Urinary immune biomarkers for late-onset sepsis in preterm very low birth weight neonates – a diagnostic accuracy study
-
Gayatri Morajker
, Anitha Haribalakrishna
Abstract
Objectives
To evaluate diagnostic accuracy of urinary immune biomarkers (IBM) for late-onset sepsis (LOS) in very preterm infants.
Methods
This multicenter, prospective diagnostic accuracy study, included preterm infants <32 weeks of gestation and birth weight <1,500 g, needing evaluation for suspected LOS. Urine samples obtained concurrent to blood culture and sepsis screen were evaluated for interleukin-8 (IL-8) and monocyte chemoattractant protein-1 (MCP-1) levels using enzyme immunoassay. Positive blood and/or CSF culture served as reference standards. Receiver operating characteristic curves were used to evaluate performance of urinary IBM for detection of LOS.
Results
During September 2021–September 2023, we evaluated 136 preterm, very low birth weight infants (mean gestational age: 28.2 weeks, mean birth weight: 933 g) for sepsis after 72 h of postnatal age and prior to 37 weeks postmenstrual age. Twenty four infants had culture-positive sepsis. Median IL-8 and MCP-1 levels in neonates with LOS were 86.5 pg/mL and 230.2 pg/mL and those in non-infected neonates were 66.5 pg/mL and 212.1 pg/mL. Urinary IL-8 had sensitivity and specificity of 42 and 51 % [AUC: 0.46 (0.35–0.57)] and urinary MCP-1 had sensitivity and specificity of 46 and 53 % [AUC: 0.49 (0.38–0.60)] for detection of LOS.
Conclusions
Urinary IL-8 and MCP-1 had low sensitivity and specificity for diagnosis of LOS in very preterm infants. These findings underscore the need to establish reference ranges for urinary IBM in preterm neonates.
Introduction
Late-onset sepsis (LOS) is a common morbidity in preterm, very low birth weight (VLBW) neonates. Its incidence in VLBW infants varies between 10 and 12 % in developed nations [1], 2], to over 30 % in developing countries [3]. Along with a high risk of mortality, LOS is associated with significant brain injury [4], 5] and long-term respiratory morbidity [6]. While early detection of LOS is essential to reduce sepsis-related mortality and morbidities, accuracy in diagnosing sepsis is also of paramount importance to avoid unnecessary antibiotic exposure to potentially non-infected neonates.
Conventional microbiological cultures of blood and/or cerebrospinal fluid (CSF) is the current gold standard for the diagnosis of neonatal sepsis. However, the long turnaround time, the effect of prior antibiotic exposure and small inoculation volumes on the interpretation of culture results, underscores the importance of reliable laboratory biomarkers as add-on diagnostic tests for sepsis. The currently available biomarkers have low sensitivity for detecting LOS, may not be detected in early stages of the disease and may demonstrate elevated levels in non-infective states [7]. None of the currently available biomarkers or their combinations have adequate diagnostic accuracy to be reliably used in the diagnosis of neonatal sepsis.
Interleukins (IL) are a class of pro-inflammatory cytokines that are released from macrophages at the onset of inflammation [8] and reach detectable levels in the blood much earlier than other biomarkers of sepsis. Chemokines or chemotactic cytokines are heparin-binding proteins that attract monocytes and T lymphocytes at the inflammatory site. The expression of the monocyte chemoattractant protein (MCP-1) has been reported to increase in preterm infants exposed to perinatal infections [9]. The utility of ILs for early diagnosis of sepsis has been extensively studied, although limited testing capacities, assay complexities and reagent costs have largely precluded their clinical application. Serum IL-6 and IL-8 are the most commonly studied immune biomarkers (IBM) in neonates, with a sensitivity ranging between 60 and 90 % and specificity between 50 and 85 %, for the detection of neonatal sepsis [10], 11]. Besides serum, the IBMs have been evaluated in other body fluids, such as urine, tracheal aspirate, amniotic fluid, and cord blood [12], [13], [14], [15]. Urinary IL-8 and MCP-1 have been reported as reliable biomarkers in diagnosis of sepsis, but the studies are limited to term neonates [16] or to infections localized to the urinary tract [17]. Urinary IBM estimations are non-invasive and may offset the sample volume constraints specific to serum evaluations in very premature infants. Furthermore, the correlations between serum and urinary ILs have been poorly studied and may not allow drawing of inferences on urinary IL levels, based on the serum measurements. There are limited data on the evaluation of urinary IBM in detection of culture positive sepsis in neonates. The objective of our study was to evaluate the diagnostic accuracy of urinary IL-8 and MCP-1 as immune biomarkers for LOS in very preterm infants born at <32 weeks of gestation.
Materials and methods
This multi-centre, prospective, diagnostic accuracy study was conducted at the Neonatal intensive care unit of Surya Children’s Hospital (SCH) and King Edward Memorial Hospital (KEM), Mumbai during the time period of September 2021 to September 2023. The study was approved by the Institutional Ethics Committee of both centers, EC-04/09/2021 and EC/OA-72/2022 respectively. Written consent was obtained from the parents prior to the enrolment of neonates in the study.
