Abstract
Objectives
To assess the efficacy of a mini-STR-based next-generation sequencing (NGS) method for non-invasive prenatal paternity testing (NIPPT).
Methods
Plasma DNA from 28 pregnant women was extracted and cell-free foetal DNA (cffDNA) genotyping was performed at 23 mini-STR loci using the Illumina NextSeq 500 system. For each mini-STR locus, the cffDNA genotype was validated by determining infant DNA genotype. The mini-STR loci with high concordance rates were selected for the comparison of STR genotyping results between cffDNA and biological father DNA or random male DNA for each family.
Results
The biological relationship was identified between alleged fathers and infants in all 28 families using the capillary electrophoresis (CE) method. Moreover, the concordance rates of STR genotypes D5S818, D19S253, and D21S1270 were less than 50% in 23 autosomal STR loci. The STR genotype matching probability was calculated using 20 STR loci with more than 60% concordance rate. There was a significant difference in the STR genotype matching probability between cffDNA and the DNA from the biological father (75–100%) or from random males (25–70%) (p<0.0001).
Conclusions
Our study demonstrated that mini-STR can be used for NGS-based NIPPT. Furthermore, this method can be used for crime control purposes using the STR data available from the national forensic DNA databases.
Introduction
In cases of sexual assault, pregnant women may feel anxious to undergo paternity testing in order to confirm the biological father of their foetuses and decide about their pregnancy. Approximately 5% of female victims of sexual assault got pregnant in the US annually; consequently, approximately 32,000 unintended pregnancies were recorded annually in the country [1]. Currently, the short tandem repeat (STR)-based DNA paternity testing method is widely used to determine parentage. In this method, peripheral blood or buccal cells are collected from the alleged father or mother and the child. On the contrary, the conventional prenatal paternity testing method relies on invasive amniocentesis or chorionic villus sampling, which may lead to a procedure-related risk of miscarriage of 0.35% or pregnancy-related complications [2, 3]. Therefore, non-invasive prenatal paternity testing (NIPPT) using cell-free foetal DNA (cffDNA) was developed.
Lo et al. [4] isolated cffDNA from maternal plasma in the 1990s. Since then, non-invasive prenatal diagnostic methods such as foetal RhD genotyping for RhD-negative women and Down-syndrome screening, have been widely used [5, 6]. Approximately 99% cffDNA is shorter than 313 base pairs (bp) [7]. While most of cffDNA in maternal plasma is derived from trophoblast destruction, some of it is derived from the apoptosis of foetal hematopoietic cells and transfer of foetal cell-free DNA through the placenta [8]. In a previous study, the circulating cffDNA with a mean half-life of 16.3 min was cleared in a short time post-delivery, and was undetectable 2 h post-delivery. This finding suggested that cffDNA testing is not affected by carryover from previous pregnancies [9]. Therefore, cffDNA can be used for NIPPT.
Mini-STRs were developed by redesigning the STR primer binding sites and moving them in close proximity to the repeat region to reduce the size of PCR amplicons [10]. Mini-STRs have been used for forensic identification of individuals based on degraded DNA samples. After the 9/11 World Trade Centre terrorist attacks, over 20% of DNA samples of victims were fingerprinted solely using mini-STRs [11]. Owing to the advantage of amplifying short DNA templates for mini-STRs, in this study, a next-generation sequencing (NGS)-based NIPPT method was evaluated with 23 autosomal mini-STR markers.
Materials and methods
Sample collection
Samples were collected from 28 families receiving prenatal tests at the Dalian Blood Centre from April 2018 to December 2019. The pregnant women were 22–41 years old with a gestational age of 12–38 weeks (18 women in the first trimester, 5 in the second trimester, and 5 in the third trimester). Peripheral blood samples were collected from the pregnant women and their husbands, and buccal swabs were collected from the infants after delivery. In addition, peripheral blood samples from 28 random males were used. All participants were informed about sample collection, and their written informed consent was obtained. The Dalian Blood Centre Ethics Committee approved the study protocol. The registration number is DBC00802015. The date of approval was 18 March 2018.
DNA extraction
A total of 5 mL of peripheral blood was collected from pregnant women in 10 mL EDTA Vacutainer tubes. Plasma was isolated from the whole blood samples using two-step centrifugation at 1,500×g for 10 min and 13,000×g for 10 min [12]. The supernatant was collected and stored at −80 °C until DNA extraction. Plasma DNA was extracted from 2 mL of cell-free maternal plasma using the MagPure Circulating DNA Maxi Kit (Angen Biotech, Guangzhou, China) according to the manufacturer’s instructions and eluted with 60 µL of water. The HiPure Tissue & Blood DNA Kit (Angen Biotech) was used to extract genomic DNA from the peripheral blood of pregnant women, their husbands, and random males, and from the buccal swabs of the infants. The quantity and purity of the extracted DNA were determined using sodium dodecyl-sulphate polyacrylamide gel electrophoresis (SDS-PAGE) with the Tanon 1,600 Gel Imaging System (Shanghai Tanon Science & Technology, Shanghai, China) and NanoDrop 2,000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA), respectively.
Paternity testing using a capillary electrophoresis (CE) method
Paternity testing was performed using the Microreader™ 21 ID System (Microread Genetics, Beijing, China) and Microreader™ 23sp ID System (Microread Genetics). The genomic DNA of the pregnant women, their husbands, infants, and random males was amplified using the Life ECO Thermal Cycler (Bioer Technology, Hangzhou, China), according to the manufacturer’s instructions. STR genotyping was performed using the 3,130 Genetic Analyzer system (Thermo Fisher Scientific). The genotyping results were analysed using GeneMapperTM v3.0 software (Thermo Fisher Scientific).
