Abstract
Objectives
To investigate the association between Doppler ultrasonographic parameters and maternal serum bile acid levels in pregnancies complicated by intrahepatic cholestasis of pregnancy (ICP) and to evaluate their potential role in fetal monitoring.
Methods
In this prospective observational study, 59 pregnant women diagnosed with ICP and 59 healthy controls were included. The diagnosis of ICP was based on pruritus and serum bile acid levels >10 μmol/L. Doppler assessments – performed by a blinded perinatologist – included the uterine artery pulsatility index (PI), middle cerebral artery (MCA) PI, umbilical artery (UA) PI, and cerebroplacental ratio (CPR). Participants were further stratified by bile acid levels (<10, 10–40, >40 μmol/L), and intergroup comparisons and correlation analyses were performed.
Results
The ICP cases had significantly higher uterine artery PI and lower MCA PI and CPR values compared to controls (p<0.05). Bile acid levels were positively correlated with uterine artery PI and inversely correlated with MCA PI and CPR. ICP pregnancies also demonstrated increased rates of preterm delivery, low birth weight, and neonatal intensive care unit (NICU) admissions.
Conclusions
Alterations in uterine and fetal Doppler indices in ICP pregnancies suggest underlying placental dysfunction. These findings support the clinical utility of Doppler ultrasound in the risk stratification and perinatal surveillance of patients with ICP.
Introduction
Intrahepatic cholestasis of pregnancy (ICP) is a unique liver disorder that typically arises in the second and third trimesters of pregnancy and spontaneously resolves postpartum. ICP is characterized by pruritus, typically localized to the palms and soles, and elevated serum bile acid levels and/or liver enzymes without other systemic or hepatobiliary diseases [1]. The global incidence of ICP is estimated to range from 0.2 to 2 %, with significant geographical and ethnic variability [2]. The pathogenesis of ICP is multifactorial, involving genetic, hormonal, and environmental influences. Elevated estrogen and progesterone levels during pregnancy, along with an isolated genetic predisposition, contribute to impaired bile acid transport and cholestasis [3].
The diagnosis of ICP is primarily established through clinical presentation and laboratory findings, with serum bile acid levels serving as a critical diagnostic marker. Serum bile acid levels exceeding 10 μmol/L are widely accepted as a diagnostic threshold for ICP, demonstrating high sensitivity (91 %) and specificity (93 %) according to a Cochrane systematic review [4].
International guidelines vary in their recommendations. The Royal College of Obstetricians and Gynaecologists (RCOG) Green-top Guideline No. 43 (2022) supports considering the diagnosis in women with pruritus and a peak random total serum bile acid concentration of ≥ 19 μmol/L, with resolution after delivery further supporting the diagnosis. In contrast, the Society for Maternal-Fetal Medicine (SMFM) Consult Series #53 (Am J Obstet Gynecol, 2021) notes that pruritus may precede biochemical abnormalities by several weeks, that random (non-fasting) measurements are acceptable, and that some clinicians diagnose ICP based on symptoms alone after exclusion of other causes, even if bile acids are <10 μmol/L [5], 6].
Fetal outcomes in ICP are influenced by bile acid accumulation, which is thought to contribute to placental insufficiency and fetal distress [7]. Some studies suggest that increased bile acid concentrations induce vasoconstriction in the chorionic veins, potentially compromising placental circulation and leading to fetal hypoxia. However, the relationship between ICP severity and Doppler ultrasound findings remains controversial [8]. While specific studies report alterations in umbilical and uterine artery resistance indices, others fail to demonstrate a consistent correlation between Doppler parameters and maternal bile acid levels [9]. These inconsistencies highlight the need for further research to establish whether Doppler ultrasonography can be a reliable tool for fetal monitoring and risk stratification in ICP pregnancies. This study aims to contribute to the ongoing discussion by assessing Doppler parameters about bile acid levels, which remains insufficiently explored in the current literature.
Patients and methods
This prospective observational study was conducted at Prof. Dr. Cemil Taşcıoğlu City Hospital after receiving Ethical Approval from the Clinical Research Ethics Committee. The study included pregnant women who presented to the Obstetric Emergency Unit or Perinatology Clinic between December 25, 2023, and June 25, 2024. Written informed consent was obtained from all participants before study enrollment.
A total of 118 pregnant women were enrolled, including 59 patients diagnosed with ICP and 59 healthy controls.
