Abstract
Heterobimetallic thiolate [Cu(PPh3)]4(ZnCl)2 (SEt)6 has been synthesised and characterised by X-ray crystallography as a THF solvate. It contains the first structurally characterised example of a CuI-S(R)-ZnII linkage and only the second species type to embody the CuI-S-ZnII triad.
In the search for efficient absorber materials for photovoltaic cells derived from earth-abundant elements, Cu2ZnSnS4 (CZTS) has attracted significant attention (Ito and Nakazawa, 1988; Ramasamy et al., 2012); cells with efficiencies, which have increased from ca. 7% to in excess of 11%, have now been reported (Guo et al., 2010; Todorov et al., 2010, 2013; Barkhouse et al., 2012; Repins et al., 2012; Shin et al., 2013).
While several methods for forming CZTS are known (Ramasamy et al., 2012; Kociok-Köhn et al., 2014a and references therein), deposition in thin film form has been problematic. We (Kociok-Köhn et al., 2014a) and others (Ramasamy et al., 2011) have noted the specificity of conditions required to bring at least three precursors (one for each metal, which may, or may not, incorporate the chalcogen) together to generate CZTS in the correct stoichiometry. One way to simplify this problem is the use of precursors that embody more than one metal, which thereby reduces the overall number of precursors involved in the process. To this end, we have previously reported the synthesis of several M-Cl-M′ species (M=Cu, Zn, Sn inter alia), which could plausibly act as starting points for the synthesis of M-S-M′-containing precursors (Kociok-Köhn et al., 2014b). Unfortunately, to date, our attempts to produce such species by salt metathesis of the M-Cl-M′ reagents with thiolate anions have been unsuccessful. However, we now wish to report an initial success from the combination of (Ph3P)2CuCl and [(EtS)3Zn]Na and the structural characterisation of the first CuI-S(R)-ZnII linkage.
[Cu(PPh3)]4(ZnCl)2(SEt)6 (1) was synthesised from the reaction of Na[Zn(SEt)3], prepared by partially following the methodology used to prepare [(EtS)6Zn2]Na2, (Watson et al., 1985) and Cu(PPh3)2Cl:
Although the reaction was carried out at 1:1 reagent stoichiometry, this is not translated to the ratio of metals in the final product. Moreover, the reaction involves (perhaps unsurprisingly) loss of Ph3P and also, but less anticipated, migration of a halogen from the softer copper to the harder zinc.
Crystals of 1.6THF were isolated from the solution, but microanalysis implies that the molecules of solvation are easily lost from the solid when the initially prepared compound was pumped dry. Spectroscopic data are unexceptional, and despite NMR integrals revealing the ratio of EtS:Ph3P ligands, the structure is revealed only by crystallography (Figure 1; Table 1). 1 consists of two Cu2ZnS3 rings, each in a distorted boat conformation, joined co-facially via two Cu-S [Cu(2)-S(1) 2.3650(11) Å] and two Zn-S [Zn(1)-S(1) 2.3836(11) Å] bonds. This results in a cage embodying both six-membered Cu2ZnS3 and four-membered CuZnS2 heterocycles. In addition to bonds to sulphur, each zinc is bonded to chlorine [Zn(1)-Cl(1) 2.2505(11) Å], while each copper has a Ph3P group external to the cage [Cu(1)-P(1) 2.2087(10) Å; Cu(2)-P(2) 2.2214(10) Å]. Cu(1) is three-coordinate in a trigonal planar environment, while both Cu(2) and Zn(1) are four-coordinate and tetrahedral, although the formation of fused rings requires significant bond angle distortion [angles about Zn(1) 98.45(4)–118.24(4)°; Cu(1) 108.76(4)–124.73(4)°; Cu(2) 97.75(4)–114.35(4)°]; the shorter bond lengths to Cu(1) in comparison to Cu(2) reflect this coordination number difference. S(1) and S(2) act in a μ3-bridging mode, while S(3) is only μ2 and has the shorter bond lengths to its neighbouring metals. Despite the two distinct copper (and hence phosphorus and thiolate) environments, separate signals are not seen in either the 31P or 13C NMR spectra, suggesting some structural fluxionality in solution.
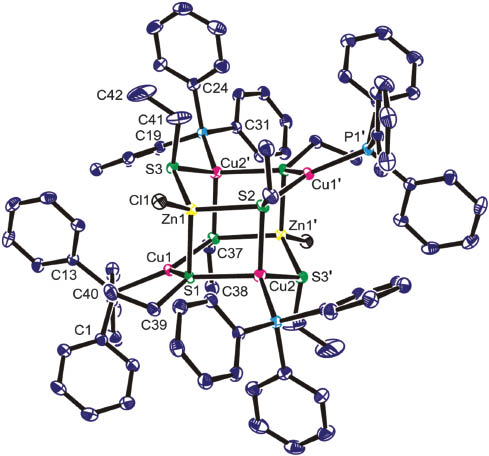
The asymmetric unit of 1 showing the labelling scheme used; thermal ellipsoids are at the 30% probability level.
