Simultaneous identification and quantification of synthetic cannabinoids (cannabimimetics) in serum, hair, and urine by rapid and sensitive HPLC tandem mass spectrometry screenings: overview and experience from routine testing
Abstract
Detection and quantification of synthetic cannabinoids (synonym: cannabimimetics) used as a substitute for natural cannabis has been a real toxicological and forensic issue since 2008. On the basis of a short overview of the pharmacological principle, chemical classification, and legal situation in Germany, the development of several analytical and screening approaches is presented. The paper further describes and validates a novel method for the simultaneous identification and quantification of JWH-018, JWH-019, JWH-073, JWH-081, JWH-122, JWH-200, JWH-210, JWH-250, WIN48,098, WIN55,212-2, AM-694 and CP47,497 by means of liquid chromatography-tandem mass spectrometry (LC-MS/MS) in human serum and hair using JWH-018-d11 and THC-d3 as internal standards. Detection limits ranging from 0.018 to 0.192 ng/mL for serum and from 0.140 to 0.820 pg/mg for hair, as well as very short retention times (up to 2.4 min), qualify this method, which presently includes 11 further compounds on a semi-quantitative basis, for rapid screening. The same method, using the deuterated 3-hydroxybutyl derivative of JWH-073, was applied for detection of 14 urinary metabolites after enzymatic hydrolysis of conjugates. Observation from routine analysis of 359 serum and 84 hair specimens reveals that concentrations of cannabimimetics in positive samples may differ by factors of at least 400 and 3000 with median values of 0.5 ng/mL and 9 pg/mg for serum and hair, respectively. Conclusions regarding consumption behaviors and strategies to decipher metabolic routes of these substances and possible implications are also discussed.
Zusammenfassung:
Der Nachweis und die Quantifizierung synthetischer Cannabinoide (Synonym: Cannabimimetika), die in Form von Räuchermischungen als Cannabisersatz in Gebrauch sind, ist seit 2008 ein aktuelles Thema der toxikologischen und forensischen Analytik. Auf der Grundlage einer kurzen Übersicht über das pharmakologische Prinzip, die chemische Klassifikation und die gesetzliche Situation in Deutschland wird die Entwicklung verschiedener Screeningmethoden dargestellt. Es wird eine neue Methode zur simultanen Quantifizierung von JWH-018, JWH-019, JWH-073, JWH-081, JWH-122, JWH-200, JWH-210, JWH-250, WIN48,098, WIN55,212-2, AM-694 und CP47,497 in Serum und Haaren mittels Flüssigchromatographie-Tandem-Massenspektrometrie (LC-MS/MS) mit JWH-018-d11 und THC-d3 als internen Standards beschrieben und validiert, die aufgrund der Nachweisgrenzen von 0.018 bis 0.192 ng/mL im Serum und 0.140 bis 0.820 pg/mg Haar sowie der besonders kurzen Retentionszeiten (maximal 2,4 Minuten) als Screeningmethode geeignet ist. Die Methode inkludiert derzeit 11 weitere Substanzen auf semiquantitativer Basis und ist nach enzymatischer Hydrolyse der Glucuronide unter Verwendung des deuterierten 3-Hydroxybutyl-Metaboliten von JWH-073 als internem Standard für die Suche nach 14 Urinmetaboliten verwendbar. Eine konsekutive Analytik der Routineanalytik von 359 Serum- und 84 Haarproben ergibt, dass die gemessenen Konzentrationen in positiven Proben um einen Faktor von 400 (Serum; Median 0.5 ng/mL) bzw. 3000 (Haare; Median 9 pg/mg) differieren können. Mögliche Rückschlüsse auf das Konsumverhalten und Ansätze zur Aufklärung von Stoffwechselrouten und ihre Bedeutung werden abschlieβend diskutiert.
Rezensierte Publikation:
Steimer W.
Introduction
Δ9-Tetrahydrocannabinol (THC), the pharmacological principle of cannabis (marijuana), is the best-known exogenous psychoactive cannabinoid with its effects being known for many centuries. As a recreational drug, THC is used in the form of chopped parts or pressed resin (hashish) of female plants [1]. For pharmaceutical purposes, THC can be extracted by solvents from respective plant material with the consequence that additional psychoactive compounds are likewise present as in, for example, nabiximols which is used as supportive treatment of spasticity in patients suffering from multiple sclerosis [2]. By contrast, semi-synthetically produced THC is used in certain countries as a pharmaceutical agent, such as dronabinol for treatment of hyperemesis and appetite loss for patients suffering from the AIDS wasting syndrome or side effects of chemotherapy [2, 3].
The search for molecular transducers has led to the detection and cloning of two subtypes of cannabinoid receptors, namely CB1 (with two subpopulations) and CB2 [3, 4]. Both are predominantly coupled to heterotrimeric inhibitory G proteins with the consequence that receptor activation results in diminished cAMP accumulation, although the receptors express significant intrinsic (or constitutive) activity due to rab-dependent endocytic cycling [5, 6]. The CB1 receptor predominates in the central nervous system where it mediates retrograde signaling at GABAergic and glutaminergic synapses [7]. It mediates psychoactive effects such as modulation of emotional control, impairment of cognition and memory, or alterations in motor control [8–10]. Neuroprotective effects are currently discussed. Furthermore, receptor activation improves appetite and weight gain, so that it can be expected that blockade of the CB1 receptor system leads to appetite loss. This has been successfully tried for overweight patients with the drug rimonabant until 2008, although obese patients did not benefit from rimonabant treatment in terms of progression of carotid atherosclerosis [11]. By contrast, CB2 receptors are mainly associated with different cells of the immune system and are expressed in the cell membrane of B lymphocytes but mostly intracellularly in T cells and monocytes [12]. Their agonistic ligands exert anti-inflammatory and immunosuppressive activity via modulation of cytokine release. A recent approach integrating both receptor systems focuses on cannabinoid modulation of neuroinflammatory diseases [13]. As could have been expected, a huge amount of non-natural cannabinoid receptor agonists (also known as cannabimimetics) were synthesized over the years in order to differentiate receptor systems by selectivities and potencies, to develop pain modulators, and, more recently, to find compounds with a potential to interact with the appetite regulation network [3, 8].
The heterogeneous expression of CB receptors enforces the presence of one or more endogenous physiological ligands. The search for candidate molecules finally resulted in the identification of anandamide (synonym: arachidonoyl ethanolamide) and related non-classical derivatives of (poly)unsaturated fatty acids such as 2-arachidonoylglycerol or oleamide [8, 14]. In addition to receptor-mediated neuroprotective and immunosuppressive effects, anandamide may similarly act through modulation of ion channels, of vanilloid TRPV receptors, or of peroxisome proliferator-activated receptors, which belong to the family of nuclear receptors [3, 8, 15]. From a pharmacological point of view, the local impact on anandamide synthesis and of anandamide degradation, which is predominantly catalyzed by fatty acid amide hydrolase [16], are promising approaches with regard to pain, appetite, and immune cell modulation [8].
Cannabimimetics have been synthesized for several years and have emerged on the market, which are officially marked “not for human consumption”, although, not officially, designed as recreational hallucinogenic drugs and distributed in headshops or, predominantly, on internet platforms. These preparations are usually herbal mixtures to which one or more synthetic cannabimimetics are added; one of the first brands was called “Spice” [17–20]. Initially, the search of active components focused on secondary plant ingredients such as salvinorin A before the principle became public [21]. The success of such preparations resulted, at least in part, from the fact that it was initially possible to circumvent positive results of routine immunoassay-based drugs of abuse testing after inhalation [22]. However, immunoassays for pretesting in urine are now available, for instance, by Mahsan Diagnostika GmbH (Reinbek, Germany), Immunalysis Corp. (Pomona, CA, USA), and Randox Laboratories (Crumlin, UK). Huge numbers of different substances have been synthesized with the consequence that active ingredients can be frequently and rapidly exchanged without relevant costs for (presumably non-European) manufacturers and (partly European) distributors. With regard to the legal situation in Germany, only a few (20 by December 12, 2012) of the relevant substances (637 according to [23]) are banned by the “Betäubungsmittelgesetz (BtmG)”, but it is presently trying to penalize the distribution of herbal mixtures according to the “Arzneimittelgesetz (AMG)”.
