Ongoing transmission of HCV: Should cesarean section be justified? Data mining discovery
-
Abd Elrazek
, Samy Saab
Abstract
Background and Objectives
Over the past few decades, cesarean section (CS) rates are steadily increasing in most of the middle- and high-income countries. However, most of the pregnant women (particularly undergoing CS) are not screened for hepatitis C virus (HCV); hence, neonates born to HCV-positive mother could be a source of future HCV infection. In this study, the role of the CS and other surgical interventions in HCV transmission in Egypt, the highest endemic country of HCV-4, was investigated.
Methods
From January to June 2016, a prospective cohort study was conducted among 3,836 pregnant women in both urban and rural areas across Egypt for HCV screening in both mothers and neonates born to HCV-positive mother. All pregnant women were screened during third trimester or just before delivery, neonates born to HCV-positive mothers were evaluated within 24-h postdelivery to record vertical transmission cases. Data mining (DM)-driven computational analysis was used to quantify the findings.
Results
Among 3,836 randomized pregnant women, HCV genotype 4 was identified in 80 women (2.08%). Out of 80 HCV-infected women, 18 have experienced surgical intervention (22.5%) and 62 CS (77.5%). HCV vertical transmission was identified in 10 neonates, 10/80 (12.5%).
Conclusion
Screening women who had experienced surgical intervention or CS during child bearing period and before pregnancy might prevent HCV mother-to-child transmission (MTCT). CS should be ethically justified to decrease global HCV transmission.
Introduction
Globally, the prevalence of hepatitis C virus (HCV) infection in pregnant women is nearly 2–3% of the general population and around 3–10% of the vertically transmitted infections in children[1, 2]. Although HCV can infect placental cells, most of the children are not regularly tested for perinatal HCV. However, a greater risk of vertical HCV transmission to the offspring may be much higher especially in HCV endemic countries such as Egypt. Egypt recorded the highest prevalence (14.7%) of HCV antibody (Ab) worldwide in 2014[3]. It is noteworthy to know that the risks involved in cesarean section (CS) delivery have a significant high impact on vertical and horizontal HCV transmission[4, 5]. The risk of transmission (one in every 20 children) delivered by chronic HCV-infected women highlights that vertical transmission likely constitutes the primary transmission route among children. These updated estimates are considered as a basis for decision making in prioritization of the research into risk-reducing measures[6-8]. Recently, data mining (DM) programming allows clinicians to extract hidden factors that lead to such disease progression or regression in an artificial intelligence manner[9]. The aim of this study was to estimate the risk of cesarean-section-associated vertical and horizontal HCV transmission in an Egyptian cohort.
Methods
We prospectively followed a cohort of 3,836 pregnant women of age between 18 and 43 years (childbearing period), attending prenatal care, unaware of HCV infection, from urban and rural areas across Egypt from January to June 2016. All the pregnant women were screened for HCV infection by third-generation ELISA and quantitative polymerase chain reaction (qPCR) tests. Patients who showed HCV-positive results were regularly followed up during the study period in randomized groups representing all Egyptian governorates. Furthermore, HCV-related complications such as cirrhosis, hepatocellular carcinoma (HCC), and esophageal varices were also investigated using noninvasive 2D ultrasound (US); additionally, routine laboratory tests were performed during pregnancy. Furthermore, all neonates born to HCV-pregnant women were investigated in the first 24 h postdelivery and before breastfeeding for both HCV Abs and HCV-RNA using ELISA and qPCR tests, respectively. Pregnant women positive to Abs and negative in qPCR test were excluded from the study. Those co-infected with HBV or HIV were also excluded.
All infected pregnant women were HCV genotype 4, the predominant genotype in Egypt. Demographic, laboratory, and clinical information were collected and entered into Microsoft Excel 2010.
Operational definitions
HCV-positive woman is defined as pregnant women who have both anti-HCV Abs and HCV-RNA or HCV core antigen detected in their blood.
Vertical HCV transmission is defined as viral transmission from the mother to the infant during pregnancy, at the time of delivery, or during the first 4 weeks after delivery.
HCV-testing: HCV status for all pregnant women was tested at the study sites on fresh plasma samples using both third-generation ELISA (for HCV Ab), and real-time PCR (for HCV-RNA).
Statistical analysis
The descriptive data were summarized as frequencies, percentages, and mean with standard deviations (SD). Chi-square test was applied for testing relationships on categorical variables. Differences were considered statistically significant when P-value is less than 0.01. The model’s discriminatory ability was verified through the operational characteristic curve, and the Hosmer– Lemeshow test was applied to check the calibration of model. All the statistical analysis were conducted using SPSS software, version 22.0 (IBM, Somers, NY, USA).