P(1) and C(7) are hidden behind C(40). Symmetry operation: 1-x, -y, 2-z.
Selected bond distances (Å) and angles (o) for 1.
| Bond lengths | |||
| Zn-S(1) | 2.3836(11) | Cu(1)-S(2′) | 2.2404(10) |
| Zn-S(2) | 2.3907(10) | Cu(2)-S(1) | 2.3650(11) |
| Zn-S(3) | 2.3000(10) | Cu(2)-S(2) | 2.4342(10) |
| Zn-Cl(1) | 2.2505(11) | Cu(2)-S(3′) | 2.3219(11) |
| Cu(1)-P(1) | 2.2087(10) | Cu(2)-P(2) | 2.2214(10) |
| Cu(1)-S(1) | 2.2204(11) | ||
| Bond angles | |||
| S(1)-Zn(1)-S(2) | 98.45(4) | S(1)-Cu(2)-S(2) | 97.75(4) |
| S(1)-Zn(1)-S(3) | 112.96(4) | S(1)-Cu(2)-S(3′) | 104.41(4) |
| S(1)-Zn(1)-Cl(1) | 103.56(4) | S(1)-Cu(2)-P(2) | 114.35(4) |
| S(2)-Zn(1)-S(3) | 107.60(4) | S(2)-Cu(2)-S(3′) | 112.21(4) |
| S(2)-Zn(1)-Cl(1) | 114.30(4) | S(2)-Cu(2)-P(2) | 113.02(4) |
| S(3)-Zn(1)-Cl(1) | 118.24(4) | S(3′)-Cu(2)-P(2) | 113.76(4) |
| S(1)-Cu(1)-S(2′) | 108.76(4) | ||
| S(1)-Cu(1)-P(1) | 126.52(4) | ||
| P(1)-Cu(1)-S(2′) | 124.73(4) |
Symmetry operation: 1-x, -y, 2-z.
1 incorporates the first examples of structurally characterised CuI-S(R)-ZnII linkages. Indeed, only one other structure in the Cambridge Crystallographic Database establishes the CuI-S-ZnII array, namely, [(Ph3P)Cu(μ-SCOR)3Zn(PPh3)] (R = Ph, thiophene), which incorporates μ2-S thiocarboxylate ligands (Singh et al., 2013).
An analogous approach in search of a similar bimetallic Cu-Sn species only generated a known compound. Thus, [(PhS)3Sn]Na, prepared by adding three equivalents of NaSPh to SnCl2 in MeOH following published methodology (Dean et al., 1985), was added to Cu(PPh3)2Cl in CH2Cl2 in a 1:1 ratio. The resulting white precipitate was recrystallised from toluene, generating, over time, red crystals of Sn[Cu(PPh3)]2(SPh)6.2CH3C6H5, in which the tin had oxidised; the product was identified crystallographically. Sn[Cu(PPh3)Cu]2(SPh)6.3H2O has been reported by others (Wang et al., 2008) from the reaction of CuCN, PPh3 and Sn(SPh)4 (1:1:1).
Experimental
Na[Zn(SEt)3] (Watson et al., 1985) (0.5 g, 1.85 mmol) was stirred with Cu(PPh3)2Cl (Reichle, 1971) (1.15 g, 1.85 mmol) in toluene (20 mL) at 80°C for 1 h, forming a pale orange precipitate. On cooling to room temperature, solution was filtered and THF (20 mL) was added to fully dissolve product. On cooling to -20°C, colourless crystals formed (0.53 g, 61%, m.p. 117–9°C). Analysis: (calculated for C84H90Cl2Cu4P4S6Zn2) C 54.1 (53.9), H 4.63 (4.85)%; 1H NMR (300 MHz, C6D6) δ ppm: 1.34–1.50 (m, 18 H), 3.55–3.60 (m, 12 H), 7.00–7.11 (m, 60 H); 13C NMR (75 MHz, C6D6) δ ppm: 26.2 (CH3), 68.2 (CH2), 129.2 (d, 3J 7.4 Hz, m-CH), 129.8 (br, p-CH), 131.4 (d, 1J 20.5 Hz, i-C), 134.7 (d, 2J 17.4 Hz, o-CH); 31P NMR (122 MHz, C6D6) δ ppm: -3.5.
