Abstract
Objectives
The SARS-CoV-2 pandemic was declared by World Health Organisation (WHO) in March 2020, causing health and economic disruptions and millions of deaths. Pregnant women represent a vulnerable group, as COVID-19 during pregnancy increases the risk of preterm birth, preeclampsia, and severe maternal illness. Nutritional status, such as vitamin D deficiency, may influence these outcomes, yet data on its status in the cohort of SARS-CoV-2 positive pregnant women as well as its association with inflammatory and angiogenic markers is scarce. The aim of this study was to evaluate the levels of vitamin D in the cohort of SARS-CoV-2 positive pregnant women and its association with inflammatory and angiogenic markers.
Methods
Prospective cohort study at Ljubljana Maternity Hospital (Oct 1, 2020–Mar 30, 2021) enrolled singleton pregnancies with PCR-confirmed SARS-CoV-2 within the last 14 days, delivering at our institution.
Results
Among 235 SARS-CoV-2-positive pregnant women 62.1 % had adequate and 21.3 % insufficient level of vitamin D and 13.6 % were vitamin D deficient. Statistical analysis revealed no significant correlations between 25-OH-vitamin D and C-reactive protein (CRP), procalcitonin (PCT), leukocyte count, soluble fms-like tyrosine kinase-1 (sFlt-1), placental growth factor (PlGF), the sFlt-1/PlGF ratio, or body mass index (BMI).
Conclusions
Our prospective cohort study revealed that high proportion of pregnant women has inadequate levels of vitamin D. Although maternal insufficiency is linked to adverse outcomes, its association with inflammatory and angiogenic markers remains unclear. Rigorous studies in pregnancy are essential to clarify vitamin D’s role in COVID-19 complications in pregnancy.
Introduction
SARS-CoV-2 emerged in the late 2019, leading to a global health crisis due to the virus rapidly spreading worldwide. Declared a pandemic by the World Health Organization (WHO) in March 2020, it disrupted daily life, overwhelmed healthcare systems, and caused millions of deaths [1]. The pandemic most significantly affected vulnerable populations, among them also pregnant women [2].
A meta-analysis has shown that COVID-19 infection during pregnancy is associated with a higher risk of preterm delivery, maternal mortality, NICU admission and neonatal death [3]. Pregnant individuals are more susceptible to severe illness due to physiological changes in all body systems, especially respiratory, immune and cardiopulmonary systems [4]. Studies have shown that COVID-19 can increase the likelihood of preterm birth, preeclampsia, cesarean delivery, and low birth weight. Severe maternal illness may also lead to intensive care admission, respiratory support, or, in rare cases, maternal mortality [3], 5], 6]. The risk is particularly elevated in those with underlying conditions such as obesity, diabetes, or hypertension [7] and the risk can be modified by certain nutritional factors, such as levels of vitamins [8].
Vitamin D is increasingly recognized for its roles beyond bone health, such as cell differentiation, immune response, and inflammation control. Deficiency may result in rickets, osteomalacia, and has been associated with increased risks of chronic diseases such as cardiovascular disorders, diabetes, as well as some cancers [9].
Vitamin D plays a vital role during pregnancy, supporting maternal bone health and fetal skeletal development by regulating calcium and phosphate metabolism [10]. Pregnant women are at increased risk of deficiency due to limited sun exposure, dietary insufficiency, or higher physiological demands. Inadequate levels have been associated with complications such as preeclampsia, gestational diabetes, low birth weight, and impaired neonatal bone growth and supplementation is often recommended, especially in regions with low sunlight or among high-risk groups [11].
Serum 25-OH-vitamin D concentration is commonly used to evaluate vitamin D status and guides supplementation or lifestyle adjustments [12]. Serum concentrations of 25-OH-vitamin D likely vary by ethnicity, age and other factors, therefore optimal levels have not been established. Nevertheless, deficiency is often considered at levels below 30 nmol/L or 12 ng/mL and supplementation to surpass this cut-off is advised (Table 1).
