Abstract
Background
It has been demonstrated that vitamin B12 determinations fail, especially in patients with pernicious anemia with high titers of intrinsic factor antibody. Consistent with this finding, we observed a case of falsely normal holotranscobalamin (HoloTC) results in a patient with pernicious anemia and severe vitamin B12 deficiency. We aimed to investigate whether such a situation can also be seen in other individuals.
Methods
Within the frameworks of the SENIORLAB study and routine samples from a mixed patient population referred to a laboratory for investigation of B12 status, we searched for study participants displaying a normal HoloTC level (≥50 pmol/L) together with a decreased total vitamin B12 level (<125 pmol/L). Thereafter, we determined whether samples with discrepant biochemical markers (i.e. low vitamin B12, normal HoloTC) also had increased functional markers of vitamin B12 deficiency (methyl malonic acid [MMA], homocysteine [Hcy]) and/or a low value of Fedosov’s combined indicator of vitamin B12 status (<−0.5).
Results
The prevalence of a normal HoloTC level and low total vitamin B12 level among the group of healthy seniors (n=1451) was 0.21% (95% confidence interval [CI], CI, 0.08–0.6%). Among the 106,635 routine samples with concurrent HoloTC and total vitamin B12 determination, 176 (i.e. 0.17%, 95% CI, 0.14–0.19%) had discrepant biochemical markers. Among them, 24 who were identified as having discrepant biochemical markers and a diagnosis of vitamin B12 deficiency could be confirmed with functional markers.
Conclusions
Initial and isolated screening for vitamin B12 deficiency using a HoloTC cut-off of ≥50 pmol/L in a small subset of patients may reveal false-negative (normal) results, meaning that patients with vitamin B12 deficiency may remain undetected.
Introduction
Vitamin B12 deficiency is a relatively frequently encountered disorder [1]. There are several laboratory parameters available for the diagnosis of B12 deficiency, including total B12, holotranscobalamin (HoloTC), methyl malonic acid (MMA) and homocysteine (Hcy) [2]. There is no single best parameter for the diagnosis of the disease [3]. Nevertheless, there are several stepwise testing strategies available to identify patients, most frequently with vitamin B12 or HoloTC as an initial test before further testing [2], [4], [5], [6], [7], [8], [9]. It has been demonstrated, however, that total vitamin B12 determinations can fail, especially in patients with pernicious anemia with a high titer of intrinsic factor antibody, which results in false normal or even increased total B12 results [10], [11]. Here, we report a case of falsely normal HoloTC results in a patient with pernicious anemia and investigated whether such a condition can also be seen in other individuals. This is in line with the insight that individual discrepancies between HoloTC and B12 results have rarely been analyzed by investigators [12].
Subjects and methods
Study population
Based on the characteristics of a subject with vitamin B12 deficiency and pernicious anemia, who had normal HoloTC and decreased vitamin B12 levels, we looked at two different populations. First, we investigated a cohort of subjectively healthy seniors recruited for the SENIORLAB study. This prospective study aimed to determine reference intervals for seniors and included 1467 subjects residing in Switzerland, 1451 of whom had both vitamin B12 and HoloTC results available. The detailed study protocol and characteristics regarding vitamin B12 metabolism have been described elsewhere [13], [14]. The SENIORLAB study was performed in accordance with the Declaration of Helsinki and was approved by the cantonal institutional review board (KEK-Bern 166/08). All the participants provided written informed consent. In addition, we analyzed our routine measurement results from the investigation of vitamin B12 status during a period from December 1, 2006 to January 31, 2018. From these routine measurements, we analyzed 773,641 clinical samples referred for either isolated or simultaneous determination of HoloTC, vitamin B12, Hcy, or MMA. Of these samples, 108,086 had a concurrent determination of HoloTC and vitamin B12. Of these 108,086 samples, a request for MMA and/or Hcy determination had been made for 18,847. The flow chart for inclusion of samples is shown in Figure 1. The study protocol for the retrospective analysis of anonymized health data was verified by the cantonal institutional review board (KEK Bern), and the need for informed consent was waived (BASEC-Nr: Req-2018-00090).