The diagnosis of ICP was based on the presence of generalized pruritus without primary skin lesions in the second or third trimester, along with either elevated total serum bile acids (TSBA) or elevated aminotransferases. Other causes of pruritus and abnormal liver function tests, such as viral hepatitis, biliary obstruction, gallstones, and other pregnancy-related liver disorders, were ruled out first. TSBA levels were measured in the non-fasting state. Due to ongoing variability in diagnostic thresholds across international guidelines, TSBA was analyzed both as a continuous variable and in three categories: <10 μmol/L, 10–40 μmol/L, and >40 μmol/L. This approach allowed the inclusion of women with typical clinical features and elevated aminotransferases despite TSBA levels below 10 μmol/L, consistent with published criteria [4], 5].
This definition aligns with the SMFM guidance, which allows diagnosis based on typical symptoms and exclusion of other causes even when bile acids are <10 μmol/L, and accepts random (non-fasting) testing. While the RCOG recommends a threshold of ≥ 19 μmol/L as supportive of the diagnosis, we adopted the SMFM-inclusive approach to capture a broader clinical spectrum of ICP.
The control group comprised consecutive eligible pregnant women with similar gestational ages to the ICP group and without chronic illnesses, pregnancy-related comorbidities, or maternal/fetal abnormalities. All participants were referred to a single perinatologist for Doppler assessment. Importantly, the perinatologist was blinded to group allocation and performed Doppler evaluations without knowledge of whether the patient belonged to the ICP or control group.
During enrollment, demographic data, medical history, laboratory findings, and clinical parameters were recorded for all participants. Doppler ultrasound measurements were performed using the Mindray Resona seven ultrasound system (Shenzhen Mindray Bio-Medical Electronics Co., Ltd., China) with an SC6-1U convex transducer (1.2–6.0 MHz) by a single specialist (M. Özalp), who was blinded to the demographic and laboratory data of the patients. The measurements were conducted according to the International Society of Ultrasound in Obstetrics and Gynecology guideline [10]. The mean pulsatility index (PI) of the bilateral uterine arteries was calculated by taking the average of the PI values from both the right and left uterine arteries. The cerebroplacental ratio (CPR) was determined by dividing the middle cerebral artery (MCA) PI by the umbilical artery (UA) PI.
Statistical analyses were conducted using SPSS 15.0 for Windows. Descriptive statistics were reported as frequencies and percentages for categorical variables, while numerical variables were summarized as mean, standard deviation, minimum, maximum, median, and interquartile range (IQR). The normality of numerical data was evaluated using the Kolmogorov-Smirnov test.
The Student’s t-test was applied when the normality assumption was met to compare numerical variables between two independent groups. At the same time, the Mann-Whitney U test was used when this assumption was not satisfied. Comparisons among more than two independent groups were conducted using one-way analysis of variance (ANOVA) when normality was maintained. In contrast, the Kruskal-Wallis test was employed when normality was not upheld. Post-hoc subgroup analyses for nonparametric tests were performed using the Mann-Whitney U test, with Bonferroni correction applied for multiple comparisons.
Categorical variables were compared between groups using the Chi-square test. Correlations between numerical variables were analyzed using Spearman’s correlation analysis since the assumptions for parametric tests were not satisfied.
A p-value of less than 0.05 was deemed statistically significant.
Results
A total of 118 pregnant women participated in the study, including 59 patients diagnosed with ICP and 59 healthy controls.
There was no statistically significant difference between the study groups regarding maternal age, gravida, parity, gestational week at blood sampling, ultrasound examination, prothrombin time (PT), or maternal fasting glucose levels. However, maternal serum levels of aspartate aminotransferase (AST), alanine aminotransferase (ALT), gamma-glutamyl transferase (GGT), and total bilirubin were significantly higher in the ICP group compared to the control group (Table 1).
Comparison of demographic characteristics and laboratory parameters of the study groups.