Levels of serum 25-OH-vitamin D and associated health status.
| nmol/L | ng/mL | Health status |
|---|---|---|
| <30 | <12 | Vitamin D deficiency, which can lead to rickets in infants and osteomalacia in adults. |
| 30–50 | 12–20 | Generally considered inadequate for bone and overall health in healthy individuals. |
| 50–125 | 20–50 | Generally considered adequate for bone and overall health in healthy individuals. |
| >125 | >50 | Linked to potential adverse effects. |
| Particularly >150 | Particularly >60 |
The most recent meta-analysis of five randomised controlled studies conducted between 2016 and 2025 revealed a significant reduction in the risk of preeclampsia among pregnant women receiving vitamin D supplementation (RR=0.61, 95 % CI: [0.50–0.75], p<0.001), supporting its protective role [13]. However, the effect among the SARS-CoV-2 positive pregnant women is yet to be determined.
Data on the influence of vitamin D deficiency on inflammatory and angiogenic marker values, which could partially explain seemingly increased risk of certain adverse events in pregnancy, is scarce. However, there has been some research done showing that low vitamin D levels are associated with higher levels of CRP, IL-6, ferritin, TNF-α, in both healthy individuals and those with conditions like obesity or COVID-19 [14], 15].
The aim of this study was to explore the prevalence of vitamin D deficiency among SARS-CoV-2 positive pregnant women in Slovenia and the effect of level of vitamin D on certain inflammatory and angiogenic markers.
Subjects and methods
A prospective cohort study was conducted at the Ljubljana Maternity Hospital between October 1st 2020, and March 30th 2021. Our institution was the center for the management of SARS-CoV-2 pregnant women during the pandemic. The study included all consecutive pregnant women with confirmed SARS-CoV-2 infection, verified by positive polymerase chain reaction (PCR) test performed within 14 days prior to the clinical examination. Only singleton pregnancies that later resulted in delivery at the study institution were considered eligible for inclusion. Data was collected prospectively from medical records and structured interviews, and included demographic characteristics (maternal age, maternal body mass index (BMI), smoking), preexisting chronic disease, gestational age at positive test (weeks), clinical course of COVID-19 (asymptomatic, symptomatic and complicated course of COVID-19, which was defined as the need for hospital admission and oxygen supplementation), laboratory results (procalcitonin (PCT) (g/L), C-reactive protein (CRP) (mg/L), leukocytes (×109/L), hemoglobin (g/L), soluble fms-like tyrosine kinase-1 (sFlt-1) (pg/mL), placental growth factor (PlGF) (pg/mL), sFlt-1/PlGF ratio, interleukin-6 (IL-6) (pg/mL)), pregnancy outcomes (miscarriages, live births, gestational age at delivery (weeks), mode of delivery (spontaneous vaginal, operative vaginal, cesarean section), and neonatal (birth weight (g)).
Descriptive statistics were used to summarize demographic and clinical characteristics of the study population. Continuous variables were expressed as medians with ranges. Categorical variables were presented as counts and percentages. We used multivariable logistic regression or multiple linear regression to assess the association between the variables as appropriate. Statistical significance was defined as a p-value <0.05. All analyses were conducted using SPSS Statistics version 20.
This study was performed in line with the principles of the Declaration of Helsinki and was approved by Medical Ethics Committee of the Republic of Slovenia (permit no. 0120-196/2020-18).
Results
A total of 235 pregnant women across all trimesters of pregnancy with confirmed SARS-CoV-2 infection met inclusion criteria.
The median gestational age at the time of the positive test was 33.0 weeks (range 7.0–40.6 weeks), and the median maternal age was 31 years (range 20–42 years). The median interval between the initial positive test and assessment at our institution was 5 days (range 0–14 days).
