Definition, classification and diagnostics of diabetes mellitus
-
Astrid Petersmann
and Kommission für Labordiagnostik der Diabetologie der Deutschen Diabetes Gesellschaft (DDG) und der Deutschen Gesellschaft für Klinische Chemie und Laboratoriumsmedizin (DGKL) (Commission for Laboratory Diagnostics in Diabetology of the German Diabetes Association [DDG] and the German Association for Clinical Chemistry and Laboratory Diagnostics)
Abstract
This recommendation aims to provide up-to-date information and the latest consensus on diabetes diagnosis from a group of experts in Germany. The disease diabetes mellitus is classified and its different types are described briefly. Options for diagnosis are presented including current cut-off values as well as reference intervals to recognize glucose utilization disorders like impaired fasting glucose or impaired glucose tolerance. Special attention is paid to the measurement value imprecision. The minimal difference (MD) is introduced as an excellent measure to distinguish measurement results which are analytically different from each other. Potential caveats considering pre-analytics in glucose measurement and limitations of HbA1c assessment for diagnosis of diabetes mellitus are presented. Taken together, this recommendation provides a comprehensive overview of the state of the art in diabetes mellitus diagnosis and also critically evaluates potential pitfalls.
The aim of recommendations like this one issued by the German Diabetes Association is to provide the diabetologist and his team the information he needs for his daily practice. These recommendations are updated annually. They are written by a group of experts, but they are not evidence-based guidelines. This specific recommendation for diabetes diagnosis briefly describes the diabetes types and the different options for diagnosis. Also the caveats and the practical procedure are presented.
Explaining introduction
Diabetes is one of the major health burdens throughout the world and belongs to the four priority noncommunicable diseases. Globally, it is estimated that 422 million adults were living with diabetes in 2014 which is almost four times as many as in 1980, and the global prevalence nearly doubled since then. According to the results of the current study by the Robert Koch Institute, diabetes was diagnosed in 7.2% of all adults between 18 and 79 years in Germany which is a 2% increase in prevalence compared to the “Bundes-Gesundheitssurvey 1998”. Moreover, unrecognized diabetes has to be supposed in 2% of all adults. Up to 95% of these are suffering from type 2 diabetes which occurs more often with increasing age and is associated with higher body weight and insufficient physical activity.
Diabetes mellitus that is unrecognized or untreated over longer time periods or is insufficiently treated is associated with increased risk of cardiovascular disease, kidney dysfunction, blindness and foot amputation. As reported by the Emerging Risk Factors Collaboration who analyzed 123,205 deaths among 820,900 people in 97 prospective studies, the life expectancy is drastically affected, i.e. a 50-year-old male diabetes patient has a 5.8-year reduced life span compared to a 50-year-old man without diabetes. In women, the reduction is 6.5 years in this age-group. In addition, in the elderly, hypoglycemia has been recognized as another emerging complication pointing to the importance of accurately and tightly controlled blood glucose levels in these patients.
The present recommendation is supposed to provide the latest consensus in diabetes diagnosis to improve patient care and aid the practitioners’ daily work.
Definition of diabetes mellitus
Diabetes mellitus is a general term for a group of metabolic disorders with the main feature of chronic hyperglycemia. It results from either impaired insulin secretion or impaired insulin efficacy or, most often, both.
Gestational diabetes
A glucose utilization disorder which occurs and is diagnosed during pregnancy for the first time.
Classification
Type 1 diabetes
β Cell destruction which leads to a total insulin deficiency
In most cases resulting from immunological factors
Latent autoimmune diabetes in adults (LADA)
Type 2 diabetes
Can range from a predominant insulin resistance with relative insulin deficiency to an extensive secretory defect with insulin resistance.
Is often associated with other disorders (e.g. metabolic syndrome).
