specific cardiac tissues and disease modeling. Cell 2019;176:913–27. https://doi.org/10.1016/j.cell.2018.11.042.Search in Google Scholar PubMed PubMed Central
30. Xiao, Y, Zhang, B, Liu, H, Miklas, JW, Gagliardi, M, Pahnke, A, et al.. Microfabricated perfusable cardiac biowire: a platform that mimics native cardiac bundle. Lab Chip 2014;14:869–82. https://doi.org/10.1039/c3lc51123e.Search in Google Scholar PubMed PubMed Central
31. Thavandiran, N, Dubois, N, Mikryukov, A, Massé, S, Beca, B, Simmons, CA, et al.. Design and formulation of functional pluripotent stem cell-derived cardiac microtissues. Proc Natl Acad Sci USA 2013;110:E4698–707. https://doi.org/10.1073/pnas.1311120110.Search in Google Scholar PubMed PubMed Central
32. Lee, M, Kim, YS, Park, J, Choe, G, Lee, S, Kang, BG, et al.. A paintable and adhesive hydrogel cardiac patch with sustained release of ANGPTL4 for infarcted heart repair. Bioact Mater 2024;31:395–407. https://doi.org/10.1016/j.bioactmat.2023.08.020.Search in Google Scholar PubMed PubMed Central
33. Köhne, M, Behrens, CS, Stüdemann, T, Bibra, CV, Querdel, E, Shibamiya, A, et al.. A potential future Fontan modification: preliminary in vitro data of a pressure-generating tube from engineered heart tissue. Eur J Cardio Thorac Surg 2022;62:ezac111. https://doi.org/10.1093/ejcts/ezac111.Search in Google Scholar PubMed PubMed Central
34. Goldfracht, I, Protze, S, Shiti, A, Setter, N, Gruber, A, Shaheen, N, et al.. Generating ring-shaped engineered heart tissues from ventricular and atrial human pluripotent stem cell-derived cardiomyocytes. Nat Commun 2020;11:75. https://doi.org/10.1038/s41467-019-13868-x.Search in Google Scholar PubMed PubMed Central
35. Zimmermann, WH, Schneiderbanger, K, Schubert, P, Didie, M, Munzel, F, Heubach, JF, et al.. Tissue engineering of a differentiated cardiac muscle construct. Circ Res 2002;90:223–30. https://doi.org/10.1161/hh0202.103644.Search in Google Scholar PubMed
36. Eder, A, Vollert, I, Hansen, A, Eschenhagen, T. Human engineered heart tissue as a model system for drug testing. Adv Drug Deliv Rev 2016;96:214–24. https://doi.org/10.1016/j.addr.2015.05.010.Search in Google Scholar PubMed
37. MacQueen, LA, Sheehy, SP, Chantre, CO, Zimmerman, JF, Pasqualini, FS, Liu, X, et al.. A tissue-engineered scale model of the heart ventricle. Nat Biomed Eng 2018;2:930–41. https://doi.org/10.1038/s41551-018-0271-5.Search in Google Scholar PubMed PubMed Central
38. Saleem, U, van Meer, BJ, Katili, PA, Mohd Yusof, NAN, Mannhardt, I, Garcia, AK, et al.. Blinded, multicenter evaluation of drug-induced changes in contractility using human-induced pluripotent stem cell-derived cardiomyocytes. Toxicol Sci 2020;176:103–23. https://doi.org/10.1093/toxsci/kfaa058.Search in Google Scholar PubMed PubMed Central
39. Arefin, A, Mendoza, M, Dame, K, Garcia, MI, Strauss, DG, Ribeiro, AJS. Reproducibility of drug-induced effects on the contractility of an engineered heart tissue derived from human pluripotent stem cells. Front Pharmacol 2023;14:1212092. https://doi.org/10.3389/fphar.2023.1212092.Search in Google Scholar PubMed PubMed Central
40. Hirt, MN, Hansen, A, Eschenhagen, T. Cardiac tissue engineering: state of the art. Circ Res 2014;114:354–67. https://doi.org/10.1161/circresaha.114.300522.Search in Google Scholar
41. Hasenfuss, G, Mulieri, LA, Blanchard, EM, Holubarsch, C, Leavitt, BJ, Ittleman, F, et al.. Energetics of isometric force development in control and volume-overload human myocardium. Comparison with animal species. Circ Res 1991;68:836–46. https://doi.org/10.1161/01.res.68.3.836.Search in Google Scholar PubMed
42. Tzatzalos, E, Abilez, OJ, Shukla, P, Wu, JC. Engineered heart tissues and induced pluripotent stem cells: macro- and microstructures for disease modeling, drug screening, and translational studies. Adv Drug Deliv Rev 2016;96:234–44. https://doi.org/10.1016/j.addr.2015.09.010.Search in Google Scholar PubMed PubMed Central
43. Esser, TU, Trossmann, VT, Lentz, S, Engel, FB, Scheibel, T. Designing of spider silk proteins for human induced pluripotent stem cell-based cardiac tissue engineering. Mater Today Bio 2021;11:100114. https://doi.org/10.1016/j.mtbio.2021.100114.Search in Google Scholar PubMed PubMed Central
44. Park, SE, Georgescu, A, Huh, D. Organoids-on-a-chip. Science 2019;364:960–5. https://doi.org/10.1126/science.aaw7894.Search in Google Scholar PubMed PubMed Central
45. Yadid, M, Lind, JU, Ardona, HAM, Sheehy, SP, Dickinson, LE, Eweje, F, et al.. Endothelial extracellular vesicles contain protective proteins and rescue ischemia-reperfusion injury in a human heart-on-chip. Sci Transl Med 2020;12:eaax8005. https://doi.org/10.1126/scitranslmed.aax8005.Search in Google Scholar PubMed PubMed Central
46. Arslan, U, Brescia, M, Meraviglia, V, Nahon, DM, van Helden, RWJ, Stein, JM, et al.. Vascularized hiPSC-derived 3D cardiac microtissue on chip. Stem Cell Rep 2023;18:1394–404. https://doi.org/10.1016/j.stemcr.2023.08.012.Search in Google Scholar PubMed PubMed Central
47. Yin, X, Mead, BE, Safaee, H, Langer, R, Karp, JM, Levy, O. Engineering stem cell organoids. Cell Stem Cell 2016;18:25–38. https://doi.org/10.1016/j.stem.2015.12.005.Search in Google Scholar PubMed PubMed Central
48. Liu, H, Bolonduro, OA, Hu, N, Ju, J, Rao, AA, Duffy, BM, et al.. Heart-on-a-Chip model with integrated extra- and intracellular bioelectronics for monitoring cardiac electrophysiology under acute hypoxia. Nano Lett 2020;20:2585–93. https://doi.org/10.1021/acs.nanolett.0c00076.Search in Google Scholar PubMed
49. Lee, J, Mehrotra, S, Zare-Eelanjegh, E, Rodrigues, RO, Akbarinejad, A, Ge, D, et al.. A heart-breast cancer-on-a-chip platform for disease modeling and monitoring of cardiotoxicity induced by cancer chemotherapy. Small 2021;17:e2004258. https://doi.org/10.1002/smll.202004258.Search in Google Scholar PubMed PubMed Central