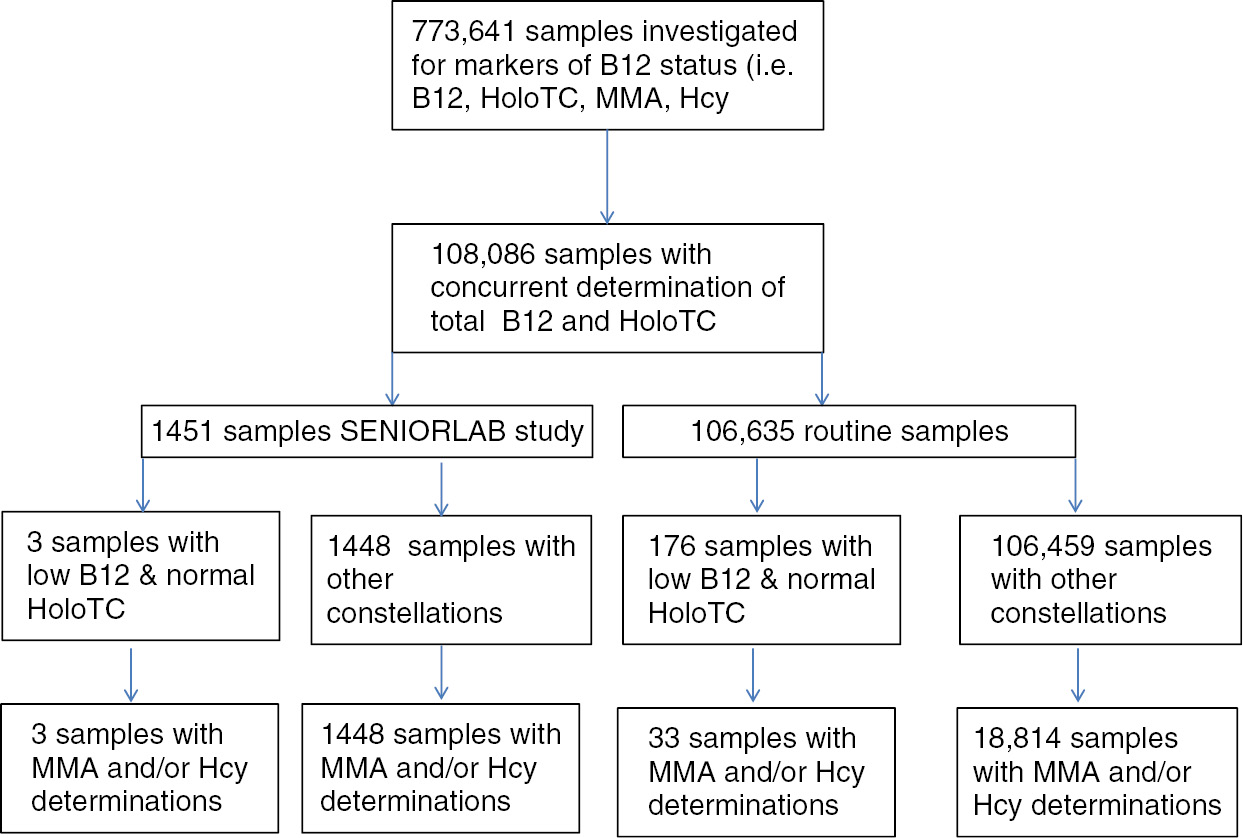
Flow chart for the determination of frequencies of samples with low vitamin B12 and normal HoloTC levels.
Laboratory methods
HoloTC was assayed either on an Axsym analyzer (until 30.11.2012) or an Architect 2000 (since 01.12.2012; Abbott Diagnostics, Baar, Switzerland). Total vitamin B12 was assayed on an Abbott Architect system (Abbott Architect, Baar, Switzerland; until 31.10.2011) or on a Beckman Unicel DxI800 (Beckman Coulter, Nyon, Switzerland; until 25.3.2012), as well as on a Cobas 6000/Cobas 8000 instrument (Roche Diagnostics, Rotkreuz, Switzerland). Hcy was measured on an Immulite 2000 instrument (Siemens, Zurich, Switzerland; until 24.11.2011) and on a Cobas 6000 instrument (since 25.11.2011). MMA was analyzed on a SCIEX API 4000 LC-MS/MS system (AB Sciex, Brugg, Switzerland). The performance characteristics of these methods are described elsewhere [14], [15]. The following cut-offs were employed for the analysis: low vitamin B12 was defined as a concentration <125 pmol/L; normal HoloTC was defined as a concentration of ≥50 pmol/L; and the increased functional parameters were defined as ≥270 nmol/L for MMA and ≥13 μmol/L for Hcy [16], [17]. A combined indicator of vitamin B12 status, cB12, was calculated as described by Fedosov and colleagues. This method integrates biochemical markers (HoloTC and vitamin B12) and functional markers of vitamin B12 deficiency (MMA and Hcy) together with age based on models obtained from large empirical investigations [18], [19], [20].
cB12 can be obtained according to the following equation [20]:
where the biochemical markers (HoloTC and vitamin B12) are in pmol/L and the functional markers (MMA and Hcy) are in μmol/L.
This method can also be used to determine cB12 when one marker (e.g. MMA or Hcy) is missing [19]. A cB12 of ≤−0.5 was defined as an indicator of low vitamin B12 with at least potential subclinical manifestations of vitamin B12 deficiency [19]. It has been recommended that individuals with a cB12 of <−0.5 should consider supplementation with vitamin B12 [19].
Statistical methods
Percentages are presented together with the 95% confidence interval (CI). Associations between two parameters were assessed using the Spearman rank correlation. Ratios were compared using the chi-square (χ2) test. p-Values <0.05 were considered statistically significant. The computer program MedCalc Version 18 (MedCalc Software bvba, Mariakerke, Belgium) was used for calculations. GraphPad Prism Version 5.04 (GraphPad Software Inc., Alameda, CA, USA) was used for graphical work.