Specific diabetes types with known causes
Diseases involving a pancreatic exocrine deficiency (e.g. pancreatitis, cystic fibrosis, hemochromatosis)
Endocrinopathies (e.g. Cushing syndrome, acromegaly, pheochromocytoma)
Drug or chemically induced (e.g. glucocorticoids, neuroleptics, interferon alpha, pentamidine), genetic defects of the β cell function (e.g. MODY types)
Genetic defects of insulin action
Other genetic syndromes which can be associated with diabetes
Infections
Rare forms of autoimmune-mediated diabetes
Gestational diabetes
Diagnostic criteria for diabetes mellitus
Random plasma glucose value of ≥200 mg/dL (≥11.1 mmol/L) or
Fasting plasma glucose value of ≥126 mg/dL (≥7.0 mmol/L) or
2-h oral glucose tolerance test (oGTT) value in venous plasma ≥200 mg/dL (≥11.1 mmol/L)
glycated hemoglobin (HbA1c) ≥6.5% (≥48 mmol/mol Hb)
Impaired fasting glucose values
Impaired fasting glucose (IFG) for the interval of fasting glucose from 100 to 125 mg/dL (5.6–6.9 mmol/L) in venous plasma.
Impaired glucose tolerance
Impaired glucose tolerance (IGT) corresponds to a 2-h plasma glucose value in oGTT in the interval from 140 to 199 mg/dL (7.8–11.0 mmol/L) with fasting glucose values <126 mg/dL (<7.0 mmol/L).
IFG and IGT are also present in many people with a glucose utilization disorder.
Diagnostic approach
The recommended diagnostic procedure is shown in Figure 1 and the differential diagnostic approach criteria are shown in Table 1.
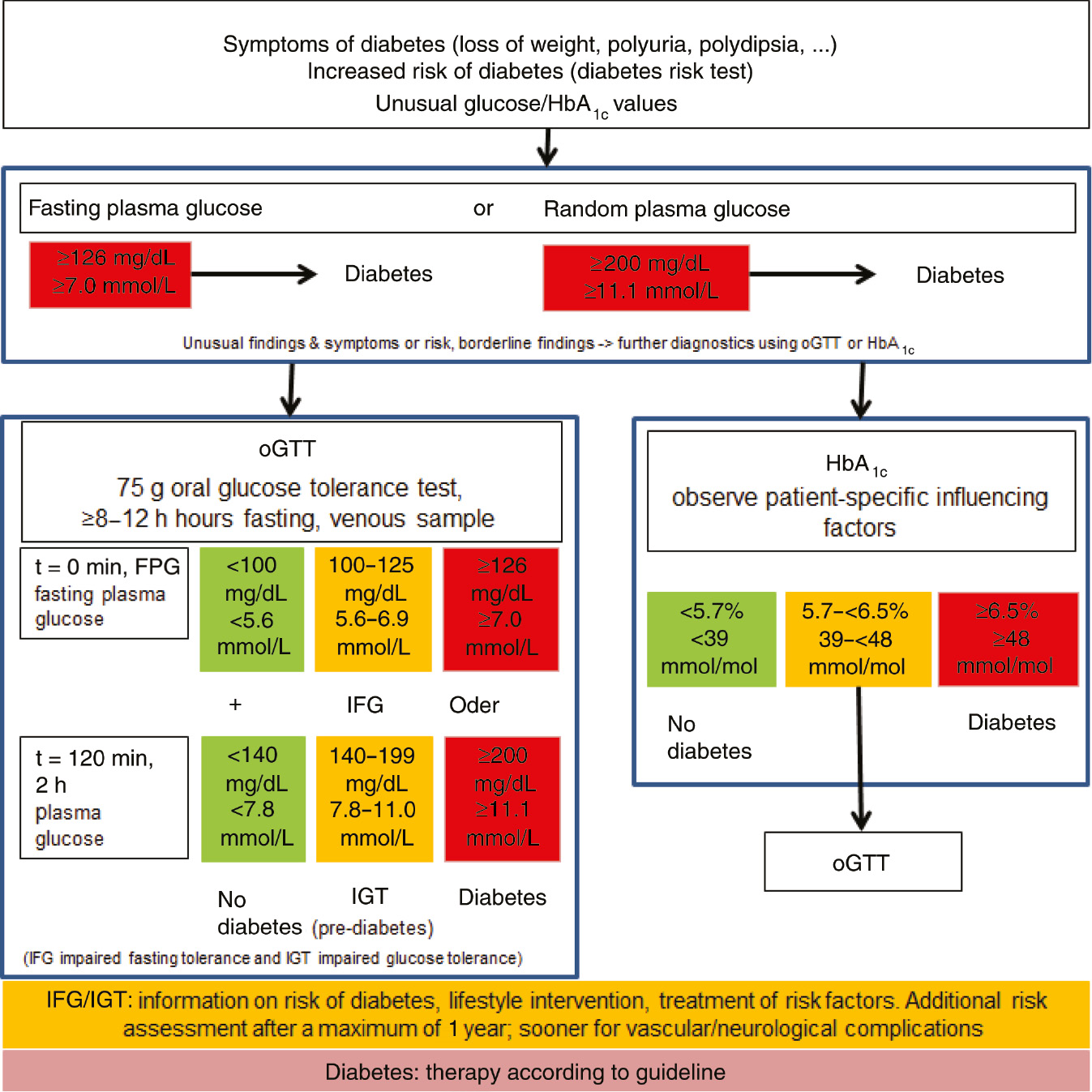
Diagnostic approach for diagnosing diabetes.
Differential diagnostic criteria for patients with type 1 or type 2 diabetes for diagnosing diabetes.
| Type 1 diabetesa | Type 2 diabetes | MODYs | |
|---|---|---|---|
| Etiology | Autoimmune, genetic predisposition | Genetic predisposition, multifactorial | Monogenic |
| Heredity | Variable | Variable | Autosomal dominant; diabetes in ≧3 generations |
| Percentage of all diabetes types | 5–10% | 90–95% | ca. 2% |
| Pathogenesis | Autoantibodies, absolute insulin deficiency | Insulin resistance and insulin secretion disorder up to insulin deficiency | Mutation of genes of transcription factors or glucokinase of the β cells |