Participants
Consecutively admitted preterm infants with gestational age <32 weeks and birth weight of <1,500 g, needing evaluation for suspected sepsis after 72 h of postnatal age were included in the study.
Infants presenting with one or more of clinical signs, including fever >100 °C, tachypnoea (respiratory rate of >60 per minute) with respiratory distress, apnoea requiring positive pressure ventilation, need for invasive ventilation, feeding intolerance (defined as clinical status that leads to interruption of feeds for a period of not less than 24 h), abdominal distension, lethargy with refusal to feed, seizures, hypotension (mean arterial pressure in mmHg less than the gestational age in weeks) or oliguria (urine output less than 1 mL/kg/h), were included for sepsis evaluation [18].
Neonates were excluded if they had serious congenital anomalies, surgical conditions or post-inflammatory states like necrotizing enterocolitis. Infants treated with antibiotics for risk factors of EOS (within 1st 48 h of age) were excluded if an antibiotic-free interval of at least 96 h was not achieved prior to inclusion in our study. Assessments of urinary biomarker was performed only for the first evaluation of LOS in eligible infants.
Index test: urinary immune biomarkers (IL-8 and MCP-1)
Given the lack of normative data for urinary IBM in neonates and the wide variation in the positivity threshold reported in literature [19], we estimated urinary levels of IL-8 and MCP-1 in a control sample of 22 healthy preterm neonates. These included inborn preterm neonates born <32 weeks of gestation with a birth weight of <1500 g and a postnatal age of >7 days and without clinical signs suspicious of sepsis.
We chose the 95th centile of the distribution of the data to define the cut-off for positivity (92 pg/mL for IL-8 and 257 pg/mL for MCP-1). These infants were not part of the study population.
Approximately 5–10 mL of urine was collected from infants included in the study, in urine collection bags during evaluation of sepsis and within 4 h of initiation of antibiotics. Infants were catheterized if urine cultures also had to be sent as part of sepsis evaluation. Urine samples were frozen and stored at −20 °C in the laboratory of the two participating hospitals (SCH and KEM) and sent frozen to the testing laboratory at the Institute of Chemical Technology (ICT), Mumbai. Samples were aliquoted and stored at −80 °C until testing.
The urinary interleukins were analyzed in 96-well plates, using sandwich ELISA kits (GENLISA Human MCP-1/CCL2 and Human IL-8/CXCL8, Krishgen Biosystems, Mumbai, India). The assay sensitivity of the test kit was set to <9.375 pg/mL for MCP-1 and <15 pg/mL for IL-8. Both biomarkers had a standard calibration range of 15.6–1,000 pg/mL and an intra-assay coefficient of variation of <10 %. The urine samples and standards were incubated with monoclonal antibodies in pre-coated microwells. Results were obtained in 5 h. The details of immunoassay have been included in the Supplementary Material.
Reference standard: blood or CSF culture (BACTEC method)
Study infants were sampled simultaneously for sepsis screen (white cell count, absolute neutrophil count, platelet count and C-reactive protein (CRP)), and blood culture. Approximately 1 mL of blood was collected for cultures and was processed using the BACTEC method. White blood cell and platelet counts were measured using automated cell counter and CRP was measured using rapid immunoturbidometric method. Additional investigations for sepsis, such as urine analysis, endotracheal aspirate cultures, serum procalcitonin, and chest and abdominal X-rays were ordered at the discretion of the clinician.
Intravenous antibiotic was commenced in the study infants until culture reports were available. Antibiotics were administered for a duration of 14 days in culture positive sepsis. Antibiotics were ceased once blood cultures were reported to be sterile (approximately three days). Infants whose cultures were negative, but had abnormal laboratory markers (CRP>10 mg/L or white blood cell count<4,500 or >24,000/cu mm or absolute neutrophil count<1,000/cu mm or platelet count<100,000 cells/cu mm) were treated with antibiotics for a duration of 5 days.
Target condition
LOS was defined by the presence of positive blood and/or CSF culture after 72 h of postnatal age. Isolation of bacteria or fungi from urine, tracheal secretions and other common sites of microbial colonization were not included in the definition of sepsis. Organisms commonly considered as skin commensals were also excluded. Coagulase-negative staphylococcal infection was defined by isolation of the organism in two blood cultures drawn 24 h apart or by a positive blood culture in the presence of an intravascular line [20].
The treating physicians recorded the demographic and neonatal outcome data for each infant in a predesigned case-record form.
The blood culture reports of the study infants were masked to the researchers at ICT who reported and interpreted the IBM levels in the study infants. Blood culture reports were interpreted by the microbiologists at both the treating hospitals without the knowledge of urinary IBM values. The laboratory personnel were also masked to the clinical condition of the study infant.
