Implementation of a universal low-dose aspirin protocol for the prevention of preeclampsia in a federally qualified health center
-
Jaclyn Del Pozzo
, Insaf Kouba
Abstract
Objectives
Low-dose aspirin (LDA) prophylaxis is an evidence-based intervention that reduces preeclampsia risk, yet adherence remains suboptimal in underserved populations due to inconsistent risk assessment and communication gaps. This study evaluated the impact of a universal LDA protocol at a Federally Qualified Health Center (FQHC) serving a socially vulnerable population.
Methods
Patients initiating prenatal care between 12 and 28 weeks of gestation were included. A retrospective cohort from 2021 (pre-intervention) was compared to a prospective cohort from 2022 (post-intervention). The intervention prescribed daily LDA to all eligible patients. Adherence was defined as provider-documented patient use during at least one follow-up visit and at delivery, an indirect measure relying on patient report and provider documentation. The primary outcome was LDA adherence. Secondary outcomes included preeclampsia with and without severe features and postpartum hemorrhage requiring blood transfusion. Multivariable logistic regression adjusted for maternal age, pregestational diabetes, and chronic hypertension.
Results
Among 775 patients, LDA adherence increased from 8.7 % pre-intervention to 75.0 % post-intervention (p<0.001). The incidence of preeclampsia with severe features decreased significantly (OR 0.14, 95 % CI 0.04–0.37). In high-risk patients, adherence rose from 8.9 to 70.9 % (p<0.001) with a similar reduction in severe preeclampsia (OR 0.16, 95 % CI 0.05–0.44). No significant changes were observed in preeclampsia without severe features or postpartum hemorrhage requiring transfusion.
Conclusions
Implementation of a universal LDA protocol in a high-risk, underserved population markedly improved adherence and reduced severe preeclampsia without increasing hemorrhage risk, offering a practical, low-cost strategy to improve maternal outcomes.
Introduction
Low-dose aspirin (LDA) prophylaxis during pregnancy is an evidence-based standard of care for reducing the risk of preeclampsia [1], 2]. This pregnancy-specific disorder is characterized by new-onset hypertension and proteinuria or new-onset hypertension plus significant end-organ dysfunction with or without proteinuria in a previously normotensive patient, typically after 20 weeks of gestation. All patients with one or more high-risk factors for preeclampsia should receive LDA during pregnancy, and it should also be considered for those with multiple moderate risk factors [1], [2], [3]. Lack of awareness about patient eligibility and inadequate communication about the benefits of LDA have resulted in low patient adherence [4], 5].
Implementing a policy of universal LDA prophylaxis during pregnancy could help overcome these barriers [6], especially in certain patient care settings. For instance, low socioeconomic status is a moderate risk factor for preeclampsia, and in clinical settings where all patients share this risk factor, implementing universal LDA prophylaxis may be particularly justified and valuable. However, some have expressed concern that LDA may be associated with bleeding complications in pregnancy [7].
Our objective was to evaluate the effectiveness of a quality improvement initiative to increase adherence to LDA during pregnancy by universally prescribing the medication to all pregnant patients at a Federally Qualified Health Center (FQHC). Specifically, we aimed to compare LDA adherence before and after the protocol’s implementation, with a particular focus on women at high risk for preeclampsia. Additionally, we evaluated the protocol’s impact on the rate of preeclampsia and postpartum hemorrhage within this population.
Materials and methods
Study population
All patients included for analysis received prenatal care at a FQHC, which serves an at-risk, underserved population. All included patients delivered at our institution. Inpatient and outpatient data were collected from January 2021 to July 2023. The pre-intervention group established prenatal care in 2021, and the post-intervention group established care in 2022. A washout period of 3 months (January-March 2022) was used to account for adjustment to the new protocol and to ensure that patients who established care in 2021 were no longer at an eligible gestational age (i.e., 12–28 weeks) to start LDA. Patients were excluded if they had a medical contraindication to LDA use, experienced a spontaneous abortion, initiated prenatal care after 28 weeks of gestation, transferred obstetrical care late in pregnancy, or delivered at an outside hospital. Baseline patient demographics and clinical characteristics were extracted from two medical record systems: eClinicalWorks (Westborough, MA) for outpatient data and Sunrise Clinical Manager (Allscripts Corp., Chicago, IL) for inpatient data.