| Typical age of manifestation | Childhood to adulthood | Adulthood | Youth to early adulthood |
| Clinical manifestation | Acute polyuria, polydipsia, severe hyperglycemia, ketoacidosis | Gradual onset, secondary complications, moderate to severe hyperglycemia | Gradual onset, variable hyperglycemia |
| Comorbidities | Autoimmune thyroiditis, celiac disease | Visceral obesity, hypertension (also called metabolic syndrome) | Kidney cysts according to MODY type |
| Tendency toward ketosis | Yes | No | No |
| Weight | Normal weight | Overweight | Normal weight |
| Plasma insulin/C-peptide | Decreased to missing | Often elevated at the beginning, then decreased | Mostly decreased |
| Autoantibodies | Yes | No | No |
| Insulin resistance | No | Yes | No |
| Therapy | Insulin | Lifestyle intervention, oral antidiabetics, GLP-1-RA, insulin | Maybe none, OADs, insulin (according to MODY type) |
Table modified from the National Medical Guideline for type 2 diabetes; www.versorgungsleitlinien.de. aLADA (latent autoimmune diabetes in adults) is associated with a slow loss of β cell function. A rapid failure of oral antidiabetics can be expected with LADA. Upon suspicion of LADA: test for glutamic acid decarboxylase-antibodies is recommended. GLP-1-RA, glucagon-like peptide-1 receptor agonist; MODY, maturity onset diabetes of the young; OAD, oral antidiabetics.
Only quality-assured laboratory methods may be used when measuring venous plasma glucose and HbA1c in diabetes diagnostics. This is defined in the guidelines published by the ‘Richtlinie der Bundesärztekammer zur Qualitätssicherung laboratoriumsmedizinischer Untersuchungen (Rili-BAEK)” (Guideline of the German Medical Association on Quality Assurance in Medical Laboratory Examinations) as standard for both central laboratories and point-of-care testing (POCT). Participation in external quality assessment (EQA) schemes has not been compulsory for POCT methods used in practices in Germany. If POCT systems are approved for diagnostic purposes by the manufacturer, the successful participation in EQA schemes, however, is mandatory before they can be used in diagnostics. Currently, the gold standard for diabetes diagnosis is the measurement of glucose in venous plasma.