50. Soltantabar, P, Calubaquib, EL, Mostafavi, E, Ghazavi, A, Stefan, MC. Heart/liver-on-a-chip as a model for the evaluation of cardiotoxicity induced by chemotherapies. Organs-on-a-Chip 2021;3:100008. https://doi.org/10.1016/j.ooc.2021.100008.Search in Google Scholar
51. Baptista, LS, Porrini, C, Kronemberger, GS, Kelly, DJ, Perrault, CM. 3D organ-on-a-chip: the convergence of microphysiological systems and organoids. Front Cell Dev Biol 2022;10:1043117. https://doi.org/10.3389/fcell.2022.1043117.Search in Google Scholar PubMed PubMed Central
52. Rumsey, JW, Lorance, C, Jackson, M, Sasserath, T, McAleer, CW, Long, CJ, et al.. Classical complement pathway inhibition in a “Human-On-A-Chip” model of autoimmune demyelinating neuropathies. Adv Ther 2022;5:2200030. https://doi.org/10.1002/adtp.202200030.Search in Google Scholar PubMed PubMed Central
53. Ahmed, SM, Shivnaraine, RV, Wu, JC. FDA modernization act 2.0 paves the way to computational biology and clinical trials in a dish. Circulation 2023;148:309–11. https://doi.org/10.1161/circulationaha.123.065585.Search in Google Scholar PubMed PubMed Central
54. Bouma, BJ, Mulder, BJ. Changing landscape of congenital heart disease. Circ Res 2017;120:908–22. https://doi.org/10.1161/circresaha.116.309302.Search in Google Scholar PubMed
55. van der Linde, D, Konings, EE, Slager, MA, Witsenburg, M, Helbing, WA, Takkenberg, JJ, et al.. Birth prevalence of congenital heart disease worldwide: a systematic review and meta-analysis. J Am Coll Cardiol 2011;58:2241–7. https://doi.org/10.1016/j.jacc.2011.08.025.Search in Google Scholar PubMed
56. Liu, Y, Chen, S, Zühlke, L, Black, GC, Choy, MK, Li, N, et al.. Global birth prevalence of congenital heart defects 1970–2017: updated systematic review and meta-analysis of 260 studies. Int J Epidemiol 2019;48:455–63. https://doi.org/10.1093/ije/dyz009.Search in Google Scholar PubMed PubMed Central
57. Richards, AA, Garg, V. Genetics of congenital heart disease. Curr Cardiol Rev 2010;6:91–7. https://doi.org/10.2174/157340310791162703.Search in Google Scholar PubMed PubMed Central
58. Zhang, TN, Wu, QJ, Liu, YS, Lv, JL, Sun, H, Chang, Q, et al.. Environmental risk factors and congenital heart disease: an umbrella review of 165 systematic reviews and meta-analyses with more than 120 million participants. Front Cardiovasc Med 2021;8:640729. https://doi.org/10.3389/fcvm.2021.640729.Search in Google Scholar PubMed PubMed Central
59. Yang, S, Hu, H, Kung, H, Zou, R, Dai, Y, Hu, Y, et al.. Organoids: the current status and biomedical applications. Media Commun 2023;4:e274. https://doi.org/10.1002/mco2.274.Search in Google Scholar PubMed PubMed Central
60. Feng, W, Schriever, H, Jiang, S, Bais, A, Wu, H, Kostka, D, et al.. Computational profiling of hiPSC-derived heart organoids reveals chamber defects associated with NKX2-5 deficiency. Commun Biol 2022;5:399. https://doi.org/10.1038/s42003-022-03346-4.Search in Google Scholar PubMed PubMed Central
61. Basu, M, Garg, V. Maternal hyperglycemia and fetal cardiac development: clinical impact and underlying mechanisms. Birth Defects Res 2018;110:1504–16. https://doi.org/10.1002/bdr2.1435.Search in Google Scholar PubMed PubMed Central
62. Øyen, N, Diaz, LJ, Leirgul, E, Boyd, HA, Priest, J, Mathiesen, ER, et al.. Prepregnancy diabetes and offspring risk of congenital heart disease: a nationwide cohort study. Circulation 2016;133:2243–53. https://doi.org/10.1161/circulationaha.115.017465.Search in Google Scholar PubMed PubMed Central
63. Nees, SN, Chung, WK. Genetic basis of human congenital heart disease. Cold Spring Harbor Perspect Biol 2020;12:a036749. https://doi.org/10.1101/cshperspect.a036749.Search in Google Scholar PubMed PubMed Central
64. Ye, S, Wang, C, Xu, Z, Lin, H, Wan, X, Yu, Y, et al.. Impaired human cardiac cell development due to NOTCH1 deficiency. Circ Res 2023;132:187–204. https://doi.org/10.1161/circresaha.122.321398.Search in Google Scholar
65. Galdos, FX, Lee, C, Lee, S, Paige, S, Goodyer, W, Xu, S, et al.. Combined lineage tracing and scRNA-seq reveals unexpected first heart field predominance of human iPSC differentiation. Elife 2023;12:e80075. https://doi.org/10.7554/elife.80075.Search in Google Scholar PubMed PubMed Central
66. Zhang, JZ, Termglinchan, V, Shao, NY, Itzhaki, I, Liu, C, Ma, N, et al.. A human iPSC double-reporter system enables purification of cardiac lineage subpopulations with distinct function and drug response profiles. Cell Stem Cell 2019;24:802–11. https://doi.org/10.1016/j.stem.2019.02.015.Search in Google Scholar PubMed PubMed Central
67. Elliott, PM, Anastasakis, A, Borger, MA, Borggrefe, M, Cecchi, F, Charron, P, et al.. 2014 ESC guidelines on diagnosis and management of hypertrophic cardiomyopathy: the task force for the diagnosis and management of hypertrophic cardiomyopathy of the European Society of Cardiology (ESC). Eur Heart J 2014;35:2733–79. https://doi.org/10.1093/eurheartj/ehu284.Search in Google Scholar PubMed
68. Bozkurt, B, Colvin, M, Cook, J, Cooper, LT, Deswal, A, Fonarow, GC, et al.. Current diagnostic and treatment strategies for specific dilated cardiomyopathies: a scientific statement from the American Heart Association. Circulation 2016;134:e579–646. https://doi.org/10.1161/cir.0000000000000455.Search in Google Scholar
69. Pietsch, N, Chen, CY, Kupsch, S, Bacmeister, L, Geertz, B, Herera-Rivero, M, et al.. Reducing microtubule detyrosination improves heart function in HCM mice and human iPSC-engineered heart tissues. bioRxiv 2023:05.25.542365. https://doi.org/10.1101/2023.05.25.542365.Search in Google Scholar PubMed PubMed Central
70. Prondzynski, M, Lemoine, MD, Zech, AT, Horváth, A, Di Mauro, V, Koivumäki, JT, et al.. Disease modeling of a mutation in α-actinin 2 guides clinical therapy in hypertrophic cardiomyopathy. EMBO Mol Med 2019;11:e11115. https://doi.org/10.15252/emmm.201911115.Search in Google Scholar PubMed PubMed Central