Library preparation and NGS
DNA extracted from cell-free maternal plasma was amplified at the following 23 autosomal mini-STR loci: D16S539, D18S51, D3S1358, D5S818, D7S820, D8S1179, FGA, TPOX, CSF, D10S1435, D12S391, D13S325, D15S659, D18S535, D19S253, D1S1656, D21S1270, D3S3045, D4S2366, D5S2500, D6S1043, D6S477, and D7S3048 (Supplementary Table S2). The DNA library was prepared using the KAPA Library Amplification Kit (Illumina, San Diego, CA, USA), according to the manufacturer’s instructions. The quantity and purity of the DNA libraries were assessed using SDS-PAGE. Subsequently, the adapter-ligated templates were purified using the MagPure A3 XP beads (Angen Biotech). The libraries were quantified using the KAPA Library Quant DNA Standards & Primer Premix Kit (Illumina) with the Qubit fluorometer (Thermo Fisher Scientific). Finally, the DNA libraries were sequenced by paired-end 150-bp reads on the NextSeq 500 system (Illumina) (Figure 1).
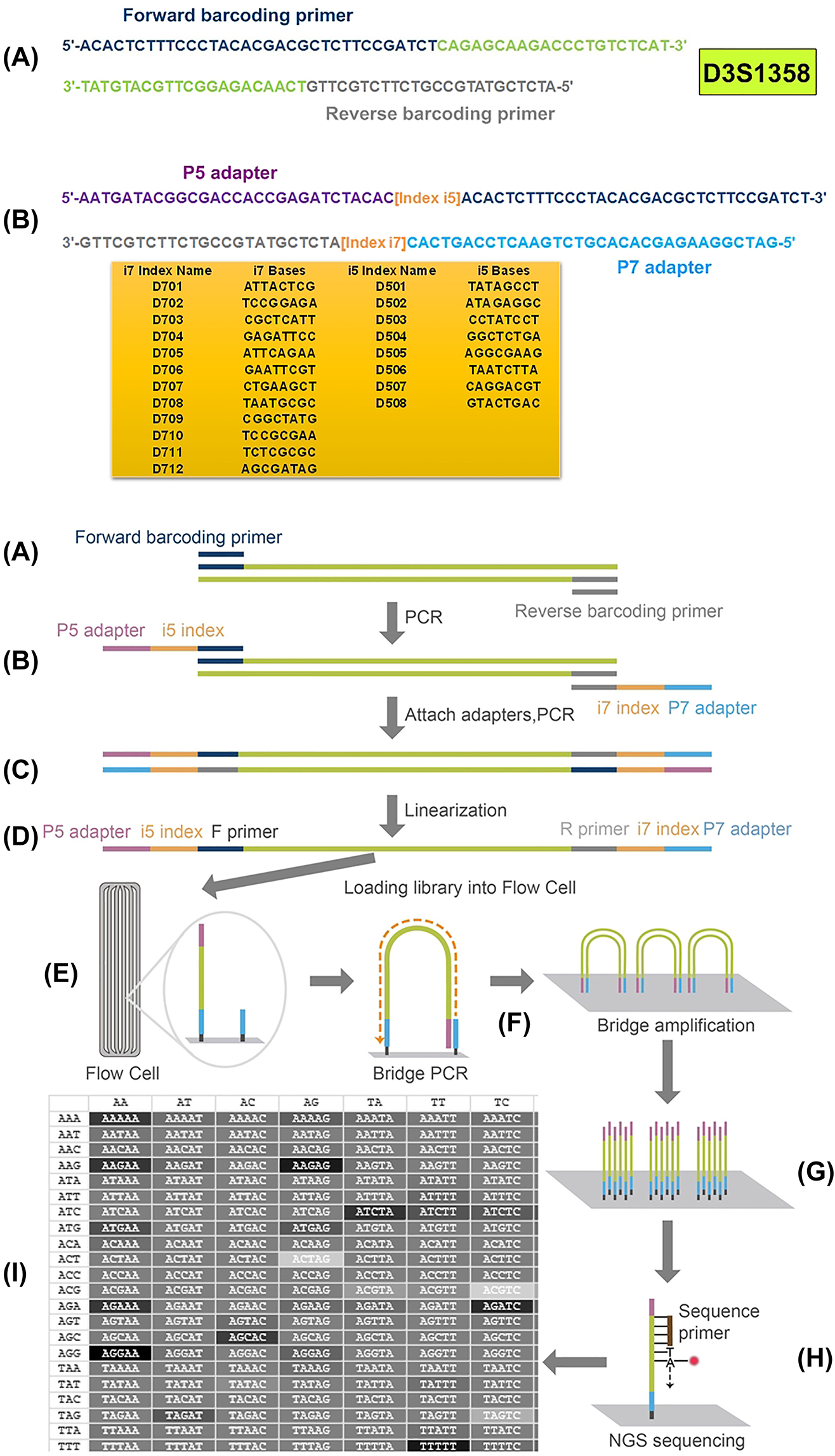
Schematic representation of library preparation and NGS sequencing.
Data for D3S1358 is shown as a representative (A) amplification of the target sequence with barcoding primers; (B) second PCR amplification by adding specialised adapters and indexes to both ends; (C) purification of the amplicon and pooling multiple libraries together; (D) linearisation of DNA libraries; (E) loading DNA libraries onto the flow cell; (F) performing bridge PCR amplification; (G) cluster generation; (H) paired-end sequencing; (I) base calling, alignment, and data analysis.