Procedures if measurement results are close to the diagnostic criteria levels
When using the fasting glucose value, a fasting time of 8–12 h is essential. The guidelines for performing an oGTT must be followed (Table 2). Using the HbA1c value for diagnosis is currently not recommended, in particular, because the allowed deviation for external quality control tests is ±18% to date in Germany. This value will be reduced to ±8% in the next years in the Rili-BAEK, then using HbA1c as a diagnostic criterion will improve significantly. The sensitivity of the laboratory tests for diagnosing diabetes is specified below in ascending order in relation to the oGTT, i.e. an oGTT should be performed to rule-out diabetes if the HbA1c values and fasting glucose values lie closely below the diagnostic criteria.
Oral glucose tolerance test (oGTT).
| 75 g oGTT in accordance with WHO guidelines |
| Performing the test in the morning – after 8–12 h fasting from food, nicotine and alcohol – after a ≥3-day diet rich in carbohydrates (≥150 g CHO per day) – sitting or lying (no muscular exertion); no smoking before or during the test |
| At point in time 0 drink 75 g glucose (or equivalent amount of hydrolysed starch) in 250–300 mL of water within 5 min – children 1.75 g/kg (maximal 75 g) – venous blood collection at points in time 0 and 120 min – proper sample processing and storage |
| Test is contraindicated for intercurrent diseases, such as status after gastrointestinal resection or gastrointestinal illness with changed resorption or if diabetes mellitus has already been diagnosed |
| Preparation of the glucose solution by the physician instead of the manufacturer is rejected by the DDG for reasons of liability and for medical reasons; see statement by KLD and AGDT on the DDG website (www.deutsche-diabetes-gesellschaft.de) |
DDG, Deutsche Diabetes Gesellschaft; WHO, World Health Organization; KLD, Kommission Labordiagnostik in der Diabetologie; AGDT, Arbeitsgemeinschaft Diabetes & Technologie.
How should the diagnostic value of a single measurement be assessed in consideration of the variability of measurement results?
For measurement results of these measurands, the question is whether the deviation from the diagnostic criterion (or cut-off value) exceeds this value to such an extent [i.e. larger than the minimal difference (see below)] that this can clearly be considered elevated. If this is the case, one individual measurement is sufficient to confirm diabetes diagnosis. For two values diverging the cut-off value (above and below), the American Diabetes Association (ADA) recommends taking the higher value. This value should be repeated and becomes the deciding factor for the diagnosis. If clinically necessary, verification should be performed in time, e.g. after 3 or 6 months. The procedure for diagnosing diabetes (as published by the ADA 2018) is as follows: “If a parameter is used and then repeated to confirm the diagnosis of diabetes, it must be ensured that each blood sample was standardized and comparably processed”. How well this is realized for plasma glucose is not simply verifiable. If the diabetes diagnosis is based on an HbA1c measurement, the confirmation measurement with the same measurand is not advisable. Due to analytical differences between laboratories the HbA1c measurement is currently not very well reproducible. Additionally, a “false” value may be obtained repeatedly due to the same patient-specific influencing factors. Independent of the measurand used for diabetes diagnosis, the result may be incorrect resulting from patient-specific influencing factors and/or insufficient measurement accuracy. A questionable diabetes diagnosis should therefore be confirmed using the other measurand (i.e. either glucose or HbA1c) in order to reduce interference or influencing factors. A measurement result which is used as a basis for diagnosis should be confirmed so that the diagnosis is made on the basis of confirmed values. It can be confirmed either by analysing a new blood sample using the same measurand (e.g. within 14 days) or by using one of the three measurands described above. If, during a second determination of the same laboratory test, there is a discrepancy in the value with regard to the cut-off value, one of the alternatives should be used for determination. If there are disparities in the results with regard to the diagnostic criterion in two different measurands, the higher value should be confirmed. If the values are close to the limit values, they should be monitored within 3–6 months again.