Statistical analysis
Categorical variables were reported as counts and percentages and continuous variables were reported as median and interquartile ranges. Urinary IBM levels and other laboratory biomarker levels were compared between those with and without proven LOS. Two sample t test/Mann-Whitney U test were used to compare the values between the groups.
We used the receiver operating characteristic (ROC) curve to determine test performance in discriminating between the infected and non-infected neonates. The area under the ROC greater than 0.75 was considered optimal. The diagnostic accuracy was reported at the prespecified cutoff for both the urinary IBM. Sensitivity, specificity, likelihood ratio (LR) of positive and negative test, were reported with 95 % confidence intervals. LR of a positive test of >5 and a negative test of <0.2 was considered to rule in or rule out sepsis with moderate probability. Statistical analysis was performed using Stata version 15.1 (StataCorp LLC).
Results
During the study period of Sep 2021 to Sep 2023, 180 infants were screened for eligibility. Urinary IBM estimation concomitant with blood culture testing was performed in 136 infants. The study enrolment process has been displayed in Figure 1. Urine samples were not available from four infants and were excluded from the analysis (inability to collect urine within 4 h of antibiotic initiation-three infants, sample contamination with feces in one infant).
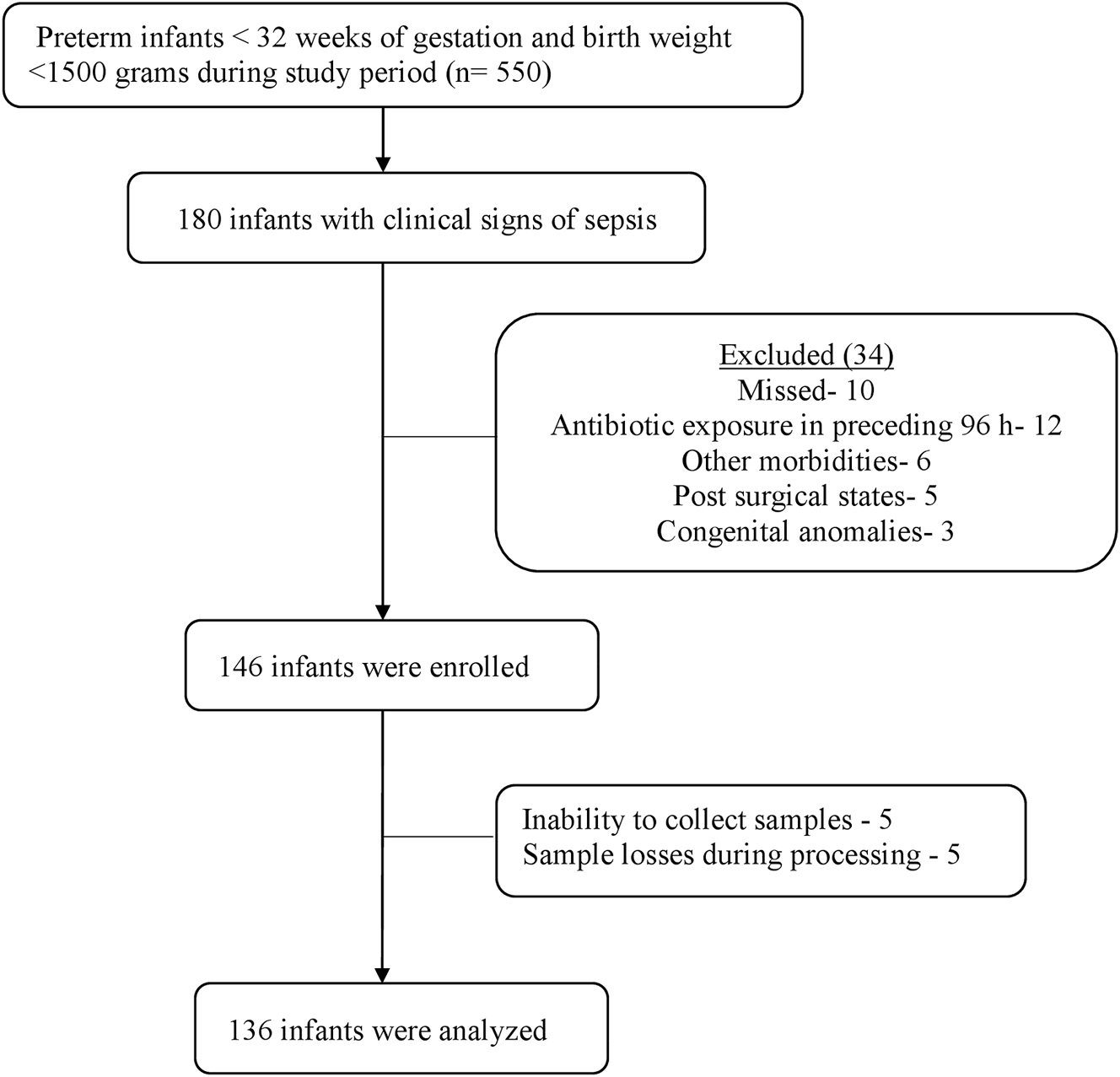
Flow diagram of study enrolment.