Data analysis
Quality control (QC) analysis of raw FASTQ data was performed using FastQC software. The FASTQ reads were mapped against the reference sequences (GRCh37/hg19) from GenBank for sequence alignment to determine the mini-STR genotypes of plasma DNA. Specific sequences of lengths 4–12 bp were selected to match the target region sequence. Compatibility with CE typing was fulfilled by the nomenclature guidelines of the International Society for Forensic Genetics and STRidER (https://strider.online/nomenclature). Based on the STR genotyping results of genomic DNA obtained via CE analysis, cffDNA with the alleles of paternal origin were distinguished from maternal plasma DNA after deducting maternally inherited alleles. For each STR locus, the mini-STR genotyping result of cffDNA was validated using the DNA genotype of the infant. The mini-STR loci with high concordance rates were selected for comparison of the STR genotyping results between cffDNA and DNA from the biological father or from random males for each family (Figure 2).
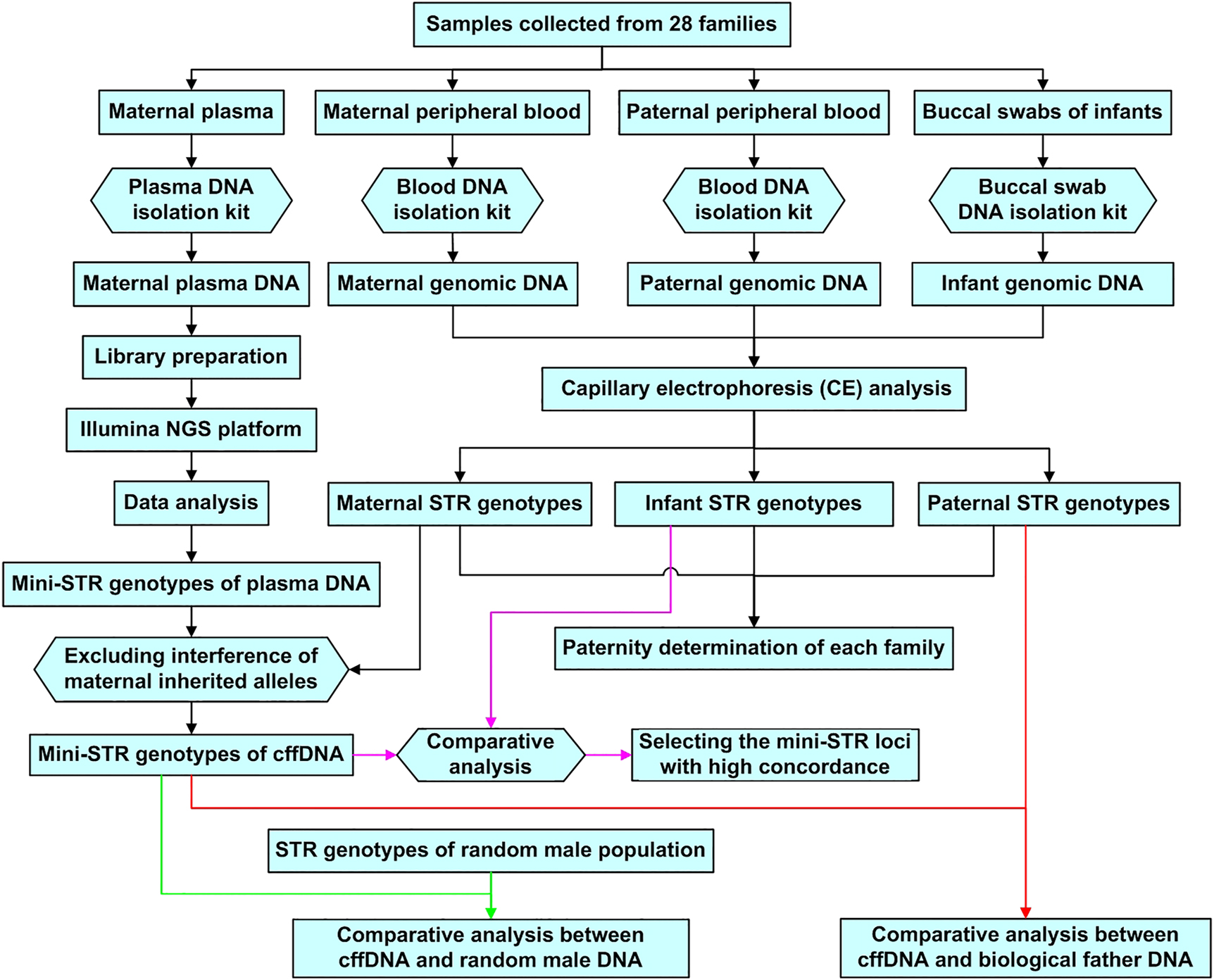
Flow chart for experimental procedures and data analysis.
Results
Paternity testing
Paternity testing based on genomic DNA from the mother, alleged father, and infant for each family was performed using the CE method. The STR genotyping results revealed the biological relationship between alleged fathers and infants of all 28 families (Supplementary Table S1).
Selection of mini-STR loci
The repeat motif and target sequence length of the 23 STR loci identified using NGS and CE methods are shown in Table 1. For these 23 loci, the mini-STR genotypes of cffDNA were validated by identifying the DNA genotypes of infants after deducing maternally inherited alleles from the 28 families (Supplementary Table S1). The concordance rates of the D5S818, D19S253, and D21S1270 STR loci were less than 50% (n=28), whereas those of the other 20 STR loci were more than 60% (Figure 3).