Results
Index case
The index case was a 62-year-old woman with moderate macrocytic anemia (hemoglobin 117 g/L) and a mean corpuscular volume of 102 fL who had been suffering from progressive neurological dysfunction (i.e. general weakness, paresthesias in all four limbs, impaired vibration sensation in the lower limbs, ataxic gait). Vitamin B12 and folate deficiency were immediately considered. Her total vitamin B12 level was very low at 44 pmol/L (cut-off for a low probability of B12 deficiency: ≥300 pmol/L), whereas folate was normal (32 nmol/L; cut-off <7 nmol/L). Measurements of HoloTC showed a normal concentration of 67 pmol/L (cut-off for a low probability of cobalamin deficiency: ≥50 pmol/L). The patient did not receive any vitamin B12 treatment at that time. MMA was elevated at 22,400 nmol/L (normal <270 nmol/L). Serum creatinine was normal at 72 μmol/L (corresponding to a normal estimated glomerular filtration rate [eGFR] of 77 mL/min/1.73 m2), excluding kidney failure as a cause of the elevated MMA concentration. The Hcy concentration was not measured. However, calculation of the Hcy concentration from MMA, HoloTC and total vitamin B12 revealed a concentration of 26.9 μmol/L [20]. Fedosov’s combined indicator of vitamin B12 status, cB12, was <−2.51, indicating probable B12 deficiency despite a normal HoloTC level [18], [19].
An intrinsic factor antibody assay was negative, but a parietal cell antibody assay was positive (1:40 titer), and an increased gastrin level of 256 pmol/L (normal <54 pmol/L) was observed. A gastroscopy conducted subsequently revealed atrophic gastritis without indication of Helicobacter pylori, which was histologically confirmed. Taking together all the symptoms and signs revealed a very high post-test probability leading to the diagnosis of pernicious anemia [21], [22]. Intramuscular cobalamin was administered, which led to progressive improvement of the clinical symptoms and signs.
Frequency among healthy seniors
To date, no data on the frequency of discrepant HoloTC and vitamin B12 results are available. Therefore, we investigated the frequency of a constellation of normal HoloTC levels (≥50 pmol/L) together with decreased total vitamin B12 levels (<125 pmol/L) and increased MMA (≥270 nmol/L) and/or Hcy (≥13 μmol/L) levels in a cohort of healthy seniors in the SENIORLAB study. We found that three of 1451 (0.21%; 95% CI, 0.08–0.6%) of the study participants had such a condition. All study participants had a C-reactive protein level <5 mg/L, an estimated eGFR >60 mL/min/1.73 m2 and a normal hemoglobin level >130 g/L.
Frequency in routine laboratory samples
Among 106,635 routine laboratory samples investigated for the diagnosis and monitoring of vitamin B12 deficiency in Switzerland, normal HoloTC with decreased total B12 was seen in 176 cases (0.17%, 95% CI, 0.14–0.19%; Figure 1). A total of 60,394 of the 106,635 samples (56.6%) originated from female patients, and the mean patient age was 52±19 years. There was no significant correlation between HoloTC and vitamin B12 in patients displaying discrepant vitamin B12 and HoloTC results (r=0.01).
Of the samples with concurrent HoloTC and vitamin B12 determinations, 18,847 also had corresponding MMA and/or Hcy measurements. Of the 176 cases with low B12 and normal HoloTC, 33 had MMA and/or Hcy data, and 24 of these cases displayed elevated MMA (≥270 nmol/L) and/or Hcy (≥13 nmol/L; Table 1). The proportion of patients with discrepant HoloTC and vitamin B12 data together with increases in one or two functional markers of vitamin B12 deficiency was 0.13% (95% CI, 0.09–0.19%, i.e. 24/18,847 samples). The data of the nine cases with low B12 and normal HoloTC in the presence of normal concentrations of MMA and Hcy are provided in Supplementary Table 1.
Samples with normal HoloTC levels (≥50 pmol/L), decreased vitamin B12 levels (<125 pmol/L) and elevation of at least one functional marker (MMA ≥270 nmol/L; Hcy ≥13 μmol/L).