The median gestational age of the included infants was 28.2 weeks and birth weight was 933 g. Seventy-six (56 %) neonates were extremely premature (<28 weeks of gestation). The median postnatal age at assessment of urinary IBM levels was 23 days. Median duration of hospital stay was 58 days. Tachypnoea and/or respiratory distress (37 %) was the most common reason for sepsis evaluation, followed by apnea requiring positive pressure ventilation (27 %) and feed intolerance (23 %). A total of six study infants died during the hospital stay (Table 1).
Baseline characteristics.
| Baseline characteristics | Neonates (n=136) |
|---|---|
| Gestational age at birth, weeks | 28.2 (26.3–30.5) |
| Birth weight, g | 933 (460–1,007) |
| Male gender | 82 (60 %) |
| Multiple births | 45 (33 %) |
| Postnatal age, days | 23 (12–45) |
| Time to urine sample collection, minutesa | 70 (45–120) |
| Duration of hospital stay, days | 58 (38–83) |
|
|
|
| Clinical signs of sepsis: | |
|
|
|
| Tachypnoea and/or respiratory distress, n, % | 50 (37 %) |
| Apnea requiring IPPV or respiratory support, n, % | 37 (27 %) |
| Abdominal distension or feed intolerance, n, % | 31 (23 %) |
| Fever >100 degree celsius, n, % | 21 (15 %) |
| Hypotension, n, % | 17 (12 %) |
| Oliguria or peripheral hypoperfusion, n, % | 8 (6 %) |
|
|
|
| Laboratory investigations: | |
|
|
|
| Thrombocytopenia, n, % | 17 (12 %) |
| Platelet count, cells/cu.mm | 2.26 x 105 (1.24–3.57) |
| White blood cell count, cells/cu.mm | 12,000 (8,510–16,800) |
| CRP, mg/L | 8.8 (2.5–35.2) |
| IL-8, pg/mL | 79.2 (16.6–357.1) |
| MCP-1, pg/mL | 230.3 (49.1–579.2) |
| Duration of antibiotic therapy, days | 5 (3–7) |
-
Data expressed as median (1st quartile, 3rd quartile) or n(%). aMedian time between suspicion of sepsis and collection of urine sample. IPPV, intermittent positive pressure ventilation; CRP, c- reactive protein; IL, interleukin; MCP, monocyte chemoattractant protein.
Culture-positive sepsis was confirmed in 24 infants (18 %). Of those, 22 had a positive blood culture, one had a positive CSF culture and one infant had both positive blood and CSF culture. Klebsiella was the most common organism (7 infants) reported among the infected neonates. E. coli sepsis was noted in four infants, followed by Acinetobacter, Pseudomonas and Enterococcal infections in three infants each. Methicillin-resistant Staphylococcus aureus was the causative organism in two infants, Streptococcus and coagulase negative staphylococcal infection were noted in one infant.
Median IL-8 levels in the studied infants were 79.2 (16.6–357.1) pg/mL and MCP-1 levels were 239 (49–579) pg/mL. The median value of IL-8 and MCP-1 was not significantly different between infants with sepsis and the non-infected neonates (Table 2).
Comparison of laboratory markers in infants with and without late onset sepsis.
| Laboratory markers | Neonates with LOS (n=24) | Neonates without LOS (n=112) | p-Value |
|---|---|---|---|
| White cell count, cells/cu.mm | 13 280 (9 600–19 290) | 11 800 (8 500–16 000) | 0.22 |
| Absolute neutrophil count, cells/cu.mm | 4 760 (1 880–8 800) | 3 850 (2 280–7 020) | 0.34 |
| Platelet count (x 105 cells/cu.mm | 2.11 (1.22–2.34) | 2.39 (1.24–3.67) | 0.25 |
| CRP, mg/L | 14.85 (6.5–61.1) | 6.5 (1.9–25.1) | 0.03 |
| Urinary IL-8, pg/mL | 86.5 (21.5–357.1) | 66.6 (10.9–330.1) | 0.45 |
| Urinary MCP-1, pg/mL | 230.2 (50.9–612.5) | 212.1 (42.9–466.1) | 0.44 |
-
Data expressed as median (1st quartile- 3rd quartile). p-Values calculated using Wilcoxon ranksum test. CRP, c- reactive protein; IL-8, interleukin 8; LOS, late onset sepsis; MCP-1, monocyte chemoattractant protein-1.
The area under the ROC was 0.46 (0.35–0.57) for IL-8 and 0.49 (0.38–0.60) for MCP-1 (Figure 2). The sensitivity and specificity of IL-8 for LOS were 42 % (95 % CI: 22–63 %) and 51 % (95 % CI: 41–60 %), using the prespecified cut-off of 92 pg/mL. The sensitivity and specificity of MCP-1 were estimated as 46 % (95 % CI: 26–67 %) and 53 % (95 % CI: 43–62 %), using the prespecified cut-off of 257 pg/mL (Table 3).