Crystallography
Experimental details relating to the single-crystal X-ray crystallographic study are summarised in Table 2. Data were collected on a Nonius Kappa CCD diffractometer (Enraf-Nonius B.V., Rotterdam, The Netherlands) at 150(2) K using Mo-Kα radiation (λ=0.71073 Å). Structure solution was followed by full-matrix least squares refinement and was performed using the WinGX-1.70 suite of programmes (Farrugia, 1999). An absorption correction (semi-empirical from equivalents) was applied. One of three THF molecules in the asymmetric unit is disordered over two sites in the ratio 1:1; bond lengths within this disorder have been restrained.
Crystal data and structure refinement for 1.
| Chemical formula | C108H138Cl2Cu4O6P4S6Zn2 |
| Formula mass | 2304.22 |
| Crystal system | Triclinic |
| a (Å) | 13.9563(3) |
| b (Å) | 14.2768(3) |
| c (Å) | 15.5890(3) |
| α (°) | 96.8507(9) |
| β (°) | 113.671(1) |
| γ (°) | 100.6253(9) |
| V (Å3) | 2730.78(10) |
| Space group | P1̅ |
| Z | 1 |
| M(Mo-kα) (mm-1) | 1.470 |
| No. of reflections measured | 39868 |
| No. of independent reflections | 12360 |
| Rint | 0.0850 |
| Final R1, wR(F2) (I>2σ(I)) | 0.0590, 0.1427 |
| Final R1, wR(F2) (all data) | 0.0843, 0.1615 |
Supporting Information
Crystallographic data for the structural analysis of 1 (in CIF format) have been deposited with the Cambridge Crystallographic Data Centre, CCDC no. 1059281. Copies of this information may be obtained from the Director, CCDC, 12 Union Road, Cambridge, CB21EZ, UK (Fax: +44-1233-336033; e-mail: deposit@ccdc.cam.ac.uk or www.ccdc.cam.ac.uk).
References
Barkhouse, D. A. R.; Gunawan, O.; Gokmen, T.; Todorov, T. K.; Mitzi, D. B. Device characteristics of a 10.1% hydrazine-processed Cu2ZnSn(Se,S)4 solar cell. Prog. Photovolt. 2012, 20, 6–11.10.1002/pip.1160Search in Google Scholar
Dean, P.W.A.; Vittal, J. J.; Payne, N.C. Synthesis and X-ray structural analysis of [(C6H5)4As][Sn(EC6H5)3], E=S and Se. Can. J. Chem. 1985, 63, 394–400.10.1139/v85-065Search in Google Scholar
Farrugia, L. J. Wingx suite for small-molecule single-crystal crystallography. J. Appl. Cryst.1999, 32, 837.10.1107/S0021889899006020Search in Google Scholar
Guo, Q.; Ford, G. M.; Yang, W.-C.; Walker, B. C.; Stach, E. A.; Hillhouse, H. W.; Agrawal, R. Fabrication of 7.2% efficient CZTSSe solar cells using CZTS nanocrystals. J. Am. Chem. Soc.2010, 132, 17384–17386.10.1021/ja108427bSearch in Google Scholar
Ito, K.; Nakazawa, T. Electrical and optical-properties of stannite-type quaternary semiconductor thin-films. Jpn. J. Appl. Phys. Part 1.1988, 27, 2094–2097.10.1143/JJAP.27.2094Search in Google Scholar
Kociok-Köhn, G.; Molloy, K. C.; Sudlow, A. L. Molecular routes to Cu2ZnSnS4: a comparison of approaches to bulk and thin-film materials. Can. J. Chem. 2014a, 92, 514–524.10.1139/cjc-2013-0497Search in Google Scholar
Kociok-Köhn, G.; Mahon, M.; Molloy, K. C.; Sudlow, A. L. Synthesis and structures of Cu-Cl-M adducts (M=Zn, Sn, Sb). Main Group Met. Chem. 2014b, 37, 11–24.10.1515/mgmc-2013-0051Search in Google Scholar
Ramasamy, K.; Malik, M.A. O’Brien, P. The chemical vapour deposition of Cu2ZnSnS4 thin films. Chem. Sci.2011, 2, 1170–1172.10.1039/c0sc00538jSearch in Google Scholar
Ramasamy, K.; Malik, M. A.; O’Brien, P. Routes to copper zinc tin sulfide Cu2ZnSnS4 a potential material for solar cells. Chem. Commun.2012, 48, 5703–5714.10.1039/c2cc30792hSearch in Google Scholar
Reichle, W. T. Preparation, physical properties, and reactions of copper(I)-triphenyl-M complexes (M=phosphorus, arsenic, antimony). Inorg. Chim. Acta. 1971, 5, 325–332.10.1016/S0020-1693(00)95939-5Search in Google Scholar