Pre-analytics of glucose measurement
Proper pre-analytic handling of the blood sample is crucial. Suitable blood collection tubes must be used to completely inhibit glycolysis in the collected blood. The addition of citrate and fluoride is necessary as fluoride alone is insufficient. The glycolytic inhibitor tubes for blood collection currently available on the German market exhibit different handling problems (Table 3). One alternative recommendation for tubes without glycolytic inhibitors is to centrifuge the sample as soon as possible after blood collection. If a period of 30 min to centrifugation is exceeded, samples should be discarded due to the occurence of glycolysis. After centrifugation, the resulting plasma supernatant must be separated from the blood cells. This occurs during centrifugation using a gel (gel tubes) or mechanical separators. It is also possible to pipette off the plasma supernatant immediately after centrifugation. Diligent and optimal pre-analytical handling of the blood collection tubes can result in a higher rate of diabetes diagnosis in practice and should not be considered over-diagnosing; however, the cut-off values used below may require adjustment and should be verified with corresponding studies.
Blood collection tubes commercially available in Germany which completely inhibit glycolysis with the addition of fluoride and citrate (current status 17.7.2017, see home pages of the respective manufacturers).
| Manufacturer | Product name | Correct filling absolutely necessary | Sufficient mixing required | Correction factor |
|---|---|---|---|---|
| Greiner bio-one | Vacuette® FC-Mix | No | 10 times | No (powder) |
| Kabe | Primavette®, KABEVETTE® | Yes | Few times | 1.16 (liquid additive) |
| Sarstedt | S-Monovette GlucoEXACT® | Yes | Few times | 1.16 (liquid additive) |
The blood collection tubes made by the company Greiner bio-one (Vacuette® FC-Mix) contain granulate. The tubes must be gently inverted 10 times to adequately dissolve the additive and mix the sample with the glycolytic inhibitors. Experience has shown that dilution errors occur when using blood collection tubes by the company Sarstedt (S-Monovette® GlucoEXACT) and Kabe (Primavette®, KABEVETTE®) if the tubes have not been filled completely. The laboratory has to identify such types of tubes to, on the one hand, identify tubes that do not meet the filling requirements specified by the manufacturer and exclude them from the analysis, and, on the other hand, take the dilution factor of 1.16 into account.
HbA1c for diagnosis purposes
Since 2010, the German Diabetes Association (“Deutsche Diabetes Gesellschaft”, DDG) has recommended the use of the HbA1c value for diabetes diagnosis (see the statement on the DDG homepage). This was made possible by improvements in measurement accuracy resulting from international standardization of HbA1c measurement methods. Simultaneously, epidemiological studies in recent years have shown that the specificity of an HbA1c value of ≥6.5% or >48 mmol/mol is sufficiently high to diagnose diabetes with a satisfactory level of certainty. At the same time, the sensitivity of an HbA1c value of <5.7% (<39 mmol/mol Hb) is sufficiently high to rule out the diagnosis of diabetes. For patients with HbA1c values in the range of 5.7 to <6.5% (39 to <48 mmol/mol Hb) or with a high clinical risk (see Screening), the diagnosis of diabetes and its early stages can only be excluded by measuring plasma glucose in accordance with the usual criteria including an oGTT. The HbA1c value is not suitable for diagnosing diabetes if values can be expected to be influenced or falsified (Table 4; see Practical Recommendation Glucose Monitoring and Control Testing for details on methodology). It is also important to note that regardless of successful standardization, measurement accuracy of HbA1c can vary considerably depending on the measurement method. In our opinion, this problem significantly limits the exclusive use of HbA1c for the diagnosis of diabetes; for more details, see the practical recommendations next to Table 4 in the legend to Figure 1. In particular, the increase of HbA1c that is associated with increasing age independent of diabetes, which can be up to an absolute value of 0.4–0.7% (4–8 mmol/mol Hb) constitutes another limitation in addition to differences due to methodology for using HbA1c to diagnose diabetes especially in the order below 7.0% (53 mmol/mol Hb).
