Establishment of high-sensitivity cardiac troponin I reference interval for a hospitalized paediatric population under improved selection criteria in the Shandong area
-
Lei Chen
Abstract
Objectives
This study aimed to evaluate the distribution of plasma troponin I concentration and establish the 99th percentile reference for hs-cTnI in a hospitalized population without a cardiovascular discharge diagnosis from the Shandong area.
Methods
The hs-cTnI data of anonymous paediatric patients were collected from Qingdao University-Affiliated Yantai Yuhuangding Hospital from 2016 to 2020. Indirect methods were used to calculate the hs-cTnI 99th percentile reference of the whole population and different age groups. Fitting curves and corresponding equations were displayed to determine the relationship between age and hs-cTnI level using the analysis of covariate variance.
Results
Hs-cTnI plasma levels were highest in the first week of life and declined with age in days. This study found significant differences in the troponin reference intervals for children in different age stratification. The serum hs-cTnI concentration decreased with age in days. In some subgroups, hs-cTnI levels between genders showed a significant difference after the analysis of covariance showed that age was the only predictor of hs-cTnI plasma levels. A non-linear relationship was observed between age and hs-cTnI levels. Thus, curvilinear fitting curve equations for each group were constructed to evaluate the possible relationship between age and hs-cTnI concentration.
Conclusions
During paediatric period, the highest hs-cTnI concentrations were observed in children aged <1 year, especially those under 7 days. This study presented the 99th percentile cut-offs for different age groups in children aged 0–14 years, which can provide a certain reference value for the clinical diagnosis and treatment of myocardial injury in children.
Introduction
Myocarditis and myocardial damage in children are the most common characteristics of primary heart disease and secondary systemic diseases, such as viral and bacterial infections [1, 2]. Dynamic changes in myocardial damage markers in severe fulminant myocarditis are similar to those in acute coronary syndrome (ACS) due to massive myocardial necrosis. Cardiac troponin (cTn) plays an important role in the diagnosis, risk stratification and therapeutic monitoring of ACS in adults [3]. Over the past decade, cTn immunoassays have been improved in analytical sensitivity and precision, thereby allowing the measurement of circulating protein levels in the major part of healthy participants, even in the paediatric population [4, 5]. High-sensitivity cardiac troponin (hs‐cTn) level also allows clinicians to detect the clinical conditions that promote the cTn release in patients who do not have ACS or cardiovascular disease (CVD). However, several studies have reported the clinical significance of cTn in adults, but only a few in children.
Children are often unable to complain about their condition; therefore, cTn can be applied to reduce the rate of delayed diagnosis and misdiagnosis. Whether the detection of cTn in children can play an essential role in the clinical diagnosis of myocardial injuries depends on the availability of valuable biological reference intervals suitable for the physiological characteristics of children [6].
To date, there is no consensus on how to define the reference population. One definition of myocardial injury is the 99th percentile upper reference limit (URL) of cTn in the apparently healthy population [7]. A questionnaire-based approach for determining laboratory test reference intervals is also recommended [8]. However, simply using self-reported health data insufficiently defines the reference population of hs-cTn [9]. A clinical history of known CVD and drug use and surrogate biomarkers for diabetes, cardiac dysfunction and renal insufficiency should also be included, and an adequate sample size of a diverse population (at least 300 males and 300 females) is preferred [10]. The primary screening objective was to exclude CVD and related non-cardiac diseases, especially those that might influence the cTnI values.
Studies concerning cTnI concentrations at the paediatric age are extremely difficult due to the very low troponin concentrations in children. However, with the advancement of high-sensitivity immunoassay, some scholars have made preliminary determinations on factors affecting troponin concentration and the reference range. Caselli et al. found that age was the only predictor of hs-cTnI plasma levels [11]. They also found that cTnI plasma levels were highest in the first month of life, with a progressive decline in the next years. Based on the present 99th percentile cut-off values, Lam established three hs-cTnT age partitions (from 0 to <6 months, from 6 months to <1 year and from 1 to <19 years) [12].
However, there are still no studies that established the reference intervals for Chinese children. Meanwhile, current methods using questionnaires and alternative biological markers do not fully guarantee that the population included will be exclusively healthy individuals. The methods for establishing reference intervals are divided into direct and indirect. The direct method requires a large amount of human and material resources, whereas the indirect method does not require a specific experimental design. In this study, reference intervals were calculated using only the patient’s test data and a combination of the patient’s clinical diagnostic information using statistical methods. Thus, we aim to establish a high-sensitivity cTnI reference interval for a paediatric population with improved selection criteria based on the International Classification of Diseases (ICD) diagnosis in inpatient medical records through the indirect method.
The study also aimed to evaluate the distribution of plasma cTnI concentrations in paediatric patients aged 0–14 years without cardiac or extra-cardiac diseases that can cause troponin elevation.
