DEK-NUP214 monitoring before and after allogeneic haematopoietic stem cell transplantation for acute myeloid leukemia: A report from the TROPHY study group
-
Shuang Fan
und Xiaoxia Hu
Abstract
Background and Objectives
Acute myeloid leukaemia (AML) with the translocation of chromosome (6;9)(p23;q34) forms the DEK-NUP214 fusion mRNA, which is a rare subtype (~1%). Owing to the paucity of this AML subtype, comprehensive studies analysing allogeneic haematopoietic stem cell transplantation (allo-HSCT) outcomes are lacking.
Methods
We aimed to evaluate the dynamic evolution of DEK-NUP214 transcripts before and after allo-HSCT as well as the impact of pretransplant DEK-NUP214 status on posttransplant outcomes in AML patients in a retrospective, multicentre study (n = 14).
Results
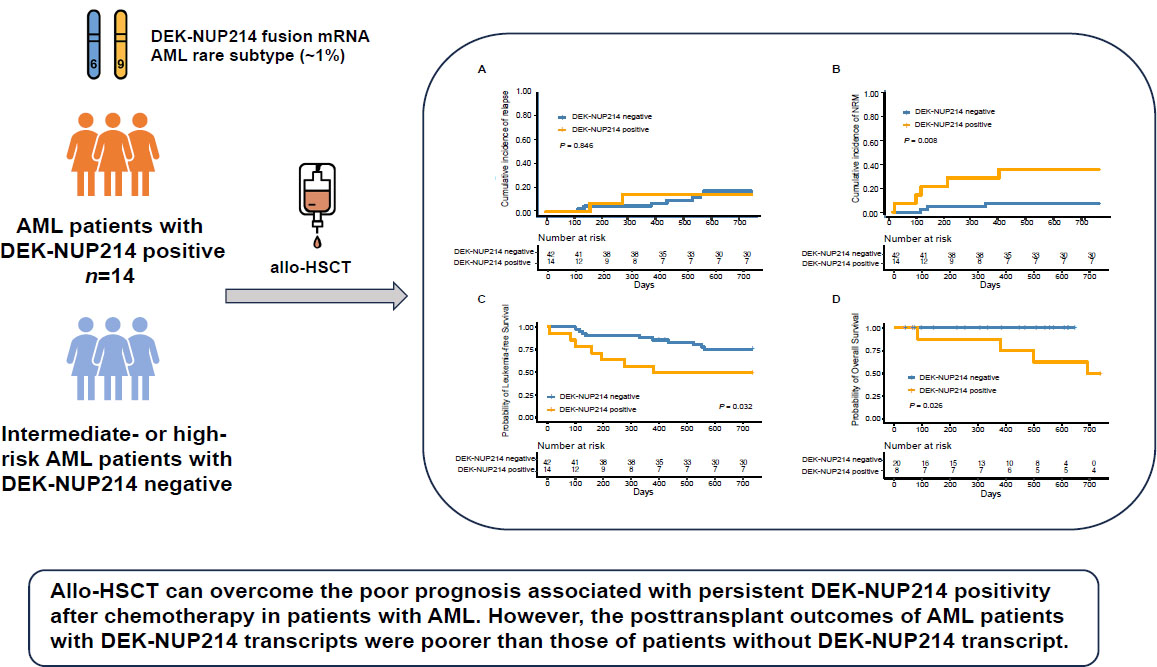
Intermediate- or high-risk AML patients without DEK-NUP214 transcripts receiving allo-HSCT during the same time period were enrolled as controls. Ten (71.4%) patients showed DEK-NUP214 positivity before allo-HSCT. Except for one patient who died early after allo-HSCT, 7 out of the other 9 patients (77.8%) achieved DEK-NUP214 negativity after allo-HSCT. The 2-year probabilities of relapse, non-relapse mortality (NRM), leukaemia-free survival (LFS), and overall survival (OS) were 14.3% (95% CI, 0%–33.6%), 35.7% (95% CI, 9.3%–62.1%), 50.0% (95% CI, 29.6%–84.4%), and 50.0% (95% CI, 29.6%–84.4%), respectively. The incidence of relapse was comparable between AML patients with and without DEK-NUP214 transcript, but the incidence of NRM, LFS, and OS of patients with DEK-NUP214 was poorer compared with those without DEK-NUP214 transcript.
Conclusions
Thus, this study observed that allo-HSCT could overcome the poor prognosis of persistent DEK-NUP214 positivity after chemotherapy; however, new therapies should be further identified to improve the outcomes of AML patients with DEK-NUP214.
Graphical Abstract
Introduction
Acute myeloid leukemia (AML) with translocation of chromosome (6;9) (p23; q34) forming the DEK-NUP214 fusion mRNA is rare, accounting for approximately 1% of cases. It was first recognized as a distinct entity in 2008 by the World Health Organization[1] and was classified as a high-risk AML with an unfavorable prognosis according to the European LeukaemiaNet (ELN) criteria, which confers a dismal 10-year overall survival (OS) of less than 30%.[2, 3, 4] Previous studies indicate that the clinical outcomes of intensive chemotherapy or autologous hematopoietic stem cell transplantation are poor in patients with AML and DEK-NUP214 fusion.[2,5] Therefore, these patients are recommended to receive allogeneic haematopoietic stem cell transplantation (allo-HSCT) at first complete remission (CR1).[6,7]
Owing to the paucity of this AML subtype, comprehensive studies analysing allo-HSCT outcomes are lacking.[8,9] In 2012, Ishiyama et al.[9] conducted a matched-pair analysis of de novo AML patients with and without t(6;9)(p23;q34), using data obtained from the Japanese HSCT data registry. The outcomes were comparable between the two groups, suggesting that allo-HSCT may overcome the unfavorable impact of t(6:9)(p23;q34). In a recent study, Diaz-Beya et al.[10] reported the largest cohort of 195 patients with t(6;9)(p23;q34) receiving allo-HSCT. For patients transplanted in CR1, the 2-year probabilities of leukemia-free survival (LFS) and OS were 57% and 61%, respectively, which were equivalent to the outcomes of other intermediate-risk AML patients.[11] However, these studies did not identify the dynamic evolution of measurable residual disease (MRD, i.e., DEK-NUP214 transcript) before and after allo-HSCT, or the impact of pretransplant MRD status on posttransplant outcomes.[12] In particular, whether DEK-NUP214 transcript positivity before allo-HSCT influences posttransplant clinical outcomes remain unclear.
Thus, this retrospective, multicentre study aimed to evaluate the dynamic evolution of DEK-NUP214 transcripts before and after allo-HSCT as well as the impact of pretransplant DEK-NUP214 status on posttransplant outcomes in AML patients.
Materials and methods
Patients
This is a multicenter, retrospective study. Consecutive patients with AML and DEK-NUP214 fusion receiving allo-HSCT at Institute of Haematology (i.e., TROPHY group) between March 2016 and April 2021 were enrolled. Intermediate- or high-risk AML patients without DEK-NUP214 fusion receiving allo-HSCT during the same time period were enrolled as controls and propensity-matched (1∶3) to those with DEK-NUP214 fusion using the nearest-neighbour method and a 2% calliper. Age, sex, donor type, and disease status were matched by propensity scores. The last follow-up was on May 1, 2023. The study was approved by the institutional review board of each participating hospital and was conducted in accordance with the Declaration of Helsinki. The requirement for written informed consent was waived owing to the retrospective nature of the study and lack of intervention in these patients.