The level of vitamin D was adequate in the majority of patients (146/235; 62.1 %), about a fifth had inadequate levels of 25-OH-vitamin D (50/235; 21.3 %) and 13.6 % of patients were considered vitamin-D deficient (32/235). Three percent (7/235; 3.0 %) of patients had high levels of 25-OH-vitamin D, none of them in the extreme range (Figure 1).
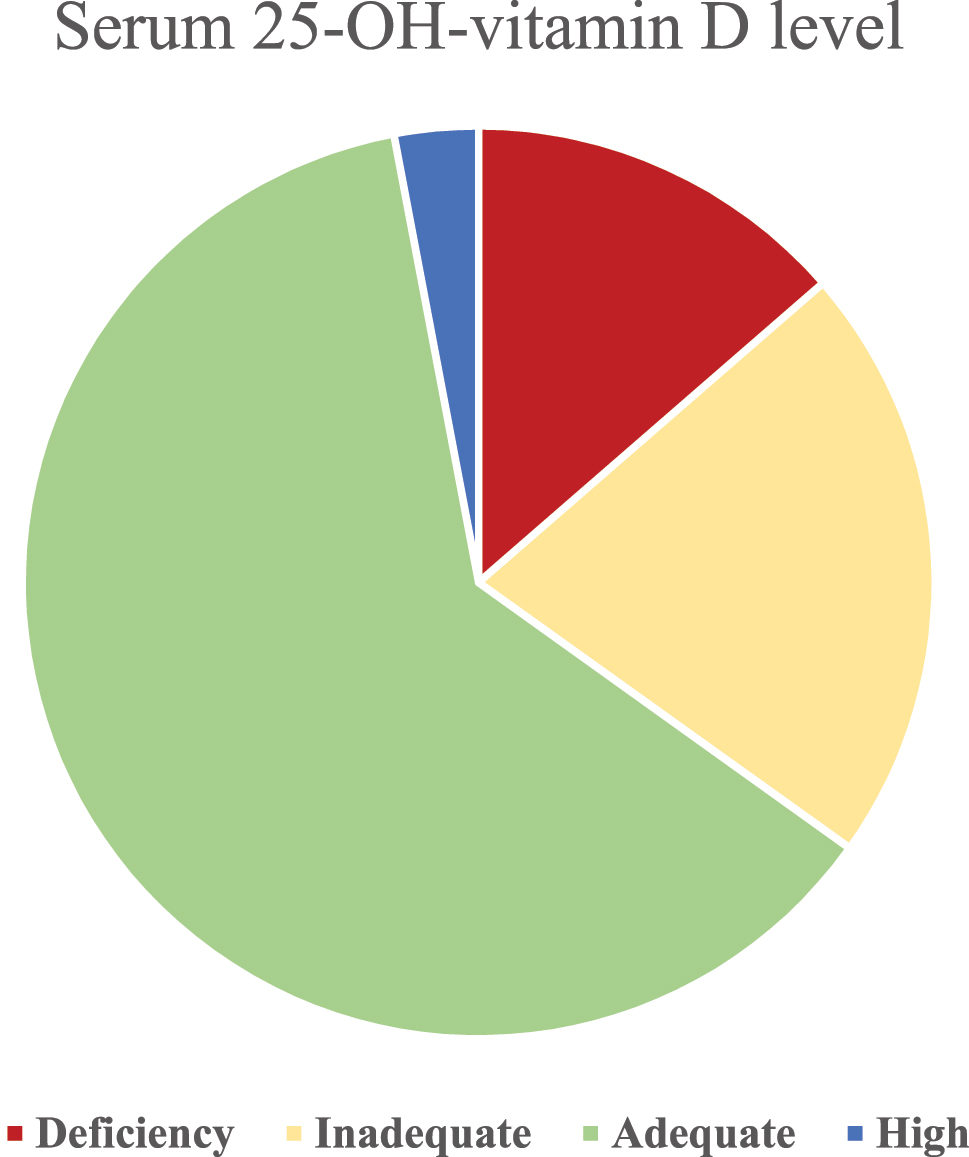
Serum levels of 25-OH-vitamin D in our cohort.