Materials and methods
Study population
From January 2017 to December 2020, a total of 13,477 paediatric patients without diseases that can cause troponin elevation through diagnosis, as confirmed by the ICD diagnosis, were identified in this study. The Ethics Committee of the Yantai Yuhuangding Hospital approved the study protocol. Inclusion criteria are as follows: (1) newborns delivered at term (37–42 weeks of gestation), with a body weight of 2.5–4.1 kg at birth and with an Apgar score of >8; (2) newborns without genetic disorders after the neonatal screening and (3) children with normal body mass index.
Furthermore, the following exclusion criteria were strictly used: (1) the presence or history of CVD is confirmed by a detailed medical history inquiry, careful clinical examination or by echocardiography and (2) the presence or history of acute/chronic extra-cardiac diseases that can cause troponin elevation including Kawasaki disease, severe hand-foot-mouth disease, severe acute bronchiolitis, perinatal asphyxia, paediatric cancer, medication with cardiotoxicity, perinatal asphyxia, acute kidney disease, advanced chronic kidney disease, type 1 diabetes severe anaemia, sepsis, severe pulmonary diseases, and trauma. Finally, 11,093 paediatric patients were included in the final analysis. The study patients were divided into six groups: Group 1 (perinatal period, 0–7 days), Group 2 (infancy period1, from 1 week to 2 months), Group 3 (infancy period2, from 2 months to 1 year), Group 4 (early childhood, 1–3 years), Group 5 (preschool period, 3–7 years) and Group 6 (school age, 7–14 years). Figure 1 summarises the specific screening process and exact exclusion numbers during each step. Outliers were further removed in each group by median absolute deviation. A total of 10,358 persons were analysed for reference interval in different age groups (n=10,358).
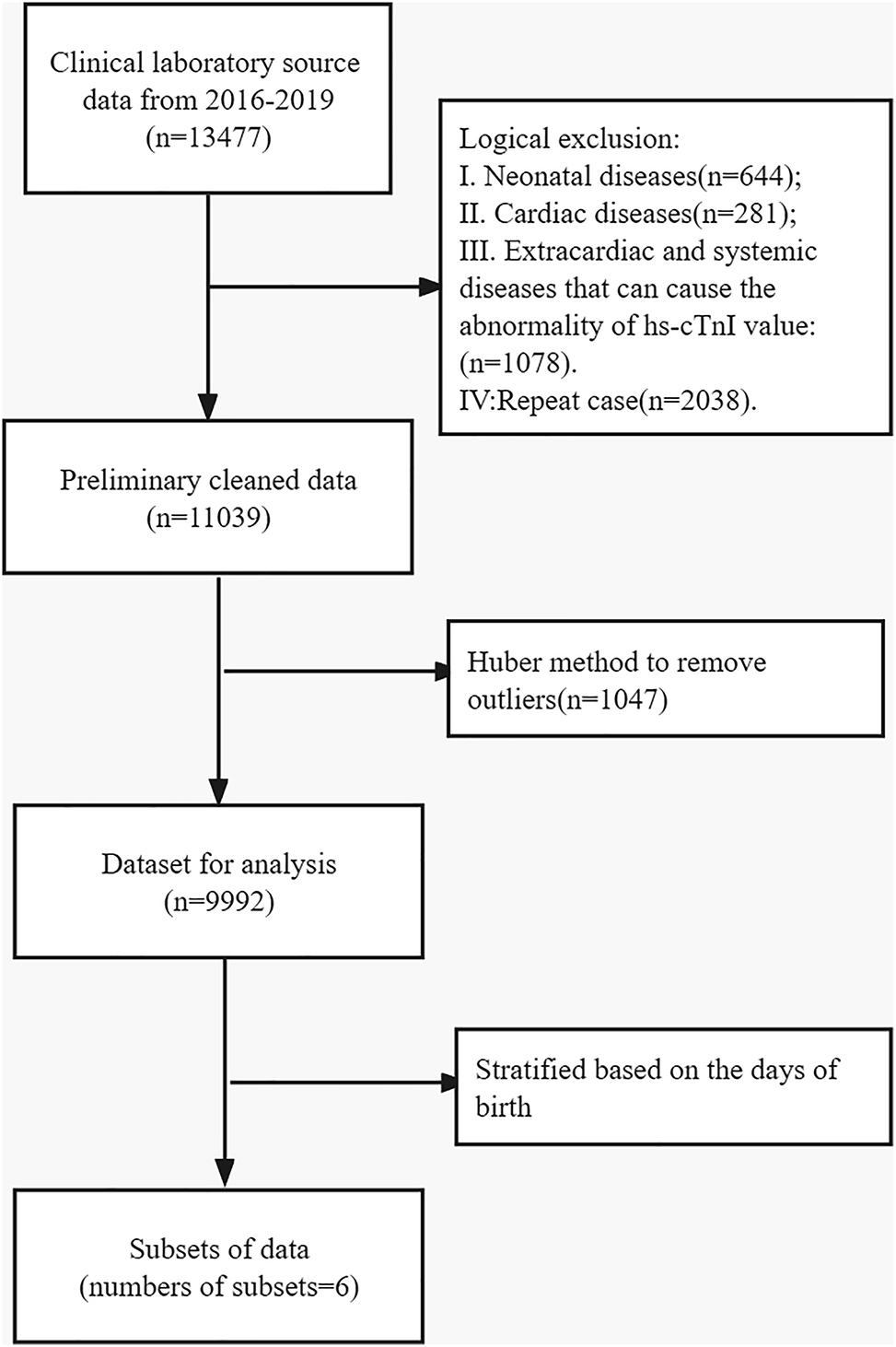
Selection procedures of individuals to establish the reference interval.