Contributing factors leading to an influence (labeled with a) or falsification (labeled with b) of the HbA1c value.
|
Quality control checks
Internal quality control must be performed every working day with suitable control material. Successful participation in EQA is required once per quarter. This applies to all laboratory systems as well as to POCT “unit use” systems (single test strips or cuvettes as per the definition of Rili-BAEK) which are designated appropriate for diagnosis by the manufacturer.
Minimal difference
In order to meet the clinical requirements, the analytical variability of the absolute values at decision limits should be specified. The “minimal difference (MD)” is a simple tool to demonstrate the significance of random errors to the user and is calculated from the standard deviation (SD) (MD=2×SD) (Figure 2). The MD gives specific concentrations as absolute values to specify if a measurement result differs from a cut-off value with diagnostic relevance. The MD should not be larger than 12.6 mg/dL (0.7 mmol/L) for a fasting glucose limit value of 126 mg/dL (7.0 mmol/L). Accordingly, for the HbA1c cut-off value of 48 mmol/mol Hb, the MD should not be larger than 1.9 mmol/mol Hb. A statement on the subject of MD can be found on the DDG homepage and will be published separately soon.
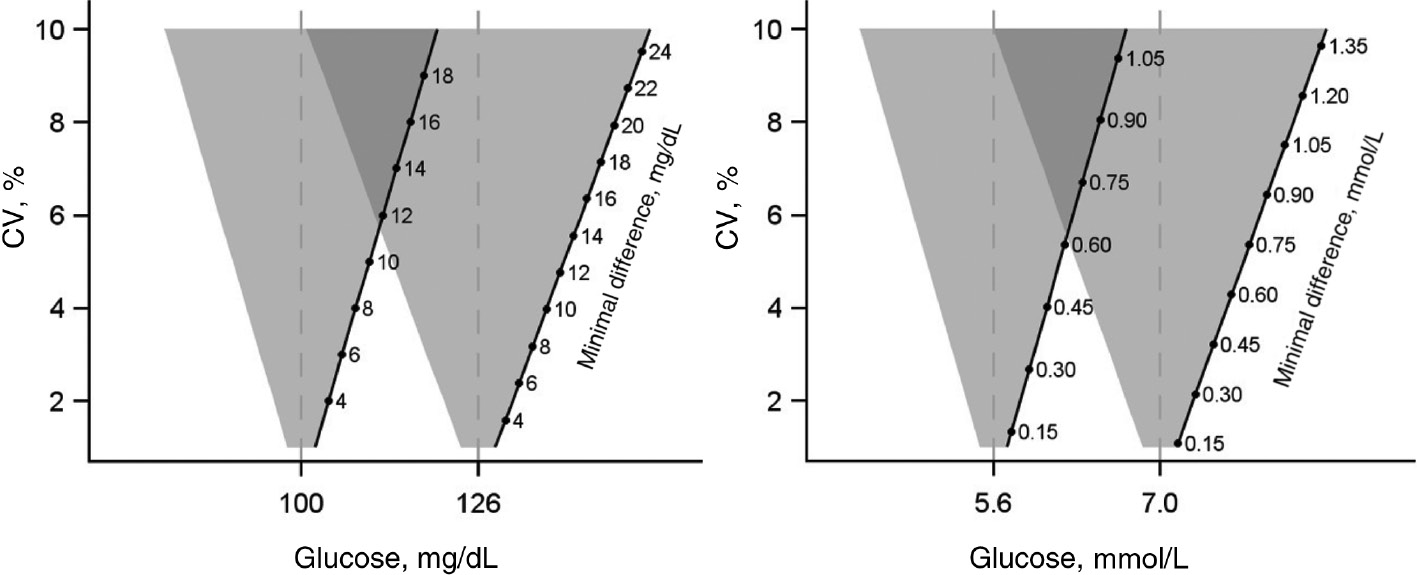
Minimal difference, given in the unit of glucose determination (mg/dL or mmol/L) for the considered diagnostic cut-off value depending on the coefficients of variation.