Data on the severity of the symptoms was available for all patients included. 225 (95.7 %) were symptomatic and two (0.8 %) experienced a complicated disease course. Fifty-six women (23.7 %) had at least one preexisting chronic condition, most commonly asthma (n=19; 8.1 %) and thyroid disease (n=13; 5.5 %). Median BMI was 23.9 (range 16.8–49.3). Current smoking was uncommon (17/235; 7.2 %).
Laboratory evaluation showed uniformly negative PCT levels with only one value of exceeding the cut off. CRP was positive in about half of the patients (109/235; 46.4 %). Median values for key biomarkers were as follows: leukocyte count 7.9 × 109/L (range 3.4–15.8 × 109/L), hemoglobin 123 g/L (range 92–151 g/L), sFlt-1 2,670 pg/mL (range 632.1–15,217 pg/mL), PlGF 256.75 pg/mL (range 12.1–3,263 pg/mL), sFlt-1/PlGF ratio 10.9 (range 0.6–188), and IL-6 3.55 pg/mL (range 1.5–153.1 pg/mL).
Regarding pregnancy outcomes, there was one miscarriage at 21 weeks’ gestation and 234 live births. We excluded the pregnancy which ended in miscarriage from further analysis. Among the live births, the median gestational age at delivery was 39.5 weeks (range 27.6–41.5 weeks), and the median birth weight was 3430 g (range 720–4720 g). Delivery modes included 167 spontaneous vaginal births (71.4 %), six operative vaginal births (2.6 %), 33 (14.1 %) elective cesarean sections, and 23 (9.8 %) emergency cesarean sections. Data describing the cohort is shown in Table 2.
Characteristics of the cohort of 235 pregnant women having had a positive SARS-CoV-2 PCR swab within 14 days of the assessment.
| Parameter | Value (median (range)) |
|---|---|
| Maternal demographics | |
|
|
|
| Total SARS-CoV-2-positive women | 235 |
| Maternal age, years | 31 (20–42) |
| Maternal BMI, kg/m2 | 23.9 (16.8–49.3) |
| Gestational age at positive test, weeks | 33.0 (7.0–40.6) |
| Interval positive test → assessment, days | 5 (0–14) |
| Symptomatic | 225/235 (95.7 %) |
| Critical disease course | 2/235 (0.8 %) |
| ≥1 pre-existing chronic disease | 56 (23.7 %) |
|
|
|
| Laboratory parameters | |
|
|
|
| Procalcitonin positive | 1 (0.4 %) |
| CRP (mg/L) positive | 109 (46.4 %); |
| Median among positive 10 (range 5–112) | |
| Leukocytes (×109/L) | 7.9 (3.4–15.8) |
| Hemoglobin, g/L | 123 (92–151) |
| sFlt-1, pg/mL | 2,670 (632.1–15,217) |
| PlGF, pg/mL | 256.8 (12.1–3,263) |
| sFlt-1/PlGF ratio | 10.9 (0.6–188) |
| IL-6, pg/mL | 3.55 (1.5–153.1) |
|
|
|
| Pregnancy outcomes | |
|
|
|
| Miscarriages | 1 (0.4 %) |
| Live births | 234 (99.6 %) |
| Gestational age at delivery (weeks) n=234 | 39.5 (27.6–41.5) |
| Birth weight (g) n=234 | 3,430 (720–4,720) |
| Mode of delivery n=234 | |
| Spontaneous vaginal | 167 (71.4 %) |
| Operative vaginal | 6 (2.6 %) |
| Elective cesarean section | 33 (14.1 %) |
| Emergency cesarean section | 23 (9.8 %) |
-
BMI, body mass index; CRP, C-reactive protein; sFlt-1, soluble fms-like tyrosine kinase-1; PLGF, placental growth factor; IL6, interleukin 6.