Blood sampling and laboratory analysis
cTnI concentration was measured at the Yantai Yuhuangding Hospital with the STAT Architect hs-TnI using the Architect i2000SR platform (Abbott Diagnostics). All of the procedures were followed by the manuscript. The limit of blank (LoB) and the limit of detection (LoD) were established by the manufacturer, i.e. 0.7 and 1.62 ng/L, respectively (Table 1). In addition, the LoQ was 8,272 ng/L within the range of the manufacturer.
LoB, LoD and LoQ values of hs-cTnI STAT Architect immunoassay for cTnI.
| Reference | LoB, ng/L | LoD, ng/L | LoQ, ng/L |
|---|---|---|---|
| Manufacturer | 0.7–1.3 | 1.1–1.9 | 4.0–10.0 |
| Krintus et al. [13][krintus2014european] | 0.7–1.3 | 1.1–1.9 | 4.6–8.1 |
| Present study | 0.7 | 1.62 | 8.272 |
Internal quality control was also performed. From January 1, 2016 to July 31, 2020, a total of 5 batches and 2 concentrations of indoor quality control products from the third-party company were used, and each level was tested at 2 concentrations every 24 h. In general, the CV% was less than 6 % and most of them were less than 5 %.
Statistical analysis
Because hs-cTnI circulating levels are not normally distributed, both non-parametric and parametric tests after a logarithmic transformation of data were used for statistical analysis. Outliers were identified from the total population and in each group using the Median Absolute Deviation. The mean (standard deviation) or median (min, max) are expressed for continuous variables. We used uni- and multi-variate linear regression models to determine the correlation between hs-cTnI and age along with gender. The 99th percentile URLs and 95 % confidence intervals were calculated using a non-parametric method. Data were analysed using the statistical software package R (http://www.R-project.org, The R Foundation). All statistical tests were two-sided, and a p-value <0.05 was considered statistically significant.
Results
Data distribution characteristics
Based on Spearman’s correlations, hs-cTnI levels were significantly correlated with age (p<0.001) (r, correlation coefficient). The distribution of hs-cTnI levels based on age is shown in Figure 2. Hs-cTnI concentration levels gradually declined in the peripheral period with age and to a relatively low level after 1 year (Figure 2A). The hs-cTnI level does not conform to the normal distribution. In this study, a negative correlation was found between the child’s age in days and the 99th percentile of troponin, and a curvilinear correlation was also observed. Figure 2B and Table 2 display the very high concentration of hs-cTnI in neonatal serum, as high as 98.5 pg/mL. Significant differences were observed in the troponin reference intervals for children at different age stratifications (Table 2).

Relationship between hs-cTnI and age.
Characteristics of the study population.