Risk stratification
Patients were classified as high risk for preeclampsia if they met the American College of Obstetricians and Gynecologists (ACOG) criteria for recommending LDA prophylaxis. This classification requires the presence of either one or more high-risk factors or at least two moderate-risk factors [2]. High-risk factors include history of preeclampsia, multifetal gestation, chronic hypertension, type 1 or 2 diabetes, renal disease, and autoimmune disease (systemic lupus erythematosus, antiphospholipid syndrome). Moderate-risk factors include nulliparity, obesity, family history of preeclampsia, sociodemographic characteristics (Black race, low socioeconomic status), age 35 year or greater, and personal history factors (e.g. low birthweight or small for gestational age, previous adverse pregnancy outcome, more than 10-year pregnancy interval). It should be noted that all patients receiving prenatal care at our FQHC were automatically considered to have one moderate-risk factor: low socioeconomic status. Patients were classified as low risk for preeclampsia if their only documented risk factor was low socioeconomic status.
Intervention
A quality improvement initiative was co-developed by obstetrician-gynecologists at a FQHC and maternal-fetal medicine specialists at our institution with the goal of increasing prenatal use of LDA. The intervention included prescribing once daily LDA to all patients who attended at least one prenatal care visit between 12 and 28 weeks of gestational age. Adherence was defined as documentation of patient’s LDA use during at least one follow-up prenatal visit and at the time of delivery. This definition accounted for variations in gestational age at initiation, the number of subsequent prenatal visits, and the timing of delivery. This definition also represents an indirect method of adherence assessment, relying on provider documentation of patient-reported use during prenatal visits and at delivery hospitalization. No direct measures – such as pill counts, pharmacy refill data, or electronic monitoring – were employed, given the resource limitations of the FQHC setting. The initiative also aimed to improve awareness and understanding through healthcare professional and patient education, distribution of informational materials, and updates to clinical protocols and workflows (see supplementary materials). These updates were designed to streamline prescribing, enhance documentation accuracy, and ensure seamless electronic transmission of LDA prescriptions to pharmacies, ultimately promoting adherence and reducing the risk of preeclampsia. Information materials were available in English and Spanish.
Outcomes
The primary outcome was LDA adherence, a binary variable (yes/no), defined above. Secondary outcomes included preeclampsia with and without severe features, and postpartum hemorrhage requiring blood transfusion. These outcomes were evaluated for the overall cohort and for the high-risk subpopulation at increased risk of developing preeclampsia.
Statistical analysis
Descriptive statistics were used to characterize the study population, with categorical variables presented as frequencies and percentages and continuous variables as means with standard deviations. Categorical data was analyzed using the Fisher’s exact test, while continuous data were compared using the Mann-Whitney or t-test, based on data distribution. For the high-risk and low-risk cohorts, univariate logistic regression was performed to evaluate the relationship between the universal LDA protocol and the primary and secondary outcomes. Odds ratios (ORs) and 95 % confidence intervals (CIs) were estimated to quantify the strength and direction of associations. For the overall cohort, multivariable logistic regression was performed to evaluate the relationship between the universal LDA protocol and the primary and secondary outcomes, adjusting for advanced maternal age, pregestational diabetes, and chronic hypertension. Statistical significance was defined as p<0.05. All analyses were performed in R version 4.3.1.
The Institutional Review Board determined that this study was exempt from formal review because it was designed to evaluate a local clinical protocol designed to meet a recognized, evidence-based standard of care for the purposes of quality improvement; Human Subjects determination request number is HSRD22-0009-SH). This study followed the SQUIRE 2.0 guideline for reporting on quality improvement initiatives [8].