To date, these substances can be assigned to five major chemical groups: (i) so-called classical cannabinoids based on the dibenzopyran ring such as HU-210 (where HU stands for Hebrew University); (ii) non-classical cannabinoids based on the cyclohexylphenol series such as CP47,497; (iii) hybrid cannabinoids combining structural elements of both classical and non-classical cannabinoids such as AM-4030 (where AM stands for Alexandros Makriyannis); (iv) aminoalkylindoles (subgroups consist of naphthoylindoles, phenylacetylindoles, naphthylmethylindoles, and benzoylindoles) such as JWH-018 (where JWH stands for J.W. Huffman and colleagues); and (v) miscellaneous compounds such as oleamide [17, 18, 20]. Some of these structures are shown in Figure 1. Whereas numerous analogs have binding affinities to CB1 receptors comparable to that of THC (i.e., Ki∼10–8 mol/L), others express Ki value as low as 10–10 mol/L [18]. Because the pharmacokinetic behavior of most of these substances is still unknown, individual effects are rarely predictable, and, accordingly, severe intoxications and exacerbations of psychotic episodes have been described [18, 24, 25] with anxiety as one prominent symptom. It is interesting to note that, according to the United Nations Office on Drugs and Crime Report on Synthetic Cannabinoids in Herbal Products, the majority (68%) of the 19 compounds mentioned have been first identified in Germany [17].
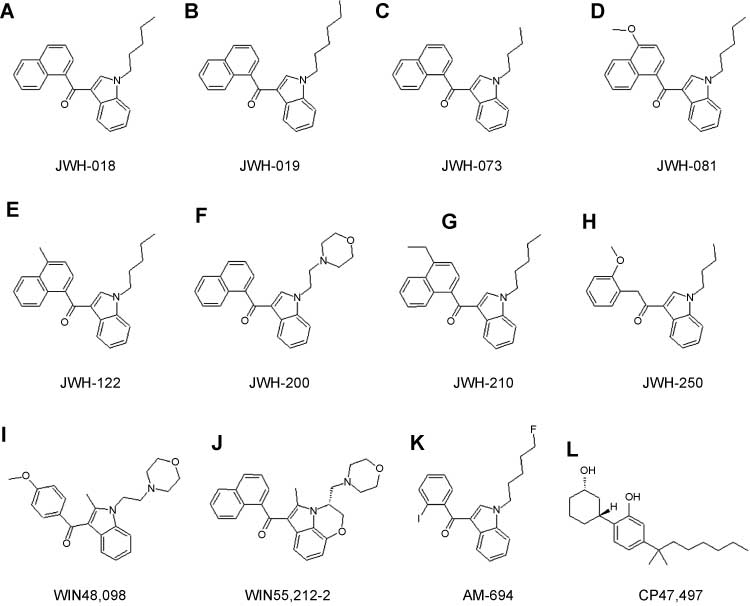
Chemical structures of the synthetic cannabinoids used for method validation in this work.
In view of the increasing consumption of herbal mixtures containing synthetic cannabinoids and continuous release of novel constituents with slightly modified substituents or side chains, it appears necessary to have methods available which are suitable for rapid screening to detect both short- and long-term abuse (e.g., in emergency as well as in detoxification units, for investigations of driving qualification, and in occupational health problems). In addition to analyses of the mixtures themselves, a few (but continuously increasing in number) methodological approaches to human matrices have been published to date. These include HPLC-MS/MS (high-pressure liquid chromatography-tandem mass spectrometry) for JWH-018 in human serum [26], HPLC-MS/MS for eight JWH substances and methanandamide in serum [27], HPLC-MS/MS for JWH-018, JWH-073, JWH-019, and JWH-250 in human blood [28], as well as HPLC-MS/MS for CP47,497-C8 and five species of the JWH series in hair [29]. Pursuing the single-sample/multiple injection strategy for simultaneous detection of great numbers of drugs by HPLC-tandem mass spectrometry [30, 31], it has become recently possible to analyze 30 synthetic cannabinoids in serum [32] and 22 substances in hair [33].
The analysis of synthetic cannabinoids in urine constitutes a particular challenge. This results from the fact that the original compounds are hardly detectable in urine. Rather, a variety of hydroxylated and eventually carboxylated derivatives are to be expected with the exact position of substituents usually not yet defined. Most of the hydroxylated derivatives are further conjugated by hepatic phase II biotransformation. Nevertheless, several methodological approaches have recently been developed for urine matrix as well [22, 34–37]. In this context, we felt forced to develop and validate a screening method comprising a variety of common synthetic cannabinoids in serum and hair, as well as corresponding hydroxyl metabolites in urine with particular emphasis to further shorten necessary run times.
Materials and methods
Specimens
Serum, hair, and/or urine specimens were sent from other laboratories, hospitals, or withdrawal clinics. Control material was provided by volunteers, including the authors, from the laboratory.
Chemicals, analytical standards, and reagents
Standard substances used for complete method validation in serum and hair, that is, JWH-018, JWH-019, JWH-073, JWH-081, JWH-122, JWH-200, JWH-210, JWH-250, WIN48,098, WIN55,212-2, AM-694, and CP47,497 (Figure 1) were purchased from THC Pharm (Frankfurt, Germany). The deuterated internal standard JWH-018-d11 was from Chiron AS (Trondheim, Norway), whereas THC-d3 was from Radian International (Austin, TX, USA) and supplied by LGC Standards (Wesel, Germany). Further standard substances used for semi-quantitative reports due to incomplete validation, that is, UR-144, AM-1220, JWH-398, AB-001, AM-2201, RCS-04, RCS-08, JWH-307, MAM-2201, AM-1248, and AKB-48, as well as all hydroxylated urine metabolites (Table 1) were from Cayman Chemical Company (Ann Arbor, MI, USA) and purchased from LGC Standards. Acetic acid (anhydrous for analysis), methanol (for analysis), sodium carbonate, and sodium hydroxide were supplied by Merck (Darmstadt, Germany), ethyl acetate and n-hexane by Sigma-Aldrich (Taufkirchen, Germany), and LC-MS grade methanol by VWR International (Darmstadt, Germany).
Monohydroxylated metabolite standards used for analysis of cannabimimetics in urine matrix.
| Parent compound | Metabolite |
|---|---|
| JWH-018 | (1-(4-Hydroxypentyl)-1H-indol-3-yl)(naphthalen-1-yl)methanone |
| JWH-019 | (1-Hexyl-5-hydroxy-1H-indol-3-yl))naphthalen-1-yl)methanone |
| JWH-073 | (1-(3-Hydroxybutyl)-1H-indol-3-yl)(naphthalen-1-yl)methanone |
| JWH-081 | (1-(5-Hydroxypentyl)-1H-indol-3-yl)(4-methoxynaphthalen-1-yl)methanone |
| JWH-122 | (1-(4-Hydroxypentyl)-1H-indol-3-yl)(4-methylnaphthalen-1-yl)methanone |
| JWH-200 | 4-Hydroxy(1-(2-(4-morpholinyl)ethyl)-1H-indol-3-yl)-1-naphthalenyl-methanone |
| JWH-210 | (4-Ethylnaphthalen-1-yl)(1-(4-hydroxypentyl)-1H-indol-3-yl)methanone |
| JWH-250 | 1-(1-(4-Hydroxypentyl)-1H-indol-3-yl)-2-(2-methoxyphenyl)ethanone |
| JWH-398 | (4-Chloronaphthalen-1-yl)(1-(5-hydroxypentyl)-1H-indol-3-yl)methanone |
| RCS-4 | (1-(4-Hydroxypentyl)-1H-indol-3-yl)(4-methoxyphenyl)methanone |
| AM-2201 | (1-(5-Fluoro-4-hydroxypentyl)-1H-indol-3-yl)(naphthalene-1-yl)methanone |
| XLR-11 | (1-(5-Fluoro-4-hydroxypentyl)-1H-indol-3-yl)(2,2,3,3-tetramethylcyclopropyl)methanone |
| UR-144 | (1-(4-Hydroxypentyl)-1H-indol-3-yl)(2,2,3,3-tetramethylcyclopropyl)-methanone |
| MAM-2201 | (1-(5-Fluoro-4-hydroxypentyl)-1H-indol-3-yl)(4-methylnaphthalene-1-yl)methanone |
| JWH-073-d5a | (1-(3-Hydroxybutyl)-1H-indol-3-yl)(naphthalen-1-yl)methanone-2,4,5,6,7-d5 |
aDepicts the deuterated internal standard.
Preparation of standard solutions and calibrators
As-received stock solutions of JWH-018, JWH-019, JWH-073, JWH-081, JWH-122, JWH-200, JWH-210, JWH-250, WIN48,098, WIN55,212-2, AM-649, and CP47,497 and further compounds (usually c=1 mg/mL methanol) were diluted in methanol (1:10). Then, 10 μL of each of these 12 solutions was further added to 1.88 mL methanol to give a standard working solution with a concentration of 500 ng/mL for each analyte. From this working solution (which was also produced if compounds were provided as dry substance), two more working solutions were prepared with final concentrations of 50 and 5 ng/mL, respectively. Similarly, JWH-018-d11 and THC-d3 were used as internal standards by further diluting the respective stock solutions to a final concentration of 100 ng/mL methanol.