| Variables | ICP (n=59) | Control group (n=59) | p-Value |
|---|---|---|---|
| Age, years | 29.5 ± 6.4 (19–43) | 29.3 ± 5.7 (20–43) | 0.855 |
| Gravida | 2.5 ± 1.5 (1–7) | 2.8 ± 1.4 (1–7) | 0.212 |
| Parity | 1.1 ± 1.0 (0–3) | 1.2 ± 1.0 (0–4) | 0.627 |
| BMI, kg/m2 | 27.8 ± 3.5 (20.3–35.6) | 29.4 ± 4.8 (2.05–39.2) | 0.060 |
| Mean arterial pressure | 85.5 ± 5.9 (70–110) | 85.1 ± 4.6 (80–93.3) | 0.751 |
| Gestational age at blood sampling, weeks | 30.6 ± 2.4 (23–34) | 30.6 ± 1.1 (28–33) | 0.136 |
| Gestational age at ultrasound examination, weeks | 31.5 ± 2.2 (23–35) | 31.6 ± 1.2 (30–35) | 0.312 |
| AST, U/L | 95.8 ± 86.9 (13–400) | 20.1 ± 5.3 (10–34) | <0.001 |
| ALT, U/L | 134.7 ± 161.9 (8–734) | 12.2 ± 6.4 (3–34) | <0.001 |
| GGT, U/L | 30.7 ± 50.9 (2–346) | 9.8 ± 5.3 (3–27) | <0.001 |
| PT, s | 12.3 ± 1.3 (8.8–17) | 11.8 ± 0.7 (9.5–13.7) | 0.088 |
| Total bilirubin, mg/dL | 0.52 ± 0.33 (0.01–2.12) | 0.37 ± 0.13 (0.09–0.63) | 0.009 |
| Fasting glucose, mg/dL | 92.3 ± 44.2 (60–380) | 80.1 ± 10.9 (55–100) | 0.242 |
-
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; GGT, gamma-glutamyl transferase; ICP, intrahepatic cholestasis of pregnancy; PT, prothrombin time; s, seconds; TSBA, total serum bile acids; U/L, units per liter. The bold values represent statistically significant results (p < 0.05).
The mean uterine artery PI was significantly higher in the ICP group compared to the control group. However, CPR and MCA PI were substantially lower in the ICP group. The groups had no significant difference in UA PI (Table 2).
Comparison of Doppler parameters between the study groups.
| Doppler parameter | ICP (n=59) | Control group (n=59) | p-Value |
|---|---|---|---|
| Uterine artery PI, mean | 0.75 ± 0.10 (0.58–0.96) | 0.67 ± 0.09 (0.51–0.86) | <0.001 |
| CPR | 1.82 ± 0.55 (0.86–4) | 1.98 ± 0.23 (1.52–2.2) | <0.001 |
| MCA PI | 1.69 ± 0.27 (1.01–2.9) | 1.79 ± 0.09 (1.58–1.95) | <0.001 |
| UA PI | 0.98 ± 0.24 (0.47–1.91) | 0.92 ± 0.10 (0.69–1.16) | 0.070 |
-
CPR, cerebroplacental ratio; ICP, intrahepatic cholestasis of pregnancy; MCA PI, middle cerebral artery pulsatility index; PI, pulsatility index; UA PI, umbilical artery pulsatility index. The bold values represent statistically significant results (p < 0.05).
When uterine artery PI values were expressed as gestational age–adjusted centiles, the control group had a mean centile of 37.64, whereas the ICP group had a mean centile of 60.52. Stratification of the ICP group by bile acid levels showed a progressive increase in centiles: 45.91 for <10 μmol/L (n=20, 33.9 %), 65.16 for 10–40 μmol/L (n=30, 50.8 %), and 76.66 for >40 μmol/L (n=9, 15.3 %). These findings indicate that, even when absolute PI values fall within conventional reference ranges, there is a clear upward shift relative to gestational age, which becomes more pronounced with higher bile acid concentrations.
In the subgroup analysis, patients with serum bile acid levels exceeding 40 μmol/L showed significantly higher uterine artery PI values and lower MCA PI values than other groups. However, no significant differences were observed in the CPR or UA PI among the subgroups (Table 3).
Subgroup analysis of Doppler parameters based on serum bile acid levels.
| Doppler parameter | <10 μmol/L (n=20) | 10–40 μmol/L (n=30) | >40 μmol/L (n=9) | p-Value |
|---|---|---|---|---|
| Uterine artery PI, mean | 0.67 ± 0.05 (0.58–0.75) | 0.77 ± 0.08 (0.58–0.94) | 0.89 ± 0.05 (0.81–0.96) | <0.001 |
| CPR | 1.93 ± 0.66 (0.86–4) | 1.81 ± 0.54 (0.88–3.22) | 1.58 ± 0.16 (1.29–1.90) | 0.106 |
| MCA PI | 1.70 ± 0.15 (1.42–2.13) | 1.72 ± 0.35 (1.01–2.90) | 1.54 ± 0.02 (1.51–1.56) | 0.001 |
| UA PI | 0.96 ± 0.30 (0.47–1.91) | 1.00 ± 0.23 (0.53–1.88) | 0.98 ± 0.10 (0.80–1.17) | 0.172 |
-
CPR, cerebroplacental ratio; MCA PI, middle cerebral artery pulsatility index; PI, pulsatility index; UA PI, umbilical artery pulsatility index. The bold values represent statistically significant results (p < 0.05).