71. Loiben, AM, Chien, WM, Friedman, CE, Chao, LS, Weber, G, Goldstein, A, et al.. Cardiomyocyte apoptosis is associated with contractile dysfunction in stem cell model of MYH7 E848G hypertrophic cardiomyopathy. Int J Mol Sci 2023;24:4909. https://doi.org/10.3390/ijms24054909.Search in Google Scholar PubMed PubMed Central
72. Pioner, JM, Vitale, G, Steczina, S, Langione, M, Margara, F, Santini, L, et al.. Slower calcium handling balances faster cross-bridge cycling in human MYBPC3 HCM. Circ Res 2023;132:628–44. https://doi.org/10.1161/circresaha.122.321956.Search in Google Scholar
73. Yang, KC, Breitbart, A, De Lange, WJ, Hofsteen, P, Futakuchi-Tsuchida, A, Xu, J, et al.. Novel adult-onset systolic cardiomyopathy due to MYH7 E848G mutation in patient-derived induced pluripotent stem cells. JACC Basic Transl Sci 2018;3:728–40. https://doi.org/10.1016/j.jacbts.2018.08.008.Search in Google Scholar PubMed PubMed Central
74. Dutsch, A, Wijnker, PJM, Schlossarek, S, Friedrich, FW, Krämer, E, Braren, I, et al.. Phosphomimetic cardiac myosin-binding protein C partially rescues a cardiomyopathy phenotype in murine engineered heart tissue. Sci Rep 2019;9:18152. https://doi.org/10.1038/s41598-019-54665-2.Search in Google Scholar PubMed PubMed Central
75. Wijnker, PJ, Friedrich, FW, Dutsch, A, Reischmann, S, Eder, A, Mannhardt, I, et al.. Comparison of the effects of a truncating and a missense MYBPC3 mutation on contractile parameters of engineered heart tissue. J Mol Cell Cardiol 2016;97:82–92. https://doi.org/10.1016/j.yjmcc.2016.03.003.Search in Google Scholar PubMed
76. Wang, BZ, Nash, TR, Zhang, X, Rao, J, Abriola, L, Kim, Y, et al.. Engineered cardiac tissue model of restrictive cardiomyopathy for drug discovery. Cell Rep Med 2023;4:100976. https://doi.org/10.1016/j.xcrm.2023.100976.Search in Google Scholar PubMed PubMed Central
77. Vermeer, MC, Bolling, MC, Bliley, JM, Arevalo Gomez, KF, Pavez-Giani, MG, Kramer, D, et al.. Gain-of-function mutation in ubiquitin-ligase KLHL24 causes desmin degradation and dilatation in hiPSC-derived engineered heart tissues. J Clin Invest 2021;131:e140615. https://doi.org/10.1172/jci140615.Search in Google Scholar PubMed PubMed Central
78. Cuello, F, Knaust, AE, Saleem, U, Loos, M, Raabe, J, Mosqueira, D, et al.. Impairment of the ER/mitochondria compartment in human cardiomyocytes with PLN p.Arg14del mutation. EMBO Mol Med 2021;13:e13074. https://doi.org/10.15252/emmm.202013074.Search in Google Scholar PubMed PubMed Central
79. Bremner, SB, Mandrycky, CJ, Leonard, A, Padgett, RM, Levinson, AR, Rehn, ES, et al.. Full-length dystrophin deficiency leads to contractile and calcium transient defects in human engineered heart tissues. J Tissue Eng 2022;13:20417314221119628. https://doi.org/10.1177/20417314221119628.Search in Google Scholar PubMed PubMed Central
80. Kerr, JP, Robison, P, Shi, G, Bogush, AI, Kempema, AM, Hexum, JK, et al.. Detyrosinated microtubules modulate mechanotransduction in heart and skeletal muscle. Nat Commun 2015;6:8526. https://doi.org/10.1038/ncomms9526.Search in Google Scholar PubMed PubMed Central
81. McKenna, WJ, Maron, BJ, Thiene, G. Classification, epidemiology, and global burden of cardiomyopathies. Circ Res 2017;121:722–30. https://doi.org/10.1161/circresaha.117.309711.Search in Google Scholar
82. Schick, R, Mekies, LN, Shemer, Y, Eisen, B, Hallas, T, Ben Jehuda, R, et al.. Functional abnormalities in induced Pluripotent Stem Cell-derived cardiomyocytes generated from titin-mutated patients with dilated cardiomyopathy. PLoS One 2018;13:e0205719. https://doi.org/10.1371/journal.pone.0205719.Search in Google Scholar PubMed PubMed Central
83. McNair, WP, Ku, L, Taylor, MR, Fain, PR, Dao, D, Wolfel, E, et al.. SCN5A mutation associated with dilated cardiomyopathy, conduction disorder, and arrhythmia. Circulation 2004;110:2163–7. https://doi.org/10.1161/01.cir.0000144458.58660.bb.Search in Google Scholar
84. Onkal, R, Mattis, JH, Fraser, SP, Diss, JK, Shao, D, Okuse, K, et al.. Alternative splicing of Nav1.5: an electrophysiological comparison of ‘neonatal’ and ‘adult’ isoforms and critical involvement of a lysine residue. J Cell Physiol 2008;216:716–26. https://doi.org/10.1002/jcp.21451.Search in Google Scholar PubMed
85. Murphy, LL, Moon-Grady, AJ, Cuneo, BF, Wakai, RT, Yu, S, Kunic, JD, et al.. Developmentally regulated SCN5A splice variant potentiates dysfunction of a novel mutation associated with severe fetal arrhythmia. Heart Rhythm 2012;9:590–7. https://doi.org/10.1016/j.hrthm.2011.11.006.Search in Google Scholar PubMed PubMed Central
86. Wauchop, M, Rafatian, N, Zhao, Y, Chen, W, Gagliardi, M, Masse, S, et al.. Maturation of iPSC-derived cardiomyocytes in a heart-on-a-chip device enables modeling of dilated cardiomyopathy caused by R222Q-SCN5A mutation. Biomaterials 2023;301:122255. https://doi.org/10.1016/j.biomaterials.2023.122255.Search in Google Scholar PubMed PubMed Central
87. Wang, G, McCain, ML, Yang, L, He, A, Pasqualini, FS, Agarwal, A, et al.. Modeling the mitochondrial cardiomyopathy of Barth syndrome with induced pluripotent stem cell and heart-on-chip technologies. Nat Med 2014;20:616–23. https://doi.org/10.1038/nm.3545.Search in Google Scholar PubMed PubMed Central
88. Moat, SJ, Bradley, DM, Salmon, R, Clarke, A, Hartley, L. Newborn bloodspot screening for Duchenne muscular dystrophy: 21 years experience in Wales (UK). Eur J Hum Genet 2013;21:1049–53. https://doi.org/10.1038/ejhg.2012.301.Search in Google Scholar PubMed PubMed Central
89. Marini, V, Marino, F, Aliberti, F, Giarratana, N, Pozzo, E, Duelen, R, et al.. Long-term culture of patient-derived cardiac organoids recapitulated Duchenne muscular dystrophy cardiomyopathy and disease progression. Front Cell Dev Biol 2022;10:878311. https://doi.org/10.3389/fcell.2022.878311.Search in Google Scholar PubMed PubMed Central