Results
Out of 1,227 new obstetric visits at the FQHC during the study period, 155 occurred during the 3-month washout period, and an additional 297 met other exclusion criteria. A total of 775 patients were included for analysis: 367 (47.4 %) in the pre-intervention group and 408 (52.6 %) in the post-intervention group. Hispanic patients constituted the largest race and ethnicity group (89.8 %). Most patients were Spanish speaking (84.3 %), multiparous (60.1 %), and younger than 35 years of age (62.5 %). Baseline characteristics of the study population are presented in Table 1.
Baseline characteristics.
| Characteristics | Pre-intervention (n=367) | Post-intervention (n=408) | p-Value |
|---|---|---|---|
| Maternal age, years | 30.6 (±6.5) | 29.4 (±6.6) | 0.02 |
| ≥35 | 112 (30.5) | 179 (43.9) | <0.001 |
| Race and ethnicity | |||
| Non-hispanic white | 3 (0.8) | 9 (2.2) | 0.04 |
| Non- hispanic black | 12 (3.3) | 15 (3.7) | |
| Hispanic | 332 (90.5) | 364 (89.2) | |
| Asian or Pacific islander | 2 (0.5) | 0 (0) | |
| American Indian or Alaska native | 15 (4.1) | 12 (2.9) | |
| Other or multiracial | 0 (0) | 7 (1.7) | |
| Declined or unknown | 3 (0.8) | 1 (0.2) | |
| Preferred language | |||
| English | 56 (15.3) | 57 (14.0) | 0.29 |
| Spanish | 309 (84.2) | 344 (84.3) | |
| Other | 2 (0.6) | 7 (1.6) | |
| Parity | |||
| Nulliparous | 114 (31.1) | 195 (47.8) | <0.001 |
| Multiparous | 253 (68.9) | 213 (52.2) | |
| Maternal comorbidities | |||
| History of preeclampsia | 21 (5.7) | 39 (9.6) | 0.05 |
| Chronic hypertension | 12 (3.3) | 12 (2.9) | 0.80 |
| Pregestational diabetes | 6 (1.6) | 10 (2.5) | 0.59 |
| Gestational diabetes | 27 (7.4) | 38 (9.3) | 0.32 |
| Autoimmune disorders | 11 (3.0) | 1 (0.2) | 0.002 |
| Multiple gestation | 3 (0.8) | 5 (1.2) | 0.60 |
| ACOG preeclampsia risk assessment | |||
| High (1 or more high risk factors)a | 80 (21.8) | 105 (25.7) | 0.01 |
| Moderate (2 or more moderate risk factors)b | 145 (39.5) | 184 (45.1) | |
| Low | 142 (38.7) | 119 (29.2) | |
| Gestational age at delivery, weeks | 39 (38.1–39.5) | 39 (38.0–39.5) | 0.74 |
-
ACOG, American College of Obstetricians and Gynecologists. Data are n (%), mean (± standard deviation), and median (interquartile range). aHistory of preeclampsia, multifetal gestation, chronic hypertension, type 1 or 2 diabetes, renal disease, autoimmune disease (systematic lupus erythematosus, antiphospholipid syndrome). bNulliparity, obesity, family history of preeclampsia (mother or sister), sociodemographic characteristics (African American race, low socioeconomic status), age 35 or older, personal history factors (e.g. low birthweight or small for gestational age, previous adverse pregnancy outcome, more than 10-year pregnancy interval).
Overall cohort
In the overall study population, the rate of LDA use increased significantly from 8.7 % (32/367) in the pre-intervention group to 75.0 % (306/408) in the post-intervention group (p<0.001; Table 2). Patients in the post-intervention group were significantly less likely to develop preeclampsia with severe features compared to those in the pre-intervention group (OR 0.14, 95 % CI 0.04–0.37). No change in the overall rate of preeclampsia or postpartum hemorrhage requiring blood transfusion was observed after protocol implementation.