In a multivariable logistic regression adjusted for age, BMI, chronic disease status, and gestational age at delivery, S-25-(OH)-vitamin D was not associated with CRP positivity. The regression coefficient for S-25-(OH)-vitamin D was B=−0.001, standard error=0.005, p=0.857, with an odds ratio of 0.999 and 95 % confidence interval 0.990–1.008. BMI was independently associated with higher odds of CRP positivity (B=0.049, SE=0.024, p=0.039, OR=1.05, 95 % CI 1.002–1.099). Age and chronic disease status did not reach statistical significance in this model.
In the analogous multivariable logistic model for PCT positivity adjusted for the same covariates, age was a strong and significant predictor of PCT positivity (B=0.126, SE=0.041, p=0.002, OR=1.13, 95 % CI 1.046–1.229). PCT, BMI, chronic disease status, and gestational age were not significant predictors in this model.
Multiple linear regression models adjusted for age, BMI, chronic disease status, and gestational age found no statistically significant association between S-25-(OH)-vitamin D and the following outcomes: total leukocyte count, sFlt-1, PlGF, the sFlt-1/PlGF ratio, and IL-6 after adjustment. Coefficients for S-25-(OH)-vitamin D were small and non-significant across these models.
Discussion
Vitamin D is a liposoluble element obtained from diet and sun exposure and is an essential micronutrient that among others exerts anti-inflammatory and immunomodulatory effects [16]. In the recent years it has been shown that vitamin D has diverse biological actions and its effect on the human body has been underestimated [17]. Vitamin D deficiency can result from a range of causes and is common around the globe, affecting approximately 1 billion people. In the U.S., 61 % of older adults are deficient, compared to 90 % in Turkey, 96 % in India, 72 % in Pakistan, and 67 % in Iran [18]. In our study only about 60 % of participants had appropriate level of serum 25-OH-vitamin D, despite the fact that pregnant women are considered to be mostly young, active and healthy. Our study was conducted in winter, which is a known risk factor for vitamin D deficiency due to limited sun exposure [19], 20]. Our results therefore highlight the importance of testing vitamin D levels especially in winter and appropriate supplementation if necessary.
We failed to demonstrate significant association between 25-OH-vitamin D and with inflammatory (leukocytes, CRP, IL-6, PCT) or angiogenic markers (sFlt-1, PlGF).
Data on the influence of vitamin D deficiency on inflammatory and angiogenic marker values is scarce. Some studies have shown that low vitamin D levels are associated with higher levels of CRP, IL-6, ferritin, TNF-α, in both healthy individuals as well as in obese patients and patients with COVID-19 [14], 15].
The lack of statistically significant association of vitamin D deficiency and the values of inflammatory and angiogenic markers might be the consequence of generally very mild course of the disease in our cohort with low median levels of inflammatory markers as the median CRP level was 10, maximum CRP reached 112, and only 1 patient had positive PCT. The interval between the positive SARS-CoV-2 tests and the assessment at our institution was short (median of 5 days), which highlights the good access to health care, however the disease might not have developed in full yet.
In terms of association of vitamin D deficiency and angiogenic factors the evidence in the literature is scarce. Although vitamin D deficiency due to vitamin D’s immunomodulatory effect as well as sFlt1/PlGF are independently linked to a higher risk of preeclampsia, research has not consistently demonstrated a direct correlation between vitamin D levels and the sFlt-1/PlGF ratio [21]. There was no significant association between vitamin D and sFlt-1 or PlGF in our study. The most recent meta-analysis of the five randomised controlled studies conducted between 2016 and 2025 revealed a significant reduction in the risk of pre-eclampsia among pregnant women receiving vitamin D supplementation (RR=0.61, 95 % CI: [0.50–0.75], p<0.001), supporting its protective role [13]. However, the effect among the SARS-CoV-2 positive pregnant women is yet to be determined. The pathophysiology of preeclampsia involves incomplete formation of the trophoblast early in pregnancy. The median gestational age at which the patients in our study tested positive for SARS-CoV-2 was 33 weeks and most of them were diagnosed in the second half of the pregnancy, when the formation of the placenta is complete. Further studies on the association of vitamin D deficiency in the 1st trimester of pregnancy with the levels of angiogenic markers and the subsequent development of preeclampsia would be warranted.