90. Gilbert, G, Kadur Nagaraju, C, Duelen, R, Amoni, M, Bobin, P, Eschenhagen, T, et al.. Incomplete assembly of the dystrophin-associated protein complex in 2D and 3D-cultured human induced pluripotent stem cell-derived cardiomyocytes. Front Cell Dev Biol 2021;9:737840. https://doi.org/10.3389/fcell.2021.737840.Search in Google Scholar PubMed PubMed Central
91. Frangogiannis, NG. Cardiac fibrosis. Cardiovasc Res 2021;117:1450–88. https://doi.org/10.1093/cvr/cvaa324.Search in Google Scholar PubMed PubMed Central
92. Moore-Morris, T, Guimarães-Camboa, N, Banerjee, I, Zambon, AC, Kisseleva, T, Velayoudon, A, et al.. Resident fibroblast lineages mediate pressure overload-induced cardiac fibrosis. J Clin Invest 2014;124:2921–34. https://doi.org/10.1172/jci74783.Search in Google Scholar PubMed PubMed Central
93. Nielsen, SH, Mouton, AJ, DeLeon-Pennell, KY, Genovese, F, Karsdal, M, Lindsey, ML. Understanding cardiac extracellular matrix remodeling to develop biomarkers of myocardial infarction outcomes. Matrix Biol 2019;75–76:43–57. https://doi.org/10.1016/j.matbio.2017.12.001.Search in Google Scholar PubMed PubMed Central
94. Manabe, I, Shindo, T, Nagai, R. Gene expression in fibroblasts and fibrosis: involvement in cardiac hypertrophy. Circ Res 2002;91:1103–13. https://doi.org/10.1161/01.res.0000046452.67724.b8.Search in Google Scholar PubMed
95. Richards, DJ, Li, Y, Kerr, CM, Yao, J, Beeson, GC, Coyle, RC, et al.. Human cardiac organoids for the modelling of myocardial infarction and drug cardiotoxicity. Nat Biomed Eng 2020;4:446–62. https://doi.org/10.1038/s41551-020-0539-4.Search in Google Scholar PubMed PubMed Central
96. Mastikhina, O, Moon, BU, Williams, K, Hatkar, R, Gustafson, D, Mourad, O, et al.. Human cardiac fibrosis-on-a-chip model recapitulates disease hallmarks and can serve as a platform for drug testing. Biomaterials 2020;233:119741. https://doi.org/10.1016/j.biomaterials.2019.119741.Search in Google Scholar PubMed
97. Wang, EY, Rafatian, N, Zhao, Y, Lee, A, Lai, BFL, Lu, RX, et al.. Biowire model of interstitial and focal cardiac fibrosis. ACS Cent Sci 2019;5:1146–58. https://doi.org/10.1021/acscentsci.9b00052.Search in Google Scholar PubMed PubMed Central
98. Leask, A. Getting to the heart of the matter: new insights into cardiac fibrosis. Circ Res 2015;116:1269–76. https://doi.org/10.1161/circresaha.116.305381.Search in Google Scholar
99. Sager, HB, Hulsmans, M, Lavine, KJ, Moreira, MB, Heidt, T, Courties, G, et al.. Proliferation and recruitment contribute to myocardial macrophage expansion in chronic heart failure. Circ Res 2016;119:853–64. https://doi.org/10.1161/circresaha.116.309001.Search in Google Scholar PubMed PubMed Central
100. Suku, M, Forrester, L, Biggs, M, Monaghan, MG. Resident macrophages and their potential in cardiac tissue engineering. Tissue Eng Part B 2022;28:579–91. https://doi.org/10.1089/ten.teb.2021.0036.Search in Google Scholar
101. Lu, RXZ, Rafatian, N, Zhao, Y, Wagner, KT, Beroncal, EL, Li, B, et al.. Heart-on-a-chip model of immune-induced cardiac dysfunction reveals the role of free mitochondrial DNA and therapeutic effects of endothelial exosomes. bioRxiv 2023:08.09.552495. https://doi.org/10.1101/2023.08.09.552495.Search in Google Scholar PubMed PubMed Central
102. Landi, F, Barillaro, C, Bellieni, A, Brandi, V, Carfì, A, D’Angelo, M, et al.. The new challenge of geriatrics: saving frail older people from the SARS-COV-2 pandemic infection. J Nutr Health Aging 2020;24:466–70. https://doi.org/10.1007/s12603-020-1356-x.Search in Google Scholar PubMed PubMed Central
103. Pan, A, Liu, L, Wang, C, Guo, H, Hao, X, Wang, Q, et al.. Association of public health interventions with the epidemiology of the COVID-19 outbreak in Wuhan, China. JAMA 2020;323:1915–23. https://doi.org/10.1001/jama.2020.6130.Search in Google Scholar PubMed PubMed Central
104. Marchiano, S, Hsiang, TY, Khanna, A, Higashi, T, Whitmore, LS, Bargehr, J, et al.. SARS-CoV-2 infects human pluripotent stem cell-derived cardiomyocytes, impairing electrical and mechanical function. Stem Cell Rep 2021;16:478–92. https://doi.org/10.1016/j.stemcr.2021.02.008.Search in Google Scholar PubMed PubMed Central
105. Bojkova, D, Wagner, JUG, Shumliakivska, M, Aslan, GS, Saleem, U, Hansen, A, et al.. SARS-CoV-2 infects and induces cytotoxic effects in human cardiomyocytes. Cardiovasc Res 2020;116:2207–15. https://doi.org/10.1093/cvr/cvaa267.Search in Google Scholar PubMed PubMed Central
106. Mills, RJ, Humphrey, SJ, Fortuna, PRJ, Lor, M, Foster, SR, Quaife-Ryan, GA, et al.. BET inhibition blocks inflammation-induced cardiac dysfunction and SARS-CoV-2 infection. Cell 2021;184:2167–82. https://doi.org/10.1016/j.cell.2021.03.026.Search in Google Scholar PubMed PubMed Central
107. Arhontoulis, DC, Kerr, CM, Richards, D, Tjen, K, Hyams, N, Jones, JA, et al.. Human cardiac organoids to model COVID-19 cytokine storm induced cardiac injuries. J Tissue Eng Regen Med 2022;16:799–811. https://doi.org/10.1002/term.3327.Search in Google Scholar PubMed PubMed Central
108. Bailey, AL, Dmytrenko, O, Greenberg, L, Bredemeyer, AL, Ma, P, Liu, J, et al.. SARS-CoV-2 infects human engineered heart tissues and models COVID-19 myocarditis. JACC Basic Transl Sci 2021;6:331–45. https://doi.org/10.1016/j.jacbts.2021.01.002.Search in Google Scholar PubMed PubMed Central
109. Knoll, R, Schultze, JL, Schulte-Schrepping, J. Monocytes and macrophages in COVID-19. Front Immunol 2021;12:720109. https://doi.org/10.3389/fimmu.2021.720109.Search in Google Scholar PubMed PubMed Central
110. Guney, C, Akar, F. Epithelial and endothelial expressions of ACE2: SARS-CoV-2 entry routes. J Pharm Pharmaceut Sci 2021;24:84–93. https://doi.org/10.18433/jpps31455.Search in Google Scholar PubMed