Data mining analysis
DM analysis is a computational machine learning process of examining large amount of data to create an applicable algorithm. Conventional statistics is used to examine a certain hypothesis. In this context, DM is superior as it makes computerized algorithms using the decision tree method created by the power of computational intelligence. Additionally, 10-fold cross-validation using naïve Bays applications are generally used to predict the performance of a model on a valid action set using computation analysis superior to statistical performance.
Ethical clearance
The study protocol was approved by Ethical Committee of Aswan School of Medicine, Aswan University, Egypt. Written informed consent was obtained from each pregnant women enrolled in the study.
Results
Among 3836 pregnant women screened for HCV, 620 were nulliparous (16.1%), 1,891 (49.3%) monoparous, and 1,325 (34.5 %) multiparous. Additionally, 3,682 (96%) were free of any medical diseases, 68 (1.7%) had history of hypertension, 46 (1.0%) had history of diabetes mellitus, 27 (0.7%) had history of cardiac diseases, and only 11 pregnant women (0.3%) had history of thyroid disorders [Table 1]. A case wise identification of HCV vertical transmission among study population was shown in Figure 1.
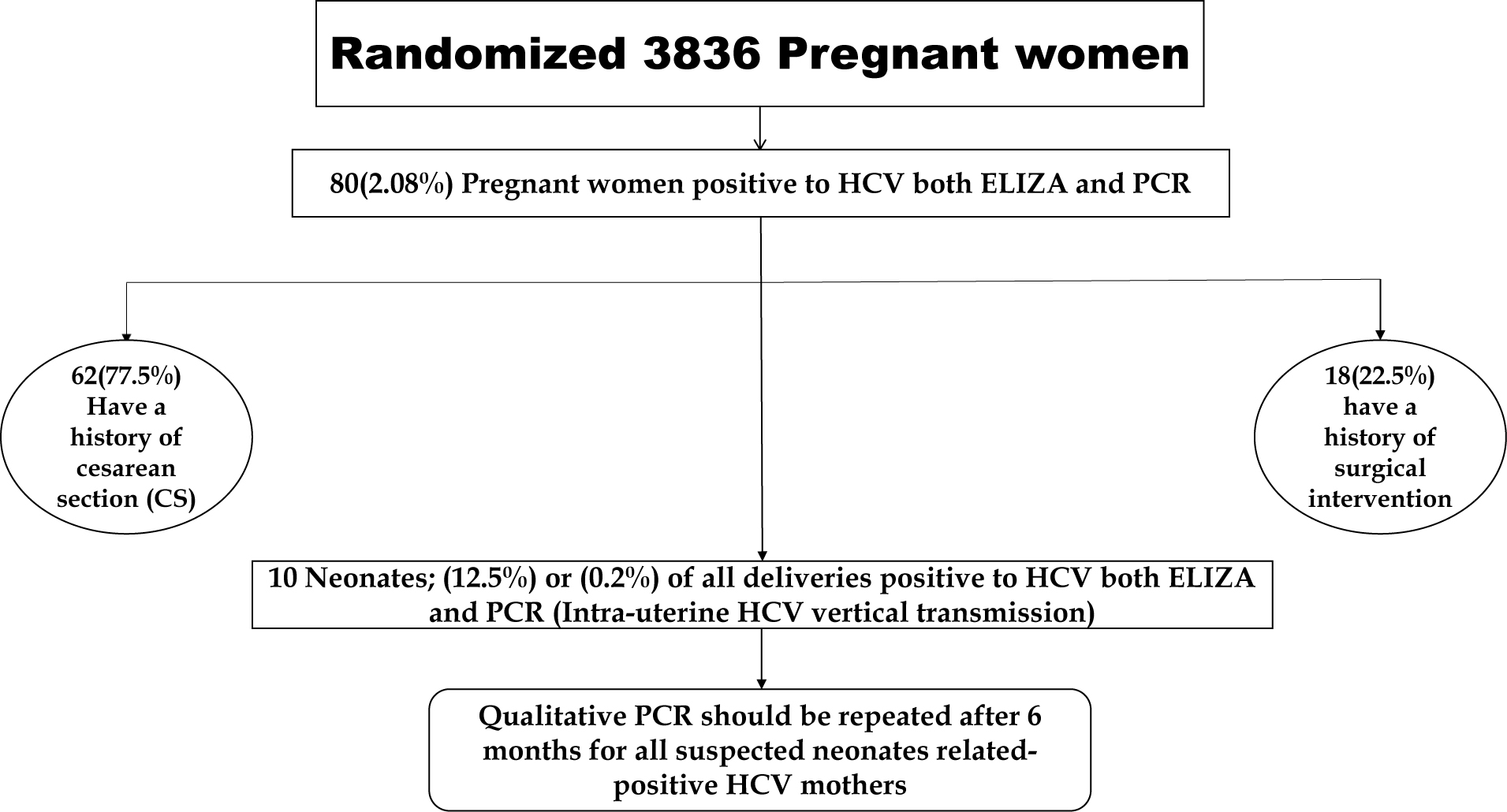
Modified decision tree modified by Rapid I, Berlin, Germany, showing the most independent factor for HCV ongoing transmission. PCR: polymerase chain reaction; HCV: hepatitis C virus; ELISA: enzyme-linked immunosorbent assay.