Correspondence of repeat motif and target sequence length at 23 STR loci between the NGS and CE methods.
| Locus | Cytogenetic location | NGS | CE | ||
|---|---|---|---|---|---|
| Repeat motif | Target sequence length, bp | Repeat motif | Target sequence length, bp | ||
| D5S818 | 5q23.2 | [AGAT]n | 97–121 | [AGAT]n | 136–189b |
| D18S51 | 18q21.33 | [AGAA]n | 105–139 | [AGAA]n | 271–362b |
| D6S1043 | 6q16.1 | [CTAT]n | 68–91 | [ATCT]n a | 370–445b |
| D3S1358 | 3p21.31 | TCTA[TCTG]n[TCTA]m | 133–149 | [TCTA]n a | 115–160 |
| D7S820 | 7q21.11 | [GATA]n | 68–88 | [GATA]n | 210–255b |
| D16S539 | 16q24.1 | [GATA]n | 103–123 | [GATA]n | 257–310b |
| CSF1PO | 5q33.1 | [CTAT]n | 114–126 | [AGAT]n | 312–363b |
| D8S1179 | 8q24.13 | [TCTA]n | 80–128 | [TCTA]n a | 197–259b |
| TPOX | 2p25.3 | [AATG]n | 79–95 | [AATG]n | 261–306b |
| D12S391 | 12p13.2 | [AGAT]8–17[AGAC]6–10[AGAT]0−1 | 110–126 | [AGAT]n a | 141–205b |
| FGA | 4q28 | [TTTC]n | 127–155 | [TTTC]n a | 277–444b |
| D6S477 | 6p24.6 | [TATC]mTGTC[TATC]n | 111–131 | [(TCTA)2TA]0−1[TCTA]2−3TA[TCTA]1[TCTG]0−1[TCTA]m | 100–158 |
| D18S535 | 18q12.5 | [GATA]n | 113–135 | [GATA]GACA[GATA]GAT[GATA]n | 160–208b |
| D19S253 | 19p13.1 | [TATC]n | 68–109 | [TATC]n | 212–260b |
| D15S659 | 15q20 | [TCTA]n | 102–126 | [TATC]n | 262–318b |
| D1S1656 | 1q42.3 | [TAGA]n | 104–132 | [TATC]n | 118–172 |
| D7S3048 | 7p17.5 | [TATC]m[TACC]n[CACC]k[TGTC]1 | 102–133 | [TCTA]n[TACC]m | 314–374b |
| D4S2366 | 4p16.1 | [ATAG]k[ATTG]m[ATAG]n | 105–123 | [ATAG]k[ATTG]m[ATAG]n | 80–123 |
| D21S1270 | 21q22.11 | [TAGA]n | 103–128 | [GATA]n | 125–170 |
| D13S325 | 13q13.7 | [CTAT]n | 113–125 | [TCTA]n | 174–245b |
| D3S3045 | 3q13.1 | [ATAG]AG[ATAG]3 AT[ATAG]n | 112–136 | [ATAG]AG[ATAG]3AT[ATAG]n | 306–350b |
| D10S1435 | 10p15.3 | [TATC]n | 107–123 | [TATC]n | 85–140.8 |
| D5S2500 | 5q11.2 | [ATAG]n | 81–102 | [ATAG]n | 344–420b |
-
aSTR locus with a complex repeat pattern; bmean target sequence length >150 bp.
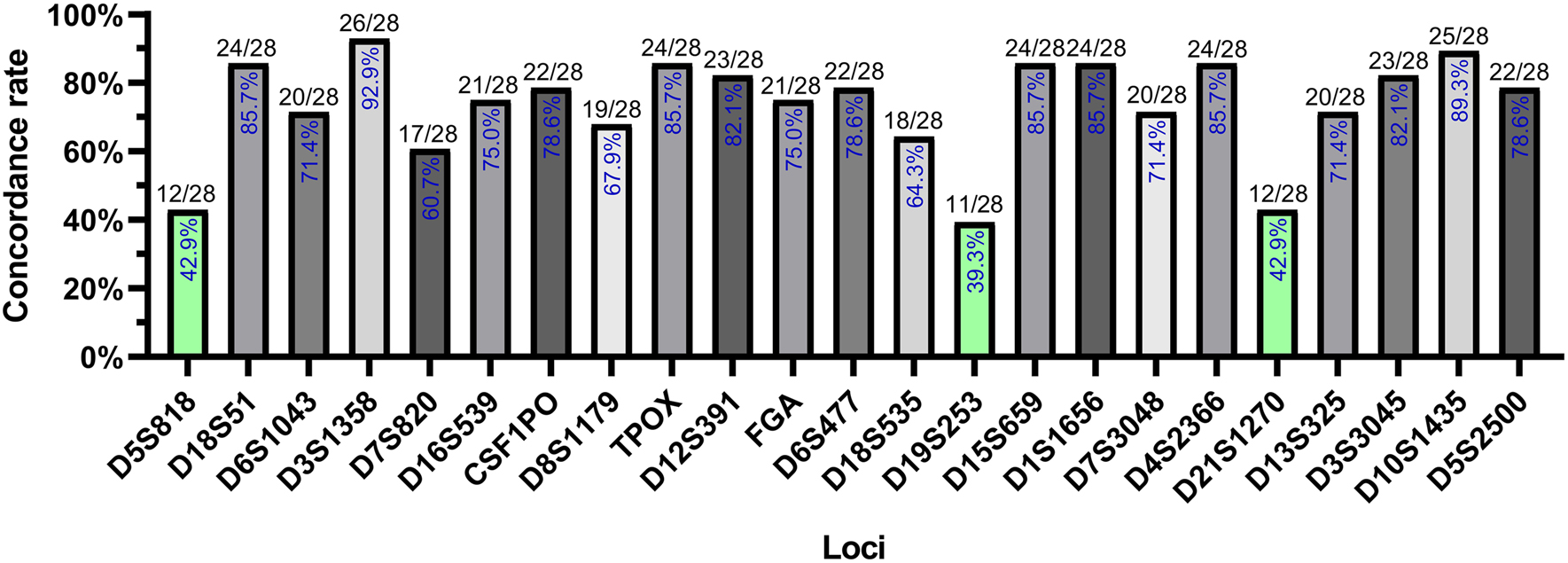
Concordance rates of the validation results at 23 STR loci.