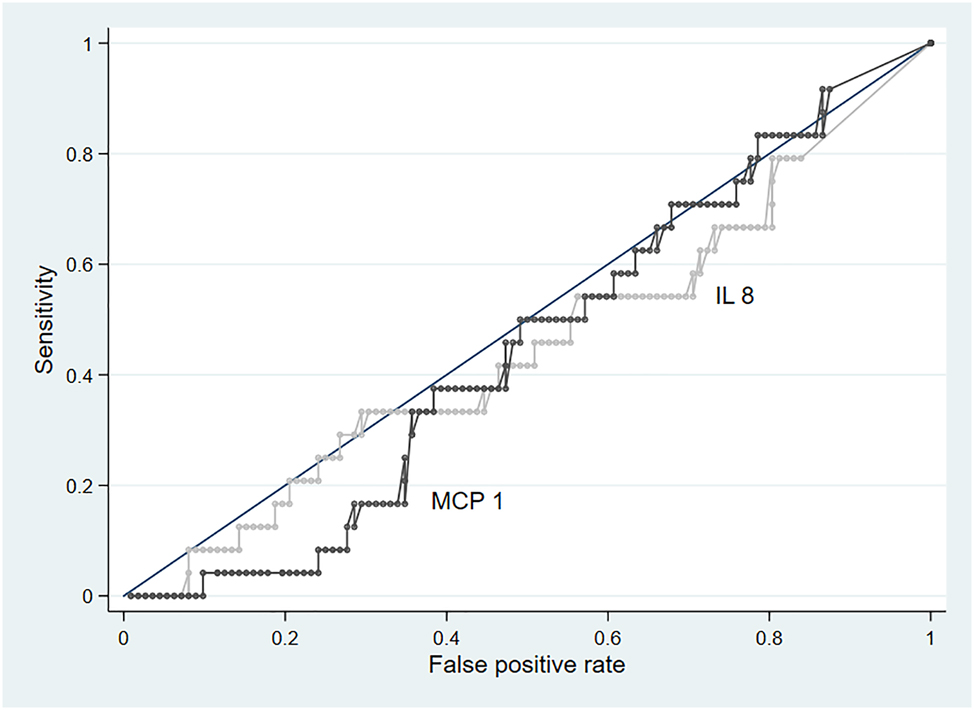
Receiver operating characteristic curves of urinary interleukin-8 (IL-8) and monocyte chemoattractant protein-1 (MCP-1) for the diagnosis of late-onset sepsis in very preterm infants. The figure illustrates the diagnostic performance of IL-8 and MCP-1 for various threshold levels. AUC, area under the curve; IL-8, 0.46 and MCP-1, 0.49. IL-8, Interleukin-8; MCP-1, monocyte chemoattractant Protein-1.
Diagnostic accuracy of biomarkers.
| Urinary biomarker | Prespecified cut-off | Sensitivity, % | Specificity, % | LR of a positive test | LR of a negative test | AUC |
|---|---|---|---|---|---|---|
| Interleukin- 8 | 92 pg/mL | 42 (22–63) | 51 (41–60) | 0.85 (0.51–1.41) | 1.15 (0.78–1.68) | 0.46 (0.35–0.57) |
| Monocyte chemoattractant Protein-1 | 257 pg/mL | 46 (26–67) | 53 (43–62) | 0.97 (0.60–1.56) | 1.03 (0.68–1.55) | 0.49 (0.38–0.60) |
-
AUC, area under the curve; LR, likelihood ratio.
Discussion
Our study shows that urinary IBM (IL-8 and MCP-1) had poor sensitivity and specificity (<50 %) and may not be reliable in diagnosing LOS in very preterm infants, of <32 weeks of gestation. The area under ROC <0.5 also suggested poor performance of the urinary interleukins over a range of decision thresholds. A randomly chosen infected neonate, as compared to a non-infected neonate is less likely to be correctly diagnosed with sepsis based on urinary interleukin levels.
Interleukins contribute to the early phase of immune activation in response to inflammatory process. The serum IL-6 and IL-8 levels peak at 6 h after onset of bacteremia and decline over next 24 h [21], 22], making them promising biomarkers for early detection of sepsis. Serum MCP-1 has also been reported as a potential biomarker for differentiating between sepsis and systemic inflammatory response syndrome in critically ill children [23].
Very limited literature exists on the molecular kinetics of urinary IBM and its role in the diagnosis of neonatal infections. While the significance of urinary interleukins has been commonly studied in the context of urinary tract infections [17], [24], [25], [26], Bentlin et al. evaluated the reliability of urinary IL-8 for diagnosis of LOS in 56 premature infants [27]. They reported that at optimized thresholds, the sensitivity of urinary IL-8 (92 %) was higher than that of serum IL-8 (69 %) for detection of LOS. However, their analysis included neonates with either clinical or culture-positive sepsis after 48 h of age. Another study reported significantly elevated urinary levels of IL-8, inducible protein-10 and MCP-1 levels in 15 term infants with presumed sepsis as compared to 15 healthy control neonates admitted to neonatal nursery[16]. The results of our study were in contrast to these previous studies. It is possible that urinary IBM levels may be reflective of local inflammatory response than systemic infection. We measured cytokine levels at a single time point in the early phase of infection. The probability of capturing peak levels of cytokines could have been higher with serial evaluations of interleukins.