Clinical characteristics of pregnant women (N = 3,836)
| Characteristics | N (%) or mean (SD) |
|---|---|
| Age (years) | 27.3 ± 4.8 |
| BMI (kg/m2) | 24.17 ± 3.23 |
| Parity | |
| p0 + 0 | 620 (16.1) |
| p1-3 | 1,891 (49.3) |
| >p3 | 1,325 (34.5) |
| Past medical history | |
| Free | 3,682 (96) |
| Hypertension | 68 (1.7) |
| Diabetes mellitus | 46 (1.0) |
| Cardiac | 27 (0.7) |
| Thyroid | 11 (0.3) |
| Gallbladder stones | 2 (–) |
| Past surgical history | 1,241 ( 32.3) |
| Caesarean section | 961 (25.0) |
| Blood transfusion | 28 (0.7) |
SD: standard deviation; BMI: body mass index
Eighty pregnant women (2.08%) showed positive HCV infection by both ELISA and real-time PCR HCV-RNA during third trimester or just before delivery. However, positive HCV antibodies were noticed in 99 pregnant women (2.6%). All HCV-positive women had history of surgical intervention (22.5%) or CS (77.5%). The mean (±SD) HCV-RNA identified by real-time PCR is 878,371 ±153,182.9 in HCV-positive women, ranging from 11,400 to 5,000,000. Ten neonates (21.5%) of all 80 deliveries showed positive HCV by both ELISA and PCR RNA within the first 24 h after delivery.
In the multivariate model, the area under the curve (AUC) value of the receiver operating characteristic curve was 0.709 (95% CI = 0.638–0.781). Calibration of the model by the Hosmer–Lemeshow test showed no significance (P = 1.000), which indicates that the model was correctly calibrated.
DM analysis showed that the only independent risk factor for such HCV transmission was CS; however, high viral load was an additional factor for vertical HCV transmission.
Discussion
HCV can cause acute and chronic hepatitis. Chronic HCV often progresses over many years, resulting in cirrhosis, HCC, liver cell failure, and the need for liver transplantation [10]. However, a considerable number of HCV-positive women are giving birth and most of their children are not being tested perinatally for HCV infection. The overall rate of mother-to-child transmission (MTCT) is 3–5%; however, coinfection with HIV increases the MTCT rate of HCV significantly. Mode of delivery does not represent significant risk factor for HCV vertical transmission; hence, infected HCV pregnant women not advised to have CS unless indicated for other causes, whatever if minded theoretically, exposure to HCV-infected blood as the infant passes through the birth canal during normal vaginal delivery (NVD) may lead to HCV transmission. However, exposure to maternal blood can also occur with CS; accordingly, there is no reason to offer elective caesarean section to HCV-infected parturients[11-14], with respect to special circumstances when CS is preferred to NVD, such as membranous rupture and internal fetal monitoring are confirmed to be associated with HCV transmission.
CS and its complications were the main cause of death in many developing countries [15-18]. Egypt has the highest HCV prevalence worldwide; therefore, our results showed that the prevalence of HCV in a sample of Egyptian pregnant women was 2.08% (80 of 3,836 women), based on HCV Ab and PCR testing. All HCV genotype 4 pregnant women had history of surgical operations, likely the route of HCV transmission. Furthermore, Data suggest that CS, which accounted for 77.5% of all surgical interventions, can transmit HCV. In addition, 10 in 80 neonates (12.5%) showed HCV positivity by ELISA and PCR during the first 24 hours after delivery, confirming intrauterine HCV transmission. Our current study showed the significance of CS over other surgical procedures in overall HCV transmission. In addition, CS is more associated with maternal complications than NVD. Furthermore, some morbidity in childhood-associated cesareans seems more significant than what was believed. A recent article from Italy recommended that the most direct way for reducing CS in the country could be the formal prohibition of CS section based on maternal request [19-21]. There is little information about the timing of MTCT; currently, no interventions to decrease transmission rates have been identified yet [22, 23]. However, all vertical transmissions might occurred during intrauterine life because all children are evaluated during the first 24 h after delivery. HCV-RNA should be positive until 1–2 weeks following MTCT, when levels of HCV viremia reach the optimal threshold.