| Subgroup | Perinatal period (0–7 days) | Infant period1 (1 week to 2 months) | Infant period2 (2 months to 1 year) | Early childhood (1–3 years) | Preschool period (3–7 years) | School-age (7–14 years) | p-Value |
|---|---|---|---|---|---|---|---|
| Hs-cTnI | |||||||
| Number | 388 | 614 | 1,534 | 2,439 | 4,039 | 2,079 | |
| Mean | 220.4 | 95.0 | 17.6 | 4.7 | 6.9 | 14.5 | 0.001 |
| SD | 2,264.6 | 472.2 | 102.0 | 55.7 | 156.7 | 244.1 | |
| Median | 19.6 | 19.9 | 3.6 | 1.2 | 0.6 | 0.4 | |
| Q1–Q3 | 9.2–61.8 | 11.9–45.2 | 1.7–9.6 | 0.6–2.4 | 0.2–1.2 | 0.0–0.9 | |
| Age, days | |||||||
| Number | 388 | 614 | 1,534 | 2,439 | 4,039 | 2,079 | |
| Mean | 2.3 | 32.8 | 197.1 | 677.5 | 1,691.5 | 3,473.5 | 0.001 |
| SD | 1.7 | 17.1 | 93.1 | 217.6 | 424.2 | 637.9 | |
| Median | 1.7 | 36.5 | 195.5 | 634.3 | 1,608.7 | 3,411.5 | |
| Q1–Q3 | 1.0–3.3 | 14.0–48.2 | 108.5–278.4 | 482.5–866.7 | 1,321.5–2,029.7 | 2,906.9–3,952.4 | |
| Sex | <0.001 | ||||||
| Male | 235 (60.6 %) | 344 (56.0 %) | 927 (60.4 %) | 1,314 (53.9 %) | 2,205 (54.6 %) | 1,163 (55.9 %) | |
| Female | 153 (39.4 %) | 270 (44.0 %) | 607 (39.6 %) | 1,125 (46.1 %) | 1,834 (45.4 %) | 916 (44.1 %) |
Gender differences
When all patients (including the outliners) were considered, no significant difference was observed in the hs-cTnI concentration between boys and girls (Table 3). Moreover, no significant difference in hs-cTnI concentration was observed between gender in groups during the perinatal period, neonatal, early childhood and school-age periods. A significant hs-cTnI difference was observed during infancy and preschool periods (p<0.05). Meanwhile, the age difference between gender was also observed. Analysis of covariance showed that age was a predictor of hs-cTnI plasma levels in some subgroups, whereas hs-cTnI levels did not significantly differ statistically between genders (Table 4).
Hs-cTnI plasma levels (pg/mL) in different age groups between different genders.
| Groups | n | Male media | IQR | n | Female media | IQR | p-Value | |
|---|---|---|---|---|---|---|---|---|
| All | Hs-cTnI | 6,188 | 0.80 | (0.30, 2.00) | 4,905 | 0.80 | (0.30, 2.00) | 0.701 |
| Age, days | 1,218.0 | (410.5, 2,191.0) | 1,205.0 | (379.3, 2,165.0) | 0.055 | |||
| Perinatal period | Hs-cTnI | 235 | 17.6 | (2.51–13.10) | 153 | 16.3 | (9.0, 51.8) | 0.703 |
| Age, days | 344 | 1.8 | (1.0, 3.5) | 1.5 | (0.8, 2.7) | 0.05 | ||
| Infancy period1 | Hs-cTnI | 20.2 | (11.5, 36.9) | 270 | 16.9 | (10.9, 29.6) | 0.098 | |
| Age, days | 36.6 | (15.5, 47.3) | 35.6 | (12.7, 48.5) | 0.569 | |||
| Infant period | Hs-cTnI | 927 | 3.0 | (1.5, 7.3) | 607 | 3.5 | (1.8, 8.2) | 0.004 |
| Age, days | 195.5 | (108.5, 278.4) | 200.5 | (116.2, 281.5) | 0.012 | |||
| Early childhood | Hs-cTnI | 1,163 | 0.6 | (0.2, 1.1) | 916 | 0.6 | (0.2, 1.1) | 0.277 |
| Age, days | 1,607.0 | (1,321.0, 2,030.0) | 1,607.0 | (1,318.0, 2,012.0) | 0.31 | |||
| Preschool period | Hs-cTnI | 2,205 | 0.3 | (0.0, 0.8) | 1,834 | 0.3 | (0.0, 0.8) | 0.029 |
| Age, days | 3,458.0 | (2,921.0, 3,959.0) | 3,354.0 | (2,886.0, 3,937.0) | 0.119 | |||
| School age | Hs-cTnI | 1,312 | 0.6 | (0.2, 1.1) | 1,125 | 0.6 | (0.2, 1.1) | 0.277 |
| Age, days | 1,607.0 | (1,318.0, 2,012.0) | 1,612.0 | (1,325.0, 2,041.0) | 0.31 |
-
Bold values: statistically significant.
Gender difference in hs-cTnI level between subgroups.
| Male vs. female | Non-adjusted | Adjusted | |||
|---|---|---|---|---|---|
| Mean and 95 % CI | p-Value | Mean and 95 % CI | p-Value | ||
| Infant period | Sex | 0.85 (0.10, 1.60) | 0.03 | 0.10 (−0.12, 1.20) | 0.11 |
| Age, days | −0.034 (−0.12, 1.21) | <0.001 | |||
| Preschool period | Sex | 0.04 (−0.01, 0.09) | 0.14 | 0.04 (−0.006, 0.093) | 0.09 |
| Age, days | −2.3 × 10−3 (−2.9 × 10−3, 1.7 × 10−3) | <0.001 | |||
-
Bold values: statistically significant.