The percentage in each bar represents the concordance rates of STR genotypes across all families divided by the total number of families (28). Green bars represent concordance rates below 50%.
STR genotype-matching analysis between the biological father and random male
The STR genotype-matching probability of the 28 families was calculated from 20 STR loci with more than 60% concordance rate. For each family, the STR genotype match between the cffDNA and genomic DNA from the biological father or from a random male was calculated. A significant difference in the matching probability was recorded between the biological (75–100%) and non-biological (25–70%) relationship groups (p<0.0001) (Figure 4).
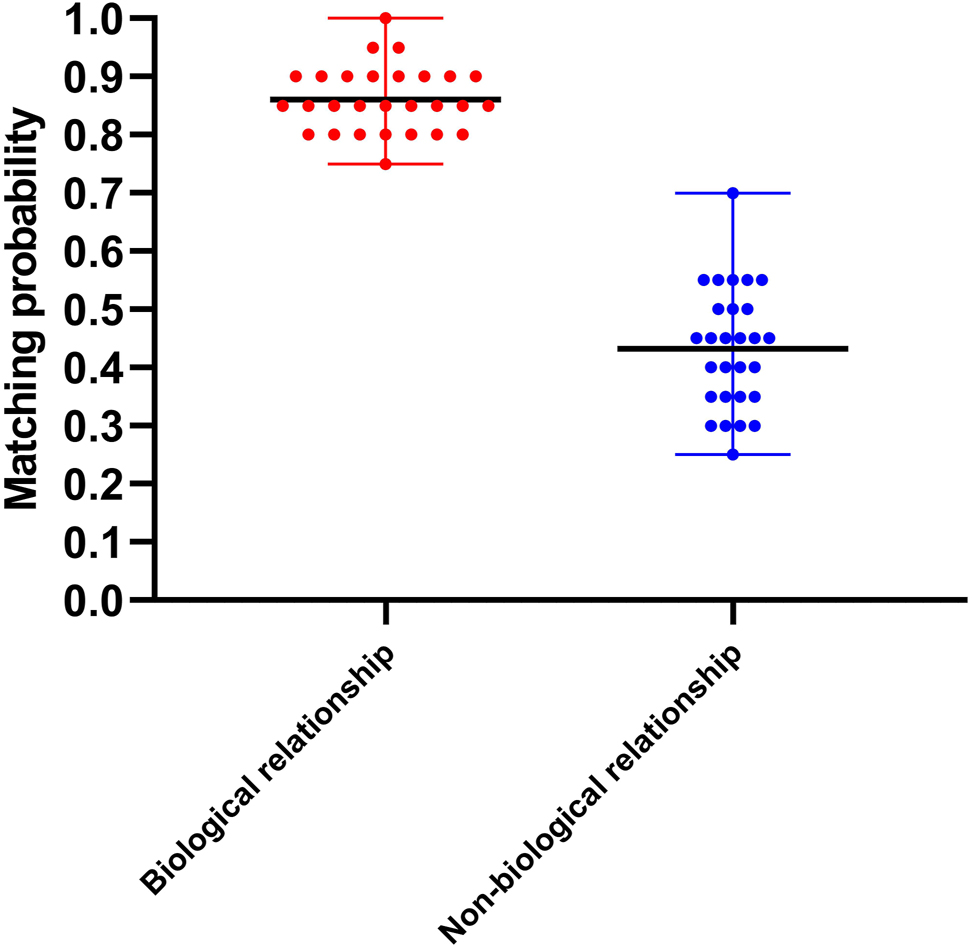
Comparative analysis of STR genotype matching probability between the biological relationship group and the non-biological relationship group.
Biological relationship group: the matching probability between cffDNA and biological father DNA ranged from 75 to 100% with a mean value of 86% (black line in the centre). Non-biological relationship group: the matching probability between cffDNA and random male genomic DNA ranged from 25 to 70% with a mean value of 43% (black line in the centre).