Transplant regimen
The protocols for the preconditioning regimen, graft-versus-host disease (GVHD) prophylaxis and treatment, and infection prophylaxis have been previously reported in detail.[7,13,14,15,16,17,18,19]
MRD monitoring protocols
The MRD status was monitored before transplantation, at 1, 2, 3, 4.5, 6, 9, and 12 months after transplantation, and at 6-month intervals thereafter.[20, 21, 22] Quantitative PCR was used for DEK-NUP214 fusion monitoring.[23] Leukemia-associated aberrant immunophenotypes (LAIPs) were identified by multicolor flow cytometry (MFC), and 0.1% was used as the threshold to distinguish MRD-positive patients.[24]
Definition
Relapse was defined as recurrence of > 5% bone marrow (BM) blasts, reappearance of blasts in peripheral blood, development of extramedullary disease, or recurrence of pretransplantation chromosomal abnormalities. Non-relapse mortality (NRM) was defined as death without disease progression or relapse. LFS was defined as survival with continuous CR. OS events were defined as death from any cause.
Statistical analysis
Frequencies and percentages were used to describe patient characteristics. The Kaplan–Meier estimator was used to calculate the probabilities of survival, and a cumulative incidence function was adopted to calculate the incidence of engraftment, GVHD, relapse, and NRM using a competing risk analysis.[25] Two-sided P values were considered statistically significant. Statistical analyses were performed using R software 4.2.0 (https://www.r-project.org) and the Statistical Package for the Social Sciences 26.0 (SPSS Inc., IBM, Armonk, NY, USA).
Results
Fourteen patients were enrolled in the study (Table 1 and Table 2). The distribution of other molecular abnormalities is shown in Figure 1A and 1B. The median follow-up duration was 716 days (range, 7–2562 days). The clinical outcomes of the patients were as follows.
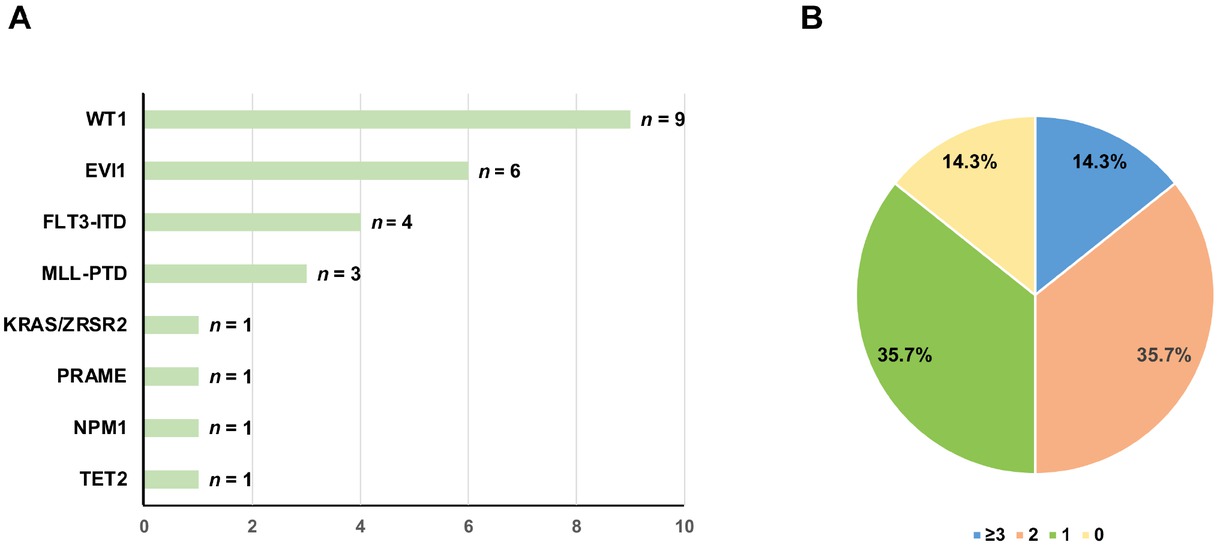
The distribution of partner genes for DEK-NUP214 (A) and types of other molecular abnormalities (B).
Patients characteristics
| Patient | Age (yr) | Gender | FLT3-ITD mutations at diagnosis | Other molecular abnormalities at diagnosis | Cytogenetic at diagnosis | Number of courses of induction for first CR | Disease status before allo-HSCT | Donor type | Conditioning regimen | Number of HLA disparity (HLA-A, HLA-B, HLA-DR) | Mononuclear cell counts, ×108/kg | CD34+cell counts, ×106/kg | Blood group disparity | Donor-recipient gender match |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 17 | Male | Negative | WT1, EVI1 | 46,XY,t(6;9) (p23;q34) [17]/46,XY[3] | 1 | CR1 | HLA-haploidentical related donor | Chemotherapy-based regimen | 3 | 14.53 | 4.13 | Matched | Male-male |
| 2 | 33 | Female | Negative | WT1, EVI1 | 46,XX,t(6;9) (p23;q34)[5] | 1 | CR1 | HLA-haploidentical related donor | Chemotherapy-based regimen | 3 | 12.65 | 1.79 | Matched | Male-female |
| 3 | 25 | Female | Negative | WT1, EVI1 | 46, XX,t(6;9) (p23;q34)[7]/46,XX[4] | 2 | CR1 | HLA-haploidentical related donor | Chemotherapy-based regimen | 3 | 8.86 | 3.30 | Matched | Male-female |
| 4 | 30 | Female | Positive | FLT3-ITD, TET2 | 46, XX | 2 | CR1 | HLA-haploidentical related donor | Chemotherapy-based regimen | 3 | 9.74 | 2.95 | Major mismatched or minor and major mismatched | Male-female |
| 5 | 34 | Male | Negative | WT1, EVI1 | 46, XY,t(6;9) (p23;q34)[12] | 2 | CR1 | HLA-haploidentical related donor | Chemotherapy-based regimen | 3 | 7.50 | 0.78 | Minor mismatched | Female-male |
| 6 | 50 | Male | Negative | 0 | 46, XY | 2 | CR1 | HLA-haploidentical related donor | Chemotherapy-based regimen | 3 | 8.72 | 1.63 | Minor mismatched | Male-male |
| 7 | 49 | Female | Negative | WT1, MLL-PTD, NPM1 | 46, XX | 1 | CR1 | HLA-haploidentical related donor | Chemotherapy-based regimen | 3 | 7.24 | 3.46 | Minor mismatched | Male-female |
| 8 | 33 | Male | Negative | WT1 | 46, XY,t(6;9) (p23;q34)[3] | 3 | CR1 | HLA-haploidentical related donor | Chemotherapy-based regimen | 3 | 7.01 | 2.74 | Matched | Female-male |
| 9 | 53 | Male | Negative | WT1, EVI1, MLLPTD, PRAME | 46,XY,der(6) t(6;9) (p23;q34),der(9) del(9)(q13q22) t(6;9) | 1 | CR1 | HLA-identical sibling donor | Chemotherapy-based regimen | 0 | 9.39 | 1.14 | Matched | Female-male |
| 10 | 51 | Male | Positive | FLT3-ITD, DEKCAN, WT1, EVI1, MLL-PTD | 46, XY[4] | 2 | CR1 | HLA-identical sibling donor | TBI | 0 | 10.38 | 2.54 | Minor mismatched | Male-male |
| 11 | 32 | Male | Negative | KRAS/ZRSR2 | 46, XY,t(6;9) (p22;q34)[3] | 3 | CR1 | HLA-haploidentical related donor | Chemotherapy-based regimen | 2 | 12.63 | 5.20 | Matched | Female-male |
| 12 | 24 | Female | Positive | FlT3-ITD | 46, XY,t(6;9) (p22;q34)[13] | 2 | CR1 | HLA-haploidentical related donor | Chemotherapy-based regimen | 3 | 14.22 | 4.56 | Matched | Male-female |
| 13 | 15 | Female | Negative | WT1 | 46 XX[5] | NA | NR | HLA-haploidentical related donor | Chemotherapy-based regimen | 3 | 15.00 | 7.88 | Matched | Male-female |
| 14 | 48 | Female | Positive | FLT3-ITD | 46,XY,t(6;9) (p22;q34)[11] | 2 | CR1 | HLA-haploidentical related donor | Chemotherapy-based regimen | 2 | 12.47 | 5.26 | Matched | Male-female |
Allo-HSCT: allogeneic hematopoietic stem cell transplantation; MRD: measurable residual disease; MFC: multicolor flow cytometry; N/A: not applicable.