The study’s prospective design constitutes a key strength, as it minimized both recall and selection biases. This approach enabled real-time monitoring of pregnancy outcomes and afforded researchers greater control over the data collection process. An important strength of the study is also the inclusion of a large cohort of pregnant women with mild COVID-19 during winter, a gap in the existing literature, which enhances the statistical power and generalizability of the findings. The median levels of inflammatory and angiogenic markers across the cohort were relatively low, potentially constraining the ability to detect associations or draw robust conclusions regarding these biomarkers. Future investigations with a more heterogeneous sample in terms of disease severity and biomarker expression could provide deeper insights. The cohort was large as it consisted of 235 SARS-CoV-2 pregnant women as proven by PCR testing, however the levels of inflammatory and angiogenic markers were generally not significantly increased and there were only two patients with complicated course of the disease, which made the comparison difficult. One notable limitation of the study is that it was conducted exclusively during the winter months, a period typically associated with reduced sunlight exposure and consequently lower serum levels of vitamin D in the population. This seasonal constraint may have introduced a bias, limiting the generalizability of the findings to other times of the year when vitamin D levels are naturally higher. To ensure a more comprehensive understanding of the variable under investigation, future research should aim to include data collection across multiple seasons. Such an approach would allow for the assessment of potential seasonal variations and enhance the robustness of the conclusions drawn.
We acknowledge the lack of power analysis, which raises the concern that non-significant findings may be due to underpowered comparisons – especially for outcomes like IL-6 or sFlt-1.
Despite us not having confirmed the correlation between increased inflammatory or angiogenic markers and vitamin D deficiency, there is growing evidence that maternal serum vitamin D levels influence pregnancy outcome with deficiency of vitamin D being associated with increased risks of complications such as preeclampsia, gestational diabetes, preterm birth, low birth weight, and hypertensive disorders, as well as adverse neonatal outcomes like small for gestational age and impaired bone development in the child [22], [23], [24]. Therefore, additional studies are warranted to confirm or dispute the role of vitamin D deficiency in pregnancy as well as in patients with COVID-19.
Conclusions
Vitamin D deficiency was common among pregnant women in Slovenia. In view of multiple proven benefits of vitamin D, the deficiency should be looked for and addressed in this population.
There was no correlation between the levels of serum 25-OH-vitamin D and inflammatory (CRP, PCT, leukocytes, IL6) or angiogenic (sFlt-1, PlGF) markers in pregnant women.
Despite not having proven the association between vitamin D deficiency and raised values of inflammatory or angiogenic markers, further studies to assess vitamin D deficiency on the values of inflammatory and angiogenic markers as well as pregnancy complications are warranted in view of the role of vitamin D in the immune modulation.
-
Research ethics: This study was performed in line with the principles of the Declaration of Helsinki and was approved by Medical Ethics Committee of the Republic of Slovenia (permit no. 0120–196/2020-18).
-
Informed consent: Informed consent was obtained from all individuals included in this study, or their legal guardians or wards.
-
Author contributions: Conceptualization MD, TPS; methodology IV; data curation VAM; writing – original draft preparation VAM; writing – review and editing MD, TPS, GK, AŠ. All authors have read and approved the submitted version of the manuscript.
-
Use of Large Language Models, AI and Machine Learning Tools: None declared.
-
Conflict of interest: The authors state no conflict of interest.
-
Research funding: None declared.
-
Data availability: The data reported in this study are available from the corresponding author upon reasonable request.