| Sex | Age, years | HoloTC, pmol/L | Vitamin B12, pmol/L | MMA, nmol/L | Hcy, μmol/L | cB12 | cB12* | PCA | IF-AB | Origin |
|---|---|---|---|---|---|---|---|---|---|---|
| F | 62 | 67 | 44 | 22,400 | 26.9a | −2.98 | −3.25 | pos | neg | 1 |
| F | 26 | 67 | 102 | 238 | 13.3 | −0.44 | −0.71 | n.a. | n.a. | 2 |
| F | 30 | 54 | 112 | 191 | 19.5 | −0.56 | −0.74 | n.a. | n.a. | 2 |
| F | 34 | 67 | 124 | 350 | 10.3 | −0.30 | −0.67 | n.a. | n.a. | 2 |
| F | 55 | 59 | 91 | 262 | 19.2 | −0.67 | −0.89 | n.a. | n.a. | 2 |
| F | 55 | 56 | 114 | 243 | 20.5 | −0.59 | −0.78 | n.a. | n.a. | 2 |
| F | 58 | 51 | 113 | 735 | 38.8 | −1.38 | −1.53 | n.a. | n.a. | 2 |
| F | 58 | 54 | 114 | 197 | 16.6 | −0.41 | −0.59 | neg | neg | 2 |
| F | 61 | 68 | 104 | 262a | 13 | −0.36 | −0.63 | n.a. | n.a. | 2 |
| F | 62 | 50 | 90 | 311a | 14.6 | −0.67 | −0.82 | pos | neg | 2 |
| F | 62 | 53 | 105 | 276 | 11.7 | −0.43 | −0.60 | n.a. | n.a. | 3 |
| F | 65 | 50 | 121 | 290 | 10.9a | −0.37 | −0.51 | n.a. | n.a. | 2 |
| F | 65 | 62 | 121 | 389 | 13.5 | −0.50 | −0.73 | n.a. | n.a. | 3 |
| F | 68 | 54 | 122 | 388 | 12.7 | −0.51 | −0.69 | neg | neg | 2 |
| F | 70 | 57 | 120 | 159 | 13.8 | −0.13 | −0.33 | n.a. | n.a. | 2 |
| F | 75 | 51 | 123 | 259 | 13.9 | −0.35 | −0.50 | n.a. | n.a. | 2 |
| F | 77 | 60 | 119 | 347 | 17.8 | −0.52 | −0.74 | n.a. | n.a. | 3 |
| F | 79 | 50 | 91 | 187 | 30 | −0.66 | −0.80 | n.a. | n.a. | 2 |
| F | 91 | 68 | 114 | 345 | 14.3 | −0.28 | −0.56 | n.a. | n.a. | 2 |
| M | 32 | 64 | 109 | 234a | 15.4 | −0.48 | −0.73 | n.a. | n.a. | 2 |
| M | 49 | 55 | 112 | 405 | 16.6 | −0.76 | −0.95 | neg | neg | 2 |
| M | 57 | 63 | 121 | 271 | 21 | −0.56 | −0.81 | n.a. | n.a. | 2 |
| M | 69 | 51 | 98 | 340a | 16.7 | −0.69 | −0.84 | n.a. | n.a. | 2 |
| M | 71 | 67 | 118 | 174 | 18.4 | −0.23 | −0.50 | n.a. | n.a. | 2 |
| M | 72 | 53 | 105 | 201 | 29.3 | −0.64 | −0.81 | n.a. | n.a. | 2 |
| M | 73 | 61 | 122 | 266 | 26.8 | −0.59 | −0.82 | n.a. | n.a. | 2 |
| M | 79 | 53 | 76 | 217 | 16 | −0.50 | −0.67 | n.a. | n.a. | 2 |
A cB12 value<−0.5 denotes a low B12 concentration requiring clinical action [10]. cB12*=cB12 is calculated by substituting a “falsely normal” HoloTC with a HoloTC concentration of 36 pmol/L. Origin of the described cases: 1, index case report; 2, routine laboratory samples; 3, SENIORLAB cohort. aMeasurement not performed and concentration calculated according to [10]. PCA, anti-parietal cell antibody; IF-AB, anti-intrinsic factor antibody; n.a., not available.
Because the HoloTC in these cases would be “falsely normal”, cB12 can also be calculated (cB12*) by substituting the HoloTC concentration with an assumed HoloTC concentration of 36 pmol/L, which is the upper limit of an indication for B12 supplementation [19]. As shown in Table 1, all but one of the 24 samples had a cB12* ≤−0.5, indicating low vitamin B12 status. Based on these results, it can be assumed that falsely normal HoloTC occurs at a low frequency among healthy seniors and daily clinical routine investigations in a mixed patient population with an estimated frequency of approximately 0.1–0.2%.
Demographic characteristics of discrepant samples
The results of samples with low B12 and normal HoloTC levels combined with low cB12 and/or increased MMA and/or Hcy levels are summarized in Table 1. Samples exhibiting vitamin B12 deficiency and normal HoloTC levels were more frequently found in women (16/24, 66.6% [95% CI 46.5, 82.0] for women vs. 8/24, 33.3% [95% CI 18.0, 53.5] for men; p=0.022). Furthermore, the majority of the samples (19/24, i.e. 79.2% [95% CI 59.3, 90.6]) originated from individuals aged ≥50. Among the samples with data available on intrinsic factor antibodies and/or parietal cell antibodies, discrepant samples were obtained from patients with and without serological evidence of atrophic autoimmune gastritis (Table 1).
Discussion
In the index case exhibiting a classical clinical presentation of pernicious anemia, a discrepantly normal HoloTC measurement result with otherwise clearly pathological parameters of B12 metabolism was observed. Such falsely normal or even elevated HoloTC results, causing missed B12 deficiency diagnosis in pernicious anemia patients, have been reported for competitive binding luminescence assays (CBLAs) measuring total B12 concentrations [10], [11]. To the best of our knowledge, this is the first case of a normal HoloTC result in the presence of severe vitamin B12 deficiency due to pernicious anemia.