Discussion
The primary goal of NIPPT is the detection of foetal-specific markers, the paternal genetic material, from maternal plasma. As Y-specific cffDNA can be easily distinguished from abundant maternal DNA signals in the maternal plasma, markers on the Y-chromosome were used earlier for paternity analysis of male foetuses [13, 14]. Differences in the methylation status of placental DNA and maternal blood cell DNA in maternal plasma have also been used to detect foetal genotypes [15]. SNP-based prenatal paternity testing combined with microarrays has been increasingly reported [16]. The SNP-based prenatal paternity testing relies on the differences in paternally inherited foetal SNP alleles from maternal SNP alleles. In this study, we used STRs with a high genetic polymorphism instead of SNPs with a low genetic polymorphism. The STR genotypes from the genomic DNA of 28 families were analysed at 23 autosomal mini-STR loci using NGS or CE. First, the genotyping accuracy for each mini-STR locus was verified by comparing the mini-STR genotype of cffDNA with the DNA genotype of infants, followed by calculation of the concordance rate. The quantitative criteria for concordance rate were personalised owing to a lack of relevant reports. Through comprehensive consideration of accurate genotyping and discrimination capacity, a 60% concordance rate was considered a reasonable standard for STR locus selection in our study. The D5S818, D19S253, and D21S1270 STR loci showed the lowest concordance rates, which might be caused by competitive inhibition of multiplex amplifications. Second, 20 STR loci with over 60% concordance rate showed a significant difference in the genotype matching probability between the biological relationship group (75–100%) and the non-biological relationship group (25–70%). Our study demonstrated that NGS-based mini-STR genotyping of cffDNA can be effectively used for NIPPT. Autosomal STR-based prenatal paternity testing is not used frequently owing to the requirement of short target sequences and maternal DNA contamination. To analyse highly degraded DNA samples, mini-STR was redesigned with primers flanking the repeat region and the amplicon length was reduced to less than 150 bp [17]. The amplicon length of the 23 mini-STR loci in our study ranged from 68 to 155 bp (Table 1). Maternal DNA contamination is unavoidable in all non-invasive prenatal diagnosis studies. Foetal fraction determination might be less meaningful for allele detection at each STR locus. As the circulating cffDNA is highly degraded, different DNA fractions may include different STR loci. Consequently, the concentration of each STR locus considerably varies in certain foetal fractions. In our study, excluding the results where no allele was detected, approximately 54.8% of the results showed definite detection of cffDNA, whereas approximately 43.8% of the results showed foetal and maternal DNA sharing the same alleles. The results with foetal and maternal DNA sharing the same alleles do not contribute to prenatal paternity determination. Accordingly, the accuracy of NIPPT increases with the number of STR loci included in the analysis. Furthermore, to solve the problem of interference of stutter signals, we evaluated the signal noise and stutter ratio of each STR marker independently. Stutter peaks primarily appear where one repeat less than the true allele, because strand slippage occurs during DNA synthesis. The detection threshold (peak height >50) was high to avoid both sequencing noise and detection of drop-in signals.
The major advantage of this study is the simultaneous determination of genotypes at several STR loci from highly degraded cffDNA by decreasing the target sequence length. The average lengths of foetal and maternal DNA fragments in maternal plasma are 145 and 166 bp, respectively [18]. The length of commonly used amplicons by forensic paternity testing kits based on the CE method is more than 150 bp (Table 1). Such amplicons were not suitable for cffDNA analysis. Multiple STR loci have to be genotyped for prenatal paternity testing. Although STR primers with short amplicons can be designed for the CE method, the limited availability of fluorescent dyes makes them unsuitable for genotyping multiple STR loci from small amounts of plasma DNA. By simultaneously genotyping 23 loci using array-based sequencing, we reduced the quantity of plasma DNA required and increased testing capacity. Another advantage of our study is that the STR genotyping results enabled systematic comparison with data in the national forensic DNA database. Current studies on prenatal paternity analysis rely on targeted sequencing of SNPs [13, 19, 20]. Paternal SNPs can be detected from maternal plasma using SNP analysis; however, this method requires millions of SNPs to be analysed because of a low genetic polymorphism. Moreover, the routine use of SNPs in forensics is doubtful because some SNPs might be present in the coding regions or are modified due to disease association [21]. In contrast, highly polymorphic STRs have been widely adopted for routine forensic application [22]. STRs are almost non-coding in nature, and therefore, no gene expression occurs at STR loci. A low genetic mutation is an important consideration in the selection of STRs for commercial paternity testing kits [23]. According to the International Society for Forensic Genetics, a minimal set of 12 STR loci can be used for paternity testing [24]. All 23 STR loci used in this study are covered by both paternity testing kits commonly used in our laboratory. Nine of these 23 loci are CODIS core loci included in national forensic DNA databases worldwide [25]. Although differences in the target sequence can result in differences in STR nomenclature between the NGS and CE methods, such differences can be calibrated based on the corresponding transformation relationship between the two methods [26]. Accordingly, non-invasive prenatal STR genotyping can be used to not only determine paternity relationship between foetuses and potential fathers, but also provide DNA evidence of suspects in rape cases without injuring pregnant women and foetuses. However, this approach has some limitations. First, ethnic diversity should be considered in the application of our method, especially in the calculation of combined paternity index. Second, we cannot exclude consanguinity between the involved families and random male population in spite of an extremely small probability. Finally, a small number of participants may affect the reliability of the results and increase the margin of error. They need to be improved or considered in the application of our method.
Conclusions
In summary, NGS-based mini-STR genotyping of cffDNA can be used for NIPPT. This method can also be potentially applied for crime control using information available from the national forensic DNA databases. However, some experimental variables, including evaluation strategy of stutter and sequencing noise, and sample size must be optimised to improve the efficacy of this method for routine NIPPT. In addition, the following strategies will be considered in future studies. First, as the paternal and maternal DNA might share identical alleles, it was not clear whether the paternal allele was masked by the maternal allele or dropped out. The number of mini-STR loci analysed will be increased to improve data accuracy. Second, Dorum et al. [27] have established a method to characterise complex DNA mixtures from pedigree-based individuals. This method can be developed to calculate the combined paternity index for NIPPT in the future.