A statistically significant correlation was observed between maternal serum bile acid levels and Doppler indices. Specifically, increasing bile acid levels correlated with higher uterine artery PI (r=0.764, p<0.001) and higher UA PI (r=0.336, p=0.009), whereas increasing bile acid levels correlated with lower MCA PI (r=−0.457, p<0.001) and lower CPR (r=−0.354, p=0.006). These relationships are visualized in Figure 1, with detailed coefficients presented in (Table 4).
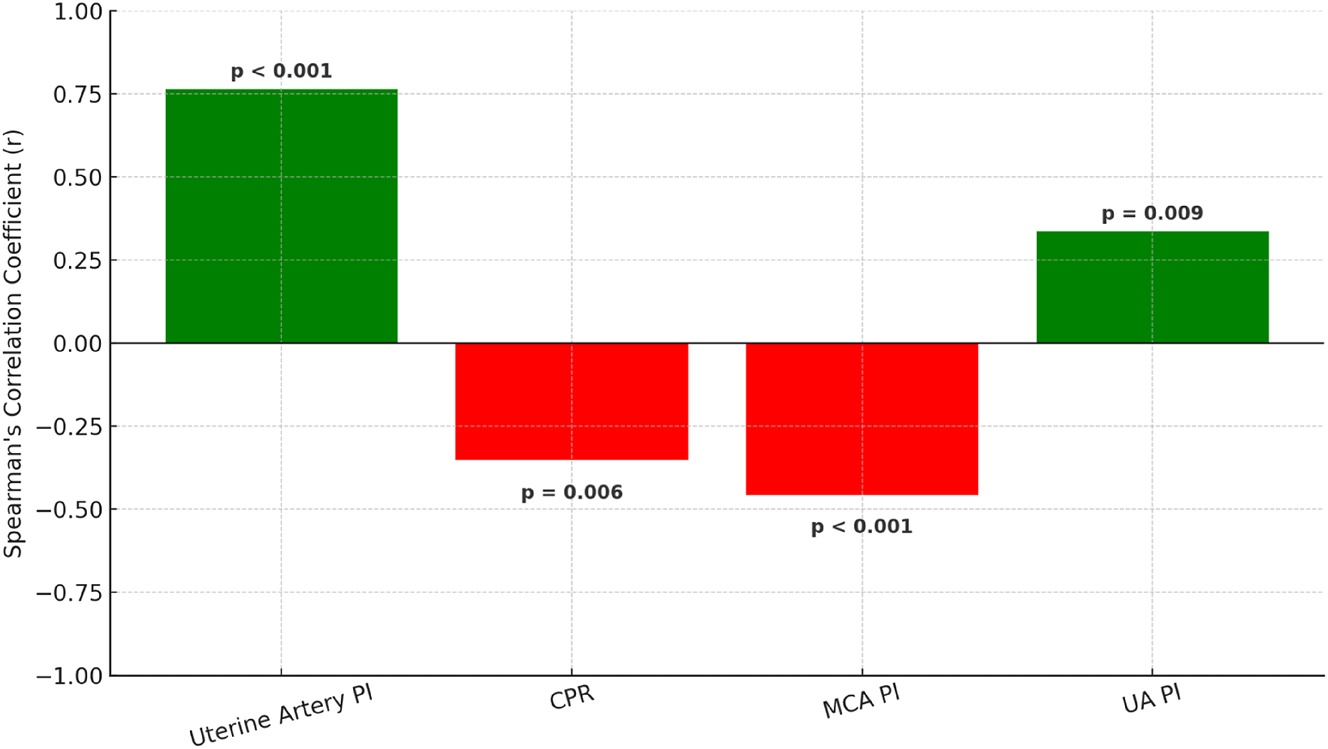
Spearman correlation coefficients between maternal serum bile acid levels and Doppler parameters. Positive correlations (green) were observed with uterine artery pulsatility index (uterine artery PI) and umbilical artery pulsatility index (UA PI), while negative correlations (red) were found with cerebroplacental ratio (CPR) and middle cerebral artery pulsatility index (MCA PI). All associations were statistically significant. See Table 4 for exact values. PI, pulsatility index; UA, umbilical artery; CPR, cerebroplacental ratio; MCA, middle cerebral artery.