Sample preparation: serum
For calibration, standard solutions (six points ranging from 50 pg to 2 ng absolute amount yielding final concentrations of 0.25, 0.5, 1.0, 2.5, 5.0, and 10 ng/mL, plus zero value) together with 20 μL of internal standard mixture were transferred into 2-mL polypropylene vials and gently evaporated to dryness. Analyte-free serum (200 μL) and 100 μL of saturated sodium bicarbonate solution were added. Similarly, 200 μL of unknown specimen plus 100 μL sodium bicarbonate were added to evaporated internal standard. Liquid-liquid extraction was then performed twice with 900 μL hexane/ethyl acetate (9+1 by volume) by rigorous shaking for 3 min. After centrifugation and evaporation of the organic phase, samples were reconstituted in 100 μL of methanol/ammonium acetate buffer (5 mmol/L, pH 3.9) 4+1 by volume and transferred to autosampler vials, from which 10 μL was injected into the LC-MS/MS.
Sample preparation: hair
Hair was washed by sonicating in acetone for 10 min, in distilled water for 5 min, again in acetone for 5 min, and finally dried at 50°C. All extracts were stored separately. Aliquots of fine-cut hair (∼50 mg) were weighed into 10-mL tubes. Known analyte-free hair was spiked with the respective standard solutions (six points ranging from 250 pg to 1.5 ng absolute amount yielding final concentrations of 5, 10, 15, 20, 25, and 30 pg/mg, plus zero value), and 10 μL of internal standard solution was added to all samples. Then, 1 mL sodium hydroxide (1 mol/L) was added, and the solution was heated under reflux at 96°C for 10 min. The fluid phase was extracted with 5 mL hexane/ethyl acetate (9+1 by volume) by mixing for 2 min. After centrifugation and evaporation of the organic phase under nitrogen, samples were reconstituted in 100 μL methanol/ammonium acetate buffer and transferred to autosampler vials, from which 10 μL was injected into the LC-MS/MS.
Sample preparation: urine
To 1.5 mL of urine, 50 μL β-glucuronidase (type H2 from Helix pomatia, Sigma-Aldrich) in phosphate buffer (pH 6.0) together with 25 ng of JWH-073 N-(3-hydroxybutyl)-d5 as the internal standard (Cayman Chemical Company, supplied by LGC Standards) were added. Hydrolysis of conjugates was achieved by incubation at 56°C for 1 h. After cooling, samples were adjusted to pH 9.0 and extracted with diethyl ether. The organic phase was transferred to a glass vial and processed as described above. All biological specimens were handled in a separate room with no contamination by stock standard solutions.
Apparatus and conditions
LC-MS/MS analyses were performed using an API 4000 tandem mass spectrometer (Applied Biosystems/Sciex, Darmstadt, Germany) with coupled turbo ion spray interface in both positive and negative MRM mode. The HPLC system consisted of a 1100 series binary pump (Agilent, Waldbronn, Germany) and a HTC-PAL autosampler (CTC-Analytics, Zwingen, Switzerland). Chromatographic separation was accomplished on an Onyx Monolithic C18, 50 mm×4.6 mm column (Phenomenex, Aschaffenburg, Germany). Data acquisition and evaluation were done using Analyst V.1.4 software (Applied Biosystems/Sciex).
Eluents were composed of methanol/1% acetic acid with a phase composition of 80+20 (by volume) for positive, and 90+10 (by volume) for negative MRM mode, respectively. General adjustments for MS were as follows: curtain gas, 30 units; nitrogen gas 1, 40 units; nitrogen gas 2, 50 units; and turbo spray temperature, 450°C. In positive and negative (for CP47,497 solely) MRM modes, CAD gas (nitrogen) was set to 4 and 6 units, ionization voltage was 4600 and –4500 V, entrance potential was 10 and –10 V, and dwell time was 20 and 50 ms, respectively. The optimized parameters for each (validated and non-validated) analyte, together with the MRM transitions chosen for the qualifier and quantifier transitions, are listed in Table 2. For routine analyses in serum and hair, triple injections were necessary: the first one for the 11 validated compounds in the positive MRM mode, the second one for the 11 non-validated compounds in the positive MRM mode (in order to achieve sufficient registration points per chromatographic signal), and the third one for CP-47,497 in the negative MRM mode. Overall run times are 5.0 min for serum and hair extracts (last eluting substance at 2.4 min) and 8.5 min for urine extracts (last eluting substance at 5.8 min).
Acquisition parameters: mass transitions and retention times for validated analytes (group I), non-validated analytes (group II), and internal standards (group III).
| Analyte | Qualifier transition | Quantifier transition | DP, V | CE, V | CXP, V | tR, min |
|---|---|---|---|---|---|---|
| Group I | ||||||
| JWH-018 | 342→127 | 342→155 | 36 | 65/35 | 22/8 | 1.63 |
| JWH-019 | 356→127 | 356→155 | 111 | 65/35 | 6/10 | 1.93 |
| JWH-073 | 328→127 | 328→155 | 81 | 61/31 | 8/10 | 1.40 |
| JWH-081 | 372→127 | 372→185 | 86 | 77/37 | 8/12 | 1.75 |
| JWH-122 | 356→141 | 356→169 | 101 | 63/35 | 10/8 | 1.90 |
| JWH-200 | 385→114 | 385→155 | 111 | 35/33 | 18/34 | 0.92 |
| JWH-210 | 370→155 | 370→183 | 96 | 55/37 | 14/12 | 2.19 |
| JWH-250 | 336→91 | 336→121 | 51 | 65/33 | 4/8 | 1.37 |
| WIN48,098 | 379→114 | 379→135 | 76 | 51/31 | 8/24 | 0.88 |
| WIN55,212-2 | 427→127 | 427→155 | 61 | 75/33 | 8/8 | 1.15 |
| AM-694 | 436→203 | 436→231 | 96 | 63/37 | 8/16 | 1.06 |
| CP47,497 | 317→245 | 317→299 | –140 | –45/–35 | –4/–6 | 1.12 |
| Group II | ||||||
| UR-144 | 312→125 | 312→55 | 101 | 33/61 | 8/8 | 1.99 |
| AM-1220 | 383→112 | 383→98 | 131 | 33/45 | 4/16 | 0.72 |
| JWH-398 | 376→189 | 376→126 | 126 | 39/101 | 10/22 | 2.31 |
| AB-001 | 351→135 | 351→93 | 91 | 41/65 | 10/16 | 2.87 |
| AM-2201 | 360→155 | 360→127 | 96 | 37/69 | 8/6 | 1.18 |
| RCS-04 | 322→135 | 322→77 | 96 | 35/73 | 6/12 | 1.39 |
| RCS-08 | 376→121 | 376→91 | 96 | 35/75 | 20/16 | 2.09 |
| JWH-307 | 386→155 | 386→127 | 96 | 29/73 | 8/22 | 1.80 |
| MAM-2201 | 374→169 | 374→141 | 116 | 37/61 | 10/24 | 1.32 |
| AM-1248 | 391→135 | 391→112 | 141 | 45/45 | 8/20 | 0.75 |
| AKB-48 | 366→135 | 366→93 | 96 | 27/73 | 10/16 | 3.18 |
| Group III | ||||||
| JWH-018 d11a | None | 353.4→155 | 96 | /33 | /12 | 1.58 |
| THC d3a | None | 315.9→247.9 | –120 | –36 | –13 | 1.29 |
DP, declustering potential (first number stands for qualifier, second number stands for quantifier transitions, respectively); CE, collision energy; CXP, collision cell exit potential; tR, retention time; adepicts deuterated internal standards. Retention times may differ slightly between different column charges.
Method validation
Limits of detection and of quantification
The limit of detection (LOD) and limit of quantification (LOQ) were evaluated in parallel by signal-to-noise ratio and a validation software called “B.E.N.” which is derived from the German Industry Standard DIN32645 and is certified by the “Gesellschaft für Toxikologische und Forensische Chemie” [38]. LOD and LOQ were calculated from the triple of the noise value for the qualifier transitions and the decuple of the noise value for the quantifier transitions at concentration of 0.25 ng/mL for serum and 2.5 pg/mg for hair. Software-based calculations were performed using five calibrators ranging from 0.05 to 0.5 ng/mL for serum and from 0.5 to 5 pg/mg for hair, respectively.