111. Khan, AO, Reyat, JS, Hill, H, Bourne, JH, Colicchia, M, Newby, ML, et al.. Preferential uptake of SARS-CoV-2 by pericytes potentiates vascular damage and permeability in an organoid model of the microvasculature. Cardiovasc Res 2022;118:3085–96. https://doi.org/10.1093/cvr/cvac097.Search in Google Scholar PubMed PubMed Central
112. Wolf, CM, Berul, CI. Inherited conduction system abnormalities – one group of diseases, many genes. J Cardiovasc Electrophysiol 2006;17:446–55. https://doi.org/10.1111/j.1540-8167.2006.00427.x.Search in Google Scholar PubMed
113. Brandão, KO, Tabel, VA, Atsma, DE, Mummery, CL, Davis, RP. Human pluripotent stem cell models of cardiac disease: from mechanisms to therapies. Dis Model Mech 2017;10:1039–59. https://doi.org/10.1242/dmm.030320.Search in Google Scholar PubMed PubMed Central
114. Grant, AO. Cardiac ion channels. Circ Arrhythm Electrophysiol 2009;2:185–94. https://doi.org/10.1161/circep.108.789081.Search in Google Scholar
115. van der Velden, J, Klein, LJ, van der Bijl, M, Huybregts, MA, Stooker, W, Witkop, J, et al.. Isometric tension development and its calcium sensitivity in skinned myocyte-sized preparations from different regions of the human heart. Cardiovasc Res 1999;42:706–19. https://doi.org/10.1016/s0008-6363(98)00337-x.Search in Google Scholar PubMed
116. Schotten, U, Verheule, S, Kirchhof, P, Goette, A. Pathophysiological mechanisms of atrial fibrillation: a translational appraisal. Physiol Rev 2011;91:265–325. https://doi.org/10.1152/physrev.00031.2009.Search in Google Scholar PubMed
117. Al-Khatib, SM. Atrial fibrillation. Ann Intern Med 2023;176:Itc97–itc112. https://doi.org/10.7326/aitc202307180.Search in Google Scholar PubMed
118. Cha, YM, Dzeja, PP, Shen, WK, Jahangir, A, Hart, CY, Terzic, A, et al.. Failing atrial myocardium: energetic deficits accompany structural remodeling and electrical instability. Am J Physiol Heart Circ Physiol 2003;284:H1313–20. https://doi.org/10.1152/ajpheart.00337.2002.Search in Google Scholar PubMed
119. Mayr, M, Yusuf, S, Weir, G, Chung, YL, Mayr, U, Yin, X, et al.. Combined metabolomic and proteomic analysis of human atrial fibrillation. J Am Coll Cardiol 2008;51:585–94. https://doi.org/10.1016/j.jacc.2007.09.055.Search in Google Scholar PubMed
120. Krause, J, Nickel, A, Madsen, A, Aitken-Buck, HM, Stoter, AMS, Schrapers, J, et al.. An arrhythmogenic metabolite in atrial fibrillation. J Transl Med 2023;21:566. https://doi.org/10.1186/s12967-023-04420-z.Search in Google Scholar PubMed PubMed Central
121. Mamoshina, P, Rodriguez, B, Bueno-Orovio, A. Toward a broader view of mechanisms of drug cardiotoxicity. Cell Rep Med 2021;2:100216. https://doi.org/10.1016/j.xcrm.2021.100216.Search in Google Scholar PubMed PubMed Central
122. Fonoudi, H, Burridge, PW. Cellular model systems to study cardiovascular injury from chemotherapy. J Thromb Thrombolysis 2021;51:890–6. https://doi.org/10.1007/s11239-020-02299-x.Search in Google Scholar PubMed PubMed Central
123. Muniyandi, P, O’Hern, C, Popa, MA, Aguirre, A. Biotechnological advances and applications of human pluripotent stem cell-derived heart models. Front Bioeng Biotechnol 2023;11:1214431. https://doi.org/10.3389/fbioe.2023.1214431.Search in Google Scholar PubMed PubMed Central
124. Sharma, A, Burridge, PW, McKeithan, WL, Serrano, R, Shukla, P, Sayed, N, et al.. High-throughput screening of tyrosine kinase inhibitor cardiotoxicity with human induced pluripotent stem cells. Sci Transl Med 2017;9:eaaf2584. https://doi.org/10.1126/scitranslmed.aaf2584.Search in Google Scholar PubMed PubMed Central
125. Burridge, PW, Li, YF, Matsa, E, Wu, H, Ong, SG, Sharma, A, et al.. Human induced pluripotent stem cell-derived cardiomyocytes recapitulate the predilection of breast cancer patients to doxorubicin-induced cardiotoxicity. Nat Med 2016;22:547–56. https://doi.org/10.1038/nm.4087.Search in Google Scholar PubMed PubMed Central
126. Arzt, M, Gao, B, Mozneb, M, Pohlman, S, Cejas, RB, Liu, Q, et al.. Protein-encapsulated doxorubicin reduces cardiotoxicity in hiPSC-cardiomyocytes and cardiac spheroids while maintaining anticancer efficacy. Stem Cell Rep 2023;18:1913–24. https://doi.org/10.1016/j.stemcr.2023.08.005.Search in Google Scholar PubMed PubMed Central
127. Wu, X, Chen, Y, Luz, A, Hu, G, Tokar, EJ. Cardiac development in the presence of cadmium: an in vitro study using human embryonic stem cells and cardiac organoids. Environ Health Perspect 2022;130:117002. https://doi.org/10.1289/ehp11208.Search in Google Scholar PubMed PubMed Central
128. Zhou, Y, Wu, Q, Li, Y, Feng, Y, Wang, Y, Cheng, W. Low-dose of polystyrene microplastics induce cardiotoxicity in mice and human-originated cardiac organoids. Environ Int 2023;179:108171. https://doi.org/10.1016/j.envint.2023.108171.Search in Google Scholar PubMed
129. Ma, J, Wang, NY, Jagani, R, Wang, HS. Proarrhythmic toxicity of low dose bisphenol A and its analogs in human iPSC-derived cardiomyocytes and human cardiac organoids through delay of cardiac repolarization. Chemosphere 2023;328:138562. https://doi.org/10.1016/j.chemosphere.2023.138562.Search in Google Scholar PubMed PubMed Central
130. Ingber, DE. Human organs-on-chips for disease modelling, drug development and personalized medicine. Nat Rev Genet 2022;23:467–91. https://doi.org/10.1038/s41576-022-00466-9.Search in Google Scholar PubMed PubMed Central
131. Xu, D, Wang, Y, Sun, L, Luo, Z, Luo, Y, Wang, Y, et al.. Living anisotropic structural color hydrogels for cardiotoxicity screening. ACS Nano 2023;17:15180–8. https://doi.org/10.1021/acsnano.3c04817.Search in Google Scholar PubMed
132. Virani, SS, Alonso, A, Benjamin, EJ, Bittencourt, MS, Callaway, CW, Carson, AP, et al.. Heart disease and stroke statistics-2020 update: a report from the American Heart Association. Circulation 2020;141:e139–596. https://doi.org/10.1161/cir.0000000000000757.Search in Google Scholar