Our study had certain strengths. Over 50 % of our study population comprised of extremely low birth weight infants with high risk of sepsis attributable mortality. We did not include a control sample of healthy infants for the diagnostic accuracy analysis. Those infants may not require screening for sepsis in real world setting and their inclusion could have impacted the generalization of our results. Our reference standard did not include clinically diagnosed sepsis based on abnormalities in other biomarkers of sepsis, thereby reducing the misclassification of infected and non-infected neonates. Prospective study design, blinding of test interpretation by clinical and microbiology team, pairing of urinary interleukin assessments with blood cultures in all included infants may potentially reduce selection bias in the interpretation of our results. Lower rates of participant exclusions and sample losses are some of the other strengths. Urine samples were stored at −20 °C at the treating hospitals for a period of 48–72 h prior to transfer to −80 °C storage conditions at the testing laboratory. Stability of urinary biomarkers has been reported at a storage temperature of 4 °C over a period of 3–7 days[28], 29]. The impact of pre-analytical storage temperatures on biomarker levels in our study is less likely.
Our study had some limitations. We chose a convenience sample size of 136 infants due to funding constraints. Assuming a 20 % prevalence of LOS in our unit, a minimally acceptable sensitivity of 90 % for the biomarker assay and a precision of ± 15 %, a sample size of approximately 350 would be required for such evaluations [30]. The test threshold selected for the study may not necessarily be optimal to distinguish sepsis from its close differential diagnosis. Wide variations in urinary IBM levels among infants with confirmed LOS precluded the identification of an optimum threshold for positivity based on our study data. Large-scale evaluations in healthy term and preterm infants are required to derive normative ranges of these biomarkers, standardize the decision thresholds and to study the influence of gestational age, postnatal age and other morbidities on these levels. We did not concurrently measure serum levels of IL-8 to verify concordance with the urinary levels. Although serum IL-8 is reported to have moderate diagnostic accuracy [31], it is important to note that most of these evaluations have been conducted in the context of early-onset sepsis and the diagnostic cut-off has varied from 0.65 to 300 pg/mL in clinical studies. Reports also indicate that urinary interleukin levels may not correlate with the serum levels in neonates.
It is possible that these tests may have better diagnostic properties on serial evaluations or when combined with other biomarkers [32]. While such assessments may facilitate monitoring of response to treatments and enable prediction of disease severity and prognosis, it remains important to ascertain the ability of these biomarker combinations to discriminate between infants with and without infection, particularly in the early phase.
Conclusions
Urinary IBM (IL-8 and MCP-1) were not reliable in diagnosis of LOS in very preterm infants. Validation of biomarker levels in infected and non-infected neonates across different gestations and postnatal ages would be required to reliably estimate the diagnostic accuracy of these urinary IBMs.
Funding source: Grand Challenges in Global Health
Award Identifier / Grant number: GCE-India/R5/2019/007
-
Research ethics: The study was approved by the Institutional Ethics Committee of Surya Children Hospital and Seth GS Medical College and KEM Hospital, EC-04/09/2021 and EC/OA-72/2022 respectively.
-
Informed consent: Informed consent was obtained from the legal guardians of all individuals included in this study.
-
Author contributions: Ashwini Patil and Gayatri Morajker contributed equally to this work and share first authorship. All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Use of Large Language Models, AI and Machine Learning Tools: None declared.
-
Conflict of interest: The author state no conflict of interest.
-
Research funding: This study was funded by the Bill and Melinda Gates Foundation and was supported by grant - GCE-India/R5/2019/007.
-
Data availability: All data generated or analyzed during this study are included in this published article.