If the measured values lie in the overlapping areas of the inverted triangles, the diagnostic limit values cannot be differentiated from each other and the measured values cannot be used for diagnosis.
Screening
For primary screening for diabetes either a “Diabetes Risk Test” (http://www.dife.de/diabetes-risiko-test/) or occasional measurement results using venous plasma is recommended. In the case of elevated risk scores detected by this questionnaire, documented cardiovascular disease, presence of excess weight with additional risk factors such as hypertension, dyslipidemia (increased triglyceride values or low HDL cholesterol value) or a positive family history of type 2 diabetes in first-degree relatives, gestational diabetes or polycystic ovarian (PCO) syndrome or non-alcoholic fatty liver disease proceed as described in Figure 1. Alternatively, the FINDRISK questionnaire can also be used (https://www.diabetesstiftung.de/findrisk).
Gestational diabetes
The cut-off values for oGTT stated in Table 5 are based on the results of the hyperglycemia and adverse pregnancy outcome (HAPO) Study. They differ only slightly from the previously used decision limits. However, now already one value exceeding the decision limit suffices to confirm the diagnosis while previously two elevated values were required.
Diagnosis of gestational diabetes.
| Venous plasma | ||
| mg/dL | mmol/L | |
| Fasting | ≥92 | ≥5.1 |
| 60 min | ≥180 | ≥10.0 |
| 120 min | ≥153 | ≥8.5 |
Diabetes is confirmed if one criterion is met. For the pre-analytics of glucose determination, reference is made to the guideline for gestational diabetes; a sufficient inhibition of glycolysis is necessary.
Internet addresses
http://www.deutsche-diabetes-gesellschaft.de
Current version of evidence-based guidelines
Information systems on diabetes
Summary
The present recommendation summarizes the current knowledge and state of the art for the diagnosis of diabetes mellitus. With emphasis on well-described but often neglected pitfalls like measurement imprecision, pre-analytical considerations of glucose measurement and limitations of HbA1c assessment, this work is intended to improve patient care and to draw attention of physicians to potential drawbacks in diabetes diagnosis.
Author contributions: All the authors have accepted responsibility for the entire content of this submitted manuscript and approved submission.
Research funding: None declared.
Employment or leadership: None declared.
Honorarium: None declared.
Competing interests: The funding organization(s) played no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; or in the decision to submit the report for publication.
Article note:
This article is an extended translation of the German-language recommendation published in Diabetologie 2017;12(Suppl 2):S94–100. This article is published with kind permission by Thieme.
©2018 Walter de Gruyter GmbH, Berlin/Boston
Articles in the same Issue
- Frontmatter
- Mini Review
- Is the hemolysis index always suitable for monitoring phlebotomy performance?
- Original Articles
- Definition, classification and diagnostics of diabetes mellitus
- Laboratory medicine contributions to patient blood management concepts
- Infectiology and Microbiology
- Genetic characterization of influenza A(H3N2) viruses from 2014 to 2017 in Yantai, east of China
- Allergy and Autoimmunity
- Assessment of dynamic thiol/disulfide homeostasis in patients with asthma
Articles in the same Issue
- Frontmatter
- Mini Review
- Is the hemolysis index always suitable for monitoring phlebotomy performance?
- Original Articles
- Definition, classification and diagnostics of diabetes mellitus
- Laboratory medicine contributions to patient blood management concepts
- Infectiology and Microbiology
- Genetic characterization of influenza A(H3N2) viruses from 2014 to 2017 in Yantai, east of China
- Allergy and Autoimmunity
- Assessment of dynamic thiol/disulfide homeostasis in patients with asthma