We further demonstrated that this constellation of test results can also be encountered in samples originating from other patients. The frequency among healthy seniors as well as routine samples was estimated to be 0.1–0.2%. Vitamin B12 deficiency can be assessed by several methods, e.g. total vitamin B12 measurement, HoloTC measurement describing the biologically active form of vitamin B12 bound to transcobalamin and measurement of the functional markers MMA and Hcy [3]. Furthermore, a combined indicator of vitamin B12, cB12, incorporates all of these parameters into an integrated estimator of vitamin B12 status [18]. The latter indicator can also be calculated when one of the four parameters is lacking [19]. In samples from patients who also have functional markers of cobalamin deficiency, a substantial proportion showed evidence of subclinical cobalamin deficiency. However, we also identified samples with results that indicated possible or probable clinical cobalamin deficiency [19]. A substantial proportion of the samples with discrepant biochemical results with falsely normal HoloTC levels were demonstrated to be vitamin B12 deficient, as shown by functional markers.
These findings raise the question of how a situation with discrepant biochemical markers and falsely normal HoloTC levels may occur. Such a situation does not follow Herbert’s classical conceptualization of how cobalamin deficiency is thought to develop [23], [24]. From a state of subclinical cobalamin deficiency due to vitamin B12 depletion with a decrease only in HoloTC levels, a more advanced stage of subclinical damage is characterized by an increase in the functional markers MMA and Hcy, which then develops into overt cobalamin deficiency, characterized by decreased vitamin B12 concentrations and clinical signs in the most severe disease stage [23]. The present investigation suggests that there may be factors that render the HoloTC assay unreliable in a subset of patients. Further information regarding low, intermediate and normal marker concentrations of HoloTC or vitamin B12 in the presence of a low HoloTC or low vitamin B12 can be found in Supplementary Table 2 [16], [17], [25].
The discrepant biochemical markers of cobalamin deficiency could be explained by the following: (a) autoantibodies interfering with the HoloTC assay; (b) intake of vitamin B12 supplements; (c) decreased expression of haptocorrin, the transport protein for the biologically inactive form of cobalamin; or (d) an increased concentration of HoloTC due to polymorphisms in the transcobalamin II gene, which encodes the transport protein for the biologically active form of cobalamin [3], [10].
There have been many reported cases of cobalamin assay failures involving pernicious anemia with CBLA, usually with high serum titers of intrinsic factor antibody. These false results have been attributed to high levels of intrinsic factor-blocking antibodies interfering with the assay and leading to falsely normal or even elevated results of total vitamin B12 measurements [11]. Vitamin B12 assays apply a method based on competitive binding of serum cobalamin to an intrinsic factor contained in the reagents. The exact mechanism of this intrinsic factor interference has not yet been elucidated.
The HoloTC microparticle enzyme immunoassay (MEIA) test consists of a capture antibody specific to vitamin B12 bound to transcobalamin II [26] and a labeling antibody, alkaline phosphatase (ALP)-marked anti-transcobalamin. HoloTC concentrations in the sample are directly proportional to the fluorescent signal. It is possible that anti-transcobalamin antibodies lead to the formation of an immune complex linking transcobalamin II to bound HoloTC immune complexes, ultimately leading to falsely increased apparent levels of HoloTC [27]. Despite the fact that anti-transcobalamin II antibodies have been characterized in patients with and without pernicious anemia, nothing is known regarding the interference of anti-transcobalamin II antibodies with HoloTC measurements [27], [28]. The present case report represents the first possible indication of such interference. A limitation of the present study is that we did not assess the presence of such antibodies. The fact that such a constellation of results is seen more frequently in women and the elderly is consistent with the theory that autoimmune processes contribute to this phenomenon, as some autoimmune diseases are more frequently seen in women and the elderly [29], [30].
Recently, a test for enteral cobalamin resorption, i.e. the CobaSorb test, has been described [31], [32]. This test is better suited to short-term oral ingestion of vitamin B12 than the total vitamin B12 test. Oral supplementation leads to an increase in HoloTC rather than in the total vitamin B12. Theoretically, it is possible that orally ingested supplements are responsible for normal HoloTC levels while total vitamin B12 concentrations are reduced. Although we were limited by the fact that we did not have records of oral supplementation or diet associated with the routine samples, three healthy seniors as well as the index case did not take a supplement and did not follow a special (i.e. vegetarian) diet. This limitation renders a discrepancy due to oral vitamin B12 supplementation improbable.
Another possibility is that there could be physiological circumstances that lower the expression of haptocorrin or their binding to vitamin B12, leading to low total B12 levels despite a normal supply of B12, as seen in pregnant women, patients with genomic haptocorrin mutations or patients with HIV [33], [34], [35], [36]. The fact that we could identify a substantial proportion of the samples as having low total B12 and normal HoloTC levels with increased functional markers or low cB12 is more consistent with falsely normal HoloTC levels than falsely low total B12 levels. Furthermore, the fact that the majority of the samples originated from men and women aged ≥50 makes pregnancy as a cause of falsely low vitamin B12 concentrations improbable. As we were limited by not having further clinical or genetic information on the routine samples, we were not able to identify other pathophysiological reasons for decreased vitamin B12 levels. Together, our data suggest that the discrepancy among the biochemical markers is due to falsely normal HoloTC rather than falsely low vitamin B12.