Establishment of the reference interval and fitting equation
After remove the outliners, the 99th, 97.5th, 95th, 90th and 50th percentile values were calculated (99th %, 97.5th %, 95th %, 75th % and 25th %, respectively). This method was used to estimate the mean value of each quantile 100,000 times. Detailed results are shown in Table 5 and Figure 3. The age-dependent 99th percentiles of hs-cTnI were as follows: 210.28 pg/mL for 0–7 days, 166.25 pg/mL for from 1 week to 2 months, 32.73 pg/mL for 1–3 years, 6.60 pg/mL for 3–7 years and 2.85 pg/mL for 7–14 years. We found a non-linear relationship between age and hs-cTnI levels. Thus, curvilinear fitting curve equations for each group were constructed to evaluate the possible relationship between age and hs-cTnI concentration (Figure 2). A negative correlation between age and hs-cTnI levels was found, and equations for different subgroups were established.
Hs-cTnI plasma levels (pg/mL) in groups of samples subdivided according to age.
| Groups | n | Mean | Median | 99th percentile | 95 % confidence interval of the 99th percentiles |
|---|---|---|---|---|---|
| All | 10,358 | 1.91 | 0.84 | 13.84 | (13.04, 30.31) |
| Perinatal period | 362 | 38.11 | 16.685 | 210.28 | (175.76, 226.31) |
| Infancy period1 | 563 | 30.21 | 18.00 | 166.25 | (133.38, 171.80) |
| Infant period | 1,423 | 5.98 | 3.26 | 32.73 | (29.22, 34.29) |
| Early childhood | 2,278 | 1.54 | 1.14 | 6.60 | (5.77, 7.29) |
| Preschool period | 3,804 | 0.75 | 0.57 | 3.63 | (2.98, 3.90) |
| School age | 1,928 | 0.51 | 0.31 | 2.85 | (2.19, 3.10) |
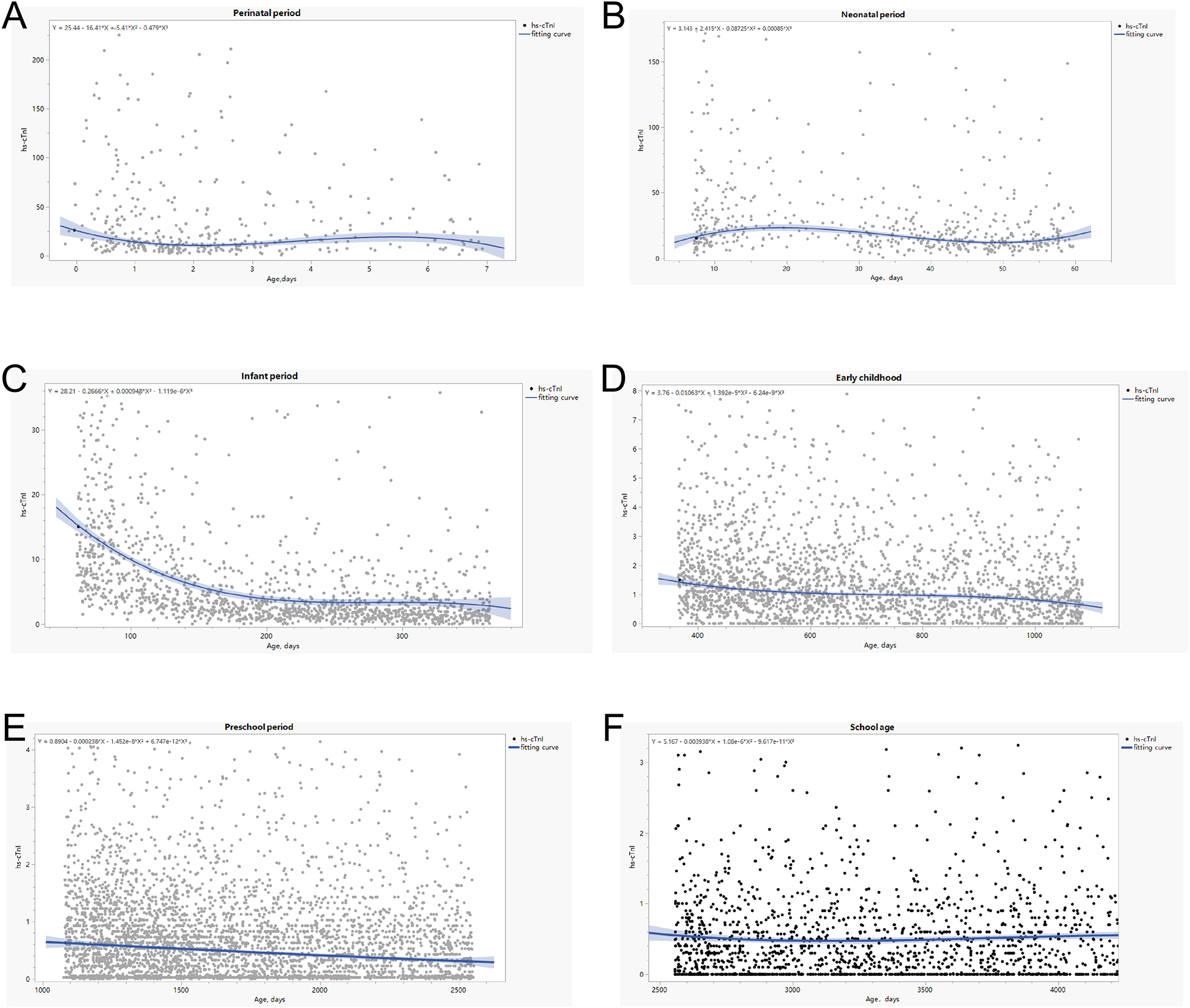
Hs-cTnI plasma levels (pg/mL) in groups of samples subdivided according to age.