Imprecision and inaccuracy
Intra-day and inter-day imprecision experiments were performed for both serum and hair to determine repeatability with at least two different concentrations (10, 2.0, and, in certain series, 0.5 ng/mL serum, and 25 and 5 pg/mg hair) with ten samples per concentration analyzed either consecutively within one run or at different working days spanning a period of 5 weeks. On the basis of the individual data, inaccuracies were calculated as deviation of the respective matrix-based target value.
Selectivity and linearity
For selectivity, it was investigated whether concentrations of cannabimimetics in ten different serum and six different hair specimens obtained from volunteers were below the LOD. With respect to the wide range of possible results, linearities were determined in serum and hair using two measuring ranges. For this purpose, serum samples were spiked with either nine concentrations ranging from 0.01 to 1.0 ng/mL (range I, final concentrations) or six concentrations ranging from 0.25 to 10 ng/mL (range II). Similarly, hair specimens were spiked with either nine concentrations ranging from 0.1 to 10 pg/mg (range I) or six concentrations ranging from 5 to 30 pg/mg (range II).
Matrix effect and recovery
Matrix effect, recovery, and process efficiency were evaluated according to the method of Matuszewski et al. [39] by comparing the peak areas of differently prepared batches, that is, standard solutions in methanol (batch A), different lots of human serum or hair spiked with analytes after extraction (batch B), and human serum or hair spiked before extraction (batch C). Values were calculated at two concentration levels (2 and 10 ng/mL serum and 5 and 25 pg/mg hair) over an average of five samples per batch, where matrix effect (ME) (%) is defined as batch B/batch A×100, recovery (R) or extraction efficiency (%) is defined as batch C/batch B×100, and process efficiency (PE) (%) is defined as batch C/batch A×100. Alternatively, the term “ion suppression” instead of matrix effect can be used, which is – according to Buhrman et al. [40] – defined (in %) as 100 – batch B/batch A. Generally, identity was approved by comparing signal intensities of the quantifier and qualifier ions (Table 2) with a tolerance of ±20%.
Results and discussion
Chromatographic separation and proof of negative specimens
Separation of synthetic cannabinoids was performed by chromatography and mass transitions. When compared to data for serum (Figure 2) and hair samples (Figure 3) spiked with 11 compounds plus internal standard (respective panels A), peak areas in blank samples spiked with internal standard only (respective panels B) were all below the detection limit. Irrespective of the short retention times, an unequivocal assignment of signals was always possible (Figures 2 and 3, Table 2).
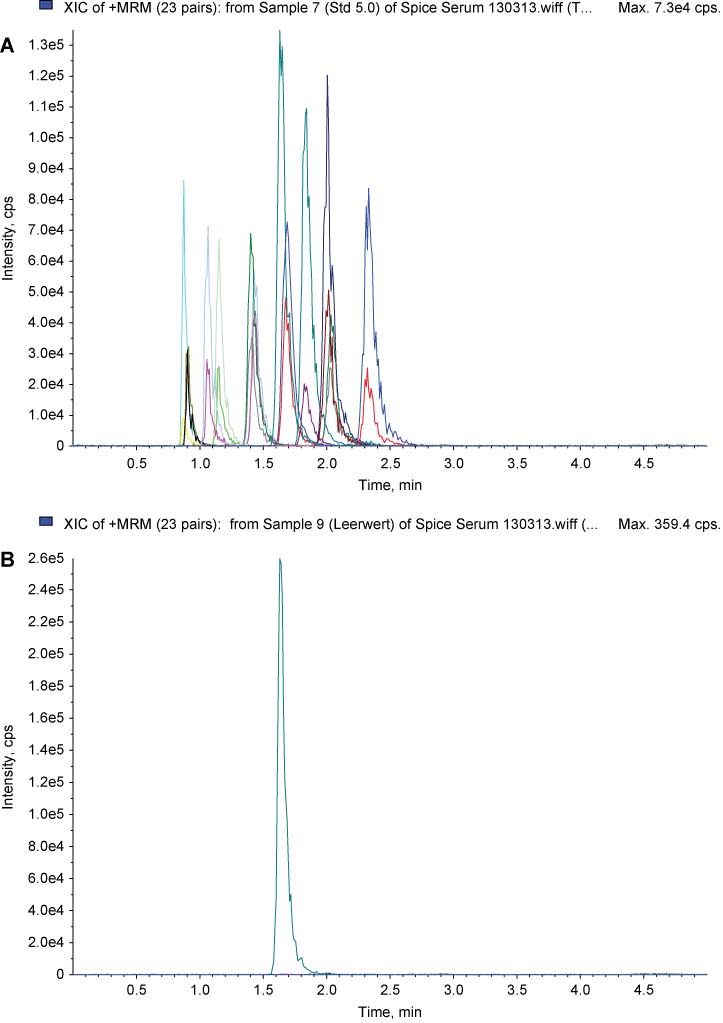
Extracted ion chromatograms of 23 positive MRM pairs (quantifier ions, qualifier ions, and internal standard JWH-018-d11) in pool serum spiked with 11 synthetic cannabinoids, 5.0 ng/mL each (A), and in pool serum blank with internal standard JWH-018-d11 (B).
For chromatographic conditions and structures of the synthetic cannabinoids, see text, Figure 1, and Table 2.
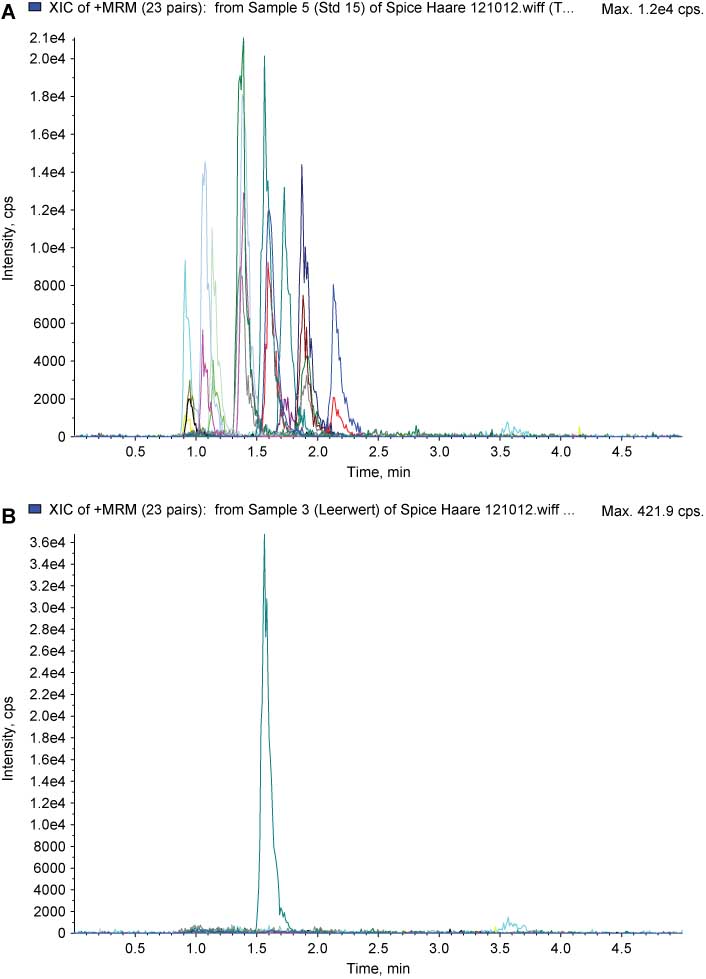
Extracted ion chromatograms of 23 positive MRM pairs (quantifier ions, qualifier ions, and internal standard JWH-018-d11) in hair sample spiked with 11 synthetic cannabinoids, 15 pg/mg each (A), and in hair sample blank with internal standard JWH-018-d11 (B).
For chromatographic conditions and structures of the synthetic cannabinoids, see text, Figure 1, and Table 2.
Thorough analyses were performed to exclude any possibility of false-positive results. Following conditions set forth by the German Society for Toxicological and Forensic Chemistry (GTFCh), especially spatial and personal separation of sample and standard handling, no positive results in control serum, hair, or urine specimens were detected. Several hair coloring/dying techniques, based on anamnestic exploration of volunteers, did not produce interfering signals. By nature, generation of false-negative results due to hair coloring cannot be excluded.