Outcomes after preemptive and maintenance therapies
| Patient | Maintenance therapies after allo-HSCT | Achieved MRD- negative at least once after allo-HSCT | Achieved MFC- negative at least once after allo-HSCT | Relapse | qPCR status before relapse | MFC status before relapse | Time from HSCT to relapse (days) | Site of relapse | Outcomes |
|---|---|---|---|---|---|---|---|---|---|
| 1 | NO | Yes | Yes | NO | Negative | Negative | N/A | N/A | Leukemia-survival free |
| 2 | NO | Yes | Yes | NO | Negative | Negative | N/A | N/A | Died infection from |
| 3 | NO | Yes | Yes | NO | Negative | Negative | N/A | N/A | Leukemia-survival free |
| 4 | NO | Yes | Yes | NO | Negative | Negative | N/A | N/A | Leukemia-survival free |
| 5 | NO | Yes | Yes | NO | Negative | Negative | N/A | N/A | Leukemia-survival free |
| 6 | NO | Yes | Yes | NO | Negative | Negative | N/A | N/A | Died infection from |
| 7 | NO | Yes | Yes | NO | Negative | Negative | N/A | N/A | Leukemia-survival free |
| 8 | NO | Yes | Yes | NO | Negative | Negative | N/A | N/A | Leukemia-survival free |
| 9 | NO | NO | Yes | NO | Negative | Negative | N/A | N/A | Died heart from failure |
| 10 | NO | NO | Yes | Yes | Yes | Yes | 157 | Bone Marrow | Died relapse of |
| 11 | HMA | Yes | Yes | NO | Negative | Negative | N/A | N/A | Leukemia-survival free |
| 12 | sorafnib | Yes | Yes | NO | Negative | Negative | N/A | N/A | Died infection from |
| 13 | NO | Yes | Yes | NO | Negative | Negative | N/A | N/A | Died from cerebral hemorrhage |
| 14 | sorafnib | Yes | Yes | Yes | Negative | Negative | 273 | Bone Marrow | Died relapse of |
Engraftment
Thirteen (92.8%) patients achieved neutrophil engraftment, and the median time from transplantation to neutrophil engraftment was 12 days (range, 11–20) days. Thirteen (92.8%) patients achieved platelet engraftment, and the median time from transplantation to platelet engraftment was 14 days (range, 9–83 days). The 60-day cumulative incidence of platelet engraftment after allo-HSCT was 92.3% (95% confidence interval [CI], 73.6%–100%).
Graft versus host disease
Ten (71.4%) patients experienced acute graft versus host disease GVHD (aGVHD) after allo-HSCT, and 5 (50.0%), 4 (40.0%), 0, and 1 (10.0%) patients experienced grade I, II, III, and IV aGVHD, respectively. The cumulative incidences of grade I–IV and grade II–IV aGVHD 100 days after allo-HSCT were 71.4% (95% CI, 45.5%–97.3%) and 35.7% (95% CI, 9.3%–62.1%), respectively.
Six (42.9%) patients developed chronic GVHD (cGVHD) after allo-HSCT, and 0 and 4 (28.6%) patients experienced moderate and severe cGVHD, respectively. The cumulative incidences of cGVHD and severe cGVHD at 2 years after allo-HSCT were 35.7% (95% CI, 8.7%–62.7%) and 21.4% (95% CI, 0%–44.3%), respectively.
Evolution and relapse of measurable residual disease
Three (21.4%) patients received maintenance therapy after allo-HSCT (Table 2). Two (14.3%) patients with FLT3 mutations received FLT3 inhibitors as maintenance therapy, and one (7.1%) patient received hypomethylation agents as maintenance therapy.
Ten (71.4%) patients showed DEK-NUP214 positivity before allo-HSCT, with a median transcript level of 4.17% (range 0.03%–143.80%). Except for one patient who died early after allo-HSCT, seven of the other nine patients (77.8%) achieved negativity after allo-HSCT, and four (57.1%), two (28.6%), and one (14.3%) achieved MRD negativity at 1, 2, and 3 months after allo-HSCT, respectively.
Two patients exhibited persistent MRD positivity after allo-HSCT. In addition, 1 patient showed MRD positivity again after MRD negativity. Thus, 3 patients showed MRD positivity after allo-HSCT. One of them received both DLI and IFN-α treatment as preemptive intervention but failed. The other two patients died of infection and did not receive preemptive therapies. One patient experienced hematologic relapse without MRD positivity.
Eight (80.0%) patients showed both MFC positivity and DEK-NUP214 positivity before allo-HSCT, with a median level of MFC-MRD of 0.55% (range 0.03%–4.50%). All patients achieved MFC negativity, and seven (87.5%) and one (22.5%) achieved MFC negativity at 1 and 2 months after allo-HSCT, respectively.