133. Bergmann, O, Zdunek, S, Felker, A, Salehpour, M, Alkass, K, Bernard, S, et al.. Dynamics of cell generation and turnover in the human heart. Cell 2015;161:1566–75. https://doi.org/10.1016/j.cell.2015.05.026.Search in Google Scholar PubMed
134. Weinberger, F, Eschenhagen, T. Cardiac regeneration: new hope for an old dream. Annu Rev Physiol 2021;83:59–81. https://doi.org/10.1146/annurev-physiol-031120-103629.Search in Google Scholar PubMed
135. Funakoshi, S, Miki, K, Takaki, T, Okubo, C, Hatani, T, Chonabayashi, K, et al.. Enhanced engraftment, proliferation, and therapeutic potential in heart using optimized human iPSC-derived cardiomyocytes. Sci Rep 2016;6:19111. https://doi.org/10.1038/srep19111.Search in Google Scholar PubMed PubMed Central
136. Laflamme, MA, Chen, KY, Naumova, AV, Muskheli, V, Fugate, JA, Dupras, SK, et al.. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat Biotechnol 2007;25:1015–24. https://doi.org/10.1038/nbt1327.Search in Google Scholar PubMed
137. Weinberger, F, Breckwoldt, K, Pecha, S, Kelly, A, Geertz, B, Starbatty, J, et al.. Cardiac repair in Guinea pigs with human engineered heart tissue from induced pluripotent stem cells. Sci Transl Med 2016;8:363ra148. https://doi.org/10.1126/scitranslmed.aaf8781.Search in Google Scholar PubMed
138. Shiba, Y, Gomibuchi, T, Seto, T, Wada, Y, Ichimura, H, Tanaka, Y, et al.. Allogeneic transplantation of iPS cell-derived cardiomyocytes regenerates primate hearts. Nature 2016;538:388–91. https://doi.org/10.1038/nature19815.Search in Google Scholar PubMed
139. Liu, YW, Chen, B, Yang, X, Fugate, JA, Kalucki, FA, Futakuchi-Tsuchida, A, et al.. Human embryonic stem cell-derived cardiomyocytes restore function in infarcted hearts of non-human primates. Nat Biotechnol 2018;36:597–605. https://doi.org/10.1038/nbt.4162.Search in Google Scholar PubMed PubMed Central
140. Robey, TE, Saiget, MK, Reinecke, H, Murry, CE. Systems approaches to preventing transplanted cell death in cardiac repair. J Mol Cell Cardiol 2008;45:567–81. https://doi.org/10.1016/j.yjmcc.2008.03.009.Search in Google Scholar PubMed PubMed Central
141. Zhang, J, Zhu, W, Radisic, M, Vunjak-Novakovic, G. Can we engineer a human cardiac patch for therapy? Circ Res 2018;123:244–65. https://doi.org/10.1161/circresaha.118.311213.Search in Google Scholar
142. Wang, L, Liu, Y, Ye, G, He, Y, Li, B, Guan, Y, et al.. Injectable and conductive cardiac patches repair infarcted myocardium in rats and minipigs. Nat Biomed Eng 2021;5:1157–73. https://doi.org/10.1038/s41551-021-00796-9.Search in Google Scholar PubMed
143. Munarin, F, Kant, RJ, Rupert, CE, Khoo, A, Coulombe, KLK. Engineered human myocardium with local release of angiogenic proteins improves vascularization and cardiac function in injured rat hearts. Biomaterials 2020;251:120033. https://doi.org/10.1016/j.biomaterials.2020.120033.Search in Google Scholar PubMed PubMed Central
144. Lesman, A, Habib, M, Caspi, O, Gepstein, A, Arbel, G, Levenberg, S, et al.. Transplantation of a tissue-engineered human vascularized cardiac muscle. Tissue Eng 2010;16:115–25. https://doi.org/10.1089/ten.tea.2009.0130.Search in Google Scholar PubMed
145. Gao, L, Gregorich, ZR, Zhu, W, Mattapally, S, Oduk, Y, Lou, X, et al.. Large cardiac muscle patches engineered from human induced-pluripotent stem cell-derived cardiac cells improve recovery from myocardial infarction in swine. Circulation 2018;137:1712–30. https://doi.org/10.1161/circulationaha.117.030785.Search in Google Scholar PubMed PubMed Central
146. Miyagawa, S, Kainuma, S, Kawamura, T, Suzuki, K, Ito, Y, Iseoka, H, et al.. Case report: transplantation of human induced pluripotent stem cell-derived cardiomyocyte patches for ischemic cardiomyopathy. Front Cardiovasc Med 2022;9:950829. https://doi.org/10.3389/fcvm.2022.950829.Search in Google Scholar PubMed PubMed Central
147. Liang, S, Zhang, Y, Wang, H, Xu, Z, Chen, J, Bao, R, et al.. Paintable and rapidly bondable conductive hydrogels as therapeutic cardiac patches. Adv Mater 2018;30:e1704235. https://doi.org/10.1002/adma.201704235.Search in Google Scholar PubMed
148. Yee, K, Malliaras, K, Kanazawa, H, Tseliou, E, Cheng, K, Luthringer, DJ, et al.. Allogeneic cardiospheres delivered via percutaneous transendocardial injection increase viable myocardium, decrease scar size, and attenuate cardiac dilatation in porcine ischemic cardiomyopathy. PLoS One 2014;9:e113805. https://doi.org/10.1371/journal.pone.0113805.Search in Google Scholar PubMed PubMed Central
149. Emmert, MY, Wolint, P, Winklhofer, S, Stolzmann, P, Cesarovic, N, Fleischmann, T, et al.. Transcatheter based electromechanical mapping guided intramyocardial transplantation and in vivo tracking of human stem cell based three dimensional microtissues in the porcine heart. Biomaterials 2013;34:2428–41. https://doi.org/10.1016/j.biomaterials.2012.12.021.Search in Google Scholar PubMed
150. Kawaguchi, S, Soma, Y, Nakajima, K, Kanazawa, H, Tohyama, S, Tabei, R, et al.. Intramyocardial transplantation of human iPS cell-derived cardiac spheroids improves cardiac function in heart failure animals. JACC Basic Transl Sci 2021;6:239–54. https://doi.org/10.1016/j.jacbts.2020.11.017.Search in Google Scholar PubMed PubMed Central
151. Tabei, R, Kawaguchi, S, Kanazawa, H, Tohyama, S, Hirano, A, Handa, N, et al.. Development of a transplant injection device for optimal distribution and retention of human induced pluripotent stem cell‒derived cardiomyocytes. J Heart Lung Transplant 2019;38:203–14. https://doi.org/10.1016/j.healun.2018.11.002.Search in Google Scholar PubMed
152. Chong, JJ, Yang, X, Don, CW, Minami, E, Liu, YW, Weyers, JJ, et al.. Human embryonic-stem-cell-derived cardiomyocytes regenerate non-human primate hearts. Nature 2014;510:273–7. https://doi.org/10.1038/nature13233.Search in Google Scholar PubMed PubMed Central