Significantly higher concentrations of HoloTC with no difference in vitamin B12 levels are more often observed in elderly subjects displaying the CC polymorphism at position 775 of the transcobalamin II gene compared with the GC and GG genotypes [37], [38]. The difference in HoloTC concentration between GG and the other genotypes is approximately 30 pmol/L [37]. It has been hypothesized that this genotype may be more efficient in delivering B12 to tissues, resulting in an enhanced B12 functional status with lower MMA concentrations [37]. The frequency of the CC genotype is between 30% and 44.2% [37], [38]. Although such a difference may account for the differences in the subclinical cases of B12 deficiency, it cannot explain the difference between vitamin B12 and HoloTC in the index case. This study is limited by the fact that we did not assess the 775 G→C polymorphism in the transcobalamin II gene. In consequence, we could not assess whether the discrepant biochemical parameters are due to the occurrence of transcobalamin II polymorphisms.
Our study has strengths and limitations. A lack of clinical data and dietary and genetic information in the routine samples means that we could not elucidate the pathophysiology of how the discrepancy in biochemical marker results may arise. Additionally, we were unable to retrieve further samples from the index case to clarify whether autoantibodies against transcobalamin II may interfere with the HoloTC assay and cause falsely normal HoloTC. A strength of this study is that we could investigate two rather large populations, allowing us to estimate the frequency of the occurrence of discrepant biochemical markers of B12 deficiency. A further strength of the study is the fact that a considerable proportion of cases had functional marker data, enabling the determination of the most comprehensive indicator of vitamin B12 deficiency, cB12. Our results support the hypotheses that autoantibodies against transcobalamin II, an unknown cause of decreased haptocorrin, or an increased expression of transcobalamin II due to genetic polymorphisms can cause discrepant biochemical markers of vitamin B12. Supplementation with vitamin B12 is unlikely to cause this discrepancy.
We demonstrated that samples with low total B12 and normal HoloTC levels can be associated with a serious underlying disease (i.e. pernicious anemia). We also demonstrated that such a scenario can also be seen in healthy seniors from the SENIORLAB study with subclinical B12 deficiency. Finally, routine clinical samples demonstrated that a proportion of the samples with this constellation of findings showed vitamin B12 deficiency with falsely normal HoloTC levels. The frequency of occurrence of this constellation of findings is rather low (0.1–0.2%). Our results corroborate the insight that there is no single best marker for detection of vitamin B12 deficiency. In conclusion, initial and isolated screening for vitamin B12 deficiency with HoloTC may result in a lack of detection of vitamin B12 deficiency. In the case of clinical suspicion of vitamin B12 deficiency, a normal HoloTC result ≥50 pmol/L should prompt further investigation to avoid missing clinically significant disease. Further work is needed to understand how normal HoloTC levels can occur in the presence of vitamin B12 deficiency.
Acknowledgments
We acknowledge the very helpful comments of Professor Ralph Carmel on the reported index case. Furthermore, the work of Walter Frehner and Toni Schönenberger in data extraction is gratefully acknowledged.
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved its submission.
Research funding: None declared.
Employment or leadership: None declared.
Honorarium: None declared.
Competing interests: The funding organization(s) played no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the report for publication.
References
1. Koenig V, Stanga Z, Zerlauth M, Bernasconi L, Risch M, Huber A, et al. Prevalence of vitamin B(12) depletion and deficiency in Liechtenstein. Public Health Nutr 2014;17:241–7.10.1017/S1368980012005289Search in Google Scholar PubMed
2. Langan RC, Goodbred AJ. Vitamin B12 deficiency: recognition and management. Am Fam Physician 2017;96:384–9.Search in Google Scholar
3. Green R, Allen LH, Bjorke-Monsen AL, Brito A, Gueant JL, Miller JW, et al. Vitamin B12 deficiency. Nat Rev Dis Primers 2017;3:17040.10.1038/nrdp.2017.40Search in Google Scholar PubMed
4. Herrmann W, Obeid R. Utility and limitations of biochemical markers of vitamin B12 deficiency. Eur J Clin Invest 2013;43:231–7.10.1111/eci.12034Search in Google Scholar PubMed