Differences in characteristics between patients with and without DEK-NUP214 transcript
| Characteristics | With DEK-NUP214 transcript (n = 14) | Without DEK-NUP214 transcript (n = 42) | P value |
|---|---|---|---|
| Median age at allo-HSCT, years (range) | 33.2 (15–53) | 33.4 (14–57) | 0.985 |
| Gender, n (%) | 1.000 | ||
| Male | 7 (50) | 21 (50) | |
| Female | 7 (50) | 21 (50) | |
| Induction courses for first CR, median (range) | 2 (1–3) | 1 (1–2) | 0.007 |
| Disease status before allo-HSCT, n (%) | 0.250 | ||
| CR1 | 13 (98.2) | 42 (100.0) | |
| > CR1 | 1 (1.8) | 0 (0) | |
| HCT-CI scores before allo-HSCT, n (%) | 0.009 | ||
| 0 (low risk) | 11 (78.6) | 30 (71.4) | |
| 1–2 (intermediate risk) | 2 (14.3) | 10 (23.8) | |
| ≥ 3 (high risk) | 1 (7.1) | 2 (4.8) | |
| ELN risk | 0.001 | ||
| Intermediate risk | 2 | 28 | |
| High risk | 12 | 14 | |
| Conditioning regimen, n (%) | 0.250 | ||
| Chemotherapy-based regimen | 13 (92.9) | 42 (100.0) | |
| TBI-based regimen | 1 (7.1) | 0 (0) | |
| Donor/recipient gender matched, n (%) | 0.247 | ||
| Female donor/male recipient combination | 4 (28.6) | 6 (14.3) | |
| Others | 10 (71.4) | 36 (85.7) | |
| Donor/recipient relation, n (%) | 1.000 | ||
| Maternal donor | 0 (0) | 2 (4.8) | |
| Collateral donor | 0 (0) | 2 (4.8) | |
| Others | 14 (100.0) | 38 (90.4) | |
| Blood group disparity, n (%) | 0.196 | ||
| matched | 9 (58.9) | 24 (57.1) | |
| minor mismatched | 4 (28.6) | 6 (14.3) | |
| major mismatched or minor and major mismatched | 1 (7.1) | 12 (8.6) | |
| MNC counts in graft, median (range, ×108/kg) | 10.1 (7.0–15.0) | 8.4 (6.1–13.6) | 0.024 |
| CD34+ cell counts in graft, median (range, ×106/kg) | 3.1 (0.8–7.9) | 2.6 (0.5–9.6) | 0.241 |
| Median follow-up of survivors, days (range) | 716 (7–2562) | 1506 (99–2632) | 0.128 |
Allo-HSCT: allogeneic hematopoietic stem cell transplantation; CR: complete remission; HLA: human leukocyte antigen; HCT-CI: hematopoietic cell transplantation-specific comorbidity index; TBI: total body irradiation; MNC: mononuclear cell; ELN: European LeukaemiaNet.
Two patients experienced relapse at 157 and 273 days after allo-HSCT and died 336 and 410 days after relapse, respectively. The cumulative incidence of relapse 2 years after allo-HSCT was 14.3% (95% CI, 0%–33.6%) (Figure 2A). The 2-year cumulative incidence of relapse after allo-HSCT was 10.0% (95% CI, 0%–30.1%) and 25.0% (95% CI, 0%–74.0%) for patients with DEK-NUP214 positivity and negativity, respectively, before allo-HSCT (P = 0.528).
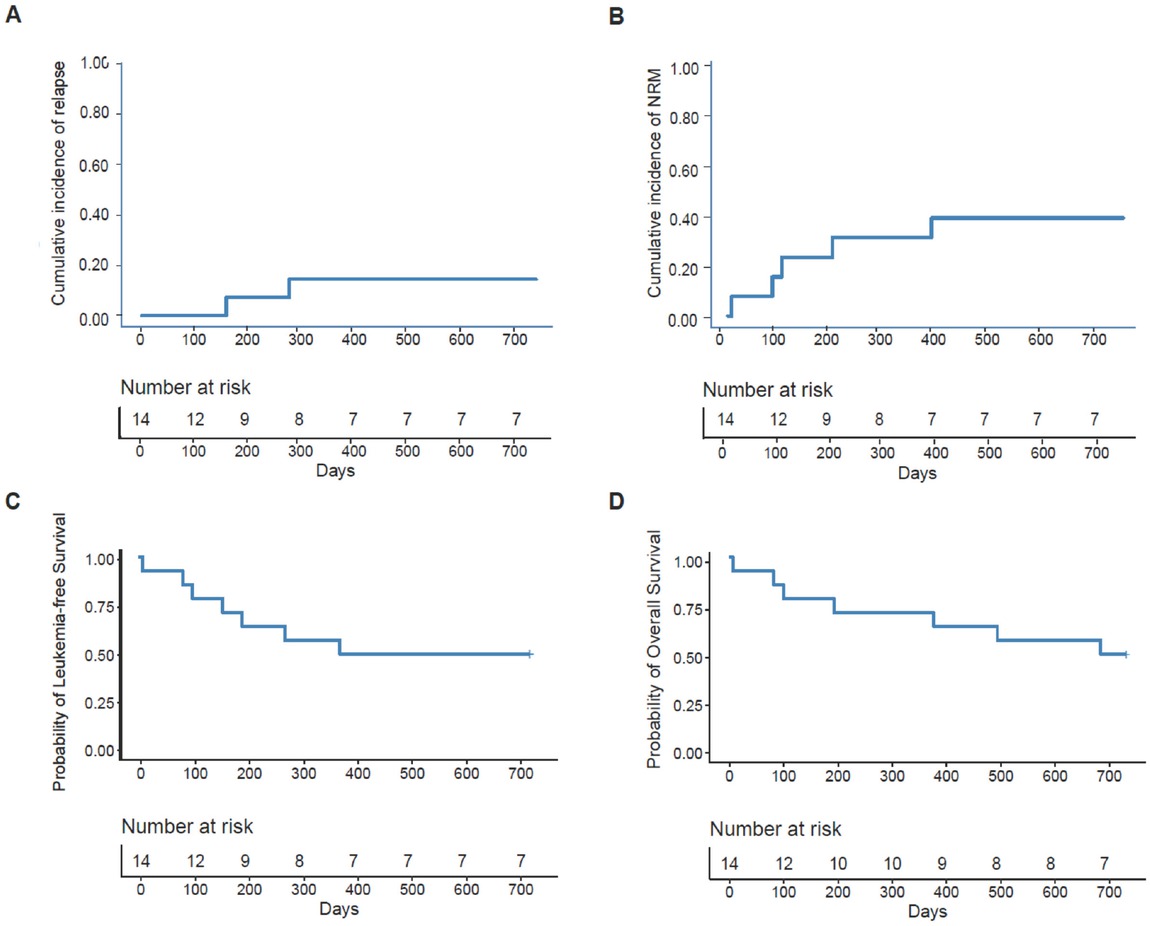
The 2-year probability of clinical outcomes after allo-HSCT, relapse (A), nonrelapse mortality (B), leukaemia-free survival (C), and overall survival (D).
NRM, LFS, and OS
Five patients died of NRM. The most common cause of NRM was infection (n = 3), followed by cerebral hemorrhage (n = 1) and heart failure (n = 1). The cumulative incidence of NRM 2 years after allo-HSCT was 35.7% (95% CI, 9.3%–62.1%) (Figure 2B). The 2-year cumulative incidence of NRM after allo-HSCT was 50.0% (95% CI, 16.3%–83.7%) and 0% for patients with DEK-NUP214 positivity and negativity before allo-HSCT, respectively (P = 0.100).
The probability of LFS 2 years after allo-HSCT was 50.0% (95% CI, 29.6%–84.4%) (Figure 2C). The 2-year probabilities of LFS after allo-HSCT were 40.0% (95% CI, 18.7%–85.5%) and 75.0% (95% CI, 42.6%–100.0%) for patients with DEK-NUP214 positivity and negativity, respectively, before allo-HSCT (P = 0.230).