Correlation analysis between maternal serum bile acid levels and Doppler parameters.
| Doppler parameter | R | p-Value |
|---|---|---|
| Uterine artery PI, mean | 0.764 | <0.001 |
| CPR | −0.354 | 0.006 |
| MCA PI | −0.457 | <0.001 |
| UA PI | 0.336 | 0.009 |
-
CPR, cerebroplacental ratio; MCA PI, middle cerebral artery pulsatility index; PI, pulsatility index; UA PI, umbilical artery pulsatility index; R, Spearman correlation coefficient. Direction of effect: Increasing bile acid levels are associated with ↑ uterine artery PI and ↑ UA PI, and with ↓ MCA PI and ↓ CPR. The bold values represent statistically significant results (p < 0.05).
As shown in Table 5, the gestational age at delivery was significantly lower in the ICP group compared to the control group. Similarly, the mean birth weight of neonates was significantly lower in the ICP group than in the control group.
Comparison of birth weight, delivery timing, and neonatal outcomes between the study groups.
| Variables | ICP (n=59) | Control group (n=59) | p-Value |
|---|---|---|---|
| Gestational age at delivery, weeks | 35.2 ± 3.0 (23–39) | 38.1 ± 1.3 (35–40) | <0.001 |
| Birth weight, g | 2,720.9 ± 656.7 (600–4,060) | 3,193.7 ± 532.0 (2,215–4,325) | <0.001 |
| Cesarean section rate, % | 36 (66.7) | 36 (61.0) | 0.533 |
| 5-min apgar score | 7.94 ± 1.12 (3–10) | 8.25 ± 0.78 (6–10) | 0.139 |
| Umbilical cord pH | 7.31 ± 0.08 (7.04–7.51) | 7.29 ± 0.06 (7.08–7.4) | 0.025 |
| NICU admission, % | 27 (45.6) | 13 (22.0) | 0.002 |
-
ICP, intrahepatic cholestasis of pregnancy; NICU, neonatal intensive care unit. The bold values represent statistically significant results (p < 0.05).
Neonatal intensive care unit (NICU) admission rates were significantly higher in the ICP group, suggesting an increased risk of neonatal complications in pregnancies affected by ICP. Additionally, umbilical cord pH values were significantly different between the two groups, although the clinical significance of this finding remains uncertain.
Conversely, there was no significant difference in 5-min Apgar scores between the ICP and control groups. Furthermore, cesarean section rates were comparable between the two groups, indicating that ICP did not significantly influence the mode of delivery (Table 5).
Discussion
This study demonstrates significant alterations in uteroplacental and fetal circulation in pregnancies complicated by ICP. Specifically, pregnancies with ICP exhibited significantly higher uterine artery PI and lower CPR and MCA PI compared to controls. Furthermore, maternal serum bile acid levels correlated positively with uterine artery PI and negatively with CPR and MCA PI, suggesting a potential impact of bile acid accumulation on placental and fetal hemodynamics. Interestingly, no significant difference was observed in UA PI between the ICP and control groups, implying a selective effect of ICP on certain vascular beds. Additionally, pregnancies with ICP were associated with lower gestational age at delivery, reduced birth weight, and higher rates of NICU admission, emphasizing the clinical significance of these hemodynamic changes.
The relationship between ICP and Doppler parameters has been previously explored, but findings remain inconsistent [11]. In contrast to our results, Yilmaz et al., reported no significant differences in Doppler indices between ICP and control groups [12]. One potential explanation for this discrepancy is the smaller sample size in their study (n=60 vs. our 118 participants), which may have limited their statistical power to detect hemodynamic alterations. Additionally, Yilmaz et al. assessed uterine artery Doppler parameters by analyzing the right and left arteries separately, whereas we calculated the mean uterine artery PI, potentially providing a more integrated measure of placental resistance. Furthermore, the inclusion of patients with milder ICP cases in their cohort may have resulted in less pronounced Doppler changes compared to our study, which categorized patients based on bile acid levels.
Similarly, Kurtulmuş et al. reported no significant differences in MCA PI, UA PI, or other Doppler indices between the ICP and control groups [8]. However, our study identified a significant increase in uterine artery PI and a decrease in MCA PI and CPR. These discrepancies may be attributed to methodological differences, including the timing of Doppler assessments. In our study, Doppler measurements were conducted earlier in gestation, whereas Kurtulmuş et al. primarily assessed patients in the late third trimester, during which fetal compensatory mechanisms may mitigate Doppler abnormalities. Additionally, we stratified ICP cases based on bile acid levels, allowing for a more refined analysis of disease severity, while their study treated ICP as a homogeneous condition. Another key distinction is that our study included uterine artery PI as a primary parameter, which was not considered in their analysis. This may explain why we could detect significant alterations in uteroplacental circulation.