A history of blood transfusion, CS, and other surgical interventions are significant sources of HCV in Egyptian pregnant women using both traditional statistical methods and DM computational analysis. All pregnant women included in this study were from rural or urban areas and were unaware of vertical transmission of HCV or the future complications associated with such transmission to their children; therefore, they did not disclose their status to the obstetrician. Prenatal care and screening of neonates born to HCV-positive women can identify the infected group and determine the factors leading to current HCV transmission. For all reasons, screening babies born to HCV-positive mothers may discover disease early. As a result of all mentioned obstacles and the limits of HCV screening in pregnant women, it is more likely that CS will continue to be a source of such HCV transmission worldwide, especially in developing countries where inadequately tested blood, unsterile medical injections, and un-medical indication of CS without ethical justification still remain. Failure to identify all HCV-infected women may drive the world to adopt universal prenatal HCV screening. Hence, we need a worldwide recommendation to reduce the cesarean section rate by attenuating the birth fear of patients, mass education about benefits of vaginal delivery, encouraging vaginal delivery by social media and teaching national seminars, and providing comprehensive counseling with all health providers about the medical issues of labor.
DM is a breakthrough in computational analysis overcoming mathematical and statistical analyses in space, labor, biology, engineering and different computer sciences, and, recently, medicine. The use of DM in prediction medicine should discover the important factors related