153. Yang, X, Pabon, L, Murry, CE. Engineering adolescence: maturation of human pluripotent stem cell-derived cardiomyocytes. Circ Res 2014;114:511–23. https://doi.org/10.1161/circresaha.114.300558.Search in Google Scholar
154. Tan, Y, Coyle, RC, Barrs, RW, Silver, SE, Li, M, Richards, DJ, et al.. Nanowired human cardiac organoid transplantation enables highly efficient and effective recovery of infarcted hearts. Sci Adv 2023;9:eadf2898. https://doi.org/10.1126/sciadv.adf2898.Search in Google Scholar PubMed PubMed Central
155. Tan, Y, Richards, D, Coyle, RC, Yao, J, Xu, R, Gou, W, et al.. Cell number per spheroid and electrical conductivity of nanowires influence the function of silicon nanowired human cardiac spheroids. Acta Biomater 2017;51:495–504. https://doi.org/10.1016/j.actbio.2017.01.029.Search in Google Scholar PubMed PubMed Central
156. Liu, Y, Zhang, Y, Mei, T, Cao, H, Hu, Y, Jia, W, et al.. hESCs-derived early vascular cell spheroids for cardiac tissue vascular engineering and myocardial infarction treatment. Adv Sci 2022;9:e2104299. https://doi.org/10.1002/advs.202104299.Search in Google Scholar PubMed PubMed Central
157. Ibrahim, AG, Cheng, K, Marbán, E. Exosomes as critical agents of cardiac regeneration triggered by cell therapy. Stem Cell Rep 2014;2:606–19. https://doi.org/10.1016/j.stemcr.2014.04.006.Search in Google Scholar PubMed PubMed Central
158. Gallet, R, Dawkins, J, Valle, J, Simsolo, E, de Couto, G, Middleton, R, et al.. Exosomes secreted by cardiosphere-derived cells reduce scarring, attenuate adverse remodelling, and improve function in acute and chronic porcine myocardial infarction. Eur Heart J 2017;38:201–11. https://doi.org/10.1093/eurheartj/ehw240.Search in Google Scholar PubMed PubMed Central
159. Wimmer, RA, Leopoldi, A, Aichinger, M, Kerjaschki, D, Penninger, JM. Generation of blood vessel organoids from human pluripotent stem cells. Nat Protoc 2019;14:3082–100. https://doi.org/10.1038/s41596-019-0213-z.Search in Google Scholar PubMed
160. Wimmer, RA, Leopoldi, A, Aichinger, M, Wick, N, Hantusch, B, Novatchkova, M, et al.. Human blood vessel organoids as a model of diabetic vasculopathy. Nature 2019;565:505–10. https://doi.org/10.1038/s41586-018-0858-8.Search in Google Scholar PubMed PubMed Central
161. Quan, Y, Hu, M, Jiang, J, Jin, P, Fan, J, Li, M, et al.. VGLL4 promotes vascular endothelium specification via TEAD1 in the vascular organoids and human pluripotent stem cells-derived endothelium model. Cell Mol Life Sci 2023;80:215. https://doi.org/10.1007/s00018-023-04858-w.Search in Google Scholar PubMed
162. Wang, J, Liu, S, Heallen, T, Martin, JF. The Hippo pathway in the heart: pivotal roles in development, disease, and regeneration. Nat Rev Cardiol 2018;15:672–84. https://doi.org/10.1038/s41569-018-0063-3.Search in Google Scholar PubMed
163. Monteil, V, Kwon, H, Prado, P, Hagelkruys, A, Wimmer, RA, Stahl, M, et al.. Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2. Cell 2020;181:905–13. https://doi.org/10.1016/j.cell.2020.04.004.Search in Google Scholar PubMed PubMed Central
164. Kawakami, E, Saiki, N, Yoneyama, Y, Moriya, C, Maezawa, M, Kawamura, S, et al.. Complement factor D targeting protects endotheliopathy in organoid and monkey models of COVID-19. Cell Stem Cell 2023;30:1315–30. https://doi.org/10.1016/j.stem.2023.09.001.Search in Google Scholar PubMed PubMed Central
165. Salewskij, K, Penninger, JM. Blood vessel organoids for development and disease. Circ Res 2023;132:498–510. https://doi.org/10.1161/circresaha.122.321768.Search in Google Scholar
166. Brumback, BD, Dmytrenko, O, Robinson, AN, Bailey, AL, Ma, P, Liu, J, et al.. Human cardiac pericytes are susceptible to SARS-CoV-2 infection. JACC Basic Transl Sci 2023;8:109–20. https://doi.org/10.1016/j.jacbts.2022.09.001.Search in Google Scholar PubMed PubMed Central
167. Lancaster, MA, Renner, M, Martin, CA, Wenzel, D, Bicknell, LS, Hurles, ME, et al.. Cerebral organoids model human brain development and microcephaly. Nature 2013;501:373–9. https://doi.org/10.1038/nature12517.Search in Google Scholar PubMed PubMed Central
168. McMurtrey, RJ. Analytic models of oxygen and nutrient diffusion, metabolism dynamics, and architecture optimization in three-dimensional tissue constructs with applications and insights in cerebral organoids. Tissue Eng C Methods 2016;22:221–49. https://doi.org/10.1089/ten.tec.2015.0375.Search in Google Scholar
169. Palasantzas, V, Tamargo-Rubio, I, Le, K, Slager, J, Wijmenga, C, Jonkers, IH, et al.. iPSC-derived organ-on-a-chip models for personalized human genetics and pharmacogenomics studies. Trends Genet 2023;39:268–84. https://doi.org/10.1016/j.tig.2023.01.002.Search in Google Scholar PubMed
170. Shemesh, J, Jalilian, I, Shi, A, Heng Yeoh, G, Knothe Tate, ML, Ebrahimi Warkiani, M. Flow-induced stress on adherent cells in microfluidic devices. Lab Chip 2015;15:4114–27. https://doi.org/10.1039/c5lc00633c.Search in Google Scholar PubMed
171. de Graaf, MNS, Cochrane, A, van den Hil, FE, Buijsman, W, van der Meer, AD, van den Berg, A, et al.. Scalable microphysiological system to model three-dimensional blood vessels. APL Bioeng 2019;3:026105. https://doi.org/10.1063/1.5090986.Search in Google Scholar PubMed PubMed Central
172. Jain, A, Graveline, A, Waterhouse, A, Vernet, A, Flaumenhaft, R, Ingber, DE. A shear gradient-activated microfluidic device for automated monitoring of whole blood haemostasis and platelet function. Nat Commun 2016;7:10176. https://doi.org/10.1038/ncomms10176.Search in Google Scholar PubMed PubMed Central
173. Yang, H, Yang, Y, Kiskin, FN, Shen, M, Zhang, JZ. Recent advances in regulating the proliferation or maturation of human-induced pluripotent stem cell-derived cardiomyocytes. Stem Cell Res Ther 2023;14:228. https://doi.org/10.1186/s13287-023-03470-w.Search in Google Scholar PubMed PubMed Central