Regression analysis of maternal outcomes.
| Outcome | Pre-intervention | Post-intervention | OR (95 % CI) | p-Value |
|---|---|---|---|---|
| High-risk cohorta | n=225 | n=289 | ||
| LDA adherence | 20 (8.9) | 205 (70.9) | 25.0 (15.1–43.4) | <0.001 |
| Preeclampsia with and without severe features | 30 (13.3) | 34 (11.8) | 0.87 (0.51–1.47) | 0.59 |
| Preeclampsia with severe features | 18 (8.0) | 4 (1.4) | 0.16 (0.05–0.44) | 0.001 |
| Postpartum hemorrhage requiring blood transfusion | 11 (4.9) | 14 (4.8) | 0.99 (0.44–2.28) | 0.99 |
|
|
||||
| Overall cohort b | n=367 | n=408 | ||
|
|
||||
| LDA adherence | 32 (8.7) | 306 (75.0) | 32.4 (21.3–50.8) | <0.001 |
| Preeclampsia with and without severe features | 38 (10.4) | 43 (10.5) | 0.98 (0.61–1.59) | 0.93 |
| Preeclampsia with severe features | 23 (6.3) | 4 (1.0) | 0.14 (0.04–0.37) | <0.001 |
| Postpartum hemorrhage requiring blood transfusion | 19 (5.2) | 20 (4.9) | 1.00 (0.52–1.93) | 0.99 |
-
LDA, low-dose aspirin. aIncludes patients at moderate or high risk for preeclampsia per American College of Obstetricians and Gynecologists (ACOG) preeclampsia risk assessment. bIncludes all study patients; regression models adjusted for advanced maternal age, pregestational diabetes, and chronic hypertension.
High-risk cohort
Among patients at moderate-to-high risk for preeclampsia (per ACOG), adherence to LDA increased significantly from 8.9 % (n=20/225) in the pre-intervention group to 70.9 % (n=205/289) in the post-intervention group (p<0.001; Table 2, Figure 1). After the intervention, high-risk patients had significantly lower odds of developing preeclampsia with severe features compared to before the intervention (OR 0.16, 95 % CI 0.05–0.44). There was no change in the overall rate of preeclampsia or postpartum hemorrhage requiring blood transfusion after protocol implementation.
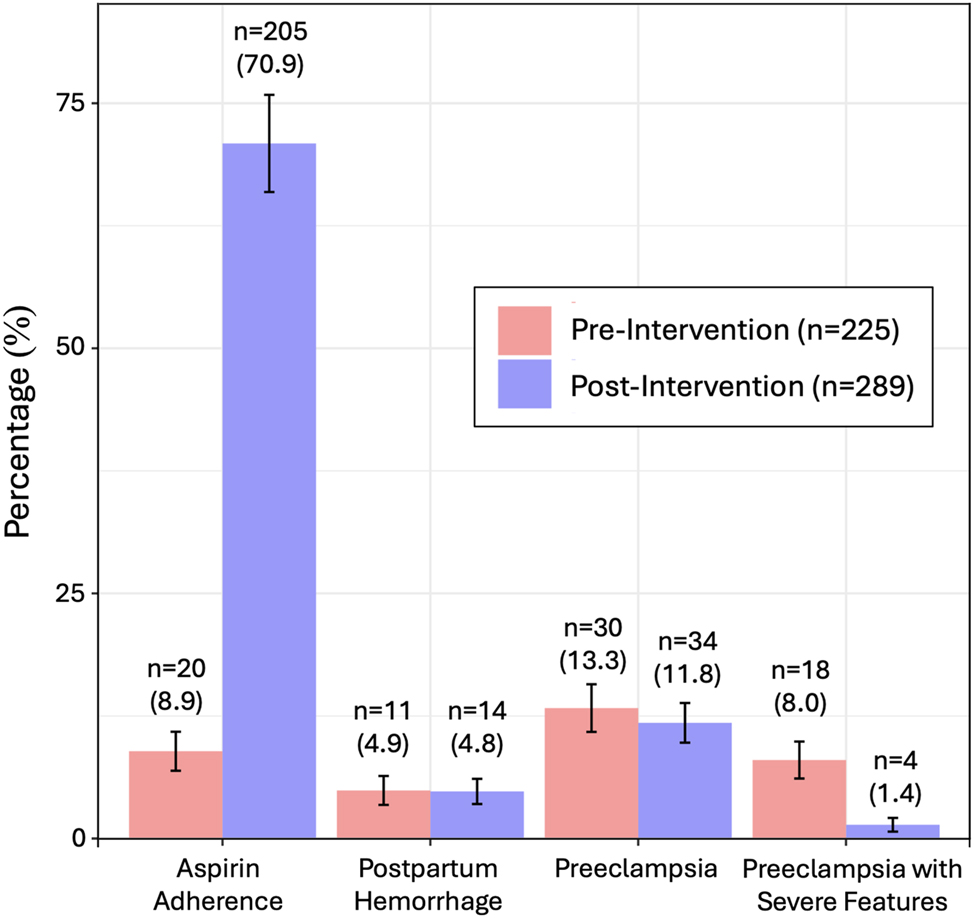
Adherence to low dose aspirin among patients at moderate-to-high risk for preeclampsia pre- and post- intervention.
Low-risk cohort
Among patients at low risk for preeclampsia, use of LDA increased from 8.5 % (n=12/142) in the pre-intervention group to 84.9 % (n=101/119) in the post-intervention group (p<0.001; Table 2). There was no change in the overall rate of preeclampsia with or without severe features or postpartum hemorrhage requiring blood transfusion after protocol implementation.
Discussion
Principal findings