to disease progression or regression by extracting hidden factors that have never been identified by the usual statistical programs. The use of DM in clinical medicine is important for morbidity and mortality prediction. For example, whenever we sign up for an Internet shopping website using VISA card, place a purchase using a credit card, or surf the Web, we are creating every purchasing data. Furthermore, these data are stored in large sets on powerful computers owned by the companies we deal with every day that they understand our mind for purchase. The same goes for physicians and researchers who understand each disease behavior [24-31].
Simple distribution Naïve Bayes and decision tree created by Rapid I version 4.6 emphasized the level of maternal HCV-related vertical transmission; > 3× 106 IU should be considered as the high level of vertical prediction, reported in our previous work [13], and CS should be likely an important route of global HCV transmission especially in developing countries [Figures 1 and 2]. However, the level of maternal viremia is still a matter of scientific debate in such vertical transmission. We have created a figure showing the global HCV transmission [Figure 3].
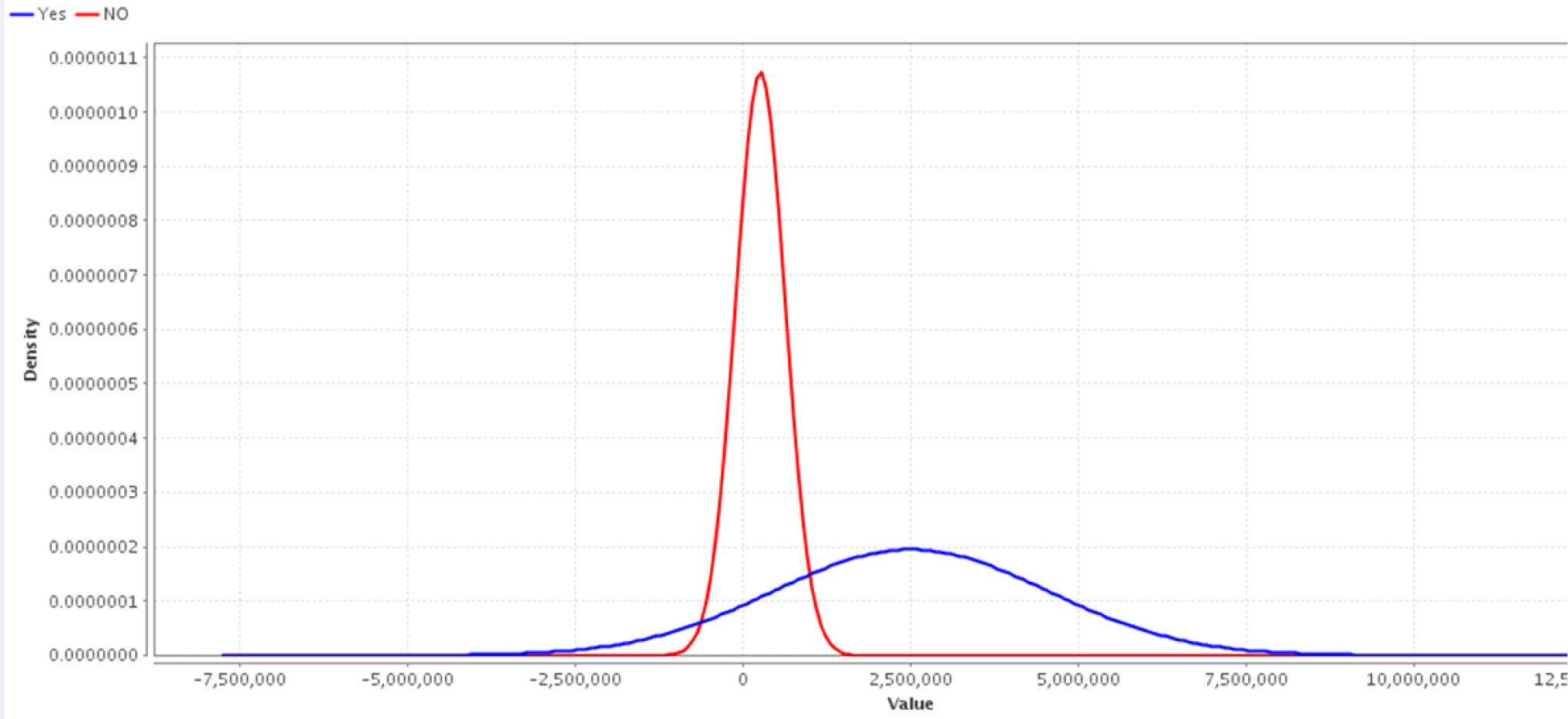
Simple distribution Naïve Bayes diagram created by Rapid I version 4.6 shows the level of maternal HCV G4 related vertical transmission; > 3× 106 IU should be considered as the high level of vertical prediction. PCR, polymerase chain reaction
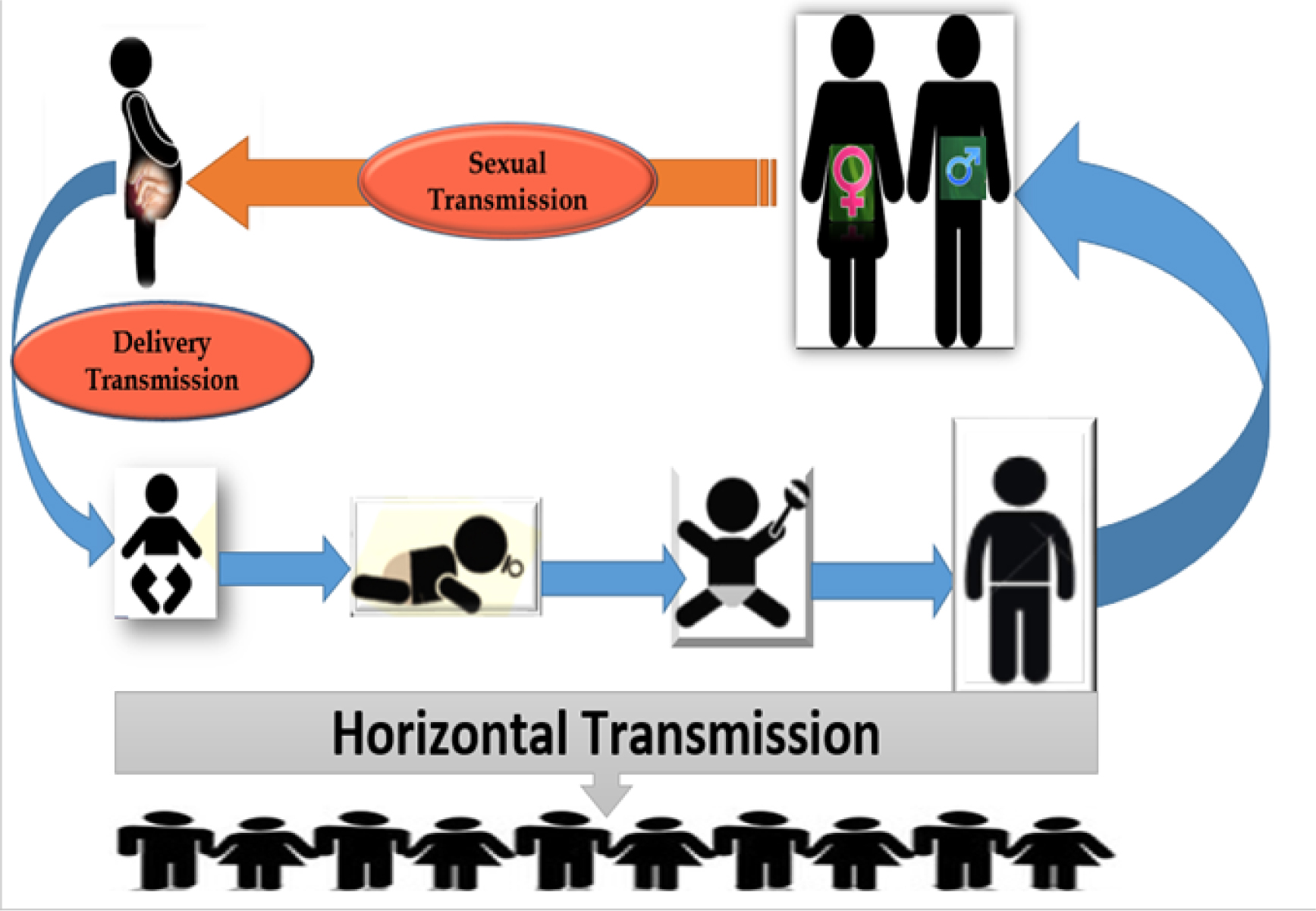
Global HCV transmission—based on MTCT.
In conclusion, screening women who had experienced surgical intervention or CS during child bearing period and before pregnancy might prevent HCV MTCT. CS should be ethically justified to decrease global HCV transmission.