Funding source: Dalian Municipal Science and Technology Bureau, Dalian, China
Award Identifier / Grant number: 2017RQ169
-
Research funding: This work was supported by the Dalian Municipal Science and Technology Bureau, China [grant numbers 2017RQ169].
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Competing interests: Authors state no conflict of interest.
-
Informed consent: Informed consent was obtained from all individuals included in this study.
-
Ethical approval: Research involving human subjects complied with all relevant national regulations, institutional policies and is in accordance with the tenets of the Helsinki Declaration (as revised in 2013), and has been approved by the authors’ Institutional Review Board (Dalian Blood Centre Ethics Committee). (Registration-Nr: DBC00802015).
References
1. Guo, X, Bayliss, P, Damewood, M. A noninvasive test to determine paternity in pregnancy. N Engl J Med 2012;366:1743. https://doi.org/10.1056/nejmc1113044.Search in Google Scholar
2. Beta, J, Lesmes-Heredia, C, Bedetti, C, Akolekar, R. Risk of miscarriage following amniocentesis and chorionic villus sampling: a systematic review of the literature. Minerva Ginecol 2018;70:215–9. https://doi.org/10.23736/s0026-4784.17.04178-8.Search in Google Scholar
3. Christiansen, SL, Jakobsen, B, Børsting, C, Udengaard, H, Buchard, A, Kampmann, ML, et al.. Non-invasive prenatal paternity testing using a standard forensic genetic massively parallel sequencing assay for amplification of human identification SNPs. Int J Leg Med 2019;133:1361–8. https://doi.org/10.1007/s00414-019-02106-0.Search in Google Scholar
4. Lo, YM, Corbetta, N, Chamberlain, PF, Rai, V, Sargent, IL, Redman, CW, et al.. Presence of fetal DNA in maternal plasma and serum. Lancet 1997;350:485–7. https://doi.org/10.1016/s0140-6736(97)02174-0.Search in Google Scholar
5. Palomaki, GE, Best, RG. Sequencing cell-free DNA in the maternal circulation to screen for Down syndrome, other common trisomies, and selected genetic disorders. In: Netto, GJ, Kaul, KL, editors. Genomic applications in pathology, 2nd ed. Cham: Springer International Publishing AG; 2019:561–82 pp.10.1007/978-3-319-96830-8_36Search in Google Scholar
6. Hyland, CA, Millard, GM, O’Brien, H, Schoeman, EM, Lopez, GH, McGowan, EC, et al.. Non-invasive fetal RHD genotyping for RhD negative women stratified into RHD gene deletion or variant groups: comparative accuracy using two blood collection tube types. Pathology 2017;49:757–64. https://doi.org/10.1016/j.pathol.2017.08.010.Search in Google Scholar PubMed
7. Chan, KC, Zhang, J, Hui, AB, Wong, N, Lau, TK, Leung, TN, et al.. Size distributions of maternal and fetal DNA in maternal plasma. Clin Chem 2004;50:88. https://doi.org/10.1373/clinchem.2003.024893.Search in Google Scholar PubMed
8. Breveglieri, G, D’Aversa, E, Finotti, A, Borgatti, M. Non-invasive prenatal testing using fetal DNA. Mol Diagn Ther 2019;23:291–9. https://doi.org/10.1007/s40291-019-00385-2.Search in Google Scholar PubMed
9. Lo, YMD, Zhang, J, Leung, TN, Lau, TK, Chang, AMZ, Hjelm, NM. Rapid clearance of fetal DNA from maternal plasma. Am J Hum Genet 1999;64:218. https://doi.org/10.1086/302205.Search in Google Scholar PubMed PubMed Central
10. Butler, JM, Shen, YB. The development of reduced size STR amplicons as tools for analysis of degraded DNA. J Forensic Sci 2003;48:1054–64. https://doi.org/10.1520/jfs2003043.Search in Google Scholar
11. Biesecker, LC, Bailey-Wilson, JE, Ballantyne, J, Baum, H, Bieber, FR, Brenner, C, et al.. DNA identifications after the 9/11 World trade center attack. Encephale 2005;310:1122–3. https://doi.org/10.1126/science.1116608.Search in Google Scholar
12. Song, W, Zhou, S, Shao, L, Wang, N, Pan, L, Yu, W. Non-invasive fetal ABO genotyping in maternal plasma using real-time PCR. Clin Chem Lab Med 2015;53:1943–50. https://doi.org/10.1515/cclm-2015-0011.Search in Google Scholar
13. Tam, JCW, Chan, YM, Tsang, SY, Yau, CI, Yeung, SY, Au, KK, et al.. Noninvasive prenatal paternity testing by means of SNP-based targeted sequencing. Prenat Diagn 2020;40:497–506. https://doi.org/10.1002/pd.5595.Search in Google Scholar
14. Barra, GB, Santa Rita, TH, Chianca, CF, Velasco, LFR, de Sousa, CF, Nery, LFA, et al.. Fetal male lineage determination by analysis of Y-chromosome STR haplotype in maternal plasma. Forensic Sci Int Genet 2015;15:105–10. https://doi.org/10.1016/j.fsigen.2014.11.006.Search in Google Scholar
15. Ou, X, Wang, H, Qu, D, Chen, Y, Gao, J, Sun, H. Epigenome-wide DNA methylation assay reveals placental epigenetic markers for noninvasive fetal single-nucleotide polymorphism genotyping in maternal plasma. Transfusion 2014;54:2523. https://doi.org/10.1111/trf.12659.Search in Google Scholar