Among pregnant patients receiving prenatal care at the FQHC, implementation of a universal LDA protocol was associated with increased medication adherence and a reduced rate of preeclampsia with severe features. There was no change in the rate of preeclampsia without severe features or the frequency of postpartum hemorrhage.
Results in the context of what is known
The findings of this study support the notion that universal LDA prescribing can improve medication adherence and maternal outcomes compared to targeted prescribing, especially in a high-risk, socially vulnerable population. By eliminating the process of risk stratification, which often leads to misclassification or inconsistent provider practices, an emphasis can be placed on consistent implementation [6], 9]. Universal administration via an opt-out approach enhances adherence more effectively than opt-in strategies, potentially due to greater provider-patient engagement and reduced decision-making barriers [6]. This study adds to the literature by focusing on a socially vulnerable population, demonstrating that universal LDA prescribing can mitigate health disparities in maternal outcomes. Moreover, it is reassuring that we did not observe any change in the rate of clinically meaningful postpartum hemorrhage, since some previous studies have reported an increased risk for this complication associated with prenatal LDA use [7], 10], 11]. While prior studies have identified LDA as a cost-effective and safe intervention for preeclampsia prevention, our findings further endorse its application as a universal protocol in underserved populations [9]. Our findings align with prior studies showing that LDA significantly reduces severe preeclampsia but has less consistent impact on mild disease, likely reflecting differences in pathophysiology and effects on placentation between these forms [12]. The preventive benefit appears greatest for preterm and severe preeclampsia – particularly when initiated at or before 16 weeks’ gestation – with more variable effects on term or mild disease [13].
Clinical implications
The findings of this study suggest that universal LDA protocols may be beneficial for socially vulnerable populations. Universal LDA prescribing was associated with a significant increase in adherence rates and a reduction in severe preeclampsia, suggesting a practical strategy to improve maternal outcomes in high-risk populations. Any effort toward adopting such a protocol should incorporate patient and provider education and workflow optimization to ensure consistent medication use. These approaches can help address health disparities, improve pregnancy outcomes, and support evidence-based care in underserved communities.