Discussion
The detection of high-sensitivity troponin concentration and its values are vital for diagnosing CVD and monitoring myocardial injury in paediatric population. Whether the reference interval provided by the laboratory to the clinic is reliable determines whether clinicians can effectively use this method in providing accurate diagnosis and treatment services. This study preliminarily investigated the distribution of serum hs-cTnI levels in children aged 0–14 years in Yantai City, Shandong Province, China. The reference interval of hs-cTnI for infants and young children was not similar to that of adults obtained from the manufacturers. In this study, a negative correlation was found between the child’s age in days and the 99th percentile of troponin, and a curvilinear correlation was also observed. Meanwhile, we found significant differences in the troponin reference intervals for children at different age stratifications. Compared to reference values in older children, newborns had significantly higher values in the first 7 days of life, which emphasises the need for caution when using hs-cTnI to diagnose neonatal patients.
Hs-cTnI is a strong prognostic predictor for adverse cardiac events [14]. To date, no standard normal reference intervals have been established for hs-cTnI in Chinese children, and different regions and ethnicities can affect hs-cTnI concentrations, which have an impact on the medical diagnosis. Therefore, reference intervals for adults have long been inapplicable to children in our region. Establishing or validating laboratory reference intervals using direct methods is time-consuming and laborious [15]. Therefore, this study focused on using indirect methods as recommended by the International Federation of Clinical Chemistry [16]. In this study, we used clinical big data to establish a regional population reference interval for high-sensitivity troponin, which can provide some guidance for the clinical diagnosis and treatment. As this is a single‐centre study, our research programmes are more representative based on big data but not on volunteers or blood donors, which avoids selection bias. Another strength of our study is that the study population was derived from patients without intra- and extra-cardiac factors causing troponin level increase via ICD diagnosis.
In the present study, the highest hs-cTnI serum concentrations were observed in the neonatal group. We speculate that this may be related to the renewal of neonatal cardiomyocytes, reperfusion after transient neonatal hypoxia and mode of delivery and development, the mechanisms that should be further investigated in a large sample. Similarly, Jehlicka conducted a statistical analysis on 241 newborns (0–28 days). The median hs-cTn concentration was 38.2 ng/L, and the 97.5th percentile reached 83.0 ng/L [17]. Mary Kathryn Bohn studied sex- and age-specific factors in the CALIPER cohort of children and adolescents, comprising approximately 600 individuals. The 99 % upper limit of hs-cTn concentrations for 0–6 months, from 6 months to 1 year, 1–19 years (female) and 1–19 years (male) was 93.0, 21.0, 11.0 and 14.0 ng/L, respectively [18]. These findings are inconsistent with our findings, which may be related to the sample type and population. However, these studies also showed that the hs-cTn concentrations in newborns and young infants have higher reference levels.
From the clinical perspective, these data may suggest that hs-cTnI reference values for paediatric patients should be determined and standardised based on age. Many challenges have been encountered in establishing accurate reference intervals in the paediatric setting. Accurate laboratory tests and age- and sex-specific reference intervals are essential for incorporating biomarkers into the medical assessment, management and care of paediatric patients [19]. In our study, the analysis of covariance showed that age in days was a predictor of hs-cTnI plasma levels in some subgroups, whereas hs-cTnI levels did not significantly differ statistically between genders. For further study of reference values on paediatric patients, accurate age in days should be considered. Cardiomyocyte proliferation (the cardiac growth and regeneration mechanism) may play a role in the development, which contributes to the growth of young hearts [20]. Therefore, children and adolescents have been hypothesised to may have the capacity for myocardial regeneration [21].
Through the study of the indirect hs-cTnI reference interval, we found the specificity of age, but not gender, which not only helps clinicians in making accurate diagnoses and identifying treatments of cardiac diseases but also provides reflections for the establishment of accurate hs-cTnI reference interval in laboratory medicine. With the advent of the digital era, we should use the big data indirect method to investigate the reference intervals of more test items.