16. Zhang, S, Han, S, Zhang, M, Wang, Y. Non-invasive prenatal paternity testing using cell-free fetal DNA from maternal plasma: DNA isolation and genetic marker studies. Leg Med 2018;32:98–103. https://doi.org/10.1016/j.legalmed.2018.03.009.Search in Google Scholar
17. Bai, X, Li, S, Cong, B, Li, X, Guo, X, He, L, et al.. Construction of two fluorescence-labeled non-combined DNA index system mini STR multiplex systems to analyze degraded DNA samples in the Chinese Han population. Electrophoresis 2010;31:2944–8. https://doi.org/10.1002/elps.201000163.Search in Google Scholar
18. Whittle, M, Francischini, C, Sumita, D. Routine implementation of noninvasive prenatal paternity testing with STRs. Forensic Sci Int Genet Suppl Ser 2017;6:e233–4. https://doi.org/10.1016/j.fsigss.2017.09.101.Search in Google Scholar
19. Dhallan, R, Guo, X, Emche, S, Damewood, M, Bayliss, P, Cronin, M, et al.. A non-invasive test for prenatal diagnosis based on fetal DNA present in maternal blood: a preliminary study. Lancet 2007;369:474–81. https://doi.org/10.1016/s0140-6736(07)60115-9.Search in Google Scholar
20. Yang, D, Liang, H, Gao, Y, Lin, S, He, Z, Gao, J, et al.. Noninvasive fetal genotyping of paternally inherited alleles using targeted massively parallel sequencing in parentage testing cases. Transfusion 2017;57:1505–14. https://doi.org/10.1111/trf.14077.Search in Google Scholar PubMed
21. Jiang, H, Xie, Y, Li, X, Ge, H, Deng, Y, Mu, H, et al.. Noninvasive prenatal paternity testing (NIPAT) through maternal plasma DNA sequencing: a pilot study. PLoS One 2016;11:e0159385. https://doi.org/10.1371/journal.pone.0159385.Search in Google Scholar
22. Zhou, Z, Shao, C, Xie, J, Xu, H, Sun, K. Genetic polymorphism and phylogenetic analyses of 21 non-CODIS STR loci in a Chinese Han population from Shanghai. Mol Genet Genomic Med 2020;8:e1083. https://doi.org/10.1002/mgg3.1083.Search in Google Scholar
23. Wyner, N, Barash, M, McNevin, D. Forensic autosomal short tandem repeats and their potential association with phenotype. Front Genet 2020;11:884. https://doi.org/10.3389/fgene.2020.00884.Search in Google Scholar
24. Morling, N, Allen, RW, Carracedo, A, Geada, H, Guidet, F, Hallenberg, C, et al.. Paternity testing commission of the international society of forensic genetics: recommendations on genetic investigations in paternity cases. Forensic Sci Int 2002;129:148–57. https://doi.org/10.1016/s0379-0738(02)00289-x.Search in Google Scholar
25. Stanley, UN, Khadija, AM, Bukola, AT, Precious, IO, Davidson, EA. Forensic DNA profiling: autosomal short tandem repeat as a prominent marker in crime investigation. Malays J Med Sci 2020;27:22. https://doi.org/10.21315/mjms2020.27.4.3.Search in Google Scholar PubMed PubMed Central
26. Liu, Q, Ma, G, Du, Q, Lu, C, Cong, B. Development of an NGS panel containing 42 autosomal STR loci and the evaluation focusing on secondary kinship analysis. Int J Leg Med 2020;134:2005–14. https://doi.org/10.1007/s00414-020-02295-z.Search in Google Scholar PubMed
27. Dørum, G, Kaur, N, Gysi, M. Pedigree-based relationship inference from complex DNA mixtures. Int J Leg Med 2017;131:629–41. https://doi.org/10.1007/s00414-016-1526-x.Search in Google Scholar PubMed
Supplementary Material
The online version of this article offers supplementary material (https://doi.org/10.1515/labmed-2021-0191).
© 2022 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.
Articles in the same Issue
- Frontmatter
- Original Articles
- Automated assessment of immunofixations with deep neural networks
- Non-invasive prenatal paternity testing using mini-STR-based next-generation sequencing: a pilot study
- The effect of short post-apnea time on plasma triglycerides, lipoprotein and cholesterol derived oxysterols levels
- Assessment of the diagnostic ability of RIFLE and SOFA scoring systems in comparison with protein biomarkers in acute kidney injury
- Diagnostic value of long noncoding RNA LINC01060 in gastric cancer
- Novel GLDC variants causing nonketotic hyperglycinemia in Chinese patients
- Congress Abstracts
- German Congress of Laboratory Medicine: 17th Annual Congress of the DGKL and 4th Symposium of the Biomedical Analytics of the DVTA e.V.
Articles in the same Issue
- Frontmatter
- Original Articles
- Automated assessment of immunofixations with deep neural networks
- Non-invasive prenatal paternity testing using mini-STR-based next-generation sequencing: a pilot study
- The effect of short post-apnea time on plasma triglycerides, lipoprotein and cholesterol derived oxysterols levels
- Assessment of the diagnostic ability of RIFLE and SOFA scoring systems in comparison with protein biomarkers in acute kidney injury
- Diagnostic value of long noncoding RNA LINC01060 in gastric cancer
- Novel GLDC variants causing nonketotic hyperglycinemia in Chinese patients
- Congress Abstracts
- German Congress of Laboratory Medicine: 17th Annual Congress of the DGKL and 4th Symposium of the Biomedical Analytics of the DVTA e.V.