5. Heil SG, de Jonge R, de Rotte MC, van Wijnen M, Heiner-Fokkema RM, Kobold AC, et al. Screening for metabolic vitamin B12 deficiency by holotranscobalamin in patients suspected of vitamin B12 deficiency: a multicentre study. Ann Clin Biochem 2012;49:184–9.10.1258/acb.2011.011039Search in Google Scholar PubMed
6. Hvas AM, Nexo E. Diagnosis and treatment of vitamin B12 deficiency – an update. Haematologica 2006;91:1506–12.Search in Google Scholar
7. Hvas AM, Nexo E. Holotranscobalamin – a first choice assay for diagnosing early vitamin B deficiency? J Intern Med 2005;257:289–98.10.1111/j.1365-2796.2004.01437.xSearch in Google Scholar PubMed
8. Harrington DJ. Laboratory assessment of vitamin B12 status. J Clin Pathol 2017;70:168–73.10.1136/jclinpath-2015-203502Search in Google Scholar PubMed
9. Hannibal L, Lysne V, Bjorke-Monsen AL, Behringer S, Grunert SC, Spiekerkoetter U, et al. Biomarkers and algorithms for the diagnosis of vitamin B12 deficiency. Front Mol Biosci 2016;3:27.10.3389/fmolb.2016.00027Search in Google Scholar PubMed PubMed Central
10. Carmel R, Agrawal YP. Failures of cobalamin assays in pernicious anemia. N Engl J Med 2012;367:385–6.10.1056/NEJMc1204070Search in Google Scholar PubMed
11. Carmel R. Chemiluminescence-based cobalamin assay errors: background and perspectives. Clin Chem Lab Med 2013;51:e253–6.10.1515/cclm-2013-0586Search in Google Scholar PubMed
12. Carmel R. Holotranscobalamin: not ready for prime time. Clin Chem 2012;58:643–5; author reply 645–6.10.1373/clinchem.2011.174516Search in Google Scholar PubMed
13. Risch M, Meier DW, Sakem B, Medina Escobar P, Risch C, Nydegger U, et al. Vitamin B12 and folate levels in healthy Swiss senior citizens: a prospective study evaluating reference intervals and decision limits. BMC Geriatr 2015;15:82.10.1186/s12877-015-0060-xSearch in Google Scholar PubMed PubMed Central
14. Risch M, Nydegger U, Risch L. SENIORLAB: a prospective observational study investigating laboratory parameters and their reference intervals in the elderly. Medicine (Baltimore) 2017;96:e5726.10.1097/MD.0000000000005726Search in Google Scholar PubMed PubMed Central
15. Conen D, Schon T, Aeschbacher S, Pare G, Frehner W, Risch M, et al. Genetic and phenotypic determinants of blood pressure and other cardiovascular risk factors (GAPP). Swiss Med Wkly 2013;143:w13728.10.4414/smw.2013.13728Search in Google Scholar PubMed
16. Aparicio-Ugarriza R, Palacios G, Alder M, Gonzalez-Gross M. A review of the cut-off points for the diagnosis of vitamin B12 deficiency in the general population. Clin Chem Lab Med 2015;53:1149–59.10.1515/cclm-2014-0784Search in Google Scholar PubMed
17. Herrmann W, Obeid R. Causes and early diagnosis of vitamin B12 deficiency. Dtsch Arztebl Int 2008;105:680–5.10.3238/arztebl.2008.0680Search in Google Scholar PubMed PubMed Central
18. Fedosov SN. Metabolic signs of vitamin B(12) deficiency in humans: computational model and its implications for diagnostics. Metabolism 2010;59:1124–38.10.1016/j.metabol.2009.09.036Search in Google Scholar PubMed
19. Fedosov SN, Brito A, Miller JW, Green R, Allen LH. Combined indicator of vitamin B12 status: modification for missing biomarkers and folate status and recommendations for revised cut-points. Clin Chem Lab Med 2015;53:1215–25.10.1515/cclm-2014-0818Search in Google Scholar PubMed
20. Fedosov SN. Biochemical markers of vitamin B12 deficiency combined in one diagnostic parameter: the age-dependence and association with cognitive function and blood hemoglobin. Clin Chim Acta 2013;422:47–53.10.1016/j.cca.2013.04.002Search in Google Scholar PubMed
21. Annibale B, Lahner E, Fave GD. Diagnosis and management of pernicious anemia. Curr Gastroenterol Rep 2011;13:518–24.10.1007/s11894-011-0225-5Search in Google Scholar PubMed
22. Cattan D. Pernicious anemia: what are the actual diagnosis criteria? World J Gastroenterol 2011;17:543–4.10.3748/wjg.v17.i4.543Search in Google Scholar PubMed PubMed Central
23. Herbert V. Staging vitamin B-12 (cobalamin) status in vegetarians. Am J Clin Nutr 1994;59:1213S–22S.10.1093/ajcn/59.5.1213SSearch in Google Scholar
24. Golding PH. Holotranscobalamin (HoloTC, Active-B12) and Herbert’s model for the development of vitamin B12 deficiency: a review and alternative hypothesis. Springerplus 2016;5:668.10.1186/s40064-016-2252-zSearch in Google Scholar
25. Herrmann W, Obeid R, Schorr H, Geisel J. The usefulness of holotranscobalamin in predicting vitamin B12 status in different clinical settings. Curr Drug Metab 2005;6:47–53.10.2174/1389200052997384Search in Google Scholar
26. Brady J, Wilson L, McGregor L, Valente E, Orning L. Active B12: a rapid, automated assay for holotranscobalamin on the Abbott AxSYM analyzer. Clin Chem 2008;54:567–73.10.1373/clinchem.2007.096784Search in Google Scholar