174. Bock, C, Kiskinis, E, Verstappen, G, Gu, H, Boulting, G, Smith, ZD, et al.. Reference Maps of human ES and iPS cell variation enable high-throughput characterization of pluripotent cell lines. Cell 2011;144:439–52. https://doi.org/10.1016/j.cell.2010.12.032.Search in Google Scholar PubMed PubMed Central
175. Burrows, CK, Banovich, NE, Pavlovic, BJ, Patterson, K, Gallego Romero, I, Pritchard, JK, et al.. Genetic variation, not cell type of origin, underlies the majority of identifiable regulatory differences in iPSCs. PLoS Genet 2016;12:e1005793. https://doi.org/10.1371/journal.pgen.1005793.Search in Google Scholar PubMed PubMed Central
176. Miyazaki, T, Futaki, S, Suemori, H, Taniguchi, Y, Yamada, M, Kawasaki, M, et al.. Laminin E8 fragments support efficient adhesion and expansion of dissociated human pluripotent stem cells. Nat Commun 2012;3:1236. https://doi.org/10.1038/ncomms2231.Search in Google Scholar PubMed PubMed Central
177. Braam, SR, Zeinstra, L, Litjens, S, Ward-van Oostwaard, D, van den Brink, S, van Laake, L, et al.. Recombinant vitronectin is a functionally defined substrate that supports human embryonic stem cell self-renewal via alphavbeta5 integrin. Stem Cell 2008;26:2257–65. https://doi.org/10.1634/stemcells.2008-0291.Search in Google Scholar PubMed
178. Zhong, Q, He, Y, Teng, L, Zhang, Y, Zhang, T, Zhang, Y, et al.. Self-assembly vascularized human cardiac organoids model cardiac diseases in petri dishes and in mice. bioRxiv 2023:08.26.554935. https://doi.org/10.1101/2023.08.26.554935.Search in Google Scholar
179. Pham, MT, Pollock, KM, Rose, MD, Cary, WA, Stewart, HR, Zhou, P, et al.. Generation of human vascularized brain organoids. Neuroreport 2018;29:588–93. https://doi.org/10.1097/wnr.0000000000001014.Search in Google Scholar
180. Sun, XY, Ju, XC, Li, Y, Zeng, PM, Wu, J, Zhou, YY, et al.. Generation of vascularized brain organoids to study neurovascular interactions. Elife 2022;11:e76707. https://doi.org/10.7554/elife.76707.Search in Google Scholar
181. Cakir, B, Xiang, Y, Tanaka, Y, Kural, MH, Parent, M, Kang, YJ, et al.. Engineering of human brain organoids with a functional vascular-like system. Nat Methods 2019;16:1169–75. https://doi.org/10.1038/s41592-019-0586-5.Search in Google Scholar PubMed PubMed Central
182. Maggiore, JC, LeGraw, R, Przepiorski, A, Velazquez, J, Chaney, C, Streeter, E, et al.. Genetically engineering endothelial niche in human kidney organoids enables multilineage maturation, vascularization and de novo cell types. bioRxiv 2023:05.30.542848. https://doi.org/10.1101/2023.05.30.542848.Search in Google Scholar PubMed PubMed Central
183. Olmsted, ZT, Paluh, JL. A combined human gastruloid model of cardiogenesis and neurogenesis. iScience 2022;25:104486. https://doi.org/10.1016/j.isci.2022.104486.Search in Google Scholar PubMed PubMed Central
184. Litvinukova, M, Talavera-Lopez, C, Maatz, H, Reichart, D, Worth, CL, Lindberg, EL, et al.. Cells of the adult human heart. Nature 2020;588:466–72. https://doi.org/10.1038/s41586-020-2797-4.Search in Google Scholar PubMed PubMed Central
185. Usui-Ouchi, A, Giles, S, Harkins-Perry, S, Mills, EA, Bonelli, R, Wei, G, et al.. Integrating human iPSC-derived macrophage progenitors into retinal organoids to generate a mature retinal microglial niche. Glia 2023;71:2372–82. https://doi.org/10.1002/glia.24428.Search in Google Scholar PubMed
186. Ormel, PR, Vieira de Sá, R, van Bodegraven, EJ, Karst, H, Harschnitz, O, Sneeboer, MAM, et al.. Microglia innately develop within cerebral organoids. Nat Commun 2018;9:4167. https://doi.org/10.1038/s41467-018-06684-2.Search in Google Scholar PubMed PubMed Central
187. Wang, Z, Wu, Z, Wang, H, Feng, R, Wang, G, Li, M, et al.. An immune cell atlas reveals the dynamics of human macrophage specification during prenatal development. Cell 2023;186:4454–71. https://doi.org/10.1016/j.cell.2023.08.019.Search in Google Scholar PubMed
188. Erturk, A, Becker, K, Jahrling, N, Mauch, CP, Hojer, CD, Egen, JG, et al.. Three-dimensional imaging of solvent-cleared organs using 3DISCO. Nat Protoc 2012;7:1983–95. https://doi.org/10.1038/nprot.2012.119.Search in Google Scholar PubMed
189. Hayes, HB, Nicolini, AM, Arrowood, CA, Chvatal, SA, Wolfson, DW, Cho, HC, et al.. Novel method for action potential measurements from intact cardiac monolayers with multiwell microelectrode array technology. Sci Rep 2019;9:11893. https://doi.org/10.1038/s41598-019-48174-5.Search in Google Scholar PubMed PubMed Central
© 2024 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International License.
Articles in the same Issue
- Frontmatter
- Editorials
- A new year, a renewed dedication: greetings from Medical Review
- The complex interplay between aging and cancer: unraveling the clues
- Reviews
- Nanotechnology-based delivery systems to overcome drug resistance in cancer
- The role of Down syndrome cell adhesion molecule in Down syndrome
- Evidence-based rehabilitation medicine: definition, foundation, practice and development
- Therapeutic robots for post-stroke rehabilitation
- The new era of cardiovascular research: revolutionizing cardiovascular research with 3D models in a dish
Articles in the same Issue
- Frontmatter
- Editorials
- A new year, a renewed dedication: greetings from Medical Review
- The complex interplay between aging and cancer: unraveling the clues
- Reviews
- Nanotechnology-based delivery systems to overcome drug resistance in cancer
- The role of Down syndrome cell adhesion molecule in Down syndrome
- Evidence-based rehabilitation medicine: definition, foundation, practice and development
- Therapeutic robots for post-stroke rehabilitation
- The new era of cardiovascular research: revolutionizing cardiovascular research with 3D models in a dish