Gajzlerska et al. emphasized the importance of Doppler assessment in ICP pregnancies, stating that conventional methods of fetal monitoring may be insufficient to detect fetal compromise [9]. They suggested that bile acids may have a vasoconstrictive effect on placental circulation, potentially leading to fetal hypoxia. Our findings align with this hypothesis, as we observed a significant increase in uterine artery PI and a decrease in MCA PI and CPR, indicating altered placental and cerebral circulation. Additionally, we demonstrated a significant correlation between bile acid levels and Doppler indices, further supporting the hypothesis that bile acids may contribute to vascular dysfunction.
The methodological differences between our study and that of Toprak & Kafadar may also explain the contrasting findings [13]. While their study evaluated Doppler parameters using the Systolic/Diastolic (S/D) ratio for the UA and MCA, we employed PI and CPR, which are considered more reliable indicators of vascular resistance. The S/D ratio primarily reflects diastolic flow but may be less sensitive in detecting subtle changes in vascular impedance, particularly in cases of altered placental or fetal circulation [14]. Conversely, PI accounts for the overall pulsatile nature of blood flow and remains a robust index even when the end-diastolic flow is diminished or absent [15]. Furthermore, unlike Toprak & Kafadar, who did not observe significant Doppler differences between ICP and control groups, our findings suggest that ICP is associated with increased uterine artery PI and decreased MCA PI and CPR, indicating alterations in uteroplacental and fetal hemodynamics.
Our results reinforce the importance of Doppler ultrasonography in the clinical management of ICP pregnancies. The observed increase in uterine artery PI and decrease in MCA PI and CPR suggest potential placental insufficiency and fetal hypoxia. Therefore, Doppler assessment may provide valuable information for fetal monitoring and risk stratification in ICP pregnancies, particularly in those with bile acid levels exceeding 40 μmol/L.
Furthermore, the selective vascular impact of ICP, evidenced by the lack of significant changes in UA PI, suggests that Doppler parameters beyond umbilical artery indices should be considered in fetal surveillance protocols. Given the increased risk of preterm birth and NICU admission in ICP pregnancies, integration of Doppler findings into clinical decision-making could aid in optimizing delivery timing and perinatal outcomes. Our findings align with those of Huang et al. and Odabaş et al., who demonstrated that ICP is significantly associated with earlier gestational age at delivery, lower birth weight, and increased NICU admission rates [16], 17]. However, unlike these meta-analyses, which primarily focused on obstetric outcomes, our study directly correlates bile acid levels with Doppler changes, emphasizing the potential role of Doppler in risk assessment.
Further research is warranted to establish standardized Doppler reference values for ICP pregnancies and to determine the predictive value of these parameters for adverse perinatal outcomes. Large-scale, multicenter prospective studies are needed to validate our findings and explore the longitudinal progression of Doppler changes in ICP. Additionally, investigations into the mechanistic role of bile acids in modulating vascular resistance could provide further insights into the pathophysiology of ICP-related fetal compromise.
Serial Doppler assessments throughout pregnancy may help elucidate the temporal evolution of hemodynamic changes and improve risk stratification models. Given the discrepancies among previous studies, future research should focus on standardizing Doppler methodologies, assessing fetal cardiac function in addition to vascular indices, and integrating biochemical markers to refine risk prediction models.
Strengths and limitations
This study has several strengths. The prospective design and blinded Doppler assessment minimize measurement and recall bias, enhancing the reliability of the findings. Unlike previous studies that primarily focused on umbilical artery indices, this study provides a comprehensive Doppler evaluation, including uterine artery PI, middle cerebral artery PI, and cerebroplacental ratio, offering a more complete assessment of maternal and fetal hemodynamics. Another notable strength is the correlation with bile acid levels, which allows for a nuanced analysis of the severity of ICP and its impact on vascular resistance. The robust statistical methodology, including subgroup analyses and correction for multiple comparisons, further strengthens the validity of the results. Clinically, the study provides important insights into the role of Doppler ultrasonography in ICP pregnancies, emphasizing its potential utility in risk stratification and fetal monitoring.