Recommendations
A. Ongoing HCV vertical transmission may force the world to adopt universal recommendations for screening survey of all women planning for pregnancy, especially in resource-limited settings; furthermore, HCV-infected women should delay pregnancy till sustained virologic response achievement.
B. Probably, we need a trial research study to use anti-HCV therapy during pregnancy for those infected with HCV or coinfected with HIV/HBV to prevent MTCT, such as anti-HIV and hepatitis B during pregnancy approved by US Food and Drug Administration (FDA).
C. Using DM in clinical medicine should be encouraged to shed light on the disease progression or regression using advanced computer intelligence.
HCV in pregnant women (N = 3,836)
| N (%) or mean (SD) | P value | |
|---|---|---|
| HCV-RNA (positive) | 80 (2.1) | <0.001 |
| HCV antibodies (positive) | 99 (2.6) | <0.001 |
| HCV-RNA (Real-time PCR) | 878,371 ± 153,182.9 (range: 11,400–5,000,000) | 0.002 |
Significance levels at P < 0.01. SD: standard deviation; HCV: hepatitis C virus; PCR: polymerase chain reaction.
Limitation of the study
The study approved intrauterine HCV transmission and detected HCV viremia in neonates’ born to HCV-positive mothers in the first 24 h postdelivery; however, some neonates showed viremia later on (3–6 months) postdelivery because of very low HCV viremia at the stage of delivery; additionally, in many of the HCV-infected neonates, the virus was totally cleared 6 months postdelivery (ongoing research).
Conflict of interest
All authors declare no conflict of interest
Acknowledgment
We would like to thank Prof. Mansour Kabbash, President of Aswan University, for his gratitude assistance for scientific publication promotion activity at Aswan, Egypt.
References
1 MohdHanafiah K, Groeger J, Flaxman AD, Wiersma ST. Global epidemiology of hepatitis C virus infection: new estimates of age-specific antibody to HCV seroprevalence. Hepatol 2013; 57:1333–42.10.1002/hep.26141Search in Google Scholar PubMed
2 Cottrell EB, Chou R, Wasson N, Rahman B, Gulse JM. Reducing risk for mother-to-infants of Hepatitis C virus: A systematic review for the U.S preventive services task force. Ann Intern Med 2013; 158:109-13.10.7326/0003-4819-158-2-201301150-00575Search in Google Scholar PubMed
3 AbdElrazek AE, Bilasy SE, Elbanna AE, Elsheriff AE. Prior to the Oral therapy, what do we know about HCV-4 in Egypt. A randomized study using data mining computational analysis. Medicine (Baltimore) 2014; 93: e20410.1097/MD.0000000000000204Search in Google Scholar PubMed PubMed Central
4 Tosone G, Maraolo AE, Mascolo S, Palmiero G, Tambaro O, Orlando R. Vertical hepatitis C virus transmission: main questions and answers. World J Hepatol 2014; 6:538-48.10.4254/wjh.v6.i8.538Search in Google Scholar PubMed PubMed Central
5 an CQ, Zou HB, Chen Y, Zhang XH, Zhang H, Li J, et al. Cesarean section reduces perinatal transmission of hepatitis B virus infection from hepatitis B surface antigen-positive women to their infants. Clin Gastroenterol Hepatol 2013; 11: 1349–55.10.1016/j.cgh.2013.04.026Search in Google Scholar PubMed
6 Benova L, Mohamoud YA, Calvert C, Abu-Raddad L. Vertical transmission of hepatitis C virus: A systematic review and meta-analysis. Clin Infect Dis 2014; 9:765-73.10.1093/cid/ciu447Search in Google Scholar PubMed PubMed Central
7 Suzuki M, Tajiri H, Tanaka Y, Takano T, Miyoshi Y, Murakami J, et al. Peginterferon therapy in children with chronic hepatitis C: A Nationwide, Multi-center Study in Japan, 2004-2013. Pediatr Gastroenterol Nutr 2016; 63: 88-93.10.1097/MPG.0000000000001120Search in Google Scholar PubMed
8 Guo X, Yang G, Yuan J, Ruan P, Zhang M, Chen X, et al. Genetic Variation in Interleukin 28B and Response to Antiviral Therapy in Patients with Dual Chronic Infection with Hepatitis B and C Viruses. PLoS One 2013; 8: e77911.10.1371/journal.pone.0077911Search in Google Scholar PubMed PubMed Central
9 Abd Elrazek Abd Elrazek, Ahmed Elbanna. Validation of Data mining in clinical medicine. Appl Math Inf Sci 2016; 10: 1637-40.10.18576/amis/100443Search in Google Scholar
10 Tovo PA, Calitri C, Scolfaro C, Gabiano C, Garazzino S. Vertically acquired hepatitis C virus infection: Correlates of transmission and disease progression. World J Gastroenterol 2016; 22: 1382-92.10.3748/wjg.v22.i4.1382Search in Google Scholar PubMed PubMed Central