27. Carmel R, Tatsis B, Baril L. Circulating antibody to transcobalamin II causing retention of vitamin B12 in the blood. Blood 1977;49:987–1000.10.1182/blood.V49.6.987.987Search in Google Scholar
28. Marcoullis G, Parmentier Y, Nicolas JP. Blocking and binding type antibodies against all major vitamin B12-binders in a pernicious anaemia serum. Br J Haematol 1979;43:15–26.10.1111/j.1365-2141.1979.tb03715.xSearch in Google Scholar
29. Cooper GS, Stroehla BC. The epidemiology of autoimmune diseases. Autoimmun Rev 2003;2:119–25.10.1016/S1568-9972(03)00006-5Search in Google Scholar
30. Broten L, Avina-Zubieta JA, Lacaille D, Joseph L, Hanly JG, Lix L, et al. Systemic autoimmune rheumatic disease prevalence in Canada: updated analyses across 7 provinces. J Rheumatol 2014;41:673–9.10.3899/jrheum.130667Search in Google Scholar PubMed
31. Hvas AM, Morkbak AL, Hardlei TF, Nexo E. The vitamin B12 absorption test, CobaSorb, identifies patients not requiring vitamin B12 injection therapy. Scand J Clin Lab Invest 2011;71:432–8.10.3109/00365513.2011.581389Search in Google Scholar PubMed
32. Hvas AM, Morkbak AL, Nexo E. Plasma holotranscobalamin compared with plasma cobalamins for assessment of vitamin B12 absorption; optimisation of a non-radioactive vitamin B12 absorption test (CobaSorb). Clin Chim Acta 2007;376:150–4.10.1016/j.cca.2006.08.009Search in Google Scholar PubMed
33. Morkbak AL, Hvas AM, Milman N, Nexo E. Holotranscobalamin remains unchanged during pregnancy. Longitudinal changes of cobalamins and their binding proteins during pregnancy and postpartum. Haematologica 2007;92:1711–2.10.3324/haematol.11636Search in Google Scholar PubMed
34. Koebnick C, Heins UA, Dagnelie PC, Wickramasinghe SN, Ratnayaka ID, Hothorn T, et al. Longitudinal concentrations of vitamin B(12) and vitamin B(12)-binding proteins during uncomplicated pregnancy. Clin Chem 2002;48:928–33.10.1093/clinchem/48.6.928Search in Google Scholar
35. Hansen M, Gimsing P, Ingeberg S, Jans H, Nexo E. Cobalamin binding proteins in patients with HIV infection. Eur J Haematol 1992;48:228–31.10.1111/j.1600-0609.1992.tb01590.xSearch in Google Scholar PubMed
36. Carmel R, Parker J, Kelman Z. Genomic mutations associated with mild and severe deficiencies of transcobalamin I (haptocorrin) that cause mildly and severely low serum cobalamin levels. Br J Haematol 2009;147:386–91.10.1111/j.1365-2141.2009.07855.xSearch in Google Scholar PubMed
37. Miller JW, Ramos MI, Garrod MG, Flynn MA, Green R. Transcobalamin II 775G>C polymorphism and indices of vitamin B12 status in healthy older adults. Blood 2002;100:718–20.10.1182/blood-2002-01-0209Search in Google Scholar PubMed
38. Refsum H, Johnston C, Guttormsen AB, Nexo E. Holotranscobalamin and total transcobalamin in human plasma: determination, determinants, and reference values in healthy adults. Clin Chem 2006;52:129–37.10.1373/clinchem.2005.054619Search in Google Scholar PubMed
Supplementary Material
The online version of this article offers supplementary material (https://doi.org/10.1515/labmed-2018-0023).
©2018 Walter de Gruyter GmbH, Berlin/Boston
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Articles in the same Issue
- Frontmatter
- Editorial
- Challenges of laboratory diagnostics in the elderly
- Geriatric Laboratory
- The SENIORLAB study in the quest for healthy elderly patients
- Laboratory diagnostics in dementia
- Red blood cell counts and indices in the elderly German population
- Original Article
- Failure of the holotranscobalamin assay in vitamin B12-deficient patients
- Endocrinology
- Serum vitamin D receptor levels in gestational diabetes mellitus
- Infectiology and Microbiology
- Detection of CagA, VacA, IceA1 and IceA2 virulent genes in Helicobacter pylori isolated from gastric ulcer patients
- Congress Abstracts
- German Congress of Laboratory Medicine: 15th Annual Congress of the DGKL (German Society of Clinical Chemistry and Laboratory Medicine) and the 3rd Symposium on Biomedical Analysis
Articles in the same Issue
- Frontmatter
- Editorial
- Challenges of laboratory diagnostics in the elderly
- Geriatric Laboratory
- The SENIORLAB study in the quest for healthy elderly patients
- Laboratory diagnostics in dementia
- Red blood cell counts and indices in the elderly German population
- Original Article
- Failure of the holotranscobalamin assay in vitamin B12-deficient patients
- Endocrinology
- Serum vitamin D receptor levels in gestational diabetes mellitus
- Infectiology and Microbiology
- Detection of CagA, VacA, IceA1 and IceA2 virulent genes in Helicobacter pylori isolated from gastric ulcer patients
- Congress Abstracts
- German Congress of Laboratory Medicine: 15th Annual Congress of the DGKL (German Society of Clinical Chemistry and Laboratory Medicine) and the 3rd Symposium on Biomedical Analysis