Methodological aspects: limits, imprecisions, linearities, and matrix effects for serum and hair matrix
LOD and LOQ values as calculated from signal-to-noise ratio and B.E.N. software are shown in Table 3. According to DIN32645, LOD and LOQ ranged from 0.018 to 0.192 ng/mL (0.084 if JWH-210 is considered as outlier) and from 0.062 to 0.708 ng/mL serum (0.304 if JWH-210 is considered as outlier). In tendency, LOD values calculated on the basis of the signal-to-noise ratio were slightly lower in most cases. In hair, variability between substances was similar with LOD and LOQ ranging from 0.140 to 0.820 pg/mg and from 0.760 to 2.870 pg/mg hair, respectively. With certain analytes, values for LOQ were lower than the actual values for LOD; in those cases, LOD and LOQ could be assigned to the same (higher) value. As a consequence, preliminary cut-off values (decision levels) were set at 0.1 ng/mL serum and 1 pg/mg hair. Results of the intra-day imprecision measurements (expressed as coefficient of variation=% relative standard deviation) are listed in Table 4. With the only exception of WIN48,098, all results met the acceptance criterion of <15%. Similarly, inter-assay imprecisions (CV) with serum ranged from 7.7% to 14.5% at 0.5 ng/mL, from 5.4% to 14.4% at 2.0 ng/mL, and from 5.7% to 14.6% at 10 ng/mL for tested cannabinoids, respectively, whereas inaccuracies varied from –1% to +24% (over all tested concentrations) with no systematic dependence on target concentrations, the only exception being again JWH-210 where inaccuracy ranged from 33% to 36%. At present, no explanation for this behavior is obvious; a possible contribution of interfering substances could be excluded by selectivity experiments. Values for hair matrix were in the same order of magnitude with inaccuracies between –27% and +3% (details not shown).
LOD and LOQ as calculated from signal-to-noise ratio and B.E.N. software according to DIN32645 for validated substances.
| Analyte | Calculated from | Serum | Hair | ||
|---|---|---|---|---|---|
| LOD, ng/mL | LOQ, ng/mL | LOD, pg/mg | LOQ, pg/mg | ||
| JWH-018 | DIN 32645 | 0.031 | 0.112 | 0.400 | 1.460 |
| s/n ratio | 0.014 | 0.094 | 0.497 | 1.471 | |
| JWH-019 | DIN 32645 | 0.063 | 0.216 | 0.250 | 0.860 |
| s/n ratio | 0.012 | 0.221 | 0.160 | 0.820 | |
| JWH-073 | DIN 32645 | 0.047 | 0.164 | 0.260 | 0.950 |
| s/n ratio | 0.036 | 0.200 | 0.355 | 1.553 | |
| JWH-081 | DIN 32645 | 0.065 | 0.240 | 0.140 | 0.760 |
| s/n ratio | 0.106 | (0.063) | 0.543 | 0.573 | |
| JWH-122 | DIN 32645 | 0.040 | 0.140 | 0.670 | 2.300 |
| s/n ratio | 0.023 | 0.027 | 0.118 | 0.450 | |
| JWH-200 | DIN 32645 | 0.018 | 0.062 | 0.490 | 1.740 |
| s/n ratio | 0.027 | 0.179 | 0.497 | 1.263 | |
| JWH-210 | DIN 32645 | 0.192 | 0.708 | 0.820 | 2.870 |
| s/n ratio | 0.040 | 0.046 | 0.862 | (0.497) | |
| JWH-250 | DIN 32645 | 0.053 | 0.186 | 0.220 | 0.830 |
| s/n ratio | 0.047 | 0.192 | 1.705 | (0.806) | |
| WIN48,098 | DIN 32645 | 0.084 | 0.304 | 0.650 | 2.260 |
| s/n ratio | 0.024 | 0.094 | 0.186 | 3.623 | |
| WIN55,212-2 | DIN 32645 | 0.070 | 0.242 | 0.210 | 0.780 |
| s/n ratio | 0.051 | 0.137 | 0.974 | 1.263 | |
| AM-694 | DIN 32645 | 0.050 | 0.171 | 0.350 | 1.240 |
| s/n ratio | 0.019 | 0.026 | 0.397 | 1.131 | |
| CP47,497 | DIN 32645 | 0.065 | 0.226 | 0.440 | 1.510 |
| s/n ratio | 0.035 | 0.177 | 0.560 | 0.602 | |
Values in parentheses represent the originally calculated LOQ value in cases whenever these were lower than the corresponding LOD value.
Imprecision within series for validated compounds (coefficient of variation,%).
| Analyte | Serum | Hair | ||
|---|---|---|---|---|
| 2 ng/mL | 10 ng/mL | 5 pg/mg | 25 pg/mg | |
| JWH-018 | 4.4 | 3.3 | 6.7 | 5.5 |
| JWH-019 | 5.7 | 5.1 | 3.7 | 5.5 |
| JWH-073 | 3.3 | 7.7 | 11.3 | 6.7 |
| JWH-081 | 4.9 | 2.5 | 11.3 | 4.0 |
| JWH-122 | 3.8 | 5.0 | 10.9 | 5.0 |
| JWH-200 | 11.6 | 11.0 | 9.6 | 11.0 |
| JWH-210 | 8.8 | 8.4 | 14.4 (n=9) | 7.5 |
| JWH-250 | 3.7 | 8.7 | 8.9 | 6.3 |
| WIN48,098 | 18.1 | 14.7 | 10.7 | 16.0 |
| WIN55,212-2 | 7.5 | 14.6 (n=9) | 10.5 | 11.8 |
| AM-694 | 5.2 | 6.8 (n=9) | 11.9 | 6.6 |
| CP47,497 | 13.3 | 5.7 | 7.0 | 8.1 |
Number of analyses per intra-assay was n=10 for all analytes. Differing numbers result from elimination of outliers defined on the basis of the mean ±4 s criterion and are given in parentheses.
As compared to data from the literature reported to date, imprecision measures reported herein are grossly similar to those obtained by Teske et al. and Kacinko et al. [26, 28] at least for JWH-018. Using a similar methodological approach, LOD values reported by Neukamm et al. [29] were, on average, by a factor 3 lower for hair matrix but by a factor of 10 higher for serum matrix as related to the method described here. It appears that – without any intention – the method developed in our laboratory, using the API4000 MS/MS instrument, favors analytical sensitivity for the serum rather than for the hair matrix, which appears useful in view of the concentrations reached in real samples (see below). In this context, it is worth mentioning that the technical approach developed herein allows fast runs (retention times up to around 2 min for extracts of serum and hair, and up to 6 min for urine metabolites) on the API4000 system. Previously documented methods are working with retention times ranging from 3 to 8 min [26, 28, 29, 32].
Testing for linearity yielded correlation coefficients of r>0.99 for both concentration ranges I and II in both serum and hair matrix (details not shown).
The absolute matrix effect for serum at low concentration (2 ng/mL) is, at least with the present approach, characterized by moderate ion suppression with matrix effect ranging from 67.5% to 87.8% for all analytes. At higher concentration (10 ng/mL), absolute matrix effects increase slightly, ranging from 100.7% to 118.2% indicating a slight decrease of ion suppression. Whereas the ion suppression effect with the method described herein for JWH-018 in serum is significantly lower than reported previously [26], the analyte concentration-dependent change in matrix effect has also been observed in previous studies [26, 28], In hair matrix, however, only ion suppression was found. Even at a low concentration (5 pg/mg) absolute matrix values range from 15.4% to 71.5%. Whether this is indeed a systematic effect needs to be clarified; nevertheless, deviations between the five different specimens used for each matrix were characterized by a relative standard deviation of <16%. Recoveries (i.e., extraction efficiencies) ranged from 33.7% to 93.7% for serum and from 61.6% to 104.9% for hair (values combined from two concentrations each for both matrices).
Results from real serum, hair, and urine specimens
Within the time period of October 2011 to April 2013, a total of 359 routine serum samples were analyzed. Of these, only 31 tested positive (values above the decision limit defined above) with a total of 52 positive results for individual cannabimimetics. The continuous evaluation of serial analyses reveals that most of the serum samples tested positive contain only one of the compounds considered, but around one-third contain two or more cannabimimetics (Figure 4). This finding may either result from consumption of a single product containing different active ingredients or from consumption of different products within a very short period. With this or similar statistics it has, however, to be realized that the spectrum of compounds detected by analytical procedures becomes continuously broader possibly leading to a “dilution” of detection probabilities for individual compounds. By contrast, analytical progress, legislative initiatives, and press or internet releases may influence consumers’ behavior and substance availability on the market. Respective changes, such as the disappearance of JWH-210 in samples by May 2012, or the appearance and disappearance of AB-001 between May and August 2012, can be followed by serial follow-up of results with the limitation that client populations are not randomly distributed over time (details not shown).