References
1. Hornik, CP, Fort, P, Clark, RH, Watt, K, Benjamin, DK, Smith, PB, et al.. Early and late onset sepsis in very-low-birth-weight infants from a large group of neonatal intensive care units. Early Hum Dev 2012;88:S69–74. https://doi.org/10.1016/s0378-3782-12-70019-1.Search in Google Scholar
2. Köstlin-Gille, N, Härtel, C, Haug, C, Göpel, W, Zemlin, M, Müller, A, et al.. Epidemiology of early and late onset neonatal sepsis in very low birthweight infants: data from the German neonatal network. Pediatr Infect Dis J 2021;40:255–9. https://doi.org/10.1097/inf.0000000000002976.Search in Google Scholar PubMed
3. Investigators of the Delhi Neonatal Infection Study (DeNIS) collaboration. Characterisation and antimicrobial resistance of sepsis pathogens in neonates born in tertiary care centres in Delhi, India: a cohort study. Lancet Global Health 2016;4:e752–760. https://doi.org/10.1016/S2214-109X-16-30148-6.Search in Google Scholar
4. Adams-Chapman, I, Stoll, BJ. Neonatal infection and long-term neurodevelopmental outcome in the preterm infant. Curr Opin Infect Dis 2006;19:290–7. https://doi.org/10.1097/01.qco.0000224825.57976.87.Search in Google Scholar PubMed
5. Stoll, BJ, Hansen, NI, Adams-Chapman, I, Fanaroff, AA, Hintz, SR, Vohr, B, et al.. Neurodevelopmental and growth impairment among extremely low-birth-weight infants with neonatal infection. JAMA 2004;292:2357–65. https://doi.org/10.1001/jama.292.19.2357.Search in Google Scholar PubMed
6. Flannery, DD, Edwards, EM, Coggins, SA, Horbar, JD, Puopolo, KM. Late-onset sepsis among very preterm infants. Pediatr 2022;150:e2022058813. https://doi.org/10.1542/peds.2022-058813.Search in Google Scholar PubMed PubMed Central
7. Brown, JVE, Meader, N, Wright, K, Cleminson, J, McGuire, W. Assessment of C-reactive protein diagnostic test accuracy for late-onset infection in newborn infants. JAMA Pediatr 2020;174:260–8. https://doi.org/10.1001/jamapediatrics.2019.5669.Search in Google Scholar PubMed PubMed Central
8. Janeway, CA, Medzhitov, R. Innate immune recognition. Annu Rev Immunol 2002;20:197–216. https://doi.org/10.1146/annurev.immunol.20.083001.084359.Search in Google Scholar PubMed
9. Petrakou, E, Mouchtouri, A, Levi, E, Lipsou, N, Xanthou, M, Fotopoulos, S. Interleukin-8 and monocyte chemotactic protein-1 mRNA expression in perinatally infected and asphyxiated preterm neonates. Neonatol 2007;91:107–13. https://doi.org/10.1159/000097127.Search in Google Scholar PubMed
10. Eichberger, J, Resch, E, Resch, B. Diagnosis of neonatal sepsis: the role of inflammatory markers. Front Pediatr 2022;10:840288. https://doi.org/10.3389/fped.2022.840288.Search in Google Scholar PubMed PubMed Central
11. Boskabadi, H, Zakerihamidi, M. Evaluate the diagnosis of neonatal sepsis by measuring interleukins: a systematic review. Pediatr Neonatol 2018;59:329–38. https://doi.org/10.1016/j.pedneo.2017.10.004.Search in Google Scholar PubMed
12. Krueger, M, Nauck, MS, Sang, S, Hentschel, R, Wieland, H, Berner, R. Cord blood levels of interleukin-6 and interleukin-8 for the immediate diagnosis of early-onset infection in premature infants. Biol Neonate 2001;80:118–23. https://doi.org/10.1159/000047130.Search in Google Scholar PubMed
13. Wang, H, Oei, J, Lui, K, Henry, R. Interleukin-16 in tracheal aspirate fluids of newborn infants. Early Hum Dev 2002;67:79–86. https://doi.org/10.1016/s0378-3782-01-00257-2.Search in Google Scholar
14. Baud, O, Emilie, D, Pelletier, E, Lacaze-Masmonteil, T, Zupan, V, Fernandez, H, et al.. Amniotic fluid concentrations of interleukin-1beta, interleukin-6 and TNF-alpha in chorioamnionitis before 32 weeks of gestation: histological associations and neonatal outcome. Br J Obstet Gynaecol 1999;106:72–7. https://doi.org/10.1111/j.1471-0528.1999.tb08088.x.Search in Google Scholar PubMed
15. Tandoi, FM, Nelle, M, Raio, L, Ghezzi, F, Malek, A, Cromi, A, et al.. 253 Interleukin-6 and C reactive protein in serum and urine of neonates. Pediatr Res 2004;56:507. https://doi.org/10.1203/00006450-200409000-00276.Search in Google Scholar
16. Suguna, NS, Hendricks-Muñoz, KD, Borkowsky, W, Mally, P. Usefulness of urinary immune biomarkers in the evaluation of neonatal sepsis: a pilot project. Clin Pediatr 2013;52:520–6. https://doi.org/10.1177/0009922813482751.Search in Google Scholar PubMed
17. Nuri, N, Ramayani, OR, Ramayati, R, Nelly, Siregar, RS, Siregar, B. The accuracy of Interleukin-6 urine compared to urine culture to diagnose pyelonephritis in neonates; 2016. [cited 2023 May 17]; Available from https://dupakdosen.usu.ac.id/handle/123456789/3127.10.19080/JOJUN.2017.01.555574Search in Google Scholar