11 Kuncio DE, Newbern EC, Johnson CC, Viner KM. Failure to Test and Identify Perinatally Infected Children Born to Hepatitis C-Virus-Infected Women. Clin Infect Dis 2016; 62: 980-5.10.1093/cid/ciw026Search in Google Scholar PubMed
12 Prasad MR, Honegger JR. Hepatitis C virus in pregnancy. Am J Perinatol 2013; 30:149-59.10.1055/s-0033-1334459Search in Google Scholar PubMed PubMed Central
13 Elrazek A, Amer M, El-Hawary B, Salah A, Bhagavathula AS, Alboraie M, et al. Prediction of HCV vertical transmission: what are factors should be optimized using data mining computational analysis. Liv Int 2016; June 16. 10.1111/liv.13146.Search in Google Scholar PubMed
14 Tovo PA, Palomba E, Ferraris G, Principi N, Ruga E, Dallacasa P, et al. Increased risk of maternal-infant hepatitis C virus transmission for women coinfectedwith human immunodeficiency virus type 1. Italian Study Group for HCV Infection in Children. Clin Infect Dis 1997; 25: 1121–4.10.1086/516102Search in Google Scholar PubMed
15 Nokhodian Z, Yazdani MR, Yaran M, Shoaei P, Mirian M, Ataei B, et al., Prevalence and risk factors of HIV, syphilis, hepatitis B and C among female prisoners in Isfahan, Iran. Hepat Mon 2012; 12: 442–7.10.5812/hepatmon.6144Search in Google Scholar PubMed PubMed Central
16 Mohamoud YA, Mumtaz GR, Riome S, Miller D, Abu-Raddad LJ. The epidemiology of hepatitis C virus in Egypt: a systematic review and data synthesis. BMC Infect Dis 2013; 13: 288.10.1186/1471-2334-13-288Search in Google Scholar PubMed PubMed Central
17 Le Campion A, Larouche A, Fauteux-Daniel S, Soudeyns H. Pathogenesis of hepatitis C during pregnancy and childhood. Viruses 2012; 4: 3531-50.10.3390/v4123531Search in Google Scholar PubMed PubMed Central
18 Mast EE. Mother-to-infant hepatitis C virus transmission and breastfeeding. Adv Exp Med Biol 2004; 554: 211-6.10.1007/978-1-4757-4242-8_18Search in Google Scholar PubMed
19 Vignier N, Esmat G, Elsharkawy A, Hassany M, Bonnard P, Delarocque-Astagneau E, et al. Reproducibility of liver stiffness measurements in hepatitis C virus (HCV)-infected patients in Egypt. J Viral Hepat 2011; 18: e358–65.10.1111/j.1365-2893.2010.01433.xSearch in Google Scholar PubMed
20 Abdel-Rahman M, El-Sayed M, lRaziky M, Elsharkawy A, Elakel W, Ghoneim H, et al. Coinfection with hepatitis C virus and schistosomiasis: Fibrosis and treatment response. World J Gastroentrol 2013; 19: 2691-6.10.3748/wjg.v19.i17.2691Search in Google Scholar PubMed PubMed Central
21 Alboraie M, Khairy M, Elsharkawy M, Asem N, Elsharkawy A, Esmat G. Value of Egy-Score in diagnosis of significant, advanced hepatic fibrosis and cirrhosis compared to aspartate aminotransferase-to-platelet ratio index, FIB-4 and Forns’ index in chronic hepatitis C virus. Hepatol Res 2015; 45: 560-70.10.1111/hepr.12385Search in Google Scholar PubMed
22 Baroncelli S, Pirillo MF, Amici R, Tamburrini E, Genovese O, Ravizza M, et al. HCV-HIV coinfected pregnant women: data from a multicenter study in Italy. Infect 2016; 44: 235–42.10.1007/s15010-015-0852-0Search in Google Scholar PubMed
23 Attallah AM, Abdallah SO, El-Far M, Omran MM, Tabll AA, Ghaly MF, et al. Perinatal transmission of hepatitis C antigens: envelope 1, envelope 2 and non-structural 4. Infect Dis (Lond) 2015; 47: 568-74.10.3109/23744235.2015.1042035Search in Google Scholar PubMed
24 Lu S, Jin B, Cowart LA, Lu X. From Data towards Knowledge: Revealing the architecture of Signaling Systems by Unifying Knowledge Mining and Data Mining of Systematic Perturbation Data. PlOS One 2013; 8: e61134.10.1371/journal.pone.0061134Search in Google Scholar PubMed PubMed Central
25 Abd Elrazek AE, Mahfouz HM, Metwally AM, ElShamy AM. Mortality prediction of nonalcoholic patients presenting with upper gastrointestinal bleeding usingdata mining. Eur J Gastroenterol Hepatol 2014; 26: 187-91.10.1097/MEG.0b013e328365c3b0Search in Google Scholar PubMed
26 Abd Elrazek MA, Mahfouz H, Afifi M, Nafady M, Fathy Ael W, El azeem KA, et al. Detection of risky esophageal varices by two-dimensional ultrasound: when to perform endoscopy. Am J Med Sci 2014; 347: 28-33.10.1097/MAJ.0b013e3182750ce8Search in Google Scholar PubMed