The probability of OS 2 years after allo-HSCT was 50.0% (95% CI, 29.6%–84.4%) (Figure 2D). The 2-year probabilities of OS after allo-HSCT were 40.0% (95% CI, 18.7%–85.5%) and 75.0% (95% CI, 42.6%–100.0%) for patients with DEK-NUP214 positivity and negativity, respectively, before allo-HSCT (P = 0.200).
Comparison between AML patients with and without DEK-NUP214
The characteristics of patients with the DEK-NUP214 transcript and intermediate-or high-risk AML patients without the DEK-NUP214 transcript are shown in Table 3. The 2-year cumulative incidence of relapse after allo-HSCT was comparable between the two cohorts (Figure 3A). However, the 2-year cumulative incidence of NRM after allo-HSCT in patients with DEK-NUP214 transcripts was higher than that in patients without DEK-NUP214 transcripts (Figure 3B). The 2-year probabilities of OS and LFS after allo-HSCT in patients with DEK-NUP214 transcripts were poorer than those in patients without DEK-NUP214 transcripts (Figure 3C and 3D).
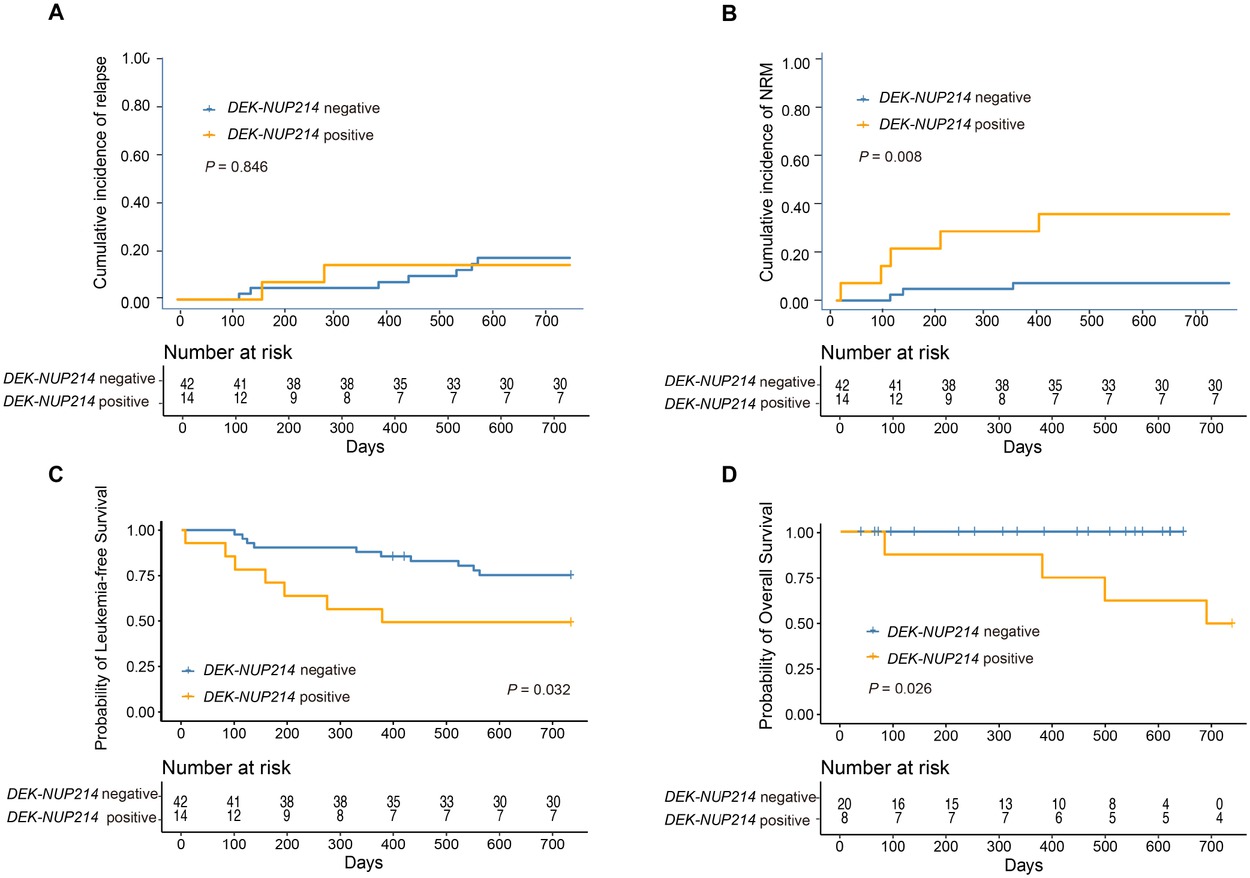
The 2-year probability of clinical outcomes after allo-HSCT according to patients with and without DEK-NUP214 transcript, relapse (A), non-relapse mortality (B), leukaemia-free survival (C), and overall survival (D).
Discussion
Owing to the rarity of AML in patients with DEK-NUP214, multicenter cooperative efforts are required to analyze the specific outcomes and the results of allo-HSCT. In the present study, we observed that the 2-year probabilities of relapse, NRM, LFS, and OS after allo-HSCT were 14.3%, 35.7%, 50.0%, and 50.0%, respectively, in AML patients with DEK-NUP214 transcripts. In particular, we observed that more than 75% of patients achieved MRD negativity after allo-HSCT and that pretransplant DEK-NUP214 positivity did not influence posttransplant outcomes. To our knowledge, this is the first and largest study focusing on the dynamic evolution of DEK-NUP214 positivity before and after allo-HSCT in patients with AML and DEK-NUP214 fusion.
Garcon et al.[5] reported that patients with AML with t(6;9) (p23;q34) who achieved consistent molecular remission, as assessed by real-time quantitative polymerase chain reaction (PCR), showed better survival than patients with persistent DEK-NUP214 positivity, implying a pivotal role for MRD monitoring in this AML subtype. In particular, they observed that all patients with persistent DEK-NUP214 positivity after chemotherapy died of refractory disease, except for one patient who died of toxicity.[5] In contrast, in the present study, we observed that most patients achieved MRD negativity after allo-HSCT, suggesting that allo-HSCT could overcome the poor prognosis of persistent DEK-NUP214 positivity after chemotherapy. This may be attributed to the graft-versus-leukemia (GVL) effect.[26] In addition, several studies have reported that allo-HSCT, particularly HID HSCT, could overcome the negative impact of pretransplant MRD positivity on posttransplant outcomes.[27, 28, 29, 30] In the present study, nearly 85% of the patients underwent HID HSCT, which may have also contributed to the high conversion of MRD positivity to negativity.