This study has several limitations that should be considered when interpreting the results. First, the study population was limited to children from the Shandong area and may not be representative of other populations. Second, the selection criteria for a regional population paediatric population may have excluded some children with underlying conditions that could affect cardiac troponin I levels. Third, the use of a single high-sensitivity cardiac troponin I assay kit may limit the generalizability of the results to other assay platforms. Fourthly, we used ICD as a scanning rule in this study. However, ECGs could also be considered in the future exploration. Fifthly, the study design did not allow for the assessment of dynamic changes in cardiac troponin I levels in the study population. Finally, due to the difficulties of obtaining sufficiently large healthy population of children, the approach of current study may be acceptable to obtain some guidance for age-dependent cTnI decision limits in children in clinical routine in our region.
Conclusions
We established the reference interval of hs-cTnI concentration in children aged 0–14 years in Yantai City, Shandong Province, China, which can provide a certain reference value for the clinical diagnosis and treatment of myocarditis and myocardial (micro) injury in children.
-
Research ethics: The Ethics Committee of the Yantai Yuhuangding Hospital approved the study protocol.
-
Informed consent: Informed consent was obtained from all individuals included in this study.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Competing interests: The authors state no conflict of interest.
-
Research funding: None declared.
-
Data availability: The datasets used and analysed during the current study are available from the corresponding author on reasonable request.
References
1. Yoldaş, T, Örün, UA. What is the significance of elevated troponin I in children and adolescents? A diagnostic approach. Pediatr Cardiol 2019;40:1638–44. https://doi.org/10.1007/s00246-019-02198-w.Search in Google Scholar PubMed
2. Rodriguez-Gonzalez, M, Perez-Reviriego, AA, Castellano-Martinez, A. Current role of cardiac biomarkers in extra-cardiac diseases in children. Biomarkers Med 2020;14:1183–7. https://doi.org/10.2217/bmm-2020-0232.Search in Google Scholar PubMed
3. Morrow, DA, Cannon, CP, Jesse, RL, Newby, LK, Ravkilde, J, Storrow, AB, et al.. National academy of clinical biochemistry laboratory medicine practice guidelines: clinical characteristics and utilization of biochemical markers in acute coronary syndromes. Clin Chem 2007;53:552–74. https://doi.org/10.1373/clinchem.2006.084194.Search in Google Scholar PubMed
4. Apple, FS, Collinson, PO. IFCC task force on clinical applications of cardiac biomarkers. Analytical characteristics of high-sensitivity cardiac troponin assays. Clin Chem 2012;58:54–61. https://doi.org/10.1373/clinchem.2011.165795.Search in Google Scholar PubMed
5. Correale, M, Nunno, L, Ieva, R, Rinaldi, M, Maffei, G, Magaldi, R, et al.. Troponin in newborns and pediatric patients. Cardiovasc Hematol Agents Med Chem 2009;7:270–8. https://doi.org/10.2174/187152509789541927.Search in Google Scholar PubMed
6. Giannitsis, E, Katus, HA. Cardiac troponin level elevations not related to acute coronary syndromes. Nat Rev Cardiol 2013;10:623–34. https://doi.org/10.1038/nrcardio.2013.129.Search in Google Scholar PubMed
7. Thygesen, K, Alpert, JS, Jaffe, AS, Chaitman, BR, Bax, JJ, Morrow, DA, et al.. Fourth universal definition of myocardial infarction (2018). J Am Coll Cardiol 2018;72:2231–64. https://doi.org/10.1161/cir.0000000000000617.Search in Google Scholar
8. Henny, J, Vassault, A, Boursier, G, Vukasovic, I, Mesko Brguljan, P, Lohmander, M, et al.. Working Group Accreditation and ISO/CEN standards (WG-A/ISO) of the EFLM, recommendation for the review of biological reference intervals in medical laboratories. Clin Chem Lab Med 2016;54:1893–900. https://doi.org/10.1515/cclm-2016-0793.Search in Google Scholar PubMed
9. Koerbin, G, Abhayaratna, WP, Potter, JM, Apple, FS, Jaffe, AS, Ravalico, TH, et al.. Effect of population selection on 99th percentile values for a high sensitivity cardiac troponin I and T assays. Clin Biochem 2013;46:1636–43. https://doi.org/10.1016/j.clinbiochem.2013.08.004.Search in Google Scholar PubMed
10. Sandoval, Y, Apple, FS. The global need to define normality: the 99th percentile value of cardiac troponin. Clin Chem 2014;60:455–62. https://doi.org/10.1373/clinchem.2013.211706.Search in Google Scholar PubMed