27 Abd Elrazek AE1, Eid KA, El-Sherif AE, Abd El Al UM. Screening Esophagus during routine U/S; medical and cost benefits. Eur J Gastroenterol Hepatol 2015; 27: 8-12.10.1097/MEG.0000000000000196Search in Google Scholar PubMed
28 Abd Elrazek AE. How Can Data Mining Improve Health Care? Appl Math Inform Sci 2017; 11: 585-8.10.18576/amis/110230Search in Google Scholar
29 North M. Data mining for the amsses. Global text projects, 2012. Available at: http://www.worldcat.org/title/data-mining-for-the-masses/oclc/812381894.html. Accessed on March 5, 2017.Search in Google Scholar
30 Hashem S, Esmat G, Elakel W, Habashy S, Abdel Raouf S, Darweesh S, et al., Accurate Prediction of Advanced Liver Fibrosis Using the Decision Tree Learning Algorithm in Chronic Hepatitis C Egyptian Patients. Gastroenterol Res Pract 2016; 2016: 2636390.10.1155/2016/2636390Search in Google Scholar PubMed PubMed Central
31 Elsharkawy A, Fouad R, El Akel W, El Raziky M, Hassany M, Shiha G, et al. Sofosbuvir-based treatment regimens: real life results of 14 409 chronic HCV genotype 4 patients in Egypt. Aliment Pharmacol Ther 2017; 45: 681-7.10.1111/apt.13923Search in Google Scholar PubMed
© 2017 Abd Elrazek, Samy Saab, Mahmoud Foad, Elsayed A. Elgohary, Mohammad M. Sallam, Abdallah Nawara, Ali Ismael, Samar S. Morsi, Altaher Salah, Mohamed Alboraie, Akshaya Srikanth Bhagavathula, Marwa Zayed, Hossam Elmasry, Tamer Z. Salem
This article is distributed under the terms of the Creative Commons Attribution Non-Commercial License, which permits unrestricted non-commercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
Articles in the same Issue
- Editorial
- Diagnosis and treatment of chronic hepatitis C with concomitant extrahepatic manifestations deserves a closer look
- Guideline Interpretation
- Endoscopic diagnosis and treatment of precancerous colorectal lesions in patients with inflammatory bowel disease: How does the latest SCENIC international consensus intersect with our clinical practice?
- Review Articles
- Direct acting anti-hepatitis C virus drugs: Clinical pharmacology and future direction
- Review Articles
- Protracted inhibition of vascular endothelial growth factor signaling improves survival in metastatic colorectal cancer: A systematic review
- Original Article
- Ongoing transmission of HCV: Should cesarean section be justified? Data mining discovery
- Original Article
- A single-step multiplex quantitative real time polymerase chain reaction assay for hepatitis C virus genotypes
- Original Article
- Utility of electronic medical recordbased fibrosis scores in predicting advanced cirrhosis in patients with hepatitic C virus infection
- Original Article
- Early detection of liver damage in Mexican patients with chronic liver disease
- Original Article
- Clinical outcomes in patients with cancer of unknown primary site treated by gastrointestinal oncologists
- Case Report
- Hepatic encephalopathy in liver cirrhosis
Articles in the same Issue
- Editorial
- Diagnosis and treatment of chronic hepatitis C with concomitant extrahepatic manifestations deserves a closer look
- Guideline Interpretation
- Endoscopic diagnosis and treatment of precancerous colorectal lesions in patients with inflammatory bowel disease: How does the latest SCENIC international consensus intersect with our clinical practice?
- Review Articles
- Direct acting anti-hepatitis C virus drugs: Clinical pharmacology and future direction
- Review Articles
- Protracted inhibition of vascular endothelial growth factor signaling improves survival in metastatic colorectal cancer: A systematic review
- Original Article
- Ongoing transmission of HCV: Should cesarean section be justified? Data mining discovery
- Original Article
- A single-step multiplex quantitative real time polymerase chain reaction assay for hepatitis C virus genotypes
- Original Article
- Utility of electronic medical recordbased fibrosis scores in predicting advanced cirrhosis in patients with hepatitic C virus infection
- Original Article
- Early detection of liver damage in Mexican patients with chronic liver disease
- Original Article
- Clinical outcomes in patients with cancer of unknown primary site treated by gastrointestinal oncologists
- Case Report
- Hepatic encephalopathy in liver cirrhosis