Research implications
Unanswered questions remain about the generalizability of our findings to other populations, the cost-effectiveness of universal protocols compared to targeted approaches, and the scalability of implementation across diverse healthcare systems. Additionally, further research is needed to explore the role of targeted patient education and its impact on adherence. Efforts should also focus on understanding why over one-quarter of high-risk patients did not adhere to LDA use despite a universal prescribing protocol. Future research should focus on validating these findings in other clinical settings, investigating cost-effectiveness and safety, and developing best practices for integrating universal LDA protocols into prenatal care. Further refinement of patient education strategies may help to maximize effectiveness.
Limitations
This study has several limitations. Its single-site design may limit generalizability, and reliance on retrospective pre-intervention data could introduce bias from incomplete documentation or missing information. Although the intervention was well defined and implemented in an underserved population at high risk for preeclampsia, the fact that all participants had at least one moderate-risk factor (low socioeconomic status) limits the applicability of our findings to populations with different baseline risk profiles. The three-month washout period might not fully eliminate overlap between pre- and post-intervention cohorts. However, combining retrospective and prospective data enabled pre- and post-intervention comparisons, while a washout period and strict exclusion criteria ensured clear cohort distinctions. Adherence was measured using an indirect method based on provider-documented patient self-report during prenatal visits and at delivery, which may be subject to recall bias, social desirability bias, and inconsistent documentation practices. The absence of direct measures such as pill counts, pharmacy refill records, or electronic medication monitoring limits the ability to confirm actual ingestion and may result in overestimation of adherence rates. Future studies should incorporate both direct and indirect measures to provide a more accurate assessment. Secondary outcomes were narrowly focused, potentially omitting other risks of LDA. Limited healthcare access among the FQHC population led to significant variation in the number of prenatal visits, potentially affecting adherence and outcome assessment. Furthermore, chart review provided confirmation of LDA prescription, not necessarily initiation of treatment. Multivariable logistic regression improved validity by accounting for population changes over time and differences between pre- and post-intervention groups, while adherence to SQUIRE 2.0 guidelines ensured methodological rigor [8]. While adjustments were made for some confounders, other influential factors were not addressed, and the exclusion of patients transferring care or delivering outside the system may limit completeness. Finally, the universal LDA protocol raises questions about cost-effectiveness and resource optimization compared to risk-based approaches, which warrant further exploration.
Conclusions
Implementation of a universal LDA protocol in a socially vulnerable population improved medication adherence and reduced the incidence of preeclampsia with severe features. Universal prescribing eliminates the need for individualized risk stratification and addresses challenges such as provider inconsistency, patient misclassification, and communication gaps about eligibility. Given its low-cost, wide availability, and favorable safety profile, universal prescribing of LDA is a practical strategy to mitigate disparities in pregnancy outcomes.
Acknowledgments
Preliminary research findings were presented at the Society for Maternal-Fetal Medicine 44th Annual Pregnancy Meeting in National Harbor, MD in February 2024.
-
Research ethics: The local Institutional Review Board deemed the study exempt from review.
-
Informed consent: Not applicable.
-
Author contributions: All authors have accepted responsibility for the entire content of this manuscript and approved its submission.
-
Use of Large Language Models, AI and Machine Learning Tools: None declared.
-
Conflict of interest: The authors state no conflict of interest.
-
Research funding: None declared.
-
Data availability: Not applicable.