18. Valentine, GC, Wallen, LD. Neonatal bacterial sepsis and meningitis. In: Gleason, CA, Sawyer, T, editors. Avery’s diseases of the newborn, 11th ed. Chicago: Elsevier; 2023:439–49 pp.10.1016/B978-0-323-82823-9.00033-7Search in Google Scholar
19. Meem, M, Modak, JK, Mortuza, R, Morshed, M, Islam, MS, Saha, SK. Biomarkers for diagnosis of neonatal infections: a systematic analysis of their potential as a point-of-care diagnostics. J Glob Health 2011;1:201–9.Search in Google Scholar
20. Jean-Baptiste, N, Benjamin, DK, Cohen-Wolkowiez, M, Fowler, VG, Laughon, M, Clark, RH, et al.. Coagulase-negative staphylococcal infections in the neonatal intensive care unit. Infect Control Hosp Epidemiol 2011;32:679–86. https://doi.org/10.1086/660361.Search in Google Scholar PubMed PubMed Central
21. Gilfillan, M, Bhandari, V. Biomarkers for the diagnosis of neonatal sepsis and necrotizing enterocolitis: clinical practice guidelines. Early Hum Dev 2017;105:25–33. https://doi.org/10.1016/j.earlhumdev.2016.12.002.Search in Google Scholar PubMed
22. Ng, PC, Lam, HS. Biomarkers for late-onset neonatal sepsis: cytokines and beyond. Clin Perinatol 2010;37:599–610. https://doi.org/10.1016/j.clp.2010.05.005.Search in Google Scholar PubMed PubMed Central
23. Hassuna, NA, Elgezawy, E, Mousa, SO, AbdelAziz, RA, Ibrahem, RA, Wahed, WYA, et al.. Diagnostic value of monocyte chemoattractant protein-1, soluble mannose receptor, presepsin, and procalcitonin in critically ill children admitted with suspected sepsis. BMC Pediatr 2021;21:458. https://doi.org/10.1186/s12887-021-02930-7.Search in Google Scholar PubMed PubMed Central
24. Roilides, E, Papachristou, F, Gioulekas, E, Tsaparidou, S, Karatzas, N, Sotiriou, J, et al.. Increased urine interleukin-6 concentrations correlate with pyelonephritic changes on 99mTc-dimercaptosuccinic acid scans in neonates with urinary tract infections. J Infect Dis 1999;180:904–7. https://doi.org/10.1086/314960.Search in Google Scholar PubMed
25. Miklaszewska, M, Korohodai, P, Kwinta, P, Tomasik, T, Zachwieja, K, Klich, B, et al.. Clinical validity of urinary interleukin 18 and interleukin 6 determinations in preterm newborns. Przegl Lek 2015;72:589–96.Search in Google Scholar
26. Krzemień, G, Szmigielska, A, Turczyn, A, Pańczyk-Tomaszewska, M. Urine interleukin-6, interleukin-8 and transforming growth factor β1 in infants with urinary tract infection and asymptomatic bacteriuria. Cent Eur J Immunol 2016;41:260–7. https://doi.org/10.5114/ceji.2016.63125.Search in Google Scholar PubMed PubMed Central
27. Bentlin, MR, de Souza Rugolo, LMS, Júnior, AR, Hashimoto, M, Lyra, JC. Is urine Interleukin-8 level a reliable laboratory test for diagnosing late onset sepsis in premature infants? J Trop Pediatr 2007;53:403–8. https://doi.org/10.1093/tropej/fmm054.Search in Google Scholar PubMed
28. Grenier, FC, Ali, S, Syed, H, Workman, R, Martens, F, Liao, M, et al.. Evaluation of the ARCHITECT urine NGAL assay: assay performance, specimen handling requirements and biological variability. Clin Biochem 2010;43:615–20. https://doi.org/10.1016/j.clinbiochem.2009.12.008.Search in Google Scholar PubMed
29. Chang, C, Obeid, W, Thiessen-Philbrook, H, Parikh, CR. Sample processing and stability for urine biomarker studies. J Appl Lab Med 2021;6:1628–34. https://doi.org/10.1093/jalm/jfab082.Search in Google Scholar PubMed PubMed Central
30. Buderer, NM. Statistical methodology: I. Incorporating the prevalence of disease into the sample size calculation for sensitivity and specificity. Acad Emerg Med Off J Soc Acad Emerg Med 1996;3:895–900. https://doi.org/10.1111/j.1553-2712.1996.tb03538.x.Search in Google Scholar PubMed
31. Zhou, M, Cheng, S, Yu, J, Lu, Q. Interleukin-8 for diagnosis of neonatal sepsis: a meta-analysis. PLoS One 2015;10:e0127170. https://doi.org/10.1371/journal.pone.0127170.Search in Google Scholar PubMed PubMed Central
32. Dillenseger, L, Langlet, C, Iacobelli, S, Lavaux, T, Ratomponirina, C, Labenne, M, et al.. Early inflammatory markers for the diagnosis of late-onset sepsis in neonates: the nosodiag study. Front Pediatr 2018;6:346. https://doi.org/10.3389/fped.2018.00346.Search in Google Scholar PubMed PubMed Central
Supplementary Material
This article contains supplementary material (https://doi.org/10.1515/jpm-2025-0342).
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.