We observed that the 2-year cumulative incidence of relapse after allo-HSCT was only 14.3% in AML patients with DEK-NUP214, which was comparable to that in intermediate- or high-risk AML patients without DEK-NUP214. Previous studies showed that the relapse rate of AML patients with DEK-NUP214 was > 80% and the 5-year survival rate was only 9% for those who did not receive allo-HSCT.[2,31] Wang et al.[32] reported that the incidence of relapse was approximately 16% in adult patients with AML receiving allo-HSCT at CR1. Thus, allo-HSCT can overcome the effect of DEK-NUP214 on relapse in patients with AML. However, the 2-year cumulative incidence of NRM in the DEK-NUP214 group was 35.7%, which was higher than that in the group without DEK-NUP214. In previous studies, the cumulative incidence of NRM in HID HSCT was 11.6%–34.0%.[28,33,34,35] Considering that most patients received HID HSCT in the present study and the bias in the small sample size, our NRM incidence was not significantly higher than that in those who enrolled HID HSCT recipients. On the other hand, we found that the DEK-NUP214 group had a higher ELN risk (P = 0.001). Therefore, we believe it is possible that the high NRM incidence is associated with a high ELN risk baseline in the DEK-NUP214 group.
Our cohort showed that concomitant FLT3-ITD mutations were present in 28.5% of patients in the present cohort, which is lower than the results of previous studies by Oyarzo et al.[36] This may also contribute to the fact that only 3 patients received FLT3 inhibitors as maintenance therapy in our study. Although concomitant FLT3-ITD mutations may provide a therapeutic target, it is unclear whether FLT3 inhibitor maintenance therapy provides a survival benefit in AML patients with DEK-NUP214 transcripts after allo-HSCT.[37] In our study, one patient still relapsed despite the use of sorafenib as maintenance therapy. In a large cohort study of adult AML patients with DEK-NUP214, seven patients who had relapsed either after allo-HSCT or post-chemotherapy were treated with TKIs in isolation or in combination with chemotherapy, which failed to achieve a response in the majority of patients.[31] These findings suggest that novel treatment strategies are needed to improve patient outcomes. Because NUP214 is part of the nuclear pore complex and is a critical player in the nuclear export of proteins and mRNA, several inhibitors of nuclear export proteins, such as CRM1 and XPO1, may have potential therapeutic effects in patients with AML and DEK-NUP214 fusion.[38,39]
The maintenance of sorafenib therapy after allo-HSCT could further decrease the incidence of relapse and improve LFS[40,41] of AML patients with FLT3 mutation; however, Xuan et al.[40] reported that male patients who were MRD positivity before or after allo-HSCT could benefit from sorafenib maintenance therapy. Similarly, in MORPHO study,[42] the researchers also observed that the benefits of gilteritinib maintenance was restricted in patients with MRD positivity before allo-HSCT. Thus, the efficacy of FLT3 inhibitor maintenance for patients who were MRD negativity before allo-HSCT should be further identified. Lastly, patients received MRD monitoring regularly after allo-HSCT. Thus, patients who had positive MRD received preemptive therapy in the present study, which might help to prevent relapse in those without maintenance therapies.
This study had some limitations. Firstly, it was a retrospective study. In addition, due to the small sample size, some trends did not reach statistical significance. Further prospective studies or larger multicentre retrospective studies are needed to confirm and supplement our present study findings.
In summary, our study demonstrated that allo-HSCT can overcome the poor prognosis associated with persistent DEK-NUP214 positivity after chemotherapy in patients with AML. However, the posttransplant outcomes of AML patients with DEK-NUP214 transcripts were poorer than those of patients without DEK-NUP214 transcript. Future studies should identify better therapeutic strategies to improve the clinical outcomes of DEK-NUP214 subtype in AML.
Funding statement: This work was supported by the National Key Research and Development Program of China (2022YFC2502600, 2022YFC2502606, 2021YFA1101503), the National Natural Science Foundation of China (82170206, 82170208 and 81973998), CAMS Innovation Fund for Medical Sciences (2019-I2M-5-034, 2022-I2M-C&T-B-121), Peking University People’s Hospital Research and Development Funds (RZ2022-02), National High Level Hospital Clinical Research Funding (BJ-2022-169), and Shanghai Municipal Health Commission Project of Disciplines of Excellence (20234Z0002).
Acknowledgements
None.
-
Author Contributions
Mo XD and Hu XX designed the protocol; Fan S, Yang Y and Lu SY wrote the manuscript. Fan S and Yang Y performed the analysis; all authors contributed patients and provided clinical and laboratory data; all authors revised, corrected, and approved the manuscript. Fan S, Yang Y and Lu SY contributed equally to this manuscript.
-
Ethical Approval
This is a retrospective study. The study was conducted in accordance with the Declaration of Helsinki as well as the relevant guidelines and regulations.
-
Informed Consent
The need for informed consent was waived by IRB of Peking University People’s Hospital, Wuhan Tongji Hospital and Shanghai RuiJin Hospital because of the retrospective nature of the study.
-
Conflict of Interest
The authors declare no competing interests.
-
Use of Large Language Models, AI and Machine Learning Tools
None declared.
-
Data Availability Statement
The datasets generated during the analysis of the current study are available from the corresponding author on reasonable request.
References
1 Vardiman JW, Thiele J, Arber DA, Brunning RD, Borowitz MJ, Porwit A, et al. The 2008 revision of the World Health Organization (WHO) classification of myeloid neoplasms and acute leukemia: rationale and important changes. Blood 2009;114:937-951.10.1182/blood-2009-03-209262Suche in Google Scholar PubMed
2 Slovak ML, Gundacker H, Bloomfield CD, Dewald G, Appelbaum FR, Larson RA, et al. A retrospective study of 69 patients with t(6;9)(p23;q34) AML emphasizes the need for a prospective, multicenter initiative for rare ‘poor prognosis’ myeloid malignancies. Leukemia 2006;20:1295-1297.10.1038/sj.leu.2404233Suche in Google Scholar PubMed
3 Grimwade D, Hills RK, Moorman AV, Walker H, Chatters S, Goldstone AH, et al. Refinement of cytogenetic classification in acute myeloid leukemia: determination of prognostic significance of rare recurring chromosomal abnormalities among 5876 younger adult patients treated in the United Kingdom Medical Research Council trials. Blood 2010;116:354-365.10.1182/blood-2009-11-254441Suche in Google Scholar PubMed