Repins, I.; Beall, C.; Vora, N.; DeHart, C.; Kuciauskas, D.; Dippo, P.; To, B.; Mann, J.; Hsu, W.-C.; Goodrich, A.; Noufi, R. Co-evaporated Cu2ZnSnSe4 films and devices. Sol. Energy Mater. Sol. Cells.2012, 101, 154–159.10.1016/j.solmat.2012.01.008Search in Google Scholar
Shin, B.; Gunawan, O.; Zhu, Y.; Bojarczuk, N. A.; Chey, S. J.; Guha, S. Thin film solar cell with 8.4% power conversion efficiency using an earth-abundant Cu2ZnSnS4 absorber. Prog. Photovolt.2013, 21, 72–76.10.1002/pip.1174Search in Google Scholar
Singh S.; Chaturvedi, J.; Aditya, A. S.; Rajasekhar Reddy, N., Bhattacharya, S. Syntheses and structural studies of heterobimetallic thiocarboxylate complexes containing zinc and copper. Inorg. Chim. Acta. 2013, 396, 6–9.10.1016/j.ica.2012.11.022Search in Google Scholar
Todorov, T. K.; Reuter, K. B.; Mitzi, D. B. High-efficiency solar cell with earth-abundant liquid-processed absorber. Adv. Mater.2010, 22, E156–E159.10.1002/adma.200904155Search in Google Scholar
Todorov, T. K.; Tang, J.; Bag, S.; Gunawan, O.; Gokmen, T.; Zhu, Y.; Mitzi, D. B. Beyond 11% efficiency: characteristics of state-of-the-art Cu2ZnSn(S,Se)4 solar cells. Adv. Energ. Mater.2013, 3, 34–38.10.1002/aenm.201200348Search in Google Scholar
Wang, L.-S.; Sheng, T.-L.; Wang, X.; Chen, D.-B.; Hu, S.-M.; Fu, R.-B.; Xiang, S.-C.; Wu, X.-T. Self-assembly of luminescent Sn(IV)/Cu/S clusters using metal thiolates as metalloligands. Inorg. Chem. 2008, 47, 4054–4059.10.1021/ic701741mSearch in Google Scholar
Watson, A. D; Pulla Rao, C. H.; Dorfman, J. R.; Holm, R. H. Systematic stereochemistry of metal(II) thiolates: synthesis and structures of [M2(SC2H5)6]2- (M=Mn(II), Ni(II), Zn(II), Cd(II)). Inorg. Chem.1985, 24, 2820–2826.10.1021/ic00212a024Search in Google Scholar
©2015 by De Gruyter
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Articles in the same Issue
- Frontmatter
- Research Articles
- Amino-functionalised metal xanthates
- The disilanes Cp*SiCl2SiH3 and Cp*SiH2SiH2Cp*
- Synthesis and characterization of gallium(III) dithiocarbamates as suitable nano-gallium(III) sulfide precursors
- Synthesis, structure and in vitro cytotoxic activity of two organotin complexes of 2-phenyl-1,2, 3-triazole-4-carboxylic acid
- Synthesis and structure of [2-((L)-menthoxycarbonyl)ethyl]diphenyltin halides
- A low-temperature, environment-friendly approach to the synthesis of magnesium borates using magnesium waste scraps
- Short Communications
- Synthesis and crystal structure of a new 1D Pb(II) coordination polymer containing salicylate and 2,2′-bipyridine ligands
- Molecular structure of novel heterobimetallic thiolate [Cu(PPh3)]4(ZnCl)2(SEt)6.6THF
- Mercury(I) chloride in vivo oxidation: a thermodynamic study
Articles in the same Issue
- Frontmatter
- Research Articles
- Amino-functionalised metal xanthates
- The disilanes Cp*SiCl2SiH3 and Cp*SiH2SiH2Cp*
- Synthesis and characterization of gallium(III) dithiocarbamates as suitable nano-gallium(III) sulfide precursors
- Synthesis, structure and in vitro cytotoxic activity of two organotin complexes of 2-phenyl-1,2, 3-triazole-4-carboxylic acid
- Synthesis and structure of [2-((L)-menthoxycarbonyl)ethyl]diphenyltin halides
- A low-temperature, environment-friendly approach to the synthesis of magnesium borates using magnesium waste scraps
- Short Communications
- Synthesis and crystal structure of a new 1D Pb(II) coordination polymer containing salicylate and 2,2′-bipyridine ligands
- Molecular structure of novel heterobimetallic thiolate [Cu(PPh3)]4(ZnCl)2(SEt)6.6THF
- Mercury(I) chloride in vivo oxidation: a thermodynamic study