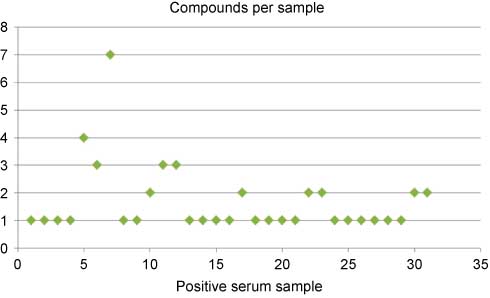
Number of different cannabimimetics in serum samples tested positive in consecutive time order within the period of October 2011 to April 2013. Around 9% of all routine specimens received were positive.
In serum samples, 11 different cannabimimetics have been found to date (Figure 5). Concentrations span a very wide range up to around 40 ng/mL. This value represents a dynamic range of a factor 400 relative to the decision level. Although such high levels are rare, they should be expected and must be covered by the analytical approach chosen. Including all substances detected to date, the median concentration amounts to around 0.5 ng/mL; this value is by a factor 5 higher than the decision level and by a factor 75 lower than peak concentrations.
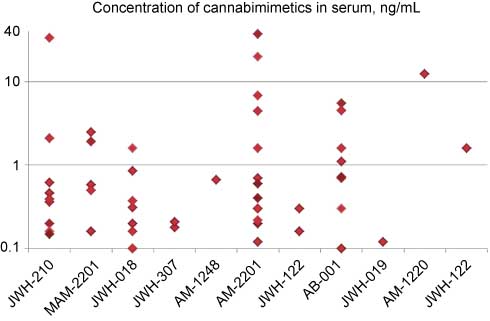
Concentrations of cannabimimetics in positive serum samples (same data pool as for Figure 4). Owing to the wide range, a logarithmic scale was applied for ordinate values.
In a parallel manner, a total of 84 hair samples have been tested between August 2011 and April 2013, of which 45 were positive with a total of 154 positive results for individual cannabimimetics. The finding that up to 13 different compounds can be detected in one individual sample attracts special attention (Figure 6). This observation allows the conclusion that consumers may change products rather frequently, either dependent on availability in headshops or electronic shops or “recommendations” in internet-based communities or personally by other users. From a clinical point of view, this finding draws attention to a high-risk behavior as far as consumers obviously change products rapidly and are presumably not aware of the highly different receptor affinities of the psychoactive components (although consumption behaviors would probably not change if consumers were aware of this risk). A limitation in the interpretation of positive hair samples results from the fact that, at least at present, only parent compounds are detected with the consequence that external airborne contamination cannot be excluded. This is in contrast to other drugs such as ethanol or cocaine, where a hepatic first-pass effect or a passage through the organism can be proven by the presence of the metabolites ethylglucuronide or benzoylecgonin (even more specific: norcocaine) in hair, respectively.
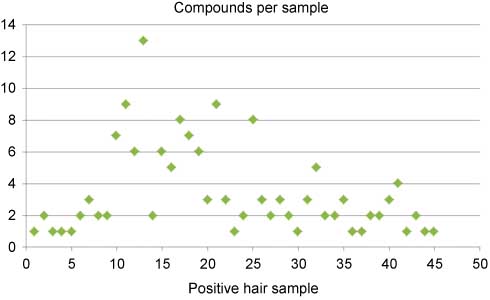
Number of different cannabimimetics in hair samples tested positive in consecutive time order within the period August 2011 to April 2013. Around 54% of all routine specimens received were positive.
Sixteen different cannabimimetics have been detected over time in hair specimens (Figure 7). Extremely high values are likewise rare, but it has to be realized that a dynamic range of a factor 3000 is obviously needed. Including all substances detected to date, the median concentration amounts to around 9 pg/mg; this value is by a factor 9 higher than the decision level and by a factor 300 lower than peak concentrations.
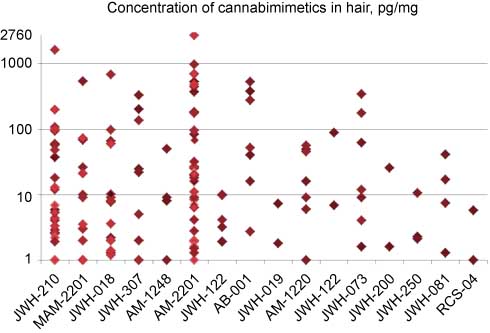
Concentrations of cannabimimetics in positive hair samples (same data pool as for Figure 6). Owing to the wide range, a logarithmic scale was applied for ordinate values.
With the exception of specimens sent for inter-laboratory comparison after positive immunoassay-based pretesting, routine urine samples received to date were negative with respect to the 14 compounds listed in Table 1 at a preliminary but not yet validated decision level of 0.5 ng/mL.
Metabolic aspects
Metabolism of cannabinoids is a matter of interest for at least two particular reasons. First, from a pharmacological point of view, knowledge of metabolic routes (qualitative aspect) and conversion rates (quantitative aspect) is essential in assessing duration and profile of a clinical effect. In contrast to general opinion, metabolites of CB receptor ligands are not necessarily inactive. Even in THC-COOH, one of the final end products of THC obtained after consecutive [41] cytochrome P450 (CYP)-catalyzed hydroxylations, analgesic properties remain whereas the psychoactive effect is lost [42]. The endocannabinoid, anandamide, is hydroxylated by CYP3A4 and CYP4F2 in human liver and brain. The CYP-catalyzed formation of epoyeicosatrienoic acid-ethanolamine from anandamide can be interpreted as a bioactivation mechanism or as a bioinactivation mechanism dependent on the specific target considered because this epoxide formation shifts receptor selectivity from CB1 to CB2 [8]. In parallel, there is increasing evidence that primary and secondary metabolites of synthetic cannabinoids express pharmacological activities. For instance, monohydroxylated metabolites of JWH-018 and JWH-073 bind agonistically to CB2 receptors but are more effective in G protein activation than the respective parent compounds [43], whereas certain monohydroxylated JWH-073 metabolites as ligands of the CB1 receptor exhibit neutral antagonist to partial agonist activity [44].
From an analytical point of view, metabolism of cannabimimetics is challenging because for one individual compound, there are multiple sites accessible for CYP-dependent hydroxylations. Owing to the structural similarity of many of these compounds as outlined in Figure 1 and Table 1, this has the consequence that it may become difficult or impossible to assign a mass fragment to a specific parent compound. For instance, members of the JWH-series can be hydroxylated at different positions of the aliphatic side chain, at the aromatic ring of the indol system, or at the naphthoyl ring system. Furthermore, N-dealkylation and hydroxyl group oxidation to carboxylic acid have been described at least for JWH-018 [45]. Similarly, eight possible hydroxylated and oxygenated CP-47,497 metabolites [46], and even 24 possible metabolites of HU-210 [47] have been identified. Those wide metabolite spectra may result from the combination of different metabolic reactions (hydroxylations, dealkylations) as shown for RCS-4 [36].
There are two general strategies to identify metabolic routes. The first one (which may be called the in vitro approach) is based on incubation of the substance in question with human liver microsomes [45–47] or specifically overexpressed CYP systems indicating that CYP2C9 and 1A2 predominantly catalyze JWH-018 and AM-2201 oxidations, whereas CYP 3A4, 2C19, and 2D6 are involved to a minor degree [48]. This approach leads to the identification of metabolites which can then be used as specific targets for urine analysis. The second strategy (which may be called the in vivo approach) is based on complete analysis of urine from customers using one defined substance by GC-MS (gas chromatography-mass spectrometry) or HPLC-MS/MS techniques. Again beginning with JWH-018 and JWH-073 [35], the technique has been further optimized to include metabolite spectra from seven or eight different parent synthetic cannabinoids [22, 34]. In turn, this approach may help to identify possible enzyme-catalyzed routes for cannabimimetic metabolism.
In conclusion, HPLC-MS/MS analysis, including the technique presented in this paper, of synthetic cannabinoids and their metabolites in different matrices is useful to further decipher biotransformation processes, clarify duration of drug action for prognostic purposes in practical medicine, and may eventually be applied to anti-doping programs as recently suggested [49].
We are grateful to Annika Bensemann and Meike Ahlers-Frömling for their excellent technical assistance, to Ute Hellwinkel for data extraction, and to Udo Holz and Wolfram Schoeder for data processing.
Conflict of interest statement
Authors’ conflict of interest disclosure: The authors stated that there are no conflicts of interest regarding the publication of this article.
Research funding: None declared.
Employment or leadership: None declared.
Honorarium: None declared.