However, certain limitations should be acknowledged. As a single-center study, the findings may not be generalizable to broader populations with diverse demographic characteristics. While the overall sample size is adequate, the subgroup analysis of patients with bile acid levels >40 μmol/L includes a limited number of participants, which may reduce statistical power. Additionally, the study lacks serial Doppler assessments, preventing an evaluation of the progression of hemodynamic changes over time. Another limitation is the absence of fetal cardiac function markers, such as myocardial performance index or venous Doppler assessments, which could provide further insights into fetal compromise.
Controls were chosen consecutively instead of randomly, which could introduce potential selection bias. This limitation was partly alleviated by the blinded Doppler assessments, which lowered the risk of measurement bias. The study also does not incorporate predictive modeling for perinatal outcomes, limiting its ability to establish independent prognostic value for Doppler parameters in ICP pregnancies. Finally, the potential impact of medical interventions, such as ursodeoxycholic acid (UDCA) treatment, on Doppler indices was not evaluated, which remains an area for further investigation.
Despite these limitations, this study contributes valuable findings to the literature by reinforcing the association between ICP and altered maternal-fetal Doppler parameters. Future large-scale, multicenter studies with longitudinal Doppler assessments and predictive modeling are needed to establish standardized reference values and optimize fetal surveillance strategies in ICP pregnancies.
Conclusions
This study provides compelling evidence that ICP is associated with significant alterations in uteroplacental and fetal circulation, as demonstrated by increased uterine artery PI and decreased CPR and MCA PI. The findings suggest a potential link between maternal bile acid levels and fetal hemodynamics, emphasizing the role of Doppler ultrasound in monitoring pregnancies complicated by ICP. Importantly, ICP pregnancies were also associated with a higher risk of adverse obstetric outcomes, including lower gestational age at delivery, reduced birth weight, and increased NICU admissions.
The results underscore the clinical relevance of Doppler ultrasonography as a potential tool for fetal surveillance and risk stratification in ICP pregnancies, particularly in cases with elevated bile acid levels. However, given the study’s limitations – including its single-center design, lack of serial Doppler assessments, and absence of predictive modeling – further large-scale, multicenter studies are needed to establish standardized Doppler reference values and assess their utility in guiding clinical management. Future research should focus on integrating Doppler parameters with biochemical and clinical markers to refine perinatal risk prediction models and optimize delivery timing in ICP pregnancies.
In conclusion, this study contributes to the growing body of evidence on the vascular implications of ICP, highlighting the need for further investigation into the role of Doppler ultrasonography in fetal monitoring. By identifying specific Doppler abnormalities associated with ICP, our findings support the development of improved surveillance strategies that may ultimately enhance perinatal outcomes in affected pregnancies.
-
Research ethics: This study was approved by the Clinical Research Ethics Committee of Istanbul Prof. Dr. Cemil Taşcıoğlu City Hospital on December 18, 2023, with the protocol number 286. The study was conducted in accordance with the Declaration of Helsinki.
-
Informed consent: Informed consent was obtained from all individuals included in this study.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission. M.E.Ş.: Conceptualization, Data collection, Data curation, Writing – Original Draft Preparation, Investigation, Visualization. M.Ö.: Conceptualization, Supervision, Project administration, Methodology, Data curation, Formal analysis. M.İ.T.: Writing – Review & Editing, Writing – Original Draft Preparation, Visualization, Formal analysis. V.M.: Supervision, Project administration, Validation, Writing – Review & Editing.
-
Use of Large Language Models, AI and Machine Learning Tools: None declared.
-
Conflict of interest: The authors state no conflict of interest.
-
Research funding: None declared.
-
Data availability: Data used in this study can be provided upon reasonable request.