4 Tarlock K, Alonzo TA, Moraleda PP, Gerbing RB, Raimondi SC, Hirsch BA, et al. Acute myeloid leukaemia (AML) with t(6;9)(p23;q34) is associated with poor outcome in childhood AML regardless of FLT3-ITD status: a report from the Children’s Oncology Group. Br J Haematol 2014;166:254-259.10.1111/bjh.12852Suche in Google Scholar PubMed PubMed Central
5 Garçon L, Libura M, Delabesse E, Valensi F, Asnafi V, Berger C, et al. DEK-CAN molecular monitoring of myeloid malignancies could aid therapeutic stratification. Leukemia 2005;19:1338-1344.10.1038/sj.leu.2403835Suche in Google Scholar PubMed
6 Wang L, Zhang C, Fan S, Mo X, Hu X. Treatment options for adult intermediate-risk AML patients in CR1: Allo-HSCT or chemotherapy? Innovation (Camb) 2023;4:100461.10.1016/j.xinn.2023.100461Suche in Google Scholar PubMed PubMed Central
7 Cao Y, Zhang C, Cao L, Mo X, Hu X. Quizartinib is a good option for AML patients with FLT3-ITD mutations. TIME 2023;1:100007.10.59717/j.xinn-med.2023.100007Suche in Google Scholar
8 Ishiyama K, Takami A, Kanda Y, Nakao S, Hidaka M, Maeda T, et al. Prognostic factors for acute myeloid leukemia patients with t(6;9) (p23;q34) who underwent an allogeneic hematopoietic stem cell transplant. Leukemia 2012;26:1416-1419.10.1038/leu.2011.350Suche in Google Scholar PubMed
9 Ishiyama K, Takami A, Kanda Y, Nakao S, Hidaka M, Maeda T, et al. Allogeneic hematopoietic stem cell transplantation for acute myeloid leukemia with t(6;9)(p23;q34) dramatically improves the patient prognosis: a matched-pair analysis. Leukemia 2012;26:461-464.10.1038/leu.2011.229Suche in Google Scholar PubMed
10 Díaz-Beyá M, Labopin M, Maertens J, Aljurf M, Passweg J, Dietrich B, et al. Allogeneic stem cell transplantation in AML with t(6;9) (p23;q34);DEK-NUP214 shows a favourable outcome when performed in first complete remission. Br J Haematol 2020;189:920-925.10.1111/bjh.16433Suche in Google Scholar PubMed
11 Mrózek K, Marcucci G, Nicolet D, Maharry KS, Becker H, Whitman SP, et al. Prognostic significance of the European LeukemiaNet standardized system for reporting cytogenetic and molecular alterations in adults with acute myeloid leukemia. J Clin Oncol 2012;30:4515-4523.10.1200/JCO.2012.43.4738Suche in Google Scholar PubMed PubMed Central
12 Cao Y, Huo W, Huang J, Yang Y, Wang Y, Chang Y, et al. MRD positivity was the poor prognostic factor for adverse-risk AML patients with allogeneic hematopoietic stem cell transplantation: a multicenter TROPHY study. Blood Cancer J 2024;14:8.10.1038/s41408-024-00976-1Suche in Google Scholar PubMed PubMed Central
13 Shen MZ, Hong SD, Lou R, Chen RZ, Zhang XH, Xu LP, et al. A comprehensive model to predict severe acute graft-versus-host disease in acute leukemia patients after haploidentical hematopoietic stem cell transplantation. Exp Hematol Oncol 2022;11:25.10.1186/s40164-022-00278-xSuche in Google Scholar PubMed PubMed Central
14 Shen MZ, Hong SD, Wang J, Zhang XH, Xu LP, Wang Y, et al. A Predicted Model for Refractory/Recurrent Cytomegalovirus Infection in Acute Leukemia Patients After Haploidentical Hematopoietic Stem Cell Transplantation. Front Cell Infect Microbiol 2022;12:862526.10.3389/fcimb.2022.862526Suche in Google Scholar
15 Fan S, Hong HY, Dong XY, Xu LP, Zhang XH, Wang Y, et al. Machine learning algorithm as a prognostic tool for Epstein-Barr virus reactivation after haploidentical hematopoietic stem cell transplantation. Blood Sci 2023;5:51-59.10.1097/BS9.0000000000000143Suche in Google Scholar PubMed PubMed Central
16 Deng DX, Fan S, Zhang XH, Xu LP, Wang Y, Yan CH, et al. Immune Reconstitution of Patients Who Recovered From Steroid-Refractory Acute Graft-Versus-Host Disease After Basiliximab Treatment. Front Oncol 2022;12:916442.10.3389/fonc.2022.916442Suche in Google Scholar PubMed PubMed Central
17 Deng D, Shen M, Zhang X, Xu L, Wang Y, Yan C, et al. Basiliximab is the potential solution for severe liver chronic GVHD: A prospective pilot study. TIME 2023;1:100009.10.59717/j.xinn-med.2023.100009Suche in Google Scholar
18 Xu Z, Mo X, Kong Y, Wen Q, Han T, Lyu M, et al. Mini-dose methotrexate combined with methylprednisolone as a first-line treatment for acute graft-versus-host disease: A phase 2 trial. J Transl Int Med 2023;11:255-264.10.2478/jtim-2023-0111Suche in Google Scholar PubMed PubMed Central
19 Liu Y, Duan W, Huang X, Lu J. A first case report of using chimeric antigen receptor T-cell immunotherapy to treat high-risk smoldering multiple myeloma. J Transl Int Med 2023;11:294-296.10.2478/jtim-2023-0097Suche in Google Scholar PubMed PubMed Central
20 Wang Y, Chen H, Chen J, Han M, Hu J, Huang H, et al. The consensus on the monitoring, treatment, and prevention of leukemia relapse after allogeneic hematopoietic stem cell transplantation in China. Cancer Lett 2018;438:63-75.10.1016/j.canlet.2018.08.030Suche in Google Scholar PubMed
21 Laboratory Diagnosis Group, Chinese Society of Hematology, Chinese Medical Association Chinese consensus on minimal residual disease detection and interpretation of patients with acute myeloid leukemia. Zhonghua Xueyexue Zazhi 2021;42:889-97.Suche in Google Scholar
22 Chinese Society of Hematology, Chinese Medical Association The consensus of allogeneic hematopoietic transplantation for hematological diseases in China (2016)-- post- transplant leukemia relapse. Zhonghua Xueyexue Zazhi 2016;37:846-51.Suche in Google Scholar
23 Gao MG, Fu Q, Qin YZ, Chang YJ, Wang Y, Yan CH, et al. Prognostic significance of DEK-NUP214 fusion gene in patients with acute myeloid leukemia after allogeneic hematopoietic stem cell transplantation. Zhonghua Neike Zazhi 2021;60(10):868-74.Suche in Google Scholar
24 Liu J, Ma R, Liu YR, Xu LP, Zhang XH, Chen H, et al. The significance of peri-transplantation minimal residual disease assessed by multiparameter flow cytometry on outcomes for adult AML patients receiving haploidentical allografts. Bone Marrow Transplant 2019;54:567-577.10.1038/s41409-018-0300-8Suche in Google Scholar
25 Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Statist Med 1999;18: 695-706.10.1002/(SICI)1097-0258(19990330)18:6<695::AID-SIM60>3.3.CO;2-FSuche in Google Scholar
26 Lv M, Shen M, Mo X. Development of allogeneic hematopoietic stem cell transplantation in 2022: Regenerating “Groot” to heal the world. Innovation (Camb) 2023;4:100373.10.1016/j.xinn.2023.100373Suche in Google Scholar
27 Buccisano F, Maurillo L, Piciocchi A, Del Principe MI, Picardi A, Cerretti R, et al. Pre-transplant persistence of minimal residual disease does not contraindicate allogeneic stem cell transplantation for adult patients with acute myeloid leukemia. Bone Marrow Transplant 2017;52:473-475.10.1038/bmt.2016.308Suche in Google Scholar