References
1. Wang, M-Y, Zhao, R, Gao, L-J, Gao, X-F, Wang, D-P, Cao, J-M. SARS-CoV-2: structure, biology, and structure-based therapeutics development. Front Cell Infect Microbiol 2020;10. https://doi.org/10.3389/fcimb.2020.587269.Search in Google Scholar PubMed PubMed Central
2. Pinkney, JA. The impact of coronavirus disease 2019 (COVID-19) on vulnerable communities. Infect Dis Clin 2025;39:331–43. https://doi.org/10.1016/j.idc.2025.02.006.Search in Google Scholar PubMed
3. Simbar, M, Nazarpour, S, Sheidaei, A. Evaluation of pregnancy outcomes in mothers with COVID-19 infection: a systematic review and meta-analysis. J Obstet Gynaecol 2023;43. https://doi.org/10.1080/01443615.2022.2162867.Search in Google Scholar PubMed
4. Horan, C. Physiological changes in pregnancy. In: Berghella, V, editor. Obstetric evidence-based guidelines, 1st ed. London: CRC Press; 2007:37–42 pp.10.3109/9780203931639-10Search in Google Scholar
5. Yang, J, D’Souza, R, Kharrat, A, Fell, DB, Snelgrove, JW, Murphy, KE, et al.. COVID-19 pandemic and population-level pregnancy and neonatal outcomes: a living systematic review and meta-analysis. Acta Obstet Gynecol Scand 2021;100:1756–70. https://doi.org/10.1111/aogs.14206.Search in Google Scholar PubMed PubMed Central
6. Bahrami, R, Schwartz, DA, Karimi-Zarchi, M, Javaheri, A, Dastgheib, SA, Ferdosian, F, et al.. Meta-analysis of the frequency of intrauterine growth restriction and preterm premature rupture of the membranes in pregnant women with COVID-19. Turk J Obstet Gynecol 2021;18:236–44. https://doi.org/10.4274/tjod.galenos.2021.74829.Search in Google Scholar PubMed PubMed Central
7. Villar, J, Ariff, S, Gunier, RB, Thiruvengadam, R, Rauch, S, Kholin, A, et al.. Maternal and neonatal morbidity and mortality among pregnant women with and without COVID-19 infection: the INTERCOVID multinational cohort study. JAMA Pediatr 2021;175:817–26. https://doi.org/10.1001/jamapediatrics.2021.1050.Search in Google Scholar PubMed PubMed Central
8. Attini, R, Laudani, ME, Versino, E, Massaro, A, Pagano, A, Petey, F, et al.. COVID-19 in pregnancy: influence of body weight and nutritional status on maternal and pregnancy outcomes – a review of literature and meta-analysis. Nutrients 2023;15:1052. https://doi.org/10.3390/nu15041052.Search in Google Scholar PubMed PubMed Central
9. Wimalawansa, SJ. Physiology of vitamin D – focusing on disease prevention. Nutrients 2024;16:1666. https://doi.org/10.3390/nu16111666.Search in Google Scholar PubMed PubMed Central
10. Karras, SN, Wagner, CL, Castracane, VD. Understanding vitamin D metabolism in pregnancy: from physiology to pathophysiology and clinical outcomes. Metabolism 2018;86:112–23. https://doi.org/10.1016/j.metabol.2017.10.001.Search in Google Scholar PubMed
11. Hynes, C, Jesurasa, A, Evans, P, Mitchell, C. Vitamin D supplementation for women before and during pregnancy: an update of the guidelines, evidence, and role of GPs and practice nurses. Br J Gen Pract 2017;67:423–4. https://doi.org/10.3399/bjgp17X692489.Search in Google Scholar PubMed PubMed Central
12. Institute of Medicine (US) Committee to Review Dietary Reference Intakes for Vitamin D and Calcium, Ross, AC, Taylor, CL, Yaktine, AL, Del Valle, HB, editors. Dietary reference intakes for calcium and vitamin D. Washington, DC: National Academies Press (US); 2011. Available from: https://www.ncbi.nlm.nih.gov/books/NBK56070/doi:%2010.17226/13050/.Search in Google Scholar