11. Li, S, Zuo, Y, Huang, W. Establishment of a reference interval for high-sensitivity cardiac troponin I in healthy adults from the Sichuan area. Medicine 2017;96:e6252. https://doi.org/10.1097/md.0000000000006252.Search in Google Scholar
12. Lam, E, Higgins, V, Zhang, L, Chan, MK, Bohn, MK, Trajcevski, K, et al.. Normative values of high-sensitivity cardiac troponin T and N-terminal pro-B-type natriuretic peptide in children and adolescents: a study from the CALIPER cohort. J Appl Lab Med 2021;6:344–53. https://doi.org/10.1093/jalm/jfaa090.Search in Google Scholar PubMed
13. Krintus, M, Kozinski, M, Boudry, P, Capell, NE, Köller, U, Lackner, K, et al.. European multicenter analytical evaluation of the Abbott ARCHITECT STAT high sensitive troponin I immunoassay. Clin Chem Lab Med 2014;52:1657–65. https://doi.org/10.1515/cclm-2014-0107.Search in Google Scholar PubMed
14. Twerenbold, R, Boeddinghaus, J, Nestelberger, T, Wildi, K, Rubini Gimenez, M, Badertscher, P, et al.. Clinical use of high-sensitivity cardiac troponin in patients with suspected myocardial infarction. J Am Coll Cardiol 2017;70:996–1012. https://doi.org/10.1016/j.jacc.2017.07.718.Search in Google Scholar PubMed
15. Ozarda, Y. Reference intervals: current status, recent developments and future considerations. Biochem Med 2016;26:5–16. https://doi.org/10.11613/bm.2016.001.Search in Google Scholar
16. Anghel, LA, Farcas, AM, Oprean, RN. An overview of the common methods used to measure treatment adherence. Med Pharm Rep 2019;92:117–22. https://doi.org/10.15386/mpr-1201.Search in Google Scholar PubMed PubMed Central
17. Jehlicka, P, Huml, M, Rajdl, D, Mockova, A, Matas, M, Dort, J, et al.. How to interpret elevated plasmatic level of high-sensitive troponin T in newborns and infants. Physiol Res 2018;67:191–5. https://doi.org/10.33549/10.33549/physiolres.933704.Search in Google Scholar
18. Bohn, MK, Higgins, V, Kavsak, P, Hoffman, B, Adeli, K. High-sensitivity generation 5 cardiac troponin T sex- and age-specific 99th percentiles in the CALIPER cohort of healthy children and adolescents. Clin Chem 2019;65:589–91. https://doi.org/10.1373/clinchem.2018.299156.Search in Google Scholar PubMed
19. Jung, B, Adeli, K. Clinical laboratory reference intervals in pediatrics: the CALIPER initiative. Clin Biochem 2009;42:1589–95. https://doi.org/10.1016/j.clinbiochem.2009.06.025.Search in Google Scholar PubMed
20. Neves, AL, Henriques-Coelho, T, Leite-Moreira, A, Areias, JC. Cardiac injury biomarkers in paediatric age: are we there yet. Heart Fail Rev 2016;21:771–81. https://doi.org/10.1007/s10741-016-9567-2.Search in Google Scholar PubMed
21. Vela, D, Buja, LM. Quest for the cardiovascular holy grail: mammalian myocardial regeneration. Cardiovasc Pathol 2008;17:1–5. https://doi.org/10.1016/j.carpath.2007.05.001.Search in Google Scholar PubMed
© 2023 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- Editorial
- Editorial to: German Society for Clinical Chemistry and Laboratory Medicine – areas of expertise – division reports from the German Congress of Laboratory Medicine 2022 in Mannheim, 13–14 October 2022
- Report
- German Society for Clinical Chemistry and Laboratory Medicine – areas of expertise: Division reports from the German Congress of Laboratory Medicine 2022 in Mannheim, 13–14 October 2022
- Original Articles
- Impact and frequency of IV fluid contamination on basic metabolic panel results using quality metrics
- A quality control procedure for central venous blood sampling based on potassium concentrations
- Establishment of high-sensitivity cardiac troponin I reference interval for a hospitalized paediatric population under improved selection criteria in the Shandong area
Articles in the same Issue
- Frontmatter
- Editorial
- Editorial to: German Society for Clinical Chemistry and Laboratory Medicine – areas of expertise – division reports from the German Congress of Laboratory Medicine 2022 in Mannheim, 13–14 October 2022
- Report
- German Society for Clinical Chemistry and Laboratory Medicine – areas of expertise: Division reports from the German Congress of Laboratory Medicine 2022 in Mannheim, 13–14 October 2022
- Original Articles
- Impact and frequency of IV fluid contamination on basic metabolic panel results using quality metrics
- A quality control procedure for central venous blood sampling based on potassium concentrations
- Establishment of high-sensitivity cardiac troponin I reference interval for a hospitalized paediatric population under improved selection criteria in the Shandong area