References
1. Mechoulam R, Hanus L. A historical overview of chemical research on cannabinoids. Chem Phys Lipids 2000;108:1–13.10.1016/S0009-3084(00)00184-5Search in Google Scholar
2. Borgelt LM, Franson KL, Nussbaum AM, Wang GS. The pharmacological and clinical effects of medical cannabis. Pharmacotherapy 2013;33:195–202.10.1002/phar.1187Search in Google Scholar PubMed
3. Howlett AC. Cannabinoid receptor signaling. Handb Exp Pharmacol 2005;168:53–79.10.1007/3-540-26573-2_2Search in Google Scholar PubMed
4. Howlett AC, Barth F, Bonner TI, Cabral G, Casellas P, Devane WA, et al. International Union of Pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol Rev 2002;54:161–202.10.1124/pr.54.2.161Search in Google Scholar PubMed
5. Demuth DG, Molleman A. Cannabinoid signalling. Life Sci 2006;78:549–63.10.1016/j.lfs.2005.05.055Search in Google Scholar PubMed
6. Leterrier C, Bonnard D, Carrel D, Rossier J, Lenkei Z. Constitutive endocytic cycle of the CB1 cannabinoid receptor. J Biol Chem 2004;279:36013–21.10.1074/jbc.M403990200Search in Google Scholar PubMed
7. Freund TF, Katona I, Piomelli D. Role of endogenous cannabinoids in synaptic signalling. Physiol Rev 2003;83:1017–66.10.1152/physrev.00004.2003Search in Google Scholar PubMed
8. Snider NT, Walker VJ, Hollenberg PF. Oxidation of the endogenous cannabinoid arachidonoyl ethanolamide by the cytochrome P450 monooxygenases: physiological and pharmacological implications. Pharmacol Rev 2010;62:136–54.10.1124/pr.109.001081Search in Google Scholar PubMed PubMed Central
9. Alger BE, Kim J. Supply and demand for endocannabinoids. Trends Neurosci 2011;34:304–15.10.1016/j.tins.2011.03.003Search in Google Scholar PubMed PubMed Central
10. Cagni P, Barros M. Cannabinoid type 1 receptor ligands WIN 55,212-2 and AM 251 alter anxiety-like behaviors of marmoset monkeys in an open-field test. Behav Brain Res 2013;240:91–4.10.1016/j.bbr.2012.11.018Search in Google Scholar PubMed
11. O’Leary DH, Reuwer AQ, Nissen SE, Després JP, Deanfield JE, Brown MW, et al. Effect of rimonabant on carotid intima-media thickness (CIMT) progression in patients with abdominal obesity and metabolic syndrome: the AUDITOR trial. Heart 2011;97:1143–50.10.1136/hrt.2011.223446Search in Google Scholar PubMed
12. Castaneda JT, Harui A, Kiertscher SM, Roth JD, Roth MD. Differential expression of intracellular and extracellular CB(2) cannabinoid receptor protein by human peripheral blood leukocytes. J Neuroimmune Pharmacol 2013;8:323–32.10.1007/s11481-012-9430-8Search in Google Scholar PubMed PubMed Central
13. Saito VM, Rezende RM, Teixeira AL. Cannabinoid modulation of neuroinflammatory disorders. Curr Neuropharmacol 2012;10:159–66.10.2174/157015912800604515Search in Google Scholar PubMed PubMed Central
14. Leggett JD, Aspley S, Beckett SR, D’Antona AM, Kendall DA, Kendall DA. Oleamide is a selective endogenous agonist of rat and human CB1 cannabinoid receptors. Br J Pharmacol 2004;141:253–62.10.1038/sj.bjp.0705607Search in Google Scholar PubMed PubMed Central
15. Diaz-Laviada I, Ruiz-Llorente L. Signal transduction activated by cannabinoid receptors. Mini Rev Med Chem 2005;5: 619–30.10.2174/1389557054368808Search in Google Scholar PubMed
16. Reggio PH. Endocannabinoid structure-activity relationships for interaction at the cannabinoid receptors. Prostanglandins Leukot Essent Fatty Acids 2002;66:143–60.10.1054/plef.2001.0343Search in Google Scholar PubMed
17. United Nations Office on Drugs and Crime. Synthetic cannabinoids in herbal products. UN Document ID Number SCITEC/24 2011:1–24. Available at: www.unodc.org/unodc/en/scientists/synthetic-cannabinoids-in-herbal-products.html. Accessed 28 February, 2013.Search in Google Scholar
18. European Monitoring Centre for Drugs and Drug Addiction. Understanding the ‘Spice’ phenomenon. EMCDDA 2009 Luxembourg: Office for Official Publications of the European Communities; 2009:1–37.Search in Google Scholar
19. Auwärter V, Dresen S, Weinmann W, Müller M, Pütz M, Ferreiros N. ‘Spice’ and other herbal blends: harmless incense or cannabinoid designer drugs? J Mass Spectrom 2009;44:832–7.10.1002/jms.1558Search in Google Scholar PubMed
20. Fattore L, Fratta W. Beyond THC: the new generation of cannabinoid designer drugs. Frontiers Behav Neurosci 2011; 5:1–12.10.3389/fnbeh.2011.00060Search in Google Scholar PubMed PubMed Central
21. McDonough PC, Holler JM, Vorce SP, Bosy TZ, Magluilo J, Past MR. The detection and quantitative analysis of the psychoactive component of salvia divinorum, salvinorin A, in human biological fluids using liquid chromatography-mass spectrometry. J Anal Toxicol 2008;32:417–21.10.1093/jat/32.6.417Search in Google Scholar PubMed
22. Hutter M, Broecker S, Kneisel S, Auwärter V. Identification of the major urinary metabolites in man of seven synthetic cannabinoids of the aminoalkylindole type present as adulterants in ‘herbal mixtures’ using LC-MS/MS techniques. J Mass Spectrom 2012;47:54–65.10.1002/jms.2026Search in Google Scholar PubMed
23. Pragst F. Molecular index of cannabimimetics. Toxichem Krimtech 2013;80:151.Search in Google Scholar
24. Müller H, Sperling W, Köhrmann M, Huttner HB, Kornhuber J, Maler JM. The synthetic cannabinoid Spice as a trigger for an acute exacerbation of cannabis induced recurrent psychotic episodes. Schizophr Res 2010;118:309–10.10.1016/j.schres.2009.12.001Search in Google Scholar PubMed
25. Schneir AB, Cullen J, Ly BT. “Spice” girls: synthetic cannabinoid intoxication. J Emerg Med 2011;40:296–9.10.1016/j.jemermed.2010.10.014Search in Google Scholar PubMed
26. Teske J, Weller JP, Fieguth A, Rothämel T, Schulz Y, Tröger HD. Sensitive and rapid quantification of the cannabinoid receptor agonist naphthalen-1-yl-(1-pentylindol-3-yl)methanone (JWH-018) in human serum by liquid chromatography-tandem mass spectrometry. J Chromatogr B 2010;878:2659–63.10.1016/j.jchromb.2010.03.016Search in Google Scholar PubMed
27. Dresen S, Kneisel S, Weinmann W, Zimmermann R, Auwärter V. Development and validation of a liquid chromatography-tandem mass spectrometry method for the quantitation of synthetic cannabinoids of the aminoalkylindole type and methanandamide in serum and its application to forensic samples. J Mass Spectrom 2011;46:163–71.10.1002/jms.1877Search in Google Scholar PubMed
28. Kacinko SL, Xu A, Homan JW, McMullin MM, Warrington DM, Logan BK. Development and validation of a liquid chromatography-tandem mass spectrometry method for the identification and quantification of JWH-018, JWH-073, JWH-019, and JWH-250 in human whole blood. J Anal Toxicol 2011;35:386–93.10.1093/anatox/35.7.386Search in Google Scholar PubMed
29. Neukamm MA, Sachs H, Mürdter TE, Fehn S, Pöhlmann-Moore T, Wehner HD, et al. Determination of ‘Spice’ cannabinoids in serum and hair by liquid chromatography-tandem mass spectrometry. Toxichem Krimtech 2011;78(Special Issue): 219–23.Search in Google Scholar
30. Kirchherr H, Kühn-Velten WN. Quantitative determination of forty-eight antidepressants and antipsychotics in human serum by HPLC tandem mass spectrometry: a multi-level, single-sample approach. J Chromatogr B 2006;843:100–13.10.1016/j.jchromb.2006.05.031Search in Google Scholar PubMed
31. Peters FT, Wissenbach DK. Systematic toxicological analysis using liquid chromatography-mass spectrometry: techniques and inter-instrument reproducibility of mass spectra. J Lab Med 2012;36:89–97.Search in Google Scholar