13. Kokkinari, A, Antoniou, E, Orovou, E, Andronikidi, PE, Tzitiridou-Chatzopoulou, M, Sarantaki, A, et al.. The role of vitamin D supplementation in preventing pre-eclampsia: a review of randomized controlled trials with meta-analysis. Healthcare 2025;13:1221. https://doi.org/10.3390/healthcare13111221.Search in Google Scholar PubMed PubMed Central
14. Hopefl, R, Ben-Eltriki, M, Deb, S. Association between vitamin D levels and inflammatory markers in COVID-19 patients: a meta-analysis of observational studies. J Pharm Pharmaceut Sci 2022;25:124–36. https://doi.org/10.18433/jpps32518.Search in Google Scholar PubMed
15. Jain, A, Chaurasia, R, Sengar, NS, Singh, M, Mahor, S, Narain, S. Analysis of vitamin D level among asymptomatic and critically ill COVID-19 patients and its correlation with inflammatory markers. Sci Rep 2020;10:20191. https://doi.org/10.1038/s41598-020-77093-z.Search in Google Scholar PubMed PubMed Central
16. DeLuca, HF. Overview of general physiologic features and functions of vitamin D. Am J Clin Nutr 2004;80:1689S–1696S. https://doi.org/10.1093/ajcn/80.6.1689S.Search in Google Scholar PubMed
17. Ismailova, A, White, JH. Vitamin D, infections and immunity. Rev Endocr Metab Disord 2022;23:265–77. https://doi.org/10.1007/s11154-021-09679-5.Search in Google Scholar PubMed PubMed Central
18. Palacios, C, Gonzalez, L. Is vitamin D deficiency a major global public health problem? J Steroid Biochem Mol Biol 2014;144:138–45. https://doi.org/10.1016/j.jsbmb.2013.11.003.Search in Google Scholar PubMed PubMed Central
19. Rabenberg, M, Scheidt-Nave, C, Busch, MA, Rieckmann, N, Hintzpeter, B, Mensink, GBM. Vitamin D status among adults in Germany – results from the German Health Interview and Examination Survey for Adults (DEGS1). BMC Public Health 2015;15:641. https://doi.org/10.1186/s12889-015-2016-7.Search in Google Scholar PubMed PubMed Central
20. Osredkar, J, Vičič, V, Hribar, M, Benedik, E, Siuka, D, Jerin, A, et al.. Seasonal variation of total and bioavailable 25-hydroxyvitamin D [25(OH)D] in the healthy adult Slovenian population. Acta Biochim Pol 2024;71. https://doi.org/10.3389/abp.2024.13108.Search in Google Scholar PubMed PubMed Central
21. Álvarez-Fernández, I, Prieto, B, Rodríguez, V, Ruano, Y, Escudero, AI, Álvarez, FV. Role of vitamin D and sFlt-1/PlGF ratio in the development of early- and late-onset preeclampsia. Clin Chem Lab Med 2015;53. https://doi.org/10.1515/cclm-2014-1039.Search in Google Scholar PubMed
22. Karras, SN, Fakhoury, H, Muscogiuri, G, Grant, WB, van den Ouweland, JM, Colao, AM, et al.. Maternal vitamin D levels during pregnancy and neonatal health: evidence to date and clinical implications. Ther Adv Musculoskelet Dis 2016;8:124–35. https://doi.org/10.1177/1759720X16656810.Search in Google Scholar PubMed PubMed Central
23. Wagner, CL, Hollis, BW. The implications of vitamin D status during pregnancy on mother and her developing child. Front Endocrinol 2018;9. https://doi.org/10.3389/fendo.2018.00500.Search in Google Scholar PubMed PubMed Central
24. Zhang, H, Wang, S, Tuo, L, Zhai, Q, Cui, J, Chen, D, et al.. Relationship between maternal vitamin D levels and adverse outcomes. Nutrients 2022;14:4230. https://doi.org/10.3390/nu14204230.Search in Google Scholar PubMed PubMed Central
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.