28 Chang YJ, Wang Y, Liu YR, Xu LP, Zhang XH, Chen H, et al. Haploidentical allograft is superior to matched sibling donor allograft in eradicating pre-transplantation minimal residual disease of AML patients as determined by multiparameter flow cytometry: a retrospective and prospective analysis. J Hematol Oncol 2017;10:134.10.1186/s13045-017-0502-3Suche in Google Scholar
29 Chang YJ, Pei XY, Huang XJ. Haematopoietic stem-cell transplantation in China in the era of targeted therapies: current advances, challenges, and future directions. Lancet Haematol 2022;9:e919-e929.10.1016/S2352-3026(22)00293-9Suche in Google Scholar
30 Cai Z, Fan S, Sun X, Mo X, Yang G. Novel microfluidic device for measurable residual disease detection in acute leukemia. Innovation (Camb) 2023;4:100408.10.1016/j.xinn.2023.100408Suche in Google Scholar
31 Kayser S, Hills RK, Luskin MR, Brunner AM, Terré C, Westermann J, et al. Allogeneic hematopoietic cell transplantation improves outcome of adults with t(6;9) acute myeloid leukemia: results from an international collaborative study. Haematologica 2020;105:161-169.10.3324/haematol.2018.208678Suche in Google Scholar
32 Wang Y, Liu QF, Xu LP, Liu KY, Zhang XH, Ma X, et al. Haploidentical vs identical-sibling transplant for AML in remission: a multicenter, prospective study. Blood 2015;125:3956-3962.10.1182/blood-2015-02-627786Suche in Google Scholar
33 Huang T, Xu L, Zhang X, Chang Y, Mo X, Sun Y, et al. Haploidentical haematopoietic stem cell transplantation for TP53-mutated acute myeloid leukaemia. Br J Haematol 2023;200:494-505.10.1111/bjh.18538Suche in Google Scholar
34 Wang Y, Liu DH, Xu LP, Liu KY, Chen H, et al. Superior graft-versus-leukemia effect associated with transplantation of haploidentical compared with HLA-identical sibling donor grafts for high-risk acute leukemia: an historic comparison. Biol Blood Marrow Transplant 2011;17:821-830.10.1016/j.bbmt.2010.08.023Suche in Google Scholar PubMed
35 Cho BS, Min GJ, Park S, Park SS, Shin SH, Yahng SA, et al. Haploidentical vs matched unrelated donor transplantation for acute myeloid leukemia in remission: A prospective comparative study. Am J Hematol 2021;96:98-109.10.1002/ajh.25993Suche in Google Scholar PubMed
36 Oyarzo MP, Lin P, Glassman A, Bueso-Ramos CE, Luthra R, Medeiros LJ. Acute myeloid leukemia with t(6;9)(p23;q34) is associated with dysplasia and a high frequency of flt3 gene mutations. Am J Clin Pathol 2004;122:348-358.10.1309/5DGB59KQA527PD47Suche in Google Scholar
37 Stahl M, Tallman MS. Outcomes of allogeneic stem cell transplantation for patients with t(6:9) AML- A strong case for allogeneic stem cell transplantation in first complete remission. Br J Haematol 2020;189:806-808.10.1111/bjh.16478Suche in Google Scholar PubMed
38 Mendes A, Fahrenkrog B. NUP214 in Leukemia: It’s More than Transport. Cells 2019;8:76.10.3390/cells8010076Suche in Google Scholar PubMed PubMed Central
39 Takeda A, Yaseen NR. Nucleoporins and nucleocytoplasmic transport in hematologic malignancies. Semin Cancer Biol 2014;27:3-10.10.1016/j.semcancer.2014.02.009Suche in Google Scholar PubMed
40 Xuan L, Wang Y, Huang F, Fan Z, Xu Y, Sun J, et al. Sorafenib maintenance in patients with FLT3-ITD acute myeloid leukaemia undergoing allogeneic haematopoietic stem-cell transplantation: an open-label, multicentre, randomised phase 3 trial. Lancet Oncol 2020;21:1201-1212.10.1016/S1470-2045(20)30455-1Suche in Google Scholar PubMed
41 Xuan L, Wang Y, Yang K, Shao R, Huang F, Fan Z, et al. Sorafenib maintenance after allogeneic haemopoietic stem-cell transplantation in patients with FLT3-ITD acute myeloid leukaemia: long-term follow-up of an open-label, multicentre, randomised, phase 3 trial. Lancet Haematol 2023;10:e600-e611.10.1016/S2352-3026(23)00117-5Suche in Google Scholar PubMed
42 Levis MJ. BMT CTN 1506/MORPHO trial which evaluated maintenance gilteritinib after allogeneic stem cell transplant in patients with FLT3-ITD–positive acute myeloid leukemia. Available from: https://www.targetedonc.com/view/key-data-from-the-bmt-ctn-1506-morpho-trialof-gilteritinib-in-flt3-itdaml.Suche in Google Scholar
© 2025 Shuang Fan, Yang Yang, Shengye Lu, Jiayu Huang, Xiaosu Zhao, Yang Cao, Xiaodong Mo, Xiaoxia Hu, published by De Gruyter on behalf of the SMP
This work is licensed under the Creative Commons Attribution 4.0 International License.
Artikel in diesem Heft
- Perspective
- Mechanisms and treatment of atrial fibrillation and stroke
- Dysfunction of monocyte in the development of HBV-ACLF: Immune activation or immune suppression?
- Induced pluripotent stem cells in the treatment of ischemic stroke
- Artificial intelligence in glycemic management for diabetes: Applications, opportunities and challenges
- Review Article
- NETosis and pyroptosis of immune cells in sepsis
- The roles of subcellular Argonaute 2 in cardiovascular diseases
- Original Article
- B10 cell-induced PD-L1/PD-1-linked macrophage polarization in periodontitis
- Is integrative therapy of traditional Chinese medicine and progesterone capsule more effective than monotherapies in oligomenorrhea and hypomenorrhea? Evidence based on a multi-center randomized controlled trial and metabolomic profile
- Definition and validation of recompensation in patients with primary biliary cholangitis-related decompensated cirrhosis treated with ursodeoxycholic acid: Based on the BAVENO VII criteria
- DEK-NUP214 monitoring before and after allogeneic haematopoietic stem cell transplantation for acute myeloid leukemia: A report from the TROPHY study group
Artikel in diesem Heft
- Perspective
- Mechanisms and treatment of atrial fibrillation and stroke
- Dysfunction of monocyte in the development of HBV-ACLF: Immune activation or immune suppression?
- Induced pluripotent stem cells in the treatment of ischemic stroke
- Artificial intelligence in glycemic management for diabetes: Applications, opportunities and challenges
- Review Article
- NETosis and pyroptosis of immune cells in sepsis
- The roles of subcellular Argonaute 2 in cardiovascular diseases
- Original Article
- B10 cell-induced PD-L1/PD-1-linked macrophage polarization in periodontitis
- Is integrative therapy of traditional Chinese medicine and progesterone capsule more effective than monotherapies in oligomenorrhea and hypomenorrhea? Evidence based on a multi-center randomized controlled trial and metabolomic profile
- Definition and validation of recompensation in patients with primary biliary cholangitis-related decompensated cirrhosis treated with ursodeoxycholic acid: Based on the BAVENO VII criteria
- DEK-NUP214 monitoring before and after allogeneic haematopoietic stem cell transplantation for acute myeloid leukemia: A report from the TROPHY study group