References
1. Pillarisetty, LSSA. Pregnancy intrahepatic cholestasis. In: StatPearls [Internet]. 2025 Jan. Aufl. Treasure Island (FL). StatPearls Publishing; 2023.Search in Google Scholar
2. Sadeghi, A. Global incidence of intrahepatic cholestasis of pregnancy: a protocol for systematic review and meta-analysis. Health Sci Rep 2024;7:e1901. https://doi.org/10.1002/hsr2.1901.Search in Google Scholar PubMed PubMed Central
3. Zöllner, J, Williamson, C, Dixon, PH. Genetic issues in ICP. Obstet Med 2024;17:157–61. https://doi.org/10.1177/1753495x241263441.Search in Google Scholar PubMed PubMed Central
4. Manzotti, C, Casazza, G, Stimac, T, Nikolova, D, Gluud, C. Total serum bile acids or serum bile acid profile, or both, for the diagnosis of intrahepatic cholestasis of pregnancy. Cochrane Database Syst Rev 2019;7:Cd012546. https://doi.org/10.1002/14651858.CD012546.pub2.Search in Google Scholar PubMed PubMed Central
5. Lee, RH, Mara, G, Metz, TD, Pettker, CM. Society for maternal-fetal medicine consult series #53: intrahepatic cholestasis of pregnancy: replaces consult #13, April 2011. Am J Obstet Gynecol 2021;224:B2–b9. https://doi.org/10.1016/j.ajog.2020.11.002.Search in Google Scholar PubMed
6. Girling, J, Knight, CL, Chappell, L. Intrahepatic cholestasis of pregnancy: green-top guideline no. 43 June 2022. BJOG 2022;129:e95-114. https://doi.org/10.1111/1471-0528.17206.Search in Google Scholar PubMed
7. Ozkan, S, Ceylan, Y, Ozkan, OV, Yildirim, S. Review of a challenging clinical issue: intrahepatic cholestasis of pregnancy. World J Gastroenterol 2015;21:7134–41. https://doi.org/10.3748/wjg.v21.i23.7134.Search in Google Scholar PubMed PubMed Central
8. Kurtulmuş S, Gür EB, Öztekin D, Güleç E, Okyay D, Gülhan İ. The impact of intrahepatic cholestasis of pregnancy on fetal cardiac and peripheral circulation. J Turk Ger Gynecol Assoc 2015;16:74–9. https://doi.org/10.5152/jtgga.2015.15173.Search in Google Scholar PubMed PubMed Central
9. Gajzlerska, E, Kozakiewicz, B, Stefaniak, M. The role of Doppler ultrasonography in intrahepatic cholestasis of pregnancy. Eur J Med Technol 2017;3:33–40.Search in Google Scholar
10. Bhide, A, Acharya, G, Baschat, A, Bilardo, CM, Brezinka, C, Cafici, D, et al.. ISUOG practice guidelines (updated): use of Doppler velocimetry in obstetrics. Ultrasound Obstet Gynecol 2021;58:331–9. https://doi.org/10.1002/uog.23698.Search in Google Scholar PubMed
11. Zimmermann, P, Koskinen, J, Vaalamo, P, Ranta, T. Doppler umbilical artery velocimetry in pregnancies complicated by intrahepatic cholestasis. J Perinat Med 1991;19:351–5. https://doi.org/10.1515/jpme.1991.19.5.351.Search in Google Scholar PubMed
12. Vural, YZ, Özdemir, O, Kurt, GY, Soysal, Ç, Yılmaz, E. The effect of intrahepatic cholestasis of pregnancy and ursodeoxycholic acid treatment on Doppler parameters of fetal and maternal circulation. Ginekol Pol 2024;95:544–8. https://doi.org/10.5603/gpl.96400.Search in Google Scholar PubMed
13. Toprak, V, Kafadar, MT. Intrahepatic cholestasis of pregnancy: is fetoplacental Doppler ultrasound useful in the diagnosis and follow-up? Ann Clin Anal Med 2021;12:87–91. https://doi.org/10.4328/ACAM.20203.Search in Google Scholar
14. Dicke, JM, Huettner, P, Yan, S, Odibo, A, Kraus, FT. Umbilical artery Doppler indices in small for gestational age fetuses: correlation with adverse outcomes and placental abnormalities. J Ultrasound Med 2009;28:1603–10. https://doi.org/10.7863/jum.2009.28.12.1603.Search in Google Scholar PubMed
15. Sotiriadis, A, Hernandez-Andrade, E, da Silva Costa, F, Ghi, T, Glanc, P, Khalil, A, et al.. ISUOG practice guidelines: role of ultrasound in screening for and follow-up of pre-eclampsia. Ultrasound Obstet Gynecol 2019;53:7–22. https://doi.org/10.1002/uog.20105.Search in Google Scholar PubMed
16. Huang, X, Gu, H, Shen, P, Zhang, X, Fei, A. Systematic review and meta-analysis: evaluating the influence of intrahepatic cholestasis of pregnancy on obstetric and neonatal outcomes. PLoS One 2024;19:e0304604. https://doi.org/10.1371/journal.pone.0304604.Search in Google Scholar PubMed PubMed Central
17. Odabaş, RK, Sökmen, Y, Dünder, E, Taşpınar, A. The incidence of intrahepatic cholestasis of pregnancy and its maternal, fetal, and neonatal adverse outcomes: a systematic review and meta-analysis. J Midwifery Womens Health 2024;69:370–82. https://doi.org/10.1111/jmwh.13640.Search in Google Scholar PubMed
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.