References
1. LeFevre, ML, U.S. Preventi, ve Services Task Force. Low-dose aspirin use for the prevention of morbidity and mortality from preeclampsia: U.S. preventive services task force recommendation statement. Ann Intern Med 2014;161:819–26. https://doi.org/10.7326/M14-1884.Search in Google Scholar PubMed
2. ACOG committee opinion no. 743: low-dose aspirin use during pregnancy. Obstet Gynecol 2018;132:e44–52. https://doi.org/10.1097/AOG.0000000000002708.Search in Google Scholar PubMed
3. Caritis, S, Sibai, B, Hauth, J, Lindheimer, M, Klebanoff, M, Thom, E, et al.. Low-dose aspirin to prevent preeclampsia in women at high risk. N Engl J Med 1998;338:701–5. https://doi.org/10.1056/NEJM199803123381101.Search in Google Scholar PubMed
4. Shanmugalingam, R, Mengesha, Z, Notaras, S, Liamputtony, P, Fulcher, I, Gaksoo, L, et al.. Factors that influence adherence to aspirin therapy in the prevention of preeclampsia amongst high-risk pregnant women: a mixed method analysis. PLoS One 2020;15:e0229622. https://doi.org/10.1371/journal.pone.0229622.Search in Google Scholar PubMed PubMed Central
5. Martinez-King, LC, Machiorlatti, M, Ogburn, T, Salcedo, J. Physician’s knowledge and practices surrounding low-dose aspirin for preeclampsia risk reduction. Am J Perinatol 2024;41:e1120–5. https://doi.org/10.1055/a-1990-2728.Search in Google Scholar PubMed
6. Lewkowitz, AK, Rouse, DJ. Miscommunication about low-dose aspirin for preeclampsia prevention – further support for universal prophylaxis. JAMA Netw Open 2021;4:e2130960. https://doi.org/10.1001/jamanetworkopen.2021.30960.Search in Google Scholar PubMed
7. White, KJ, Son, M, Lundsberg, LS, Culhane, J, Partridge, C, Reddy, U, et al.. Low-dose aspirin during pregnancy and postpartum bleeding. Am J Perinatol 2023;40:1390–7. https://doi.org/10.1055/a-2096-5199.Search in Google Scholar PubMed
8. Ogrinc, G, Davies, L, Goodman, D, Batalden, PB, Davidoff, F, Stevens, D. SQUIRE 2.0 (standards for QUality improvement reporting excellence): revised publication guidelines from a detailed consensus process. BMJ Qual Saf 2016;25:986–92. https://doi.org/10.1136/bmjqs-2015-004411.Search in Google Scholar PubMed PubMed Central
9. Mone, F, Mulcahy, C, McParland, P, McAuliffe, FM. Should we recommend universal aspirin for all pregnant women? Am J Obstet Gynecol 2017;216:141.e1–5. https://doi.org/10.1016/j.ajog.2016.09.086.Search in Google Scholar PubMed
10. Souter, V, Painter, I, Sitcov, K, Khalil, A. Propensity score analysis of low-dose aspirin and bleeding complications in pregnancy. Ultrasound Obstet Gynecol 2024;63:81–7. https://doi.org/10.1002/uog.27472.Search in Google Scholar PubMed
11. Jiang, Y, Chen, Z, Chen, Y, Wei, L, Peng, G, Zhang, J, et al.. Low-dose aspirin use during pregnancy may be a potential risk for postpartum hemorrhage and increased blood loss: a systematic review and meta-analysis. Am J Obstet Gynecol MFM 2023;5:100878. https://doi.org/10.1016/j.ajogmf.2023.100878.Search in Google Scholar PubMed
12. Roberge, S, Giguère, Y, Villa, P, Nicolaides, K, Vainio, M, Forest, JC, et al.. Early administration of low-dose aspirin for the prevention of severe and mild preeclampsia: a systematic review and meta-analysis. Am J Perinatol 2012;29:551–6. https://doi.org/10.1055/s-0032-1310527. Erratum in: Am J Perinatol. 2014;31(6):e3.Search in Google Scholar PubMed
13. Roberge, S, Bujold, E, Nicolaides, KH. Aspirin for the prevention of preterm and term preeclampsia: systematic review and metaanalysis. Am J Obstet Gynecol 2018;218:287–93.e1. https://doi.org/10.1016/j.ajog.2017.11.561.Search in Google Scholar PubMed
Supplementary Material
This article contains supplementary material (https://doi.org/10.1515/jpm-2025-0215).
© 2025 the author(s), published by De Gruyter, Berlin/Boston
This work is licensed under the Creative Commons Attribution 4.0 International License.