32. Kneisel S, Auwärter V. Analysis of 30 synthetic cannabinoids in serum by liquid chromatography-electrospray ionization tandem mass spectrometry after liquid-liquid extraction. J Mass Spectrom 2012;47:825–35.10.1002/jms.3020Search in Google Scholar PubMed
33. Hutter M, Kneisel S, Auwärter V, Neukamm MA. Determination of 22 synthetic cannabinoids in human hair by liquid chromatography-tandem mass spectrometry. J Chromatogr B 2012;903:95–101.10.1016/j.jchromb.2012.07.002Search in Google Scholar PubMed
34. DeJager AD, Warner JV, Henman M, Ferguson W, Hall A. LC-MS/MS method for the quantitation of metabolites of eight commonly-used synthetic cannabinoids in human urine – an Australian perspective. J Chromatogr B 2012;897:22–31.10.1016/j.jchromb.2012.04.002Search in Google Scholar PubMed
35. Yanes EG, Lovett DP. High-throughput bioanalytical method for analysis of synthetic cannabinoid metabolites in urine using salting-out sample preparation and LC-MS/MS. J Chromatogr B 2012;909:42–50.10.1016/j.jchromb.2012.10.013Search in Google Scholar PubMed
36. Kavanagh P, Grigoryev A, Melnik A, Simonov A. The identification of the urinary metabolites of 3-(4-methoxybenzoyl)-1- pentylindole (RCS-4), a novel cannabimimetic, by gas chromatography-mass spectrometry. J Anal Toxicol 2012; 36:303–11.10.1093/jat/bks032Search in Google Scholar
37. Wohlfarth A, Scheidweiler KB, Chen X, Liu HF, Huestis MA. Qualitative confirmation of 9 synthetic cannabinoids and 20 metabolites in human urine using LC-MS/MS and library search. Anal Chem 2013;85:3730–8.10.1021/ac3037365Search in Google Scholar
38. Herbold M, Schmitt G. B.E.N. – Programm zur Berechnung der Bestimmungsgrenze, Erfassungsgrenze und Nachweisgrenze nach DIN 32645 © 1998. Heidelberg: Institut für Rechtsmedizin und Verkehrsmedizin der Universität Heidelberg.Search in Google Scholar
39. Matuszewski BK, Constanzer ML, Chavez-Eng CM. Strategies for the assessment of matrix effect in quantitative bioanalytical methods based on HPLC-MS/MS. Anal Chem 2003;75:3019–30.10.1021/ac020361sSearch in Google Scholar
40. Buhrman D, Price P, Rudewicz P. Quantitation of SR27417 in human plasma using electrospray liquid chromatography-tandem mass spectrometry: a study of ion suppression. J Am Soc Mass Spectrom 1996;7:1099–1105.10.1016/S1044-0305(96)00072-4Search in Google Scholar
41. Kühn-Velten WN. A microcompartmentation analysis of intermediate leakage response to substrate excess in a membrane-bound bifunctional enzyme: local control of hydroxyprogesterone channeling efficiency during cytochrome P450XVII-catalysed androgen biosynthesis. J Cell Biochem 1990;43:149–59.10.1002/jcb.240430206Search in Google Scholar
42. Burstein SH. The cannabinoid acids: nonpsychoactive derivatives with therapeutic potential. Pharmacol Ther 1999;82:87–96.10.1016/S0163-7258(98)00069-2Search in Google Scholar
43. Rajasekaran M, Brents LK, Franks LN, Moran JH, Prather PL. Human metabolites of synthetic cannabinoids JWH-018 and JWH-073 bind with high affinity and act as potent agonists at cannabinoid type-2 receptors. Toxicol Appl Pharmacol 2013;269:100–8.10.1016/j.taap.2013.03.012Search in Google Scholar PubMed PubMed Central
44. Brents LK, Gallus-Zawala A, Radominska-Pandya A, Vasiljevik T, Prisinzano TE, Fantegrossi WE, et al. Monohydroxylated metabolites of the K2 synthetic cannabinoid JWH-073 retain intermediate to high cannabinoid 1 receptor (CB1R) affinity and exhibit neutral antagonist to partial agonist activity. Biochem Pharmacol 2012;83:952–61.10.1016/j.bcp.2012.01.004Search in Google Scholar PubMed PubMed Central
45. Grigoryev A, Savchuk S, Melnik A, Moskaleva N, Dzhurko J, Ershov M, et al. Chromatography-mass spectrometry studies on the metabolism of synthetic cannabinoids JWH-018 and JWH-073, psychoactive components of smoking mixtures. J Chromatogr B 2011;879:1126–36.10.1016/j.jchromb.2011.03.034Search in Google Scholar PubMed
46. Jin MJ, Lee J, In MK, Yoo HH. Characterization of in vitro metabolites of CP 47,497, a synthetic cannabinoid, in human liver microsomes by LC-MS/MS. J Forensic Sci 2013;58:195–9.10.1111/j.1556-4029.2012.02261.xSearch in Google Scholar PubMed
47. Kim U, Jin MJ, Lee J, Han SB, In MK, Yoo HH. Tentative identification of phase I metabolites of HU-210, a classical synthetic cannabinoid, by LC-MS/MS. J Pharm Biomed Anal 2012; 64–65:26–34.10.1016/j.jpba.2012.02.007Search in Google Scholar PubMed
48. Chimalakonda KC, Seely KA, Bratton SM, Brents LK, Moran CL, Endres GW, et al. Cytochrome P450-mediated oxidative metabolism of abused synthetic cannabinoids found in K2/Spice: identification of novel cannabinoid receptor ligands. Drug Metab Dispos 2012;40:2174–84.10.1124/dmd.112.047530Search in Google Scholar PubMed PubMed Central
49. Heltsley R, Shelby MK, Crouch DJ, Black DL, Robert TA, Marshall L, et al. Prevalence of synthetic cannabinoids in U.S. athletes: initial findings. J Anal Toxicol 2012;36:588–93.10.1093/jat/bks066Search in Google Scholar PubMed
©2013 by Walter de Gruyter Berlin Boston
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Articles in the same Issue
- Masthead
- Masthead
- Drug Monitoring und Toxikologie/Drug Monitoring and Toxicology
- Simultaneous identification and quantification of synthetic cannabinoids (cannabimimetics) in serum, hair, and urine by rapid and sensitive HPLC tandem mass spectrometry screenings: overview and experience from routine testing
- Allergie und Autoimmunität/Allergy and Autoimmunity
- ANCA: Gesichertes und offene Fragen
- Endokrinologie/Endocrinology
- Labordiagnostik Katecholamin-produzierender Tumoren
- Klinische Chemie und Stoffwechsel/Clinical Chemistry and Metabolism
- Kardiovaskuläre Verkalkungen im Rahmen des chronischen kardiorenalen Syndroms: welche Bedeutung haben Biomarker für Diagnostik und Risikostratifizierung?
- Infektiologie und Mikrobiologie (Schwerpunkt Bakteriologie)/Infectiology and Microbiology (Focus Bacteriology)
- Tödliche Urosepsis durch verzögerte Diagnose einer urogenitalen Melioidose
- Fatal urosepsis due to delayed diagnosis of genitourinary melioidosis1)
- Buchbesprechung/Book Review
- Effects of Herbal Supplements on Clinical Laboratory Test Results
Articles in the same Issue
- Masthead
- Masthead
- Drug Monitoring und Toxikologie/Drug Monitoring and Toxicology
- Simultaneous identification and quantification of synthetic cannabinoids (cannabimimetics) in serum, hair, and urine by rapid and sensitive HPLC tandem mass spectrometry screenings: overview and experience from routine testing
- Allergie und Autoimmunität/Allergy and Autoimmunity
- ANCA: Gesichertes und offene Fragen
- Endokrinologie/Endocrinology
- Labordiagnostik Katecholamin-produzierender Tumoren
- Klinische Chemie und Stoffwechsel/Clinical Chemistry and Metabolism
- Kardiovaskuläre Verkalkungen im Rahmen des chronischen kardiorenalen Syndroms: welche Bedeutung haben Biomarker für Diagnostik und Risikostratifizierung?
- Infektiologie und Mikrobiologie (Schwerpunkt Bakteriologie)/Infectiology and Microbiology (Focus Bacteriology)
- Tödliche Urosepsis durch verzögerte Diagnose einer urogenitalen Melioidose
- Fatal urosepsis due to delayed diagnosis of genitourinary melioidosis1)
- Buchbesprechung/Book Review
- Effects of Herbal Supplements on Clinical Laboratory Test Results

